Abstract
Tumor cells naturally live in three-dimensional (3D) microenvironments, while common laboratory tests and evaluations are done in two-dimensional (2D) plates. This study examined the impact of cultured 4T1 cancer cells in a 3D collagen–chitosan scaffold compared with 2D plate cultures. Collagen–chitosan scaffolds were provided and passed confirmatory tests. 4T1 tumor cells were cultured on scaffolds and then tumor cells growth rate, resistance to X-ray radiation, and cyclophosphamide as a chemotherapy drug were analyzed. Furthermore, 4T1 cells were extracted from the scaffold model and were injected into the mice. Tumor growth rate, survival rate, and systemic immune responses were evaluated. Our results showed that 4T1 cells infiltrated the scaffolds pores and constructed a 3D microenvironment. Furthermore, 3D cultured tumor cells showed a slower proliferation rate, increased levels of survival to the X-ray irradiation, and enhanced resistance to chemotherapy drugs in comparison with 2D plate cultures. Transfer of extracted cells to the mice caused enhanced tumor volume and decreased life span. This study indicated that collagen–chitosan nanoscaffolds provide a suitable model of tumor that would be appropriate for tumor studies.
Keywords: collagen-chitosan, immune response, in vivo model, scaffolds, tumor cells
1. Introduction
Cancer is one of the most important diseases that causes death. For instance, approximately one out of every eight women will be diagnosed with breast cancer [1]. In situ evaluation of breast cancer development and metastasis takes 5–30 years. Regarding this point, the development of a suitable model system for use in studying cancer progression at a molecular basis is highly desirable [2].
Current treatments of malignant tumors are usually based on radiotherapy, chemotherapy, and immunotherapy [3]. However, clinical usages of these treatments need to be proven by previously developed screening tests. Development of effective therapies requires a cost-effective in vitro tumor model that more accurately resembles the in vivo tumor microenvironment. Currently, breast cancer screening protocols are done in the two-dimensional (2D) culture of tumor cells [4].
Although 2D culture provides some advantages including accessibility and simplicity, the natural microenvironments are completely different with 2D cultures. In 2D conditions, cell–cell contacts, cell–matrix interaction, cell surface receptor expression, proliferation, and aggressiveness (malignancy) of tumor cells are extensively changed. Furthermore, hypoxic conditions as a typical feature in the tumor microenvironment are rarely provided in 2D cultures. Therefore, outcomes from 2D in vitro culture could not translate the exact situation of in vivo systems [5–7].
Three-dimensional (3D) culture systems are designed to bridge the gap between in vitro and in vivo cancer models. These 3D systems mimic extracellular matrix (ECM) conditions and provide a more malignant in vivo-like phenotype of tumor cells [8,9]. In addition, it has been reported that tumor cells can show partial differentiation in 3D cultures [10]. At in vivo conditions, mechanical and physicochemical support of tumor cells is provided and tumor cells can interact with the ECM. The five classes of macromolecules including collagens, laminins, fibronectins, proteoglycans, and hyaluronans establish the natural ECM. Interstitial spaces are often filled with polysaccharides and fibrous proteins [11,12]. Collagen, as one of the main components of the ECM, plays an important role in supporting and interaction with tumor cells [13]. Spaces between basement proteins are usually filled by polysaccharides. Chitosan is a biodegradable, semicrystalline polysaccharide obtained by N-deacetylation of chitin, which is harvested from the exoskeleton of marine crustaceans and is vastly used for construction of scaffolds and also for drug/ gene deliveries [14,15].
Therefore, in the present study we aimed to investigate tumor cell growth rates, resistance to X-ray radiation, and cyclophosphamide based chemotherapy in 2D and 3D cultures. Also, we evaluated in vivo tumor growth rate and mice survival as well as systemic immune responses following injection of precultured 4T1 cells to mice in 2D/3D cultures.
2. Materials and methods
2.1. Animals, cell line, and materials
Inbred Balb/c mice (6–8 weeks-old) and documented 4T1 cell line were obtained from the Pasteur Institute (Tehran, Iran). Chitosan (100–300 kDa; 75–85% degree of deacetylation) and 4-hydroxycyclophosphamide as the active form of cyclophosphamide were purchased from Sigma (St. Louis, MO, USA). Flat-bottomed plates were bought form Nunc (Kamstrup, Denmark). Mouse cytokine enzyme-linked immunosorbent assay (ELISA) kits were obtained from eBiosciences (Frankfurt, Germany). RPMI (Roswell Park Memorial Institute) 1640 and fetal bovine serum (FBS) were bought from Invitrogen (Gibco, Grand Island, NY, USA). Collagen Type I was isolated from fresh bovine tendon using a method of trypsin digestion and acetic acid dissolution described previously in Ma et al (2003) [16].
