Abstract
Pistacia lentiscus (Anacardiaceae) is commonly used in folk medicine to treat various diseases. The aim of the present study was to evaluate the hepatoprotective and antioxidant activities of extracts of P. lentiscus leaves (PL) and fruits (PF) against experimentally induced liver damage. Furthermore, characterization of extracts was attempted by a spectroscopic methodology (Fourier transform infrared spectroscopy) and high-performance liquid chromatography with diode array detection analysis. A hepatoprotective potential against paracetamol [165 mg/kg body weight (b.w.)] toxicity was noticed in mice pretreated with the same dose of PL or PF extract (125 mg/kg b.w.) or a combination of both (PL/PF 63/63 mg/kg b.w.), as revealed by an analysis of biochemical parameters (alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase activities and total bilirubin). These results were confirmed by histological examination of the liver, which revealed significant protection against paracetamol-induced hepatic necrosis. Furthermore, PF extract exhibited a promising antidiabetic activity in streptozotocin-induced diabetic rats, similar to the reference drug glibenclamide (0.91 g/L), a result confirmed by in vitro inhibition of α-amylase. We demonstrated that the leaf crude extract showed the best effect in all tested methods, compared to its fruit counterpart, probably due to the presence of higher amounts of phenolic compounds, as determined by phytochemical and Fourier transform infrared spectroscopy analyses. Moreover, high-performance liquid chromatography with diode array detection led to the identification of six compounds for each part of the plant. Gallic acid, a characteristic compound of Pistacia species, was most abundant in leaves and fruits, while luteolin was detected for the first time in fruits. Obtained activities of P. lentiscus extracts may well be due, at least in part, to the presence of the above compounds.
Keywords: α-amylase, antidiabetic, antioxidant, Fourier transform infrared spectroscopy, hepatoprotective, high-performance liquid chromatography, Pistacia lentiscus
1. Introduction
The liver, a key organ of metabolism and excretion, is continually exposed to a variety of xenobiotics and therapeutic agents. About 20,000 deaths are recorded every year due to liver disorders, caused in some cases by paracetamol, a well-known analgesic and antipyretic agent. Taken at high doses, paracetamol is hepatotoxic since it generates the highly reactive metabolite N-acetyl-p-benzoquinoneimine, which induces glutathione depletion and oxidative stress, leading to hepatic necrosis [1]. Antioxidant compounds of natural origin, mostly polyphenols found in medicinal plants, can counteract the harmful toxic side effects of modern drugs by providing hepatoprotective capacity. Preserving the liver of people suffering from diabetes is also important, since the disease induces oxidative stress resulting from glucose oxidation [2,3]. The number of diabetes cases is currently about 117 million globally and predicted to reach 336 million by the end of 2030 [4]. Indeed, a number of in vitro and in vivo studies have shown that plant polyphenols can be used as chemopreventive agents against different metabolic diseases [5]. The current modern approach to treating diabetes, including insulin and various oral antidiabetic synthetic drugs, has demonstrated its limits, with unsatisfactory results and many side effects [6]. The challenging issue for researchers was to seek natural analogs of insulin and amylin, in addition to alpha-glucosidase inhibitors, devoid of toxicity. In that perspective, more than 1200 plants were identified experimentally to be used in the treatment of diabetes, due to the interesting biological properties of their constituent polyphenols [7]. Indeed, according to the World Health Organization, approximately 80% of the inhabitants of this planet have recourse to traditional medicines. As in many countries of the world, this type of medicine is a part of the Algerian culture, as it is resourced in a rich agricultural heritage of rejuvenated herbs and food traditionally used for curing several ailments, including diabetes [7]. Nevertheless, the use of medicinal plants should be subjected to systematically screening assays, accompanied by objective data analysis to ascertain their safety, for the purpose of discovery and development of new efficient drugs [8–10].
Pistacia lentiscus, a sclerophyll evergreen shrub and a member of the Anacardiaceae family, largely distributed in extreme ecosystems of the Mediterranean basin [11,12], is widely used in phytotherapy for its sedative, antiatherogenic, and antioxidant properties [13]. The aerial parts of P. lentiscus are used in folk medicine as stimulants and diuretics, and to treat hypertension, cough, sore throat, eczema, stomach ache, and kidney stones and jaundice [14]. Fruits are consumed raw or roasted, while their oil has versatile medicinal uses either internally in the treatment of ulcers or externally to heal psoriasis [14]. In addition to their therapeutic effects, P. lentiscus parts are also used in the food industry. For example, essential oils obtained from aerial parts of P. lentiscus are used as flavoring agents in alcoholic beverages and chewing gum [15]. Moreover, the anthocyanins extracted from the fruits are exploited as food colorants, and the oil rich in mono-unsaturated and omega-3 fatty acids such as oleic acid and linolenic acid, a high quantity of phytosterols such as β-sitosterol, and vitamins may potentially be included in animal and human diets as antioxidants [16]. The protective effect of P. lentiscus can be due to the presence of flavonoids, anthocyanins, hydrolysable tannins, and flavonoid glycosides [11,17]. Hence, the present study focused on evaluating the potential hepatoprotective effect of ethanolic extracts from P. lentiscus leaves (PL) and fruits (PF) on paracetamol-induced liver injury in mice and their antidiabetic effect on rats. To contribute to the existing pool of local plants with claimed antidiabetic activity, in vivo experiments on streptozotocin (STZ)-induced diabetic rats were selected for anti-hyperglycemic activity. Antioxidant properties of PL and PF extracts were also investigated by evaluating oxygen uptake inhibition during oxidation of methyl linoleate. Finally, the subacute toxicity of the plant parts was assessed in vivo.
2. Methods
2.1. Plant material
The leaves and fruits of P. lentiscus were harvested in the forest of Tizi Neftah in the suburbs of Amizour city by the Department of Bejaia (Bejaia, Northeastern Algeria). The plant was identified in the Laboratory of Botany (University of Bejaia) where a specimen was deposited. The Laboratory of Botany (University of Bejaia) does not have a specific voucher specimen. In fact, only the characteristics of the plant as well as its place and date of harvest and the person who identified the plant are recorded. However, the same plant is recorded by one of our colleagues in the Herbarium of Natural History, Museum of Aix-en-Provence, France, under the voucher specimen (D-DH-2013-37-5) [18]. After drying, leaves and fruits of P. lentiscus were ground using an electric mill (Kika Labortechnik, Stoufen, Germany), to give, respectively, a fine powder (63 μm) and a paste.
2.2. Animals
Albino male mice (17–20 g) and Wistar rats (180–200 g) were obtained from the Center of Research and Development (CRD-SAIDAL, Algiers, Algeria). Animals were housed in cages and maintained on a 12-hour light/dark cycle, at a temperature of ~23°C, with constant humidity. They were allowed to acclimatize to laboratory conditions for a week before starting the experiment. Drinking water and food were provided ad libitum throughout the experiment, except for a short fasting period of 12 hours prior to treatment. Animals were handled according to the recommendations of the International Ethics Committee (Directive of the European Council 86/609/EC).
2.3. Extraction procedure
The extraction was conducted according to a well-established procedure [19]. Leaf powder and fruit paste were macerated separately in ethanol (1:4 w/v) for 24 hours with mechanical agitation. After evaporation, the ethanol extract was fractionated by solvent partition between ethyl acetate and water (1:3:1 w/v/v) to obtain two distinct phases, organic and aqueous phases, which were separately dried. The organic phase was further extracted using a mixture of chloroform and water (1:3:1 w/v/v) to obtain another two fractions that were separately dried. At the end, five different extracts for each part of this plant were collected and tested for various activities.
2.4. Determination of total phenols, flavonoids, and tannins
Total phenols were determined by the Folin–Ciocalteu procedure [20]. Aliquots (0.1 mL) of test solutions were transferred into test tubes and volumes were brought up to 0.5 mL by Folin–Ciocalteu phenol reagent (1:10 v/v) and 1000 μL of distilled water, followed by the addition of 1500 μL of sodium carbonate solution (20%). After incubation for 2 hours in the dark and at room temperature, the absorbance was measured at 760 nm and the results were expressed as milligrams of catechin equivalent per gram of dry weight of extract (mg C Eq/g).
Quantification of flavonoids was performed using known colorimetric methods [21] by the addition of aluminum chloride reagent (AlCl3) to the solution containing the extract. A volume of 1 mL of extract (100 μg/mL) was combined with 500 μL of aluminum chloride solution (133 mg of AlCl3 + 400 mg of CH3CO2Na) dissolved in 100 mL of distilled water. The absorbance was recorded at 430 nm, and concentrations of flavonoids were deduced from a standard curve and expressed as milligrams of rutin equivalent per gram of extract (mg R Eq/g).
Determination of tannin content in P. lentiscus extracts was performed according to the method described by Hagerman and Butler [22]. Two milliliters of a solution of bovine serum albumin (1 mg/mL prepared in 200mM acetic acid and 170mM NaCl adjusted to pH 4.9) were added to 1 mL (1 mg/mL) of extract solution. The mixture was incubated for 24 hours at 4°C for plant extract and for 15 minutes at room temperature for tannic acid used as standard, and then centrifuged at 3000g for 15 minutes. The pellet was dissolved in 4 mL of sodium dodecyl sulfate (SDS)-triethanolamine solution [1% SDS and 5% triethanolamine (v/v) in distilled water]. One milliliter of ferric chloride reagent (0.01M ferric chloride in 0.01N hydrochloric acid) was added and the solutions were mixed immediately. After 30 minutes, the absorbance was recorded at 510 nm and results were expressed as milligrams of tannic acid equivalent per gram of extract (mg TA Eq/g).
2.5. Fourier transform infrared spectroscopy analysis
Fourier transform infrared (FTIR) spectra were recorded as KBr disks on a Perkin Elmer FTIR spectrometer SPECTRUM 2000 (Waltham, Mass, USA) in the wave number range between 4000/cm and 400/cm. Finely divided extract samples (9 mg) were dispersed in a matrix of KBr (300 mg) and pressed to form pellets. This method is used to deduce information on the nature of the chemical bonds present in a compound. All comparisons were made qualitatively, due to the difficulty in defining a reference spectral band and to perform quantitative measurements [23].
2.6. High-performance liquid chromatography analysis
High-performance liquid chromatography (HPLC) analysis was carried out using an Agilent 1200 Series LC system (Agilent Technologies, Palo Alto, CA, USA) equipped with an online vacuum degasser, a thermostated autosampler (15°C), a quaternary pump, and a diode array detector (DAD). The column used for chromatographic separation was a Kinetex PFP (100 × 4.6 mm2, 2.6 μm; Phenomenex, Utrecht, The Netherlands) thermostated at 30°C.
