Abstract
This study aimed to isolate fungal pathogens and to subsequently quantify aflatoxin (AF; B1 + B2 + G1 + G2) contamination in wheat crops grown in Pakistan. Accordingly, a total of 185 wheat samples were collected from different areas of Pakistan and numerous potent fungal pathogens were isolated. AF contamination attributed to the presence of intoxicating fungal pathogens and resulting metabolic activities were quantified using a high performance liquid chromatography-fluorescence detector coupled with postcolumn derivatization. Additionally, the effect of fungal pathogens on seed germination was also examined. The results obtained showed that 50% of tested wheat samples were found to be contaminated with a diverse range of fungal species. The rate of recurrence of fungal pathogens were Aspergillus 31%, Penicillium 9%, Fusarium 8%, Rhizopus 3%, and Alternaria 2%. The presence of Tilletia indica and Claviceps purpurea species was found to be inevident in all tested wheat samples. AFB1 contamination was detected in 48 (26.0%) samples and AFB2 in 13 (7.0%) samples. AFG1 and AFG2 were not found in any of the tested samples. The contamination range of AFB1 and AFB2 was 0.05–4.78 μg/kg and 0.02–0.48 μg/kg, respectively. The total amount of AFs (B1 + B2) found in 48 (26.0%) samples had a mean level of 0.53 ± 0.40 μg/kg and a contamination range of 0.02–5.26 μg/kg. The overall results showed that in 137 (74.0%) samples, AFs were not found within detectable limits. Furthermore, in 180 (97.2%) samples, AF levels were found to be below the maximum tolerated levels (MTL) recommended by the European Union (4 μg/kg). In five (2.7%) samples, AF contamination was higher than the MTL of the European Union. However, these samples were fit for human consumption with reference to the MTL (20 μg/kg) assigned by the USA (Food and Drug Administration and Food and Agriculture Organization) and Pakistan (Pakistan Standards and Quality Control Authority). Germination rates in healthy and contaminated wheat kernels were 84.6% and 45.2%, respectively. Based on the obtained results, it was concluded that the levels of fungal pathogen and AF contamination in Pakistani-grown wheat are not a potential threat to consumer health. However, control procedures along with a strict monitoring policy are mandatory to further minimize the prevalence of fungal carriers and the potency of AFs in crops cultivated in Pakistan.
Keywords: aflatoxins, fungal pathogens, HPLC, Pakistan, wheat
1. Introduction
Wheat (Triticum aestivum L.), which belongs to the family Poaceae (Gramineae), is the most favored staple food in the world. Furthermore, wheat ranked first in the essential diet of Pakistan’s population followed by rice and maize. During the year 2013–2014, wheat was cultivated on an area of approximately 9.2 million ha with the production of 25.98 million tons. Wheat comprises 10.0% of the agricultural sector and contributes to 2.1% of the gross domestic product of Pakistan [1].
Wheat kernels can be contaminated by pathogenic fungi such as Aspergillus, Penicillium, and Fusarium during harvesting, storage, and transportation. These deleterious fungi are responsible for the production of hepatotoxic, immune-suppressive, mutagenic, and teratogenic secondary metabolites [2]. Growth and production of these fungal pathogens on plants might result in lowered seed germination and reduced seed vigor, and seed necrosis might also be affected. Finally, these fungal pathogens can cause destruction and serious diseases during the different stages of plant growth [3]. Generally, tropical conditions such as moisture, high temperature, unseasonal rains, monsoons, and flash floods lead to fungal propagation, and finally the growth of aflatoxins (AFs) [4,5].
AFs are the best known and most intensively reported mycotoxins that are produced by fungi of the genus Aspergillus species, particularly Aspergillus flavus and Aspergillus parasiticus [6]. AFs are carcinogenic, mutagenic, teratogenic, and immunosuppressive fungal metabolites. AFs could pose a potential risk to human health because of aflatoxicosis and cancer [7,8]. The most important AFs are aflatoxin B1, B2, G1, and G2. However, AFB1 is the most dangerous of all of them and frequently occurs in food commodities [7,9,10]. Economic losses due to AF contamination have been reported in developed and developing countries [11–13]. The AF problem might be due to the lack of infrastructure in developing countries (such as India, Sri Lanka, and Pakistan) to monitor and control fungal invasion and production of toxic fungal metabolites. Insufficient implementation of good harvesting practices, improper storage, inadequate transportation, and marketing conditions could also contribute to Aspergillus growth and increase the risk of AF contamination.
Several countries, including Pakistan, have put forward guidelines and acceptance levels for AFs attributed to their frequent incidence, toxicity, and potential health hazard to humans. For instance, the maximum tolerated levels (MTL) in the European Union (EU) are 2 μg/kg for AFB1 and 4 μg/kg for the sum of AFs (AFB1, AFB2, AFG1, and AFG2) [14]. In the USA (Food and Drug Administration and Food and Agriculture Organization) and Pakistan (Pakistan Standards and Quality Control Authority), the MTL for total AFs in wheat is 20 μg/kg [15,16]. Furthermore, different methods have been developed in the past for the quantification of AFs in different matrices. For instance, Campone et al [17–19] reported in 2009, 2011, and 2015, the analysis of AFs in nuts, cereal products, and dried fruits using high performance liquid chromatography (HPLC) with fluorescence detection and ultrahigh-pressure liquid chromatography–tandem mass spectrometry, respectively. Waltking and Wilson [20], Brera et al [21], and Ofitserova et al [22] quantified AFB1 and AFs in corn using HPLC with postcolumn photochemical and chemical derivatization.
Due to fetal toxicity and their effect on human health, the present study was designed to isolate and identify the type and distribution level of pathogenic fungi such as Aspergillus, Penicillium, Fusarium, Alternaria, Rhizopus, Tilletia indica, and Claviceps purpurea, and AF contamination levels in Pakistani wheat grains using HPLC with postcolumn derivatization and fluorescence detection. Additionally, the attribution of fungal pathogens on seed germination was also observed. Moreover, the contamination levels were compared with reported levels in the international community.
