Abstract
Extraction temperature can potentially affect the chemical compositions and bioactivities of the extracts obtained. The objective of this study was to investigate the effect of extraction temperature on the distribution of bioactive compounds and the bioactivities of Pleurotus citrinopileatus. The antioxidant activities (2,2-diphenyl-1-picrylhydrazyl and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)+ scavenging capabilities) and the inhibitory capabilities on pancreatic α-amylase, intestinal α-glucosidase, and hypertension-linked angiotensin-converting enzyme of hot water P. citrinopileatus extract and cold water P. citrinopileatus extract were determined. The results showed that the antioxidant capabilities and inhibitory effects on α-amylase, α-glucosidase, and angiotensin-converting enzyme of cold water P. citrinopileatus extract were significantly higher than those of hot water P. citrinopileatus extract. The cold water P. citrinopileatus extracted was further precipitated with 100% ammonium sulfate to obtain a polysaccharide fraction or with 75% ethanol to obtain a protein fraction. The inhibitory activities of the protein fraction of the cold water P. citrinopileatus extract on α-amylase, α-glucosidase, and angiotensin-converting enzyme were significantly higher than those of the polysaccharide fraction. In conclusion, the protein fraction of the cold water P. citrinopileatus extract could be responsible for its bioactivities.
Keywords: α-amylases, α-glucosidases, angiotensin-converting enzyme, Pleurotus citrinopileatus
1. Introduction
Hyperglycemia and hypertension are major health problems that can lead to serious conditions, such as diabetes and cardiovascular diseases [1]. By suppressing the activities of carbohydrate digestion-related enzymes, such as α-amylase and α-glucosidase, and angiotensin-converting enzyme (ACE) may improve the control of hyperglycemia and hypertension. A number of bioactive components isolated from maitake, berries, herbs, and edible mushrooms have been shown to exert potential inhibiting activities on the above mentioned enzymes and may serve as complementary treatments for hyperglycemia and hypertension [2–6].
Pleurotus citrinopileatus, a cultivated edible mushroom, has been shown to exert several beneficial physiological activities, such as immunomodulatory, antitumor, antihyperglycemic, antioxidant, and antihyperlipidemic effects [7–11]. Processing temperature can affect the chemical compositions and bio-activities of extracts obtained. For example, more phenolic compounds could be obtained from white tea when extracted under cold temperature [12]. The high temperature can destroy the flavonoids of onions [13]. A high temperature treatment can decrease the antibacterial activity of Hypsizigus marmoreus from 100% to 60% [14] and can reduce the capacity of Tricholoma giganteum on ACE inhibition [15]. Collectively, extraction temperature appears to be an important factor affecting the efficacy of extraction. Nevertheless, the effects of extraction temperature on the contents of bioactive components and their bioactivities of P. citrinopileatus extracts have not been investigated. Thus, the objective of this study was to evaluate the effects of extraction temperature on the inhibitory capability of P. citrinopileatus extracts against glycemic and hypertensive enzymes.
2. Methods
2.1. Materials
Protein assay dye reagent concentrate was obtained from Bio-Rad (Hercules, CA, USA). Bovine serum albumin, 2,2′-diphenyl-1-picrylhydrazyl (DPPH), gallic acid, 2(3)-t-butyl-4-hydroxyanisole (BHA), 3,5-dinitrosalicylic acid, α-glucosidase, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), p-nitrophenyl-α-D-glucopyranoside, ACE, N-hippuryl-histidylleucine, and sodium tetraborate decahydrate were obtained from Sigma-Aldrich (St. Louis, MO, USA). α-Amylase was purchased from Sigma-Aldrich (Buchs, Switzerland). Trifluoroacetic acid (TFA) was obtained from Merck Co. (Darmstadt, Germany). Acarbose was purchased from Hisum Pharmaceutical Co., Ltd. (Jiaojiang, Taizhou, China) and lisinopril was obtained from Hetero Drugs Ltd. (Hyderabad, India).
2.2. Preparation of cold and hot water extracts
Fresh fruiting bodies of P. citrinopileatus (Rich Year Farm, Puli, Taiwan) were washed with tap water, rinsed with distilled water, drained, and chopped. Chopped P. citrinopileatus was blended with three volumes of deionized water using a homogenizer (6641, Osterizer, Shelton, CT, USA) for 1 minute. For cold water extraction, a portion of homogenized mixture was agitated in a reciprocal shaking water bath (DKW-20, Deng Yng, Taipei, Taiwan) for 24 hours in a refrigerator (4°C), and centrifuged (10,400g; CT15RT centrifuge, Pantech Instrument, Taipei, Taiwan) at 4°C for 30 minutes to obtain the supernatant (PCC-24H). For hot water extraction, a portion of homogenized mixture was heated to keep boiling for either 1 hour or 2 hours, cooled to room temperature, and centrifuged (10,400g; CT15RT centrifuge, Pantech Instrument) at 4°C for 30 minutes to obtain the supernatant (PCH-1H and PCH-2H, respectively). The supernatants were freeze-dried and stored at −20°C until use.
2.3. Component analysis
Protein contents were measured according to the method of Bradford [16] using the Bio-Rad protein assay dye reagent diluted with five volumes of deionized water. The absorbances at 595 nm were measured after 10-μL samples (1 mg/mL) and 200-μL diluted Bio-Rad protein assay dye reagent were thoroughly mixed and kept for 10 minutes. Bovine serum albumin (0–500 μg/mL) was used as the standard.
