Abstract
A polyphenol-enriched extract from selenium-enriched Ziyang green tea (ZTP) was selected to evaluate its antitumor effects against human breast cancer MCF-7 cells. In ZTP, (−)-epigallocatechin gallate (28.2%) was identified as the major catechin, followed by (−)-epigallocatechin (5.7%) and (−)-epicatechin gallate (12.6%). ZTP was shown to inhibit MCF-7 cell proliferation (half maximal inhibitory concentration, IC50 = 172.2 μg/mL) by blocking cell-cycle progression at the G0/G1 phase and inducing apoptotic death. Western blotting assay indicated that ZTP induced cell-cycle arrest by upregulation of p53 and reduced the expression of CDK2 in MCF-7 cells. ZTP-caused cell apoptosis was associated with an increase in Bax/Bcl-2 ratio, and activation of caspase-3 and -9. MCF-7 cells treated with ZTP also showed an overproduction of reactive oxygen species, suggesting that reactive oxygen species played an important role in the induction of apoptosis in MCF-7 cells. This is the first report showing that ZTP is a potential novel dietary agent for cancer chemoprevention or chemotherapy.
Keywords: apoptosis, breast cancer, MCF-7 cells, polyphenols, Ziyang green tea
1. Introduction
Tea derived from the leaves of the plant Camellia sinensis is the second most widely consumed beverage in the world. Tea ingestion is claimed to have antitumor beneficial effects, which are mediated by green tea polyphenols (catechins) [1,2]. Interestingly, the increasing evidence in green tea consumption also suggests that most of the biological effects, including antineoplastic activities, are attributed to the antioxidant and free radical-scavenging properties of tea [3–6]. For a long time, catechins, a group of polyphenolic compounds, have been considered as the active ingredients responsible for the antioxidant effects of tea [3,5,6]. The major catechins in tea are (−)-epigallocatechin gallate (EGCG), (−)-epigallocatechin (EGC), and (−)-epicatechin gallate (ECG), and their antioxidant activities have been demonstrated in the following order: catechin < epicatechin < EGC < ECG < EGCG [7]. Recently, interest in flavonoids and other phenolic compounds has increased owing to their potential health benefits including anticancer property.
A specific C. sinensis is named as selenium-enriched Ziyang tea in China and is widely distributed in the second seleniferous region, Ziyang County, in northern China. It is a very popular selenium-enriched food because it is claimed to promote health and alleviate the severity of many disorders [8]. In fact, previous studies suggested that antioxidant activity of selenium-enriched green tea was higher than that of regular green tea [9–13]. At present, selenium-enriched Ziyang green tea is recommended as an important dietary source of health-promoting compounds because of its chemical composition and antioxidant and anticancer properties [13]. Accordingly, further studies on anticancer activities of Ziyang tea polyphenols are necessary.
The majority of recent mechanistic studies mainly focus on the anticancer properties of specific pure phenolic compounds, such as quercetin, 5-o-caffeoylquinic acid, and catechins [14,15]. Nevertheless, combinations of polyphenols naturally found in fruits and vegetables have been suggested to be most favorable for cancer prevention and their anticarcinogenic effects [16,17]. Therefore, the main endeavor of the present study was to investigate the potential antiproliferative activity of the polyphenols derived from selenium-enriched Ziyang green tea against human breast cancer MCF-7 cells. For this purpose, a polyphenol-enriched extract (ZTP) from selenium-enriched Ziyang green tea was prepared, and its phenolic profiles were determined by high-performance liquid chromatography (HPLC). Furthermore, we assessed ZTP-induced cellular viability, cell-cycle distribution, apoptosis, and changes in the protein levels involved in the control of cell cycle and apoptosis, including the expression of Bax and Bcl-2, and the activation of the caspase cascade in human breast cancer MCF-7 cells. Moreover, we also elucidated the possible involvement of intracellular reactive oxygen species (ROS) in the antitumor activity of the promising bioactive tea polyphenols. As far as we know, it is the first time that the antiproliferative properties of the polyphenolic extract derived from selenium-enriched Ziyang tea against human breast cancer MCF-7 cells have been evaluated.
2. Methods
2.1. Materials and reagents
Folin–Ciocalteu (99%), EGC (98%), ECG (98%), EGCG (95%), catechin (95%), and gallic acid (98%) were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). In addition, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dimethyl sulfoxide, bovine serum albumin, EDTA, propidium iodide (PI), phenylmethyl-sulfonyl fluoride, RNase-A, Tris-HCl, glycine, dodecyl sulfate sodium salt (SDS), and nonfat milk powder were purchased from Sigma-Aldrich (St Louis, MO, USA). The annexin V-FITC/PI apoptosis detection kit was obtained from BestBio (Shanghai, China). The poly-vinylidene difluoride (PVDF) membrane was from Millipore (Bedford, MA, USA). The mouse monoclonal antibodies against Bax, Bcl-2, p53, CDK2, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and the horseradish peroxidase-conjugated goat antimouse secondary antibody were obtained from BioVision, Inc. (Milpitas, CA, USA). Dichlorofluorescein diacetate (DCFH-DA) was obtained from BestBio Co. (Shanghai, China). Ultrapure water was prepared by the Millipore Milli Q-Plus system (Millipore) in our own laboratory.
2.2. Sample preparation and extraction
Fresh leaves of Ziyang tea cultivar were harvested in June 2012 from Ziyang County of Shaanxi Province, China. Tea leaves were shattered by an electric grinder after dehydration, which was carried out in a hot air dryer (Changzhou Far Yu Drying Equipment Co., Ltd, Changzhou, China) at 45°C. For ethanolic extract preparation [18,19], 50 g of dried tea leaves were thoroughly mixed with 500 mL of 80% ethanol, and were extracted three times at 70°C for 60 minutes for each extraction while being vigorously shaken. The obtained supernatant was further concentrated and precipitated by 1.0M ZnCl2 (pH 6.2), and 0.4M hydrochloric acid was used to dissolve the sediments. The resultant solution was further extracted by ethyl acetate to isolate and enrich lipophilic polyphenols. After the drying process of ethyl acetate fraction, the tea polyphenol-enriched extract was obtained and defined as ZTP.
2.3. Determination of total flavonoids in ZTP
Total flavonoids in ZTP were measured as gallic acid equivalents (GAEs) using a modified aluminum chloride colorimetric method [20], expressed as milligrams of GAEs per gram of extract [19]. The data were presented as the average of triplicate assays.
2.4. HPLC analysis of phytochemicals in ZTP
Phytochemicals in the polyphenol-enriched ZTP were identified by a HPLC method [18,19]. The analysis was carried out using a reversed-phase HPLC column (4.6 mm i.d. × 250 mm, 5 μm, Inertsil ODS-SP; GL Sciences Inc., Tokyo, Japan) on a Shimadzu LC-2010A HPLC system equipped with an UV detector and an autosampler (Shimadzu, Kyoto, Japan). Gradient elution was performed by varying the proportion of solvent A (water, containing 0.2% acetic acid) to solvent B (acetonitrile). The gradient program was as follows: 0–3 minutes with 90% solvent A; 3–5 minutes from 90% to 80% solvent A; 5–10 minutes from 80% to 60% solvent A; and 10–40 minutes with 60% solvent A. The flow rate of the mobile phase was 1 mL/min, the UV detection wavelength was 280 nm, and the sample injection volume was 20 μL at a 30°C column temperature.
2.5. Cell lines and cell culture
All the cell lines were purchased from Cell Bank of Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China), including human breast carcinoma cell line (MCF-7) and normal mammary epithelial cell line (MCF-10A). In addition, all the purchased cells were cultivated in a growth medium consisting of Dulbecco’s modified Eagle’s Medium (DMEM) with 2-oxopropanoic acid sodium salt (10mM), L-glutamine (4.0mM), 10% heat-inactivated fetal bovine serum, 100 units/mL of penicillin, and 100 μg/mL streptomycin at 37°C in an incubator with 5% CO2 and 95% air.
