Abstract
Atherosclerosis plays a key role in the development of cardiovascular diseases, and is often associated with oxidative stress and local inflammation. Thymol, a major polyphenolic compound in thyme, exhibits antioxidant and anti-inflammatory properties. In this study, we measured the in vitro antioxidant activity of thymol, and investigated the effect of thymol on high-fat-diet-induced hyperlipidemia and atherosclerosis. New Zealand white rabbits were fed with regular chow, high-fat and high-cholesterol diet (HC), T3, or T6 (HC with thymol supplementation at 3 mg/kg/d or 6 mg/kg/d, respectively) for 8 weeks. Aortic intimal thickening, serum lipid parameters, multiple inflammatory markers, proinflammatory cytokines, and atherosclerosis-associated indicators were significantly increased in the HC group but decreased upon thymol supplementation. In summary, thymol exhibits antioxidant activity, and may suppress the progression of high-fat-diet-induced hyperlipidemia and atherosclerosis by reducing aortic intimal lipid lesion, lowering serum lipids and oxidative stress, and alleviating inflammation-related responses.
Keywords: antioxidant, atherosclerosis, inflammatory markers, oxidative stress, thymol
1. Introduction
Cardiovascular diseases continue to be the leading cause of deaths worldwide, with atherosclerosis often being the primary underlying cause [1]. Oxidative stress is the putative mechanism involved in the pathogenesis of endothelial dysfunction, an early key event in the progression of atherosclerosis [2,3]. Oxidized low-density lipoprotein (ox-LDL) stimulates endothelial cells to produce a number of proinflammatory cytokines, including interleukin-1 (IL-1) and tumor necrosis factor-α (TNF-α), which, in turn, induce the expression of adhesion molecules and chemotactic factors, resulting in monocyte adherence and migration [4,5]. Monocytes migrate into the arterial wall and differentiate into macrophages to facilitate the uptake of ox-LDL. Over time, these macrophages not only transform into foam cells but also release multiple proinflammatory cytokines that stimulate smooth muscle cell (SMC) migration and proliferation. These macrophage foam cells contribute to the formation of early and mature fatty streaks [6]. In summary, increased oxidative stress and local inflammation may play a pivotal role in the pathogenesis of endothelial dysfunction, and consequently, atherosclerosis.
Matrix metalloproteinases (MMPs), a family of zinc- and calcium-dependent endopeptidases, mainly include collagenases, gelatinases, stromelysins, and membrane-type MMPs [7]. MMPs cleave the components of extracellular matrix, thereby facilitating migration of vascular SMCs [8]. The production of MMP and the migration of SMCs may contribute to the pathogenesis of neointima formation and atherosclerosis. In particular, the inducible expression of gelatinase MMP-9 has been shown to be directly involved in cancer cell invasion and SMC migration through the vascular wall [9,10].
The leafy parts and essential oils of thyme (Thymus vulgaris) have been widely used in foods for flavoring and improving aroma and/or preservation capacity [11]. Thyme has also been used in folk medicine [11]. Studies have shown that thymol, the most abundant component of thyme, exhibits antioxidant as well as antibacterial and anti-inflammatory properties [11–13]. The antioxidant property may be related to its phenolic structure, as phenolic compounds often exhibit redox properties, which may adsorb and neutralize free radicals [14–17]. Previous studies have suggested that thymol may become an important antioxidant food supplement [18].
Atherosclerosis is associated with many risk factors, such as dyslipidemia, and there is increasing emphasis on preventing or slowing the progression of atherosclerosis with natural supplements, healthy eating, and exercise [1,19]. Endothelial dysfunction is a reversible disorder; therefore, modifying cardiovascular risk factors with lipid-lowering, antioxidant, and anti-inflammatory dietary supplements may improve endothelial function and, consequently, reduce the risk of atherosclerosis [1,19]. Compared with other laboratory animals, rabbits are sensitive to the manipulation of dietary cholesterol and saturated fat, which can quickly develop hyperlipidemia and prominent aortic lesions [20]. In addition, rabbits exhibit similar lipo-protein metabolism as humans and appear to be the ideal model for human atherosclerosis in translational medicine [20,21]. This study examined the in vitro free radical-scavenging activities of thymol, and the effect of thymol in rabbits fed a high-fat and high-cholesterol diet (HC). In vitro trolox equivalent antioxidant capacity (TEAC) and serum total antioxidant status (TAS) were measured to assess the ability of thymol to inhibit the oxidation of 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid), ABTS, as a readout of its antioxidant potential [22]. Serum malondialdehyde (MDA) level, a marker indicating the oxidation status of lipids, was measured to determine the degree of lipid peroxidation [23]. Our findings show that thymol possesses strong antioxidant activity and may suppress the progression of atherosclerosis by lowering serum lipid, oxidative stress, and inflammatory-related gene expression as well as by reducing aortic intimal thickening in hyperlipidemic rabbits. These data indicate a potential translational application of thymol in treating atherosclerosis.
2. Materials and methods
2.1. Reagents and chemicals
All reagents and chemicals (reagent grade) were obtained from Sigma, Inc. (St. Louis, MO, USA) unless stated otherwise. Thymol (≥99.0%) in white powder was mixed with the laboratory chow for the animal study.
2.2. In vitro antioxidant activity of thymol
The 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity of thymol was examined. In brief, 1 volume (vol) of acetate buffer (100 mmol/L, pH 5.5), 1 vol of ethanol, and 0.5 vol of freshly prepared DPPH ethanolic solution (500 nmol/L) were mixed. After adding the test compound, the mixture was incubated at 25°C for 90 minutes. The change in absorbance at 517 nm was then determined [24].