2. Materials and methods
2.1. Chemicals and reagents
HPLC standards of AFBl (2 μg/mL; catalogue number 002017), AFB2 (0.5 μg/mL; catalogue number 002018), AFGl (2 μg/mL; catalogue number 002019), and AFG2 (0.51 μg/mL; catalogue number 002020) in acetonitrile (ACN) were procured from Biopure (Vienna, Austria). Ready to use potato dextrose agar (PDA) media [potato extract 4.0 g, glucose 20.0 g, and agar 15.0 g in 1 L deionized (DI) H2O, pH 5.6 ± 0.2] and phosphate buffered saline (PBS) tablets (pH 7.3 ± 0.2) were purchased from Oxoid (Hampshire, UK). ACN and methanol (MeOH) were obtained from Merck (Darmstadt, Germany). Ethanol (EtOH), nitric acid (HNO3), and potassium bromide (KBr) were obtained from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals and solvents used were of analytical grade from BDH (Poole, England) and Merck. Highly purified water (Resistivity = 18 MΩ·cm) was prepared by processing DI water through a Purelab Ultra Option water purification system (Model No. DV 25; ELGA, Buckinghamshire, UK).
2.2. Apparatus
An autoclave (Model Number LS-2D) was acquired from Rexmed (Kaohsiung, Taiwan). Purelab Ultra Option (DV 25) water purification system was purchased from ELGA. Explosion-proof blender (Model Number 8018) was obtained from Ebarch (New York, NY, USA). The HPLC system consisted of a pump (Model Number L-2130) from VWR-Hitachi (Tokyo, Japan), an auto-sampler (Model Number L-2200) from Merck-Hitachi (Tokyo, Japan), a column thermostat from Jones-Chromatography (Wales, UK), a LiChroCART 100Å RP-18 (5 μm, 250 × 4.0 mm) column from Merck, and a fluorescence detector (Model Number L-2480) from VWR-Hitachi. Kobra Cell and Easi-Extract AF immunoaffinity columns (IACs; Catalogue Number RP70N) were purchased from R-Biopharm (Glasgow, Scotland). All other glassware such as conical flasks, beakers, measuring cylinders, Petri plates, and forceps were sterilized by autoclaving at 121°C for 15 minutes.
2.3. Sample collection
A total of 185 wheat grain samples were collected from different areas of Pakistan during the period of January 2014 to December 2014. It is well recognized that AFs occur in high concentrations and are heterogeneously dispersed throughout food and feed commodities. Therefore, the sampling procedure was based on the method explained in the Association of Official Analytical Chemists official method number 977.16 [23]. Briefly, a minimum sample size of 0.5–1.0 kg was blended thoroughly for 10 minutes. Each homogenized and representative sample was divided into three identical portions. One portion of sample was employed for the blotter paper method and the second portion of sample was put in PDA for the isolation and identification of fungal pathogens. The third portion of sample was pulverized into particles ≤1 mm using a sample grinder (Cyclotec 1093 Mill; Tecator, Höganes, Sweden) and subsampled to a final quantity of 100 g. Finally, all pulverized samples were kept in separate air tight opaque polyethylene bags and stored at −20°C until further AF analysis.
2.4. Prevalence of fungal pathogens in wheat samples
The isolation of fungi from wheat grains was assessed using two methods: (1) blotter paper method; and (2) agar plate method as recommended by the International Seed Testing Association [24].
2.4.1. Blotter paper method
A total of 400 grains from each wheat sample was placed on three layers of water-soaked blotter paper already kept in 9-cm sterilize Petri plates (10 grains per plate). The Petri plates were then incubated at 25 ± 2°C for 7 days in the dark. The growth of fungal species in each Petri plate was observed on the basis of morphological and microscopic characterization which included colony color, conidiophores, phialids, presence of vesicles, and size of vesicles [25]. The growth of fungal species was calculated using the following equation:
| (1) |
2.4.2. Agar plate method
A total of 400 grains per sample were plated (10 grains/plate) on Petri plates containing PDA (39 g/L; pH 5.6 ± 0.2). The plates were incubated for 7 days at 25 ± 2°C in the dark. The growth of fungal species in each Petri plate was observed on the basis of morphological and microscopic characteristics. However, the growth of fungal species was calculated using Eq. (1).
2.5. Seed germination
The germination rate of wheat seeds was assessed using the standard blotter paper method [24]. A total of 100 seeds of each sample were placed between two layers of blotter paper. The plates were incubated for 15 days at 25 ± 2°C in the dark. The final counting of normal and abnormal seedling rate was recorded 15 days after planting and the total percent of germination was calculated as recommended by the International Seed Testing Association [26].
| (2) |
2.6. Analysis of AFs
AF contamination in wheat samples was quantified using HPLC coupled with postcolumn derivatization and fluorescence detector [27]. Briefly, the entire procedure consisted of three major steps: (1) sample preparation (extraction of sample); (2) sample clean-up; (3) HPLC analysis.
2.6.1. Sample preparation (extraction of sample)
Fifty grams of each homogenized and pulverized wheat sample was extracted in 100 mL MeOH:H2O (80:20 v/v). The sample suspensions were blended using an explosion-proof blender at 1950 relative centrifugal force for 2 minutes. The blended extracts were filtered through Whatman Number 1 filter paper and clear supernatants were collected in separate airtight amber vials.
2.6.2. Immunoaffinity clean-up
The sample clean-up was carried out using IACs. Briefly, 2 mL of each sample extract was diluted with 14 mL PBS and passed through IACs at a flow rate of about 1–2 drops/s. IACs were washed with 20 mL of PBS at a flow rate of approximately 5 mL/min and rapidly dried by passing air. AFs were eluted with 1.5 mL of methanol followed by 1.5 mL of DI H2O and collected in separate amber vials for subsequent chromatographic analysis.