2.6. Determination of cell viability
The MTT assay was applied to determine cell viability [21]. MCF-7 cells were seeded onto 96-well plates at a density of 1 × 104 cells/well, and treated with different doses of ZTP at a concentration of 0 μg/mL, 10 μg/mL, 50 μg/mL, 100 μg/mL, 200 μg/mL, and 400 μg/mL at 37°C for 24 hours, 48 hours, and 72 hours. After removal of media, the cells were washed by phosphate buffer solution (PBS) and incubated for an additional 4 hours in a medium containing 0.5 mg/mL MTT. Subsequently, the formed crystal formazan was dissolved in 150 μL of solution (10% SDS plus 0.01M HCl and 5% isobutyl alcohol). After complete dissolution of crystals, absorbance of the solution was measured by an enzyme-linked immunosorbent assay reader (Rayto-RT6000; Rayto, Guangdong, China). The percentage of cell survival was expressed as follows [22]:
| (1) |
2.7. Morphological evaluation
A 12-well plate containing 5 × 104 MCF-7 cells/well was incubated at 37°C for 24 hours, and then various doses of ZTP were added and the culture was continued for 48 hours. Subsequently, the cells were washed with PBS for further observation. The morphology of cells was visualized by fluorescence microscopy (Leica DMIL LED; Leica, Solms, Germany) [23].
2.8. Cell-cycle analysis
Cell-cycle analysis was performed by PI staining (Sigma-Aldrich). The cells were treated with different concentrations of ZTP (50 μg/mL and 100 μg/mL) for 24 hours, and it was fixed in 70% ethanol, incubated with 0.1% RNase A in PBS at 37°C for 30 minutes, and resuspended in PBS containing 25 μg/mL PI for 30 minutes at room temperature. The stained cells were analyzed by flow cytometry (FACSCalibur; Becton Dickinson, Franklin Lakes, NJ, USA). The Modifit program (version 5.7.2, Verity Software House Inc., Topsham, ME, USA) was used to calculate the percentage of cells in the G0/G1, S, and G2/M phases [24].
2.9. Apoptosis assay
According to the method of cell-cycle analyses described above, cell culture and treatment were carried out. Annexin V-FITC/PI double staining method was employed to analyze cell apoptosis using commercial detection kit (BestBio Co.) following the manufacturer’s instructions [18].
2.10. Western blot analysis
MCF-7 cells were incubated in a 25 cm2 cell culture flask for 24 hours at a density of 5 × 105 cells/flask. After incubation for 24 hours, the cells were treated with different concentrations of ZTP (0 μg/mL, 50 μg/mL, and 100 μg/mL) for 48 hours, or treated with 100 μg/mL ZTP for different time periods (0 hours, 24 hours, 48 hours). The total proteins of collected MCF-7 cells were extracted in lysis buffer (150mM NaCl, 50mM Tris at pH 7.4, 1mM EDTA, 1% Triton X-100, 0.5% SDS, and 0.01% protease inhibitor cocktail) and quantified using the bicinchoninic acid (BCA) method. Equal amounts of lysate samples were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) for 90 minutes at 110 V and transferred to the PVDF membrane for 2 hours at 300 mA for assessment of the levels of Bax, Bcl-2, CDK2, p53, and GAPDH. Subsequently, the PVDF membranes were incubated overnight at 4°C with the antibodies at the following concentrations: mouse anti-Bax (1:750), mouse anti-Bcl-2 (1:750), rabbit anti-CDK2 (1:750), rabbit anti-p53 (1:750), and rabbit anti-GAPDH (1:1000). After the membrane was washed by PBST (0.1% Tween-20 in PBS buffer), followed by incubation with horseradish peroxidase-conjugated goat antimouse or horseradish peroxidase-rabbit secondary antibody (1:5000) at 37°C for 2 hours. Protein bands were visualized using ECL detection reagent (Pierce) and detected by ImageJ software (version 1.43, National Institutes of Health, Bethesda, MD, USA) [18,25,26].
2.11. Assay for caspase-3 and -9 activities
MCF-7 cells were treated with 0–100 μg/mL ZTP, and caspase-3 and -9 activities were assessed by the caspase activity assay kit (BestBio Inc.) according to the manufacturer’s instructions. Control or treated cells were lysed in 100 μL of cold lysis buffer containing 1M dithiothreitol (10 μL/mL buffer) and incubated on ice for 15 minutes. After centrifugation (1000g), the supernatant containing about 20–50 μg protein was mixed with 90 μL detection buffer and 10 μL catalytic substrate (Ac-DEVD-pNA specific for caspase-3 and Ac-LEHD-pNA for caspase-9) in a 96-well microplate [27]. All samples were incubated at 37°C for 2 hours. The absorbance, representing the activity of cas-pases, was measured at 405 nm with an enzyme-linked immunosorbent assay (ELISA) reader (Rayto-RT6000; Rayto). Data are expressed as percentage of the control, which is designated as 1.0.
2.12. Measurement of intracellular reactive oxygen species (ROS)
MCF-7 cells were initially seeded in six-well plates at a density of 1 × 105 cells/well in triplicate and incubated at 37°C for 24 hours. The cells were then treated with or without ZTP or H2O2 as a positive control for 12 hours. Subsequently, the cells were washed twice with PBS and exposed to 10μM DCFH-DA probe at 37°C for 30 minutes. ROS generation was quantified with flow cytometry (excitation 485 nm, emission 525 nm), and ROS content was calculated as percentages relative to the oxidative stress of the control cells (100%) [18].
2.13. Statistical analysis
Values of test results in triplicate were expressed as means and standard deviation. IC50 of ZTP was calculated using a linearizing regression analysis, which was performed by Excel 2003 (Microsoft, Redmond, WA, USA). Statistical analysis was performed by SPSS 16.0, and the difference among treatment groups was analyzed with Duncan’s multiple range tests. A significant difference was considered for p < 0.05.
3. Results
3.1. ZTP inhibited proliferation of human breast cancer cells
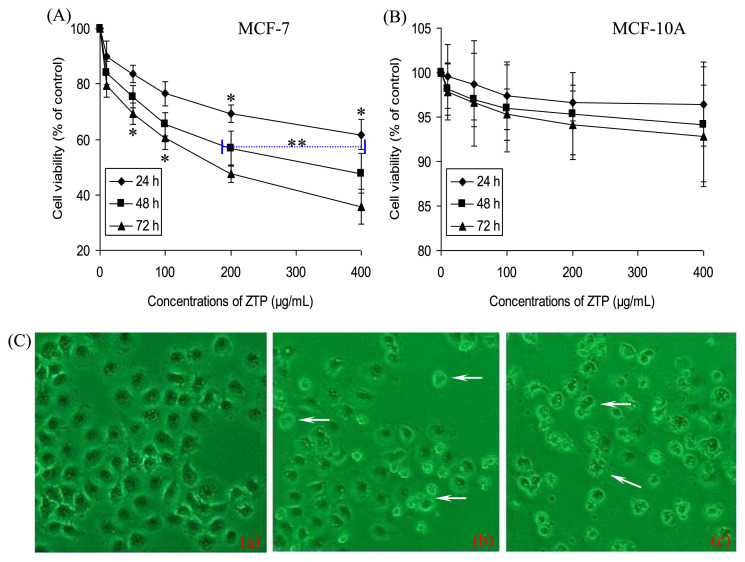
As shown in Figure 1, ZTP at 10 μg/mL, 50 μg/mL, 100 μg/mL, 200 μg/mL, and 400 μg/mL, with an IC50 value of 172.2 μg/mL, significantly inhibited cellular proliferation of MCF-7 cells for 72 hours in a dose- and time-dependent manner, when compared with the untreated cells (p < 0.05, Figure 1A). Interestingly, no effect was observed in normal mammary epithelial MCF-10A cells (Figure 1B), suggesting that ZTP specifically targeted cancer cells while sparing normal cells. As observed under a microscope (Figure 1C), MCF-7 cells also showed great changes in the morphology after treatment with ZTP at 50 μg/mL and 200 μg/mL. The shape of untreated normal cells was regular (a in Figure 1C), whereas MCF-7 cells treated with ZTP exhibited morphological alterations, such as cell shrinkage and blebbing, disorganization, and elongation of membrane (b and c in Figure 1C). At the same time, some of them lost their ability to adhere to the plate surface, and detached from the bottom, aggregated, and floated in the medium, leading to a decrease in the density of ZTP-treated cells. The extent of the changes in cell morphology and density depended on the concentrations of ZTP.
