Abstract
The profiles of bioactive compounds (including phenolics and flavonoids in free and bound fractions, anthocyanins, proanthocyanidins, vitamin E, and γ-oryzanol) of outer and inner rice bran from six colored rice samples collected from local markets were investigated. Proanthocyanidins could only be detected in red rice bran but not in black rice bran. The free fraction of the extracts dominated the total phenolics (72–92%) and the total flavonoids (72–96%) of colored rice bran. Most of the phenolic acids (83–97%) in colored rice bran were present in the bound form. Protocatechualdehyde was identified for the first time in the bound fraction of red rice bran by high performance liquid chromatography-photodiode array/electrospray ionization tandem mass spectrometry. The antioxidative activities of the free fraction of the colored rice bran were attributed to the proanthocyanidins in red colored rice and anthocyanins in black rice, while that of the bound fraction was mainly due to the phenolic acids.
Keywords: antioxidative activity, bran, colored rice, protocatechualdehyde
1. Introduction
Rice (Oryza sativa L.) is the most important cultured crop in Asia that has been used as a staple food for more than half of the population in the world. Some special rice cultivars have pigments in their pericarp and seed coat which exhibit colors of the pigments on the surface. These rice cultivars with unusual colors, such as red and black, are known as colored rice or pigmented rice. Although the colored rice has been considered as a nutritious food for weak people in traditional Chinese medicine, the breeding and cultivation of colored rice was discouraged and prohibited since early 19th century in Taiwan due to the agricultural policy of rice variety improvement [1].
Anthocyanins (ACNs) and proanthocyanidins (PA)/ condensed tannins are common pigments in black rice and red rice, respectively [2–4]. In addition, the health beneficial components of common rice, including sterols, γ-oryzanol, tocopherols, tocotrienols, and phenolic compounds, can be found in colored rice bran as well [5,6]. The major phenolic acids in rice include ferulic acid, p-coumaric acid, and diferulate, which exist especially in the outer layer of grains such as pericarp and aleurone [7]. Both types, bound and free form, of phenolics can be found in rice bran. The free-form phenolics can be simply extracted with methanol or other aqueous-organic solvents. The bound-form phenolics are bound with cell wall polysaccharides and lignins through ester or ether linkages, which need to be hydrolyzed with alkaline or acid for releasing [4,8].
The beneficial effects of whole grains (including colored rice) consumption have been widely reported in this decade. The antioxidative activity, body fat reduction, antiinflammatory, cardioprotective, and antiatherogenic effects of colored rice have been proved in vivo tests [9–12]. In Taiwan, the cultivation and consumption of colored rice are getting popular for local farmers and people for health concerns. In this study, six commercially-available colored rice (3 black and 3 red) in local markets were studied. The objective of this study is to establish the profiles of bioactive compounds (including phenolics and flavonoids in free and bound fractions, ACNs, PA, vitamin E, and γ-oryzanol) of outer and inner rice bran from these six colored rice. The information revealed in this study can provide the distribution and contents of bioactive compounds in colored rice bran that are important for the applications of colored rice.
2. Materials and methods
2.1. Rice bran
Colored rice bran from six rice samples collected from the local markets were obtained by polishing the colored rice using a laboratory rice mill (VP-31T, Yamamoto Co. Ltd., Tendu, Japan) with the setting of 5 for flow. Four of the six colored rice, including two black and two red rice, were domestically grown. Two colored rice grown in Eastern Taiwan, Taibalang black waxy rice (HB) and Taibalang red waxy rice (HR), were purchased from Hualien KuangFeng Farmer’s Association (Hualien, Taiwan) and another one grown in Eastern Taiwan, Guangfu red rice (GR), was purchased from a local farmer (Hualien, Taiwan). A black rice (WB) cultivated in western Taiwan was provided by Yeedon Enterprise Co. Ltd. (Taipei, Taiwan). Two imported rice samples were from Thailand, one black rice (TB) and one red rice (TR), and provided by Yeedon Enterprise Co. Ltd. Rice bran collected from the first pass and from the second to forth passes of the polishing process were defined as RB-1st (outer bran) and RB-2nd (inner bran), respectively. The bran samples were stored at 4°C until analyses.