The phenolic compounds were separated at a flow rate of 0.1 mL/min using the following gradient composed of water with 0.1% formic acid (eluent A) and acetonitrile with 0.1% formic acid (eluent B): 5% B at 0 minutes, 5% B at 6 minutes, 20% B at 13 minutes, 60% B at 17 minutes, 100% at 20 minutes; and a final reconditioning cycle of 6 minutes at 5% B. The injection volume was 5 μL. The compounds separated were monitored with the DAD at 250 nm, 280 nm, 315 nm, and 370 nm, and peak spectra were acquired from 200 nm to 400 nm. All analyses were carried out in triplicate (n = 3). Constituent phenols were identified by comparing retention times with known commercial pure standards purchased from Sigma-Aldrich (St. Louis, MO, USA) (gallic acid, catechin, syringic acid, ellagic acid, 3,4-dihydroxyhydro-cinnamic acid, benzoic acid, salicylic acid, quercetin 3-O-rhamnoside, and luteolin). Quantification was equally performed by external standardization using solutions (5 × 10−3–1 mg/mL) of each pure commercial compound.
2.7. Evaluation of antioxidant activity
Antioxidant properties of PL and PF extracts were investigated by evaluating oxygen uptake inhibition during oxidation of methyl linoleate [24]. Oxidation was induced by molecular oxygen in a gas-tight borosilicate glass apparatus (60°C), using butan-1-ol. Initial conditions inside the vessel were as follows: methyl linoleate (99%; Fluka, Buchs, Switzerland), 0.4 mol/L; 2,2′-azodiisobutyronitrile (98%; Fluka), 9 × 10−3 mol/L; plant extract, 0.4 g/L; and oxygen pressure, 200 hPa. Oxygen uptake was monitored continuously by a pressure transducer. Without any additive, oxygen uptake is roughly linear and constitutes the control. In the presence of an antioxidant, oxygen consumption is slower, and the antioxidant capacity of extract (ACE) was estimated by comparing oxygen uptake at a chosen time (150 minutes), in the presence and absence of extract, according to the following formula:
| (1) |
ACE is 0 for an inactive extract and 100% for a perfect antioxidant.
2.8. Subacute toxicity study
Wistar albino rats, starved overnight, were divided into five groups (n = 5) and were given oral daily doses of P. lentiscus ethanol leaf and fruit extracts [50 mg/kg body weight (b.w.) and 250 mg/kg b.w.], except for the last group (control), which received distilled water. Food was provided to the rats approximately 1 hour after treatment. Observations for signs of toxicity were conducted daily within the first 6 hours of the treatment, which included weight measurements and visual recordings of mortality, behavior, changes in physical appearance, injury, pain, and signs of illness during the 28 days of treatment period.
At the end of the study, blood was collected from the retro-orbital sinuses and centrifuged at 3500g for 15 minutes, and sera were collected and used for the analysis of biochemical parameters [glucose, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total bilirubin, cholesterol, triglycerides, and creatinine]. Animals were sacrificed and quickly dissected, and their livers and kidneys were removed and fixed in 10% formalin. The organs were dehydrated in ascending grades of ethanol, cleared in xylene, and embedded in paraffin. The hematoxylin–eosin staining method was used before thorough observations under the microscope for histological analysis.
2.9. Hepatoprotective activity
The method described by Nithianantham et al [9] was employed, with some minor modifications. Mice (18–20 g) were divided into five groups (n = 7). Negative and positive control groups (GI and GII, respectively) orally received a dose of 10 mL/kg b.w. of saline (0.9%) and paracetamol (165 mg/kg b.w. per day), respectively. Animals of Groups III, IV, and V (GIII–GV) received orally 125 mg/kg b.w. of P. lentiscus leaf ethanol extract (PL), 125 mg/kg b.w. of fruit ethanol extract (PF) extract, and leaf (63 mg/kg b.w.) + fruit (63 mg/kg b.w.) extracts, respectively, 3 hours after administration of paracetamol. The treatments were sustained for 3 days, which were followed 24 hours later by sacrificing and dissecting all animals.
2.9.1. Assessment of hepatoprotective activity
After sacrifice, blood was collected and centrifuged at 3500g for 15 minutes, and sera were separated and stored at −20°C until use for the analysis of biochemical parameters (AST, ALT, and bilirubin).
2.9.2. Sample preparation of histological sections
For histological studies, livers of all animal groups were removed, rinsed with cold saline (0.9%), and fixed in 10% formalin solution.
Histopathological parameters for liver and kidney tissues were scored as described by Eidi et al [25] and Arsad et al [26], with a score of 0 showing no changes, and 1, 2, 3, and 4 indicating mild, moderate, extensive, and severe changes, respectively. The scores for histopathological parameters for liver and kidney can be explained as follows: Score 0 = no visible cell damage; Score 1 = focal cells damage on < 30% of the tissue; Score 2 = focal cells damage on 30–50% of the tissue; Score 3 = extensive, but focalized cell lesions, more than 50%; and Score 4 = global cells necrosis.
2.9.3. Lipid peroxidation inhibitory activity
Lipid peroxidation was assayed spectrophotometrically by measuring malondialdehyde (MDA) levels [27]. Excised livers from all groups were homogenized in ice-cold KCl (1.15%; 10% w/v), and the resulting homogenate was centrifuged at 2500g for 10 minutes to remove cellular debris. The supernatant (0.5 mL) was added to 0.5 mL of Trichloroacetic acid (TCA) (20%) and 1 mL of Thiobarbituric acid (TBA) (0.67%), and the mixture was heated at 100°C for 15 minutes, cooled, and supplemented with 4 mL of n-butanol. After centrifugation for 15 minutes at 1500g, the optical density of the supernatant was determined using a spectrophotometer at 532 nm and the MDA levels were expressed as nmol MDA/mg of liver tissue, calculated on the basis of the absorbance coefficient of the TBA–MDA complex (ɛ = 1.56 × 105/cm M).
2.10. Antidiabetic activity
2.10.1. In vivo antidiabetic activity
In this study, we investigated the possible antidiabetic effect of the crude extract in STZ-induced diabetic rats. Animals were fasted for 16 hours, after which they received a single intraperitoneal injection of STZ (60 mg/kg b.w.) [28], freshly prepared in 0.9% cold saline. Three days later, fasting blood glucose was determined and the rats that developed diabetes with glucose levels above 200 mg/dL were selected for the study [29]. Animals were divided into six groups (n = 5): Group I (GI, diabetic control rats) received saline 0.9%; Group II (GII) received glibenclamide (50 mg/kg b.w.), a known antidiabetic drug; Groups III (GIII) and IV (GIV) received PL ethanol extract (50 mg/kg b.w. and 125 mg/kg b.w., respectively); and Groups V (GV) and VI (GVI, diabetic rats) received PF ethanol extract (50 mg/kg b.w. and 125 mg/kg b.w., respectively). Extracts and glibenclamide were given orally and blood was collected twice (1 hour and 2 hours after treatment) through retro-orbital sinus of rat eyes. Serum was separated by centrifugation at 2500g for 10 minutes, and blood glucose was estimated by the glucose oxidase kit (cat. no-1001191; Spinreact, St Esteve de Bas, Girona, Spain).
2.10.2. In vitro antidiabetic activity: α-amylase inhibitory assay
The assay was carried out following the standard protocol with slight modifications [30]. Starch azure (2 mg) was suspended in 0.2 mL of 0.5M Tris–HCl buffer (pH 6.9) containing 0.01M CaCl2 (substrate solution). The tubes containing substrate solution were boiled for 5 minutes and then pre-incubated at 37°C for 5 minutes. Ethanol extracts of P. lentiscus leaves and fruits were dissolved in dimethyl sulfoxide in order to obtain concentrations of 20 μg/mL, 60 μg/mL, and 100 μg/mL. Then, 0.2 mL of plant extract was added to the tube containing the substrate solution, followed by the addition of 0.1 mL of Aspergillus oryzae α-amylase (4 units/mL; Sigma-Aldrich) prepared in Tris–HCl buffer (2 units/mL). The mixture was incubated at 37°C for 10 minutes, after which 0.5 mL of 50% acetic acid were supplemented to stop the reaction. The solution was centrifuged at 1500g for 5 minutes at 4°C, and the absorbance of the resulting supernatant was measured at 595 nm. Acarbose, a known α-amylase inhibitor, was used as a standard drug. The α-amylase inhibitory activity (AIA) was calculated using the following formula:
| (2) |
where Ac+, Ac−, As, and Ab are defined as the absorbencies of 100% enzyme activity (only solvent with enzyme), 0% enzyme activity (only solvent without enzyme), test sample (with enzyme), and blank (test sample without enzyme), respectively. The concentration of acarbose and plant extracts required to inhibit 50% of α-amylase activity was defined as the IC50 value.
2.11. Statistical analysis
In vitro results were obtained from three independent experiments and were expressed as mean ± standard deviation. The one-way analysis of variance (ANOVA) was performed using GraphPad Prism followed by Newman–Keuls multiple comparison test. In vivo results were presented as mean ± standard error of the mean. The analysis of variance was performed using GraphPad Prism followed by Dunnett test. The results are considered statistically significant at p < 0.05, p < 0.01, and p < 0.001.
3. Results and discussion
3.1. Determination of total phenols, flavonoids, and tannins
Solvent extraction is frequently used for isolation of antioxidants in plants taking into account the fact that the extraction yield is dependent on the solvent and method of extraction, and that different polarity may influence the different antioxidant potentials of the constituent compounds. It was established that higher extraction yields of phenolic compounds were obtained with an increase in the polarity of the solvent [31], in accordance with our findings. In fact, the highest amounts of polyphenols were recorded in the aqueous chloroform extracts of both leaves and fruits, followed by in ethanolic and aqueous ethyl acetate extracts (Table 1). Our results also showed that P. lentiscus is rich in tannins and not in flavonoids, as reported previously [19] for the same plant. Conversely, total amounts of phenolics and flavonoids of leaf crude extracts (517.512 ± 5.53 mg C Eq/g and 108.67 ± 0.5 mg R Eq/g, respectively) were significantly (p < 0.01) higher than those found in fruit crude extracts (254.9 ± 5.04 mg C Eq/g and 3.49 ± 1.19 mg R Eq/g, respectively). Quantification of total polyphenols (35 mg TA Eq/g of extract) in a related species, Pistacia vera [31], showed the highest levels in aqueous extracts, in agreement with our results and with those of previous investigators who reported that 75% of phenolic compounds of P. lentiscus are hydrolysable tannins (galloylated tannins) [32], mostly soluble in aqueous solvents. However, it has also been stated that any discrepancies in phenolic concentrations may be due to the collection period as well as soil conditions [33].
Table 1.