Figure 1.
Effects of ZTP on cellular viability and morphology in human breast cancer MCF-7 cells. Cells were treated with or without different concentrations of ZTP at 0 μg/mL, 10 μg/mL, 50 μg/mL, 100 μg/mL, 200 μg/mL, and 400 μg/mL for 24 hours, 48 hours, and 72 hours, and then the cell viabilities of (A) MCF-7 cells and (B) the normal human breast cells (MCF-10A) were assessed by MTT assay. Data represent mean ± SD of three independent experiments. (C) MCF-7 cells in response to various concentrations of ZTP for 48 hours showed morphological changes, which indicated ZTP-induced cell death (original microscope magnification, 200×). * p < 0.05 indicates statistically significant difference versus the control group. ** p < 0.01 indicates statistically significant difference versus the control group. MTT = 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; SD = standard deviation.
3.2. ZTP perturbed cell-cycle progression and modulated cell-cycle regulatory molecules
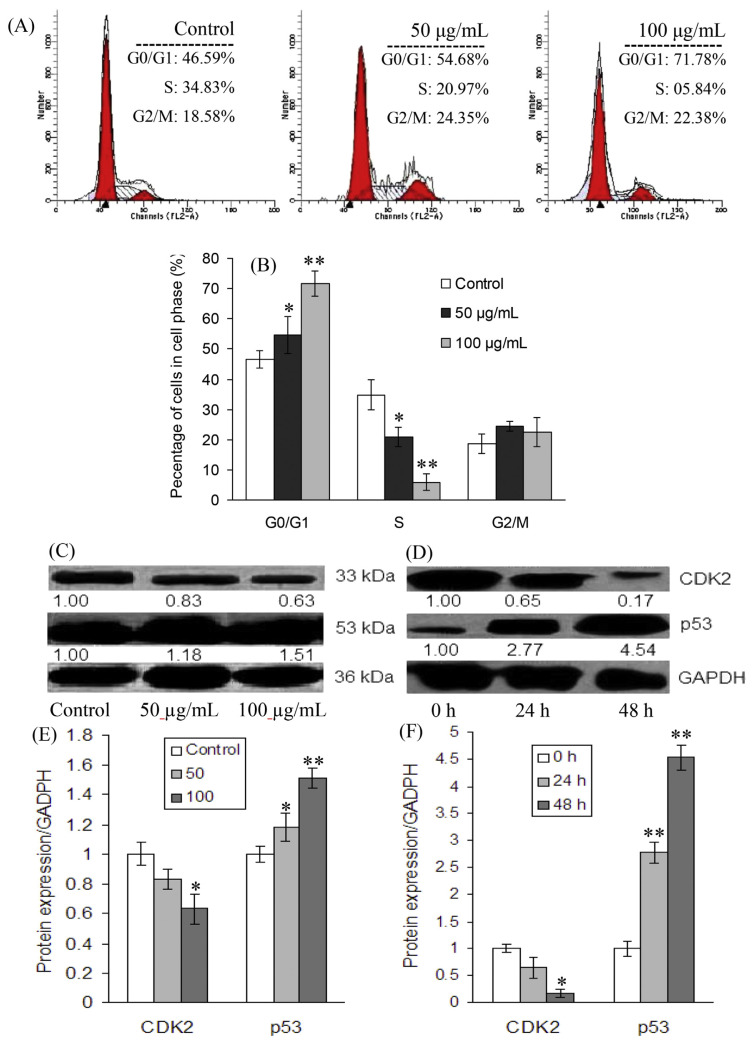
To determine the cellular mechanism of growth inhibitory effects of ZTP on MCF-7 cells, we investigated cell-cycle progression after ZTP treatment. As shown in Figure 2, the treatment of MCF-7 cells with 50 μg/mL and 100 μg/mL ZTP caused, respectively, 8.1% and 25.2% increases of cells in the G0/G1 phase after 24 hours of exposure in comparison with untreated MCF-7 cells, accompanied by a decrease in the percentage of cells in the S phase from 34.8% to 21.0% and 5.8%, respectively. At the tested concentrations, ZTP did not induce significant change in the G2/M phase (Figures 2A and 2B), suggesting that the treated cells were subjected to a blockage at the G1 phase of the cell cycle.
Figure 2.
Regulative effects of ZTP on cell-cycle arrest and the expression of correlative proteins in MCF-7 cells. (A) Representative histograms of DNA content in MCF-7 cells treated for 24 hours and (B) the distribution of cell cycles in ZTP-treated cells are shown. The protein expression of p53 and CDK2 was determined in MCF-7 cells (C, E) with or without ZTP (control, 50 μg/mL, and 100 μg/mL) for 24 hours, and (D, F) 100 μg/mL ZTP for 24 hours and 48 hours by Western blot assay. All values are expressed as mean ± SD of three independent experiments. The asterisks indicate a significant difference between control and ZTP-treated cells. * p < 0.05. ** p < 0.01. SD = standard deviation.
To further test whether ZTP-induced protein expression was associated with G0/G1 phase blockage in MCF-7 cells, the levels of cell-cycle-regulating proteins p53 and CDK2 were determined by immunoblotting. The levels of GAPDH served as an internal control. It was found that the treatment of MCF-7 cells with ZTP at 50 μg/mL and 100 μg/mL resulted in downregulation of CDK2 protein and upregulation in p53 levels. As depicted in Figures 2C and 2D, p53 expression significantly increased up to 2.77- and 4.54-fold above that in untreated control MCF-7 cells (p < 0.01) following the treatment of MCF-7 cells with 100 μg/mL of ZTP for 24 hours and 48 hours, respectively; however, CDK2 expression decreased 0.65- and 0.17-fold with respect to control cells (p < 0.05), respectively (Figures 2E and 2F), suggesting that ZTP blocked the progression of the cell cycle at the G0/G1 phase by modifying p53 and CDK2 expression.
3.3. ZTP induced mitochondria-mediated intrinsic apoptosis
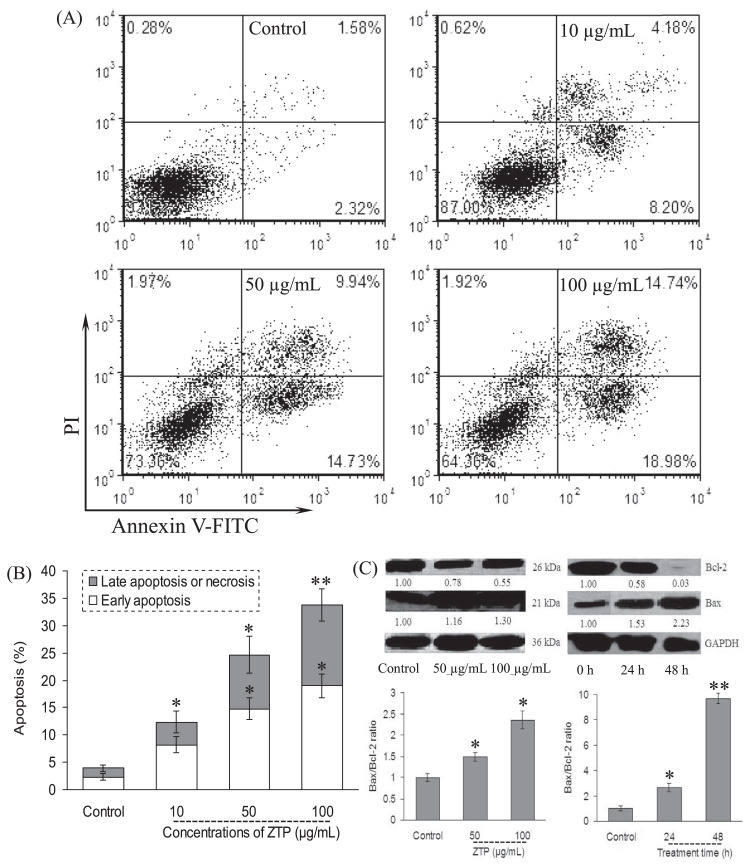
Cell-cycle deregulation and apoptosis are closely related events, and disruption of cell-cycle progression may ultimately lead to apoptotic/necrotic death [28]. In this study, phosphatidylserine translocation was assessed to determine the apoptosis of the ZTP-treated MCF-7 cells by staining with FITC-conjugated annexin V. As shown in Figure 3A, annexin V-positive cells were remarkably increased in MCF-7 cells in a dose-dependent manner. In comparison with vehicle-treated cells within 48 hours, ZTP induced early and late apoptosis in 12.4%, 24.7%, and 33.7% of MCF-7 cells exposed to 10 μg/mL, 50 μg/mL, and 100 μg/mL, respectively (Figure 3B).