Total antioxidant capacity of thymol was measured by TEAC as previously described [22]. The TEAC values of the samples under investigation are defined as a concentration equivalent to 1 mmol/L of trolox, a water-soluble analog of α-tocopherol, required for scavenging ABTS radical cations (ABTS•+ radicals).
2.3. Animals and treatment
A total of 24 4- to 6-week-old New Zealand white (NZW) male rabbits (Society for Laboratory Animal Science, Taiwan) with an average body weight of 1.5 kg were housed in individual cages with an environmentally controlled atmosphere (25 ± 1°C) and a 12-hour light/dark cycle. Food and water were provided ad libitum. The animal use protocol (CJCU-99-005) has been reviewed and approved by the Institutional Animal Care and Use Committee at Chang Jung Christian University in accordance with the Care and Use of Lab Animals for Scientific Application enacted by the Legislative Yuan, Taiwan. After a 1-week adaptation period, animals were randomly assigned to one of the four dietary manipulation groups (six rabbits/group) as follows: control group, high-fat and high-cholesterol group (HC group), T3 group, and T6 group. The control group (normal; N) was fed with regular laboratory rabbit chow (100 g/d). The HC group was fed with regular chow enriched with 10% lard and 1% cholesterol. The T3 and T6 groups were fed with HC supplemented with thymol (3 mg/kg/d and 6 mg/kg/d, respectively). The dietary manipulation continued for 8 weeks. At the end of the 8-week study period, all animals were killed under CO2 anesthesia. Blood samples were collected by cardiac puncture, and the aortas were removed and longitudinally cut for morphological and gene expression analyses.
2.4. Biochemical measurements
The blood samples were centrifuged at 1800g for 10 minutes at 4°C, and the serum was harvested. Serum total cholesterol (TC), triacylglycerol (TG), LDL-cholesterol (LDL-C), high-density lipoprotein-cholesterol (HDL-C), and TAS were analyzed using commercial kits (Fortress Diagnostics Limited, Antrim, United Kingdom) according to the manufacturer’s instructions. Serum TAS is defined by the ability of antioxidants in the serum sample to inhibit the oxidation of ABTS to ABTS•+ radicals by metmyoglobin (a peroxidase) [22]. The amount of radical generation can be monitored by reading the absorbance at 600 nm. Serum C-reactive protein (CRP) level was measured using an enzyme-linked immunosorbent assay kit (Immunology Consultants Laboratory, Newberg, OR, USA).
2.5. Measurement of serum MDA level
Serum MDA level was determined according to the method suggested previously [23], but with minor modifications. The assay mixture, comprising 25 μL of serum, 2.5 μL of 60 mM CuSO4, and 22.5 μL of H2O, was allowed to react for 4 hours at 37°C. The mixture was then added to 0.35 mL of 20% trichloroacetic acid and 0.35 mL of 0.67% thiobarbituric acid, and heated for 30 minutes at 70°C. After centrifugation at 13,750g for 2.5 minutes, the supernatant was assayed spectrophotometrically at 540 nm. Serum MDA content was expressed as nanomoles/milliliter. Tetramethoxypropane was used as the standard.
2.6. Morphological analysis
The thoracic aortic arch region was dissected longitudinally and divided into eight equal segments, fixed with 10% buffered neutral formalin, embedded in paraffin, and then cut at 5-μm thickness for hematoxylin and eosin (HE) staining and photographed. The intimal lipid lesions in each segment were examined quantitatively by estimating the percentage of the HE staining regions (lipid infiltration) in photographs (100×). The sum of lipid infiltration in eight segments was the intimal thickening for each rabbit. All groups received the same microscopic quantification procedures to estimate the aortic intimal thickening.
2.7. RNA isolation and real-time polymerase chain reaction
Total RNA of the aortic arch was extracted using PureLink RNA Mini Kit (Invitrogen, Carlsbad, CA, USA), and complementary DNA was synthesized from the RNA samples using the SuperScript III First-Strand Synthesis SuperMix for quantitative reverse transcription-polymerase chain reaction (Invitrogen). Real-time polymerase chain reaction assays were performed using SYBR Green SuperMix (Invitrogen) and iQ5 Multicolor Real-Time PCR Detection System (BioRad, Hercules, CA, USA). The primers for inflammation-related genes are shown in Table 1, as previously reported [25]. β-actin was used as an internal control. Amplification was performed under the following conditions: 40 cycles of 95°C for 15 seconds and at a specific annealing temperature (60°C) for 60 seconds. The specificity of the amplification was verified by melting curve analysis [26].
Table 1.
Primers used for real-time polymerase chain reaction assay.
| Matrix metalloproteinase-9 | Sense | 5′-TTGGTGGTCTTCCCAGGAGAG-3′ |
| Antisense | 5′-GCAGGTCTTCGGAGTAGTTTTGG-3′ | |
| Vascular cell adhesion molecule-1 | Sense | 5′-GAACACTCTTACCTGTGTACAGC-3′ |
| Antisense | 5′-CTCACATTAATTGCTATGAGGATGG-3′ | |
| Monocyte chemotactic protein-1 | Sense | 5′-GTCTCTGCAACGCTTCTGTGCC-3′ |
| Antisense | 5′-AGTCGTGTGTTCTTGGGTTGTGG-3′ | |
| Tumor necrosis factor-α | Sense | 5′-GCTCACGGACAACCAGCT-3′ |
| Antisense | 5′-TCCCAAAGTAGACCTGCCC-3′ | |
| Interleukin-1β | Sense | 5′-CCACAGTGGCAATGAAAATG-3′ |
| Antisense | 5′-AGAAAGTTCTCAGGCCGTCA-3′ | |
| Interleukin-6 | Sense | 5′-CTTCAGGCCAAGTTCAGGAG-3′ |
| Antisense | 5′-AGTGGATCGTGGTCGTCTTC-3′ | |
| Tumor necrosis factor-β | Sense | 5′-CTCCGCCACGGCTTCTC-3′ |
| Antisense | 5′-AACCACCTGCGAGTACACGAA-3′ |
2.8. Statistical analysis
All data were expressed as mean ± standard deviation. Comparisons between the four groups were made with the Kruskal–Wallis test. Duncan grouping was used to analyze significant effects. A p value of 0.05 was used as the threshold for statistical significance.
