Abstract
Acarbose is an antidiabetic drug which acts by inhibiting α-amylase and α-glucosidase activities but with deleterious side effects. Gallic acid (GA) is a phenolic acid that is widespread in plant foods. We therefore investigated the influence of GA on α-amylase and α-glucosidase inhibitory properties of acarbose (in vitro). Aqueous solutions of acarbose and GA were prepared to a final concentration of 25μM each. Thereafter, mixtures of the samples (50% acarbose + 50% GA; 75% acarbose + 25% GA; and 25% acarbose + 75% GA) were prepared. The results revealed that the combination of 50% acarbose and 50% GA showed the highest α-glucosidase inhibitory effect, while 75% acarbose + 25% GA showed the highest α-amylase inhibitory effect. Furthermore, all the samples caused the inhibition of Fe2+-induced lipid peroxidation (in vitro) in rat pancreatic tissue homogenate, with the combination of 50% acarbose and 50% GA causing the highest inhibition. All the samples also showed antioxidant properties (reducing property, 2,2′-azino-bis (-3-ethylbenzthiazoline-6-sulphonate [ABTS*] and 1,1-diphenyl-2-picrylhydrazyl [DPPH] free radicals scavenging abilities, and Fe2+ chelating ability). Therefore, combinations of GA with acarbose could be employed as antidiabetic therapy, with a possible reduction of side effects of acarbose; nevertheless, the combination of 50% acarbose and 50% GA seems the best.
Keywords: acarbose, antidiabetic, combination therapy, gallic acid, plant foods
1. Introduction
Diabetes Mellitus (DM) commonly referred to as diabetes is a group of metabolic diseases characterized by hyperglycemia (high blood glucose levels over a prolonged period), either because the pancreas does not produce enough insulin, or because the cells do not respond to the insulin that is produced [1]. The main symptoms of high blood glucose include polyuria (frequent urination), polydipsia (increased thirst), and polyphagia (increased hunger). If left untreated, this chronic disease can cause many complications [2]. Type 2 DM (T2DM) is the most common form of diabetes [3]; it is characterized by insulin resistance, which may be combined with relatively reduced insulin secretion [1], leading to hyperglycemia and ultimately malfunctioning of the pancreatic β-cells. Prolonged hyperglycemia results in increased generation of reactive oxygen species (ROS) and impairment of endogenous antioxidants [4]. Oxidative stress resulting from the hyperglycemic condition in T2DM has been implicated in the impairment of the pancreatic β-cells and diabetes complications such as diabetes nephropathy [5], diabetes retinopathy [6,7], and diabetes neuropathy [8].
A practical approach to reducing the postprandial hyperglycemia is to retard the absorption of carbohydrates after food intake [9]. This could be achieved through the inhibition of α-amylase and α-glucosidase present in the gastrointestinal tract [10]. Inhibitors of these enzymes slow down carbohydrate digestion time, causing a reduction in the rate of glucose absorption and consequently blunting the postprandial plasma glucose rise [11]. The dietary polysaccharides are first broken down to monosaccharides by certain gastrointestinal enzymes, since only monosaccharides can be absorbed from the intestinal lumen. Polysaccharides are hydrolyzed to oligosaccharides and disaccharides by α-amylase and intestinal α-glucosidase further hydrolyzes it to glucose before being absorbed into the intestinal epithelium entering the blood circulation [12]. Therefore, therapeutic inhibitors of α-glucosidase and α-amylase such as acarbose, miglitol, voglibose, nojirimycin, and 1-deoxynojirimycin have been developed for the management of hyperglycemia and T2DM [9].
Acarbose is an oral α-glucosidase and α-amylase inhibitor used in the management of T2DM [13,14]. Acarbose is a complex oligosaccharide known to reduce and slow down the intestinal absorption of glucose, which subsequently reduces the postprandial rise of blood glucose levels in T2DM patients [15]. T2DM patients are often placed on a recommended acarbose starter dose of 50 mg thrice daily, which may be increased to a maintenance dose of 100–200 mg thrice daily after 6–8 weeks if necessary [13]. Nevertheless, the use of acarbose is not without its attendant side effects; the major ones being diarrhea and flatulence as a result of prolonged inhibition of starch hydrolysis [16]. Furthermore, excessive pancreatic α-amylase inhibition as a result of prolonged use of acarbose can result in the accumulation of undigested carbohydrate in the colon and serving as substrate for bacteria fermentation [16,17]; the consequences of this are severe gastrointestinal complications such as flatulence, diarrhea, and abdominal distention [16].
In recent years, there has been an increased interest in the potential applications of antioxidants for medicinal purposes, as information linking the pathogenesis of several human diseases to oxidative stress abounds [18]. Natural food sources are known to contain antioxidant compounds that can scavenge free radicals and these dietary antioxidants have generated particular interest as defenses against degenerative diseases [19,20]. Phenolic compounds are found abundant in many plant foods and most of them have shown antioxidant properties in both in vitro [21] and in vivo studies [22]. Gallic acid or 3,4,5-trihydroxybenzoic acid (Figure 1) is a phenolic acid which is abundantly distributed in many food sources such as fruits, vegetables, spices, and tea [23]. Recent studies have documented that gallic acid and its esters [e.g., (−)-epigallocatechin-3-gallate] exert antioxidant, anticancer, antiviral, and many other biological effects [24,25]. Consequently, this study investigated the effect of gallic acid on the inhibitory properties of acarbose on the activities of key enzymes (α-glucosidase and α-amylase) linked to T2DM (in vitro).
Figure 1.
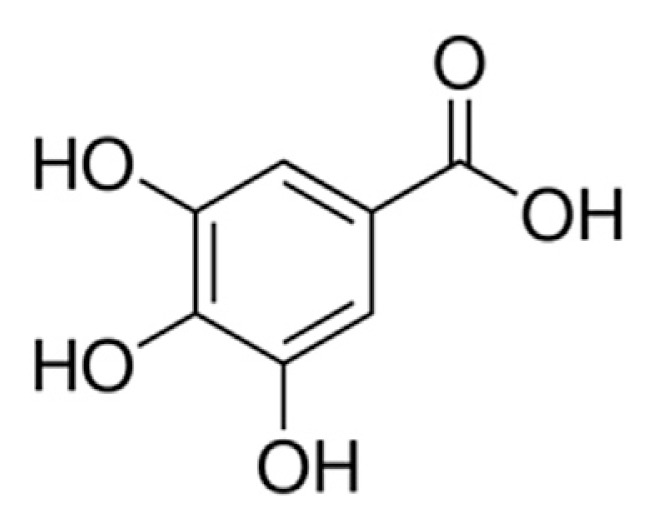
Structural formula of gallic acid (3,4,5-trihydroxybenzoic acid).
