Abstract
Allium chinense is a medicinal plant and nutritional food commonly used in Eastern Asia. In this study, we investigated the in vitro antioxidant activity (scavenging of α,α-diphenyl-β-picrylhydrazyl free radical, total phenol content, reducing power, and total antioxidant activity) and constituents of various extracts from A. chinense. Moreover, we also studied the in vivo hypolipidemic effects of extracts on high-fat-diet Wistar rats. Ethanol extracts from A. chinense showed notable antioxidant activity, and its high-dose essential-oil extract both significantly reduced serum and hepatic total cholesterol, triglyceride, and low-density lipoprotein levels and increased serum high-density lipoprotein levels in high-fat-diet Wistar rats compared with those observed following treatment with the control drug probucol. Additionally, visceral fat in high-fat-diet Wistar rats was reduced. Furthermore, groups with high doses of essential-oil and residue extracts showed protective effects associated with histopathological liver alteration. These results suggested that A. chinense is a valuable plant worthy of further investigation as a potential dietary supplement or botanical drug.
Keywords: Allium chinense, antihyperlipidemic, antioxidant, essential oil, flavonoid
1. Introduction
Both reactive oxygen species (ROS) and oxidative stress play a central role in the pathology and progression of many related human diseases. Therefore, enormous efforts to scientifically investigate potential endogenous and exogenous antioxidants have arisen [1]. For estimating actual oxidative defense or stress status, a selected group of nutrients and antioxidant biomarkers, such as superoxide dismutase, catalase, glutathione peroxidase, and NADPH (nicotinamide adenine dinucleotide phosphate) oxidase, are measured. In general, appropriate ROS levels result in transmission of intracellular and defense signals against pathogens. However, increasing ROS levels lead to the development of several human diseases, including cancer, diabetes, atherosclerosis, ischemia, and endocrine dysfunction. Therefore, moderating ROS levels and lowering the amount of serum lipids are important in disease treatment.
Distributed in Eastern Asia, Allium chinense (synonyms: Allium bakeri; common name: rakkyo, Chinese onion) is a perennial herb native to China that is usually harvested from March to May. A. chinense is commonly cultivated for nutritional and medicinal purposes, and is often pickled and served as a side dish or as a nutritional supplement. The plant is traditionally used to treat stenocardia, heart asthma, and antiplatelet aggregation [2]. Its naturally occurring sulfur-containing compounds responsible for its onion-like flavor were proven to influence plasma cholesterol and atherosclerosis in vitro [3,4]. Another study also reported the ability of its steroidal constituents to prevent cardiac injuries induced by oxidative stress. Although more than 20 different steroidal compounds have been identified, including furostanol saponins [5], spirostanol saponins [6], and xiebai-saponins [7], only one study investigated the antioxidant activity of A. chinense [8]. Because its antihyperlipidemic activity has not been previously studied, we investigated the target activity and chemical constituents of A. chinense.
In this study, A. chinense bulbs cultivated in Taiwan were investigated for their principal components and organic acids. The amount of quercetin and rutin in extracts was analyzed by high-performance liquid chromatography (HPLC), while volatile oil compounds were analyzed by gas chromatography mass spectrometry (GC-MS). Moreover, extracts of A. chinense bulbs were tested in a series of in vitro antioxidation assays, as well as in an in vivo antihyperlipidemic rat model. Evidence reported herein for antioxidative and antihyperlipidemic effects of A. chinense extracts show that the plant material is a promising candidate for counteracting oxidative stress-related diseases and atherosclerosis.
2. Materials and methods
2.1. Chemicals
α,α-Diphenyl-β-picrylhydrazyl free radical (DPPH), linoleic acid, butylated hydroxyanisole (BHA), butylated hydroxytoluene, α-tocopherol, bovine serum albumin, thiobarbituric acid, ferrozine, lecithin, SDS (sodium dodecyl sulfate), ammonium thiocyanate, ferric chloride, KH2PO4, and K2HPO4 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Sodium dihydrogen phosphate, disodium hydrogen phosphate, NaBr, and trichloacetic acid were obtained from Merck & Co. Inc. (Kenilworth, JN, USA). Tween 20 was obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). HCl, NaCl, and copper sulfate were purchased from the Tokyo Chemical Industry Co. Ltd. (Tokyo, Japan). EDTA was purchased from Mallinckrodt Pharmaceuticals (Raleigh, NC, USA). Ferrous chloride, Coomassie brilliant blue G-250, n-butanol, and phosphotungstic acid were bought from Avantor Performance Materials (Baker analyzed reagents; Center Valley, PA, USA).
2.2. Plant material and the preparation of the extracts
A. chinense was bought and cultured from Puli Town in Nantou County, Taiwan (April 2013). The bulb portion of the plant was washed and dried in the shade. The water extract of A. chinense bulb was prepared by a plant-to-water ratio of (1:4), and then a blender was used to smash the bulb. Finally, the Likens-Nickerson apparatus was used to heat the extract with solvent (n-hexane-to-ether ratio: 1:1) for 3 hours. Later, nitrogen gas was used to dry the extract and obtain the essential oil (ABO). The remaining residue was filtered and freeze dried to obtain the supernatant (ABW) and residue (ABR) layers.
2.3. Analysis of principal components
2.3.1. Determination of water content, ash content, crude-fat content, crude-protein content, crude-fiber content, and carbohydrate content
The targeting components indicated were detected using methods approved by the Association of Official Agricultural Chemists.
2.3.2. Determination of organic acid
Sample powder (500 mg) was extracted with 50 mL 80% ethanol. This suspension was shaken for 45 minutes at room temperature and filtered through Whatman No. 4 filter paper. The residue was re-extracted five times with an additional 25 mL 80% ethanol. The combined filtrate was then rotary evaporated at 40°C and redissolved in deionized water to a final volume of 10 mL. The aqueous extract was filtered using a 0.45-mm polyvinylidene fluoride membrane filter (Millipore, Billerica, MA, USA) and analyzed using high-performance liquid chromatography (HPLC). The HPLC system consisted of a Hitachi L-2130 pump (Tokyo, Japan), a Rheodyne 7725i injector (Rohnert Park, CA, USA), a 20-mL sample loop, a Hitachi L-2400 UV detector, and an RP-18 GP250 column (4.6 mm × 250 mm; Mightysil, Kanto Chemical Co., Tokyo, Japan). The mobile phase was acetonitrile/deionizer water [75:25 (v/v)] at a flow rate of 0.8 mL/min, and UV detection was at 300 nm. Each organic acid was identified using the authentic organic acid (all from Sigma-Aldrich) and quantified by its respective calibration curve.