Figure 3.
ZTP induced apoptosis through the initiation of the mitochondrial pathway. MCF-7 cells were treated with ZTP at 10 μg/mL, 50 μg/mL, and 100 μg/mL for 48 hours, and after harvesting, MCF-7 cells were double stained with annexin V-FITC and PI, and then 10,000 cells were analyzed by flow cytometry. (A) Representative dot plots of annexin V/PI staining. (B) Column bar graph of apoptotic cells. (C) After MCF-7 cells were further treated with ZTP at 50 μg/mL and 100 μg/mL for 48 hours, and at 100 μg/mL for 24 hours and 48 hours, the expression levels of Bcl-2 family protein and the corresponding ratio of Bax/Bcl-2 are assayed, respectively. Western blot analysis was performed in triplicate per experimental point, and the relative expression of protein was quantified densitometrically; GAPDH was used as a reference control. The number under each band in the immunoblot indicates the relative intensity of the corresponding band. * p < 0.05, as compared with the control cells. ** p < 0.01, as compared with the control cells. PI = propidium iodide.
Bcl-2 protein has been associated with apoptosis inhibition, whereas the expression of Bax has been associated with apoptosis induction [28]; thus, the Bax/Bcl-2 ratio is important in apoptosis [29]. In this study, we next measured the change of the expression of the Bcl-2 family protein and the activation of caspases. Immunoblot analysis showed that the treatment of MCF-7 cells with ZTP at 50 μg/mL and 100 μg/mL increased Bax protein levels after 48 hours of exposure (Figure 3C). By contrast, ZTP decreased Bcl-2 levels, leading to a sharp increase in the proapoptotic/antiapoptotic Bax/Bcl-2 ratio (p < 0.01, Figure 3C).
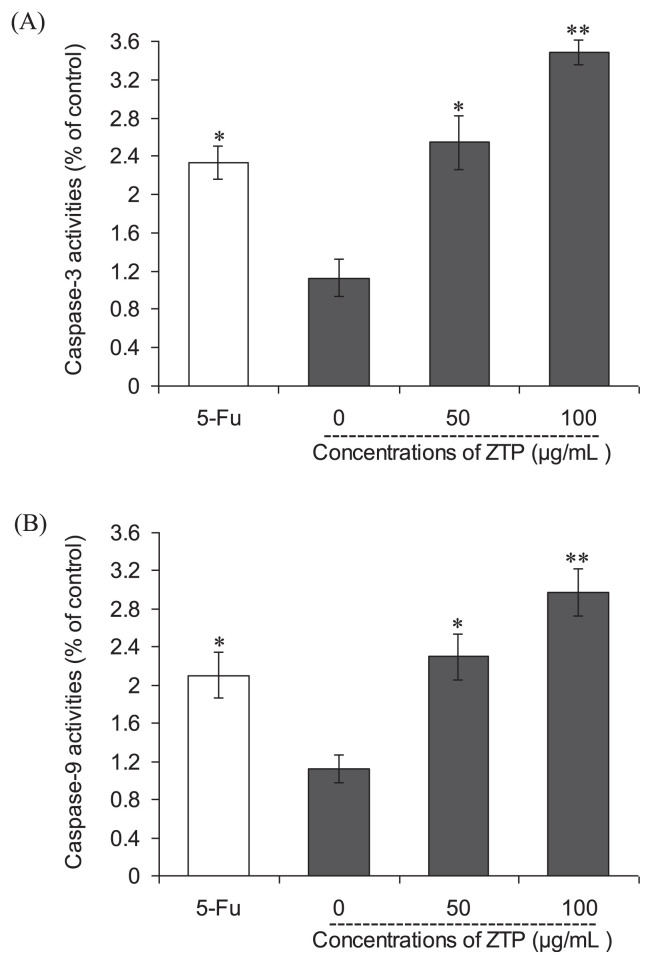
To further examine the involvement of caspases in apoptosis induction of ZTP, the activities of caspase-3 and -9 were also examined subsequently, as shown in Figures 4A and 4B, respectively. The results showed that both caspase-3 and -9 were activated significantly after ZTP treatment for 48 hours in MCF-7 cells. ZTP at a concentration of 50 μg/mL caused 1.26- and 1.04-fold increases in the activities of caspase-3 and -9 in MCF-7 cells, respectively (p < 0.01); a higher concentration of ZTP (100 μg/mL) induced further enhancements in the activation of caspase-3 and -9—increments of 2.10- and 1.65-fold above control cells (p < 0.01), respectively. This finding indicates that mitochondrial pathway is involved in ZTP-induced apoptosis.
Figure 4.
Effects of ZTP on the activities of (A) caspase-3 and (B) caspase-9 in MCF-7 cells. The values of optical density at 405 nm were determined by an ELISA reader. 5-FU (100 μg/mL) was used as positive control. Values are means ± SD of three independent experiments. * p < 0.05 versus untreated cells. ** p < 0.01 versus untreated cells. ELISA = enzyme-linked immunosorbent assay; SD = standard deviation; 5-FU = 5-fuorouracil.
3.4. Involvement of intracellular ROS in ZTP-induced apoptosis
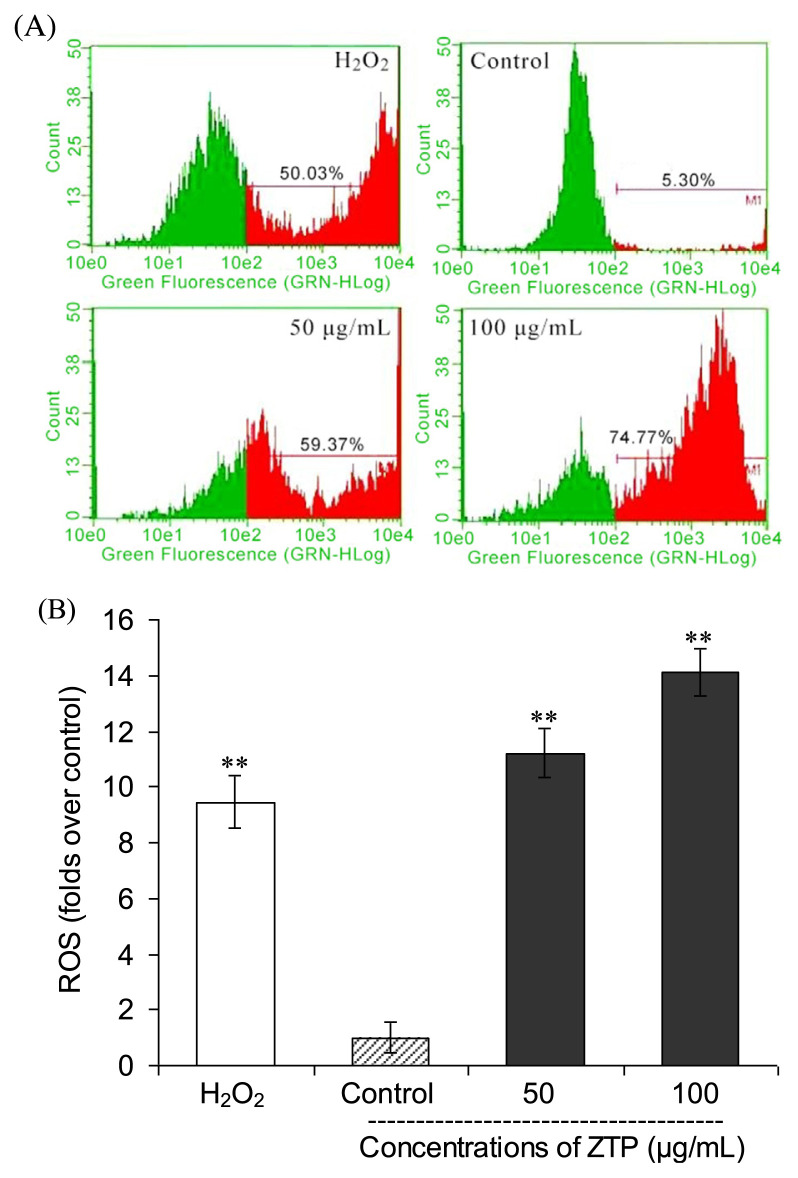
To determine whether intracellular ROS is involved in the cellular mechanism of growth inhibitory effects of ZTP on MCF-7 cells, we employed flow cytometry to further detect the changes of 2′,7′-Dichlorodihydrofluorescein diacetate (H2DCFDA) fluorescence intensity in MCF-7 cells exposed to ZTP (50 μg/mL and 100 μg/mL) for 12 hours. As shown in Figure 5A, the treatment with ZTP increased the proportion of cells with elevated green fluorescence intensity in a dose-dependent manner, indicating that ZTP induced the accumulation of intracellular ROS in the mitochondria of MCF-7 cells. As displayed in Figure 5B, ZTP at 50 μg/mL caused a 11.2-fold increase in ROS accumulation in MCF-7 cells, and a further treatment of MCF-7 cells with 100 μg/mL of ZTP led to a concentration-dependent accumulation of intracellular ROS (an increase of 14.1-fold) relative to untreated control cells (p < 0.01). A similar increase (by 9.4-fold) in green fluorescence intensity of MCF-7 cells was also obtained via incubation with H2O2 serving as a positive control for measuring the effects of oxidative stress (Figure 5A, p < 0.01 vs. control). These results indicate that ZTP induces growth inhibition and apoptosis through enhancing intracellular oxidative stress of breast cancer MCF-7 cells.