Determination of total phenols, flavonoids, and tannins in extracts of Pistacia lentiscus leaves and fruits.
| Extracts | Total phenols (mg C Eq/g extract) | Flavonoids (mg R Eq/g extract) | Tannins (mg TA Eq/g extract) | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Leaves | Fruits | Leaves | Fruits | Leaves | Fruits | |
| Ethanol | 517.512 ± 5.53 | 254.9 ± 5.04b | 108.67 ± 0.5 | 3.49 ± 1.19c | 409.87 ± 6.9 | 309.45 ± 6.88 |
| Ethyl acetate | ||||||
| Organic fraction | 399.423 ± 9.33*** | 100.7 ± 1.06b,*** | 115.14 ± 1.52*** | 7.41 ± 1.27c,* | 171.47 ± 13.03*** | 116.30 ± 14.08*** |
| Aqueous fraction | 587.292 ± 28.8*** | 281.84 ± 8.29b,*** | 36.91 ± 0.39*** | 6.71 ± 2.16c,* | 383.07 ± 5.17* | 65.45 ± 3.01c,*** |
| Chloroform | ||||||
| Organic fraction | 247.786 ± 2.79*** | 69.32 ± 3.67c,*** | 15.00 ± 1.00*** | 6.11 ± 0.46a,* | 115.65 ± 10.05*** | 69.68 ± 6.08c,*** |
| Aqueous fraction | 1104.603 ± 6.7*** | 366.04 ± 10.54c,*** | 253.82 ± 0.46*** | 16.57 ± 1.11c,*** | 479.35 ± 22.22*** | 226.95 ± 0.89b,*** |
Data are presented as mean ± SD, n = 3; one-way ANOVA followed by Newman–Keuls test. Values in the same column sharing different letters are significantly different.
p < 0.05,
p < 0.01,
p < 0.001 compare all pairs of columns of different solvents versus ethanol.
p < 0.05,
p < 0.01,
p < 0.001 compare all pairs of columns of fruits versus leaves.
ANOVA = analysis of variance; C Eq/g extract = catechin equivalent per gram of extract; R Eq/g extract = rutin equivalent per gram of extract; SD = standard deviation; TA Eq/g extract = mg tannic acid equivalent per gram of extract.
3.2. FTIR analysis
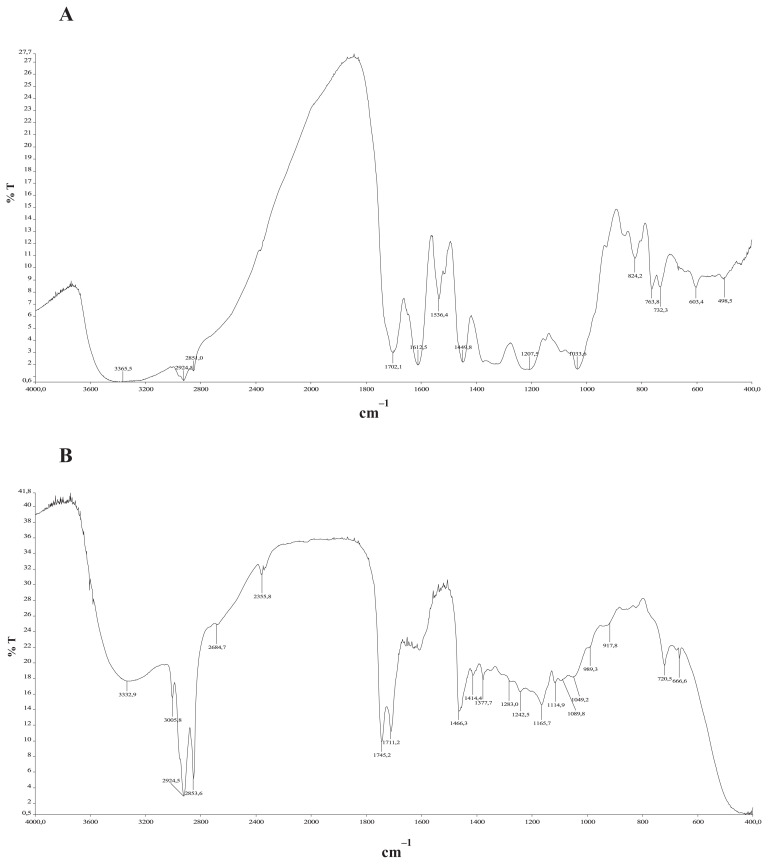
The FTIR spectra of a potassium bromide (KBr) pellet of crude extracts of P. lentiscus (leaves and fruits) are shown in Figure 1. The spectra (Figure 1) show the presence of two bands of strong intensity at 2853/cm and 2924/cm, characteristic of C–H bonds of aliphatic CH2 and CH3 groups, respectively. Moreover, characteristic carbonyl group (C==O) bands around 1700/cm (1701/cm, 1711/cm, and 1745/cm) are indicative of the presence of phenolic acids. In fact, the low-value bands are probably due to the presence of conjugated double bonds or aromatic moieties in the vicinity of carboxylic acids. At around 1600/cm, we found more aromatic cycles for both leaf and fruit extracts. Vibrations of deformation of aliphatic groupings CH2 and CH3 are found at 1377/cm and 1414/cm. The absorption bands of the C–H bonds of an aromatic ring or a double bond are around 3005/cm. Finally, the C–N function of an amine is determined by two absorption bands at 1207/cm and 1033/cm, featuring an aminophenol. Between 690/cm and 900/cm, we detected a grouping OH-bond, most probably due to the presence of an aromatic cycle. The detection of these characteristic absorption bands confirms the richness of fruit and especially leaf extracts in phenolic compounds.
Figure 1.
FTIR spectra of the crude extracts of (A) Pistacia lentiscus leaves and (B) fruits. FTIR = Fourier transform infrared spectroscopy.
3.3. Identification and quantification of individual phenolics
The phenolic profiles of PL and PF extracts obtained by comparing retention times and UV spectra at 280 nm (Table 2) with those of respective known standards (Table S1 and Figure S1) allowed the identification of six phenolic compounds for each part of the plant: gallic acid, catechin, syringic acid, ellagic acid, quercetin-3-O-rhamnoside, and luteolin in leaves, and gallic acid, catechin, 3,4-dihydroxyhydro-cinnamic acid, benzoic acid, salicylic acid, and luteolin in fruits. The presence of gallic acid and quercetin-3-O-rhamnoside in the leaves of P. lentiscus was previously reported [34]. Gallic acid, identified in both leaves and fruits, constitutes the basic unit of galloyl tannins and is a hallmark of Pistacia species.
Table 2.
Quantitative and qualitative analyses of phenolics in PL and PF ethanolic extracts by HPLC-DAD.
| Identified compounds | Calibration curve | R 2 | λmax (nm) | r.t. (min) | PL (mg/g d.w.) | PF (mg/g d.w) |
|---|---|---|---|---|---|---|
| Gallic acid | y = 14,538x | 0.9986 | 280 | 1.64 | 161.67 ± 35.27 | 5.67 ± 0.017*** |
| Catechin | y = 12,453x | 0.9869 | 280 | 9.059 | 31.79 ± 5.76 | 0.2 ± 0.001*** |
| Syringic acid | y = 66,229x | 0.9914 | 280 | 10.149 | 52.66 ± 7.23 | ND |
| Ellagic acid | y = 160,888x | 0.9986 | 250 | 13.636 | 9.54 ± 0.63 | ND |
| 3,4-Dihydroxyhydro-cinnamic acid | y = 16,515x | 0.9839 | 280 | 5.895 | ND | 0.07 ± 0.001 |
| Benzoic acid | y = 8505.7x | 0.9963 | 250 | 11.417 | ND | 0.6 ± 0.0 |
| Salicylic acid | y = 23,187x | 0.9972 | 315 | 13.29 | ND | 0.22 ± 0.007 |
| Quercetin 3-O-rhamnoside | y = 75,791x | 0.9991 | 370 | 16.020 | 0.12 ± 0.053 | ND |
| Luteolin | y = 19,014x | 0.9984 | 250 | 16.074 | 0.61 ± 0.01 | 2.97 ± 0.1*** |
Data are presented as means ± SD (n = 3), one-way ANOVA, followed by Newman–Keuls test.
p < 0.05 comparing all pairs of columns.
p < 0.01 comparing all pairs of columns.
p < 0.001 comparing all pairs of columns.
ANOVA = analysis of variance; d.w. = dry extract; HPLC-DAD = high-performance liquid chromatography with photodiode array detection; ND = not detected; PF = P. lentiscus fruits; PL = P. lentiscus leaves; r.t. = retention time; SD = standard deviation.
Quantification of the identified compounds, detected in the leaves and fruits, was accomplished by external standardization. For this purpose, nine standard calibration curves were established using the following commercial standards: gallic acid, catechin, syringic acid, ellagic acid, 3,4-dihydroxyhydro-cinnamic acid, benzoic acid, salicylic acid, quercetin 3-O-rhamnoside, and luteolin, within the concentrations tested (5 × 10−3−1 mg/mL). The amount of each identified phenolic compound [expressed as mg/g of dry weight (d.w.)] is reported in Table 2. The data revealed both in terms of qualitative and quantitative profiles, a significant difference between Pistacia leaf and fruit extracts.
In fact, in PL, gallic acid (161.67 ± 35.27 mg/g) was found to be the most abundant compound, followed by syringic acid (52.66 ± 7.23 mg/g), catechin (31.79 ± 5.76 mg/g), and finally ellagic acid (9.54 ± 0.63 mg/g). The presence of the latter compound in PL is unprecedented. By contrast, fruits were a poor source of phenolics, with gallic acid (5.67 ± 0.017 mg/g) and luteolin (2.97 ± 0.1 mg/g) being the most abundant compounds. However, it is important to mention that the present study is the first to identify luteolin in P. lentiscus fruits.
High concentrations of compounds such as gallic acid and catechin, which have shown important biological activities [35], make the plant worthy of further research, offering important therapeutic potentials against diabetes, as an anti-inflammatory agent, or in alleviating aspects of the aging process [34]. Among the molecules identified in P. lentiscus, quercetin and catechin, isolated from Rhododendron groenlandicum were proved to be active against diabetes [36]. The identified phenolic compounds are known to be potent anti-oxidant agents, which confers them strong biological properties including antidiabetic [3].
Moreover, identification of the plant’s active compounds paves the way to their use for pharmacokinetic studies and in the development of new therapeutic agents for the treatment of metabolic diseases.
3.4. Antioxidant activity
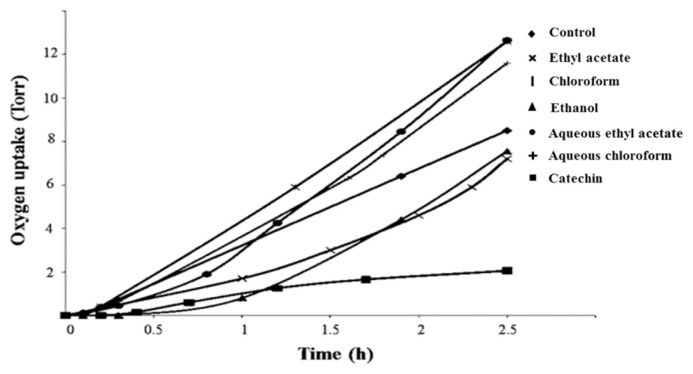
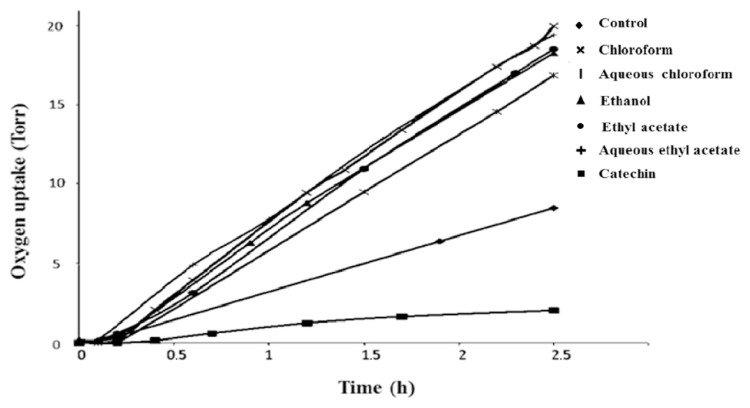
The antioxidant properties of P. lentiscus were determined by the oxygen consumption method. The oxygen uptake profile during auto-oxidation of methyl linoleate is shown in Figures 2 and 3, for PL and PF extracts, respectively. Only two extracts (ethanol and aqueous chloroform leaf extracts) reduced the rate of oxygen consumption by 15% and 11%, respectively, less than that of catechin (76%), but in agreement with previous results [24,37]. The highest amounts of total phenols for ethanol and chloroform aqueous extracts (517.51 ± 5.53 mg C Eq/g extract and 1104.6 ± 6.7 mg C Eq/g extract, respectively) could be responsible for the antioxidant properties of the extracts. Polyphenols were demonstrated to have an important role in stabilizing lipid peroxidation [38,39] and are associated with a wide range of biological activities including antioxidant activity, due to their redox properties, as reducing agents or hydrogen atom donors. It has already been reported that chloroform aqueous fraction of PL extract strongly inhibited (99% at 100 μg/mL; IC50 = 0.84 μg/mL) linoleic acid peroxidation [19]. In corroboration with our findings, the leaves of P. lentiscus contain antioxidant flavonol glycosides such as quercetin-3-O-rhamnoside [40] that contribute to the high antioxidant potential established in vitro [41] and in vivo [42].