2. Methods
2.1. Sample collection
Acarbose was purchased from Glenmark Generics Pharmaceutical Europe Limited, Mumbai, India. Gallic acid was purchased from Sigma Aldrich Co. (St. Louis, MO, USA).
2.2. Chemicals and reagents
Chemical reagents such as Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), 2, 2-diphenyl-1picrylhydrazyl (DPPH), thiobarbituric acid, gallic acid, porcine pancreatic α-amylase, and 1,10-phenanthroline were procured from Sigma Aldrich Co. Trichloroacetic acid was sourced from Sigma Aldrich, Chemie GmbH (Steinheim, Germany). Hydrogen peroxide, methanol, acetic acid, hydrochloric acid, aluminium chloride, potassium acetate, sodium dodecyl sulfate, iron (II) sulfate, potassium ferrycyanide, and ferric chloride were sourced from BDH Chemicals Ltd., (Poole, England, UK). Ascorbic acid and starch were products of Merck (Darmstadt, Germany). Except stated otherwise, all other chemicals and reagents were of analytical grades and the water used was glass distilled.
2.3. Sample preparation
Acarbose and gallic acid were dissolved in distilled water to a final concentration of 25μM. Thereafter, sample mixtures were prepared as follows: S1 = 100% acarbose (25μM); S2 = 100% gallic acid (25μM); S3 = 50% acarbose + 50% gallic acid; S4 = 75% acarbose + 25% gallic acid; and S5 = 25% acarbose + 75% gallic acid. All samples were kept in the refrigerator at 4°C for subsequent analysis.
2.4. α-Glucosidase activity assay
Appropriate dilution of the sample (50 μL) and 100 μL of α-glucosidase solution (effective concentration 3.2.1.20; 1.0 U/ mL) in 0.1M phosphate buffer (pH 6.9) was incubated at 25°C for 10 minutes. Thereafter, 50 μL of 5mMp-nitrophenyl-α-D-glucopyranoside solution in 0.1M phosphate buffer (pH 6.9) was added. The mixtures were incubated at 25°C for 5 minutes and the absorbance was read at 405 nm in the spectrophotometer (Jenway 6305 model, Bibby Scientific, Staffordshine, United Kingdom). The α-glucosidase inhibitory activity was expressed as percentage inhibition [26].
2.5. α-Amylase activity assay
The aqueous sample dilution (500 μL) and 500 μL of 0.02M sodium phosphate buffer (pH 6.9 with 0.006M NaCl) containing 0.5 mg/mL Hog pancreatic α-amylase (effective concentration 3.2.1.1) were incubated at 25°C for 10 minutes. Thereafter, 500 μL of 1% starch solution in 0.02M sodium phosphate buffer (pH 6.9 with 0.006M NaCl) was added to each reaction mixture. The reaction mixtures were incubated at 25°C for 10 minutes and stopped with 1.0 mL of dinitrosalicylic acid color reagent. Thereafter, the mixture was incubated in a boiling water bath for 5 minutes, and cooled to room temperature. The reaction mixture was then diluted by adding 10 mL of distilled water, and the absorbance was measured at 540 nm. The reference samples included all other reagents and the enzyme with the exception of the test sample. The percentage enzyme inhibitory activity of the extract was subsequently calculated [27].
2.6. Fe2+ chelation assay
The Fe2+ chelating ability of the samples was determined using the modified method of Puntel et al [28]. Freshly prepared 500 μmol/L FeSO4 (150 μL) was added to a reaction mixture containing 168 μL of 0.1 mol/L Tris-HCl (pH 7.4), 218-μL saline, and the extract (0–100 μL). The reaction mixture was incubated for 5 minutes before the addition of 13 μL of 0.25% 1:10 phenanthroline (weight/volume). The absorbance was subsequently measured at 510 nm in a spectrophotometer. The Fe2+ chelating ability of the extract was subsequently calculated as percentage of the control.
2.7. Lipid peroxidation assay
2.7.1. Experimental animals
Albino rats weighing 200–210 g were purchased from the breeding colony of Department of Veterinary Medicine, University of Ibadan, Ibadan, Nigeria. Rats were maintained at 25°C, on a 12 hour light/12 hour dark cycle, with free access to food and water. They were acclimatized under these conditions for 1 week before the experiment.
2.7.2. Preparation of tissue homogenate
The rat was immobilized by cervical dislocation and the pancreatic tissue was rapidly isolated and placed on ice and weighed. This tissue was subsequently homogenized in cold saline (1/10 weight/volume) with about 10-up-and-down strokes at approximately 1200 rev/min in a Teflon glass homogenizer (Mexxcare, mc14 362, Aayushi Design Pvt. Ltd., Uttar Pradesh, India). The homogenate was centrifuged for 10 minutes at 3000g in a refrigerated centrifuge (KX3400C, KEN-XIN Intl. Co., Hong Kong) at 4°C to yield a pellet that was discarded, and a low-speed supernatant (LSS), which was kept for a lipid peroxidation assay [29].
2.7.3. Inhibition of lipid peroxidation and thiobarbituric acid reactions
The lipid peroxidation assay was carried out using the modified method of Ohkawa et al [30]. Briefly 100 μL of LSS fraction was mixed with a reaction mixture containing 30 μL of 0.1M pH 7.4 Tris-HCl buffer, extract (0–100 μL), and 30 μL of 250μM freshly prepared FeSO4. The volume was made up to 300 μL by water before incubation at 37°C for 1 hour. The color reaction was developed by adding 300 μL 8.1% sodium dodecyl sulfate to the reaction mixture containing LSS and this was subsequently followed by the addition of 600 μL of acetic acid/HCl (pH 3.4) mixture and 600-μL 0.8% thiobarbituric acid. This mixture was incubated at 100°C for 1 hour. The thiobarbituric acid reactive species produced was measured at 532 nm in a UV/visible spectrophotometer (Jenway 6305 model, Jenway 6305 model, Bibby Scientific, Staffordshine, United Kingdom) and expressed using malondialdehyde (MDA) equivalent.