The total polysaccharide contents were determined following the method of Chaplin and Kennedy [17]. A 200-μL sample, 1-mL concentrated sulfuric acid, and 200-μL phenol (5%) were mixed and kept at room temperature for 10 minutes. The absorbance of the samples at 490 nm was measured after keeping at room temperature for an additional 30 minutes. Glucose (0–100 μg/mL) was used as the standard to establish a calibration curve.
The concentration of total phenolic compounds was determined spectrophotometrically using Folin–Ciocalteu’s reagent and the method of Quettier-Deleu et al [18]. The absorbance of the samples at 760 nm was determined after mixing 0.2-mL sample, 1-mL Folin–Ciocalteu’s phenol reagent, 0.8-mL sodium carbonate (7.5%), and incubated for 30 minutes. Gallic acid (0–100 μg/mL) was used as the standard for a calibration curve and the results were expressed as μg of gallic acid equivalents per mg of sample (μg GAE/mg).
2.4. Evaluation of antioxidant characteristics
2.4.1. Scavenging capability of DPPH free radical
The scavenging capability of DPPH free radicals was determined following the method of Shimada et al [19]. One milliliter samples and 5-mL DPPH solution (0.1mM) were vigorously mixed and incubated at room temperature for 50 minutes. The absorbance of the samples at 517 nm was measured. BHA and Trolox were used as standards. The percentage of DPPH scavenging capability was calculated according to the equation:
| (1) |
2.4.2. Determination of Trolox equivalent antioxidant capacity
The Trolox equivalent antioxidant capacity (TEAC) of the samples was assayed using the method of Arnao et al [20] and Miller and Rice-Evans [21]. A mixture of 1.5-mL deionized water, 0.25-mL peroxidase (44 units/mL), 0.25-mL H2O2 (0.5mM), and 0.25-mL ABTS (1mM) were vortexed and set in dark for 1 hour until a stable and blue–green colored ABTS+ radical cation was formed. The absorbance of the samples at 734 nm was determined after mixing with 0.2-mL samples. A standard calibration curve was constructed with Trolox.
2.5. Assays of enzyme inhibition
2.5.1. Inhibition of α-amylase
Inhibition of α-amylase was determined according to the method described by Pinto et al [5] with modifications. A mixture of 0.2-mL sample (dissolved in 0.02M sodium phosphate buffer, pH 6.9) and 0.2-mL α-amylase (10 U/mL in the same buffer) was kept at 37°C with occasionally shaking for 45 minutes. Then, 0.4-mL starch (0.5%) was added and incubated at 37°C for 5 minutes, before the addition of 2-mL 3,5-dinitrosalicylic acid (1%) to terminate the reaction. The absorbance of the samples at 540 nm was read after the samples were heated in boiling water for 10 minutes, cooled to room temperature, and mixed with 3-mL buffer. The buffer without sample was used as a blank. The percentage of α-amylase inhibition was calculated according to the equation:
| (2) |
where ACα and ASα were the absorbances of buffer or sample reacted with α-amylase, respectively; AC and AS were the absorbances of buffer or sample reacted without α-amylase, respectively.
2.5.2. Inhibition of α-glucosidase
Inhibition of α-glucosidase was determined according to the method described by Pinto et al [5] with modifications. The reaction was carried out in 96-well microplates. To each well, a 50-μL sample and 50-μL buffer (with or without 0.13-U/mL α-glucosidase) were thoroughly mixed and incubated at 37°C for 30 minutes. After the addition of 50-μL p-nitrophenyl-α-D-glucopyranoside (3mM), the mixture was incubated at 37°C for another 30 minutes. The absorbance at 405 nm was read with an enzyme-linked immunosorbent assay reader (MQX200, uQuant, Bio-Tek Instruments Inc., Winooski, VT, USA). The percentage of α-glucosidase inhibition was calculated according to the equation:
| (3) |
where ACα and ASα were the absorbances of buffer (control) or sample reacted with α-glucosidase, respectively; AC and AS were the absorbances of buffer or sample reacted without α-glucosidase, respectively.
2.5.3. Inhibition of ACE
Inhibition of ACE was determined according to the method described by Cushman and Cheung [22] with modifications. After incubating 45-μL Hip-His-Leu (3mM in pH 8.3 sodium borate buffer) and 10-μL sample or lisinopril (used as the standard) at 37°C for 10 minutes, 45-μL ACE (33 mU/mL in pH 8.3 sodium borate buffer) was added to react for 30 minutes, and then 150-μL TFA (0.1%) was added to terminate the reaction. While Hip-His-Leu was converted into hippuric acid (HA) and histidylleucine by ACE, HA concentration was determined using a high performance liquid chromatographer (L-6000, Hitachi, Tokyo, Japan) equipped with a Lichrospher 100 RP-18 column (Merck, Darmstadt, Germany). The methanol containing 0.1% TFA as an eluting solvent was at a flow rate of 0.6 mL/min and HA was detected at 228 nm. All of these determinations were repeated three times.
ACE inhibition was calculated according to the following equation:
| (4) |
where AC and AS were the peak areas of buffer (control) or sample, respectively.
2.6. Preparation of polysaccharide-rich and protein-rich fractions and bioactivity assays
To investigate which the principal components (polysaccharides or proteins) were responsible for the bioactivities of PCC-24H, the polysaccharide-rich and protein-rich fractions (PCC-Ps and PCC-Pr, respectively) were prepared from the PCC-24H. PCC-Ps was obtained by mixing PCC-24H with 75% ethanol and removing the supernatant. PCC-Pr was prepared by mixing PCC-24H with saturated ammonium sulfate and discarding the supernatant. The precipitates were freeze-dried and stored at −20°C until analysis. The analyses of protein and polysaccharide contents, antioxidant activities, as well as the enzyme inhibitions were conducted according to the methods described in the previous sections.