Figure 2.
Oxygen uptake during the autoxidation of methyl linoleate induced by AIBN in the presence of different Pistacia lentiscus leaf extracts and the reference (catechin). AIBN = 2,2′-azodiisobutyronitrile.
Figure 3.
Oxygen uptake during the autoxidation of methyl linoleate induced by AIBN in the presence of different Pistacia lentiscus fruit extracts and the reference (catechin). AIBN = 2,2′-azodiisobutyronitrile.
3.5. Subacute toxicity study
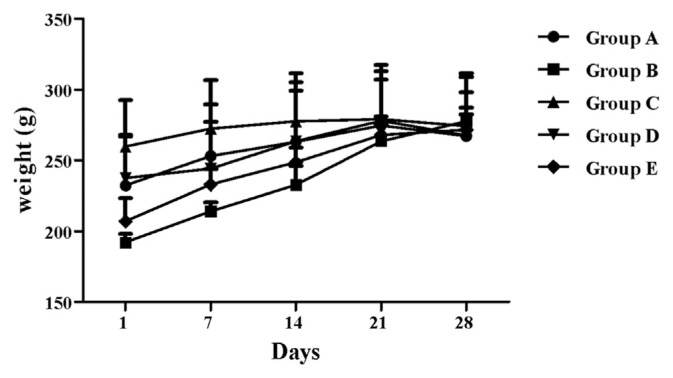
General behavior and body weight are among the critical parameters for the evaluation of the first signs of toxicity [43]. Results did not reveal any significant changes in body weight in the treated rats, compared with the control rats (Figure 4). Both control and treated groups appeared healthy throughout the period of study, although those receiving a high dose (250 mg/kg b.w.) of either PL or PF extract exhibited minor diarrhea, probably due to the laxative effect of the plant [44] or due to its high content of tannins, which, by forming complexes with proteins, interfere with their digestion [45]. However, no death was recorded in all experimental groups, suggesting that the LD50 (lethal dose, 50%) for chronic oral dosing with P. lentiscus was much higher than 250 mg/kg b.w.
Figure 4.
Rat weight versus time. All values are expressed as mean ± SEM, n = 5. Group A is the normal control group; Group B, 50 mg/kg b.w. Pistacia lentiscus leaf crude extract-treated group; Group C, 250 mg/kg b.w. P. lentiscus leaf crude extract-treated group; Group D, 50 mg/kg b.w. P. lentiscus fruit crude extract-treated group; and Group E, 250 mg/kg b.w. P. lentiscus fruit crude extract-treated group. b.w. = body weight; SEM = standard error of the mean.
Biochemical parameters (cholesterol, glucose, and bilirubin) showed no significant changes in the treated rats compared with the control rats (Table 3), suggesting that P. lentiscus extracts had no effect on lipid and carbohydrate metabolisms of the rats. However, a considerable increase (p < 0.001) in triglyceride levels was noticed at high concentrations of PL and PF extracts (250 mg/kg b.w.), which may be related to the presence of a high level of glycosylated flavonoids in these extracts [40]. Moreover, high concentrations of PL extract caused a slight increase in ALT and a significant (p < 0.001) increase in AST and ALP levels. By contrast, a significant (p < 0.001) decrease in creatinine production in both groups treated with leaf and fruit extracts at 250 mg/kg b.w. was observed (Table 3), probably correlated to a reduction in muscle mass due to weight loss, but indicating no impairment in kidney function.
Table 3.
Blood parameter levels of different rat groups treated with ethanolic extracts of Pistacia lentiscus leaves and fruits.a
| Parameters | Group A | Group B (50 mg/kg b.w.) | Group C (250 mg/kg b.w.) | Group D (50 mg/kg b.w.) | Group E (250 mg/kg b.w.) |
|---|---|---|---|---|---|
| Glucose (g/L) | 1.066 ± 0.05 | 1.298 ± 0.12 | 1.380 ± 0.08 | 1.293 ± 0.07 | 1.502 ± 0.13* |
| ALT (IU/L) | 29.8 ± 1.16 | 30.6 ± 5.03 | 35.1 ± 1.30 | 31.12 ± 2.77 | 33.8 ± 3.64 |
| AST (IU/L) | 85.4 ± 6.14 | 71.4 ± 6.15 | 135.02 ± 3.51*** | 66.48 ± 3.59 | 91.2 ± 2.32* |
| ALP (IU/L) | 218 ± 13.27 | 227.6 ± 22.41 | 371.8 ± 23.13** | 222.2 ± 39.4 | 389.6 ± 28.91*** |
| TB (mg/L) | 1.1 ± 0.04 | 1.02 ± 0.05 | 0.7 ± 0.08 | 0.752 ± 0.22 | 0.648 ± 0.01* |
| CHOL (g/L) | 0.47 ± 0.03 | 0.442 ± 0.05 | 0.344 ± 0.01* | 0.532 ± 0.04 | 0.486 ± 0.01 |
| TRG (g/L) | 0.442 ± 0.04 | 0.488 ± 0.07 | 1.465 ± 0.15*** | 0.4866 ± 0.05 | 1.38 ± 0.19*** |
| CREA (mg/L) | 9 ± 0.30 | 9.8 ± 0.83 | 0.388 ± 0.01*** | 4.16 ± 0.16*** | 0.41 ± 0.01*** |
Data are presented as means ± SEM, n = 5. One-way ANOVA followed by Dunnett t test.
p < 0.05 versus control group.
p < 0.01 versus control group.
p < 0.001 versus control group.
ALP = alkaline phosphatase; ALT = alanine aminotransferase; ANOVA = analysis of variance; AST = aspartate aminotransferase; b.w. = body weight; CHOL = cholesterol; CREA = creatinine; SEM = standard error of the mean; TB = total bilirubin; TRG = triglyceride.
Animal groups are as follows: Group A, control group; Group B, 50 mg/kg b.w. P. lentiscus leaf ethanolic extract-treated group; Group C, 250 mg/kg b.w. P. lentiscus leaf ethanolic extract-treated group; Group D, 50 mg/kg b.w. P. lentiscus fruits ethanolic extract-treated group; and Group E, 250 mg/kg b.w. P. lentiscus fruit ethanolic extract-treated group.
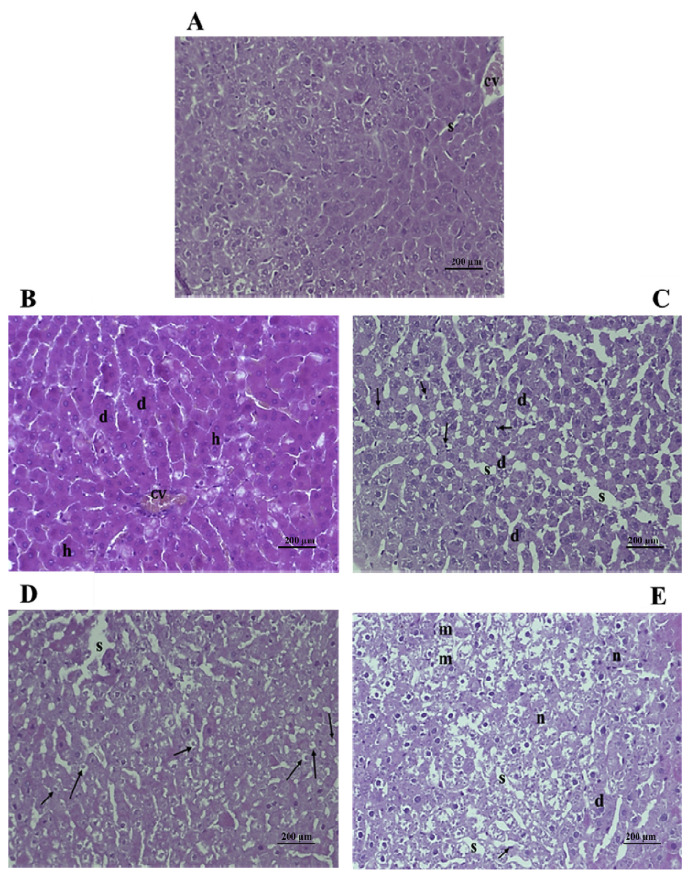
3.5.1. Histopathological examination
Histological sections supplemented by the histological injury score (Table 4) have led to the following observations. Histopathological studies of kidneys and livers of rats exposed to subchronic treatment with PL and PF extracts (50 mg/kg b.w. and 250 mg/kg b.w., respectively) did not show any major aberrations in their structural integrity. The liver is the main target organ of toxicity, which is exposed to foreign substances absorbed in intestines and metabolized to other compounds that may or may not be hepatotoxic to the rats [46]. In this study, photomicrographs of rat livers subchronically exposed to PL and PF extracts (50 mg/kg) showed subnormal structure surrounded by inflated hepatocytes and degeneration in hepatic plates (Figures 5B and 5D).
Table 4.
Histological injury score of liver and kidney after treatment for 28 days with ethanolic extracts of Pistacia lentiscus leaves and fruits.a
| Groups | Injury scoreb | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Liver | Kidney | |||||||
|
|
|
|||||||
| Necrosis | Infiltration of inflammatory cells | Fatty degeneration | Degenerated hepatocyte | Sinusoidal dilatation | Dilated hypocellular tubules | Intratubular debris | Coagulate necrosis of tubular epithelial cells | |
| Group A (control) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Group B (50 mg/kg b.w.) | 0 | 0 | 0 | 3 | 0 | 1 | 1 | 2 |
| Group C (250 mg/kg b.w.) | 0 | 0 | 3 | 1 | 2 | 4 | 0 | 0 |
| Group D (50 mg/kg b.w.) | 0 | 0 | 3 | 0 | 2 | 1 | 0 | 1 |
| Group E (250 mg/kg b.w.) | 1 | 2 | 4 | 1 | 2 | 2 | 0 | 3 |
b.w. = body weight.
