Abstract
The chemical compositions of seven essential oils from Taiwan were analyzed by gas chromatography-mass spectrometry. The eluates were identified by matching the mass fragment patents to the National Institute of Standards and Technology (NIST) 08 database. The quantitative analysis showed that the major components of lemon verbena are geranial (26.9%) and neral (23.1%); those of sweet marjoram are γ-terpinene (18.5%), thymol methyl ether (15.5%), and terpinen-4-ol (12.0%); those of clove basil are eugenol (73.6%), and β-(Z)-ocimene (15.4%); those of patchouli are carvacrol (47.5%) and p-cymene (15.2%); those of rosemary are α-pinene (54.8%) and 1,8-cineole (22.2%); those of tea tree are terpinen-4-ol (33.0%) and 1,8-cineole (27.7%); and those of rose geranium are citronellol (28.9%) and 6,9-guaiadiene (20.1%). These components are somewhat different from the same essential oils that were obtained from other origins. Lemon verbena has the same major components everywhere. Tea tree, rose geranium, and clove basil have at least one major component throughout different origins. The major components and their amounts in sweet marjoram, patchouli, and rosemary vary widely from one place to another. These results demonstrate that essential oils have a large diversity in their composition in line with their different origins. The antibacterial activity of essential oils against Escherichia coli was evaluated using the optical density method (turbidimetry). Patchouli is a very effective inhibitor, in that it completely inhibits the growth of E. coli at 0.05%. Clove basil and sweet marjoram are good inhibitors, and the upper limit of their minimum inhibitory concentration is 0.1%.
Keywords: antibacterial activity, chemical composition, Escherichia coli, essential oil, gas chromatography-mass, spectrometry
1. Introduction
Essential oils have a long history of being used by people in their daily lives for both spiritual and practical reasons. Plant products were the principal sources of pharmaceutical agents used in traditional medicine. Some medicinal plants are rich in antimicrobial reagents [1]. Several essential oils derived from varieties of medicinal plants are known to possess insecticidal, antifungal, anti-inflammatory, and antioxidant activities [2–5]. Using essential oils from flowering plants (angiosperms) as food preservatives can be traced back to the ancient Egyptians [6]. In modern society, pure chemicals are developed for use as insecticides, food preservatives, and antibacterial reagents. The practical functions of essential oils have been completely replaced by industrial products. However, in recent years, many concerns have been raised regarding the overuse of chemicals as preservatives and additives in food products. The urge to search for healthy chemical substitutes has drawn massive attention to essential oils [7]. Addition of essential oils into food, drugs, and cosmetics is now desired in various products. Many remarkable results have been made possible by the advent of essential oil products. It is therefore important to scientifically evaluate the possible applications of essential oils.
One of the intriguing properties of essential oils is their antibacterial property. Depending on the plants’ growth condition, such as location and climate, the chemical composition of the same essential oils may vary. Hence, it is important to determine the chemical composition of each essential oil. Documentation of the contents of essential oils not only makes the investigation of the influence between essential oils and claims possible, but it also builds a common ground for discussion.
In this paper, we report the chemical compositions of seven essential oils that are extracted from the plants that were grown and harvested in Ji-an Town, Hualien, Taiwan, and the potential of these essential oils for inhibition against Escherichia coli.
2. Materials and methods
2.1. Chemicals
Ethyl acetate was ACS grade and was purchased from Mallinckrodt (Dublin, Ireland). Normal paraffin C7, C8, C9, C10 mix and C10, C12, C14, C16 mix were purchased from Aldrich (St. Louis, USA). Sodium chloride was purchased from Merck (Darmstadt, Germany). Tryptone (casein hydrolysate) enzymatic digest, yeast extract, and agar were purchased from USB Corporation (Cleveland, USA). All reagents were used as received without further purification.