2.7. Statistical analysis
Data were expressed as mean ± standard deviation from triplicate analysis. One-way analysis of variance (ANOVA) and Duncan’s new multiple range test were used to compare the means of antioxidant activities and enzyme inhibitory capabilities among PCC-24H, PCH-1H, and PCH-2H samples. Student t test was used to compare the differences between PCC-Pr and PCC-Ps fractions. All statistical analyses were performed with the SPSS for Windows, version 12.0 (SPSS Inc., Chicago, IL, USA). A p value < 0.05 was considered statistically significant.
3. Results and discussion
3.1. Effects of extraction temperature on yield and bio-compound contents
Previous studies have demonstrated that extraction temperature could lead to varying degrees of physiological activities of extracted compounds [23]. For example, cold extracts of Lignosus rhinocerotis exhibited distinctively cellular toxicity due to the cytotoxic components being only found in the cold extracts [24]. The high temperature could decompose or convert phenolic compounds of white tea [12]. Compared with hot water extraction, cold water extraction was more effective in controlling the growth of fungal pathogens of Hibiscus sabdariffa [25]. In the present study, the yields and component analysis of cold and hot water extracts from P. citrinopileatus are listed in Table 1. The yields of PCC-24H, PCH-1H, and PCH-2H were 1.89%, 1.12%, and 1.19%, respectively. A higher yield could be obtained from the cold water extraction. Moreover, a longer extraction time of 2 hours, compared with 1 hour, in hot water did not increase the extraction yield. Extract temperature was also able to affect the extraction efficiency of protein, polysaccharide, and total phenolics. The contents of protein, polysaccharide, and total phenolics of hot water extracts were only 52–75% of those obtained in the cold water extract.
Table 1.
Yield and component contents of extracts and precipitates of cold and hot water extracts from Pleurotus citrinopileatus.
| Sample | Yield (%) | Protein content (μg/mg) | Polysaccharide content (μg/mg) | Total phenolics content (μg GAE/mg) |
|---|---|---|---|---|
| Cold water extracts | ||||
| PCC-24H | 1.89 | 75.4 ± 1.5 | 238.6 ± 1.9 | 27.5 ± 0.3 |
| Hot water extracts | ||||
| PCH-1H | 1.12 | 42.3 ± 1.1 | 176.1 ± 1.3 | 14.2 ± 0.3 |
| PCH-2H | 1.19 | 49.3 ± 0.7 | 179.2 ± 1.0 | 16.4 ± 0.5 |
| 100% Ammonium sulfate precipitates | ||||
| PCC-Pr | 0.13 | 271.7 ± 1.7 | 142.8 ± 1.7 | 25.1 ± 0.3 |
| 75% Ethanol precipitates | ||||
| PCC-Ps | 0.21 | 38.4 ± 0.7 | 539.8 ± 2.3 | 5.0 ± 0.1 |
GAE = gallic acid equivalent.
3.2. Effects of extraction temperature on antioxidant activities
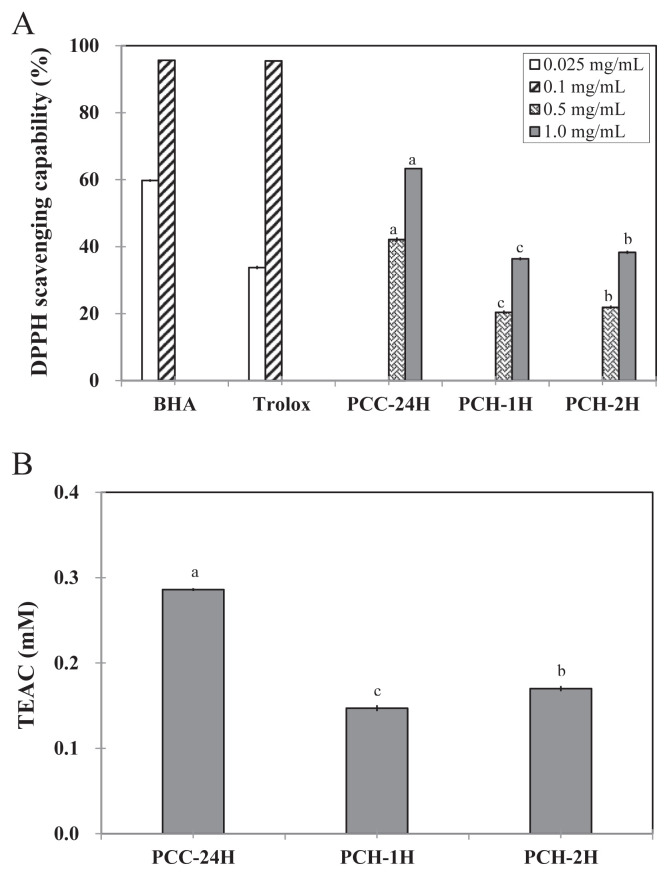
With regard to the DPPH scavenging capabilities, PCC-24H exerted a stronger capability than those of PCH-1H and PCH-2H at either 0.5 mg/mL or 1.0 mg/mL (Figure 1A). However, the DPPH scavenging capability of PCC-24H was significantly lower (p < 0.05) than those of BHA and Trolox at a concentration of 0.1 mg/mL. While the IC50 values for DPPH scavenging capabilities of BHA and Trolox were 0.032 mg/mL and 0.02 mg/mL, respectively, those of PCC-24H, PCH-1H, and PCH-2H were 0.6 mg/mL, 2.1 mg/mL, and 1.8 mg/mL, respectively (Table 2). Similar results were found in the TEAC assay. The TEAC values of PCC-24H, PCH-1H, and PCH-2H at 500 μg/mL were 0.29mM, 0.15mM, and 0.17mM, respectively (Figure 1B).