2.6.3. HPLC analysis
Chromatographic analysis of AFs was performed using a HPLC system with postcolumn derivatization and fluorescence detector. An aliquot of 99 μL of each AF standard and sample was injected in to the auto-sampler. The mobile phase consisted of MeOH:ACN:H2O (65:17.5:17.5 v/v) containing 119 mg/L of KBr and 154 μl/L of HNO3 and the flow rate was 1 mL/min. The elution was performed in isocratic mode. The excitation and emission wavelength was adjusted at 362 nm and 425 nm in the fluorescence detector. The column temperature was maintained at 40°C and the current source in KobraCell (R-Biopharm) was adjusted at 100 μA. All four AFs were well resolved within a total run time of about 20 minutes.
2.7. Method validation
The validation of the HPLC method was carried out in accordance with the EU commission regulation number 1881/2006 [13]. Briefly, the performance of the method was evaluated in terms of linearity, precision, accuracy, limit of detection (LOD), limit of quantification (LOQ), and recovery. The linearity of the method was estimated in terms of coefficient of determination (R2). A sequence of each AF in final concentrations of 0.0125 ng/mL, 0.025 ng/mL, 0.125 ng/mL, 0.25 ng/ mL, 0.625 ng/mL, 1.25 ng/mL, 2.5 ng/mL, 5 ng/mL, 10 ng/mL, 15 ng/mL, and 20 ng/mL was injected to the HPLC system and chromatograms were recorded. Each concentration was analyzed in triplicate. Finally, calibration curves for AFB1, AFB2, AFG1, and AFG2 were separately prepared by plotting the mean area versus the relevant concentration and the R2 value was then calculated.
The intraday and interday precision and accuracy of the HPLC method were evaluated using quality control (QC) samples [21]. Briefly, three different concentrations of each AF (0.25 ng/mL, 2.5 ng/mL, and 10 ng/mL) of QC samples were analyzed using the HPLC system and precision was calculated in terms of relative standard deviation (RSD). Furthermore, the accuracy was calculated as the relative mean error (RME). The LOD and LOQ of the HPLC method were estimated according to the International Council on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use guidelines. Briefly, the LOD and LOQ were calculated from the calibration curves at the concentration with a peak area ratio of signal-to-noise ratio not less than 3 and 10, respectively [28]. The efficacy of the extraction and chromatography procedure was assessed using sample fortification. Briefly, 50 g of AF-free sample (wheat) was spiked with AF solution at least 1 hour before analysis. The final spiked concentration of each AF (AFB1, AFB2, AFG1, or AFG2) was 0.5 μg/kg, 5 μg/kg, and 10 μg/kg. AFs were extracted and liquid chromatography was performed according to the protocol as described above. Finally, the actual and measured concentrations of spiked AFs were compared and the percent recovery of each AF was evaluated.
2.8. Statistical data analysis
Statistical data analysis was carried out using the Student t test with p < 0.05 as the minimal level of significance unless indicated otherwise. All values were expressed as the means ± standard deviation.
3. Results and discussion
3.1. Method validation
All analytics were performed in triplicate to verify the accuracy of the method performance. In addition, the performance of the HPLC method was also evaluated in terms of linearity, precision and accuracy, LOD, LOQ, and recovery studies. All relevant parameters regarding method validation are presented in Table 1. The results illustrated that AF concentrations were found to be proportional to the related areas. The calibration curves were linear over the evaluated range of 0.0125–20 ng/mL. The R2 values for AFB1, AFB2, AFG1, and AFG2 ranged between 0.9992 and 0.9997. The precision (% RSD) and the accuracy (% RME) of the HPLC method were estimated using QC samples and reported in Table 2. The results showed that the method had an excellent RSD and RME. The intraday and interday precision and accuracy were found to be below 6% for the all QC samples. The % RSD of intraday and interday assessments ranged from 0.20% to 2.20% and 0.31% to 2.48%, respectively. The % RME of intraday and interday ranged from −5.20% to 2.20% and −3.45% to 3.24%, respectively.
Table 1.
Method validation for the quantification of aflatoxins (AFs; AFB1, AFB2, AFG1, and AFG2) in wheat.
| Toxins | Correlation coefficient (R2) | LOD (μg/kg) | LOQ (μg/kg) | Recovery range (%) | RSD (%) (n = 20) | Measurement uncertainty (μg/kg) |
|---|---|---|---|---|---|---|
| AFB1 | 0.9995 | 0.031 | 0.093 | 94.8–97.2 | 1.17 | 0.08 |
| AFB2 | 0.9994 | 0.022 | 0.066 | 95.1–98.1 | 1.01 | 0.10 |
| AFG1 | 0.9992 | 0.032 | 0.096 | 94.4–96.1 | 1.18 | 0.06 |
| AFG2 | 0.9997 | 0.028 | 0.084 | 93.2–95.6 | 0.72 | 0.10 |
| Total AFs | 0.9994 | 0.091 | 0.273 | 93.2–97.2 | 1.12 | 0.09 |
HPLC = high-performance liquid chromatography; LOD = limit of detection; LOQ = limit of quantification; RSD = relative standard deviation.
Table 2.
Intraday and interday precision and accuracy for the determination of aflatoxins (AFs; AFB1, AFB2, AFG1, and AFG2) in wheat samples.a
| Spiked concentration (μg/kg) | AFB1 | AFB2 | AFG1 | AFG2 | Precision (% RSD) | Accuracy (% RME) | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mean ± SD (μg/kg) | |||||||
| Intraday | 0.25 | 0.259 ± 0.017 | 0.244 ± 0.017 | 0.253 ± 0.014 | 0.252 ± 0.014 | 0.25–2.2 | −1.48 to 1.43 |
| 2.5 | 2.442 ± 0.028 | 2.524 ± 0.018 | 2.483 ± 0.016 | 2.403 ± 0.021 | 0.54–2.11 | −3.48 to 2.20 | |
| 10 | 9.749 ± 0.018 | 9.817 ± 0.029 | 10.14 ± 0.208 | 9.779 ± 0.040 | 0.68–0.63 | −5.20 to 1.45 | |
| Interday | 0.25 | 0.253 ± 0.014 | 0.240 ± 0.021 | 0.254 ± 0.020 | 0.251 ± 0.031 | 0.67–1.68 | −2.65 to 3.24 |
| 2.5 | 2.483 ± 0.035 | 2.523 ± 0.040 | 2.520 ± 0.040 | 2.453 ± 0.035 | 1.47–2.48 | −1.94 to 0.80 | |
| 10 | 9.780 ± 0.020 | 9.817 ± 0.028 | 9.910 ± 0.062 | 9.853 ± 0.011 | 0.31–1.14 | −3.45 to 1.45 | |
RME = relative mean error; RSD = relative standard deviation; SD = standard deviation.