2.8. Determination of ferric reducing antioxidant property
The reducing property of the samples was determined by assessing its ability to reduce a FeCl3 solution as described by Pulido et al [31]. A 2.5-mL aliquot of the extract was mixed with 2.5 mL of 200mM sodium phosphate buffer (pH 6.6) and 2.5 mL of 1% potassium ferricyanide. The mixture was incubated at 50°C for 20 minutes and thereafter 2.5 mL of 10% trichloroacetic acid was added. This mixture was centrifuged at 805g for 10 minutes; 5 mL of the supernatant was mixed with an equal volume of water and 1 mL of 0.1% ferric chloride. The absorbance was measured at 700 nm in the spectrophotometer after allowing the solution to stand for 30 minutes. The reducing property was subsequently calculated using an ascorbic acid equivalent (AAE).
2.9. 2, 2′-Azino-bis(-3-ethylbenzthiazoline-6-sulphonate ) scavenging ability
Trolox equivalent antioxidant capacity (TEAC) of the samples was determined by their 2, 2′-azino-bis(-3-ethylbenzthia zoline-6-sulphonate (ABTS+) scavenging ability according to the method described by Re et al [32]. The ABTS+ was generated by reacting (7 mmol/L) ABTS+ aqueous solution with K2S2O8 (2.45 mmol/L, final concentration) in the dark for 16 hours and adjusting the absorbance at 734 nm to 0.700 in a spectrophotometer with ethanol. An appropriate dilution of 0.2 mL extract was added to 2.0 mL ABTS+ solution and the absorbance was measured at 734 nm in a spectrophotometer after 15 minutes. The Trolox equivalent antioxidant capacity was subsequently calculated using Trolox as the standard.
2.10. 1, 1-Diphenyl–2-picrylhydrazyl) free radical scavenging ability
The scavenging ability of the extracts against 1, 1-diphenyl–2-picrylhydrazyl (DPPH) free radical was evaluated as described by Gyamfi et al [33] with slight modifications. One milliliter of 0.4mM DPPH in methanol was mixed with 0.05 mL of the extract. The mixture was left in the dark for 30 minutes and the absorbance was measured at 516 nm in the spectrophotometer. The DPPH free radical scavenging ability was subsequently calculated as percentage of the control.
2.11. Statistical analysis
The results of replicate readings were pooled and expressed as mean ± standard deviation and the least significance difference [34]. One-way analysis of variance was used to compare the mean and significance was accepted at p < 0.05.
3. Results
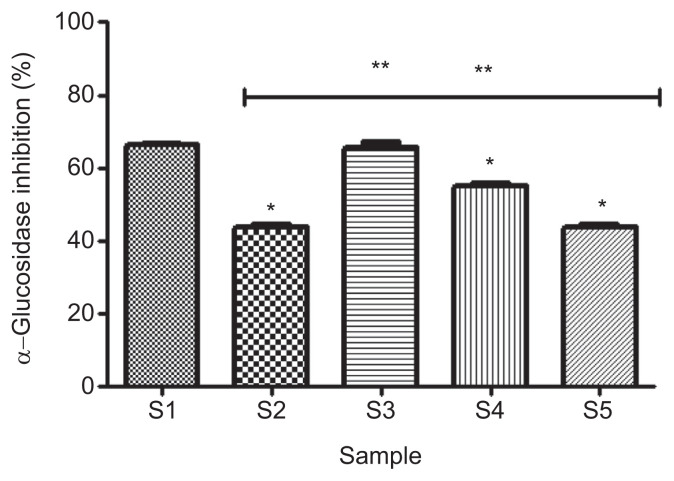
The effect of gallic acid on α-glucosidase inhibitory properties of acarbose (Figure 2) revealed that 100% acarbose (S1) had significantly higher (p < 0.05) inhibitory effect (66.2 ± 0.7%) than 100% gallic acid (S2; 43.9 ± 0.7%). However, considering the combinations, a combination of 50% acarbose and 50% gallic acid (S3) showed the highest significant (p < 0.05) inhibitory effect (65.7 ± 1.4%), which was not significantly different (p > 0.05) from the inhibitory effect of 100% acarbose.
Figure 2.
Effect of gallic acid on α-glucosidase inhibitory ability of acarbose (in vitro). Bars represent mean ± standard deviation of triplicate readings (n =3). * Mean values are significantly different (p < 0.05) compared with S1. ** Mean values are significantly different (p < 0.05) compared with S2. S1 =100% acarbose (25μM); S2 =100% gallic acid (25μM); S3 =50% acarbose +50% gallic acid; S4 =75% acarbose +25% gallic acid; S5 =25% acarbose +75% gallic acid.
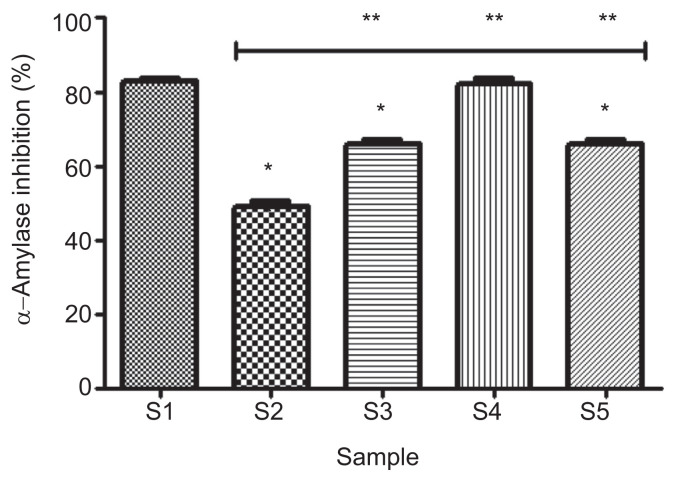
The effect of gallic acid on α-amylase inhibitory activities of acarbose is presented in Figure 3. The result revealed that 100% acarbose (S1) had significantly higher (p < 0.050 enzyme inhibitory effect (82.8 ± 0.7%) than 100% gallic acid (S2; 49.0 ± 1.4%). It also showed that of the various combinations, 75% acarbose and 25% gallic acid (S4) had the highest significant (p < 0.05) inhibitory effect (82.2 ± 1.6%); there was however, no significant difference (p > 0.05) in the inhibitory effect of S4 and S1.
Figure 3.