2.2. Proximate composition analysis
The contents of moisture, crude lipid, crude protein, ash, and dietary fiber of colored rice bran were analyzed according to American Association of Cereal Chemists international approved methods 44-15A, 30-25, 46-11A, 08-01, and 32-07, respectively [13].
2.3. Determination of total phenolics and total flavonoids
2.3.1. Extraction and fractionation
Free and bound phenolics were extracted and fractionated basically following the methods of Dvořáková et al [8] and Lin and Lai [4] with some modifications. One hundred milligrams of rice bran were extracted with 2 mL of cold 80% ethanol for 10 minutes at room temperature with continuous stirring. After centrifugation (10,000 g, 10 minutes, 4°C), the supernatant and the precipitate were separated by decantation. The precipitate was extracted again as described above one more time. The supernatants were combined, brought to a total volume of 5 mL with 80% ethanol, and defined as crude free phenolics extract (CFPE) which were later used for the analyses of total phenolics (TP), total flavonoids (TF), and anti-oxidative activity.
Four milliliters of the CFPE was evaporated with a rotary evaporator at 40°C to eliminate ethanol and then adjusted to pH 2 with 1N HCl. The acidified CFPE was extracted with ethyl acetate (v/v = 1:1) three times. The ethyl acetate extract (organic layer) was collected and dehydrated with anhydrous sodium sulfate. After filtration with a Whatman Number 2 filter paper, the extract was brought to dryness with a rotary evaporator and redissolved in 0.5 mL of 80% ethanol. This extract was defined as free phenolic acids extract (FPAE) of which the specific phenolic acid was analyzed with high performance liquid chromatography (HPLC).
The residue of rice bran left after extraction with 80% ethanol in the beginning was hydrolyzed with 20 mL of 2N NaOH and stirred for 4 hours at room temperature under nitrogen. The solution was acidified to pH 5 with 6N HCl and mixed with 95% ethanol (v/v = 1:4) to precipitate polysaccharides overnight. After centrifugation (10,000 g, 10 minutes, 4°C), the supernatant was collected and evaporated to remove ethanol. The concentrated solution was adjusted to pH 2 with 1N HCl and then extracted with ethyl acetate by the same procedure as extraction of FPAE. The dried extract was redissolved with 1 mL of 80% ethanol, and this fraction was called bound phenolics extract (BPE).
2.3.2. Determination of TP
The amounts of TP in CFPE were determined using the Folin–Ciocalteu colorimetric method. The appropriate diluted extract (50 μL) was mixed with 1 mL of 2% sodium carbonate, then 100 μL of 50% Folin–Ciocalteu reagent was added in 2 minutes later. The mixed solution was incubated at room temperature for 30 minutes in the dark. The absorbance at 750 nm was measured using a spectrophotometer (UA-160A, Shimadzu, Kyoto, Japan) and the TP of CFPE were expressed as ferulic acid equivalent [mg FAE/g dry matter (DM)].
2.3.3. Determination of TF
For the assay of TF, the appropriate diluted extract (100 μL) was mixed with 400 μL of distilled water and 30 μL of 5% sodium nitrite, and then reacted with 30 μL of 10% aluminum chloride. After adding 1N NaOH (400 μL), 240 μL of distilled water was added. The absorbance at 510 nm of the well-mixed mixture was measured. TF was calibrated with a standard curve of catechin and expressed as catechin equivalent (mg CE/g DM).
2.4. Assay of antioxidative activity
2.4.1. 2,2-diphenyl-1-picrylhydrazyl) radical scavenging activity
The antioxidative activity of colored rice bran was estimated with 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity and reducing power, both of which were determined with CFPE (free fraction) and BPE (bound fraction). For the DPPH radical scavenging activity assay, an appropriate concentration of the extract (40 μL) was added with an equal volume of 50mM DPPH methanol solution, then diluted with 1.92 mL of methanol. After the solution was kept at room temperature for 30 minutes, the absorbance at 519 nm was measured with a UV spectrophotometer [14]. The DPPH radical scavenging activity was calibrated with the standard curve of 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (trolox) and expressed as trolox equivalent (mg TE/g DM).