Figure 5.
ZTP-mediated accumulation of intracellular ROS in MCF-7 cells. MCF-7 cells were preincubated with or without various concentrations of ZTP (0 μg/mL, 50 μg/mL, and 100 μg/mL) for 12 hours, and then the intracellular ROS level was measured by a DCFH-DA probe (A) and the folds over control was calculated (B). H2O2 (50μM) was used as a positive control. The results represent the mean ± SD of three independent experiments. ** p < 0.01 indicates statistically significant difference with untreated cells. ROS = reactive oxygen species; SD = standard deviation.
3.5. Identification of bioactive phytochemicals in ZTP
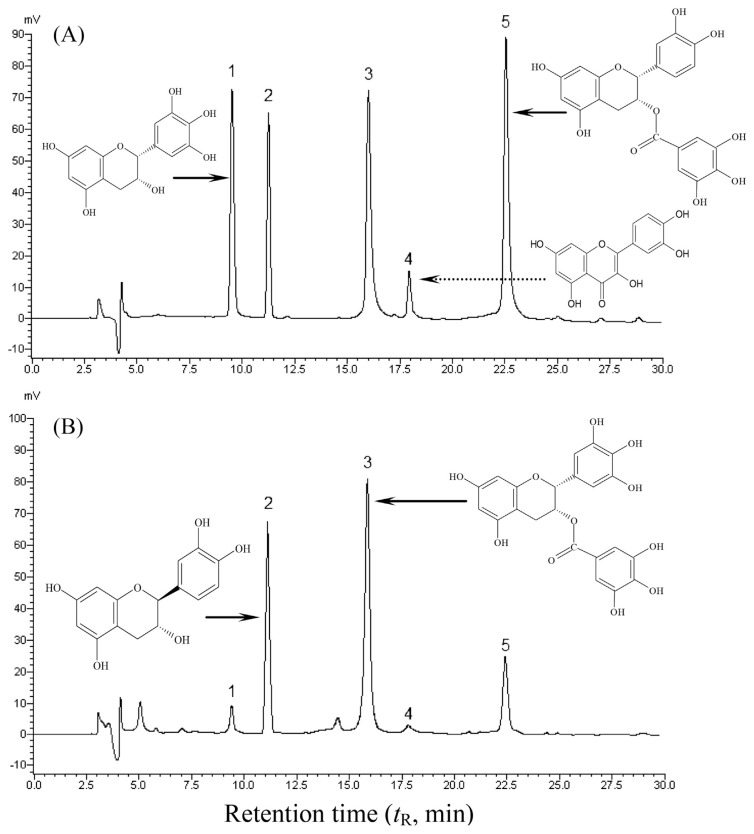
Given the significant activity of ZTP, we next attempted to examine and identify its bioactive constituents. Herein, we successively extracted the polyphenol-enriched ZTP with a multistep purification procedure. With this method, the extraction yield of ZTP from Ziyang green tea could reach 3.7% (w/w) of the dried tea. The content of total flavonoids was 27.6 μg GAEs/mg extract, suggesting that ZTP was a polyphenol-enriched extract. To further characterize composition information, ZTP was also subjected to compositional determination of monomeric phytochemical compounds by a HPLC technique. A representative HPLC chromatogram of authentic standards is shown in Figure 6, and the identified phytochemicals of ZTP by HPLC are illustrated in Table 1. The identification of bioactive constituents was performed according to the retention time (tR) obtained from authentic standards under identical HPLC conditions. As depicted in Figure 6, five peaks corresponding to authentic standards were identified in the order of EGC (9.5 minutes), catechin (11.2 minutes), EGCG (16.0 minutes), quercetin (18.5 minutes), and ECG (22.5 minutes). In this study, linear regression was assessed for the content calculation, and the assay had excellent linearity between Y (peak area of the standard polyphenols) and X (concentration of the polyphenols) with the correlation coefficients (R2) in the range 0.9892–0.9994, and the quantitative data were calculated from their respective calibration curves (Table 1). As shown in Figure 6 and Table 1, ZTP was characterized by the presence of four flavonoids, and (−)-epigallocatechin-3-gallate (EGCG, 28.2%) was presented in the highest content in ZTP, followed by catechin (17.3%), (−)-epicatechin-3-gallate (ECG, 12.6%), and EGC (5.7%), and only a trace amount of quercetin (2.8 μg/mg) was detected. The results obtained in this test clearly indicate that the tea extract ZTP presents a characteristic phenolic profile with noticeable amounts of lipophilic flavonoids, which are most probably responsible for the remarkable antiproliferative activity of Ziyang green extract against human breast cancer MCF-7 cells.
Figure 6.
Typical HPLC chromatograms for the separation and analysis of (A) authentic standards and (B) catechins found in ZTP, as detected by absorbance at 280 nm. Chemical formulas of the major active flavonoids were marked. HPLC analysis was performed as described in the experimental section. Assignment of peaks is as follows: (1) (−)-epigallocatechin, (2) catechin, (3) (−)-epigallocatechin gallate, (4) quercetin, and (5) (−)-epicatechin gallate. HPLC = high-performance liquid chromatography.
Table 1.
Calibration curves and contents of the identified polyphenols in ZTP from Ziyang green tea by HPLC.
| Peaks | Assigned identitya | tR (min) | Content (μg/mg) | Equation of regression (y = ax + b) | R 2 |
|---|---|---|---|---|---|
| 1 | EGC | 9.51 ± 0.12 | 56.8 | y = 0.33085x + 0.09659 | 0.9892 |
| 2 | Catechin | 11.23 ± 0.09 | 172.8 | y = 2.21653x + 0.01551 | 0.9952 |
| 3 | EGCG | 16.01 ± 0.06 | 281.5 | y = 1.19199x + 0.03923 | 0.9991 |
| 4 | Quercetin | 18.50 ± 0.11 | 2.8 | y = 0.98562x + 0.01295 | 0.9987 |
| 5 | ECG | 22.52 ± 0.15 | 126.3 | y = 1.90724x + 0.06391 | 0.9994 |
ECG = (−)-epicatechin gallate; EGC = (−)-epigallocatechin; EGCG = (−)-epigallocatechin gallate; HPLC = high-performance liquid chromatography.