Effect of gallic acid on α-amylase inhibitory ability of acarbose (in vitro). Bars represent mean ± standard deviation of triplicate readings (n =3). * Mean values are significantly different (p < 0.05) compared with S1. ** Mean values are significantly different (p < 0.05) compared with S2. S1 =100% acarbose (25μM); S2 =100% gallic acid (25μM); S3 =50% acarbose +50% gallic acid; S4 =75% acarbose +25% gallic acid; S5 =25% acarbose +75% gallic acid.
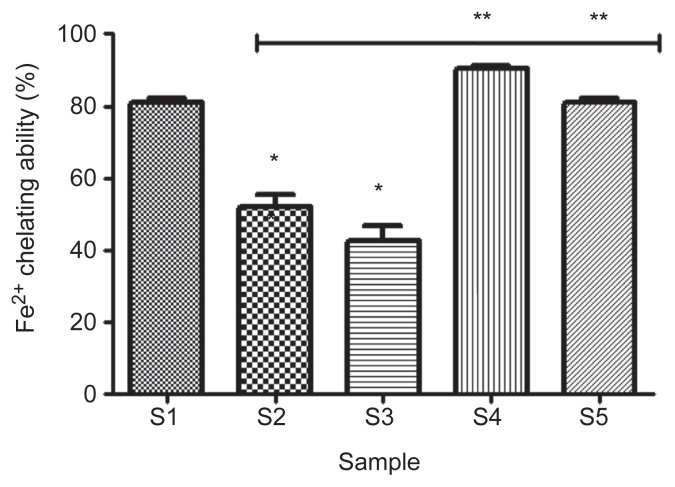
The result of the Fe2+ chelating ability, as shown in Figure 4, revealed that 100% acarbose (S1) had significantly higher (p < 0.05) chelating ability (80.9 ± 1.3%) compared with 100% gallic acid (S2; 52.3 ± 3.2%). However, considering the combinations, a combination of 75% acarbose and 25% gallic acid (S4) had the highest chelating ability (90.5 ± 0.6%) compared with other combinations, but was not significantly different (p > 0.05) from the chelating ability (80.9 ± 1.3%) of the combination of 25% acarbose and 75% gallic acid (S5).
Figure 4.
Effect of gallic acid on Fe2+ chelating ability of acarbose (in vitro). Bars represent mean ± standard deviation of triplicate readings (n =3). * Mean values are significantly different (p < 0.05) compared with S1. ** Mean values are significantly different (p < 0.05) compared with S2. S1 =100% acarbose (25μM); S2 =100% gallic acid (25μM); S3 =50% acarbose +50% gallic acid; S4 =75% acarbose +25% gallic acid; S5 =25% acarbose +75% gallic acid.
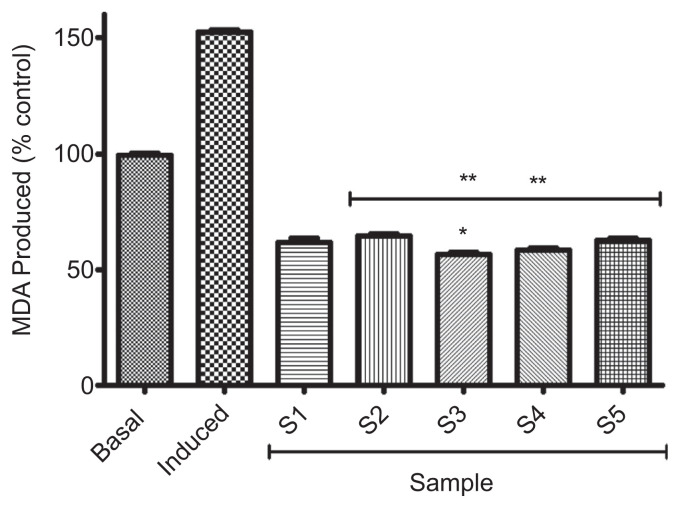
Incubation of rat’s pancreatic tissue homogenate in the presence of 250μM FeSO4 induced a significant (p < 0.05) increase (152.6 ± 0.7%) in the MDA content (Figure 5). However, introduction of all the samples (S1–S5) inhibited lipid peroxidation in the pancreatic tissue homogenates by causing a significant (p < 0.05) reduction in the MDA content; nevertheless, there was no significant difference (p > 0.05) in the inhibitory effects of 100% acarbose (S1; 61.7 ± 2.2%) and 100% gallic acid (S2; 64.8 ± 0.7%). Considering the combinations, a combination of 50% acarbose and 50% gallic acid (S3) caused the highest significant (p < 0.05) reduction in the MDA content (56.6 ± 0.7%).
Figure 5.
Effect of gallic acid on inhibition of Fe2+ induced lipid peroxidation in rat’s pancreas (in vitro) by acarbose. Bars represent mean ± standard deviation of triplicate readings (n =3). * Mean values are significantly different (p < 0.05) compared with S1. ** Mean values are significantly different (p < 0.05) compared with S2. MDA =malondialdehyde; S1 =100% acarbose (25μM); S2 =100% gallic acid (25μM); S3 =50% acarbose +50% gallic acid; S4 =75% acarbose +25% gallic acid; S5 =25% acarbose +75% gallic acid.
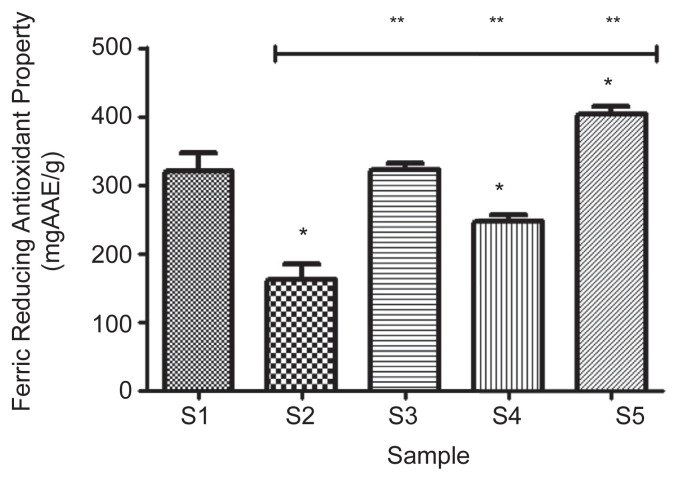
The ferric reducing antioxidant properties of the samples (S1–S5) were presented as AAE in Figure 6. The result revealed that 100% acarbose had the highest significant (p < 0.05) reducing property (319.7 ± 26.9 mgAAE/g) compared with 100% gallic acid (162.1 ± 22.5 mgAAE/g). However, a combination of 25% acarbose and 75% gallic acid (S5) had the highest significant (p < 0.05) reducing property (403.2 ± 11.4 mgAAE/g) compared with the other combinations, while the reducing property of the combination of 50% acarbose and 50% gallic acid (S3; 321.9 ± 10.1 mgAAE/g) showed no significant difference (p > 0.05) to that of 100% acarbose.