2.2. Plant material
Fresh leaves of Lippia citrodora (Paláu) Kunth (lemon verbena), Origanum majorana L. (sweet marjoram), Ocimum gratissimum L. (clove basil), Pogostemon cablin Benth. (patchouli), Rosmarinus officinalis L. (rosemary), Melaleuca alternifolia Cheel (tea tree), and Pelargonium graveolens L’Hér. (rose geranium) were collected from Ji-an Town, Hualien, Taiwan. The plant samples were identified, and the voucher specimens were deposited at the Herbarium of the Department of Life Science, National Taiwan Normal University. The voucher numbers are as follows: P. cablin Benth (TNU055241); P. graveolens L’Hér (TNU055242); O. majorana L. (TNU055243); O. gratissimum L. (TNU055246); M. alternifolia Cheel (TNU055247); L. citrodora (Pálau) Kunth (TNU055248); R. officinalis L. (TNU055249). All seven essential oils were extracted from air-dried leaves of plants by hydrodistillation for 40 minutes using a Clevenger-type apparatus. The oil samples obtained were dried over anhydrous sodium sulfate and stored in sealed vials in a cool and dark place prior to analyses. Essential oils were volumetrically diluted to a thousand times in ethyl acetate prior to gas chromatography (GC) injection.
2.3. GC and GC-mass spectrometry analysis
GC was performed on Agilent Technologies 6850 Series II equipped with a flame ionization detector. The capillary column was HP-5MS cross bond (5% diphenyl-polysiloxane and 95% dimethyl-polysiloxane; 30 m × 250 μm × 0.25 μm). The injector and detector temperatures were both set at 250°C. Nitrogen was used as the carrier gas, and the flow rate was set to constant mode (1 mL/min). Injection volume was 1 μL and the injection mode was splitless. The temperature was raised by a step gradient that starts at 60°C for 15 minutes then quickly rises up to 80°C in 4 minutes, followed by a slow rise to 135°C in 55 minutes, and finally a rapid rise to 260°C in 8 minutes and hold for 2 minutes. GC-mass spectrometry (GC-MS) analysis was performed using a Hewlett-Packard 6890 Gas Chromatograph and Hewlett-Packard 5973 Mass Selective Detector. The capillary column was also HP-5MS cross bond. The injector and detector temperatures were set at 250°C and 230°C, respectively. Helium was used as the carrier gas, and the flow rate was set to constant mode (1 mL/min). The injection volume was 1 μL, and the injection mode was split with a 50:1 ratio. The temperature gradient was the same as in the GC condition. Ionization voltage was 70 eV by electron impact. The acquisition mass range was set to 30–650 amu. The mass spectra were searched and compared through the database of National Institute of Standards and Technology (NIST) 08 libraries for characterization (the matching quality between the experimental data and the database is more than 90%). For further confirmation, Kovats retention index (RI) was calculated relative to standard n-alkanes of n-paraffin mix C7, C8, C9, C10 and C10, C12, C14, C16. The calculated RI was compared with those reported by Pino et al [8] and Pitarokili et al [9]. The relative percentage amounts were calculated on the basis of peak areas for both GC and GC-MS analysis, and the results were very similar. To confirm the high percentage components, standards were used to spike into the sample.
2.4. Antibacterial test
Sterilized lysogeny broth (LB) was prepared for the growth of E. coli. Frozen stock of strain ATCC 13676 was grown at 37°C on a 1.5% agar plate overnight. An E. coli stock solution was prepared by suspending a selected colony to 1 mL sterilized LB [10]. For each test, tubes with 5 mL sterilized LB were inoculated with 40 μL of E. coli stock solution. Essential oils were added to the tubes directly to give the appropriate concentrations: 0.01%, 0.05%, and 0.1% (v/v). The antibacterial activity of the essential oils against E. coli was evaluated using the optical density method (turbidimetry) [11]. During the incubation (37°C and 150 rev/min), the optical density of the inoculated broths at 600 nm was measured using Amersham Pharmacia Biotech Novaspec II Visible Spectrophotometer every hour. The process was carried out for 10 hours. After 10 hours, the spectra were taken at 24 and 28 hours. All spectra were taken against a blank solution of LB medium only.