3. Results
3.1. In vitro antioxidative capacity of thymol
Based on inhibitory concentration 50% values, approximately 121.4 μM of thymol could scavenge 50% of DPPH-free radicals. According to the TEAC assay, thymol was about 2.83 times more effective than trolox (a water-soluble form of vitamin E) at scavenging ABTS•+ radicals. Therefore, in accordance with literature [27–29], thymol exhibited free radical-scavenging activity and appeared as a strong antioxidant.
3.2. Effect of thymol on serum lipid profiles and aortic arches in high-fat-diet-induced hyperlipidemic rabbits
Body weight changes among the four groups, N, HC, T3, and T6, at the end of 8-week dietary manipulation period were 2.85 ± 0.24 kg, 3.21 ± 0.37 kg, 2.98 ± 0.36 kg, and 3.07±0.42 kg, respectively. No statistically significant difference was found among the four groups with respect to body weight gain and food intake at the end of the study. As shown in Table 2, serum levels of TC, TG, LDL-C, and MDA were significantly increased in the HC group as compared with the N (normal diet) group (p < 0.05), whereas these readings were significantly decreased upon thymol supplementation, particularly in the T6 group, as compared with the HC group. TAS and HDL-C concentration were significantly enhanced in the T6 group as compared with the HC and T3 groups (p < 0.05). Serum CRP level was significantly increased in the HC group as compared with the N (normal diet) group, but decreased in the T3 and T6 groups when compared with the HC group (p < 0.05).
Table 2.
Effect of thymol on serum lipid profiles and C-reactive protein in high-fat diet-induced hyperlipidemic rabbits.
| Normal diet | High-fat and high-cholesterol diet | T3 | T6 | |
|---|---|---|---|---|
| Triglyceride (mmol/L) | 1.3 ± 0.7c | 12.0 ± 4.6a | 10.3 ± 1.6a,b | 8.9 ± 4.4b |
| Total cholesterol (mmol/L) | 1.7 ± 0.4c | 24.3 ± 2.5a | 19.6 ± 6.3b | 19.2 ± 7.5b |
| Low-density lipoprotein cholesterol (mmol/L) | 0.8 ± 0.3c | 19.9 ± 1.8a | 15.0 ± 6.0b | 13.6 ± 6.8b |
| High-density lipoprotein cholesterol (mmol/L) | 0.7 ± 0.3c | 2.0 ± 1.8b | 2.5 ± 3.9b | 3.8 ± 5.0a |
| Malondialdehyde (μmol/L) | 4.8 ± 2.1b | 8.2 ± 1.3a | 4.2 ± 1.6b | 4.1 ± 1.9b |
| C-reactive protein (ng/mL) | 20.7 ± 18.9c | 88.3 ± 19.1a | 62.3 ± 15.0b | 52.1 ± 20.0b |
| Total antioxidant status (mmol/L) | 1.6 ± 2.4c | 22.2 ± 11.2b | 34.3 ± 22.1b | 53.1 ± 10.7a |
Data with different superscripts in the same row are significantly different at p < 0.05.
All data were expressed as mean ± standard deviation. p < 0.05 was used as the threshold for statistical significance (n = 6).
HC = high-fat and high-cholesterol diet; T3 = HC supplemented with thymol 3 mg/kg/d; T6 = HC supplemented with thymol 6 mg/kg/d.
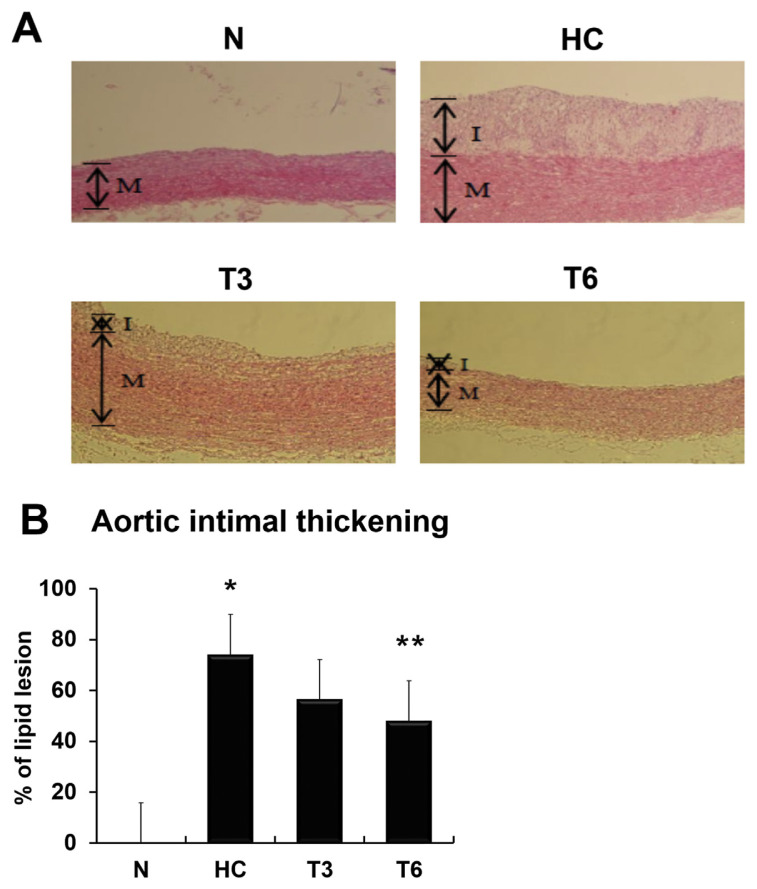
The effect of thymol on thoracic aortic intimal thickening in NZW rabbits is shown in Figure 1. Analysis of the percentage of lipid lesions showed that intimal thickening was significantly increased to about 75% in the HC group as compared with the N group, but decreased to 60% (p > 0.05) and 50% (p < 0.05) in the T3 and T6 groups, respectively, when compared with the HC group. These data indicate that thymol supplementation may suppress the atherogenic diet-induced increase in TG, TC, LDL-C, MDA, and CRP levels, and attenuate the atherosclerotic damage caused by high-fat diet.