Content of each component in ZTP is expressed as μg/mg dried extract.
4. Discussion
Breast cancer is one of the most common cancers in the world. No effective chemotherapeutic strategy is available to treat breast cancer successfully [22]. Natural products have become more popular for the prevention or treatment of cancer. Tea with high levels of catechins has been consumed as a favorite beverage worldwide and is regarded as health drinks with cancer preventative properties [30]. Some scientists believe that isolated pure compounds do not have the same health benefits, owing to the loss of synergic or additive biological effects, as a compound that is present in whole, vegetables, fruits, or tea [31,32]. Chinese selenium-enriched Ziyang green tea, a new cultivar of C. sinensis, is used as an important dietary source of natural antioxidants, and the phenolic catechins can affect cancerous cells through cell-cycle arrest and activation of apoptotic signal transduction pathways [33]. Our analysis showed that the ZTP contained 0.39 μg/g selenium, which was evaluated by our established method [34]. In this regard, ZTP may have a high breast carcinoma-inhibitory activity. In this study, we demonstrated for the first time that ZTP exhibited remarkably high cell growth inhibition, cell-cycle arrest, induction of apoptosis, and ROS production against human breast cancer MCF-7 cells, but did not exert any significant toxicity on normal breast cells. Our finding contributes to the understanding of the strong positive relationship between total flavonoid content in ZTP and antitumor activity, and also makes it a promising polyphenolic fraction for the development of novel effective cancer preventive or therapeutic agents.
It is well known that there is a balance between cell-cycle arrest and cell death through apoptosis in response to genomic damage and cellular stress in proliferating cells [35]. Defects in this balance lead to the development of cancer and other pathological conditions [36]. In the present study, we have characterized the mechanisms of the ZTP-induced inhibitory effect of MCF-7 cells by inducing G0/G1 cell-cycle arrest, and consequently apoptosis in a dose- and time-dependent manner. It is well known that the eukaryotic cell cycle is strictly regulated by different cyclins/cyclin-dependent kinase (CDK) complexes, and p53 exerts its effects through transcriptional activation of target genes such as CDK inhibitor p21 and then mediates both G1 and G2/M phase arrest [37]; therefore, induction of cell-cycle arrest has become an appreciated target for the management of cancer [38,39]. Our results suggested that ZTP blocked proliferation of tumor cells by arresting the cells in the G0/G1 phase of the cell cycle. Previous works suggested that EGCG (main in ZTP) upregulates the expression of p21 to inhibit cancer cell proliferation, which is regulated by p53 [40,41]. The treatment of MCF-7 cells with ZTP resulted in a significant upregulation in the expression level of p53 protein and downregulation in the expression level of CDK2 protein (Figure 2). As a result, the growth inhibition of ZTP was suggested to be associated with the G0/G1 arrest, which was implicated in p53-dependent regulation in MCF-7 cells.
Selective induction of apoptosis is a highly desirable trait of ideal chemopreventive and chemotherapeutic regimens. Mitochondria are thought to be the major pathway for apoptosis, and therefore, targeting the mitochondria is a novel strategy for cancer therapy [42–46]. Mitochondrial-mediate apoptosis is highly regulated by the Bcl-2 family proteins comprising both antiapoptotic (Bcl-2 and Bcl-xL) and proapoptotic (Bax and Bak) members, and the balance between the expression levels of pro- and antiapoptotic proteins is critical for cell survival or cell death [42–46]. We found that ZTP treatment could result in a significant increase in Bax expression and a decrease in Bcl-2 expression, suggesting that the change in the ratio of pro- and antiapoptotic Bcl-2 family proteins might largely contribute to the mitochondria-mediated apoptosis (Figure 3). In addition, the caspase protease family also plays a crucial role in the process of apoptotic signal transduction [47,48]. Caspase-9 is the initiator caspase responsible for the activation of the executioner caspases, such as downstream caspase-3, within the mitochondrial pathway of apoptosis [49]. In our study, we assessed the activity of caspase-3 and its upstream initiator caspase-9. From the tested results, it was clearly found that ZTP significantly enhanced the activities of caspase-9 and -3, suggesting that the mitochondrial pathway of apoptosis was involved in ZTP-induced apoptosis in human beast carcinoma MCF-7 cells.
Mitochondria are a source of ROS during apoptosis, and a reduced mitochondria membrane potential leads to ROS generation and apoptosis [50]. ROS has been implicated as a second messenger in multiple signaling pathways [50] and can also play an important role in apoptosis by regulating the activity of certain enzymes involved in the cell death pathway [50–53]. To investigate if the mitochondrial dysfunction in ZTP-treated MCF-7 cells was promoted by ROS production, we measured ROS levels using the cell-permeable dye DCFH-DA. As compared with the untreated control cells (Figure 5A), the accumulation of intracellular ROS of ZTP-treated cells for 24 hours was significantly elevated, suggesting that the apoptotic effect of ZTP on MCF-7 cells was associated with an elevated level of intracellular ROS and ROS production might lead to apoptotic cell death via the mitochondrial pathway. These findings are in line with the previous reports [51], showing that polyphenol-rich extracts from açai pulp and açai oil significantly inhibited cell proliferation and increased ROS generation [51]. Although tea polyphenols exhibited strong antioxidant activities in vitro [7], these antioxidant properties may not be entirely responsible for their chemopreventive effects [54]. In the presence of transitional metal ions, polyphenols as antioxidants are also considered to exhibit pro-oxidant properties [55]. Thus, the pro-oxidant properties of polyphenols may account for their anticancer properties owing to the fact that chromatin-bound endogenous transitional metal ions were mediated by polyphenols to generate ROS in apoptosis processes of cancer cells [54]. Previous studies have reported that catechin (17.3% in ZTP) exert pro-oxidant actions [54,56]. Accordingly, potential mechanisms for the chemopreventive effect of ZTP were attributed to the promotion of ROS, which in turn could lead to alternation of the Bax/Bcl-2 ratio and activation of caspases, thereby initiating cell injury/death [9,11,57]. Oxidative stress was proved to be involved in many diseases and to play an important role in indirect genotoxicity. Previous studies on the pro-oxidant activity have shown that EGCG may be converted to a phenoxyl radical after neutralizing the peroxy or other radicals [2].
Our study is the first to identify the remarkable anticancer activity of the polyphenol-enriched extract (ZTP) from Chinese Ziyang green tea against human breast cancer MCF-7 cells. We demonstrate the selective effects of ZTP in inhibiting the growth of MCF-7 cancer cells with minimal effects on normal cells. Our results also show that ZTP may be an effective ingredient for the treatment and/or prevention of breast cancer. Our data also show compelling evidence for further evaluation of ZTP as a chemopreventive regimen for breast cancer.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (C31171678); the Fundamental Research Funds for the Central Universities of Shaanxi Normal University, China (GK201103004); and the Scientific Research Foundation of Shangluo University (13SKY-FWDF007).
Funding Statement
This work was supported by the National Natural Science Foundation of China (C31171678); the Fundamental Research Funds for the Central Universities of Shaanxi Normal University, China (GK201103004); and the Scientific Research Foundation of Shangluo University (13SKY-FWDF007).