2.2. Preparation of collagen–chitosan scaffold
Collagen or chitosan was dissolved in 1% acetic acid solution to prepare 2% (w/v: 2 g/100 mL) solutions. The chitosan solution was then slowly dripped into the collagen suspension to achieve a final collagen–chitosan ratio of 9:1; thereafter, the materials were homogenized to obtain a collagen–chitosan blend. This blend was then injected into a mold (diameter = 15 mm, depth = 3 mm), frozen at −20°C for 1 hour, and then placed at −70°C. The samples were then dried in a lyophilizer (Edwards MicroModulyo, Bolton, England).
2.3. Cross-linking treatment
Lyophilized scaffolds were rehydrated and stabilized in ethanol. In this process, scaffolds were immersed in absolute ethanol for approximately 1 hour and then sequentially in 70% (v/v) and then 50% ethanol for 30-minute periods. The scaffolds were finally equilibrated in phosphate-buffered saline (PBS, pH 7.4) prior to mechanical testing.
2.4. Scanning electron microscopy of matrices
The matrices were analyzed by scanning electron microscopy (SEM). The sample was coated with a 10-nm thick gold film using a sputter coater, and then analyzed by an electron acceleration voltage of 20 KeV in a DSM 940A SEM system (Zeiss, Hamburg, Germany).
2.5. 4T1 Tumor cell line culture and action
The 4T1 cell line (NBCI code: C604), which mimics Stage IV of human breast cancer, was obtained from the Cell Bank of Pasteur Institute of Iran (Tehran, Iran). 4T1 cells were first cultured overnight in T25 culture flasks and then the cells were recovered and cultured on prepared scaffolds or routine 24-well plates adjusted to 5 × 105 cells/mL RPMI medium [now supplemented with 11mM NaHCO3, 2mM L-glutamine, 100 U penicillin/mL, 100 μg streptomycin/mL, and 5% FBS (all reagents from Gibco)]. For further evaluations, the 4T1 cells were treated with the active form of cyclophosphamide (4-hydroxycyclophosphamide; 1–100 μg/mL) and in another series the cultured cells were exposed to X-ray radiation (0.5–5 Gy). Thereafter, treated cells were incubated for 48 hours at 37°C and under 5% CO2. After this period, the cells were examined for viability.
2.6. Assessment of cell viability
4T1 cells viability was evaluated using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction assay indicative of cellular metabolic activity. At the end of the 48-hour incubation, MTT (20 μL, at 5 mg/mL in PBS) was added to each well and the plate incubated for 4 hours at 37°C. After incubation, culture supernatants were gently removed and 100 μL acidic isopropanol (0.04M HCl in isopropanol) was added in order to dissolve the formazan crystals generated within the viable cells. Absorbance in each well was then assessed at 540 nm using a Multiskan plate reader (Thermo Scientific, Vantaa, Finland). All results were expressed as survival index (SI) calculated as the Optical Density (OD540) of test cultures/OD540 of control wells.
2.7. Induction of tumor mice model
4T1 cells were cultured on prepared collagen–chitosan scaffolds and routine 2D flasks. Then, 100 μL of 1 × 107 4T1cells/mL PBS suspension in logarithmic growth phase were injected subcutaneously into Balb/c mice flank. All Balb/c mice were kept singly in separate standard cages and maintained in a pathogen-free facility at 22°C with a 12-hour light/dark cycle. Then, using a digital caliper, the tumor volume of each mouse was measured at 4-day intervals and the tumor volume estimated according to the formula V = LW2/2 (where V: tumor volume; L: large diameter; W: small diameter). Finally, changes in tumor volume between Day 10 and Day 34 were measured according to δV = V1−V2 (V1: 1st day tumor volume; V2: 20th day tumor volume). Tumor bearing mice were monitored for mortality from injection day until 100 days and the death day was recorded for survival analysis. The Tarbiat Modares University Ethical Committee for Animal Care and Use approved all aspects of this study.
2.8. In vivo splenocytes responses
In order to assess induced in vivo systemic antitumor immune responses in the treated 4T1 tumor mice model, mice were sacrificed on Day 35 and the spleen was harvested and smashed. The obtained cell suspensions were cultured 4 × 105 cells/well in 10% FBS complete RPMI 1640 in the presence of 20 μg/mL 4T1 lysate. After 48 hours of incubation in 37°C and 5% CO2, an MTT proliferation assay kit I (Roche, Basel, Switzerland) was used for cell proliferation assay and stimulation index calculated. Additionally, the supernatant of each well was collected and interferon-gamma (IFN-γ), interleukin-4 (IL-4), transforming growth factor beta (TGF-β), and IL-10 were measured using an ELISA kit (eBiosciences, Frankfurt, Germany).