3. Results and discussion
3.1. Composition analysis of essential oils
GC-MS chromatography with database NIST 08 was used to identify the chemical compositions of the essential oils. The same GC analyses with flame ionization detector were used to integrate the peak area. The chemical components of seven essential oils, ranked according to their Kovats retention indices, and their relative abundance (above 0.5%) are listed in Table 1. Although most of the essential oils have many components, clove basil and patchouli have only one dominate species each (eugenol and carvacrol, respectively). Lemon verbena has geometric isomers of citral (the common name is geranial and neral for the E and Z form, respectively) in high percentage, which together comprise the major species. Sweet marjoram contains about 18.5% of γ-terpinene, 15.5% of thymol methyl ether, and 12.0% of terpinen-4-ol. Rosemary contains about 54.8% of α-pinene and 22.2% of 1,8-cineole. Tea tree contains about 33.0% of terpinen-4-ol and 27.7% of 1,8-cineole. Rose geranium contains about 28.9% of citronellol and 20.1% of 6,9-guaiadiene. The main components mentioned above constitute at least half of the essential oils. These high percentage contents indicate potential for special applications.
Table 1.
Volatile components of the seven essential oils and their relative abundance.
| RIa | RIb | RIc | Compoundsd | Lemon verbena | Sweet marjoram | Clove basil | Patchouli | Rosemary | Tea tree | Rose geranium |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| % of totale | ||||||||||
| 916 | 923 | 905 | Tricyclene | t | ||||||
| 919 | 931 | 909 | α-Thujene | t | 1.7 | 0.7 | t | t | ||
| 925 | 939 | 918 | α-Pinene | 0.9 | 1.5 | t | 54.8 | 2.6 | ||
| 939 | 953 | 934 | Camphene | 4.7 | ||||||
| 963 | 976 | 955 | Sabinene | 2.1 | 4.8 | t | ||||
| 968 | 978 | —f | 1-Octen-3-ol | t | ||||||
| 969 | 980 | 962 | β-Pinene | t | t | 2.6 | t | |||
| 977 | 985 | — | 6-Methyl-5-hepten-2-one | t | ||||||
| 985 | 991 | 976 | β-Myrcene | t | 2.4 | 1.1 | t | t | ||
| 1005 | 1005 | 990 | α-Phellandrene | t | t | t | ||||
| 1016 | 1018 | 1005 | α-Terpinene | 8.1 | 1.9 | t | 5.8 | |||
| 1026 | 1026 | 1010 | p-Cymene | 9.2 | 15.2 | t | 6.0 | |||
| 1031 | 1031 | — | Limonene | 16.1 | 2.6 | t | 3.7 | 2.9 | ||
| 1033 | 1032 | 1024 | 1,8-Cineole | 4.6 | 2.4 | 22.2 | 27.7 | |||
| 1041 | 1040 | — | β-(Z)-Ocimene | t | 3.5 | 15.4 | ||||
| 1053 | 1050 | 1032 | β-(E)-Ocimene | 2.4 | ||||||
| 1061 | 1062 | 1043 | γ-Terpinene | t | 18.5 | 10.7 | t | 14.0 | ||
| 1075 | — | 1051 | cis-Sabinene hydrate | t | 1.9 | t | ||||
| 1087 | 1087 | 1072 | Terpinolene | 3.2 | t | 2.2 | ||||
| 1097 | — | 1086 | trans-Sabinene hydrate | 9.9 | ||||||
| 1102 | 1098 | 1088 | Linalool | t | t | t | ||||