Figure 1.
(A) 2,2-Diphenyl-1-picrylhydrazyl (DPPH) scavenging activity; (B) Trolox equivalent antioxidant capacity (TEAC) of PCC-24H, PCH-1H, and PCH-2H. Data with different letters were significantly different among extractions at same concentrations using Duncan’s new multiple range test (p < 0.05). BHA = 2(3)-t-butyl-4-hydroxyanisole.
Table 2.
The IC50 values for bioactivities, including 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging capability, inhibitory activities on α-amylase, α-glucosidase, and angiotensin-converting enzyme of extracts and fractions from Pleurotus citrinopileatus.
| Sample | IC50a (mg/mL) | |||
|---|---|---|---|---|
|
| ||||
| DPPH | α-Amylase | α-Glucosidase | ACE | |
| Positive control | ||||
| BHA | 3.2 × 10−2 | ND | ND | ND |
| Trolox | 4.0 × 10−2 | ND | ND | ND |
| Acarbose | ND | 0.5 | 1.7 | ND |
| Lisinopril | ND | ND | ND | 8.4 × 10−6 |
| Cold water extracts | ||||
| PCC-24H | 0.6 | 7.1 | 7.6 | 4.6 |
| Hot water extracts | ||||
| PCH-1H | 2.1 | NIb | 27.1 | 9.6 |
| PCH-2H | 1.8 | 61.7 | 27.7 | 7.2 |
| 100% Ammonium sulfate precipitates | ||||
| PCC-Pr | 1.3 | 2.5 | 0.8 | 2.0 |
| 75% Ethanol precipitates | ||||
| PCC-Ps | 6.4 | 4.4 | 33.6 | 8.8 |
ACE = angiotensin-converting enzyme; BHA = gallic acid; ND = not determined; NI = no inhibition.
IC50 value is defined as the inhibitor concentration to inhibit 50% of activity.
No inhibition, less than 10% inhibition at the concentration of 10 mg/mL.
3.3. Effects of extraction temperature on enzyme activities
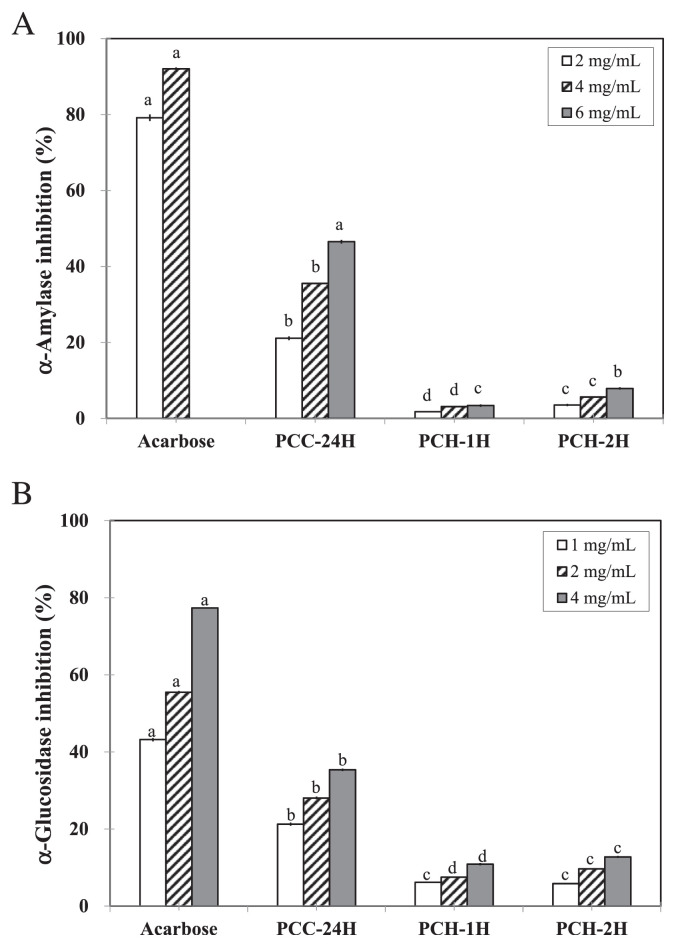
Pancreatic α-amylase and intestinal α-glucosidase are two critical enzymes for the breakdown of complex carbohydrates into simple sugars. Both the cold water extract and the two hot water extracts were able to significantly inhibit the activities of α-amylase (Figure 2A) and α-glucosidase (Figure 2B).
Figure 2.
(A) α-Amylase inhibitory activity; (B) α-glucosidase inhibitory activity of PCC-24H, PCH-1H, and PCH-2H. Data with different letters were significantly different among extractions at same concentrations using Duncan’s new multiple range test (p < 0.05).
By preventing the hydrolysis of starch into di- and monosaccharides, the extracts might be able to suppress postprandial hyperglycemia. While the IC50 value of acarbose (positive control) on α-amylase inhibition was 0.5 mg/mL, the IC50 value of PCC-24H was 7.1 mg/mL (Table 2). The inhibition of PCH-1H and PCH-2H on α-amylase was below 10% even at the concentration of 6 mg/mL (Figure 2B) and this result indicated that extracts from hot extraction was unable to inhibit the activity of α-amylase.