2.9. Statistical analysis
All in vitro experiments contained at least three to five replicates and all in vivo experiments consisted of at least five animals/group. Obtained results were analyzed using SPSS 15 (SPSS Inc., Chicago, IL, USA) and a p value < 0.05 was considered statistically significant. The Kruskal–Wallis and Mann–Whitney U tests were used for within and between group statistical differences, respectively. The Kaplan–Meier test was used for survival, and comparison of groups was determined using log-rank (Mantel–Cox).
3. Results
3.1. Scaffolds morphology and 4T1 cells growth
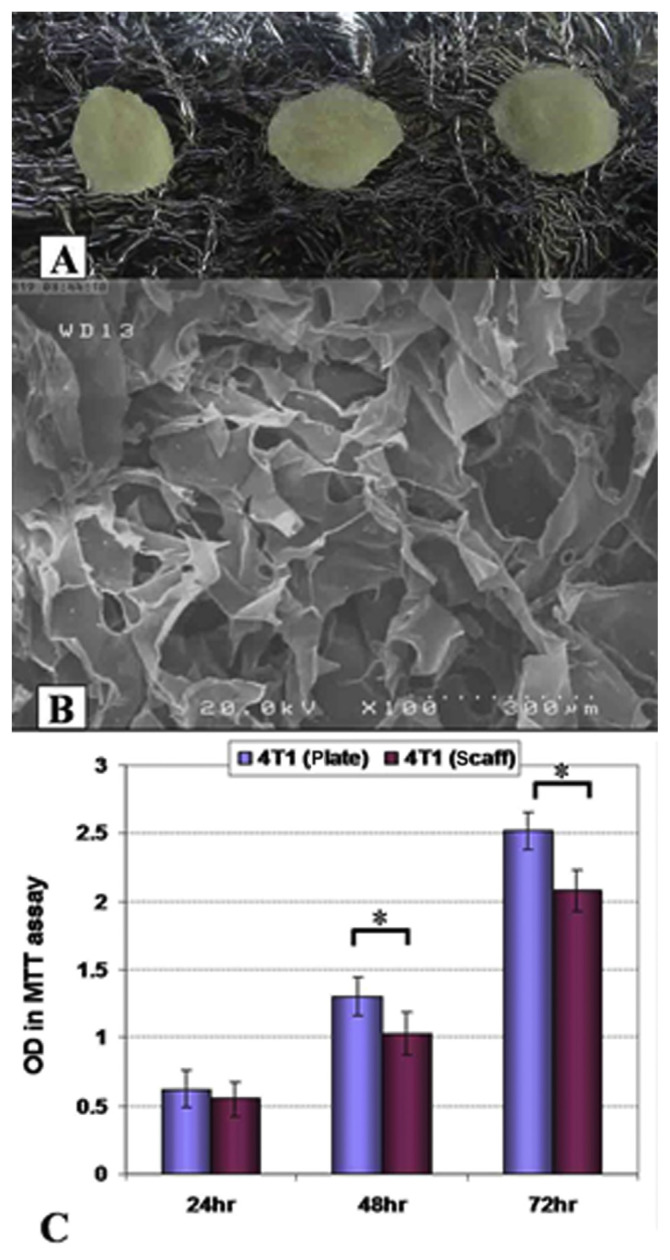
Electronic microscopy analysis demonstrated the presence of a porous surface in collagen–chitosan scaffolds that would be suitable for cells seeding and infiltrations (Figures 1A and 1B). The proliferation rates of 4T1 cells in two conditions of 2D and 3D cultures were determined by the MTT assay (Figure 1C). MTT reduction capability showed lower proliferation rate for 4T1 cells cultured on collagen–chitosan 3D culture conditions in comparison with routine 2D plate cultures after 48–72 hours of culturing (p < 0.05).
Figure 1.
(A) Macroscopic and (B) electronic microscopy structure of collagen–chitosan scaffolds. (C) 4T1 tumor growth in three-dimensional (3D) and two-dimensional conditions. Scaffolds presented an appropriate porosity for cell infiltrations. Cultured 4T1 cells showed lower growth rates on 3D scaffolds surfaces after 48 hours and 72 hours of culture (p < 0.05). OD = Optical Density; MTT = 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; scaff = scaffold, *: p < 0.05.