| 1110 | — | — | cis-Rose oxide | t | ||||||
| 1117 | — | — | Chrysanthenone | t | ||||||
| 1123 | — | — | trans-Rose oxide | t | ||||||
| 1139 | 1143 | 1136 | Camphor | t | ||||||
| 1149 | — | — | Isomenthone | t | ||||||
| 1158 | 1154 | — | Menthone | 2.4 | ||||||
| 1158 | — | — | cis-Chrysanthenol | 0.7 | ||||||
| 1164 | 1164 | 1152 | Borneol | t | 3.7 | |||||
| 1167 | — | 1162 | cis-Pinocamphone | t | ||||||
| 1174 | 1177 | 1162 | Terpinen-4-ol | t | 12.0 | t | t | 33.0 | ||
| 1177 | — | — | (E)-Isocitral | 1.1 | ||||||
| 1182 | 1173 | — | Menthol | t | ||||||
| 1189 | 1189 | 1174 | α-Terpineol | 1.0 | t | t | 5.9 | |||
| 1205 | 1204 | — | Verbenone | t | 4.5 | |||||
| 1218 | — | 1204 | Nerol | 0.4 | ||||||
| 1224 | — | — | Citronellol | t | 28.9 | |||||
| 1227 | — | — | Thymol methyl ether | 15.5 | ||||||
| 1230 | 1240 | — | Neral | 23.1 | ||||||
| 1234 | 1242 | 1229 | Carvone | 4.9 | ||||||
| 1244 | 1255 | — | Geraniol | 0.7 | t | 2.4 | ||||
| 1259 | 1270 | — | Geranial | 26.9 | ||||||
| 1267 | 1275 | — | Citronellyl formate | 11.7 | ||||||
| 1273 | 1285 | 1269 | Bornyl acetate | t | 3.9 | |||||
| 1291 | — | — | Geranyl formate | t | ||||||
| 1292 | 1298 | 1287 | Carvacrol | 2.2 | 47.5 | |||||
| 1334 | — | 1341 | α-Cubebene | t | ||||||
| 1336 | 1356 | 1342 | Eugenol | 73.6 | ||||||
| 1345 | 1354 | — | Citronellyl acetate | t | ||||||
| 1356 | 1376 | 1357 | α-Copaene | t | t | 0.9 | ||||
| 1368 | 1383 | 1365 | Geranyl acetate | 0.9 | ||||||
| 1368 | — | 1365 | β-Bourbonene | t | 1.5 | |||||
| 1378 | 1391 | — | β-Elemene | t | ||||||
| 1399 | 1409 | — | α-Cedrene | t | ||||||
| 1404 | 1418 | 1406 | β-Caryophyllene | 3.9 | 3.1 | t | 10.9 | t | 9.3 | |
| 1406 | — | — | β-Cedrene | t | ||||||
| 1424 | 1439 | 1423 | α-Guaiene | 1.3 | ||||||
| 1429 | — | — | 6,9-Guaiadiene | 20.1 | ||||||
| 1435 | — | — | cis-Muurola-3,5-diene | 1.1 | ||||||
| 1439 | 1454 | 1435 | α-Humulene | t | 2.7 | 1.8 | ||||
| 1443 | — | — | allo-Aromadendrene | t | t | |||||
| 1462 | 1477 | 1457 | γ-Muurolene | t | ||||||
| 1465 | 1480 | 1461 | Germacrene D | t | 8.3 | 4.5 | ||||
| 1472 | 1487 | 1461 | ar-Curcumene | 6.2 | ||||||
| 1472 | 1484 | 1466 | β-Selinene | t | ||||||
| 1474 | — | 1476 | Bicyclogermacrene | 3.9 | t | 1.0 | ||||
| 1486 | — | 1480 | α-Muurolene | t | ||||||
| 1490 | — | — | Isodaucene | t | ||||||
| 1499 | — | 1494 | γ-Cadinene | 0.9 | ||||||
| 1501 | — | 1492 | β-Curcumene | 1.3 | ||||||
| 1506 | 1523 | 1503 | δ-Cadinene | 2.1 | ||||||
| 1556 | 1568 | 1544 | (E)-Nerolidol | 0.6 | 1.0 | |||||
| 1557 | — | 1557 | Spathulenol | 1.8 | 1.8 | |||||
| 1561 | 1581 | 1565 | Caryophyllene oxide | 1.5 | 2.1 | 1.7 | ||||
| 1566 | — | — | Furopelargone A | 1.7 | ||||||
| 1597 | 1642 | 1592 | 1,10-di-epi-Cubenol | t | ||||||
t = Trace, <0.5%.