The inhibitory capability of PCC-24H on α-glucosidase appeared to be dose-dependent and was significantly higher than those of PCH-1H and PCH-2H (p < 0.05; Figure 2B). The IC50 values of PCC-24H and acarbose (positive control) on α-glucosidase inhibition were 7.6 mg/mL and 1.7 mg/mL, respectively (Table 2). However, the inhibition percentages of PCH-1H and PCH-2H on α-glucosidase were only 10.9 ± 0.3% and 12.8 ± 0.3% at the concentration of 4.0 mg/mL, respectively (Figure 2B).
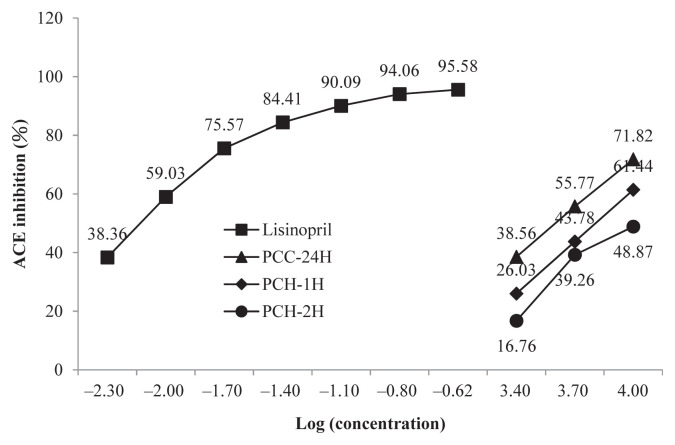
Compared with the positive control lisinopril, the ACE inhibition abilities of either the cold water or the hot water extracts were lower (Figure 3). In terms of extraction temperature, the cold water extract exerted a stronger ACE inhibition than the hot water extract. This finding is similar to that reported by Lee et al [15]. In their study, the ACE inhibition of a cold water extract from Tricholoma giganteum was higher than that of a hot water extract [15]. It is plausible that the high temperature can partially destroy the bioactive components that are responsible for the ACE inhibition effects. Nevertheless, the observation that ACE inhibition of PCC-2H was stronger than PCC-1H suggested that the inhibition capabilities were extraction time-dependent. This result is in line with the study on T. giganteum where ACE inhibition capabilities were extraction time-dependent up to 3 hours [15].
Figure 3.
Angiotensin-converting enzyme (ACE) inhibitory of PCC-24H, PCH-1H and PCH-2H. Data are mean of triplicate determinations.
3.4. Corresponding compounds of extracts on their bioactivities
Previous studies have demonstrated that phenolic contents are responsible for the antioxidant ability and carbohydrate indigestibility of plant extracts. Dudonnel et al [26] found that total phenolic contents were significantly correlated to ABTS+ and DPPH scavenging ability and ferric ion reducing activity in plant extracts. Pinto et al [4] further demonstrated that the total phenolic content of Fragaria x ananassa Duch. could affect the levels of antioxidation and α-glucosidase inhibition. Dehghan et al [27] reported a good correlation between DPPH scavenging activity and total phenolic contents in 11 herbal plants. In the present study, a higher level of phenolic contents was found in PCC-24H than in PCH-1H and PCH-2H. Consequently, PCC-24H exhibited stronger antioxidant activities and higher inhibition effects on α-amylase, α-glucosidase, and ACE (p < 0.05).
3.5. Effects of polysaccharide-rich and protein-rich fractions on the distribution of bioactive components and their bioactivities
Since relatively high levels of protein and polysaccharide were detected in PCC-24H, the phenolic content might not be the only factor contributing to the enzyme inhibition. To investigate the contribution of protein and polysaccharide on enzyme inhibition, cold water extracts (PCC-24H) were precipitated with 100% ammonium sulfate to obtain protein-rich (PCC-Pr) fraction or with 75% ethanol to obtain polysaccharide-rich (PCC-Ps) fraction. The yields of freeze-dried PCC-24H precipitated with ammonium sulfate and ethanol were 0.13% and 0.21%, respectively, indicating that more polysaccharide than protein were present in the cold water PCC-24H extract (Table 1).
The sample precipitated with ammonium sulfate increased the protein content of the cold water extract from 75.4 ± 1.5 μg/mg to 271.7 ± 1.7 μg/mg, whereas the sample precipitated with ethanol increased polysaccharide content from 238.6 ± 1.9 μg/mg to 539.8 ± 2.3 μg/mg (Table 1). While the phenolic contents of PCC-Pr (25.1 ± 0.3 μg GAE/mg) and PCC-24H (27.5 ± 0.3 μg GAE/mg) were similar, they were higher than that of PCC-Ps (5.0 ± 0.1 μg GAE/mg).