3.2. Evaluation of irradiation and 4-hydroxycyclophosphamide effect
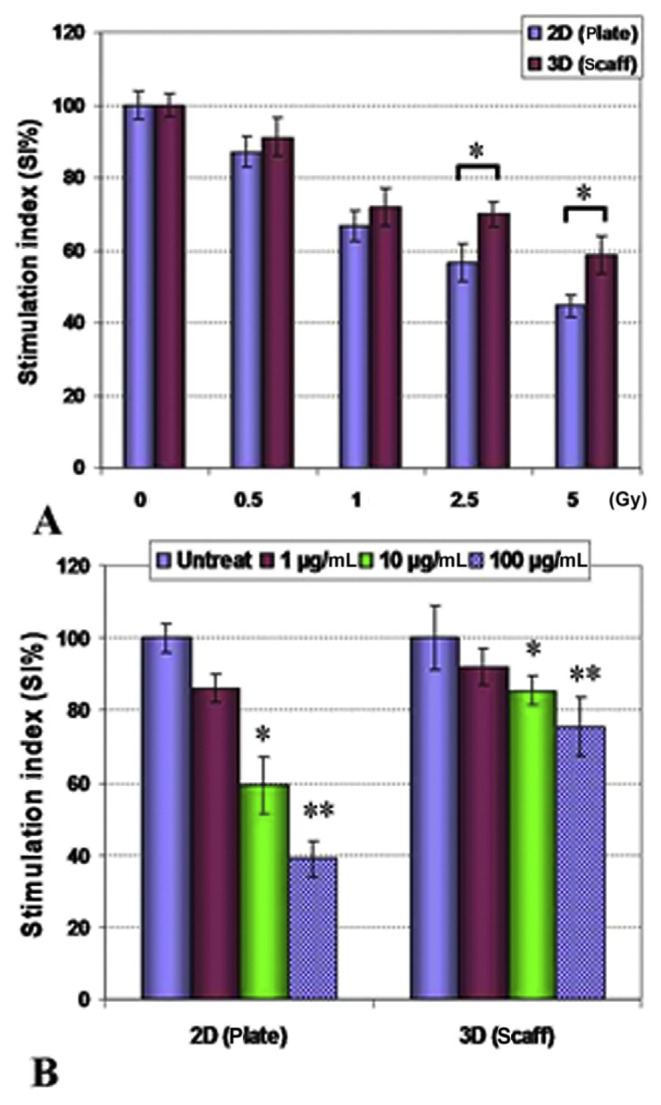
The effects of X-ray irradiation and cyclophosphamide on cultured 4T1 cells in 2D/3D conditions were assessed (Figure 2). Analysis of the results showed that cultured 4T1 cells on 3D collagen–chitosan conditions were more resistant to higher doses of irradiation (2.5–5 Gy) in comparison with cultured cells in 2D conditions (p < 0.05). Precultured cells on scaffolds also were more resistant to toxic effects of 4-hydroxycyclophosphamide (in higher examined doses of 1–100 μg/mL; p < 0.05).
Figure 2.
4T1 tumor cell line growth in scaffold three-dimensional (3D) and plate 2D conditions exposed to (A) X-ray irradiation (0.5–5 Gy) and (B) 4-hydroxycyclophosphamide (1–100 μg/mL). 4T1 cells were protected against higher doses of X-ray (2.5–5 Gy) cultured on 3D collagen–chitosan scaffolds in comparison with 2D culture conditions (p < 0.05). Tumor cells also were more resistant against 4-hydroxycyclophosphamide when they were cultured on collagen–chitosan scaffolds (p < 0.05). scaff = scaffold, *: p < 0.05, **: p < 0.01.