Triplicate analysis was performed for all measurements and reported as mean ± SD.
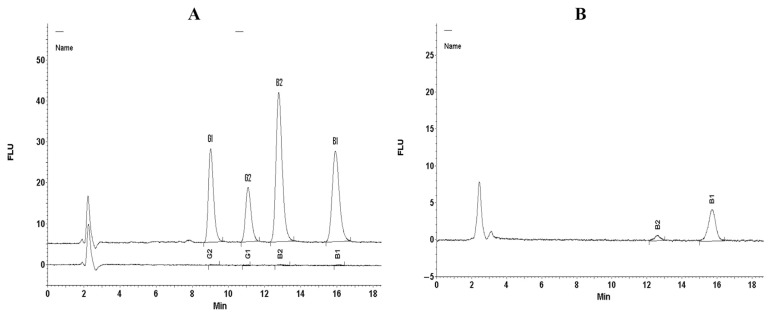
The LOD and LOD of the HPLC method were calculated from the calibration curves for each AF (AFB1, AFB2, AFG1, and AFG2). The calculated values are reported in Table 1. The LOD and LOQ of the method effectively satisfied the MTL regulated by the EU (4 μg/kg) and USA (20 μg/kg) [14,15]. The LOD and LOQ for AFB1, AFB2, AFG1, and AFG2 were 0.031/0.093 μg/kg, 0.022/0.066 μg/kg, 0.032/0.096 μg/kg, and 0.028/0.084 μg/kg, respectively. The average recoveries for each AF are summarized in Table 1 and the HPLC chromatogram is shown in Figure 1A. The mean recoveries ranged from 93.2% to 97.2%. The recoveries rates satisfy the guidelines for recoveries limits set by the Association of Official Analytical Chemists and Codex Standard [29,30].
Figure 1.
High performance liquid chromatography chromatograms of: (A) spike; and (B) naturally contaminated samples of wheat. FLU = Fluorescence.
3.2. Prevalence of pathogenic fungi in wheat samples
Repeated invasion of pathogenic fungi such as Aspergillus, Penicillium, and Fusarium within food and feed commodities are of concern to human and animal health due to the associated severe toxicity. In the present study, a total of 185 samples of wheat grains were collected from different regions of Pakistan during January 2014 to December 2014. The wheat samples were then assessed to isolate and diagnose the fungal pathogens on the basis of their microscopic and cultural characteristics. The relevant data regarding contamination of the above mentioned fungal pathogens is summarized in Table 3.
Table 3.
The incidence of wheat samples with various fungal genera detected using blotter paper and agar plate methods.
| Fungal genera | Wheat samples with fungal spp., n (%) | Difference | |
|---|---|---|---|
|
| |||
| Blotter paper method | Agar plate method | ||
| Aspergillus flavus | 24 (13.0) | 26 (14.0) | NS |
| Aspergillus niger | 26 (14.0) | 30 (16.2) | NS |
| Penicillium spp. | 15 (8.1) | 17 (9.2) | NS |
| Fusarium spp. | 13 (7.0) | 15 (8.1) | NS |
| Alternaria spp. | 4 (2.2) | 4 (2.2) | NS |
| Rhizopus spp. | 6 (3.2) | 4 (2.2) | NS |
| Tilletia indica | ND | ND | NS |
| Claviceps purpurea | ND | ND | NS |
ND = not detected; NS = not significant.
The results showed that about 50% of the samples were found to be contaminated with different fungal genera. The most common fungi isolated were A. flavus (14.0%) and Aspergillus niger (16.2%). Other fungal genera such as Penicillium (9.2%), Fusarium (8.1%), Alternaria (2.2%), and Rhizopus (3.2%) were also found in low frequency. The other nonsystemic bunt fungal pathogens such as Tilletia indica and Claviceps purpurea species were not detected in any of the wheat samples. These nonsystemic bunt fungal pathogens are supposed to be responsible for Karnal bunt disease of wheat [31]. No significant differences were seen among the blotter paper method and agar plate method and the results obtained by adapting both methods were found to be in good agreement.
3.3. AF contamination level in wheat samples
The contamination level of AFs is summarized in Table 4. The chromatogram of AFs in naturally contaminated wheat is shown in Figure 1B. The results showed that AFB1 was found in 48 (25.9%) wheat samples. The AFB1 concentration ranged between 0.05 μg/kg and 4.78 μg/kg with a mean level of 0.51 ± 1.14 μg/kg.
Table 4.
Occurrence of aflatoxins (AFs; AFB1, AFB2, AFG1, and AFG2) in wheat samples collected from different areas of Pakistan.a
| Toxins | Tested samples (n) | Positive samples, n (%) | No. and percentage of samples in concentration range (μg/kg) | Mean ± SD (μg/kg) | Range (μg/kg) | |||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| <LODb | ≤2 | 2–4c | ≥4d | |||||
| AFB1 | 185 | 48 (26.0) | 137 (74.0) | 26 (14.0) | 17 (9.2) | 5 (2.7) | 0.51 ± 0.32 | 0.05–4.78 |
| AFB2 | 13 (7.0) | 172 (93.0) | 13 (7.0) | 0 | 0 | 0.02 ± 0.08 | 0.02–0.48 | |
| AFG1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| AFG2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total AFs | 48 (26.0) | 137 (74.0) | 26 (14.0) | 17 (9.2) | 5 (2.7) | 0.53 ± 0.40 | 0.02–5.26 | |
LOD = limit of detection; SD = standard deviation.
All measurements were made in triplicate and reported as mean ± SD.