2.4. Flavonoids determination
2.4.1. Determination of the content of quercetin and rutin
One gram of the sample was extracted for 24 hours with 20 mL of ethanol in a 35°C water bath. The solution was filtered and prepared for further HPLC analysis. A Hitachi HPLC system with L-2130 pump, L-2200 autosampler, and UV-detector (214 nm and 350 nm, L-2400 UV detector) was used. A RP-18 GP250 column (250 mm × 4.6mm inner diameter, 5 μm; Mightysil, Kanto Chemical Co.) was used, with a mobile phase of phosphate buffer (H2O: 85% phosphoric acid 99.7:0.3, v/v)/acetonitrile/methanol at a flow rate of 1.0 mL/min. The injection volume of the sample was 20 μL. The analytic condition was performed using Fuleki’s method [9]. First, the standard products of quercetin and rutin were dissolved in methanol for establishment of a calibration curve, then calibration curves were constructed using the peak area (Y axis) and the concentration (mg/mL; X axis) of quercetin or rutin standards. The application of HPLC analysis of A. chinense extracts was performed, and the content of quercetin and rutin was determined under different processes associated with A. chinense.
2.4.2. Determination of essential oils by GC-MS
Essential oil was then analyzed using a gas chromatograph/mass selective detector (Hewlett-Packard 6890 GC connected to Hewlett-Packard 5973 MSD; Agilent Technologies, Santa Clara, CA, USA). A capillary column (Rtx-wax, inner diameter: 0.53 mm × 30 m, 1-μm film thickness) was used. The carrier gas (helium) was operated at a flow rate of 30 mL/min, and the air-flow rate was 30 mL/min. Qualitative and quantitative determinations of the volatile components were performed according to Majlat et al [10].
2.5. Antioxidative assay
2.5.1. Preparation of ethanol extract and water extract of A. chinense
Two batches of A. chinense powder (100 mg/batch) with the addition of 200 mL ethanol and deionized water were individually stirred at room temperature for 8 hours. The solution was filtered using Whatman No. 1 filter paper and centrifuged. The supernatant liquid was filtered with Whatman No. 6 filter paper and dried. Then, 20 mL ethanol and deionized water were used to individually dissolve the samples, and the ethanol extract (ACEE) and water extract (ACWE) were obtained and stored at −20°C.
2.5.2. DPPH free radical-scavenging activity test
DPPH free radical-scavenging activity was estimated according previous methods [11]. After 2 mL of the sample solution was added to fresh 2 mL 0.2mM DPPH in methanol, the sample was protected from light for 30 minutes, followed by absorbance measurement at 517 nm.
2.5.3. Determination of total polyphenolic compounds
The concentration of standard gallic acid or 0.2 mL sample was combined with 1 mL Folin-Ciocalteu’s phenol reagent and mixed for 3 minutes. Na2CO3 (7.5%; 0.8 mL) was added and allowed to sit for 30 minutes before measuring at 769 nm. The positive control was gallic acid. Total polyphenolic compounds were determined as a gallic acid equivalent [11].
2.5.4. Total antioxidant-activity capacity assay
Deionized water (1.5 mL), 0.25 mL peroxidase (44 U/mL), 0.25mL H2O2 (500μM), and 0.25 mL 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS; 1mM) were mixed and reacted in a dark room for 1 hour to produce blue-green ABTS. The sample was added to the mixture, and the absorbance was obtained at 734 nm. The lower value of absorbance was detected, while stronger total antioxidant activity was observed. Antioxidant activity was calculated according to the following formula:
2.5.5. Examination of reducing activity
The reducing power was analyzed according to a previously described method [12]. Different concentrations of A. chinense ethanol extract (2.5 mL) were added to 0.2M phosphate buffer (2.5 mL; pH 6.6) and 1% potassium ferricyanide (2.5 mL) and allowed to react for 20 minutes at 50°C before cooling. After adding 10% trichloroacetic acid (2.5 mL), the sample was centrifuged for 10 minutes at 1510g. Supernatant (5 mL), 5 mL deionized water, and 1 mL 0.1% ferric chloride solution was reacted for 10 minutes, and the absorbance was measured at 700 nm. BHA and α-tocopherol were regarded as positive controls. The higher the absorbance value, the stronger the reducing activity exerted. The production of Fe4 [Fe(CN)6]3 was the marker used for determination of reducing activity.
2.6. In vivo assay
2.6.1. Animal experiment
Seventy-six Wistar rats (6-weeks old) were separated into nine groups for serum lipid-regulating experiments. The control group contained 12 rats, while the other groups contained eight rats each. All treated groups were treated with A. chinense prepared from fresh raw materials with different procedures used to obtain ABO, ABW, and ABR. One group of rats was given a normal diet (C) and eight groups of rats were given a high-fat, high-cholesterol diet (H). After two weeks of high-fat inducement, the eight treated groups were separated into HP (Probucol+ H), ABON (A. chinense volatile oil-normal dose + H), ABOH (A. chinense volatile oil-high dose + H), ABWN (A. chinense extracts-normal dose + H), ABWH (A. chinense extracts-high dose + H), ABRN (A. chinense residue-normal dose + H), and ABRH (A. chinense residue-high dose + H) groups (Table 1). The temperature of the rat room was 22 ± 2°C, with relative moisture at 40–60%, and a self-timer was set up enabling light (8:00 AM–8:00 PM) and dark (8:00 PM–8:00 AM) periods. Feed and water were provided ad libitum (Table 2). During the experimental period, the amount of feed was recorded daily, the weight of the rat was measured twice/wk, and orbital blood was sampled for 2 weeks for triglyceride (TG), total cholesterol (TC), high-density lipoprotein (HDL), and low-density lipoprotein (LDL). After 12 weeks, rats were sacrificed, and the stool of the Wistar rats was collected 3 days prior. Rats fasted for 12 hours overnight before being sacrificed with ether. Viscera (liver and kidney), fatty tissue (fat around the kidneys and epididymal fat), and blood were used as specimens. Liver organs were washed twice with phosphate-buffered saline (PBS) and weighed. A portion of tissue was rapidly frozen with liquid nitrogen, then stored at −80°C for further TG and TC content analysis. Other samples were immersed in formalin solution (10% formaldehyde solution) for tissue specimens. Fatty tissues were also washed twice with PBS and weighed.
Table 1.
The animal grouping for testing serum lipid levels in Wistar rats.
| Groups | Diet model |
|---|---|
| C | Normal diet |
| HF | High-fat diet |
| HP | High-fat diet plus porbucol (10 g/kg/FW) |
| ABON | High-fat diet plus normal dose A. chinense essential oil |
| ABOH | High-fat diet plus high dose A. chinense essential oil |
| ABWN | High-fat diet plus normal dose A. chinense extracts |
| ABWH | High-fat diet plus high dose A. chinense extracts |
| ABRN | High-fat diet plus normal dose A. chinense residue |
| ABRH | High-fat diet plus high dose A. chinense residue |
Table 2.
Feed ingredients for Wistar rats.a
| Materials (%) | Groups | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| C | H | HP | ABON | ABOH | ABWN | ABWH | ABRN | ABRH | |
| Casein | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| d,l-Methionine | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Corn starch | 15 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Sucrose | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
| Cellulose | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 3.2 | 3.2 |
| Cholesterol | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Mineral mixture | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 |
| Vitamin mixture | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Choline bitartrate | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Corn oil | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Lard | — | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Probucol | — | — | 10 | — | — | — | - | - | - |
| Volatile oils | — | — | — | 0.018 | 0.036 | — | — | — | — |
| Aqueous | — | — | — | — | — | 0.090 | 0.180 | — | — |
| Residue | — | — | — | — | — | — | - | 1.180 | 2.360 |
ABOH = Allium chinense essential oil-high dose + HF; ABON = Allium chinense essential oil-normal dose + HF; ABRH = Allium chinense residue-high dose + HF; ABRN = Allium chinense residue-normal dose + HF; ABWH = Allium chinense extracts-high dose + HF; ABWN = Allium chinense extracts-normal dose + HF; C = normal diet; HF = high-fat diet; HP = Porbucol + HF.