Animal groups are as follows: Group A, control group; Group B, 50 mg/kg b.w. P. lentiscus leaf ethanolic extract-treated group; Group C, 250 mg/kg b.w. P. lentiscus leaf ethanolic extract-treated group; Group D, 50 mg/kg b.w. P. lentiscus fruit ethanolic extract-treated group; and Group E, 250 mg/kg b.w. P. lentiscus fruit ethanolic extract-treated group.
Livers and kidneys were scored for histological injury via light microscopy with Score 0 = no visible cell damage; Score 1 = damage <30% of the tissue; Score 2 = damage on 30–50% of the tissue; Score 3 = extensive, lesions >50%; and Score 4 = complete necrosis.
Figure 5.
Effect of PL and PF crude extracts on rat livers after treatment for 28 days. Histological changes in rat liver after treatment with Pistacia lentiscus leaf and fruit crude extracts are shown. Rats were administered orally 50 mg/kg b.w. and 250 mg/kg b.w. of leaf and fruit crude extracts. The liver was stained with hematoxylin and eosin method: (A) control group (normal central vein and hepatocytes); (B) 50 mg/kg b.w. PL-treated group (light steatosis); (C) 250 mg/kg b.w. PL-treated group (steatosis); (D) 50 mg/kg b.w. PF-treated group (sub-normal structure); and (E) 250 mg/kg b.w. PF-treated group (light necrosis) (magnification 200×). Each arrow indicates an adipocyte linked to a lateral nucleus membrane. cv = central vein; d = degenerated hepatocytes; h = surrounding hepatocytes; m = infiltration of inflammatory cells; n = focal necrosis; PF = P. lentiscus fruits; PL = P. lentiscus leaves; s = dilated hepatic sinusoids.
At 250 mg/kg b.w., we noted light steatosis and degenerated hepatocytes in liver sections of rats treated with leaf extracts (Figure 5C), while higher steatosis with slight inflammatory infiltrations and minor focal necrosis were noticeable in livers of rats treated with fruit extracts (Figure 5E), compared with the controls (Figure 5A). These results correspond closely to the above findings concerning biochemical parameters of ALP and AST, which did not indicate any toxicity at low levels (50 mg/kg b.w.) of PL and PF extracts. However, at high levels (250 mg/kg b.w.), a slight toxicity was noticed, which was more visible for fruit extracts than for its leaf counterparts.
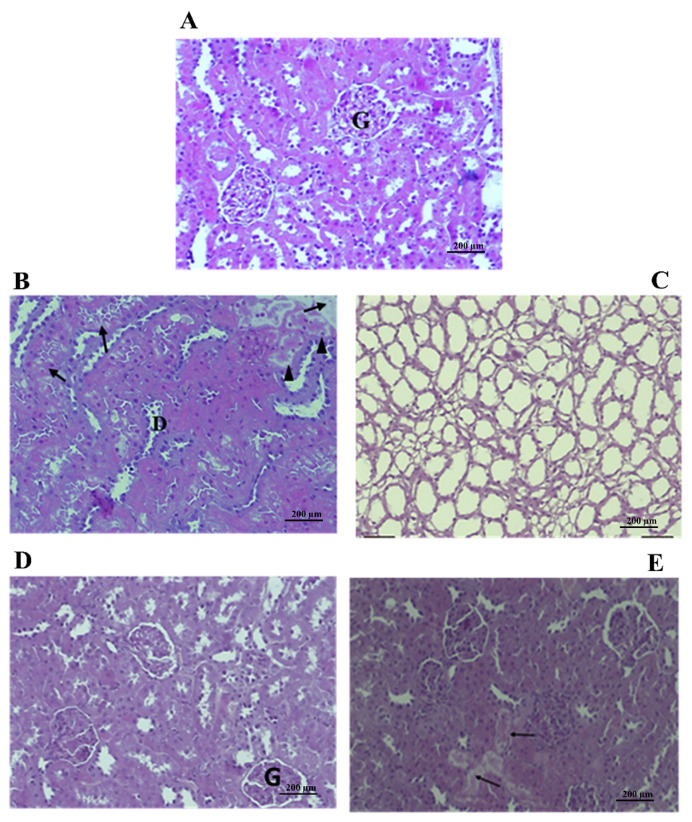
Photomicrographs of kidney sections of rats treated with 50 mg/kg b.w. of leaf extracts (Figure 6B) showed only minor necrosis of tubular epithelial cells; dilated hypocellular tubules with cells were found in the tubular lumen, whereas the kidneys of the fruit extract-treated group seemed completely normal (Figure 6D), compared with the control group (Figure 6A). At 250 mg/kg b.w., a distension of the renal tubules was observed in the case of the leaf extract-treated group (Figure 6C) and a slight necrosis for the fruit extract-treated group (Figure 6E), compared with the control group (Figure 6A). We conclude that histological examination of the organs of rats treated with P. lentiscus extracts at a dose of 50 mg/kg b.w. revealed that there was no major potential toxicity or damage to the cell structure of livers and kidneys.
Figure 6.
Effect of PL and PF crude extracts on rat kidneys after treatment for 28 days. Histological changes in kidneys of rats treated with Pistacia lentiscus leaf and fruit crude extracts are shown. Rats were administered orally 50 mg/kg b.w. and 250 mg/kg b.w. of plant extracts. Kidney tissue was stained with hematoxylin and eosin: (A) control rats group (normal kidney structure); (B) 50 mg/kg b.w. PL-treated group (minor necrosis); (C) 250 mg/kg b.w. PL-treated group (distension of the renal tubules); (D) 50 mg/kg b.w. PF-treated group (subnormal kidney structure); and (E) 250 mg/kg b.w. PF-treated group (light necrosis) (magnification 250×). Arrows indicate coagulate necrosis of tubular epithelial cells and arrow heads dilated hypocellular tubules. D = intratubular debris; G = glomeruli; PF = P. lentiscus fruits; PL = P. lentiscus leaves.
3.6. Hepatoprotective activity
3.6.1. Biochemical parameters
The effects of P. lentiscus extracts on liver marker enzymes and serum bilirubin content presented in Table 5 showed that intoxication with paracetamol (165 mg/kg/d) for 3 days generated liver damage reflected by a significant increase in AST, ALT, ALP, and bilirubin levels, compared with the normal control group. The elevated level of bilirubin is usually an indication of biliary obstruction, hemolysis, and in some cases renal failure [47,48]. However, administration of PL or PF extract, or a mixture (PL/PF) of both, attenuated serum enzyme levels and caused a subsequent recovery toward normalization, with more efficiency for PL extract. This result is indicative of a better hepatoprotective potential of leaves compared to that of fruits and was also seen in the group that received a combined treatment of leaves and fruits (75.54 IU/L, 214.14 IU/L, and 137.54 IU/L for ALT, AST, and ALP, respectively) and total bilirubin (0.54 mg/L).
Table 5.
Effect of ethanolic extracts of Pistacia lentiscus leaves and fruits on liver marker enzymes and serum bilirubin.a
| Groups | GI | GII | GIII (125 mg/kg b.w.) | GIV (125 mg/kg b.w.) | GV (63/63 mg/kg b.w.) |
|---|---|---|---|---|---|
| ALT (IU/L) | 34.5 ± 1.3c | 90.67 ± 6.5*** | 86.34 ± 4.87*** | 69.57 ± 4.05** | 75.54 ± 13.79** |
| AST (IU/L) | 78.9 ± 1.78c | 241.83 ± 28.7*** | 190.4 ± 27.04** | 225.21 ± 22.85*** | 214.14 ± 24.29*** |
| ALP (IU/L) | 77.9 ± 9.85c | 210.5 ± 4.7*** | 125.26 ± 14.27*,c | 148.43 ± 17.37**,b | 137.54 ± 11.28**,c |
| TB (mg/L) | 0.46 ± 0.19c | 2.42 ± 0.17*** | 1.23 ± 0.22*,c | 1.5 ± 0.28 **,a | 0.54 ± 0.11 **,c |
Results are expressed as mean ± SEM, n = 7; one-way ANOVA followed by Dunnett t test.
p < 0.05,
p < 0.01,
p < 0.001 compare all animals treated groups versus normal control.
p < 0.05,
p < 0.01,
p < 0.001 compare all animals treated groups versus paracetamol treated.
ALP = alkaline phosphatase; ALT = alanine aminotransferase; ANOVA = analysis of variance; AST = aspartate aminotransferase; b.w. = body weight; SEM = standard error of the mean; TB = total bilirubin.
The animal’s groups are as follows: GI, normal control; GII, 165 mg/kg b.w. paracetamol-treated group; GIII, 125 mg/kg b.w. P. lentiscus leaf ethanolic extract-treated group; GIV, 125 mg/kg b.w. P. lentiscus fruit ethanolic extract-treated group; and GV, 63/63 mg/kg b.w. P. lentiscus leaf/fruit ethanolic extract-treated group.
3.6.2. Lipid peroxidation inhibitory activity
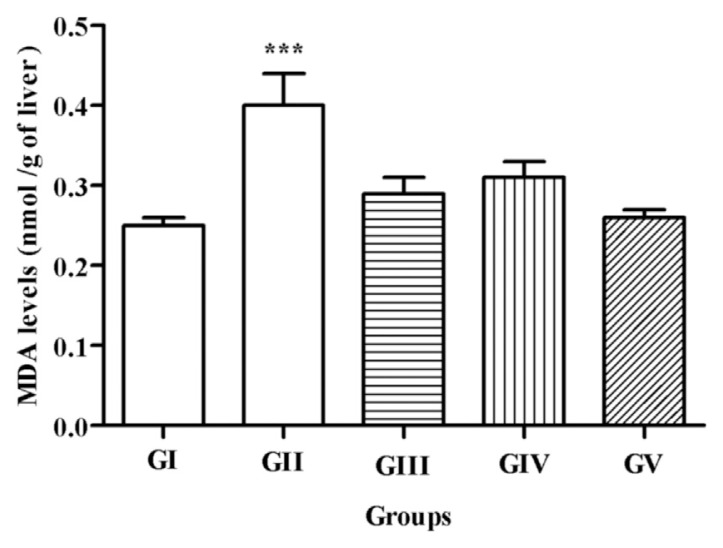
Paracetamol-treated mice showed a significant (p < 0.001) increase in serum lipid peroxidation as measured by the amount of MDA formed (0.4 ± 0.098 nmol/mg liver tissue), compared with the control group (0.25 ± 0.032 nmol/mg liver tissue). By contrast, extract-treated groups exhibited a significant and comparable decrease in MDA levels (0.33 ± 0.046 nmol/mg and 0.31 ± 0.042 nmol/mg liver tissue, for leaves and fruits, respectively), the decrease being more pronounced after the combined treatment of both parts of the plant (0.26 ± 0.031 nmol/mg liver tissue; Figure 7). The latter data are suggestive of a synergistic action between the constituents of leaf and fruit extracts. It also consolidates the above findings concerning the better efficiency of the combined treatment in reducing serum marker enzymes and those of previous findings that reported that Pistacia atlantica extract showed good antilipoperoxidation potential and protected red blood cells against CCl4-induced damage [49]. Furthermore, an earlier study demonstrated the hepatoprotective effect of extracts derived from boiled and nonboiled P. lentiscus leaf homogenates against CCl4 cytotoxicity by reducing the activity of ALP, ALT, and AST, as well as bilirubin level [50]. In agreement with previous studies that demonstrated that flavonoids increase the intracellular glutathione concentrations [51], it is probable that the richness of P. lentiscus extracts in various important flavonoids such as catechin and quercetin glycoside prevents the exhaustion of glutathione by N-acetyl-p-benzoquinoneimine, thereby enhancing the hepatoprotective potential of leaf extract.