2.4.2. Reducing power
The reducing power was determined by the method reported by Yen and Chen [15] with some modifications. The 80 μL of appropriately diluted bran extract was added with 200 μL of 0.2M phosphate buffer (pH 6.6) and 200 μL of 1% potassium ferricyanide [K3Fe(CN)6] solution. The mixture was incubated in a water bath at 50°C for 20 minutes. Two hundred microliters of 10% trichloroacetic acid was added to the heated mixture, which was then centrifuged at 786 g for 10 minutes. The supernatant (0.5 mL) was mixed with 0.5 mL of distilled water and 100 μL of 1% ferric chloride solution. The absorbance at 700 nm of the mixture was measured. The reducing power of colored rice bran was calibrated by the standard curve of ascorbic acid and expressed as ascorbic acid equivalent (mg AAE/g DM).
2.5. Identification of phenolic acids by HPLC/HPLC-tandem mass spectrometry
A HPLC system equipped with a Hitachi L-2130 pump (Hitachi Ltd., Tokyo, Japan), an Atlantis dC18 column (250 mm × 4.6 mm, 5-μm particle size, Waters, Milford, MA, USA), and a photodiode array (PDA) detector (Hitachi L-7455) were used to identify the types of phenolic acids. The extracts (FPAE and BPE) were diluted with distilled water and filtered through a hydrophilic polypropylene membrane filter (0.45 μm). The filtered sample (20 μL) was injected and the mobile phase was consisted of 0.1% formic acid (A) and methanol (B) with a flow rate of 1.0 mL/min. The linear gradient elution program was as follows: 0 minutes 25% B; 20 minutes 25% B; 30 minutes 35% B; 40 minutes 100% B; 42 minutes 100% B; 50 minutes 25% B. The absorbance was monitored between 220 nm and 400 nm. The area of peaks of chromatogram at 280 nm was integrated individually. Protocatechuic, chlorogenic, p-hydroxybenzoic, vanillic, caffeic, syringic, p-coumaric, ferulic, and sinapic acids were quantified based on the calibration curve of each standard.
The Thermo Finnigan LXQ liquid chromatography tandem mass spectrometer (LC-MS/MS; Thermo Fisher Scientific, San Jose, CA, USA) was used for qualitative analysis of the unknown peaks in the HPLC chromatogram. The extract was purified with a Strata-X solid phase extraction (SPE) column (6 mL, 500 mg; Phenomenex Inc., Torrance, CA, USA) which was preconditioned with 2 V of methanol and then 3 V of distilled water. One milliliter of extract was diluted with 4 mL of distilled water and loaded into the preconditioned SPE column. After loading, the SPE column was dried with an aspirator (Eyela A-3S, Tokyo Rililai Co. Ltd., Tokyo, Japan) for 1 minute. The column was then eluted with 5 mL of 60% methanol, following with 70% methanol until the 10 mL of effluent was collected with a volumetric flask. The collected effluent sample was filtered and injected into the LC/MS/MS system equipped with a Hydrosphere C18 HS-3C2 column (150 mm × 2.0 mm, 5 μm, YMC Co., Ltd., Kyoto, Japan). The eluent was composed of 0.1% formic acid aqueous solution (A) and 0.1% formic acid methanol solution (B). The linear gradient elution program, at a flow rate of 0.2 mL/min, was as follows: 0 minutes 20% B; 20 minutes 35% B; 30 minutes 100% B; 38 minutes 100% B; 40 minutes 20% B; 50 minutes 20% B. The absorbance response was monitored with a PDA detector in a wavelength range of 200–600 nm. The molecules were ionized by an electrospray ionization (ESI) source. An ion trap mass spectrometry analyzer (Finnigan LXQ, Thermo Fisher Scientific) was used and operated in negative-ion mode. The dissociation of a parent ion was induced with 35 eV of collision to obtain the fragment ions. The mass analyzer scanned the m/z in a range of 100–400.