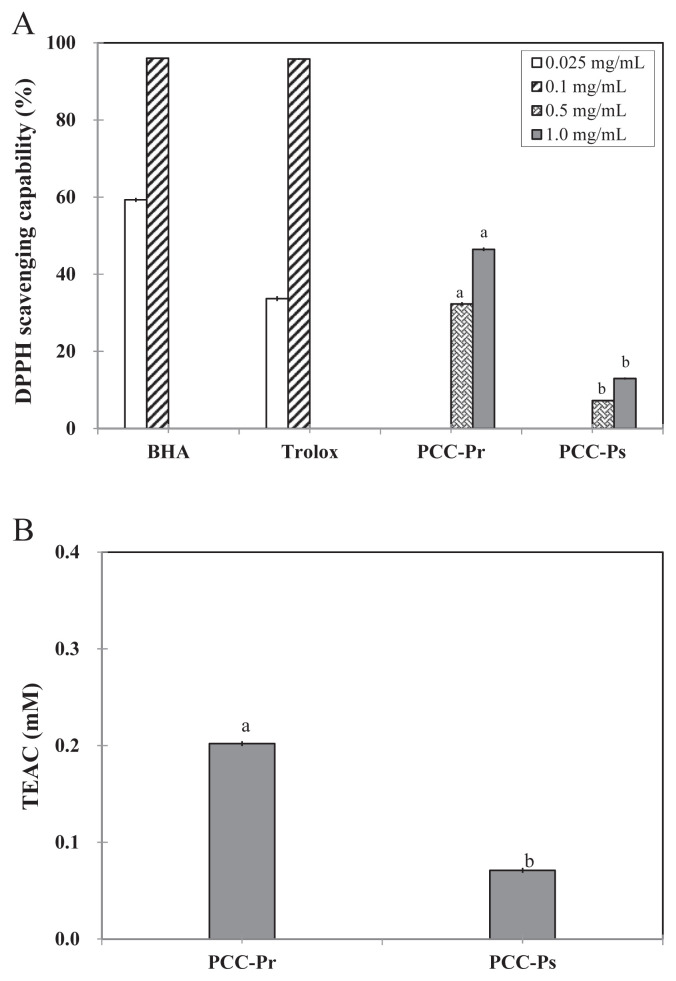
The DPPH scavenging capabilities of both fractions were dose-dependent (Figure 4A). The IC50 values for DPPH scavenging of PCC-Pr and PCC-Ps were 1.3 mg/mL and 6.4 mg/mL, respectively (Table 2). The TEAC of PCC-Pr was also significantly higher than that of PCC-Ps, with Trolox equivalents for PCC-Pr and PCC-Ps at 500 μg/mL equaled to 0.20mM and 0.07mM, respectively (Figure 4B). The phenolic content also correlated well with DPPH scavenging and TEAC abilities.
Figure 4.
(A) 2,2-Diphenyl-1-picrylhydrazyl (DPPH) scavenging activity; (B) Trolox equivalent antioxidant capacity (TEAC) of PCC-Pr and PCC-Ps. Data with different letters were significantly different between PCC-Pr and PCC-Ps at same concentrations by Student t test (p < 0.05). BHA = 2(3)-t-butyl-4-hydroxyanisole.
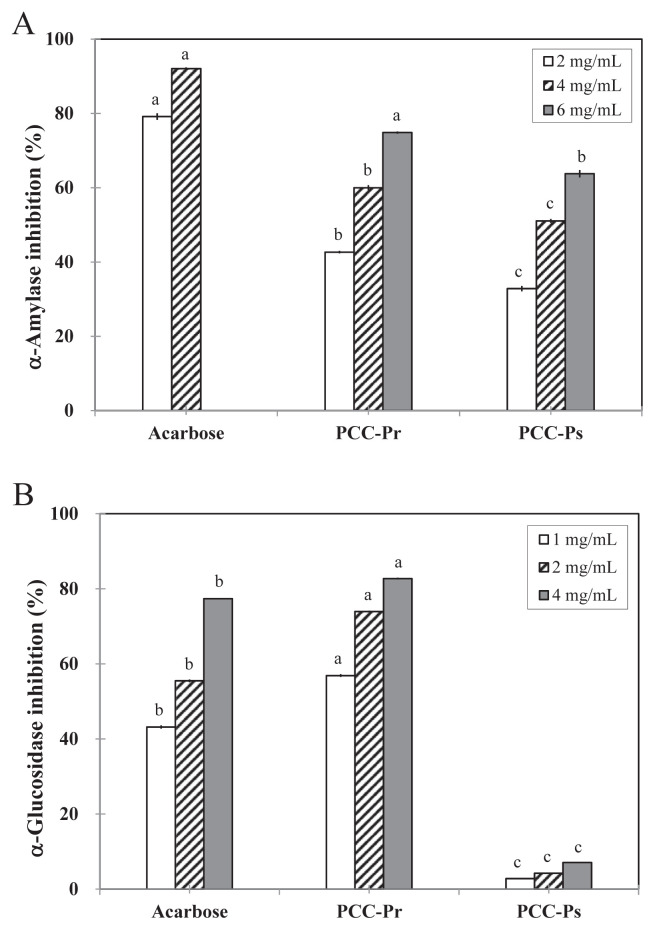
The dose-dependent inhibitory abilities of PCC-Pr on α-amylase (Figure 5A) and α-glucosidase (Figure 5B) were significantly higher than those of PCC-Ps. Fractionation improved the α-amylase inhibition, especially for the protein-rich fraction. The IC50 values of PCC-24H, PCC-Pr, and PCC-Ps on α-amylase inhibition were 7.1 mg/mL, 2.5 mg/mL, and 4.4 mg/mL, respectively (Table 2). Compared with the inhibition of the positive control acarbose, the inhibition of PCC-24H and PCC-Pr was about 7.5% and 20.8% of the positive control, respectively. Moreover, fractionation with ammonium sulfate, but not ethanol, improved the capability of inhibition on α-glucosidase. The IC50 values on α-glucosidase inhibition were 7.6 mg/mL, 0.8 mg/mL, and 33.6 mg/mL for PCC-24H, PCC-Pr, and PCC-Ps, respectively (Table 2). The effect of PCC-Pr on α-glucosidase inhibition was even better than that of acarbose in all the concentrations tested. However, the inhibition of PCC-Ps was only 10%. The higher inhibition effect of PCC-Pr on α-glucosidase suggested that further studies should be conducted regarding its potential to be used as a hypoglycemic agent.
Figure 5.
(A) α-Amylase and (B) α-glucosidase inhibitory activity of PCC-Pr and PCC-Ps. Data with different letters were significantly different among fractions and control at same concentrations by Student t test (p < 0.05).