Below the limit of detection.
As per European Union standard (≤4 μg/kg).
Below the Food and Drug Administration, Food and Agriculture Organization, and Pakistan Standards and Quality Control Authority maximum tolerated level (≤20 μg/kg).
The contamination level of AFB1 was significantly lower than the MTL (2 μg/kg) for AFB1 assigned by the EU. AFB2 was found only in 13 (7.0%) samples with a mean level of 0.02 ± 0.08 μg/kg. The AFB2 contamination ranged between 0.02 μg/kg and 0.48 μg/kg. AFG1 and AFG2 were not detected in any of the analyzed samples. Total AFs (B1 + B2) were found in 48 (25.9%) wheat samples ranging from 0.05 μg/kg to 5.26 μg/kg with a mean level of 0.53 ± 0.40 μg/kg. The overall results indicated that AFs were not found within detectable limits (>0.091 μg/kg) in 137 (74.0%) samples. Out of 185 samples analyzed, 180 (97.3%) samples contained AF levels below the MTL of 4 μg/kg for total AFs as recommended by the EU. Furthermore, only five (2.7%) samples showed AF contamination more than the MTL of the EU. However, these samples were fit for human consumption with reference to the MTL (20 μg/kg) assigned by the USA (Food and Drug Administration and Food and Agriculture Organization) and Pakistan (Pakistan Standards and Quality Control Authority).
The fungal growth may not only change the chemical and physical properties of the food products but is also responsible for the deterioration of nutrient contents of the grains. The quality and germination rate of seeds is directly and indirectly affected by the seed-borne pathogens. Seed-borne diseases have been found to affect the growth and productivity of crop plants as the liable pathogens attack and destroy the seedlings [32]. Furthermore, Karim [33] reported that the Fusarium and Alternaria spp. are also responsible for reducing the germination rate and inducing seedling blight. The results of the present study were found to be in good agreement with the above-mentioned earlier studies. The seed germination rate was lower in contaminated wheat in comparison to healthy wheat samples. For instance, the seed germination rates in healthy and contaminated wheat samples were 84.6% and 45.2%, respectively.
The pathogenic fungi are responsible for producing carcinogenic compounds such as AFs, ochratoxin A, deoxynevalenone, and fumonisin. Therefore, a number of authors reported the presence of numerous pathogenic fungal species in wheat samples (Table 5). Joshaghani et al [34] tested 34 samples of wheat seeds in Iran. The incidence of contamination by A. flavus, A. niger, Fusarium, Alternaria, and Penicillium was 10%, 21%, 18%, 27%, and 9%, respectively. Kolawole et al [35] reported from Nigeria that out of 400 samples, about 17%, 12%, 16%, 6%, 22%, and 10% of samples were found to be contaminated with A. flavus, A. niger, Fusarium, Alternaria, Penicillium, and Rhizopous species, respectively. The findings obtained in the present study were found to be in close association with the previous study. However, Fusarium and Penicillium species were found to be higher in the Nigerian study. Furthermore, Anand et al [36] reported from India that 43% and 35% samples of wheat were contaminated with A. flavus and Fusarium species, respectively. El-Shanshoury et al [37] detected incidence rates of A. flavus (60%), A. niger (60%) Fusarium (60%), Alternaria (50%), Penicillium (60%), and Rhizopous (40%) in 10 samples of wheat in Egypt. Al-Kahtani [38] reported from Saudi Arabia that the incidence rates of Aspergillus species, Fusarium, and Alternaria were 24%, 7%, and 60%, respectively. In the present study, the contamination levels of fungal pathogens were found to be lower in comparison with the above-mentioned studies.
Table 5.
Rate of recurrence of fungal pathogens in wheat samples from different countries.
| Country | Tested samples (n) | Types of isolates, n (%) | Methods used | Authors | |||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Aspergillus flavus | Asergillus. niger | Fusarium | Alternaria | Penicillium | Rhizopous | ||||
| Iran | 34 | 6 (11) | 11 (21) | 11 (18) | 15 (27) | 5 (9) | 2 (3) | Agar plate | Joshaghani et al [34] (2013) |
| Nigeria | 400 | 70 (17) | 50 (12) | 63 (16) | 25 (6) | 90 (22) | 40 (10) | Agar plate | Kolawole et al [35] (2013) |
| India | 30 | 13 (43) | NR | 10 (35) | NR | NR | NR | Standard plate count | Anand et al [36] (2009) |
| Egypt | 10 | 6 (60) | 6 (60) | 6 (60) | 5 (50) | 6 (60) | 4 (40) | Agar plate | El-Shanshoury et al [37] (2014) |
| Saudi Arabia | 16 | 4 (24) | 1 (7) | 11 (69) | NR | NR | Agar plate | Al-Kahtani [38] (2014) | |
| Pakistan | 185 | 24 (13) | 26 (14) | 13 (7) | 4 (2) | 15 (8) | 6 (3) | Blotter paper | Current study |
| 26 (14) | 30 (16) | 15 (8) | 4 (2) | 17 (9) | 4 (2) | Agar plate | Current study | ||
NR = not reported.
It has been reported that, the AFB1 and AFB2 are the most common contaminants in comparison to AFG1 and AFG2 in Pakistan [9]. The basic reason is that the temperature and relative humidity for AFB1 and AFB2 production is favorable in Pakistan. Schroeder and Hein [39] reported that the suitable temperatures for AF production ranged between 20°C and 35°C. Elevation of temperature up to 40°C or a decline down to 10°C could result in reduced AF production. A high temperature (30–35°C) within the optimal range favors the production of aflatoxin B (B1 and B2). In contrast, low temperature (15–20°C) favors the production of aflatoxin G (G1 and G2). Pakistan is located in a region which has a hot and humid climate, with high temperatures averaging 23.9°C and a double maxima rainfall pattern (489 mm) [40]. These hot and humid climatic conditions are considered to be very favorable for the production of AFs [41]. The findings of the present study support the above-mentioned facts and figures.