Figure 1.
(A) Effect of thymol on thoracic aortic intimal thickening in New Zealand white rabbits. In the control (normal diet) group (N), rabbits were fed with regular rabbit chow; in the high-fat and high-cholesterol diet group (HC), rabbits were fed with rabbit chow plus 10% lard and 1% cholesterol; in the T3 and T6 groups, rabbits were fed as the HC group but also supplemented with 3 mg/kg/d and 6 mg/kg/d of thymol, respectively (magnification: 100×). (B) The percentage of lipid lesion was calculated; all data were expressed as mean ± standard deviation; p < 0.05 was used as the threshold for statistical significance (n = 6). *p < 0.05 as compared with the N group; **p < 0.05 as compared with the HC group. I = intima; M = media. T3 = HC supplemented with thymol 3 mg/kg/d; T6 = HC supplemented with thymol 6 mg/kg/d.
3.3. Effect of thymol on inflammation-related genes
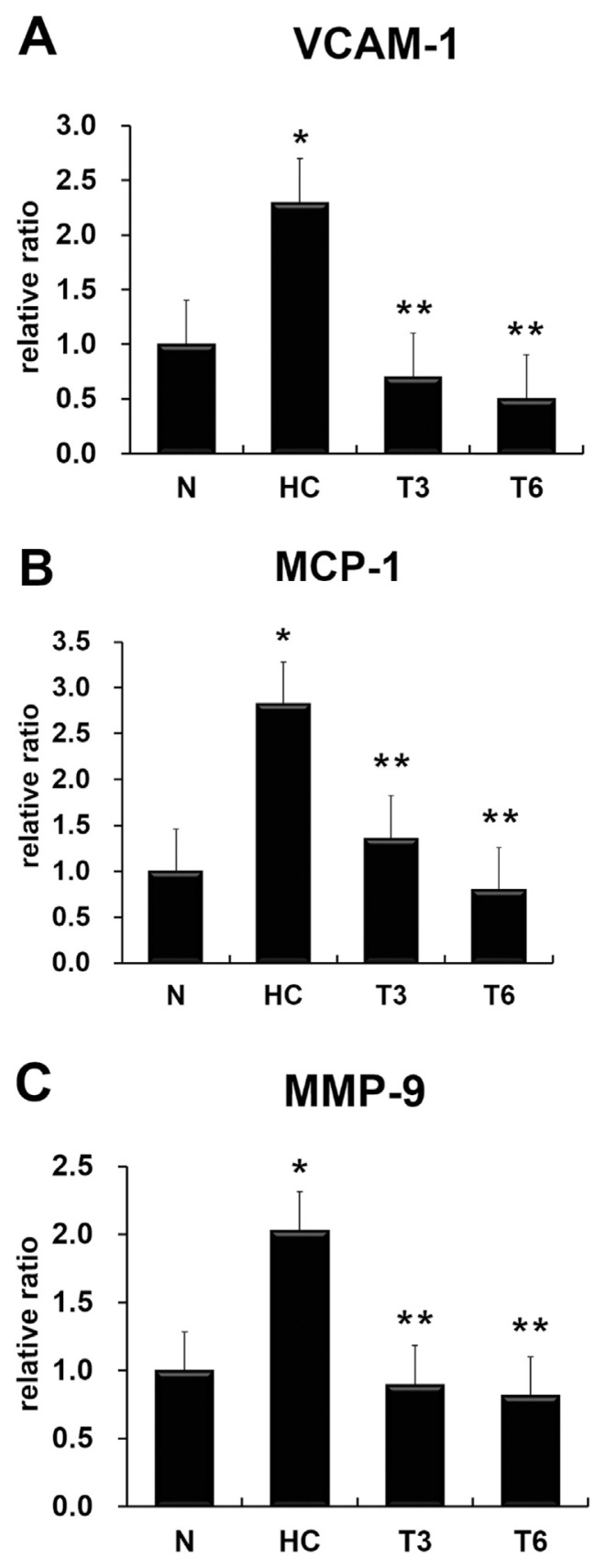
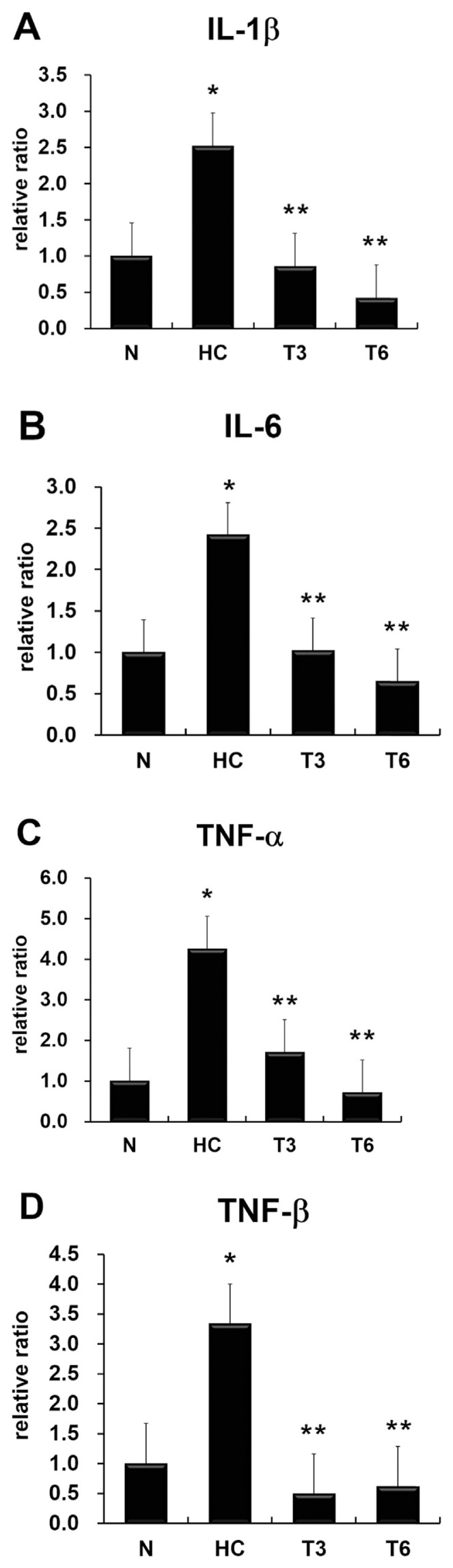
The expression levels of atherosclerosis-associated indicators, namely, vascular cell adhesion molecule-1 (VCAM-1), monocyte chemotactic protein-1 (MCP-1), and MMP-9 (Figure 2), and proinflammatory cytokines IL-1β, IL-6, TNF-α, and TNF-β (Figure 3) were significantly increased in the HC group as compared with the N group, but decreased in the T3 and T6 groups when compared with the HC group (p < 0.05).
Figure 2.
Effect of thymol on gene expression of (A) vascular cell adhesion molecule-1 (VCAM-1), (B) monocyte chemotactic protein-1 (MCP-1), and (C) matrix metalloproteinase-9 (MMP-9). All data were expressed as mean ± standard deviation; p < 0.05 was used as the threshold for statistical significance (n = 6). *p < 0.05 as compared with the normal diet (N) group; **p < 0.05 as compared with the high-fat high-cholesterol group (HC). T3 = HC supplemented with thymol 3 mg/kg/d; T6 = HC supplemented with thymol 6 mg/kg/d.
Figure 3.
Effect of thymol on gene expression of (A) interleukin-1β (IL-1β), (B) IL-6, (C) tumor necrosis factor-α (TNF-α), and (D) TNF-β. All data were expressed as mean ± standard deviation; p < 0.05 was used as the threshold for statistical significance (n = 6). *p < 0.05 as compared with the normal diet (N) group; **p < 0.05 as compared with the high-fat high-cholesterol group (HC). T3 = HC supplemented with thymol 3 mg/kg/d; T6 = HC supplemented with thymol 6 mg/kg/d.