On the basis of the American Institute of Nutrition-76 formula.
2.6.2. Measurements of animal intake and food efficiency
Daily feed intake was measured and recorded. Food utilization (feed efficiency) was calculated at the end of the experiment using the following formula:
2.6.3. Preparation of serum samples from Wistar rats
After sacrifice, blood was collected into 15-mL centrifuge tubes with a 10-mL syringe. Tubes were centrifuged (800g, 15 minutes) to completely separate the serum and blood cells. The supernatant (serum) was stored at 7°C for further testing of TC, TG, HDL, and LDL.
2.6.3.1. Measurement of serum TC
Serum samples (2 mL) were added along with 200 μL cholesterol reagent to a 96-well plate, mixed, and incubated at room temperature for 10 minutes, and absorbance was measured at 500 nm. TC concentration was calculated according to the formula of the products:
2.6.3.2. Measurement of serum TG
Serum samples (2 mL) were added along with 200 μL cholesterol reagent to a 96-well plate, mixed, and incubated at room temperature for 10 minutes, and absorbance was measured at 500 nm. TG concentration was calculated according to the formula of the products:
2.6.3.3. Measurement of serum LDL
Serum samples (50 μL) were added along with 500 μL LDL reagent, mixed, and incubated at room temperature for 15 minutes prior to centrifugation at 1500g. The supernatant (12.5 μL) was mixed with 250 μL cholesterol reagent and incubated for 10 minutes, followed by measurement of the absorbance at 500 nm. LDL concentration was calculated according to the formula of the products:
2.6.3.4. Measurement of serum HDL
Serum samples (200 μL) were added along with 500 mL HDL reagent, mixed, and incubated at room temperature for 10 minutes prior to centrifugation for 10 minutes at 1500g. Supernatant (25 μL) was mixed with 250 μL cholesterol reagent and incubated for 10 minutes, followed by measurement of the absorbance at 500 nm. HDL concentration was calculated according to the formula of the products:
2.6.4. Preparation of hepatic samples from Wistar rats
Rat liver (0.5 g) was washed three times with 1× PBS, and added to 60 mL chloroform with 30 mL methanol (2:1) to make a homogenate for lipid extraction. The extract was filtered (No. 2; Toyo Screen Kyogo Co., Ltd., Tokyo, Japan).
2.6.4.1. Measurement of hepatic TG
Liver homogenate (50 μL) and 50 μL Triton X-100 were placed in a dark room to dry for 4 hours at 50°C. The dried sample (2 μL) was transferred to a 96-well plate with TG buffer, mixed, and incubated at room temperature for 10 minutes. The absorbance was measured at 500 nm, and the TG concentration was calculated according the formula of the products:
2.6.4.2. Measurement of hepatic TC
Liver homogenate (50 μL) and 50 μL Triton X-100 were placed in a dark room to dry for 4 hours at 50°C. The dried sample (2 μL) was transferred to a 96-well plate with cholesterol buffer, mixed, and incubated at room temperature for 10 minutes. The absorbance was measured at 500 nm, and the TG concentration was calculated according the formula of the products:
2.6.5. Measurement of total neutral cholesterol in feces
Two possible metabolism pathways associated with cholesterol include the conversion to bile acids through bile and neutral-steroid metabolism, followed by excretion with feces. Therefore, determination of fecal neutral sterols could indicate the effects of galangal acetate content on cholesterol absorption and/or increased cholesterol discharge. A modified Folch’s method was used for this determination [13]. The collected feces were dried to a constant weight and powdered. Feces (0.5 g) were mixed with 1 mL chloroform and methanol (2:1), extracted for 12 hours, filtered, and the extract liquid (0.1 mL) obtained. The extract liquid was dried by nitrogen gas, and 1 mL was added to Libermann-Burchard reagent [acetic anhydride:sulfuric acid:acetic acid (20:1:10)] and reacted for 20 minutes at room temperature. The absorbance was measured at 550 nm. Standard cholesterol was used as the control for quantitation of fecal neutral-sterol content.
2.6.6. Measurement of food intake and efficiency
Daily feed intake was measured and recorded. The formula used to calculate food utilization (feed efficiency) at the end of the experiment was as follows:
2.6.7. Measurement of animal body weight
During the trial period, rats were weighed once/wk on a scale and observed for changes in weight.
2.6.8. Measurement of the total fat in animals
After the animals were sacrificed, the peritoneal cavity near the epididymis and the fat around the kidney were weighed.
2.6.9. Histopathology
The liver and fat cells of H rats were extracted, the tissues were fixed in 10% neutral-buffered formalin and samples were dehydrated, cleared, infiltrated, embedded, and sectioned. Representative sections were stained using hematoxylin and eosin [12].
2.6.10. Statistical analysis
Samples were tested in triplicate, and results were presented as mean ± standard deviation (SD) of three independent experiments. The data were analyzed by one-way analysis of variance using SPSS statistical software version 16.0 (SPSS Inc, Chicago, IL, USA). For the mean separation, Duncan’s multiple-range tests were used to determine the mean difference at the level of p < 0.05.
3. Results and discussion
3.1. Analysis of principal components and organic acids
Fresh A. chinense bulbs contained 81.4% water and 12.3% carbohydrates (Table 3). Other constituents included crude fiber (3.01%), crude protein (2.02%), ash (0.80%), and fats (0.50%). Moreover, the composition of organic acids was examined utilizing HPLC analysis. As shown in Table 3, there was only 8.06 mg/g of organic acid in the extracts, with malic acid, a strong ocular irritant [14], constituting the majority (4.05 mg/g). Malic acid is responsible for the acidity of bulbs. Additionally, the bulbs contained 1.07 mg/g of succinic acid, suggesting that A. chinense exhibits anti-inflammatory activity [15].
Table 3.
Components of fresh Allium chinense bulbs.
| Item | Content (wt %) |
|---|---|
| Principal component analysis | |
| Moisture | 81.4 ± 0.03 |
| NFEa | 12.3 ± 0.05 |
| Crude ash | 0.80 ± 0.12 |
| Crude fat | 0.50 ± 0.11 |
| Crude fiber | 3.01 ± 0.45 |
| Crude protein | 2.02 ± 1.30 |
| Organic acid analysis | |
| Oxalic acid | 1.06 ± 0.03 |
| Tartaric acid | 0.67 ± 0.02 |
| Lactate | 0.26 ± 0.01 |
| Citric Acid | 0.95 ± 0.03 |
| Succinic acid | 1.07 ± 0.06 |
| Malic acid | 4.05 ± 0.15 |
| Flavonoids analysis | |
| Quercetin | 0.14 ± 0.01 |
| Rutin | 0.07 ± 0.00 |
NFE = nitrogen-free extract.