Figure 7.
MDA levels in different treated groups. All values are expressed as mean ± SEM, n = 7. One-way ANOVA followed by Dunnett multiple comparison test was used for statistical significance. The animal groups are as follows: GI, normal control group; GII, 165 mg/kg b.w. paracetamol-treated group; GIII, 125 mg/kg b.w. Pistacia lentiscus leaves crude extract-treated group; GIV, 125 mg/kg b.w. P. lentiscus fruits crude extract-treated group; and GV, 63/63 mg/kg b.w. P. lentiscus leaves/fruits crude extract-treated group. * p < 0.05, when compared with normal control values. ** p < 0.01, when compared with normal control values. *** p < 0.001, when compared with normal control values. ANOVA = analysis of variance; b.w. = body weight; MDA = malondialdehyde; SEM = standard error of the mean.
3.6.3. Histopathological analysis
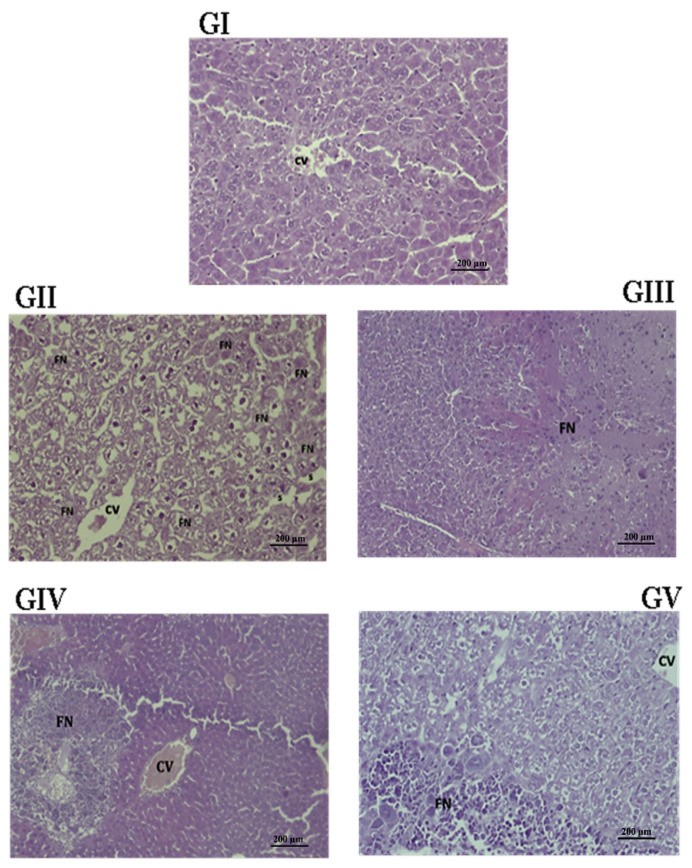
The present study underlines the potential hepatoprotective activity of P. lentiscus against hepatic injury caused by paracetamol in mice (Table 6). Paracetamol is a well-known antipyretic and analgesic agent, safe in therapeutic doses, but can produce fatal hepatic necrosis in humans, rats, and mice at high doses [52]. Histopathological studies (Figure 8) revealed major damage in the liver of animals intoxicated with paracetamol (GII), compared with the normal control group (GI). This toxicity may be due to depletion of glutathione stores or free radical generation initiating lipid peroxidation [1], the latter being revealed by the higher MDA levels found above (0.4 ± 0.098 nmol/mg liver tissue). Liver biopsy (Figure 8) revealed cell necrosis, deeply stained nuclei, sinusoidal dilatation, and degeneration of hepatocytes with lymphocyte infiltration in the sections of livers of mice injected with paracetamol at a dose of 165 mg/kg (Figure 8, GII). In the leaf extract-treated group (Figure 8, GIII), the liver architecture was similar to that observed in the control group (Figure 8, GI), except for slight necrosis (score = 4) (score = 2) that occupies a small area (Figure 8, GIII). In the fruit extract-treated group, the necrosis is very pronounced and localized around the congested central vein (Figure 8, GIV). However, administration of both leaf and fruit extracts prevented liver injury (Figure 8, GV), probably by reducing lipid peroxidation (0.26 ± 0.031 nmol/mg liver tissue). This effect indicates a synergistic activity of compounds present in fruits and leaves. Our findings are in strong agreement with those of a study on a related species, P. atlantica, which reported that the extract obtained from this plant stopped lipid peroxidation and protected red blood cells against CCl4-induced damage [49].
Table 6.
Histological injury score of liver after paracetamol treatment in mice with ethanolic extracts of Pistacia lentiscus leaves and fruits.a
| Groups | Injury of score of liverb | Sinusoidal dilatation | |||
|---|---|---|---|---|---|
|
| |||||
| Necrosis | Infiltration of inflammatory cells | Fatty degeneration | Degenerated hepatocyte | ||
| GI | 0 | 0 | 0 | 0 | 0 |
| GII | 4 | 3 | 4 | 2 | 3 |
| GIII (125 mg/kg b.w.) | 2 | 0 | 0 | 0 | 1 |
| GIV (125 mg/kg b.w.) | 2 | 0 | 0 | 0 | 1 |
| GV (63/63 mg/kg b.w.) | 1 | 0 | 0 | 0 | 0 |
b.w. = body weight.
The animal’s groups are as follows: GI, normal control; GII, 165 mg/kg b.w. paracetamol-treated group; GIII, 125 mg/kg b.w. P. lentiscus leaf ethanolic extract-treated group; GIV, 125 mg/kg b.w. P. lentiscus fruit ethanolic extract-treated group; and GV, 63/63 mg/kg b.w. P. lentiscus leaf/fruit ethanolic extract-treated group.
Livers were scored for hepatic injury via light microscopy with Score 0 = no visible cell damage; Score 1 = focal hepatocyte damage on <30% of the tissue; Score 2 = focal hepatocyte damage on 30–50% of the tissue; Score 3 = extensive, but focal, hepatocyte lesions >50%; and Score 4 = global hepatocyte necrosis.
Figure 8.
Effect of PL and PF crude extracts on liver pathologic analysis after paracetamol treatment in mice. Histological changes in the livers of paracetamol-treated rats that received orally PL (125 mg/kgb.w.), PF (125 mg/kg b.w.), or a mixture (PL/PF; 63 + 63 mg/kg b.w.) plant crude extracts are shown. Livers were stained with hematoxylin–eosin method. The animal groups are as follows: GI, normal control group (normal central vein and architecture); GII, paracetamol group (completely necrosed); GIII, PL-treated group (a very light necrosis); (GIV, PF-treated group [showing central vein containing blood (CV) and necrosis around the central vein] (200×); and GV, PL/PF-treated group (light necrosis, which occupies a tiny surface of the organ, but the rest of the organ is intact from both) (250×). b.w. = body weight; CV = central vein; FN = focal necrosis; PF = P. lentiscus fruits crude extract; PL = P. lentiscus leaves crude extract; PL/PF = P. lentiscus leaves/fruits crude extracts; S = dilated hepatic sinusoids.
3.7. Antidiabetic activity
3.7.1. In vivo antidiabetic activity
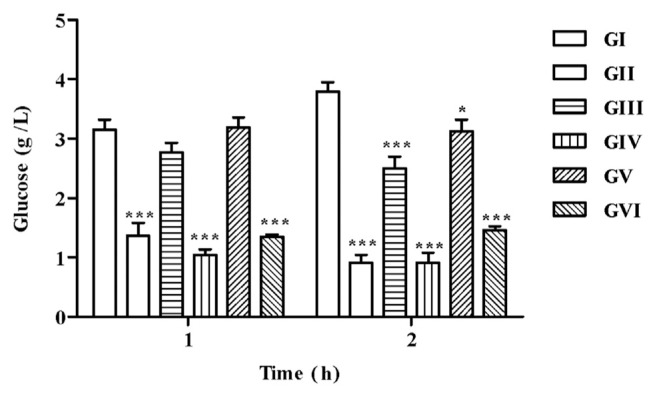
STZ is well known for its selective pancreatic β-cell cytotoxicity and has been extensively used to induce diabetes mellitus in animals [53]. Induction of diabetes in rats was confirmed by the presence of a high fasting plasma glucose level in the control animals injected with STZ, as depicted in Figure 9. The reduction in blood glucose levels in both PL and PF extract-treated rats was observed 1 hour after treatment, compared with diabetic control (3.79 ± 0.17 g/L). After two hours of treatment with PL extracts, blood glucose level was restored to normal values (0.91 ± 0.16 g/L), for both concentrations used (50 mg/kg b.w. and 125 mg/kg b.w.), as good as the reference drug glibenclamide (0.91 ± 0.13 g/L). Conversely, blood glucose level decreased but remained relatively high (1.45 ± 0.07 g/L) after treatment with fruits extracts.
Figure 9.
Glucose levels in treated groups. All values are expressed as mean ± SEM, n = 6. One-way ANOVA followed by Dunnett multiple comparison test was used for statistical significance. The animal groups are as follows: GI, diabetic control; GII, glibenclamide-treated group; GIII, 50 mg/kg b.w. PL-treated group; GIV, 125 mg/kg b.w. PL-treated group; GV, 50 mg/kg b.w. PF-treated group; and GVI, 125 mg/kg b.w. PF-treated group. * Values deviate significantly (p < 0.05) from diabetic control. *** Values deviate very significantly (p < 0.001) when compared with diabetic control values. ANOVA = analysis of variance; b.w. = body weight; PF = Pistacia lentiscus fruits; PL = P. lentiscus leaves; SEM = standard error of the mean.
Recent studies indicated that P. lentiscus leaf extracts contain several phytochemical constituents such as rutin, quercetin, isoquercetin, and myricetin [54,55], which enhance insulin release and glycogen metabolism, and decrease blood glucose levels in diabetic animals [56], while others argue in favor of inhibition of nuclear factor-kB activation and restoration of enzymatic antioxidants [57]. We have documented the presence of considerable amounts of gallic acid, catechin, and ellagic acid in PL extracts, which, as phytoconstituents of Terminalia paniculata bark, have been demonstrated to act in the management of glucose in diabetic rats [58]. In parallel, other investigators reported that gallic acid, rutin, and ellagic acid found in Eugena uniflora leaves could contribute to increasing serum insulin levels in diabetic mice [59]. These same authors have linked the observed activity to the antioxidant potential of plant extracts. In a similar study, it has been indicated that the antioxidative effect of Cleistocalyx operculatus flower buds might be responsible for the reduction in the oxidative cytotoxic status of β-cells, leading to their protection and prevention of insulin deficiency [60]. Thus, the significant antidiabetic effect of P. lentiscus ethanolic extract could be attributed to the presence of various phytoconstituents with antioxidant properties detected in the phytochemical screening, FTIR analysis, and HPLC-DAD, acting individually or in synergy. The higher contents of total polyphenols and flavonoids in leaves over their fruit counterpart (Tables 1 and 2) could account for the higher hypoglycemic activity.