3.3. Mice model splenocytes proliferation and cytokines release
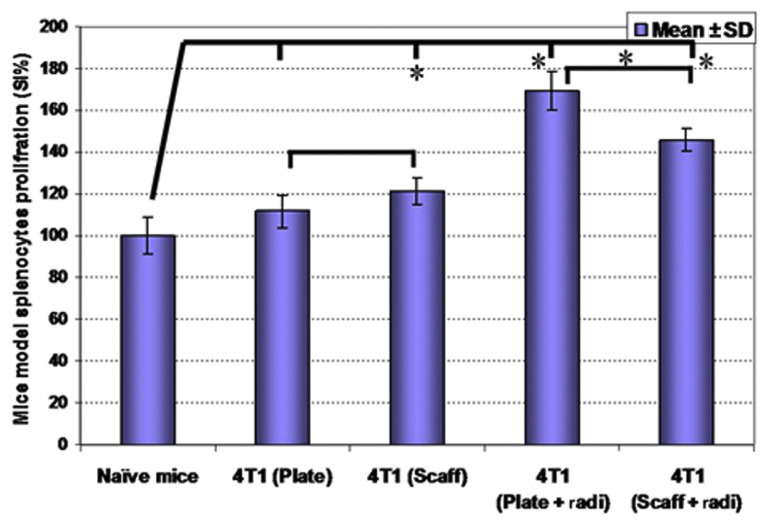
4T1 cells were cultured on collagen–chitosan scaffolds and routine plates, and also in other groups exposed to X-ray irradiation and then injected into the mice subcutaneously for in vivo evaluations. On Day 35 postinjection, mice were sacrificed and splenocytes proliferation rates (Figure 3) and cytokines release patterns (Figure 4) were evaluated. Analysis of proliferation rates showed that splenocytes from irradiated 4T1 cells and precultured 4T1 cells on collagen–chitosan scaffolds groups have higher proliferation rates in comparison with naïve mice and plate precultured 4T1 groups (p < 0.05). Further analysis indicated that splenocytes proliferation rates were higher in 4T1-plate-irradiation in comparison with 4T1-scaffold-irradiation (p < 0.05).
Figure 3.
Precultured 4T1 cells were injected into Balb/c mice then mice splenocytes proliferation rates were evaluated on Day 35. A higher splenocytes proliferation rate was observed in 4T1-scaffold precultured group and also preradiated cell groups [both two-dimensional (2D) and 3D cultured cells], in comparison with naïve mice splenocytes. * p < 0.05. radi = radiation; Scaff = scaffold; SD = standard deviation; SI = survival index.
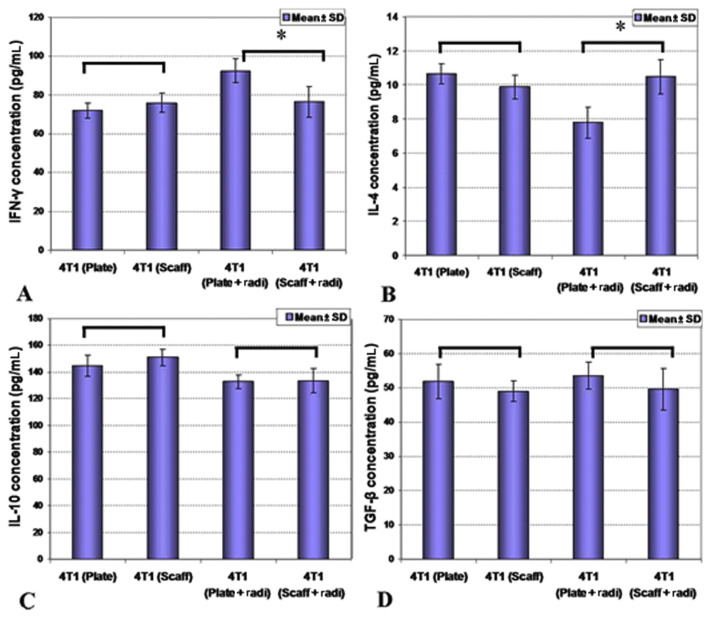
Figure 4.
Precultured 4T1 cells were injected into Balb/c mice in different groups and then mice splenocyte cytokine release was evaluated on Day 35. (A and B) While there were no differences of cytokine secretion in two-dimensional (2D) and 3D precultures (p > 0.05), (A and B) preradiation of 4T1 cells caused enhanced interferon-gamma (IFN-γ) and reduced interleukin-4 (IL-4) release in 2D culture conditions in comparison with 3D precultures. (C and D) there were no differences of cytokine secretion in two-dimensional (2D) and 3D precultures regarding IL-10 and TGF-β cytokines. IL-10 = interleukin-10; radi = radiation; Scaff = scaffold; SD = standard deviation; TGF-β = transforming growth factor beta, *: p < 0.05.
Produced cytokines on cultured splenocytes were measured. Statistical analysis between 2D versus 3D precultured groups showed that normal precultured 4T1 cells on 2D versus 3D conditions were not affected by cytokine production by splenocytes (p > 0.05), however, production of IFN-γ was induced and secretion of IL-4 was suppressed by irradiation of plate precultured 4T1 cells (p < 0.05). There was no significant difference between diverse groups regarding secretion of IL-10 and TGF-β (p > 0.05).