NFE = 100% – (% moisture + % crude ash + % crude fat + % crude fiber + % crude protein.
3.2. Flavonoid examination by HPLC
3.2.1. Quercetin and rutin
Flavonoids and polyphenolic compounds are widespread in plants possessing a wide spectrum of biological activities, including antioxidant, antiallergy, anti-inflammatory, and antitumor activities [16]. Two characteristic flavonoids, quercetin and rutin, appeared at tR 25.5 minutes and tR 36.2 minutes, respectively. Quercetin and rutin content was calculated at 0.14% and 0.07% (Table 3), respectively, demonstrating that A. chinense exhibited potential as an antioxidant agent.
3.2.2. Definition of the volatile oil by GC-MS
Allyl sulfides are the source of the characteristic odors of rakkyo in Allium species. GC-MS was used to analyze the volatile oil in A. chinense, and 23 different kinds of volatile compounds were identified. Nineteen of these were sulfide derivatives (17.03%), including dimethyl trisulfide (6.7%), dimethyl disulfide (2%), and methyl-cis-propenyl-disulfide (2%) (Table 4). These findings explained the specific odor of A. chinense.
Table 4.
Analysis of the volatile oils from Allium chinense bulbs by GC-MS.
| Compounds | MW | Content (%)a |
|---|---|---|
| Sulfide | ||
| Methanethiol | 48 | tb |
| Methyl hydrodisulfide | 80 | 0.01 |
| Allyl methyl-sulfide | 88 | 0.04 |
| Dimethyl disulfide | 94 | 2 |
| 3,4-Dimethylthiophene | 112 | 0.04 |
| Methylallyl disulfide | 120 | 0.3 |
| Methyl-cis-propenyl-disulfide | 120 | 2 |
| Methyl-trans-propenyl-disulfide | 120 | 1.8 |
| Dimethyl trisulfide | 126 | 6.7 |
| Allyl propyl disulfide | 148 | 0.1 |
| Di-2-propenyl disulfide | 146 | 0.11 |
| Dipropyl disulfide | 150 | 0.13 |
| trans-Propenyl disulfide | 148 | 0.3 |
| Methyl allyltrisulfide | 152 | 1 |
| 1-Propenyl trisulfide | 154 | 1 |
| 3-Vinyl-4H-1,2-dithiin | 152 | 1 |
| 2-Vinyl-4H-1,3-dithiane | 146 | 0.3 |
| Dimethyl tetrasulfide | 158 | tb |
| Dipropyl trisulfide | 182 | 0.2 |
| Others | ||
| Propanal | 58 | 0.1 |
| Hexanal | 100 | 0.2 |
| 2-Pentylfuran | 138 | tb |
| Limonene | 136 | 0.02 |
GC-MS = gas chromatography mass spectrometry; MW = molecular weight; t = trace.
The contents of the volatile compound (%) in dry A. chinense bulbs.
Trace amount < 0.01%.
Interestingly, there are some sulfide-related volatile oils in other Allium species. The major components of volatile oils in onions (Allium cepa L.) are dimethyl-trisulfide and methyl-propyl-trisulfide; however, the major components in garlic (Allium sativum) are diallyl trisulfide and diallyl disulfide [17,18]. These findings indicated the presence of specific odors associated with this genus and imply the presence of potential health functions. Most sulfur compounds present in onions are in the form of cysteine derivatives (e.g., S-allyl cysteine sulfoxide) that are degraded during extraction by the enzyme allinase into a variety of volatile compounds, including thiosulfonates and polysulfides [19]. Moreover, these compounds have beneficial effects, such as prevention of cancer [20,21], cardiovascular diseases [22,23], hypercholesterolemia [24], hypertension [9], and diabetes [10,25,26].
3.3. Antioxidative assays
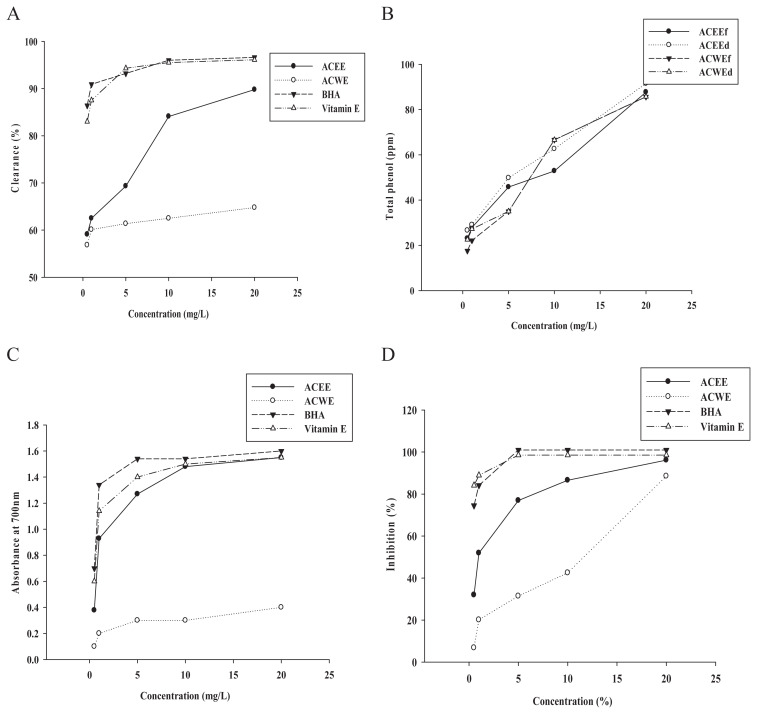
Only one related investigation reported that the steroid ingredients from ethanol extracts from fresh garlic bulbs showed potential for preventing cardiovascular diseases induced by oxidative stress [27]. Additionally, another Allium species (onions) was also tested for its total phenolic and antioxidant properties using antioxidant assays [28]. Therefore, different extraction methods and storage processes of the plant have been studied for their antioxidative effects. Here, antioxidant activity was examined in the ACEE and ACWE, and BHA and α-tocopherol were used as positive controls. At 10 mg/mL and 20 mg/mL, the ACEE showed 84.09–89.77% DPPH free radical-scavenging activity; however, the ACWE exhibited only mild activity (Figure 1A). Total polyphenolic content in A. chinense extracts prepared using four different processes, including fresh and dry bulbs of A. chinense extracted by ethanol (ACEEf and ACEEd) and water (ACWEf and ACWEd), were tested. The total polyphenolic contents of the four extracts displayed similar data, including that the ACEEd exhibited the highest percentage of total polyphenol (91.5%; Figure 1B).
Figure 1.
Antioxidative activity of Allium chinense extracts. (A) The DPPH free radical-scavenging activities; (B) total phenol component; (C) reducing activity; and (D) inhibition of lipid peroxidation (%) by A. chinense extracts. ACEE = A. chinense ethanol extract; ACWE = A. chinense water extract; BHA = Butylated hydroxyanisole; d = dried Allium chinense; DPPH = α,α-diphenyl-β-picrylhydrazyl free radical; f = fresh Allium chinense.