2.6. Determination of total proanthocyanidin content
Total proanthocyanidin content was determined with the vanillin/hydrochloric acid method. Rice bran (100 mg) was mixed with acetone/water/acetic acid (70:29.5:0.5, v/v/v) solution (2 mL) and sonicated at 37°C for 30 minutes. After centrifugation (10,000 g, 10 minutes, 20°C), the supernatant was decanted. The solid was extracted again with the above procedures. The supernatant was combined and evaporated to dryness by a rotary evaporator at 40°C, and the residue was redissolved with 2 mL of 30% methanol. The appropriate diluted sample (10 μL) was mixed with 4% vanillin methanol solution (0.6 mL) and concentrated HCl (0.3 mL), and stood for 15 minutes at room temperature in the dark. After mixing, the absorbance was measured at 500 nm. To eliminate the interference from ACNs, the blank assay was made with a mixture of 10 μL of diluted extract, 0.6 mL of methanol, and 0.3 mL of concentrated HCl. Proanthocyanidins concentration of the extract was determined against external standard of cetachin. Total proanthocyanidin content of rice bran was expressed as catechin equivalent (mg CE/g DM).
2.7. Determination of total ACN content
Total ACN content of the extract was quantified with the pH-differential method. Rice bran (0.5 g) was mixed with 16 mL of acidified methanol (1% HCl in methanol) and extracted at room temperature with stirring overnight (circa 10 hours). The mixture was centrifuged (10,000 g, 15 minutes, 4°C) and then the supernatant was collected and diluted with acidified methanol to the volume of 20 mL. Two portions of the ACN extract were respectively diluted with potassium chloride buffer solution (0.025M, pH 1.0) and sodium acetate buffer solution (0.4M, pH 4.5) to 2 mL in two microcentrifuge tubes. The tubes were shaken by hand and thereafter equilibrated in dark for 15 minutes. After centrifugation (8736 g, 5 minutes), the absorbance between 260 nm and 750 nm was measured. The total ACN content was calculated as the following equation and expressed as cyanidin-3-glucoside equivalent (mg Cy 3-glc E/g DM).
| (1) |
Where A = (A510 – A700) pH 1.0 – (A510 – A700) pH 4.5; 449.2 L/cm/mol and 26900 L/cm/mol are the molecular weight and extinction coefficient of Cy 3-glc, respectively; DF is the abbreviation of dilution factor; V is the total volume (20 mL); l is the path length (1 cm); W is the sample weight (g, DM).
2.8. Analysis of ACNs with HPLC/ESI-MS/MS
The individual ACNs in colored rice bran were quantified with HPLC and identified using ESI-MS/MS. Rice bran was extracted with acidified methanol as described above. The supernatant obtained after centrifugation was concentrated under nitrogen. The concentrate was centrifuged (10,000 g, 20 minutes, 4°C) and added with acidified methanol to make a total volume of 5 mL. This extract solution was diluted appropriately with 1% HCl aqueous solution, passed through a 0.45-μm Nylon filter, and 20 μL of the sample was injected to HPLC system for analysis. The HPLC system was equipped with a Hitachi L-6000 pump (Hitachi Ltd), a YMC-pack ODS-AQ column (250 mm × 4.6 mm, 5-μm particle size; YMC Co. Ltd., Kyoto, Japan), and a PDA detector (Hitachi Ltd.). The eluent was composed of 1% formic acid (A) and methanol (B). The ACNs were carried out at a flow rate of 1.0 mL/min by a gradient elution as follows: 0 minutes 25% B; 5 minutes 25% B; 9 minutes 33% B; 17 minutes 90% B; 18 minutes 100% B; 23 minutes 100% B. The chromatogram at the wavelength of 510 nm was used for quantification of ACNs. The ACNs separated using HPLC were collected and injected to the ESI-MS/MS system (Thermo Finigan LXQ, San Jose, CA, USA) for confirmation. The standards of Cy 3-glc, cyanidin 3-rutinoside, and peonidin 3-glucoside (Pn 3-glc) purchased from Extrasynthese Co. (Genay Cedex, France) were used for analyses.