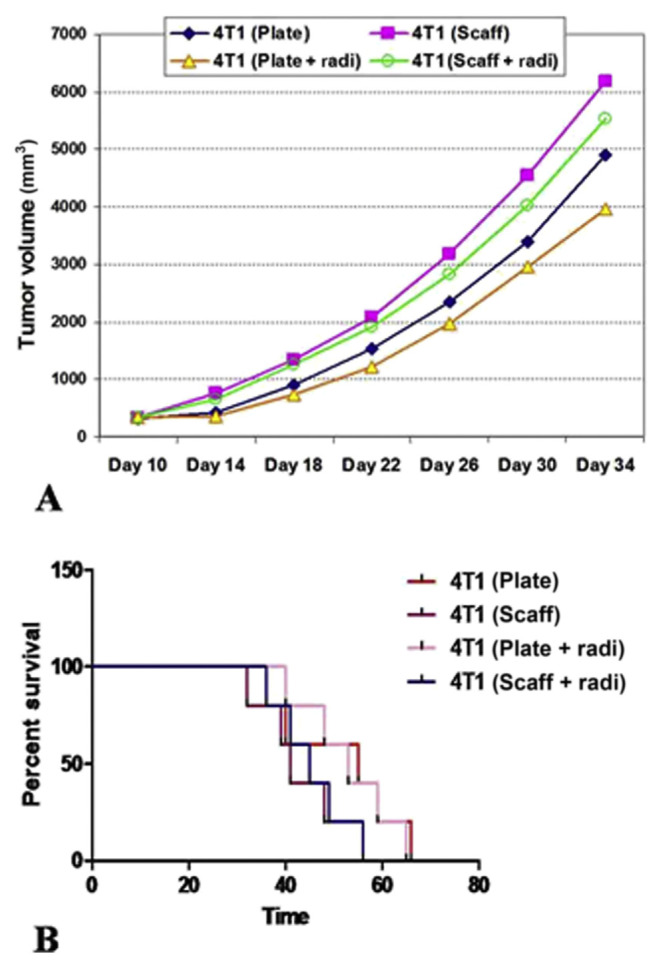
3.4. Mice model survival and tumor growth
After culturing of 4T1 cells in 2D or 3D conditions and irradiation in some groups, cells were injected to Balb/c mice subcutaneously and tumor growth and survival rates were evaluated (Figure 5). Analysis of results by the Kaplan–Meier test and comparison by log-rank (Mantel–Cox) indicated that tumor growth in collagen–chitosan scaffolds precultured 4T1 cells showed higher growth rates compared with 2D precultured groups, even after irradiation (p < 0.05). The 3D precultured group also showed a lower survival rate in comparison with mice that were injected with 2D precultured 4T1 cells (p < 0.05).
Figure 5.
Precultured 4T1 cells were injected into Balb/c mice in different groups and then (A) tumor growth and (B) mice survival rates were determined. Mice tumors from three dimensional (3D) precultures showed higher growth rates and their differences were statistically significant (p < 0.05). 3D precultured 4T1 injected mice also showed lower survival rates in comparison with mice with 2D precultured 4T1 cell injection (p < 0.05). radi = radiation; Scaff = scaffold, Time: days.
4. Discussion
Development of effective therapeutic strategies requires a cost-effective in vitro tumor model that more accurately resemble the in vivo tumor microenvironment, as standard 2D tissue culture conditions do not mimic the natural microenvironment precisely. In this study, we provided collagen–chitosan scaffolds as a 3D model and then the chemotherapy and irradiation effects on the tumor cells were evaluated. Our results showed that tumor cells suitably infiltrated the scaffolds pores and constructed a 3D interacting microenvironment. The cells in the 3D model demonstrated accelerated proliferation rates and higher levels of resistance to X-ray irradiation and 4-hydroxycyclophosphamide as a chemotherapy agent.
Incorporated cells into collagen–chitosan scaffolds showed lower growth rates than those in 2D culture. This more closely resembles the tumor behaviors. Previous studies also reported enhanced resistance of tumor cells in 3D conditions. For instance, Dhiman et al [17], showed that MCF-7 cells cultured in 3D conditions were more resistant to tamoxifen in comparison with 2D cultures [17]. In the tumor microenvironment, tumor cells need to recruit blood vessels before rapid proliferation and suffering from hypoxia and less nutrients. In 2D culture, cells are supplied with sufficient nutrients and oxygen [18]. Furthermore, 3D culture provides additional interaction between tumor cells and allows the formation of tight junctions with neighboring cells [19]. Previous studies showed that growth factors required for the establishment and maturation of blood vessels are highly expressed in 3D culture models compared with 2D culture. Therefore, the enhancement in secretion of proangiogenic growth factors overcame the lack of blood vessels within the initial days of tumor implantation [20,21].