Figure 6.
The effect of gallic acid on ferric reducing antioxidant properties of acarbose (in vitro). Bars represent mean ± standard deviation of triplicate readings (n =3). * Mean values are significantly different (p < 0.05) compared with S1. ** Mean values are significantly different (p < 0.05) compared with S2. AAE =ascorbic acid equivalent; S1 =100% acarbose (25μM); S2 =100% gallic acid (25μM); S3 =50% acarbose +50% gallic acid; S4 =75% acarbose +25% gallic acid; S5 =25% acarbose +75% gallic acid.
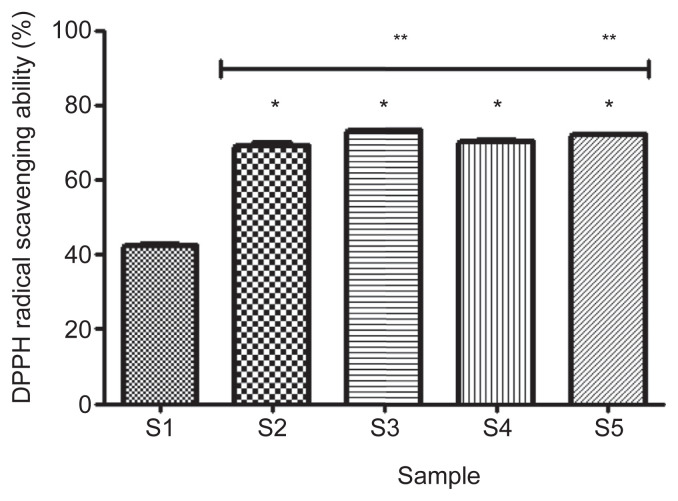
The result of the DPPH free radical scavenging ability (Figure 7) revealed that 100% gallic acid (S2) had significantly higher (p < 0.05) scavenging ability (69.2 ± 0.5%) compared with 100% acarbose (S1; 42.5 ± 0.3%). However, a combination of 50% acarbose and 50% gallic acid (S3) has the highest significant (p < 0.05) scavenging ability (73.2 ± 0.1%), but not significantly different (p > 0.05) from the scavenging ability (72.1 ± 0.1%) of the combination of 25% acarbose and 75% gallic acid (S5).
Figure 7.
Effect of gallic acid on 2, 2-diphenyl-1picrylhydrazyl (DPPH) radical scavenging ability of acarbose (in vitro). Bars represent mean ± standard deviation of triplicate readings (n =3). * Mean values are significantly different (p < 0.05) compared with S1. ** Mean values are significantly different (p < 0.05) compared with S2. S1 =100% acarbose (25μM); S2 =100% gallic acid (25μM); S3 =50% acarbose +50% gallic acid; S4 =75% acarbose +25% gallic acid; S5 =25% acarbose +75% gallic acid.
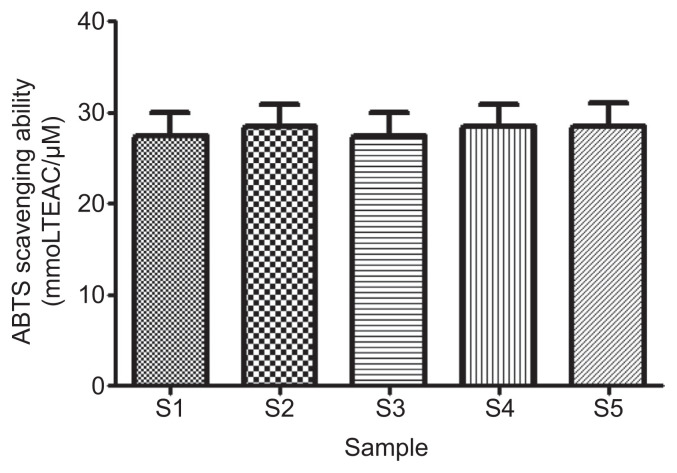
ABTS+ scavenging ability of the samples presented as Trolox equivalent antioxidatant capacity (Figure 8) revealed that there was no significant difference (p > 0.05) in the ABTS* scavenging ability of all the samples (S1 = 25.67 ± 3.55 mmol-TEAC/g; S2 = 26.61 ± 3.55 mmolTEAC/g; S3 = 25.69 ± 3.55 mmoLTEAC/g; S4 = 26.63 ± 3.55 mmoLTEAC/g; and S5 = 26.67 ± 3.55 mmoLTEAC/g).
Figure 8.
Effect of gallic acid on 2′-azino-bis(-3-ethylbenzthiazoline-6-sulfonate (ABTS) radical scavenging ability of acarbose (in vitro). Bars represent mean ± standard deviation of triplicate readings (n =3). S1 =100% acarbose (25μM); S2 =100% gallic acid (25μM); S3 =50% acarbose +50% gallic acid; S4 =75% acarbose +25% gallic acid; S5 =25% acarbose +75% gallic acid. TEAC =Trolox equivalent antioxidant capacity.
4. Discussion
Hyperglycemia is a condition of abnormal rise in blood glucose level and is an etiology of T2DM; a disease caused by insulin resistance [35,36]. Acarbose is one of the therapeutic drugs used to reduce postprandial hyperglycemia in T2DM patients, but several side effects are observed during long term use [16,17]. Although the recommended therapeutic dose of acarbose ranges from 150 mg/d to 600 mg/d depending on the stage and severity of the diabetic condition [13], studies have however, shown that the systemic absorption of acarbose is < 2% after oral dosing [15]; hence, the choice of a low concentration of acarbose in this study. We therefore hypothesize that combinations of acarbose with diets rich in antidiabetic and antioxidant phenolic phytochemicals, such as gallic acid, could help produce synergistic effects at reducing postprandial rise in blood glucose level while at the same time, offering possible reduction in the attendant side effects of this drug. This is more so because previous studies have reported the antihyperglycemic and antioxidant properties of phenolic rich food sources [37–39].