4. Discussion
Experimental animal models of human atherosclerosis are important tools to study the fundamental mechanism and to explore the therapeutic means of the disease [20]. Many types of animal models, including mice, rats, rabbits, hamsters, and guinea pigs, are available, and each model has its own advantages and limitations [21]. Rabbits are prone to hyperlipidemia and experimental atherosclerosis upon dietary manipulation of cholesterol and fat [20]. The rabbit model has been useful to study the lipid-lowering effect by diet or statins on the development of atherosclerosis [30].
Serum lipid-lowering effect on experimental atherosclerosis was found in ellagic acid, olive leaf extract, and a sesamol derivative in the rabbit models as reported previously [24,31,32]. Specific polyphenols may reduce plasma lipids by altering hepatic triglyceride secretion and fecal fat secretion as well as the activity of the 3-hydroxy-3-methylglutaryl-coenzyme A reductase and LDL receptor [24,33–35]. Our study suggests that thymol, a phenolic compound, possesses similar lipid-lowering functions.
The imbalance between cellular free radical formation and antioxidant defense leads to oxidative stress. An excessive production of free radicals may attack cellular components, such as lipids, initiating the process of atherogenesis through cell dysfunction [2]. Presumably, the administration of antioxidants could counteract excess free radicals and thus may be applicable to combat oxidative stress. In this study, serum levels of TC, TG, LDL-C, and MDA, a marker of lipid peroxidation, were significantly increased in the HC group (rabbits receiving atherogenic diet), whereas thymol supplementation effectively reduced these lipid parameters (Table 2). Although TAS and HDL-C levels were increased in the HC group, the thymol supplementation T6 group exhibited significantly higher levels than those of the HC group (p < 0.05). The in vitro DPPH and TEAC results and in vivo TAS data indicated that thymol exhibited free radical-scavenging activity. Together, these data supported the protective effects of thymol on atherogenesis. Our study suggested that thymol functioned as a strong antioxidant, with the ability to increase serum total antioxidant capacity and inhibit lipid peroxidation, thereby improving the unfavorable progression of atherosclerosis.