3.7.2. In vitro antidiabetic activity: α-amylase inhibitory assay
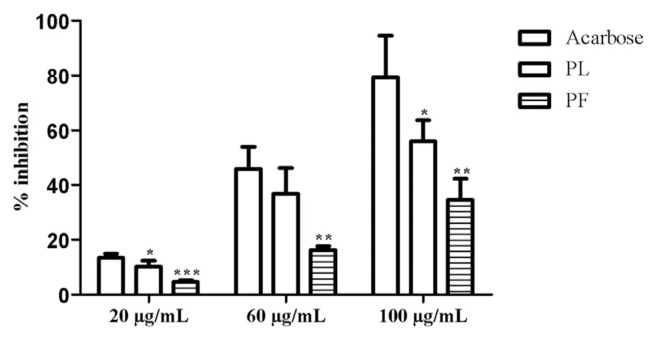
In this test, P. lentiscus ethanolic extract tested above for its in vivo antidiabetic activity was investigated for its potential to inhibit porcine pancreatic α-amylase activity. Three different concentrations (20 μg/mL, 60 μg/mL, and 100 μg/mL) of PL and PF ethanolic extracts were used, compared with acarbose (Figure 10). The latter compound (100 μg/mL) showed 79.34 ± 15.19% inhibitory effects on the α-amylase activity with an IC50 value of 64.56 μg/mL, significantly surpassing PL (55.86 ± 7.75%; IC50: 87.5 μg/mL) and PF (34.50 ± 7.74%; IC50: 144.29 μg/mL) extracts at the same concentration. However, PL extract showed higher efficiency in inhibiting alpha-amylase than its PF counterpart, which is in agreement with the in vivo antidiabetic effect.
Figure 10.
Comparison of α-amylase inhibitory effect of Pistacia lentiscus ethanolic leaf and fruit extracts at different concentrations. All values are expressed as mean ± SD, n = 3. One-way ANOVA followed by Dunnett multiple comparison test was used for statistical significance, when compared with acarbose. * p < 0.05. ** p < 0.01. *** p < 0.001. ANOVA = analysis of variance; SD = standard deviation.
A dose-dependent increase in percentage inhibitory activity implicates that the plant contains a substantial amount of compounds that have the ability to curb diabetes by inhibiting the conversion of starch to glucose. The present study has proved, in consolidation with previous reports [54], that P. lentiscus contains essential herbal bioactive compounds such as flavanone glycosides and luteolin, with inhibiting enzyme activities [61]. In addition, a clear link between polyphenols and antidiabetic activity of herbal extracts has been shown [62]. This study is the first to report a potential mode of action of P. lentiscus similar to that of other plant extracts, and suggests that the hypoglycemic effect of this plant is due, at least in part, to the inhibition of α-amylase.
4. Conclusion
It can be concluded that P. lentiscus extracts has a moderate hepatoprotective effect on paracetamol-induced acute hepatitis, as evidenced by a decrease in tissue necrosis and by reduced transaminase and MDA serum levels. Alternatively, the hypoglycemic activity along with the protective effect against STZ challenge provides the scientific rationale for the use of P. lentiscus in herbal antidiabetic therapy. The use of this plant extract will be greatly beneficial to reduce the rate of digestion and absorption of carbohydrates, thereby contributing to effective management of diabetes by decreasing postprandial hyperglycemia. Future studies will provide more insight into the molecular mechanisms by which this plant and its active compounds regulate glucose homeostasis. Finally, the observed activities can be due to the presence of a large spectrum of phytoconstituents revealed by polyphenol quantification, FTIR, and HPLC-DAD analyses.
Acknowledgments
This work was sponsored by the Algerian Ministry of Higher Education and Scientific Research (Grant No. F00620100006).
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jfda.2016.03.002.
Funding Statement
This work was sponsored by the Algerian Ministry of Higher Education and Scientific Research (Grant No. F00620100006).
Footnotes
Conflicts of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- 1. Hurkadale PJ, Shelar PA, Palled SG, Mandavkar YD, Khedkar AS. Hepatoprotective activity of Amorphophallus paeoniifolius tubers against paracetamol-induced liver damage in rats. Asian Pac J Trop Biomed. 2012;2:S238–42. [Google Scholar]
- 2. Niedowicz DM, Daleke DL. The role of oxidative stress in diabetic complications. Cell Biochem Biophys. 2005;43:289–330. doi: 10.1385/CBB:43:2:289. [DOI] [PubMed] [Google Scholar]
- 3. Shori AB. Camel milk as a potential therapy for controlling diabetes and its complications: a review of in vivo studies. J Food Drug Anal. 2014;23:609–18. doi: 10.1016/j.jfda.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Adefegha SA, Oboh G, Adefegha OM, Boligon AA, Athayde ML. Antihyperglycemic, hypolipidemic, hepatoprotective and antioxidative effects of dietary clove (Szyzgium aromaticum) bud powder in a high-fat diet/streptozotocin-induced diabetes rat model. J Sci Food Agric. 2014;94:2726–37. doi: 10.1002/jsfa.6617. [DOI] [PubMed] [Google Scholar]
- 5. Stagos D, Amoutzias GD, Matakos A, Spyrou A, Tsatsakis AM, Kouretas D. Chemoprevention of liver cancer by plant polyphenols. Food Chem Toxicol. 2012;50:2155–70. doi: 10.1016/j.fct.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Brahmachari G.Research Signpost, editor. Opportunity, challenge and scope of natural products in medicinal chemistry. Trivandrum, Kerala, India: Research Signpost; 2011. Bio-flavonoids with promising antidiabetic potentials: a critical survey; pp. 187–212. [Google Scholar]
- 7. Kambouche N, Merah B, Derdour A, Bellahouel S, Bouayed J, Dicho A, Younos C, Soulimani R. Hypoglycemic and antihyperglycemic effects of Anabasis articulata (Forssk) Moq (Chenopodiaceae), an Algerian medicinal plant. Afr J Biotechnol. 2009;8:5578–83. [Google Scholar]
- 8. Li AP. Preclinical in vitro screening assays for drug-like properties. Drug Discov Today Technol. 2005;2:179–85. doi: 10.1016/j.ddtec.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 9. Nithianantham K, Shyamala M, Chen Y, Latha LY, Jothy SL, Sasidharan S. Hepatoprotective potential of Clitoria ternatea leaf extract against paracetamol induced damage in mice. Molecules. 2011;16:10134–45. doi: 10.3390/molecules161210134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dehghan H, Sarrafi Y, Salehi P. Antioxidant and antidiabetic activities of 11 herbal plants from Hyrcania region. Iran. J Food Drug Anal. 2016;24:179–88. doi: 10.1016/j.jfda.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tattini M, Remorini D, Pinelli P, Agati G, Saracini E, Traversi ML, Massai R. Morpho-anatomical, physiological and biochemical adjustments in response to root zone salinity stress and high solar radiation in two Mediterranean evergreen shrubs, Myrtus communis and Pistacia lentiscus. New Phytol. 2006;170:779–94. doi: 10.1111/j.1469-8137.2006.01723.x. [DOI] [PubMed] [Google Scholar]
- 12. Trabelsi H, Renaud J, Herchi W, Boukhchina S, Mayer P. Triacylglycerols and aliphatic alcohols from fruits of three Tunisian Pistacia lentiscus populations. J Sci Food Agric. 2015;95:2028–32. doi: 10.1002/jsfa.6915. [DOI] [PubMed] [Google Scholar]
- 13. Dedoussis GVZ, Kaliora AC, Psarras S, Chiou A, Mylona A, Papadopoulos NG, Andrikopoulos NK. Antiatherogenic effect of Pistacia lentiscus via GSH restoration and down regulation of CD36 mRNA expression. Atherosclerosis. 2004;174:293–303. doi: 10.1016/j.atherosclerosis.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 14. Benhammou N, Bekkara FA, Panovska TK. Antioxidant and antimicrobial activities of the Pistacia lentiscus and Pistacia atlantica extracts. Afr J Pharm Pharmacol. 2008;2:22–8. [Google Scholar]
- 15. Zrira S, Elamrani A, Benjilali B. Chemical composition of the essential oil of Pistacia lentiscus L. from Morocco a seasonal variation. Flavour Fragr J. 2003;18:475–80. [Google Scholar]
- 16. Bozorgi M, Memariani Z, Mobli M, Surmaghi MHS, Shams-Ardekani MR, Rahimi R. Five Pistacia species (P. vera, P. atlantica, P. terebinthus, P. khinjuk, and P. lentiscus): a review of their traditional uses, phytochemistry, and pharmacology. Sci World J. 2013;219815:1–33. doi: 10.1155/2013/219815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Romani A, Coinu R, Carta S, Pinelli P, Galardi C, Vincieri FF, Franconi F. Evaluation of antioxidant effect of different extracts of Myrtus communis L. Free Radic Res. 2004;38:97–103. doi: 10.1080/10715760310001625609. [DOI] [PubMed] [Google Scholar]
- 18. Amessis-Ouchemoukh N, Madani K, Falé PLV, Serralheiro ML, Araújo MEM. Antioxidant capacity and phenolic contents of some Mediterranean medicinal plants and their potential role in the inhibition of cyclooxygenase-1 and acetylcholinesterase activities. Ind Crops Prod. 2014;53:6–15. [Google Scholar]
- 19. Atmani D, Chaher N, Berboucha M, Ayouni K, Lounis H, Boudaoud H, Debbache N, Atmani D. Antioxidant capacity and phenol content of selected Algerian medicinal plants. Food Chem. 2009;112:303–9. [Google Scholar]
- 20. Djeridane A, Yousfi M, Nadjemi B, Boutassouna D, Stocker P, Vidal N. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006;97:654–60. [Google Scholar]
- 21. Maksimovic Z, Malencié D, Kovacevié N. Polyphenol contents and antioxidant activity of Maydis stigma extracts. Bioresour Technol. 2005;96:873–7. doi: 10.1016/j.biortech.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 22. Hagerman AE, Butler LG. Protein precipitation method for the quantitative determination of tannins. J Agric Food Chem. 1978;26:809–12. [Google Scholar]
- 23. Mburu F, Dumarcay S, Huber F, Petrissans M, Gérardin P. Evaluation of thermally modified Grevillea robusta heartwood as an alternative to shortage of wood resource in Kenya: characterisation of physicochemical properties and improvement of bio-resistance. Bioresour Technol. 2007;98:3478–86. doi: 10.1016/j.biortech.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 24. Diouf PN, Merlin A, Perrin D. Antioxidant properties of wood extracts and colour stability of woods. Ann For Sci. 2006;63:525–34. [Google Scholar]