Similarly, the ACEE possessed reduction ability similar to the positive controls BHA and Vitamin E, and exhibited 4–5-fold stronger activity relative to the ACWE (Figure 1C). Additionally, for the total antioxidant capacity assay at concentrations of 10 mg/mL and 20 mg/mL, the ACEE displayed comparable effects 86.5–96.2% to that of BHA and Vitamin E (Figure 1D).
3.4. Effect of A. chinense-extract treatment on H Wistar rats
Garlic and related Allium species are being increasingly investigated both chemically and biologically as nutritional supplements. Naturally occurring sulfur-containing compounds and steroid saponins in the Allium family possess antihyperlipidemic effects against atherosclerosis. Species, such as A. sativum, Allium tuberosum, and Allium porrum, reduce serum cholesterol, TG, LDL, and atherogenic indices in several animal studies [12,28,29]. Although A. chinense contains functional components, such as sulfur compounds and flavonoids, there is no study on the antihyperlipidemic effect of A. chinense. Therefore, here, the effect of A. chinense extracts on lipid content in the serum and liver from rats with normal and high-fat diets were investigated.
3.4.1. Changes in body weight, organ weight, food intake, and water intake in rats
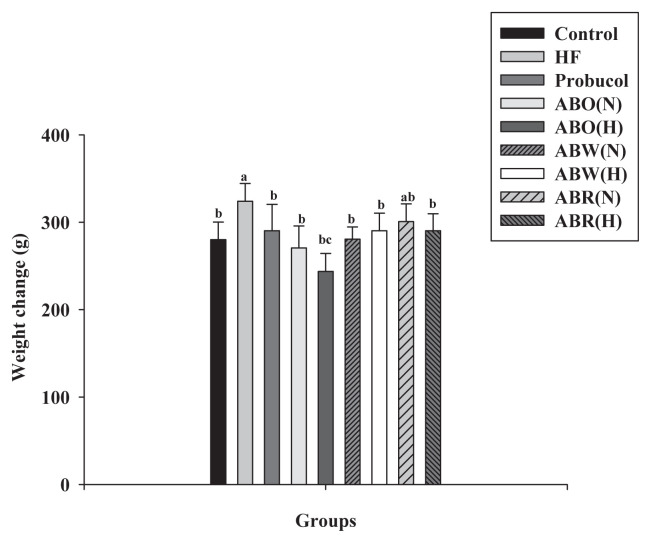
The average initial weight of a H rat was 310 g, and only H rats exhibited significantly increased body weight (p < 0.05). In the organs, the H rat had higher liver weight and the largest kidney weight due to high doses of ABOH. Additionally, consuming food and drinking water did not affect rat weight (Table 5). Figure 2 shows the weight changes of Wistar rats during different treatments. High doses of ABOH effectively slowed the weight gain observed in H rats.
Table 5.
Effect of Allium chinense extracts on body weight, liver weight, kidney weight, food intake, and water intake in Wistar rats induced by high-fat diet.a
| Dietary group | Initial body weight (g) | Final body weight (g) | Liver weight (g) | Liver/body weight | Kidney weight (g) | Kidney/body weight | Food intake (g/wk/Wistar rat) | Water intake (mL/wk/Wistar rat) |
|---|---|---|---|---|---|---|---|---|
| C | 307.7 ± 5.13 | 573.5 ± 2.9 | 14.28 ± 1.53 | 0.026 ± 0.00 | 3.2 ± 0.09 | 0.55 ± 0.01 | 24.4 ± 2.24 | 66.8 ± 18.4 |
| HF | 311.6 ± 8.22 | 630.2 ± 16.9 | 16.84 ± 2.04 | 0.026 ± 0.00 | 3.1 ± 0.27 | 0.50 ± 0.01 | 24 ± 1.59 | 76.7 ± 18.1 |
| HP | 312.4 ± 2.47 | 597.2 ± 3.7 | 13.91 ± 1.16 | 0.023 ± 0.00 | 3.1 ± 0.19 | 0.55 ± 0.02 | 23.5 ± 2.52 | 71.7 ± 16.7 |
| ABON | 306.4 ± 9.22 | 579.5 ± 1.0 | 14.22 ± 1.95 | 0.025 ± 0.00 | 3.2 ± 0.21 | 0.59 ± 0.04 | 21.6 ± 2.95 | 80.5 ± 21.1 |
| ABOH | 315.6 ± 6.41 | 550.0 ± 1.4 e | 14.52 ± 1.28 | 0.027 ± 0.00 | 3.5 ± 0.26 | 0.65 ± 0.03 | 21.3 ± 3.46 | 85.8 ± 25.7 |
| ABWN | 309.6 ± 14.3 | 619.4 ± 3.5 | 15.11 ± 1.2 | 0.025 ± 0.00 | 2.9 ± 0.29 | 0.50 ± 0.02 | 21.6 ± 2.74 | 76.2 ± 20.3 |
| ABWH | 307.0 ± 7.03 | 571.9 ± 1.1 | 15.37 ± 1 | 0.027 ± 0.00 | 3.0 ± 0.19 | 0.56 ± 0.01 | 21.2 ± 2.53 | 66.8 ± 12.4 |
| ABRN | 308.7 ± 2.82 | 582.5 ± 3.4 | 14.69 ± 2.6 | 0.025 ± 0.00 | 3.2 ± 0.23 | 0.57 ± 0.06 | 21.8 ± 2.26 | 99.3 ± 13.4 |
| ABRH | 308.4 ± 7.20 | 605.2 ± 3.2 | 15.99 ± 0.9 | 0.026 ± 0.00 | 3.0 ± 0.11 | 0.50 ± 0.01 | 23.1 ± 2.96 | 85.3 ± 16.5 |
ABOH = A. chinense essential oil-high dose + HF; ABON = A. chinense essential oil-normal dose + HF; ABRH = A. chinense residue-high dose + HF; ABRN = A. chinense residue-normal dose + HF; ABWH = A. chinense extracts-high dose + HF; ABWN = A. chinense extracts-normal dose + HF; C = normal diet; HF = high-fat diet; HP = Porbucol + HF; SD = standard deviation.
All values are expressed as mean ± SD (n = 8) for eight Wistar rats per dietary group.
Figure 2.
Effect of Allium chinense extracts on body weight in high-fat-diet Wistar rats. The columns not sharing a common superscript letter are significantly different from control.
3.4.2. Effect on serum lipid levels in H rats
The effect on serum lipid levels in H rats is illustrated in Table 6. Initially, the TC of each group was insignificant (p > 0.05), with the maximum TC content attained at 6 weeks (Table 6). During that time, the H group exhibited the highest TC of all the groups (80 mg/dL). ABWN, ABWH, and ABR groups exhibited significant inhibition of TC increase with no drawbacks.
Table 6.