We used irradiation as a common treatment in cancer therapy. Our data showed that 3D culture induced greater resistance to irradiation and chemotherapy. In 3D culture, tumor cells form clusters resulted in reduction of exposure of the tumor cells to irradiation. These data are parallel to previous studies that showed 3D culture increased tumor cells resistance to chemotherapy agents through limitation of drug diffusions into the tumor mass and may induce drug-resistant properties in tumor cells through upregulation of P-glycoprotein multidrug transporter [13,17]. In addition, the hypoxic situation in 3D culture leads to trigger cell quiescence remarks by reducing susceptibility of cells to irradiation [22].
In the second part of our evaluations, we injected 2D/3D cultured 4T1 cells into Balb/c mice and then evaluated tumor features and immune system responses. Results showed that splenocytes proliferation and cytokines secretion were not affected by 2D or 3D culturing of the cells, but interestingly, irradiation of 2D or 3D cultures affected immune system responses to the tumor cells. Irradiated 4T1 cells in the 3D model induced a low level of immune responses while irradiated 2D cultured cells released tumor antigens to stimulate mice immunity. These results simulated natural 3D microenvironment of tumor and may explain why there are many appropriate results in 2D in vitro tumor treatment studies without suitable in vivo antitumor outcomes.
Previous studies indicated that the expression and secretion of metastasis related cytokines such as RANTES, macrophage inflammatory protein 2, tumor necrosis factor-alpha, and macrophage colony-stimulating factor increased in 3D cultured cells in comparison with the matrigel culture cells and, moreover, plated tumor cells recruited T cells results to impair tumor growth [23,24]. Tsao et al [23], showed that matrigel cultured cells are more susceptible to T cells killing actions in comparison with 3D cultured tumor cells.
In another study, Kievit et al [13] showed that chitosan–alginate (CA) scaffolds were able to mimic the tumor microenvironment and glioma cells precultured on CA scaffolds induced higher levels of angiogenesis and tumor volume growth. Leung et al [20] also showed that CA scaffolds could mimic hepatocellular carcinoma. Szot et al [25] indicated that the expression of tumor promoting genes such as hypoxia-inducible factor-1α and vascular endothelial growth factor-A genes were highly induced when MDA-MB-231 human breast cancer cells were cultured on Collagen Type I based 3D scaffolds. Arya et al evaluated NCI–H460 (NSCLC), HeLa (cervical cancer), MCF-7 (breast cancer), and MG-63 (osteosarcoma) cell lines cultured on 3D culture of chitosan–gelatin scaffolds and showed that 3D cultured tumor cells expressed higher levels of tumor related genes alongside enhanced proliferation rates in comparison with 2D cultured tumor cells [26].
In conclusion, in vitro studies are an essential component of the initial screening for any anticancer therapy and this study showed that 3D culture of tumor cells on collagen–chitosan scaffolds could be considered a useful and more reliable in vitro platform than traditional 2D culture for future tumor cell line-based studies.
Footnotes
Conflicts of interest
No conflicts of interest declared.
REFERENCES
- 1. DeSantis C, Siegel R, Bandi P, Jemal A. Breast cancer statistics, 2011. CA Cancer J Clin. 2011;61:408–18. doi: 10.3322/caac.20134. [DOI] [PubMed] [Google Scholar]
- 2. Day N, Williams D, Khaw K. Breast cancer screening programmes: the development of a monitoring and evaluation system. Br J Cancer. 1989;59:954. doi: 10.1038/bjc.1989.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khatcheressian JL, Hurley P, Bantug E, Esserman LJ, Grunfeld E, Halberg F, Hantel A, Henry NL, Muss HB, Smith TJ, Vogel VG, Wolff AC, Somerfield MR, Davidson NE. Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology clinical practice guideline update. Clin Oncol. 2013;31:961–5. doi: 10.1200/JCO.2012.45.9859. [DOI] [PubMed] [Google Scholar]
- 4. Frese KK, Tuveson DA. Maximizing mouse cancer models. Nat Rev Cancer. 2007;7:654–8. doi: 10.1038/nrc2192. [DOI] [PubMed] [Google Scholar]
- 5. Smalley KS, Lioni M, Herlyn M. Life isn’t flat: taking cancer biology to the next dimension. In Vitro Cell Dev Biol Anim. 2006;42:242–7. doi: 10.1290/0604027.1. [DOI] [PubMed] [Google Scholar]