This study employed a high-fat and high-cholesterol diet to induce hyperlipidemia and atherosclerosis. The atherogenic diet resulted in hyperlipidemia and aortic fatty streaks in rabbits, which were similar to those of humans as described previously [36]. Hyperlipidemia upregulated a series of inflammation-related genes, such as the IL-1β, IL-6, TNF-α, and TNF-β, in the aortas of rabbits, whereas thymol supplementation downregulated these genes. Our histological data also demonstrated that 75% of the intima of the thoracic aorta was covered with lipid lesions in the HC group, but reduced to 60% and 50% in the T3 and T6 groups, respectively. Thymol appears to be able to decrease the gene expression of proinflammatory cytokines and, consequently, reduce the formation of fatty streaks.
CRP is synthesized primarily in the liver and its release is stimulated by IL-6 and other proinflammatory cytokines [1]. Previous studies have indicated that CRP, an inflammatory acute-phase reactant, increases remarkably during the inflammatory response to tissue injury or oxidative stress [37]. High levels of plasma CRP have also been shown to be associated with future cardiovascular events in patients with acute coronary disease [38]. In this study, serum levels of CRP and the gene expression of IL-6 declined significantly in the T3 and T6 groups, suggesting that thymol possesses antiinflammatory properties, with the ability to decrease the release of IL-6 and downregulate the synthesis of CRP in the liver.
Cellular adhesion molecules and chemotactic factors on the surface of endothelial cells have been shown to mediate the adherence of monocytes to the endothelium and their subsequent migration into the arterial wall [31]. Proin-flammatory cytokines, such as IL-1 or TNF-α, directly stimulate the production of these adhesion molecules and chemotactic factors [5]. The cell adhesion molecule VCAM-1, a member of the immunoglobulin gene superfamily, is involved in atherogenesis by favoring firm adhesion of monocytes to the vascular endothelium [39]. MCP-1, a chemotactic factor with great potency for monocytes, is synthesized and secreted by endothelial cells upon various stimuli [40]. Our study showed that the messenger RNA expression of VCAM-1 and MCP-1 increased in the HC group but decreased upon thymol administration. These findings suggest that thymol may be able to modulate the production of inflammatory cytokines, resulting in the inhibition of the induction of VCAM-1 and MCP-1 expressions in the endothelial cells, thereby inhibiting the adherence of circulating monocytes (such as leukocytes) to vascular endothelium. These results are in accordance with a previous study using LDL receptor-deficient rabbits [39].
The migration of SMCs from the tunica media to the sub-endothelial region is a key event in the development and progression of atherosclerosis [41]. MMP-9 appears to be associated with SMC migration and plaque rupture [9,10]. Previous studies have indicated that MMP-9 can be induced by proinflammatory cytokines, such as IL-1β and TNF-α [42]. Our study demonstrated that thymol may have the ability to modulate the expression of MMP-9 in vivo via an inflammation-related mechanism.
Oxidative stress and inflammatory responses are involved in the development of atherosclerosis [17]. The redox-sensitive transcription factor nuclear factor-kappa B (NF-κB) modulates inflammatory responses. In the resting state, NF-κB is present in the cytoplasm. Upon activation by various stimuli such as oxidative stress or pro-inflammatory cytokines, NF-κB translocates into the nucleus and regulates a variety of downstream target genes including inflammation-associated TNF-α, IL-1β, and IL-6, creating a positive feedback loop [43]. In addition, cellular adhesion molecule VCAM-1, chemotactic factor MCP-1, and MMP-9, which are involved in the progression of atherosclerosis, are also mediated by NF-κB [31,44]. The current study did not explore the direct role of NF-κB in experimental atherosclerosis. However, according to previous studies with similar experimental designs [31,38,44], it may be reasonable to presume that NF-κB is responsible for the upregulation of the inflammatory cytokines, IL-1β, IL-6, TNF-α, and TNF-β, as well as VCAM-1, MCP-1, and MMP-9 in the current study. Therefore, it is legitimate to suggest that thymol supplementation alleviated the upregulation of the inflammatory cytokines induced by atherogenic diet, at least partially, through downregulating NF-κB.
In conclusion, the serum levels of TC, LDL-C, TG, MDA, and CRP, and intimal thickening significantly increased in the HC group but decreased in the T3 and T6 groups. The serum levels of HDL-C and TAS significantly increased in the T6 group as compared with the HC and T3 groups. Furthermore, the messenger RNA expression of IL-1β, IL-6, TNF-α, TNF-β, VCAM-1, MCP-1, and MMP-9 significantly increased in the HC group but decreased in the T3 and T6 groups. These results suggest that thymol suppresses the progression of atherosclerosis by lowering serum lipids and reducing oxidative stress and inflammatory response in hyperlipidemic rabbits. Further investigation is necessary to reveal the exact cellular mechanisms of the effect of thymol on the endothelial dysfunction and SMC migration.
Acknowledgements
This study was supported by grants from the Ministry of Science and Technology, Taiwan (NSC 99-2320-B-309-002) to Y.-M.Y. and (NSC 102-2320-B-309-001-MY3) to M.-F.L.