Several studies have reported the contamination levels of AFs in wheat samples (Table 6). In Malaysia, Abdullah et al [42] reported that 18 (21.7%) wheat flour samples out of 83 samples were contaminated with AFs. The contamination of AFB1, AFB2, AFG1, and AFG2 were present in 1.2%, 4.8%, 3.6%, and 13% of samples ranging from LOD to 25.6 μg/kg, 11.2 μg/kg to 252.5 μg/kg, 25.0 μg/kg to 289.4 μg/kg, and 16.2 μg/kg to 436.2 μg/kg, respectively. Furthermore, Joshaghani et al [34] reported from Iran that 29% of samples were contaminated with AFB1 out of 34 samples, ranging from LOD to 6.91 μg/kg. In another study from Brazil, Trombete et al [10] analyzed 108 wheat samples and reported that 31% samples were positive for total AFs. The contamination range was between LOD and 6.2 μg/kg with a mean level of 2.2 μg/kg. In the present study, the contamination level of AFs was lower than as reported in the above-mentioned studies. However, the percentage of positive samples was comparable with the mentioned studies.
Table 6.
The levels of individual and total aflatoxins (AFs) in wheat samples reported in different countries.
| Country | Tested samples (n) | Toxin types | Positive samples, n (%) | Maximum (μg/kg) | Range (μg/kg) | Mean ± SD (μg/kg) | Year of survey | Authors |
|---|---|---|---|---|---|---|---|---|
| Malaysia | 84 | AFB1 | 1 (1.2) | 25.6 | LOD–25.6 | NR | 1998 | Abdullah et al [42] (1998) |
| AFB2 | 4 (4.8) | 252.5 | 11.2–252.5 | NR | ||||
| AFG1 | 3 (3.6) | 289.4 | 25.0–289.4 | NR | ||||
| AFG2 | 11 (13) | 436.2 | 16.2–436.2 | NR | ||||
| Algeria | 53 | AFB1 | 30 (56) | 37.4 | 0.13–37.4 | – | 2004–2006 | Riba et al [45] (2010) |
| Turkey | 100 | AFB1 | 20 (20) | 12.2 | 0.025–12.2 | 0.48 ± 0.21 | 2006 | Aydin et al [43] (2008) |
| Total AFs | 45 (45) | 14.01 | 0.05–14.0 | 0.79 ± 0.99 | ||||
| Iran | 34 | AFB1 | 10 (29) | 6.91 | LOD–6.91 | – | 2008–2009 | Joshaghani et al [34] (2013) |
| Iran | 100 | AFB1 | 77 | NR | NR | 0.53 ± 0.87 | 2010 | Taheri et al [44] (2012) |
| AFB2 | 98 | NR | NR | 0.30 ± 0.71 | ||||
| AFG1 | 85 | NR | NR | 0.55 ± 0.93 | ||||
| AFG2 | 70 | NR | NR | 0.59 ± 0.62 | ||||
| Total AFs | 99 | NR | NR | 0.99 ± 1.96 | ||||
| Brazil | 108 | Total AFs | 33 (31) | 6.2 | LOD–6.2 | 2.2 | 2013–2014 | Trombete et al [10] (2014) |
| Pakistan | 185 | AFB1 | 48 (25.9) | 4.78 | 0.05–4.78 | 0.51 ± 1.14 | 2014 | Current study |
| AFB2 | 13 (7.1) | 0.48 | 0.02–0.48 | 0.02 ± 0.08 | ||||
| AFG1 | 0 | 0 | 0 | 0 | ||||
| AFG2 | 0 | 0 | 0 | 0 | ||||
| Total AFs | 48 (25.9) | 5.26 | 0.05–5.26 | 0.53 ± 0.40 |
LOD = limit of detection; NR = not reported; SD = standard deviation.
In another study from Turkey, Aydin et al [43] analyzed 100 samples of wheat flour. AFB1 and total AFs were found in 20% and 45% of samples, respectively. For AFB1 and total AFs, the contamination range was 0.025–12.2 μg/kg and 0.05–14.0 μg/ kg with a mean level of 0.48–0.79 μg/kg, respectively. Taheri et al [44] analyzed 100 samples of wheat flour in two different seasons in Iran. The incidences rate of AFs in winter and summer were 99% and 70%, respectively. The contamination level of AFB1 was also higher than the summer seasons (77% vs. 33%). The average contamination of AFB1, AFB2, AFG1, AFG2, and total AFs were 0.53 μg/kg, 0.30 μg/kg, 0.55 μg/kg, 0.59 μg/kg, and 0.99 μg/kg, respectively. Riba et al [45] reported from Algeria that about 56% samples were positive with AFB1 and ranged between 0.13 μg/kg and 37.4 μg/kg. In the present study, the mean level of AF contamination was comparable with the above-mentioned studies. However, the percentage of positive samples was lower than the above-mentioned studies.
All the above-mentioned previous studies indicated that fungal pathogens and AF contamination frequently occur in wheat. The present study also showed that the Pakistani wheat samples were found to be contaminated with low levels of fungal pathogens and AF contamination. However, the present status of the fungal pathogens and AF levels in Pakistani wheat does not concurrently present a potential risk to human health. The frequency of positive samples indicated that there is need for further study, regular monitoring, and performance of routine analysis as per food QC measures. In order to achieve a low level of contamination, it is necessary to conduct a regular training plan, and good manufacturing and storage practices along with implementation of a hazard analysis and critical control points-based safety program.
4. Conclusion
In the present study, a total of 185 wheat samples were collected from Pakistan and investigated for the presence of fungal pathogens and AF contamination levels. The Aspergillus species was the most common fungal isolate in wheat samples. Out of 185 samples, 48 (25.9%) were positive for total AFs (B1 + B2) with contamination levels ranging between 0.05 μg/ kg and 5.26 μg/kg with a mean level of 0.53 ± 0.40 μg/kg. The frequencies of seed-borne fungi and AF levels in wheat samples were significantly lower than the permissible limits of the EU, USA, and Pakistan and fit for human consumption. Germination rates in healthy and contaminated wheat kernels were 84.6% and 45.2%, respectively. On the basis of obtained results, it was also concluded that fungal pathogens have adverse effects on the germination of wheat seeds. The detection of fungal pathogens and AF contamination warrants further investigation, regular monitoring, and routine analysis as per food QC measures. The initial approach is to take precautions and proper action by applying fungicides and biological compounds to reduce crop losses and as a result increase the quality of produce.