2.9. Determination of vitamin E and γ-oryzanol contents
Vitamin E and γ-oryzanol of colored rice bran were quantized with normal phase HPLC. Rice bran (500 mg) was extracted with 3 mL of hexane containing 0.02% butylated hydroxytoluene at 60°C for 20 minutes and agitated with a vortex for 5 minutes during extraction. The mixture was centrifuged at 786 g for 15 minutes and the supernatant was collected. The extraction procedure was repeated three times. The supernatants were combined and made a total volume of 10 mL and stored at −30°C until analysis.
The HPLC system for analysis of vitamin E and γ-oryzanol was composed of a super intelligent LC pump (ICA-5120; Toa electronics Ltd., Tokyo, Japan), a silica gel column (Inertsil SIL 100A 5 μm, 4.6 mm × 250 mm; GL Science, Kyoto, Japan), and a fluorescence detector (L-2485, Hitachi Ltd.). The HPLC system was eluted with n-hexane/ethyl acetate/isopropanol/acetic acid (97.6:0.8:0.8:0.8, v/v/v/v) at a flow rate of 0.7 mL/min. The excitation and emission wavelengths of fluorescence detector were 290 nm and 330 nm, respectively. Eight different forms of vitamin E standards, α-, β-, γ-, and δ-tocopherol, and α-, β-, γ-, and δ-tocotrienol (ChromaDex, Inc., Irvine, CA, USA), and γ-oryzanol (TCI, Japan) were used to calibrate their concentrations in rice bran.
2.10. Statistical analysis
For proximate composition analysis, moisture, ash, and crude lipid contents were conducted in triplicate; crude protein and dietary fiber content were done in duplicate. All of extractions of bioactive compounds were conducted in duplicate and quantifications were duplicated of each extract. Statistical analyses were performed using SPSS version 12.0 for Windows (SPSS Inc., Chicago, IL, USA). The data were analyzed with one-way analysis of variance and the significant differences among means were determined using the Duncan test at a level of p < 0.05. The significant differences between two groups of data were determined with a t test (p < 0.05). Pearson correlation coefficient was analyzed to investigate the relationship between two variables.
3. Results and discussion
3.1. Degrees of milling and proximate composition of colored rice bran
After the black and red rice passed through the continuous rice polisher for the first time, the polished rice still had a little bran attached so it would be remilled for one to three more time(s) until the rice kernel was near white. The degrees of milling (DOM) and proximate compositions of RB-1st and RB-2nd of six colored rice are shown in Table 1. The DOM were in the ranges of 10.3 (GR) to 19.2 (TB) and 5.6 (TR) to 11.4 (WB) of RB-1st and RB-2nd, respectively (Table 1). In general, the DOM of the nonpigmented rice ranged from 4% to 10% [16], which is lower than the ones in this study. The DOM of both the RB-1st and the sum of the RB-1st and RB-2nd of the black rice (12–19% and 24–26%, respectively) were higher than those of the red rice (10–13% and 18–21%, respectively).
Table 1.
Degree of milling (DOM) and proximate composition of colored rice bran.