Calculated Kovats retention index on the HP-5MS column.
Kovats retention index (RI) from Pino et al [8].
Kovats RI from Pitarokili et al [9].
The chemical compositions were identified by Database NIST 08.
Integral of peak area in the GC chromatograms is used.
Not found in the literature.
The growing condition of plants has a definite role in the amount of each component in a particular essential oil [12,13]. It is of interest to compare the composition of species grown in Taiwan and other species grown elsewhere. By comparing with the literature data, we found that the major components for lemon verbena are the same everywhere—that two structural isomers of citral (geranial and neral) consist of at least half essential oil [14]. Clove basil and rose geranium have at least one major component conserved around the world. Clove basil grown in Taiwan contains primarily eugenol along with other minor components, whereas that grown in Brazil contains a relative abundance of (Z)-α-bisabolene or 1,8-cineole in addition to eugenol [15,16]. Rose geranium in Taiwan is abundant in citronellol and 6,9-guaiadiene, but the one grown in United States contains citronellol and geraniol [17]. Components from Taiwan’s sweet marjoram, patchouli, and rosemary essential oils are very different from those of their counterparts in other parts of the world. The essential oil of sweet marjoram from Taiwan contains thymol methyl ether and γ-terpinene, whereas that from Greece or Germany primarily contains either carvacrol or limonene, depending on the species [18,19]. It is interesting to note that thymol and carvacrol are structural isomers that have the hydroxyl group at different positions of the aromatic ring. Patchouli essential oils from Brazil and the United States have been reported to contain α-guaiene, δ-guaiene, and patchoulol [20,21], whereas those from Taiwan contain mainly carvacrol. Although both are alcohols, the structures of carvacrol and patchoulol are very different and indicates little to no relationship between them. Rosemary essential oils have been reported to have a high percentage of 1,8-cineole (Greece) [22], three major components of camphor, limonene, and α-pinene (Serbia) [23], and α-pinene and 1,8-cineole (Taiwan). These results further demonstrate the complexity of essential oils.
M. alternifolia essential oil, commonly known as narrow-leaved tea-tree essential oil, is the primary species for commercial production of tea tree essential oil. It is native to Australia and has been cultivated around the world. It has been reported to have many desirable applications, such as smoothing, antifungal, anti-inflammatory, antimicrobial, and antioxidant [24]. This wide range of functions could be attributed to the fact that its chemical composition is dramatically different from that of other essential oils produced in other areas of the world. Table 2 summarizes the major compositions of tea trees from Australia, United States, India, Brazil, and Taiwan. Although terpinen-4-ol, γ-terpinene, and α-terpinene are three major components for all originally, except for that in Taiwan, the percentage of each major component varies widely. The most abundant component, terpinen-4-ol, is found to comprise 53.7% of the essential oil from the Brazilian species to about 35% in Australian, Indian, and Taiwanese species. It is rather remarkable to find that 1,8-cineole is abundant in the species from Taiwan in spite of its scarcity in others.
Table 2.
Comparison of major components of essential oils from tea trees of various origins.