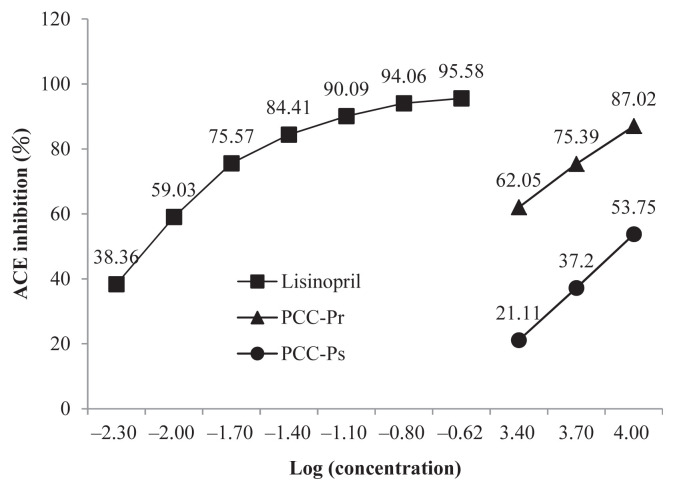
For the ACE inhibition, both fractions followed a dose-dependent pattern, 62–87% and 21–54% for PCC-Pr and PCC-Ps at concentrations between 2.5 mg/mL and 10 mg/mL, respectively (Figure 6). PCC-Pr possessed a significantly higher ACE inhibition than PCC-Ps but less than that of the positive control lisinopril.
Figure 6.
Angiotensin-converting enzyme (ACE) inhibitory activity of PCC-Pr and PCC-Ps. Lisinopril was the positive control. Data are mean of triplicate determinations.
Previous studies have shown good correlations between antioxidant ability and total phenolic content [4] and between hypoglycemic effect and the content of water soluble polysaccharides from P. citrinopileatus [11]. Other studies have also demonstrated that peptide fraction was related to the ACE inhibition [28,29]. In our study, compared with other extracts and fractions, PCC-24H contained the highest amount of total phenolics and thus, exerting the strongest antioxidant abilities. However, the inhibition capabilities on carbohydrate digestion-related enzymes appeared to be related to protein, but not the total phenolic and polysaccharide contents since the phenolic contents of PCC-24H and PCC-Pr were at approximately the same levels. Similar to the inhibition effect on carbohydrate digestion-related enzymes, PCC-Pr also exhibited a stronger ACE inhibition, indicating that it has more potential for use in the management of hypertension.
The findings from this study indicated that a low extraction temperature was the preferred choice for the extraction of P. citrinopileatus since a high extraction temperature could destroy the bioactive components and thereby affect the bioactive capabilities of P. citrinopileatus. While the antioxidant capabilities were correlated well to a higher phenolic content, the inhibitions on the carbohydrate digestion-related enzymes and ACE of PCC-Pr were significantly stronger than those of PCC-Ps. Therefore, the protein, but not the polysaccharide in PCC was more likely to be responsible for the suppression effects of P. citrinopileatus on hyperglycemia and hypertension.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
REFERENCES
- 1. Shen SC, Cheng FC, Wu NJ. Effect of guava (Fridium guajava Linn.) leaf soluble solids on glucose metabolism in type 2 diabetic rats. Phytother Res. 2008;22:1458–64. doi: 10.1002/ptr.2476. [DOI] [PubMed] [Google Scholar]
- 2. Ondetti MA, Rubin B, Cushman DW. Angiotensin I-converting enzyme inhibitory peptides from pepsin digest of maitake (Grifola frondosa) Food Sci Tech Res. 2000;6:9–11. [Google Scholar]
- 3. DiNicolantonio JJ, Bhutani J, O’Keefe JH. Acarbose: safe and effective for lowering postprandial hyperglycaemia and improving cardiovascular outcomes. Open Heart. 2015;2:e000327. doi: 10.1136/openhrt-2015-000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pinto MS, Carvalho JE, Lajolo FM, Genoverse MI, Shetty K. Evaluation of antiproliferative, antitype 2 diabetes, and antihypertension potentials of ellagitannins from strawberries (Fragaria x ananassa Duch.) using in vitro models. J Med Food. 2010;13:1027–35. doi: 10.1089/jmf.2009.0257. [DOI] [PubMed] [Google Scholar]
- 5. Pinto MS, Ghaedian R, Shinde R, Shetty K. Potential of cranberry powder for management of hyperglycemia using in vitro models. J Med Food. 2010;13:1036–44. doi: 10.1089/jmf.2009.0225. [DOI] [PubMed] [Google Scholar]
- 6. Watanabe K, Kamata K, Sato J, Takahashi T. Fundamental studies on the inhibitory action of Acanthopanan senticosus Harms on glucose absorption. J Ethnopharmacol. 2010;132:193–9. doi: 10.1016/j.jep.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 7. Li YR, Liu QH, Wang HX, Ng TB. A novel lectin with potent antitumor, mitogenic, and HIV-1 reverse transcriptase inhibitory activities from the edible mushroom Pleurotus citrinopileatus. Biochim Biophys Acta. 2008;1780:51–7. doi: 10.1016/j.bbagen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 8. Chen JN, Ma CY, Tsai PF, Wang YT, Wu JSB. In vitro antitumor and immunomodulatory effects of the protein PCP-3A from mushroom Pleurotus citinopileatus. J Agric Food Chem. 2010;58:12117–22. doi: 10.1021/jf103576r. [DOI] [PubMed] [Google Scholar]
- 9. Sheu F, Chien PJ, Wang HK, Chang HH, Shyu YT. New protein PCiP from edible golden oyster mushroom (Pleurotus citrinopileatus) activating murine macrophages and splenocytes. J Sci Food and Agric. 2007;87:1550–8. [Google Scholar]