- 25. Eidi A, Mortazavi P, Bazargan M, Zaringhalam J. Hepatoprotective activity of cinnamon ethanolic extract against CCl4-induced liver injury in rats. EXCLI J. 2012;11:495–507. [PMC free article] [PubMed] [Google Scholar]
- 26. Arsad SS, Esa NM, Hamzah H. Histopathologic changes in liver and kidney tissues from male Sprague Dawley rats treated with Rhaphidophora decursiva (Roxb.) Schott extract. J Cytol Histol. 2014;S4:001. [Google Scholar]
- 27. Ohkawa N, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 28. Bagri P, Ali M, Aeri V, Bhowmik M, Sultana S. Antidiabetic effect of Punica granatum flowers: effect on hyperlipidemia, pancreatic cells lipid peroxidation and antioxidant enzymes in experimental diabetes. Food Chem Toxicol. 2009;47:50–4. doi: 10.1016/j.fct.2008.09.058. [DOI] [PubMed] [Google Scholar]
- 29. Singh SN, Vats P, Suri S, Shyam R, Kumria MML, Ranganathan S, Sridharan K. Effect of an antidiabetic extract of Catharanthus roseuson enzymic activities in streptozotocin induced diabetic rats. J Ethnopharmacol. 2001;76:269–77. doi: 10.1016/s0378-8741(01)00254-9. [DOI] [PubMed] [Google Scholar]
- 30. Tamil IG, Dineshkumar B, Nandhakumar M, Senthilkumar M, Mitra A. In vitro study on α-amylase inhibitory activity of an Indian medicinal plant, Phyllanthus amarus. Indian J Pharmacol. 2010;42:280–2. doi: 10.4103/0253-7613.70107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goli AH, Barzegar M, Sahari MA. Antioxidant activity and total phenolic compounds of pistachio (Pistacia vera) hull extracts. Food Chem. 2005;92:521–5. [Google Scholar]
- 32. Azaizeh H, Halahleh F, Abbas N, Markovics A, Muklada H, Ungar ED, Landau SY. Polyphenols from Pistacia lentiscus and Phillyrea latifolia impair the exsheathment of gastrointestinal nematode larvae. Vet Parasitol. 2013;191:44–50. doi: 10.1016/j.vetpar.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 33. Tattini M, Traversi ML. Responses to changes in Ca+ + supply in two Mediterranean evergreens, Phillyrea latifolia and Pistacia lentiscus, during salinity stress and subsequent relief. Ann Bot. 2008;102:609–22. doi: 10.1093/aob/mcn134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rodriguez-Perez C, Quirantes-Pine R, Amessis-Ouchemoukh N, Madani K, Segura-Carretero A, Fernandez-Gutierrez A. A metabolite-profiling approach allows the identification of new compounds from Pistacia lentiscus leaves. J Pharm Biomed Anal. 2013;77:167–74. doi: 10.1016/j.jpba.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 35. Nikbakht J, Hemmati AA, Arzi A, Mansouri MT, Rezaie R, Ghafourian M. Protective effect of gallic acid against bleomycin-induced pulmonary fibrosis in rats. Pharmacol Rep. 2015;67:1061–7. doi: 10.1016/j.pharep.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 36. Eid HM, Ouchfoun M, Saleem A, Guerrero-Analco JA, Walshe-Roussel B, Musallam L, Rapinski M, Cuerrier A, Martineau LC, Arnason JT, Haddad PS. A combination of (+)-catechin and (−)-epicatechin underlies the in vitro adipogenic action of Labrador tea (Rhododendron groenlandicum), an antidiabetic medicinal plant of the Eastern James Bay Cree pharmacopeia. J Ethnopharmacol. 2016;178:251–7. doi: 10.1016/j.jep.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 37. Poaty B, Dumarçay S, Perrin D. New lipophilic catechin derivatives from an oxa-Pictet–Spengler reaction. Eur Food Res Technol. 2009;230:111–7. [Google Scholar]
- 38. El Hage R, Perrin D, Brosse N. Effect of pre-treatment severity on the antioxidant properties of ethanol Origanosolv Miscanthus × giganteus Lignin. Nat Resour J. 2012;3:29–34. [Google Scholar]
- 39. Hussin MH, Rahim AA, Ibrahim MNM, Yemloul M, Perrin D, Brosse N. Investigation on the structure and antioxidant properties of modified lignin obtained by different combinative processes of oil palm fronds (OPF) biomass. Ind Crops Prod. 2014;52:544–51. [Google Scholar]
- 40. Romani A, Pinelli P, Galardi C, Mulinacci N, Tattini M. Identification and quantification of galloyl derivatives, flavonoid glycosides and anthocyanins in leaves of Pistacia lentiscus L. phytochemical analysis. Phytochem Anal. 2002;13:79–86. doi: 10.1002/pca.627. [DOI] [PubMed] [Google Scholar]
- 41. Ljubuncic P, Azaizeh H, Portnaya I, Coganc U, Said O, Saleh KA, Bomzon A. Antioxidant activity and cytotoxicity of eight plants used in traditional Arab medicine in Israel. J Ethnopharmacol. 2005;99:43–7. doi: 10.1016/j.jep.2005.01.060. [DOI] [PubMed] [Google Scholar]
- 42. Shirazi FH, Yazdanpanah H, Khoshjoo F, Hosseinzadeh L. Pistachio extracts effects on the Aflatoxin B1 cytotoxicity in HepG2 cells. Int J Pharm. 2006;2:233–9. [Google Scholar]
- 43. Ezeja MI, Anaga AO, Asuzu IU. Acute and sub-chronic toxicity profile of a methanol leaf extract of Gouania longipetala in rats. J Ethnopharmacol. 2014;151:1155–64. doi: 10.1016/j.jep.2013.12.034. [DOI] [PubMed] [Google Scholar]
- 44. Mohd A, Hamid A, Kamal A, Mushtaq H, Mehboob A. Mastagi (Pistacia lentiscus) a Unani medicine: a review. Univers J Pharm. 2014;03:46–50. [Google Scholar]
- 45. Samanta S, Giri S, Parua S, Nandi DK, Pati BR, Mondal KC. Impact of tannic acid on the gastrointestinal microflora. Microb Ecol Health Dis. 2004;16:32–4. [Google Scholar]
- 46. Jothy SL, Zakaria Z, Chen Y, Lau YL, Latha LY, Sasidharam S. Acute oral toxicity of methanolic seed extract of Cassia fistula in mice. Molecules. 2011;16:5268–82. doi: 10.3390/molecules16065268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang X, Chowdhury JR, Chowdhury NR. Bilirubin metabolism: applied physiology. Curr Paediatr. 2006;16:70–4. [Google Scholar]
- 48. Yousef MI, Omar SA, El-Guendi MI, Abdelmegid LA. Potential protective effects of quercetin and curcumin on paracetamol-induced histological changes, oxidative stress, impaired liver and kidney functions and haemato toxicity in rat. Food Chem Toxicol. 2010;48:3246–61. doi: 10.1016/j.fct.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 49. Mirzaei A, Mirzaei M, Mirzaei N. Antioxidant activity of the Pistacia atlantica in carbon tetrachloride intoxicated rats in Iran. Clin Biochem. 2011;44:S329–30. [Google Scholar]
- 50. Janakat S, Al-Merie H. Evaluation of hepatoprotective effect of Pistacia lentiscus, Phillyrea latifolia and Nicotiana glauca. J Ethnopharmacol. 2002;83:135–8. doi: 10.1016/s0378-8741(02)00241-6. [DOI] [PubMed] [Google Scholar]
- 51. Fernandes F, Sousa C, Ferreres F, Valentao P, Remiao F, Pereira JA, Andrade PB. Kale extract increases glutathione levels in V79 cells, but does not protect them against acute toxicity induced by hydrogen peroxide. Molecules. 2012;17:5269–88. doi: 10.3390/molecules17055269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Subramanian M, Balakrishnan S, Chinnaiyan SK, Sekar VK, Chandu AN. Hepatoprotective effect of leaves of Morindat inctoria Roxb. against paracetamol-induced liver damage in rats. Drug Invent Today. 2013;5:223–8. [Google Scholar]
- 53. Arokiyaraj S, Balamurugan R, Augustian P. Anti-hyperglycemic effect of Hypericum perforatum ethyl acetate extract on streptozotocin-induced diabetic rats. Asian Pac J Trop Biomed. 2011;1:386–90. doi: 10.1016/S2221-1691(11)60085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Remila S, Atmani-Kilani D, Delemasure S, Connat JL, Azib L, Richard T, Atmani D. Antioxidant, cytoprotective, anti-inflammatory and anticancer activities of Pistacia lentiscus (Anacardiaceae) leaf and fruit extracts. Eur J Integr Med. 2015;7:274–86. [Google Scholar]
- 55. Ezuruike UF, Prieto JM. The use of plants in the traditional management of diabetes in Nigeria: pharmacological and toxicological considerations. J Ethnopharmacol. 2014;155:857–924. doi: 10.1016/j.jep.2014.05.055. [DOI] [PubMed] [Google Scholar]
- 56. Gandhi RG, Sasikumar P. Antidiabetic effect of Merremiae marginata Burm. F. in streptozotocin-induced diabetic rats. Asian Pac J Trop Biomed. 2012;2:281–6. doi: 10.1016/S2221-1691(12)60023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Huang CS, Yin MC, Chiu LC. Antihyperglycemic and antioxidative potential of Psidium guajava fruit in streptozotocin-induced diabetic rats. Food Chem Toxicol. 2011;49:2189–95. doi: 10.1016/j.fct.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 58. Ramachandran S, Rajasekaran A, Adhirajan N. In vivo and in vitro antidiabetic activity of Terminalia paniculata bark: an evaluation of possible phytoconstituents and mechanisms for blood glucose control in diabetes. ISRN Pharmacol. 2013;2013:1–10. doi: 10.1155/2013/484675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schumacher NSG, Colomeu TC, De Figueiredo D, Carvalho VC, Cazarin CBB, Prado MA, Meletti LMM, Zollner RL. Identification and antioxidant activity of the extracts of Eugenia uniflora leaves. Characterization of the anti-inflammatory properties of aqueous extract on diabetes expression in an experimental model of spontaneous Type 1 diabetes (NOD Mice) Antioxidants. 2015;4:662–80. doi: 10.3390/antiox4040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mai TT, Yamaguchi K, Yamanaka M, Lam NT, Otsuka Y, Chuyen NV. Protective and anticataract effects of the aqueous extract of Cleistocalyx operculatus. Flower buds on beta-cells of streptozotocin-diabetic rats. J Agric Food Chem. 2010;58:4162–8. doi: 10.1021/jf904304w. [DOI] [PubMed] [Google Scholar]
- 61. Kim JS, Kwon CS, Son KH. Inhibition of alpha-glucosidase and amylase by luteolin, a flavonoid. Biosci Biotechnol Biochem. 2000;64:2458–61. doi: 10.1271/bbb.64.2458. [DOI] [PubMed] [Google Scholar]
- 62. Ali H, Houghton PJ, Soumyanath A. Alpha-amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J Ethnopharmacol. 2006;107:449–55. doi: 10.1016/j.jep.2006.04.004. [DOI] [PubMed] [Google Scholar]