3.2. Antibacterial activity against E. coli
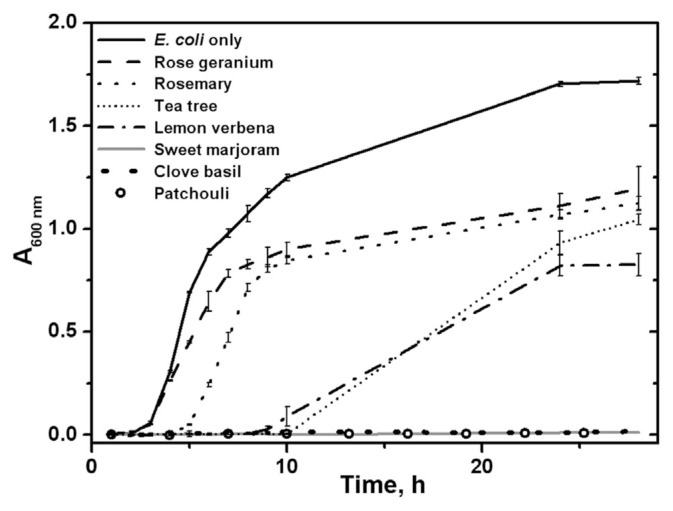
Because of the large amount of phenol-type compounds found in essential oils from Taiwan, we decided to assess their antibacterial activity. E. coli is the most widely studied prokaryotic model organism in biochemistry and is used as an indicator for bacteriological water analysis. All seven essential oils have been tested for their inhibition of E. coli growth. The growth curves of E. coli with 0.1% of seven essential oils are shown in Figure 1. The inhibitory effect is defined as:
Figure 1.
Growth curves of Escherichia coli in lysogeny broth (LB) medium in the absence and presence of 0.1% of seven essential oils.
| (1) |
where ODEO is the optical density at 600 nm in the presence of essential oil and ODcontrol is the optical density of the E. coli sample without any essential oil after 24 hours of incubation. Figure 1 shows a very high inhibitory effect for patchouli, clove basil, and sweet marjoram. To fully assay the inhibitory effect, concentrations of 0.01%, 0.05%, and 0.1% essential oils were added to the E. coli solution. The minimum inhibitory concentration (MIC) is the lowest concentration of an antibiotic that will inhibit the visible growth of a microorganism after overnight incubation [29]. Although the exact value of MIC cannot be obtained directly, it is evaluated to be between 0.01% and 0.05% for patchouli, 0.05–0.1% for clove basil and sweet marjoram, and >0.1% for lemon verbena, tea tree, rosemary, and rose geranium. Rosemary and rose geranium show a poor inhibitory effect such that even at 0.1%, E. coli growth reduces by only about 30% after an overnight incubation. Tea tree is well known for its broad-spectrum antimicrobial activity [24]. It is expected to restrain the growth of E. coli. The MIC of the Australian species was reported to be 0.25% for E. coli strain AG100 [30]. The species from Taiwan completely inhibits the E. coli growth in the first 10 hours of incubation at 0.1%. However, after 10 hours, E. coli regains its growth to about 50% of no essential oil control. This phenomenon could result from the consumption of essential oils by enzymes secreted from E. coli over time. Terpinen-4-ol is the major component in both Australian and Taiwanese species and may be the key component for inhibitory effect.
4. Conclusion
We have demonstrated that GC-MS with NIST 08 database is an ideal tool for the routine analysis of essential oils. Although seven essential oils from Taiwan show complex compositions, some of them contain a high percentage of a few compounds. These essential oils are subject to specific applications. For example, patchouli and clove basil have a high content of carvacrol and eugenol, respectively. Both compounds are very effective antibacterial reagents, and it is not surprising that patchouli and clove basil exhibit excellent inhibition to E. coli. The compositions of essential oils are highly dependent on the growing conditions of the plants. We found that tea tree essential oils from Taiwan have a high percentage of 1,8-cineole, and account for its unique flavor.
Acknowledgments
The financial support from the Ministry of Science and Technology of the Republic of China (NSC 98-2622-M-003-002-CC3) is gratefully acknowledged. Professor Y.C. Wang’s (Department of Life Science) generous offer of making laboratory facilities available for our use is very much appreciated. Professor J.C. Wang’s (Department of Life Science) help on the preparation of plant specimens is also highly appreciated.
Funding Statement
The financial support from the Ministry of Science and Technology of the Republic of China (NSC 98-2622-M-003-002-CC3) is gratefully acknowledged.