This study has revealed that acarbose, gallic acid, and their various combinations caused the inhibition of both α-amylase and α-glucosidase activities in vitro with 100% acarbose exhibiting stronger inhibitory activities than the 100% gallic acid. A previous report has shown that acarbose serves as a strong inhibitor of enzymes associated with carbohydrate hydrolysis compared with phenolic compounds [9]. Specifically, as presented in Figure 2, the combination of acarbose and gallic acid in equal proportions had a higher α-glucosidase inhibitory effect compared with 100% acarbose, a drug known to be a strong inhibitor of α-glucosidase. Therefore, this combination could be said to be synergistic, and could hence serve as a possible combinatory therapy in preference to 100% acarbose to possibly reduce the several side effects associated with the use of acarbose.
Furthermore, the inhibitory effect of acarbose, gallic acid, and their various combinations on α-amylase activity, showed that acarbose inhibits α-amylase significantly more than it inhibits α-glucosidase activity. Nevertheless, it exhibits a stronger inhibiting property compared with gallic acid, which showed mild inhibition of α-amylase. These observations could be responsible in part for the side effects of acarbose and are also in agreement with previous reports which indicated that excessive inhibition of pancreatic α-amylase could result in the abnormal bacterial fermentation of undigested carbohydrates in the colon; and, therefore, mild α-amylase inhibition activity is desirable [40]. Therefore, since the combination of acarbose and gallic acid at equal proportion serves as a mild inhibitor of α-amylase and strong inhibitor of α-glucosidase, it could be of great therapeutic importance in addressing the side effects associated with acarbose in the management of T2DM, which is linked with excess inhibition of α-amylase. Also, the combination of acarbose and gallic acid at the ratio of 75:25 shows a synergistic inhibition of α-amylase. Hence, the inhibition of these enzymes (α-glucosidase and α-amylase) using the various combinations of acarbose and gallic acid could be very effective in reducing hyperglycemia and, hence, T2DM.
In addition, all the samples were able to chelate Fe2+, with the combinations of acarbose and gallic acid in the ratios of 75:25 and 25:75 having more Fe2+ chelating ability among the various combinations. Fe2+ catalyzes one electron transfer reactions that generates ROS such as the OH, which is formed from H2O2 through the Fenton reaction. Iron causes lipid peroxidation and also decomposes the lipid peroxides, which leads to the generation of peroxyl and alkoxyl radicals, favoring the propagation of lipid peroxidation [9]. In this study, the incubation of rat pancreatic tissue homogenates in the presence of 250 μM FeSO4 caused a significant (p < 0.05) increase in the MDA content. Previous studies have also shown that incubation of rat tissues in the presence of 250 μM FeSO4 solution caused a significant increase in their MDA content [9,37]. However, the introduction of acarbose, gallic acid, and their various combinations caused significant (p < 0.05) decrease in the MDA content of the incubated pancreatic tissue homogenate as shown in Figure 5. The possible mechanism by which the samples protect against lipid peroxidation could be by Fe2+ chelation [41]. Therefore, the decrease in the pancreatic MDA content by acarbose, gallic acid, and their various contributions could be attributed to their Fe2+ chelating properties. However, a combination of 50% acarbose and 50% gallic acid had the highest inhibitory effect on MDA production.
According to Li et al [42], pancreatic β-cells are vulnerable to oxidative damage caused by ROS due to their limited anti-oxidant defense systems. Antioxidants as diets, supplements, or as nutraceuticals could help protect against the oxidative damage and thus prevent diabetes and its complications. Persistent hyperglycemia may induce free radical production via glycation and autoxidation. This free radical production contributes to β-cells destruction in T2DM [43].
The ability of phytochemicals possessing antioxidant properties to reduce oxidative species has been shown to be one of their antioxidative mechanisms [44]. The reducing property of the samples was assessed based on their ability to reduce Fe3+ to Fe2+. The result reveals that the 100% acarbose exhibits a higher ferric reducing ability than 100% gallic acid. Of the various combinations, a combination of 25% acarbose and 75% gallic acid shows the highest reducing property and hence a synergistic effect. These observations could possibly be attributed to the synergistic chemical reaction between the phenolic acid (gallic acid) and acarbose.
Furthermore, this study also revealed that the samples scavenged DPPH free radicals. One-hundred percent gallic acid exhibited a higher radical scavenging ability than 100% acarbose; nevertheless, a combination of 50% acarbose and 50% gallic acid showed the highest scavenging ability, hence portraying a possible synergy in the DPPH free radical scavenging abilities of acarbose and gallic acid. The antioxidant property of gallic acid has been previously reported [23]; hence, the presence of a phenolic compound (gallic acid) in the various combinations could have enhanced their DPPH scavenging abilities. The ability of gallic acid to act as a free radical scavenger is due to the presence of multiple hydroxyl groups in each compound which are able to donate their protons to finally break the chain reaction of the free radicals [45]. The ABTS radical scavenging ability result (Figure 8) reveals that there was no significant difference in the ABTS* scavenging ability of all the samples (acarbose, gallic acid, and their various combinations). Nevertheless, the samples were able to scavenge the ABTS+ radical.
The free radical (ABTS+ and DPPH) scavenging abilities of gallic acid, acarbose, and their various combinations could be attributed to a structure–function relationship; interactions among their hydroxyl groups could contribute significantly to their free radicals scavengign abilities [39].
5. Conclusion
Conclusively, we have been able to show that gallic acid influenced the in-vitro inhibitory properties of acarbose on key enzymes (α-glucosidase and α-amylase) linked to T2DM. The antioxidant properties of acarbose–gallic acid combinations could also be of unique therapeutic importance in managing oxidative stress which is linked to diabetes. Therefore, combination of gallic acid with acarbose could be employed in the management of T2DM, with the comparative advantage of a possible reduction of the side effects of acarbose; nevertheless, the combination of 50% acarbose and 50% gallic acid seems the best. In view of this, diets rich in gallic acid could serve as a means of managing diabetes. Furthermore, in cases of chronic diabetes where drugs are to be taken, diets rich in gallic acid could be recommended with acarbose to reduce the side effects associated with the drug; however, further in vivo studies and clinical trials are recommended.