Footnotes
Conflicts of interest
The authors declared no conflicts of interest relevant to this article.
REFERENCES
- 1.Pakistan Economic. Survey Ministry of Finance. Government of Pakistan; 2013–2014. [accessed 05, 06, 15]. http://www.finance.gov.pk/survey/chapters_15/02_Agricultre.pdf . [Google Scholar]
- 2. Bryden WL. Mycotoxins in the food chain: human health implications. Asia Pacific J Clin Nutr. 2007;16:95–101. [PubMed] [Google Scholar]
- 3. Weber R, Hrynczuk B, Runowska-Hrynczuk B, Kita W. Influence of the mode of tillage diseases of culm base in some winter wheat varieties, oats and spring wheat. J Phytopathol. 2001;149:185–8. [Google Scholar]
- 4.Bhat RV, Siruguri V. Mycotoxin food safety risk in developing countries Focus 10, Brief 3 of 17. In: Unnevehr LJ, editor. Food safety in food security and food trade 2020 vision focus. Washington D.C: International Food Policy Research Institute (IFPRI); 2003. [Google Scholar]
- 5. Turner NW, Subrahmanyam S, Piletsky SA. Analytical methods for determination of mycotoxins: a review. Anal Chim Acta. 2009;632:168–80. doi: 10.1016/j.aca.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 6. Khan MA, Asghar MA, Iqbal J, Ahmed A, Iqbal J, Shamsuddin ZA. Aflatoxins contamination and prevention in red chillies (Capsicum annuum L.) in Pakistan. Food Addit Contam Part B Surveill. 2014;7:1–6. doi: 10.1080/19393210.2013.825330. [DOI] [PubMed] [Google Scholar]
- 7. Anukul N, Vangnai K, Mahakarnchanakul W. Significance of regulation limits in mycotoxin contamination in Asia and risk management programs at the national level. J Food Drug Anal. 2013;21:227–41. [Google Scholar]
- 8. Jeffrey AM, Williams GM. Risk Assessment of DNA-reactive carcinogens in food. Toxicol Appl Pharmacol. 2005;207:628–35. doi: 10.1016/j.taap.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 9. Asghar MA, Iqbal J, Ahmed A, Khan MA. Occurrence of aflatoxins contamination in brown rice from Pakistan. Iranian J Publ Health. 2014;43:291–9. [PMC free article] [PubMed] [Google Scholar]
- 10. Trombete FM, de Ávila Moraes D, Porto YD, Santos TB, Direito GM, Fraga ME, Saldanha T. Determination of aflatoxins in wheat and wheat by-products intended for human consumption, marketed in Rio de Janeiro, Brazil. J Food Nutr Res. 2014;2:671–4. [Google Scholar]
- 11. Chen MT, Hsu YH, Wang TS, Chien SW. Mycotoxin monitoring for commercial foodstuffs in Taiwan. J Food Drug Anal. 2016;24:147–56. doi: 10.1016/j.jfda.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen YC, Liao CD, Lin HY, Chiueh LC. Survey of aflatoxin contamination in peanut products in Taiwan from 1997 to 2011. J Food Drug Anal. 2013;21:247–52. [Google Scholar]
- 13. Bhat R, Rai RV, Karim AA. Mycotoxins in food and feed: present status and future concerns. Compr Rev Food Sci F. 2010;9:57–81. doi: 10.1111/j.1541-4337.2009.00094.x. [DOI] [PubMed] [Google Scholar]
- 14. European Commission Regulation (EC) No. 165/2010 of 26 February 2010. Amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards aflatoxins. Off J Eur Union. 2010;L50:8–12. [Google Scholar]
- 15.US Food and Drug Administration. Guidance for industry: action levels for poisonous or deleterious substances in human food and animal feed. [accessed 10, 06,15]. http://www.fda.gov .
- 16. Ashiq S. Natural occurrence of mycotoxins in food and feed: Pakistan perspective. Compr Rev Food Sci Food Saf. 2015;14:159–75. doi: 10.1111/1541-4337.12122. [DOI] [PubMed] [Google Scholar]
- 17. Campone L, Piccinelli AL, Aliberti L, Rastrelli L. Application of pressurized liquid extraction in the analysis of aflatoxins B(1), B(2), G(1) and G(2) in nuts. J Sep Sci. 2009;32:3837–44. doi: 10.1002/jssc.200900329. [DOI] [PubMed] [Google Scholar]
- 18. Campone L, Piccinelli AL, Celano R, Rastrelli L. Application of dispersive liquid–liquid microextraction for the determination of aflatoxins B1, B2, G1 and G2 in cereal products. J Chromatogr A. 2011;1218:7648–54. doi: 10.1016/j.chroma.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 19. Campone L, Piccinelli AL, Celano R, Russo M, Valdés A, Ibáñez C, Rastrelli L. A fully automated method for simultaneous determination of aflatoxins and ochratoxin A in dried fruits by pressurized liquid extraction and online solid-phase extraction cleanup coupled to ultra-high-pressure liquid chromatography–tandem mass spectrometry. Anal Bioanal Chem. 2015;407:2899–911. doi: 10.1007/s00216-015-8518-4. [DOI] [PubMed] [Google Scholar]
- 20. Waltking AE, Wilson D. Liquid chromatographic analysis of aflatoxin using post-column photochemical derivatization: collaborative study. J AOAC Int. 2006;89:678–92. [PubMed] [Google Scholar]
- 21. Brera C, Debegnach F, Minardi V, Pannunzi E, De Santis B, Miraglia M. Immunoaffinity column cleanup with liquid chromatography for determination of aflatoxin B1 in corn samples: Interlaboratory study. J AOAC Int. 2007;90:765–72. [PubMed] [Google Scholar]