Footnotes
Conflicts of interest
All authors declare no competing financial interest.
REFERENCES
- 1. Mahesh B, Satish S. Antimicrobial activity of some important medicinal plant against plant and human pathogens. World J Agric Sci. 2008;4:839–43. [Google Scholar]
- 2. Lin CW, Yu CW, Wu SC, Yih KH. DPPH free-radical scavenging activity, total phenolic contents and chemical composition analysis of forty-two kinds of essential oils. J Food Drug Anal. 2009;17:386–95. [Google Scholar]
- 3. Wang HF, Yih KH, Huang KF. Comparative study of the antioxidant activity of forty-five commonly used essential oils and their potential active components. J Food Drug Anal. 2010;18:24–33. [Google Scholar]
- 4. Yen HF, Wang SY, Wu CC, Lin WY, Wu TY, Chang FR, Wang CK. Cytotoxicity, anti-platelet aggregation assay and chemical components analysis of thirty-eight kinds of essential oils. J Food Drug Anal. 2012;20:478–83. [Google Scholar]
- 5. Lee CJ, Chen LW, Chen LG, Chang TL, Huang CW, Huang MC, Wang CC. Correlations of the components of tea tree oil with its antibacterial effects and skin irritation. J Food Drug Anal. 2013;21:169–76. [Google Scholar]
- 6. Abdel-Maksoud G, El-Amin A-R. A review on the materials used during the mummification process in ancient Egypt. Mediterr Archaeol Archaeom. 2011;11:129–50. [Google Scholar]
- 7. Raut JS, Karuppayil SM. A status review on the medicinal properties of essential oils. Ind Crop Prod. 2014;62:250–64. [Google Scholar]
- 8. Pino JA, Mesa J, Munoz Y, Marti MP, Marbot R. Volatile components from mango (Mangifera indica L.) cultivars. J Agric Food Chem. 2005;53:2213–23. doi: 10.1021/jf0402633. [DOI] [PubMed] [Google Scholar]
- 9. Pitarokili D, Tzakou O, Loukis A, Harvala C. Volatile metabolites from Salvia fruticosa as antifungal agents in soilborne pathogens. J Agric Food Chem. 2003;51:3294–301. doi: 10.1021/jf0211534. [DOI] [PubMed] [Google Scholar]
- 10. Bozin B, Mimica-Dukic N, Simin N, Anackov G. Characterization of the volatile composition of essential oils of some Lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils. J Agric Food Chem. 2006;54:1822–8. doi: 10.1021/jf051922u. [DOI] [PubMed] [Google Scholar]
- 11. Ponce AG, Fritz R, del Valle C, Roura SI. Antimicrobial activity of essential oils on the native microflora of organic Swiss chard. LWT-Food Sci Technol. 2003;36:679–84. [Google Scholar]
- 12. Wang CL, Yin HW. The locational and seasonal variations of leaf essential oil from cultivated Cinnamomum osmophloeum Kaneh. Taiwan J For Sci. 1991;6:313–28. [Google Scholar]
- 13. Yin HW. Yield and composition variation of essential oil from leaves of different Cinnamomum osmophloeum Kanehira clones in Taiwan. Q J Chin For. 1991;24:83–104. [Google Scholar]
- 14. Romeo FV, De Luca S, Piscopo A, De Salvo E, Poiana M. Effect of some essential oils as natural food preservatives on commercial grated carrots. J Essent Oil Res. 2010;22:283–7. [Google Scholar]
- 15. Freire CMM, Marques MOM, Costa M. Effects of seasonal variation on the central nervous system activity of Ocimum gratissimum L. essential oil. J Ethnopharmacol. 2006;105:161–6. doi: 10.1016/j.jep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 16. Lemos JA, Passos XS, Fernandes OFL, Paula JR, Ferri PH, Souza LKH, Lemos AA, Silva MRR. Antifungal activity from Ocimum gratissimum L. towards Cryptococcus neoformans. Mem Inst Oswaldo Cruz. 2005;100:55–8. doi: 10.1590/s0074-02762005000100011. [DOI] [PubMed] [Google Scholar]