Effect of Allium chinense extracts on lipid profiles in serum and livers of Wistar rats fed a high-fat diet.a
| Groups | C | H | HP | ABON | ABOH | ABWN | ABWH | ABRN | ABRH |
|---|---|---|---|---|---|---|---|---|---|
| Serum (mg/dL) | |||||||||
| TG | 132.45 ± 2.32 | 181.25 ± 1.37 | 129.75 ± 3.66 | 124.60 ± 3.69 | 115.33 ± 2.47 | 130.26 ± 1.26 | 120.33 ± 5.32 | 121.04 ± 2.15 | 100.21 ± 1.24 |
| TC | 80.14 ± 2.33 | 81.34 ± 2.31 | 70.33 ± 3.33 | 61.33 ± 2.66 | 61.24 ± 2.32 | 60.13 ± 3.14 | 60.88 ± 2.32 | 60.35 ± 2.66 | 60.33 ± 3.66 |
| LDL-C | 41.26 ± 2.37 | 50.13 ± 5.33 | 42.16 ± 2.33 | 40.13 ± 4.53 | 42.70 ± 3.14 | 40.67 ± 3.32 | 41.32 ± 3.20 | 42.10 ± 1.03 | 40.63 ± 1.99 |
| HDL-C | 14.24 ± 2.31 | 7.23 ± 0.68 | 14.51 ± 1.36 | 12.63 ± 2.54 | 15.21 ± 2.02 | 11.33 ± 2.16 | 11.46 ± 1.59 | 11.52 ± 0.40 | 10.15 ± 2.67 |
| Liver (mg/g) | |||||||||
| TG | 32.01 ± 2.32 | 38.26 ± 2.21 | 30.56 ± 1.23 | 29.31 ± 2.11 | 28.11 ± 2.10 | 30.11 ± 1.13 | 29.52 ± 1.54 | 29.21 ± 1.36 | 29.13 ± 1.11 |
| TC | 22.32 ± 2.14 | 26.63 ± 1.15 | 22.63 ± 1.00 | 21.11 ± 1.04 | 21.34 ± 1.26 | 23.51 ± 1.03 | 22.12 ± 1.23 | 22.33 ± 1.25 | 22.56 ± 2.33 |
ABOH = A. chinense essential oil-high dose + HF; ABON = A. chinense essential oil-normal dose + HF; ABRH = A. chinense residue-high dose + HF; ABRN = A. chinense residue-normal dose + HF; ABWH = A. chinense extracts-high dose + HF; ABWN = A. chinense extracts-normal dose + HF; C = normal diet; HDL-C = high-denisty lipoprotein; HF = high-fat diet; HP = Porbucol + HF; LDL-C = low-density lipoprotein; SD = standard deviation; TC = total cholesterol; TG = triglyceride.
All values are expressed as mean ± SD (n = 8) for eight Wistar rats per dietary group.
The H group exhibited the maximum concentration (181.25 mg/dL) of serum TG during 12 weeks (Table 6). After treatment with A. chinense extracts, ABOH and ABRN groups showed the highest inhibition of serum TG production.
Changes in serum LDL in H rats are shown in Table 6. During the 1st week, each group showed insignificant differences (p > 0.05); however, an increasing trend of serum LDL was observed after 10 weeks of treatment. The H and control groups displayed higher serum LDL levels at concentrations of 50 mg/dL. However, ABWH and ARBH groups showed effective inhibition of LDL production, indicating that A. chinense may prevent risks associated with atherosclerosis.
Additionally, serum HDL variation in H rats is shown in Table 6. During the 8th week, a slight change in HDL concentration was observed, especially in the H group, which had the lowest concentration at 11 mg/dL (p < 0.05). ABO groups (normal and high doses) exhibited increased HDL concentrations to 5–7 mg/mL, demonstrating that essential oils can improve HDL production. In contrast, the H group both increased the LDL concentration and decreased the HDL concentration. These results were similar with the hypolipidemic effects of S-methyl cysteine sulfoxide from Alliumcepa Linn [23] and suggest that volatile oils can improve HDL levels [17].
3.4.3. Effect on hepatic lipids in H rats
The H group exhibited the highest TG content in the liver at 35 mmol/L (p < 0.05; Table 6). The ABO groups (ABON and ABOH) decreased TG levels as effectively as the positive-control group, demonstrating that volatile oils can improve TG accumulation. Moreover, all groups, except ABWN, exhibited the same antihyperlipedemic effect as the positive control group. Previously, sulfide components from fermented garlic was reported to protect against TG accumulation in obese rats fed a high-fat diet [17] (Table 6).
3.4.4. Effect on feed intake and efficiency in the H rat model
Total feed intake of the control and probucol-treated groups showed significant differences from those of the H and A. chinense-extract groups in H rats (Table 7). The observed decrease in total feed intake of H rats was due to the heat-equilibrium effect, which reduced the diet of the rats. The feed efficiency in the positive control group was the highest (17.47%) compared with other groups. The study on feed efficiency can help explore the health of rats and improve formulae associated with rat feed.
Table 7.
Effect of various Allium chinense extracts on feed intake and efficiency in high-fat-diet Wistar rats.a
| Groups | Feed intake (kg) | Feed efficiency (%) |
|---|---|---|
| C | 2.21 ± 0.11 | 11.77 ± 1.29 |
| HF | 1.94 ± 0.10 | 17.47 ± 2.43 |
| HP | 2.18 ± 0.11 | 12.95 ± 1.92 |
| ABON | 1.97 ± 0.08 | 13.65 ± 2.54 |
| ABOH | 1.85 ± 0.07 | 12.95 ± 2.40 |
| ABWN | 1.92 ± 0.08 | 16.25 ± 1.48 |
| ABWH | 1.86 ± 0.04 | 14.52 ± 2.53 |
| ABRN | 1.89 ± 0.15 | 14.48 ± 2.10 |
| ABRH | 2.05 ± 0.04 | 14.61 ± 1.47 |
ABOH = A. chinense essential oil-high dose + HF; ABON = A. chinense essential oil-normal dose + HF; ABRH = A. chinense residue-high dose + HF; ABRN = Allium chinense residue-normal dose + HF; ABWH = A. chinense extracts-high dose + HF; ABWN = A. chinense extracts-normal dose + HF; C = normal diet; HF = high-fat diet; HP = Porbucol + HF; SD = standard deviation.
All values are expressed as mean ± SD (n = 8) for eight Wistar rats per dietary group.
3.4.5. Effect of weight on organ fat in H rats
Table 8 shows the effect of weight on organ fat in H rats. The H group possessed the highest ratio of visceral and epididymal fat to body weight (p < 0.05), indicating that the H group had the highest visceral fat, while the ABO group had the lowest epididymal fat. Porbucol can reduce epididymal fat significantly compared with amounts observed in the H and control groups. However, the A. chinense-extract groups did not show a significant decrease in epididymal fat.
Table 8.