Funding Statement
This study was supported by grants from the Ministry of Science and Technology, Taiwan (NSC 99-2320-B-309-002) to Y.-M.Y. and (NSC 102-2320-B-309-001-MY3) to M.-F.L.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
REFERENCES
- 1. Hackam DG, Anand SS. Emerging risk factors for atherosclerotic vascular disease: a critical review of the evidence. JAMA. 2003;290:932–40. doi: 10.1001/jama.290.7.932. [DOI] [PubMed] [Google Scholar]
- 2. Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91:7A–11A. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 3. Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–75. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 4. Ehara S, Ueda M, Naruko T, Haze K, Itoh A, Otsuka M, Komatsu R, Matsuo T, Itabe H, Takano T, Tsukamoto Y, Yoshiyama M, Takeuchi K, Yoshikawa J, Becker AE. Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation. 2001;103:1955–60. doi: 10.1161/01.cir.103.15.1955. [DOI] [PubMed] [Google Scholar]
- 5. Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 6. Mizuno Y, Jacob RF, Mason RP. Inflammation and the development of atherosclerosis. J Atheroscler Thromb. 2011;18:351–8. doi: 10.5551/jat.7591. [DOI] [PubMed] [Google Scholar]
- 7. Creemers EE, Cleutjens JP, Smits JF, Daemen MJ. Matrix metalloproteinase inhibition after myocardial infarction: a new approach to prevent heart failure? Circ Res. 2001;89:201–10. doi: 10.1161/hh1501.094396. [DOI] [PubMed] [Google Scholar]
- 8. Busti C, Falcinelli E, Momi S, Gresele P. Matrix metalloproteinases and peripheral arterial disease. Intern Emerg Med. 2010;5:13–25. doi: 10.1007/s11739-009-0283-y. [DOI] [PubMed] [Google Scholar]
- 9. Cho A, Reidy MA. Matrix metalloproteinase-9 is necessary for the regulation of smooth muscle cell replication and migration after arterial injury. Circ Res. 2002;91:845–51. doi: 10.1161/01.res.0000040420.17366.2e. [DOI] [PubMed] [Google Scholar]
- 10. Galis ZS, Johnson C, Godin D, Magid R, Shipley JM, Senior RM, Ivan E. Targeted disruption of the matrix metalloproteinase-9 gene impairs smooth muscle cell migration and geometrical arterial remodeling. Circ Res. 2002;91:852–9. doi: 10.1161/01.res.0000041036.86977.14. [DOI] [PubMed] [Google Scholar]
- 11. Nabavi SM, Marchese A, Izadi M, Curti V, Daglia M, Nabavi SF. Plants belonging to the genus Thymus as antibacterial agents: from farm to pharmacy. Food Chem. 2015;173:339–47. doi: 10.1016/j.foodchem.2014.10.042. [DOI] [PubMed] [Google Scholar]
- 12. Kim DO, Lee CY. Comprehensive study on vitamin C equivalent antioxidant capacity (VCEAC) of various polyphenolics in scavenging a free radical and its structural relationship. Crit Rev Food Sci Nutr. 2004;44:253–73. doi: 10.1080/10408690490464960. [DOI] [PubMed] [Google Scholar]
- 13. Fachini-Queiroz FC, Kummer R, Estevao-Silva CF, Carvalho MD, Cunha JM, Grespan R, Bersani-Amado CA, Cuman RK. Effects of thymol and carvacrol, constituents of Thymus vulgaris L. essential oil, on the inflammatory response. Evid Based Complement Alternat Med. 2012;2012:657026. doi: 10.1155/2012/657026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ndhlala AR, Moyo M, Van Staden J. Natural antioxidants: fascinating or mythical biomolecules? Molecules. 2010;15:6905–30. doi: 10.3390/molecules15106905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lou SN, Hsu YS, Ho CT. Flavonoid compositions and antioxidant activity of calamondin extracts prepared using different solvents. J Food Drug Anal. 2014;22:290–5. doi: 10.1016/j.jfda.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chiu YW, Lo HJ, Huang HY, Chao PY, Hwang JM, Huang PY, Huang SJ, Liu JY, Lai TJ. The antioxidant and cytoprotective activity of Ocimum gratissimum extracts against hydrogen peroxide-induced toxicity in human HepG2 cells. J Food Drug Anal. 2013;21:253–60. [Google Scholar]
- 17. Do QD, Angkawijaya AE, Tran-Nguyen PL, Huynh LH, Soetaredjo FE, Ismadji S, Ju YH. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J Food Drug Anal. 2014;22:296–302. doi: 10.1016/j.jfda.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jaric S, Mitrovic M, Pavlovic P. Review of ethnobotanical, phytochemical, and pharmacological study of Thymus serpyllum L. Evid Based Complement Alternat Med. 2015;2015:101978. doi: 10.1155/2015/101978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 20. Fan J, Kitajima S, Watanabe T, Xu J, Zhang J, Liu E, Chen YE. Rabbit models for the study of human atherosclerosis: from pathophysiological mechanisms to translational medicine. Pharmacol Ther. 2015;146:104–19. doi: 10.1016/j.pharmthera.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moghadasian MH. Experimental atherosclerosis: a historical overview. Life Sci. 2002;70:855–65. doi: 10.1016/s0024-3205(01)01479-5. [DOI] [PubMed] [Google Scholar]
- 22. Miller NJ, Rice-Evans C, Davies MJ, Gopinathan V, Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Sci (Lond) 1993;84:407–12. doi: 10.1042/cs0840407. [DOI] [PubMed] [Google Scholar]