Footnotes
Conflicts of interest
The authors have no conflicts of interest to declare.
REFERENCES
- 1.David G, Gardner D. Greenspan’s basic & clinical endocrinology. 9th ed. New York: McGraw-Hill Medical; 2011. [Google Scholar]
- 2. Cooke DW, Plotnick L. Type 1 diabetes mellitus in pediatrics. Pediatr Rev. 2008;29:374–84. doi: 10.1542/pir.29-11-374. [DOI] [PubMed] [Google Scholar]
- 3. Dehghan H, Sarrafi Y, Salehi P. Antioxidant and antidiabetic activities of 11 herbal plants from Hyrcania region, Iran. J Food Drug Anal. 2016;24:179–88. doi: 10.1016/j.jfda.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ohkuwa T, Sato Y, Naoi M. Hydroxyl radical formation in diabetic fats induced by streptozotocin. Life Sci. 1995;56:1789–98. doi: 10.1016/0024-3205(95)00150-5. [DOI] [PubMed] [Google Scholar]
- 5. Wu CC, Hung CN, Shin YC, Wang CJ, Huang HP. Myrciaria cauliflora extracts attenuate diabetic nephropathy involving the Ras signaling pathway in streptozotocin/nicotinamide mice on a high fat diet. J Food Drug Anal. 2016;24:136–46. doi: 10.1016/j.jfda.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bearse A, Marcus J, Ying Han, Marilyn E, Schneck SB, Carl J, Anthony JA. Local multifocaloscillatory potential abnormalities in diabetes and early diabetic retinopathy. Invest Opthamol Vis Sci. 2004;45:3259–65. doi: 10.1167/iovs.04-0308. [DOI] [PubMed] [Google Scholar]
- 7. Hove MN, Kristensen JK, Lauritzen T, Bek T. The prevalence of retinopathy in anunselected population of type-2 diabetes patients from Arhus County,. Denmark. Acta Ophthamol Scand. 2004;82:443–8. doi: 10.1111/j.1600-0420.2004.00270.x. [DOI] [PubMed] [Google Scholar]
- 8. Seki M, Tanaka T, Nawa H, Usui T, Fukuchi T, Ikeda K, Abe H, Takei N. Involvement of brain-derived neurotrophic factor in early retinal neuropathy of streptozotocin-induced diabetes in rats: therapeutic potential of brain-derived neurotrophic factors for dopaminergic amacrine cells. Diabetes. 2004;53:2412–9. doi: 10.2337/diabetes.53.9.2412. [DOI] [PubMed] [Google Scholar]
- 9. Oboh G, Olabiyi AA, Akinyemi AJ, Ademiluyi AO. Inhibition of key enzymes linked to type 2 diabetes and sodium nitroprusside-induced lipid peroxidation in rat pancreas by water-extractable phytochemicals from unripe pawpaw fruit (Carica papaya) J Basic Clin Physiol Pharmacol. 2014;25:21–34. doi: 10.1515/jbcpp-2013-0002. [DOI] [PubMed] [Google Scholar]
- 10. Shim YJ, Doo HK, Ahn SY, Kim YS, Seong JK, Park S, Min BM. Inhibitory effect of aqueous extract from the gall of Rhuz chinensis on alpha-glucosidase activity and posprandial blood glucose. J Ethnopharmacol. 2003;85:283–7. doi: 10.1016/s0378-8741(02)00370-7. [DOI] [PubMed] [Google Scholar]
- 11.Rhabasa-Lhoret R, Chiasson JL. Alpha-glucosidase inhibition. In: Defronzo RA, Ferrannini E, Keen H, Zimmet P, editors. International text book of diabetes mellitus. 3rd ed. Hoboken, New Jersy, UK: John Wiley; 2004. pp. 901–14. [Google Scholar]
- 12. Oboh G, Ademiluyi AO, Faloye YM. Effect of combination on the antioxidant and inhibitory properties of tropical pepper against α-amylase and α-glucosidase activities in vitro. J Med Food. 2011;14:1152–8. doi: 10.1089/jmf.2010.0194. [DOI] [PubMed] [Google Scholar]
- 13. Balfour JA, McTavish D. Acarbose. Drugs. 1993;46:1025–54. doi: 10.2165/00003495-199346060-00007. [DOI] [PubMed] [Google Scholar]
- 14. Ademiluyi AO, Oboh G. Aqeuous extracts of roselle (Hibiscus sabdariffa Linn) varieties inhibits α-amylase and α-glucosidase activities in vitro. J Med food. 2013;16:88–93. doi: 10.1089/jmf.2012.0004. [DOI] [PubMed] [Google Scholar]
- 15. Li S. Pharmacodynamic bioequivalence testing. J Clin Pharm Ther. 2012;37:497–8. doi: 10.1111/j.1365-2710.2012.01338.x. [DOI] [PubMed] [Google Scholar]
- 16. Kim JG, Jo SH, Ha KS, Kim SC, Kim YC, Apostolidis E, Kwon YI. Effect of long-term supplementation of low molecular weight chitosan oligosaccharide (GO2KA1) on fasting blood glucose and HbA1c in db/db mice model and elucidation of mechanism of action. BMC Complement Altern Med. 2014;14:272. doi: 10.1186/1472-6882-14-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee KH, Ha KS, Jo SH, Lee CM, Kim YC, Chung KH, Kwon YI. Effect of long-term dietary arginyl-fructose (AF) on hyperglycemia and HbA1c in diabetic db/db mice. Int J Mol Sci. 2014;15:8352–9. doi: 10.3390/ijms15058352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Giustarini D, Dalle-Donne I, Tsikas D, Rossi R. Oxidative stress and human disease: Origin, link, measurement, mechanisms, and biomarkers. Crit Rev in Clin Lab Sci. 2009;46:241–81. doi: 10.3109/10408360903142326. [DOI] [PubMed] [Google Scholar]
- 19. Yen HF, Hsieh CT, Hsieh TJ, Chang FR, Wang CK. In vitro antidiabetic effect and chemical component analysis of 29 essential oils products. J Food Drug Anal. 2015;23:124–9. doi: 10.1016/j.jfda.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oboh G, Bello FO, Ademosun AO. Hypocholesterolemic properties of grapefruit (Citrus paradisii) and shaddock (Citrus maxima) juices and inhibition of angiotensin-1-converting enzyme activity. J Food Drug Anal. 2014;22:477–84. doi: 10.1016/j.jfda.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chiu YW, Lo HJ, Huang HY, Chao PY, Hwang JM, Huang PY, Huang SJ, Liu JY, Lai TJ. The antioxidant and cytoprotective activity of Ocimum gratissimum extracts against hydrogen peroxide-induced toxicity in human HepG2 cells. J Food Drug Anal. 2013;21:253–60. [Google Scholar]