Effect of visceral and epididymal fat in high-fat-diet Wistar rats induced by Allium chinense extracts and porbucol.a
| Groups | Visceral fat + epididymal fat/body weight (g/g) | Epididymal fat/body weight (g/g) |
|---|---|---|
| C | 0.07 ± 0.01 | 0.034 ± 0.00 |
| HF | 0.09 ± 0.01 | 0.041 ± 0.00 |
| HP | 0.07 ± 0.01 | 0.032 ± 0.01 |
| ABON | 0.07 ± 0.01 | 0.032 ± 0.00 |
| ABOH | 0.06 ± 0.01 | 0.027 ± 0.00 |
| ABWN | 0.07 ± 0.01 | 0.030 ± 0.01 |
| ABWH | 0.08 ± 0.01 | 0.036 ± 0.00 |
| ABRN | 0.08 ± 0.01 | 0.034 ± 0.01 |
| ABRH | 0.05 ± 0.01 | 0.027 ± 0.01 |
ABOH = A. chinense essential oil-high dose + HF; ABON = A. chinense essential oil-normal dose + HF; ABRH = A. chinense residue-high dose + HF; ABRN = A. chinense residue-normal dose + HF; ABWH = Allium chinense extracts-high dose + HF; ABWN = A. chi-nense extracts-normal dose + HF; C = normal diet; HF = high-fat diet; HP = Porbucol + HF; SD = standard deviation.
All values are expressed as mean ± SD (n = 8) for eight Wistar rats per dietary group.
3.4.6. Histopathology of livers and fat cells
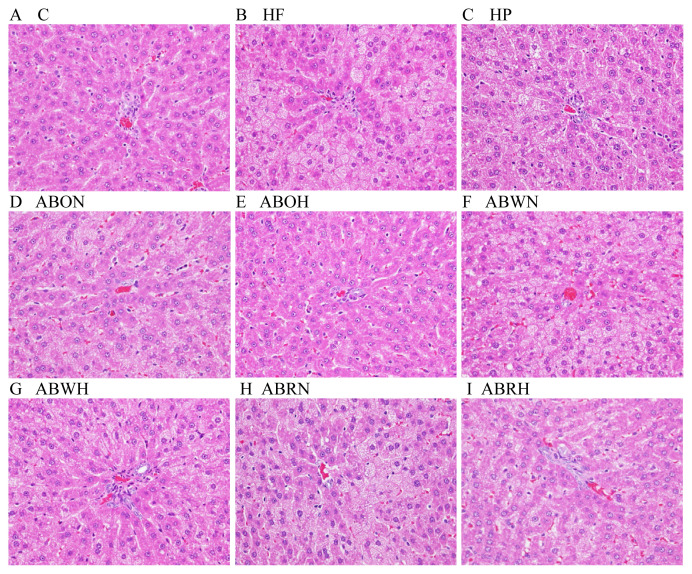
Histological examination of liver specimens (Figure 3) after high-dose treatments of essential oils (ABOH) and residue extracts (ABRH) indicated significant decreases in lesion-histopathology score (Table 9; Figure 3) in H rats compared with the probucol-treated group. In contrast, results from fat cells from H rats exhibited no significant differences between all treatment groups and the control. Fats accumulated in liver cells form microvesicular and macrovesicular changes at different stages of abnormal retention and represent fatty liver status. The histopathology scores associated with the ABOH and ABRH groups were strongly improved relative to those of the H group (Table 9).
Figure 3.
Histopathological alterations (400×) in livers treated with high-fat-diet-induced fatty liver in rats. (A) Group C; (B) Group HF; (C) Group HP; (D) Group ABON; (E) Group ABOH; (F) Group ABWN; (G) Group ABWH; (H) Group ABRN, and (I) Group ABRH. ABOH = Allium chinense essential oil-high dose + HF; ABON = Allium chinense essential oil-normal dose + HF; ABRH = Allium chinense residue-high dose + HF; ABRN = Allium chinense residue-normal dose + HF; ABWH = Allium chinense extracts-high dose + HF; ABWN = Allium chinense extracts-normal dose + HF; C = normal diet; HF = high-fat diet; HP =Porbucol + HF.
Table 9.
Effects of Allium chinense extract on histopathologic scores in high fat diet-induced fatty livers in rats.
| Group | Histopathologic score of liversa,b | ||
|---|---|---|---|
|
| |||
| Fatty liver (General degree) | Microvesicles | Macrovesicles | |
| C | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| HF | 4.33 ± 0.58 | 4.33 ± 0.58 | 2.67 ± 0.58 |
| HP | 2.33 ± 0.58 | 2.33 ± 0.58 | 1.00 ± 1.00 |
| ABON | 2.33 ± 0.58 | 2.33 ± 0.58 | 2.33 ± 0.58 |
| ABOH | 0.67 ± 0.58 | 0.67 ± 0.58 | 0.67 ± 1.15 |
| ABWN | 3.33 ± 0.58 | 3.33 ± 0.58 | 3.33 ± 0.58 |
| ABWH | 2.33 ± 1.15 | 2.33 ± 1.15 | 0.67 ± 1.15 |
| ABRN | 3.33 ± 0.58 | 3.33 ± 0.58 | 1.33 ± 1.15 |
| ABRH | 0.33 ± 0.58 | 0.33 ± 0.58 | 0.00 ± 0.00 |
ABOH = A. chinense essential oil-high dose + HF; ABON = A. chinense essential oil-normal dose + HF; ABRH = A. chinense residue-high dose + HF; ABRN = A. chinense residue-normal dose + HF; ABWH = Allium chinense extracts-high dose + HF; ABWN = A. chinense extracts-normal dose + HF; C = normal diet; HF = high-fat diet; HP = Porbucol + HF; SD = standard deviation.
Lesion degree was graded from 1 to 5 depending on severity: 1 = minimal (<1%); 2 = slight (1–25%); 3 = moderate (26–50%); 4 = moderate/severe (51–75%); 5 = severe/high (76–100%). The final numerical score was calculated by dividing the sum of the number per grade of affected rat by the total number of examined rats (n ≥ 3).
All values are expressed as mean ± SD.
In conclusion, chemical constituents, as well as antioxidant and antihyperlipidemic activities, of A. chinense were examined. Both in vitro and in vivo experiments suggested that A. chinense possessed antioxidant and serum lipid effects in H Wistar rats. These results indicated that the antioxidant activity was concentrated in the ethanol extract; however, hypolipidemic affects were produced following treatment using its essential oils and residue extracts. This is the first report investigating the antihyperlipidemic effect of A. chinense extracts on H Wistar rats. The possible mechanisms of activity associated with A. chinense are currently under investigation.
Acknowledgments
This work was supported by a research grant from the Ministry of Education, Executive Yuan of Taiwan, under the project title “Studies on the active components extraction and hypolipidemic effect evaluation of Allium chinense” (102B-29-022)”.