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
REFERENCES
- 1. Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Res. 2006;66:2500–5. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- 2. Yang CS, Maliakal P, Meng X. Inhibition of carcinogenesis by tea. Ann Rev Pharm Toxicol. 2002;42:25–54. doi: 10.1146/annurev.pharmtox.42.082101.154309. [DOI] [PubMed] [Google Scholar]
- 3. Chen HX, Zhang M, Xie BJ. Components and antioxidant activity of polysaccharide conjugate from green tea. Food Chem. 2005;90:17–21. [Google Scholar]
- 4. Gimenez B, Moréno S, López-Caballero ME, Montero P, Gómez-Guillen MC. Antioxidant properties of green tea extract incorporated to fish gelatin films after simulated gastrointestinal enzymatic digestion. LWT Food Sci Technol. 2013;53:445–51. [Google Scholar]
- 5. Wang DF, Wang CH, Zhao GW, Wei ZG, Tao Y, Liang XG. Composition, characteristic and activity of rare earth elements-bound polysaccharide from tea. Biosci Biotechnol Biochem. 2001;65:1987–92. doi: 10.1271/bbb.65.1987. [DOI] [PubMed] [Google Scholar]
- 6. Yang CS, Sang SG, Lambert JD, Hou Z, Ju JY, Lu G. Possible mechanisms of the cancer-preventive activities of green tea. Mol Nutr Food Res. 2006;50:170–5. doi: 10.1002/mnfr.200500105. [DOI] [PubMed] [Google Scholar]
- 7. Spadiene A, Savickiene N, Ivanauskas L, Jakstas V, Skesters A, Silova A, Rodovicius H. Antioxidant effects of Camellia sinensis L. extract in patients with type 2 diabetes. J Food Drug Anal. 2014;22:505–11. doi: 10.1016/j.jfda.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fang WX, Wu PW, Hu RZ. Geochemical research of the impact of Se–Cu–Mo–V-bearing coal layers on the environment in Pingli County, Shaanxi Province, China. J Geochem Explor. 2003;80:105–15. [Google Scholar]
- 9. Molan AL, Flanagan J, Wei W, Moughan PJ. Selenium containing green tea has higher antioxidant and prebiotic activities than regular green tea. Food Chem. 2009;114:829–35. [Google Scholar]
- 10. Vodnar DC, Socaciu C. Selenium enriched green tea increase stability of Lactobacillus casei and Lactobacillus plantarum in chitosan coated alginate microcapsules during exposure to simulated gastrointestinal and refrigerated conditions. LWT Food Sci Technol. 2014;57:406–11. [Google Scholar]
- 11. Xu J, Zhu SG, Yang FM, Cheng LC, Hu Y, Pan GX, Hu QH. The influence of selenium on the antioxidant activity of green tea. J Sci Food Agric. 2003;83:451–5. [Google Scholar]
- 12. Xu J, Yang FM, Chen LC, Hu QH. Effect of selenium on increasing the antioxidant activity of tea leaves harvested during the early spring tea producing season. J Agric Food Chem. 2003;51:1081–4. doi: 10.1021/jf020940y. [DOI] [PubMed] [Google Scholar]
- 13. Yu F, Sheng JC, Xu J, An XX, Hu QH. Antioxidant activities of crude tea polyphenols, polysaccharides and proteins of selenium-enriched tea and regular green tea. Eur Food Res Technol. 2007;225:843–8. [Google Scholar]
- 14. Granado-Serrano AB, Martín MA, Bravo L, Goya L, Ramos S. Quercetin induces apoptosis via caspase activation, regulation of Bcl-2, and inhibition of PI-3-kinase/Akt and ERK pathways in a human hepatoma cell line (HepG2) J Nutr. 2006;136:2715–21. doi: 10.1093/jn/136.11.2715. [DOI] [PubMed] [Google Scholar]
- 15. Kandaswami C, Lee LT, Lee PP, Hwang JJ, Ke FC, Huang YT, Lee MT. The antitumor activities of flavonoids. In Vivo. 2005;19:895–909. [PubMed] [Google Scholar]
- 16. Mertens-Talcott SU, Percival SS. Ellagic acid and quercetin interact synergistically with resveratrol in the induction of apoptosis and cause transient cell cycle arrest in human leukemia cells. Cancer Lett. 2005;218:141–51. doi: 10.1016/j.canlet.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 17. Vauzour D, Rodriguze-Mateos A, Corona G, Oruna-Concha MJ, Spencer JPE. Polyphenols and human health: prevention of disease and mechanisms of action. Nutrients. 2010;2:1106–31. doi: 10.3390/nu2111106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li T, Zhu J, Guo L, Shi X, Liu Y, Yang XB. Differential effects of polyphenols-enriched extracts from hawthorn fruit peels and fleshes on cell cycle and apoptosis in human MCF-7 breast carcinoma cells. Food Chem. 2013;141:1008–18. doi: 10.1016/j.foodchem.2013.04.050. [DOI] [PubMed] [Google Scholar]
- 19. Tian LM, Shi XL, Yu LH, Zhu J, Ma R, Yang XB. Chemical composition and hepatoprotective effects of polyphenol-rich extract from Houttuynia cordata tea. J Agric Food Chem. 2012;60:4641–8. doi: 10.1021/jf3008376. [DOI] [PubMed] [Google Scholar]
- 20. Do QD, Angkawijaya AE, Tran-Nguyen PL, Huynh LH, Soetaredjo FE, Ismadji S, Ju YH. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J Food Drug Anal. 2014;22:296–302. doi: 10.1016/j.jfda.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chiu YW, Lo HJ, Huang HY, Chao PY, Hwang JM, Huang PY. The antioxidant and cytoprotective activity of Ocimum gratissimum extracts against hydrogen peroxide-induced toxicity in human HepG2 cells. J Food Drug Anal. 2013;21:253–60. [Google Scholar]
- 22. Chang YC, Chen PN, Chu SC, Lin CY, Kuo WH, Hsieh YS. Black tea polyphenols reverse epithelial-to-mesenchymal transition and suppress cancer invasion and proteases in human oral cancer cells. J Agric Food Chem. 2012;60:8395–403. doi: 10.1021/jf302223g. [DOI] [PubMed] [Google Scholar]
- 23. Huang C, Lee SY, Lin CL, Tu TH, Chen LH, Chen YJ, Huang HC. Co-treatment with quercetin and 1,2,3,4,6-petna-O-galloyl-β-D-glucose causes cell cycle arrest and apoptosis in human breast cancer MDA-MB-231 and AU565 cells. J Agric Food Chem. 2013;61:6430–45. doi: 10.1021/jf305253m. [DOI] [PubMed] [Google Scholar]
- 24. Kim B, Kim SH, Jeong SJ, Sohn EJ, Jung JH, Lee MH, Kim SH. Brazilin induces apoptosis and G2/M arrest via inactivation of histone deacetylase in multiple myeloma U266 cells. J Agric Food Chem. 2012;60:9882–9. doi: 10.1021/jf302527p. [DOI] [PubMed] [Google Scholar]
- 25. Hsu YL, Chen CY, Hou MF, Tsai EM, Jong YJ, Hung CH, Kuo PL. 6-Dehydrogingerdione, an active constituent of dietary ginger, induces cell cycle arrest and apoptosis through reactive oxygen species/c-Jun N-terminal kinase pathways in human breast cancer cells. Mol Nutr Food Res. 2010;54:1307–17. doi: 10.1002/mnfr.200900125. [DOI] [PubMed] [Google Scholar]
- 26. Hsu JD, Kao SH, Ou TT, Chen YJ, Li YJ, Wang CJ. Gallic acid induces G2/M phase arrest of breast cancer cell MCF-7 through stabilization of p27kip1 attributed to disruption of p27kip1/skp2 complex. J Agric Food Chem. 2011;59:1996–2003. doi: 10.1021/jf103656v. [DOI] [PubMed] [Google Scholar]
- 27. Shang DJ, Li Y, Wang C, Wang XM, Yu Z, Fu X. A novel polysaccharide from Se-enriched Ganoderma lucidum induces apoptosis of human breast cancer cells. Oncol Rep. 2011;25:267–72. [PubMed] [Google Scholar]
- 28. Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–6. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 29. Raisova M, Hossini AM, Eberel J, Riebeling C, Wieder T, Sturm I, Daniel PT, Orfanos CE, Geilen CC. The bax/bcl-2 ratio determines the susceptibility of human melanoma cells to CD95/Fas-mediated apoptosis. J Invest Dermatol. 2001;117:333–40. doi: 10.1046/j.0022-202x.2001.01409.x. [DOI] [PubMed] [Google Scholar]