- 17. Peterson A, Machmudah S, Roy BC, Goto M, Sasaki M, Hirose T. Extraction of essential oil from geranium (Pelargonium graveolens) with supercritical carbon dioxide. J Chem Technol Biotechnol. 2006;81:167–72. [Google Scholar]
- 18. Daferera DJ, Tarantilis PA, Polissiou MG. Characterization of essential oils from Lamiaceae species by Fourier transform Raman spectroscopy. J Agric Food Chem. 2002;50:5503–7. doi: 10.1021/jf0203489. [DOI] [PubMed] [Google Scholar]
- 19. Šipailieneė A, Venskutonis PR, Baranauskienė R, Šarkinas A. Antimicrobial activity of commercial samples of thyme and marjoram oils. J Essent Oil Res. 2006;18:698–703. [Google Scholar]
- 20. Donelian A, Carlson LHC, Lopes TJ, Machado RAF. Comparison of extraction of patchouli (Pogostemon cablin) essential oil with supercritical CO2 and by steam distillation. J Supercrit Fluid. 2009;48:15–20. [Google Scholar]
- 21. Verma RS, Padalia RC, Chauhan A. Assessment of similarities and dissimilarities in the essential oils of patchouli and Indian Valerian. J Essent Oil Res. 2012;24:487–91. [Google Scholar]
- 22. Daferera DJ, Ziogas BN, Polissiou MG. GC-MS analysis of essential oils from some Greek aromatic plants and their fungitoxicity on Penicillium digitatum. J Agric Food Chem. 2000;48:2576–81. doi: 10.1021/jf990835x. [DOI] [PubMed] [Google Scholar]
- 23. Bozin B, Mimica-Dukic N, Samojlik I, Jovin E. Antimicrobial and antioxidant properties of rosemary and sage (Rosmarinus officinalis L. and Salvia officinalis L., Lamiaceae) essential oils. J Agric Food Chem. 2007;55:7879–85. doi: 10.1021/jf0715323. [DOI] [PubMed] [Google Scholar]
- 24. Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (tea tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev. 2006;19:50–62. doi: 10.1128/CMR.19.1.50-62.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shellie R, Marriott P, Zappia G, Mondello L, Dugo G. Interactive use of linear retention indices on polar and apolar columns with an MS-Library for reliable characterization of Australian tea tree and other Melaleuca sp. oils. J Essent Oil Res. 2003;15:305–12. [Google Scholar]
- 26. Kawakami M, Sachs RM, Shibamoto T. Volatile constituents of essential oils obtained from newly developed tea tree (Melaleuca alternifolia) clones. J Agric Food Chem. 1990;38:1657–61. [Google Scholar]
- 27. Verghese J, Jacob CV, Kartha CVK, McCarron M, Mills AJ, Whittaker D. Indian tea tree (Melaleuca alternifolia Cheel) essential oil. Flavour Frag J. 1996;11:219–21. [Google Scholar]
- 28. Silva CJ, Barbosa LCA, Maltha CRA, Pinheiro AL, Ismail FMD. Comparative study of the essential oils of seven Melaleuca (Myrtaceae) species grown in Brazil. Flavour Frag J. 2007;22:474–8. [Google Scholar]
- 29.CLSI. Twenty-Fourth Informational Supplement. CLSI document M100-S24. Wayne, PA: Clinical and Laboratory Standards Institute; 2014. Performance standards for antimicrobial susceptibility testing. [Google Scholar]
- 30. Gustafson JE, Liew YC, Chew S, Markham J, Bell HC, Wyllie SG, Warmington JR. Effects of tea tree oil on Escherichia coli. Lett Appl Microbiol. 1998;26:194–8. doi: 10.1046/j.1472-765x.1998.00317.x. [DOI] [PubMed] [Google Scholar]