- 6. Trédan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99:1441–54. doi: 10.1093/jnci/djm135. [DOI] [PubMed] [Google Scholar]
- 7. Wu XZ, Chen D, Xie GR. Extracellular matrix remodeling in hepatocellular carcinoma: effects of soil on seed? Med Hypotheses. 2006;66:1115–20. doi: 10.1016/j.mehy.2005.12.043. [DOI] [PubMed] [Google Scholar]
- 8. Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–12. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 9. Xu F, Burg KJ. Three-dimensional polymeric systems for cancer cell studies. Cytotechnology. 2007;54:135–43. doi: 10.1007/s10616-007-9065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spancake KM, Anderson CB, Weaver VM, Matsunami N, Bissell MJ, White RL. E7-transduced human breast epithelial cells show partial differentiation in three-dimensional culture. Cancer Res. 1999;59:6042–5. [PubMed] [Google Scholar]
- 11. Hayashi Y, Yamada S, Yanagi Guchi K, Koyama Z, Ikeda T. 6 Chitosan and fish collagen as biomaterials for regenerative medicine. Adv Food Nutr Res. 2012;65:107–20. doi: 10.1016/B978-0-12-416003-3.00006-8. [DOI] [PubMed] [Google Scholar]
- 12. Lee J, Cuddihy MJ, Kotov NA. Three-dimensional cell culture matrices: state of the art. Tissue Eng Part B Rev. 2008;14:61–86. doi: 10.1089/teb.2007.0150. [DOI] [PubMed] [Google Scholar]
- 13. Kievit FM, Florczyk SJ, Leung MC, Veiseh O, Park JO, Disis ML, Zhang M. Chitosan-alginate 3D scaffolds as a mimic of the glioma tumor microenvironment. Biomaterials. 2010;31:5903–10. doi: 10.1016/j.biomaterials.2010.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Younes I, Rinaudo M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar Drugs. 2015;13:1133–74. doi: 10.3390/md13031133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Elgadir MA, Uddin MS, Ferdosh S, Adam A, Chowdhury AJK, Sarker MZI. Impact of chitosan composites and chitosan nanoparticle composites on various drug delivery systems: a review. J Food Drug Anal. 2015;23:619–29. doi: 10.1016/j.jfda.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ma L, Gao C, Mao Z, Zhou J, Shen J, Hub X, Han C. Collagen/ chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials. 2003;24:4833–41. doi: 10.1016/s0142-9612(03)00374-0. [DOI] [PubMed] [Google Scholar]
- 17. Dhiman HK, Ray AR, Panda AK. Three-dimensional chitosan scaffold-based MCF-7 cell culture for the determination of the cytotoxicity of tamoxifen. Biomaterials. 2005;26:979–86. doi: 10.1016/j.biomaterials.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 18. Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–92. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 19. Lin RZ, Chang HY. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol J. 2008;3:1172–84. doi: 10.1002/biot.200700228. [DOI] [PubMed] [Google Scholar]
- 20. Leung M, Kievit FM, Florczyk SJ, Veiseh O, Wu J, Park JO, Zhang M. Chitosan-alginate scaffold culture system for hepatocellular carcinoma increases malignancy and drug resistance. Pharm Res. 2010;27:1939–48. doi: 10.1007/s11095-010-0198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Merwin JR, Anderson JM, Kocher O, Van Itallie CM, Madri JA. Transforming growth factor beta1 modulates extracellular matrix organization and cell-cell junctional complex formation during in vitro angiogenesis. J Cell Physiol. 1990;142:117–28. doi: 10.1002/jcp.1041420115. [DOI] [PubMed] [Google Scholar]
- 22. Sermeus A, Cosse J-P, Crespin M, Mainfroid V, de Longueville F, Ninane N, Raes M, Remacle J, Michiels C. Hypoxia induces protection against etoposide-induced apoptosis: molecular profiling of changes in gene expression and transcription factor activity. Mol Cancer. 2008;7:27. doi: 10.1186/1476-4598-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tsao C-T, Kievit FM, Wang K, Erickson AE, Ellenbogen RG, Zhang M. Chitosan-based thermoreversible hydrogel as an in vitro tumor microenvironment for testing breast cancer therapies. Mol Pharm. 2014;11:2134–42. doi: 10.1021/mp5002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 25. Szot CS, Buchanan CF, Freeman JW, Rylander MN. 3D in vitro bioengineered tumors based on collagen I hydrogels. Biomaterials. 2011;32:7905–12. doi: 10.1016/j.biomaterials.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arya N, Sardana V, Saxena M, Rangarajan A, Katti DS. Recapitulating tumour microenvironment in chitosan–gelatin three-dimensional scaffolds: an improved in vitro tumour model. J R Soc Interface. 2012;9:3288–302. doi: 10.1098/rsif.2012.0564. [DOI] [PMC free article] [PubMed] [Google Scholar]