Funding Statement
This work was supported by a research grant from the Ministry of Education, Executive Yuan of Taiwan, under the project title “Studies on the active components extraction and hypolipidemic effect evaluation of Allium chinense” (102B-29-022)”.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
REFERENCES
- 1. Rajendran P, Nandakumar N, Rengarajan T, Palaniswami R, Gnanadhas EN, Lakshminarasaiah U, Gopas J, Nishigaki I. Antioxidants and human diseases. Clin Chim Acta. 2014;436:332–47. doi: 10.1016/j.cca.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 2. Xu Z, Um YC, Kim CH, Lu G, Guo DP, Liu HL, Bah AA, Mao A. Effect of plant growth regulators, temperature and sucrose on shoot proliferation from the stem disc of Chinese jiaotou (Allium chinense) and in vitro bulblet formation. Acta Physiol Plant. 2008;30:521–8. [Google Scholar]
- 3. Banerjee SK, Maulik SK. Effect of garlic on cardiovascular disorders: a review. Nutr J. 2002;1:4–18. doi: 10.1186/1475-2891-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mochizuki E, Yamamoto T. Identification of Allium products using flame photometric detection gas chromatography and distribution patterns of volatile sulfur compound. J Agr Food Chem. 1998;46:5170–6. [Google Scholar]
- 5. Peng J, Yao X, Tezuka Y, Kikuchi T, Narui T. New furostanol glycosides, chinenoside IV and V, from Allium chinense. Planta Med. 1996;62:465–8. doi: 10.1055/s-2006-957941. [DOI] [PubMed] [Google Scholar]
- 6. Jiang Y, Wang NL, Yao X, Kitanaka S. A new spirostanol saponin from Allium chinense. Chinese Chem Lett. 1997;8:965–6. [Google Scholar]
- 7. Baba M, Ohmura M, Kishi N, Okada Y, Shibata S, Peng J, Yao SS, Nishino H, Okuyama T. Saponins isolated from Allium chinense G. Don and antitumor-promoting activities of isoliquiritigenin and laxogenin from the same drug. Biol Pharm Bull. 2000;23:660–2. doi: 10.1248/bpb.23.660. [DOI] [PubMed] [Google Scholar]
- 8. Ren G, Qiao HX, Yang J, Zhou CX. Protective effects of steroids from Allium chinense against H2O2-induced oxidative stress in rat cardiac H9C2 cells. Phytothe Res. 2010;24:404–9. doi: 10.1002/ptr.2964. [DOI] [PubMed] [Google Scholar]
- 9. Fuleki T. Rutin, the main component of surface deposits on pickled green asparagus. J Food Sci. 1990;64:252–4. [Google Scholar]
- 10. Majlát P, Erdös Z, Takás J. Calculation and application of the retention indices in programmed-temperature gas chromatography. J Chromatogr A. 1974;9:89–103. [Google Scholar]
- 11. Wu CP, Calcagno AM, Hladky SB, Ambudkar SV, Barrand MA. Modulatory effects of plant phenols on human multidrug-resistance proteins 1, 4 and 5 (ABCC1, 4 and 5) FEBS J. 2005;272:4725–40. doi: 10.1111/j.1742-4658.2005.04888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jung YM, Lee SH, Lee DS, Youc MJ, Chung IK, Cheon WH, Kwon YS, Leed YJ, Ku SK. Fermented garlic protects diabetic, obese rats when fed a high-fat diet by antioxidant effects. Nutr Res. 2011;31:387–96. doi: 10.1016/j.nutres.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 13. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 14. Fiume Z. Final report on the safety assessment of malic acid and sodium malate. Int J Toxicol. 2001;20:47–55. doi: 10.1080/109158101750300946. [DOI] [PubMed] [Google Scholar]
- 15. Volchegorskii IA, Pravdin EV, Uzlova TV. Effect of 3-oxypyridine and succinic acid derivatives on endometrial leucocyte infiltration and lipid peroxidation in recrudescence of inflammatory diseases of the uterus and its appendages. Eksp Klin Farmakol. 2013;76:13–8. [PubMed] [Google Scholar]
- 16. Dai F, Miao Q, Zhou B, Yang L, Liu ZL. Protective effects of flavonols and their glycosides against free radical-induced oxidative hemolysis of red blood cells. Life Sci. 2006;78:2488–93. doi: 10.1016/j.lfs.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 17. Sakai Y, Murakami T, Yamamoto Y. Antihypertensive effects of onion on NO synthase inhibitor-induced hypertensive rats and spontaneously hypertensive rats. Biosci Biotechnol Biochem. 2003;67:1305–11. doi: 10.1271/bbb.67.1305. [DOI] [PubMed] [Google Scholar]
- 18. El-Demerdash FM, Yousef MI, El-Naga NI. Biochemical study on the hypoglycemic effects of onion and garlic in alloxan-induced diabetic rats. Food Chem Toxicol. 2005;43:57–63. doi: 10.1016/j.fct.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 19. Babu PS, Srinivasan K. Influence of dietary capsaicin and onion on the metabolic abnormalities associated with streptozotocin induced diabetes mellitus. Mol Cell Biochem. 1997;175:49–57. doi: 10.1023/a:1006881027166. [DOI] [PubMed] [Google Scholar]
- 20. Kumari K, Mathew BC, Augusti KT. Antidiabetic and hypolipidemic effects of Smethyl cysteine sulfoxide isolated from Alliumcepa Linn. Indian J Biochem Biophys. 1995;32:49–54. [PubMed] [Google Scholar]
- 21. Kumari K, Augusti KT. Antidiabetic and antioxidative effects of S-methyl cysteine sulfoxide isolated from onions (Allium cepa Linn) compared to standard drugs in alloxan diabetic rats. Indian J Exp Biol. 2002;40:1005–9. [PubMed] [Google Scholar]
- 22. Matsuura H. Saponins in garlic as modifiers of the risk of cardiovascular disease. J Nutr. 2011;131:1000–5. doi: 10.1093/jn/131.3.1000S. [DOI] [PubMed] [Google Scholar]
- 23. Siddiq M, Roidoung S, Sogi DS, Dolan KD. Total phenolics, antioxidant properties and quality of fresh-cut onions (Allium cepa L.) treated with mild-heat. Food Chem. 2013;136:803–6. doi: 10.1016/j.foodchem.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 24. Movahedian H, Sadeghi A, Ghannadi MG, Azarpajooh S. Hypolipidemic activity of Allium porrum L. in cholesterol-fed rabbits. J Med Food. 2006;9:98–101. doi: 10.1089/jmf.2006.9.98. [DOI] [PubMed] [Google Scholar]
- 25. Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the autoxidation of sobean oil in cyclodextrin emulsion. J Agric Food Chem. 1992;40:945–8. [Google Scholar]
- 26. Christel QD, Bernard G, Jacques V, Thierry D, Claude B, Miches L, Micheline C, Jean CC, Francois B, Francis T. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J Ethnopharmacol. 2000;72:35–42. doi: 10.1016/s0378-8741(00)00196-3. [DOI] [PubMed] [Google Scholar]
- 27. Oyaizu M. Antioxidative activity of browning products of glucosamine fractionated byorganic solvent and thin-layer chromatography. Nippon Shokuhin Kogyo Gakkaish. 1986;35:771–5. [In Japanese, English abstract] [Google Scholar]
- 28. Richmond W. Preparation and properties of a cholesterol oxidase from Nocardia sp. and its application to the enzymatic assay of total cholesterol in serum. Clin Chem. 1973;19:1350–6. [PubMed] [Google Scholar]
- 29. Lin LY, Peng CC, Yang YL, Peng RY. Optimization of bioactive compounds in buckwheat sprouts and their effect on blood cholesterol in hamsters. J Agric Food Chem. 2008;56:1216–23. doi: 10.1021/jf072886x. [DOI] [PubMed] [Google Scholar]