- 22. Ofitserova M, Nerkar S, Pickering M, Torma L, Thiex N. Multi residue mycotoxin analysis in corn grain by column high-performance liquid chromatography with post column photochemical and chemical derivatization: single-laboratory validation. J AOAC Int. 2009;92:15–25. [PubMed] [Google Scholar]
- 23.Horwitz W, Latimer GW. Natural toxins. In: Truckess MW, editor. Official methods of analysis of AOAC International. 19th ed. Gaithersburg, MD: AOAC International; 2012. pp. 1–85. [Google Scholar]
- 24. ISTA (International Seed Testing Association) International rules for seed testing. Seed science and technology. Suppl Seed Prod. 1985;6:57–84. [Google Scholar]
- 25.Pedro WC, Verkley JM, Groenewald JZ, Samson RA. Fungal biodiversity. The Netherlands: CBS-KNAW Fungal Biodiversity Center; 2009. [Google Scholar]
- 26.ISTA (International Seed Testing Association) Zürichstrasse 50, CH-8303. 2007. Bassersdorf, Switzerland: Secretariat; 2007. International rules for seed testing. [Google Scholar]
- 27. Asghar MA, Iqbal J, Ahmed A, Khan MA, Shamsuddin ZA, Jamil K. Development and validation of a high-performance liquid chromatography method with postcolumn derivatization for the detection of aflatoxins in cereals and grains. Toxicol Ind Health. 2016 doi: 10.1177/0748233714547732. . In press. [DOI] [PubMed] [Google Scholar]
- 28.Miller JC, Miller JN. Statistics for analytical chemistry. 3rd ed. New York: Ellis Horwood PTR Prentice-Hall; 1993. [Google Scholar]
- 29.Codex standard for contaminants and toxins in food and feed. Codex standard 193–1995. 1995. [accessed 15, 06, 15]. www.fao.org/.../pdf/CXS_193e.pdf .
- 30.AOAC International. AOAC guidelines for single laboratory validation of c Methods for dietary supplements and botanicals. Gaithersburg: AOAC International; [accessed 22, 06, 15]. http://www.aoac.org/imis15_prod/AOAC_Docs/StandardsDevelopment/SLV_Guidelines_Dietary_Supplements.pdf . [Google Scholar]
- 31. Ehsan-Ul-Haq, Rattu AR, Mirza JI. Prevalence of karnal bunt of wheat in NWFP (Pakistan) Int J Agric Biol. 2002;4:150–2. [Google Scholar]
- 32. Dawson WAM, Bateman GL. Fungal communities on roots of wheat and barley and effects of seed treatments containing fluquinconazole applied to control take-all. Plant Pathol. 2001;50:575–82. [Google Scholar]
- 33.Karim M. Prevalence of fungi associated with seeds of some minor cereals. Mymensingh: Department of Plant Pathology, Bangladesh Agricultural University; 2005. [Google Scholar]
- 34. Joshaghani H, Namjoo M, Rostami M, Kohsar F, Niknejad F. Mycoflora of fungal contamination in wheat storage (silos) in Golestan province, North of Iran. Jundishapur J Microbiol. 2013;6:e6334. [Google Scholar]
- 35. Kolawole RM, Thomas BT, Adekunle AA, Oluwadun A. Postharvest pathogenic fungi of wheat circulating in Lagos State, Nigeria. Am J Res Commun. 2013;1:421–8. [Google Scholar]
- 36. Anand R, Senthil PS, Sundaramoorthi C, Bhuvaneswari K. Characterization of fungal contaminants from wheat and the speculation of mycotoxin with reference to aflatoxin. Adv Bio Tech. 2009;8:13–6. [Google Scholar]
- 37. El-Shanshoury AR, El-Sabbagh SM, Emara HA, Saba HE. Occurrence of molds, toxicogenic capability of Aspergillus flavus and levels of aflatoxins in maize, wheat, rice and peanut from markets in central delta provinces. Egypt. Int J Curr Microbiol App Sci. 2014;3:852–65. [Google Scholar]
- 38. Al-Kahtani DFM. Isolation of fungi and their mycotoxin extract from stored wheat and other grains importer in Saudi Arabia. Am J Food Tech. 2014;9:370–6. [Google Scholar]
- 39. Schroeder HW, Jr, Hein H. Aflatoxins: production of the toxins in vitro in relation to temperature. Appl Microbiol. 1967;15:441–5. doi: 10.1128/am.15.2.441-445.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Climatemps.com. [accessed 25, 06, 15]. http://www.pakistan.climatemps.com .
- 41. Cotty PJ, Jaime-Garcia R. Influences of climate on aflatoxin producing fungi and aflatoxin contamination. Int J Food Microbiol. 2007;119:109–15. doi: 10.1016/j.ijfoodmicro.2007.07.060. [DOI] [PubMed] [Google Scholar]
- 42. Abdullah N, Nawawi A, Othman I. Survey of fungal counts and natural occurrence of aflatoxins in Malaysian starch-based foods. Mycopathologia. 1998;143:53–8. doi: 10.1023/a:1006945514876. [DOI] [PubMed] [Google Scholar]
- 43. Aydin A, Gunsen U, Demirel S. Total aflatoxin, aflatoxin B1 and ochratoxin A levels in Turkish wheat flour. J Food Drug Anal. 2008;16:48–53. [Google Scholar]
- 44. Taheri N, Semnani S, Roshandel G, Namjoo M, Keshavarzian H, Chogan AG, Ghasemi Kebria F, Joshaghani H. Aflatoxin contamination in wheat flour samples from Golestan province, northeast of Iran. Iranian J Public Health. 2012;41:42–7. [PMC free article] [PubMed] [Google Scholar]
- 45. Riba A, Bouras N, Mokrane S, Mathieu F, Lebrihi A, Sabaou N. Aspergillus section Flavi and aflatoxins in Algerian wheat and derived products. Food Chem Toxicol. 2010;48:2772–7. doi: 10.1016/j.fct.2010.07.005. [DOI] [PubMed] [Google Scholar]