- 23. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 24. Yu YM, Chang WC, Wu CH, Chiang SY. Reduction of oxidative stress and apoptosis in hyperlipidemic rabbits by ellagic acid. J Nutr Biochem. 2005;16:675–81. doi: 10.1016/j.jnutbio.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 25. Matsumoto T, Watanabe H, Ueno T, Tsunemi A, Hatano B, Kusumi Y, Mitsumata M, Fukuda N, Matsumoto K, Saito S, Mugishima H. Appropriate doses of granulocyte-colony stimulating factor reduced atherosclerotic plaque formation and increased plaque stability in cholesterol-fed rabbits. J Atheroscler Thromb. 2010;17:84–96. doi: 10.5551/jat.2279. [DOI] [PubMed] [Google Scholar]
- 26. Yeh SH, Tsai CY, Kao JH, Liu CJ, Kuo TJ, Lin MW, Huang WL, Lu SF, Jih J, Chen DS, Chen PJ. Quantification and genotyping of hepatitis B virus in a single reaction by real-time PCR and melting curve analysis. J Hepatol. 2004;41:659–66. doi: 10.1016/j.jhep.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 27. Tsai ML, Lin CC, Lin WC, Yang CH. Antimicrobial, antioxidant, and anti-inflammatory activities of essential oils from five selected herbs. Biosci Biotechnol Biochem. 2011;75:1977–83. doi: 10.1271/bbb.110377. [DOI] [PubMed] [Google Scholar]
- 28. Anthony KP, Deolu-Sobogun SA, Saleh MA. Comprehensive assessment of antioxidant activity of essential oils. J Food Sci. 2012;77:C839–43. doi: 10.1111/j.1750-3841.2012.02795.x. [DOI] [PubMed] [Google Scholar]
- 29. Miguel MG, Gago C, Antunes MD, Megias C, Cortes-Giraldo I, Vioque J, Lima AS, Figueiredo AC. Antioxidant and antiproliferative activities of the essential oils from Thymbra capitata and Thymus species grown in Portugal. Evid Based Complement Alternat Med. 2015;2015:851721. doi: 10.1155/2015/851721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zaragoza C, Gomez-Guerrero C, Martin-Ventura JL, Blanco-Colio L, Lavin B, Mallavia B, Tarin C, Mas S, Ortiz A, Egido J. Animal models of cardiovascular diseases. J Biomed Biotechnol. 2011;2011:497841. doi: 10.1155/2011/497841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang L, Geng C, Jiang L, Gong D, Liu D, Yoshimura H, Zhong L. The anti-atherosclerotic effect of olive leaf extract is related to suppressed inflammatory response in rabbits with experimental atherosclerosis. Eur J Nutr. 2008;47:235–43. doi: 10.1007/s00394-008-0717-8. [DOI] [PubMed] [Google Scholar]
- 32. Ying Z, Kherada N, Kampfrath T, Mihai G, Simonetti O, Desikan R, Selvendiran K, Sun Q, Ziouzenkova O, Parthasarathy S, Rajagopalan S. A modified sesamol derivative inhibits progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:536–42. doi: 10.1161/ATVBAHA.110.219287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rea TJ, DeMattos RB, Pape ME. Hepatic expression of genes regulating lipid metabolism in rabbits. J Lipid Res. 1993;34:1901–10. [PubMed] [Google Scholar]
- 34. Kumar A, Middleton A, Chambers TC, Mehta KD. Differential roles of extracellular signal-regulated kinase-1/2 and p38(MAPK) in interleukin-1beta- and tumor necrosis factor-alpha-induced low density lipoprotein receptor expression in HepG2 cells. J Biol Chem. 1998;273:15742–8. doi: 10.1074/jbc.273.25.15742. [DOI] [PubMed] [Google Scholar]
- 35. Zern TL, Fernandez ML. Cardioprotective effects of dietary polyphenols. J Nutr. 2005;135:2291–4. doi: 10.1093/jn/135.10.2291. [DOI] [PubMed] [Google Scholar]
- 36. González-Santiago M, Martín-Bautista E, Carrero JJ, Fonollá J, Baró L, Bartolomé MV, Gil-Loyzaga P, López-Huertas E. One-month administration of hydroxytyrosol, a phenolic antioxidant present in olive oil, to hyperlipidemic rabbits improves blood lipid profile, antioxidant status and reduces atherosclerosis development. Atherosclerosis. 2006;188:35–42. doi: 10.1016/j.atherosclerosis.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 37. Pepys MB, Baltz ML. Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid A protein. Adv Immunol. 1983;34:141–212. doi: 10.1016/s0065-2776(08)60379-x. [DOI] [PubMed] [Google Scholar]
- 38. Ridker PM. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol. 2007;49:2129–38. doi: 10.1016/j.jacc.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 39. Fruebis J, Gonzalez V, Silvestre M, Palinski W. Effect of probucol treatment on gene expression of VCAM-1, MCP-1, and M-CSF in the aortic wall of LDL receptor-deficient rabbits during early atherogenesis. Arterioscler Thromb Vasc Biol. 1997;17:1289–302. doi: 10.1161/01.atv.17.7.1289. [DOI] [PubMed] [Google Scholar]
- 40. Harrington JR. The role of MCP-1 in atherosclerosis. Stem Cells. 2000;18:65–6. doi: 10.1634/stemcells.18-1-65. [DOI] [PubMed] [Google Scholar]
- 41. Maeda K, Kuzuya M, Cheng XW, Asai T, Kanda S, Tamaya-Mori N, Sasaki T, Shibata T, Iguchi A. Green tea catechins inhibit the cultured smooth muscle cell invasion through the basement barrier. Atherosclerosis. 2003;166:23–30. doi: 10.1016/s0021-9150(02)00302-7. [DOI] [PubMed] [Google Scholar]
- 42. Yu YM, Lin CH, Chan HC, Tsai HD. Carnosic acid reduces cytokine-induced adhesion molecules expression and monocyte adhesion to endothelial cells. Eur J Nutr. 2009;48:101–6. doi: 10.1007/s00394-008-0768-x. [DOI] [PubMed] [Google Scholar]
- 43. Barnes PJ, Adcock IM. NF-kappa, B a pivotal role in asthma and a new target for therapy. Trends Pharmacol Sci. 1997;18:46–50. doi: 10.1016/s0165-6147(97)89796-9. [DOI] [PubMed] [Google Scholar]
- 44. Chang WC, Chen CH, Lee MF, Chang T, Yu YM. Chlorogenic acid attenuates adhesion molecules upregulation in IL-1beta-treated endothelial cells. Eur J Nutr. 2010;49:267–75. doi: 10.1007/s00394-009-0083-1. [DOI] [PubMed] [Google Scholar]