- 10. Hu SH, Liang ZC, Chia YC, Lien JL, Chen KS, Lee MY, Wang JC. Antihyperlipidemic and antioxidant effects of extracts from Pleurotus citrinopileatus. J Agric Food Chem. 2006;54:2103–10. doi: 10.1021/jf052890d. [DOI] [PubMed] [Google Scholar]
- 11. Hu SH, Wang JC, Lien JL, Liaw ET, Lee MY. Antihyperglycemic effect of polysaccharide from fermented broth of Pleurotus citrinopileatus. Appl Microbiol Biotechnol. 2006;70:107–13. doi: 10.1007/s00253-005-0043-5. [DOI] [PubMed] [Google Scholar]
- 12. Venditti E, Bacchetti T, Tiano L, Carloni P, Creci L, Damiani E. Hot versus cold water steeping of different teas: do they affect antioxidant activity? Food Chem. 2010;119:1597–604. [Google Scholar]
- 13. Sharma K, Ko EY, Assefa AD, Ha S, Nile SH, Lee ET, Park SW. Temperature-dependent studies on the total phenolics, flavonoids, antioxidant activities, and sugar content in six onion varieties. J Food Drug Anal. 2015;23:243–52. doi: 10.1016/j.jfda.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lam SK, Ng TB. Hypsin, a novel thermostable ribosome-inactivating protein with antifungal and antiproliferative activities from fruiting bodies of the edible mushroom Hypsizigus marmoreus. Biochem Biophys Res Commun. 2001;285:1071–5. doi: 10.1006/bbrc.2001.5279. [DOI] [PubMed] [Google Scholar]
- 15. Lee DH, Kim JH, Park JS, Choi YJ, Lee JS. Isolation and characterization of a novel angiotensin I-converting enzyme inhibitory peptide derived from the edible mushroom Tricholoma giganteum. Peptides. 2004;25:621–7. doi: 10.1016/j.peptides.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 16. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 17.Chaplin MF, Kennedy JF. Carbohydrate analysis: a practical approach. 2nd ed. New York: Oxford University Press; 1994. [Google Scholar]
- 18. Quettier-Deleu C, Gressier B, Vasseur J, Dine T, Brunet C, Luyckx M, Cazin M, Cazin JC, Bailleul R, Trotin F. Potential of cranberry powder for management of hyperglycemia using in vitro models. J Med Food. 2000;13:1036–44. [Google Scholar]
- 19. Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem. 1992;40:945–8. [Google Scholar]
- 20. Arnao MB, Cano A, Acosta M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001;73:239–44. [Google Scholar]
- 21. Miller NJ, Rice-Evans CA. The relative contributions of ascorbic acid and phenolic antioxidants to the total antioxidant activity of orange and apple fruit juices and blackcurrant drink. Food Chem. 1996;60:331–7. [Google Scholar]
- 22. Cushman DW, Cheung HS. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem Pharmacol. 1971;20:1637–48. doi: 10.1016/0006-2952(71)90292-9. [DOI] [PubMed] [Google Scholar]
- 23. Cisse M, Vaillant F, Kane A, Ndiaye Q, Dornier M. Impact of the extraction procedure on the kinetics of anthocyanin and colour degradation of roselle extracts during storage. J Sci Food Agric. 2011;92:1214–21. doi: 10.1002/jsfa.4685. [DOI] [PubMed] [Google Scholar]
- 24. Lau BF, Abdullah N, Aminudin N, Lee HB. Chemical composition and cellular toxicity of ethnobotanical-based hot and cold aqueous preparations of the tiger’s milk mushroom (Lignosus rhinocerotis) J Ethnopharmacol. 2013;150:252–62. doi: 10.1016/j.jep.2013.08.034. [DOI] [PubMed] [Google Scholar]
- 25. Touba EP, Zakaria M, Tahereh E. Anti-fungal activity of cold and hot water extracts of spices against fungal pathogens of Roselle (Hibiscus sabdariffa) in vitro. Microb Pathogenesis. 2012;52:125–9. doi: 10.1016/j.micpath.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 26. Dudonnel S, Vitrac X, Coutiere P, Woillez M, Merillon JM. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J Agric Food Chem. 2009;57:1768–74. doi: 10.1021/jf803011r. [DOI] [PubMed] [Google Scholar]
- 27. Dehghan H, Sarrafi Y, Salehi P. Antioxidant and antidiabetic activities of 11 herbal plants from Hyrcania region, Iran. J Food Drug Anal. 2016;24:179–88. doi: 10.1016/j.jfda.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Samaranayaka AGP, Kitts DD, Li-Chan ECY. Antioxidative and angiotensin-I-converting enzyme inhibitory potential of a pacific hake (Merluccius productus) fish protein hydrolysate subjected to simulated gastrointestinal digestion and Caco-2 cell permeation. J Agric Food Chem. 2010;58:1535–42. doi: 10.1021/jf9033199. [DOI] [PubMed] [Google Scholar]
- 29. Segura-Campos M, Chel-Guerrero L, Betancur-Ancona D. Angiotensin-I converting enzyme inhibitory and antioxidant activities of peptide fractions extracted by ultrafiltration of cowpea Vigna unguiculata hydrolysates. J Sci Food Agric. 2010;90:2512–8. doi: 10.1002/jsfa.4114. [DOI] [PubMed] [Google Scholar]