- 22. Spadiene A, Savickiene N, Ivanauskas L, Jakstas V, Skesters A, Silova A, Rodovicius H. Antioxidant effects of Camellia sinensis L. extract in patients with type 2 diabetes. J Food Drug Anal. 2014;22:505–11. doi: 10.1016/j.jfda.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Adefegha SA, Oboh G, Ejakpovi1 II, Oyeleye SI. Antioxidant and antidiabetic effects of gallic and protocatechuic acids: a structure–function perspective. Comp Clin Pathol. 2015;24:1579–85. [Google Scholar]
- 24. Chen GH, Lin YL, Hsu WL, Hsieh SK, Tzen JT. Significant elevation of antiviral activity of strictinin from Pu’er tea after thermal degradation to ellagic acid and gallic acid. J Food Drug Anal. 2015;23:116–23. doi: 10.1016/j.jfda.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sameermahmood Z, Raji L, Saravanan T, Vaidya A, Mohan V, Balasubramanyam M. Gallic acid protects RINm5F b-cells from glucolipotoxicity by its antiapoptotic and insulin-secretagogue actions. Phytother Res. 2010;24:S83–94. doi: 10.1002/ptr.2926. [DOI] [PubMed] [Google Scholar]
- 26. Apostolidis E, Kwon YI, Shetty K. Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Innov Food Sci Emerg Technol. 2007;8:46–54. [Google Scholar]
- 27.Worthington V. Alpha amylase. In: Worthington V, editor. Worthington enzyme manual. Freehold, NJ: Worthington Biochemical Corp; 1993. pp. 36–41. [Google Scholar]
- 28. Puntel RL, Nogueira CW, Rocha JBT. Krebs cycle intermediates modulate thiobarbituric acid reactive species (TBARS) production in rat brain in vitro. Neurochem Res. 2005;30:225–35. doi: 10.1007/s11064-004-2445-7. [DOI] [PubMed] [Google Scholar]
- 29. Belle NAV, Dalmolin GD, Fonini G, Rubim MA, Rocha JBT. Polyamines reduce lipid peroxidation induced by different pro-oxidant agents. Brain Res. 2004;1008:245–51. doi: 10.1016/j.brainres.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 30. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Ann Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 31. Pulido R, Bravo L, Saura-Calixto F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J Agric Food Chem. 2000;48:3396–402. doi: 10.1021/jf9913458. [DOI] [PubMed] [Google Scholar]
- 32. Re R, Pellegrini N, Proteggente A, Pannala A, Yamg M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–7. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 33. Gyamfi MA, Yonamine M, Aniya Y. Free-radical scavenging action of medicinal herbs from Ghana: Thonningia sanguinea on experimentally-induced liver injuries. Gen Pharmacol. 1999;32:661–7. doi: 10.1016/s0306-3623(98)00238-9. [DOI] [PubMed] [Google Scholar]
- 34.Zar JH. Biostatistical analysis. Upper Saddle River, New Jersy, New Jersey: Prentice-Hall; 1984. [Google Scholar]
- 35. Oritz-Andrade RR, Garcia-Jimenez S, Castilo-Espana P, Villalobos-Molina R, Estrado-Soto S. Alpha-glucosidase inhibitory activity of the methanolic extract from Tournefinan hartwegiana: an antihyperglycemic agent. J Ethnopharmacol. 2007;109:48–53. doi: 10.1016/j.jep.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 36. Jaspinder K. Comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:1–21. doi: 10.1155/2014/943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37. Ademiluyi AO, Oboh G, Aragbaiye FP, Oyeleye SI, Ogunsuyi OB. Antioxidant properties and in vitro α-amylase and α-glucosidase inhibitory properties of phenolics constituents from different varieties of Corchorus spp. J Taibah Univ Med Sci. 2015;10:278–87. [Google Scholar]
- 38. Mbhele N, Balogun FO, Kazeem MI, Ashafa T. In vitro studies on the antimicrobial, antioxidant and antidiabetic potential of Cephalaria gigantean. Bangladesh J Pharmacol. 2015;10:214–21. [Google Scholar]
- 39. Shori AB. Camel milk as a potential therapy for controlling diabetes and its complications: A review of in vivo studies. J Food Drug Anal. 2015;23:609–18. doi: 10.1016/j.jfda.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shai LJ, Masoko P, Mokgotho MP, Magano SR, Mogale AM, Boaduo N, Eloff JN. Yeast alpha glucosidase inhibitory and antioxidant activities of six medicinal plants collected in Phalaborwa, South Africa. South African J Botany. 2010;76:465–70. [Google Scholar]
- 41. Oboh G, Puntel RL, Rocha JBT. Hot pepper (Capsicum anuum, tepin and Capsicum Chinese, Habanero) prevents Fe2+-induced lipid peroxidation in brain–in vitro. Food Chem. 2007;102:178–85. [Google Scholar]
- 42. Li N, Figerio F, Machler P. The sensitivity of pancreatic β-cells to mitochondria injuries triggered by lipotoxicity & oxidative stress. Biochem Soc Trans. 2008;36:930–4. doi: 10.1042/BST0360930. [DOI] [PubMed] [Google Scholar]
- 43. Maria M, Maria G, Corihn M, Carmen D, Alexandra T. The sources of the targets of oxidative stress in the etiology of diabetic complications. Romanian J Biophys. 2007;17:63–84. [Google Scholar]
- 44. Islam MM. Biochemistry, medicinal and food values of jute (Corchorus capsularis L. and C. olitorious L.) leaf: a review. Int J Enhanced Res Sci Technol Eng. 2013;2:135–44. [Google Scholar]
- 45. Van Acker S, Van-den Berg D, Tromp M, Griffioen D, Van-Bennekom W, Van der Vijgh W, Bast A. Structural aspects of antioxidants activity of flavonoids. Free Radic Biol Med. 1996;20:331–42. doi: 10.1016/0891-5849(95)02047-0. [DOI] [PubMed] [Google Scholar]