- 30. Hsu CP, Shih YT, Lin BR, Chiu CF, Lin CC. Inhibitory effect and mechanisms of an anthocyanins- and anthocyanidins-rich extract from purple-shoot tea on colorectal carcinoma cell proliferation. J Agric Food Chem. 2012;60:3686–92. doi: 10.1021/jf204619n. [DOI] [PubMed] [Google Scholar]
- 31. Dai J, Patel JD, Mumper RJ. Characterization of blackberry extract and its antiproliferative and anti-inflammatory properties. J Med Food. 2007;10:258–65. doi: 10.1089/jmf.2006.238. [DOI] [PubMed] [Google Scholar]
- 32. Yi W, Joan F, Gerard K, Casimir CA. Phenolic compounds from blueberries can inhibit colon cancer cell proliferation and induce apoptosis. J Agric Food Chem. 2005;53:7320–9. doi: 10.1021/jf051333o. [DOI] [PubMed] [Google Scholar]
- 33. Lin LK. Cancer chemoprevention by tea polyphenols through modulating signal transduction pathways. Arch Pharm Res. 2002;25:561–71. doi: 10.1007/BF02976924. [DOI] [PubMed] [Google Scholar]
- 34. He N, Shi X, Zhao Y, Tian L, Wang D, Yang X. Inhibitory effects and molecular mechanisms of selenium-containing tea polysaccharides on human breast cancer MCF-7 cells. J Agric Food Chem. 2013;61:579–88. doi: 10.1021/jf3036929. [DOI] [PubMed] [Google Scholar]
- 35. Oren M. Decision making by p53: life, death and cancer. Cell Death Differ. 2003;10:431–42. doi: 10.1038/sj.cdd.4401183. [DOI] [PubMed] [Google Scholar]
- 36. Allan LA, Clarke PR. Apoptosis and autophagy: regulation of caspase-9 by phosphorylation. FEBS J. 2009;276:6063–73. doi: 10.1111/j.1742-4658.2009.07330.x. [DOI] [PubMed] [Google Scholar]
- 37. Giono LE, Manfredi JJ. Mdm2 is required for inhibition of CDK2 activity by p21, thereby contributing to p53-dependent cell cycle arrest. Mol Cell Biol. 2007;27:4166–78. doi: 10.1128/MCB.01967-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hafeez BB, Siddiqui IA, Asim M, Malik A, Afaq F, Adhami VM, Saleem M, Din M, Mukhtar H. A dietary anthocyanidin delphinidin induces apoptosis of human prostate cancer PC3 cells in vitro and in vivo: involvement of nuclear factor-κB signaling. Cancer Res. 2008;68:8564–72. doi: 10.1158/0008-5472.CAN-08-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li L, Lu N, Dai Q, Wei L, Zhao Q, Li Z, He Q, Dai Y, Guo Q. GL-V9, a newly synthetic flavonoid derivative, induces mitochondrial mediated apoptosis and G2/M cell cycle arrest in human hepatocellular carcinoma HepG2 cells. Eur J Pharmacol. 2011;670:13–21. doi: 10.1016/j.ejphar.2011.08.054. [DOI] [PubMed] [Google Scholar]
- 40. Ravindranath MH, Saravanan TS, Monteclaro CC, Presser N, Ye X, Selvan SR, Brosman S. Epicatechins purified from green tea (Camellia sinensis) differentially suppress growth of gender-dependent human cancer cell lines. Evid Based Complement Alternat Med. 2006;3:237–47. doi: 10.1093/ecam/nel003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Eom DW, Lee JH, Kim YJ, Hwang GS, Kim SN, Kwak JH, Cheon GJ, Kim KH, Jang HJ, Ham J, Kang KS, Yamabe N. Synergistic effect of curcumin on epigallocatechin gallate-induced anticancer action in PC3 prostate cancer cells. BMB Rep. 2015;48:461–6. doi: 10.5483/BMBRep.2015.48.8.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–4. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 43. EI-Deiry WS, Harper JW, O’Connor PM, Velculescu VE, Canman CE, Jackman J, Pietenpol JA, Burrell M, Hill DE, Wang Y, Wiman KG, Mercer WE, Kastan MB, Kohn KW, Elledge SJ, Kinzler KW, Vogelstein B. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994;54:1169–74. [PubMed] [Google Scholar]
- 44. Papi A, Orland M, Bartolini G, Barillari J, Iori R, Paolini M, Ferroni F, Fumo MG, Pedulli GF, Valgimgli L. Cytotoxic and antioxidant activity of 4-methylthio-3-butenyl isothiocyanate from Raphanus sativus L. (Kaiware Daikon) sprouts. J Agric Food Chem. 2008;56:875–83. doi: 10.1021/jf073123c. [DOI] [PubMed] [Google Scholar]
- 45. Weng CJ, Yang YT, Ho CT, Yen GC. Mechanisms of apoptotic effects induced by resveratol, dibenzoylmethane, and their analogues on human lung carcinoma cells. J Agric Food Chem. 2009;57:5235–43. doi: 10.1021/jf900531m. [DOI] [PubMed] [Google Scholar]
- 46. Xu Y, Ge R, Du J, Xin H, Yi T, Sheng J, Wang Y, Ling C. Corosolic acid induces apoptosis through mitochondrial pathway and caspases activation in human cervix adenocarcinoma HeLa cells. Cancer Lett. 2009;284:229–37. doi: 10.1016/j.canlet.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 47. Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–6. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 48. Vaculova A, Zhivotovsky B. Caspases: determination of their activities in apoptotic cells. Method Enzymol. 2008;442:157–81. doi: 10.1016/S0076-6879(08)01408-0. [DOI] [PubMed] [Google Scholar]
- 49. Hail N, Jr, Carter BZ, Konopleva M, Andreeff M. Apoptosis effector mechanisms: a requiem performed in different keys. Apoptosis. 2006;11:889–904. doi: 10.1007/s10495-006-6712-8. [DOI] [PubMed] [Google Scholar]
- 50. Sreelatha S, Jeyachitra A, Padma PR. Antiproliferation and induction of apoptosis by Moringa oleifera leaf extract on human cancer cells. Food Chem Toxicol. 2011;49:1270–5. doi: 10.1016/j.fct.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 51. Pacheco-Palencia LA, Talcott ST, Safe S, Mertens-Talcott S. Absorption and biological activity of phytochemical-rich extracts from acai (Euterpe oleracea Mart.) pulp and oil in vitro. J Agric Food Chem. 2008;56:3593–600. doi: 10.1021/jf8001608. [DOI] [PubMed] [Google Scholar]
- 52. Zhang Y, Luo M, Zu Y, Fu Y, Gu C, Wang W, Yao L, Efferth T. Dryofragin, a phloroglucinol derivative, induces apoptosis in human breast cancer MCF-7 cells through ROS-mediated mitochondrial pathway. Chem Biol Interact. 2012;199:129–36. doi: 10.1016/j.cbi.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 53. Tripathi M, Singh BK, Kakkar P. Glycyrrhizic acid modulates t-BHP induced apoptosis in primary rat hepatocytes. Food Chem Toxicol. 2009;47:339–47. doi: 10.1016/j.fct.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 54. Sarwar T, Zafaryab M, Husain MA, Ishqi HM, Rehman SU, Moshahid Alam Rizvi M, Tabish M. Redox cycling of endogenous copper by ferulic acid leads to cellular DNA breakage and consequent cell death: a putative cancer chemotherapy mechanism. Toxicol Appl Pharmacol. 2015;289:251–61. doi: 10.1016/j.taap.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 55. Yamashita N, Murata M, Inoue S, Burkitt MJ, Milne L, Kawanishi S. Alphatocopherol induces oxidative damage to DNA in the presence of copper(II) ions. Chem Res Toxicol. 1998;11:855–62. doi: 10.1021/tx970129v. [DOI] [PubMed] [Google Scholar]
- 56. Oikawa S, Hirosawa I, Hirakawa K, Kawanishi S. Site specificity and mechanism of oxidative DNA damage induced by carcinogenic catechol. Carcinogenesis. 2001;22:1239–45. doi: 10.1093/carcin/22.8.1239. [DOI] [PubMed] [Google Scholar]
- 57. Hadi SM, Asad SF, Singh S, Ahmad A. Putative mechanism for anticancer and apoptosis-inducing properties of plant-derived polyphenolic compounds. IUBMB Life. 2000;50:167–71. doi: 10.1080/152165400300001471. [DOI] [PubMed] [Google Scholar]