| Sample | DOM | Moisture content | Crude lipid | Crude protein | Ash | Total carbohydratea | Dietary fiber | ||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| IDF | SDF | TDF | |||||||
|
|
|
|
|||||||
| % | %, as is | %, DM | |||||||
| RB-1st | |||||||||
| HB | 15.4 | 11.99 ± 0.03 bb | 13.54 ± 0.17 cd | 11.28 ± 0.83 bc | 6.13 ± 0.04 d | 69.12 ± 0.95 b | 22.92 ± 0.22 bc | 2.07 ± 1.08 ab | 24.99 ± 0.86 b |
| WB | 12.3 | 10.48 ± 0.12 e | 13.03 ± 0.22 de | 12.49 ± 0.04 b | 5.99 ± 0.07 d | 68.59 ± 0.15 bc | 20.62 ± 0.39 d | 1.52 ± 0.03 ab | 22.14 ± 0.42 d |
| TB | 19.2 | 9.80 ± 0.06 f | 12.47 ± 0.66 e | 11.77 ± 0.00 bc | 5.07 ± 0.08 e | 71.04 ± 0.15 a | 23.54 ± 0.29 b | 1.21 ± 0.22 ab | 24.75 ± 0.51 b |
| HR | 11.4 | 11.62 ± 0.04 c | 14.07 ± 0.42 c | 10.64 ± 0.13 cd | 8.12 ± 0.08 b | 67.43 ± 0.02 cd | 21.78 ± 0.06 cd | 1.02 ± 0.16 b | 22.81 ± 0.09 cd |
| GR | 10.3 | 10.81 ± 0.10 d | 16.34 ± 0.37 b | 10.71 ± 0.72 cd | 8.58 ± 0.04 a | 64.37 ± 1.03 e | 25.95 ± 0.57 a | 2.25 ± 0.14 a | 28.20 ± 0.71 a |
| TR | 12.5 | 7.99 ± 0.11 g | 17.34 ± 0.53 a | 9.64 ± 0.31 d | 7.13 ± 0.03 c | 66.18 ± 0.36 d | 23.26 ± 1.18 b | 1.19 ± 0.03 a | 24.45 ± 1.21 bc |
| RB-2nd | |||||||||
| HB | 10.0 | 11.43 ± 0.02 a | 6.21 ± 0.46 b | 8.81 ± 0.06 b | 3.80 ± 0.01 c | 81.43 ± 0.28 a | 5.88 ± 1.22 cd | 0.71 ± 0.25 b | 6.59 ± 1.46 b |
| WB | 11.4 | 10.51 ± 0.02 c | 7.60 ± 0.81 b | 9.59 ± 0.11 a | 4.32 ± 0.01 b | 78.49 ± 0.93 b | 9.13 ± 0.18 a | 0.45 ± 0.13 b | 9.58 ± 0.05 a |
| TB | 6.9 | 9.69 ± 0.04 d | 6.96 ± 0.48 b | 9.64 ± 0.11 a | 3.15 ± 0.04 e | 80.09 ± 0.68 a | 7.17 ± 0.15 bc | 0.16 ± 0.04 b | 7.33 ± 0.10 b |
| HR | 9.6 | 11.45 ± 0.06 a | 6.24 ± 0.97 b | 9.45 ± 0.14 a | 3.56 ± 0.09 d | 81.20 ± 0.44 a | 8.84 ± 0.35 ab | 0.74 ± 0.05 b | 9.58 ± 0.30 a |
| GR | 10.0 | 10.83 ± 0.02 b | 8.57 ± 0.58 a | 9.00 ± 0.07 b | 5.28 ± 0.05 a | 77.42 ± 0.30 b | 6.05 ± 0.95 cd | 0.42 ± 0.42 b | 6.46 ± 0.54 b |
| TR | 5.6 | 8.58 ± 0.02 e | 7.17 ± 0.52 b | 8.84 ± 0.04 b | 3.52 ± 0.04 d | 80.75 ± 0.19 a | 5.19 ± 0.86 d | 1.44 ± 0.41 a | 6.62 ± 1.28 b |
DM = dry matter; HB = Taibalang black waxy rice; WB = black rice western Taiwan; TB = black rice Thailand; HR = Taibalang red waxy rice; GR = Guangfu red rice; TR = red rice Thailand; RB-1st = outer bran; RB-2nd = inner bran; IDF = insoluble dietary fiber; SDF = soluble dietary fiber; TDF = total dietary fiber.
Total carbohydrate was estimated with the content of nitrogen-free extract. Total carbohydrate (%, DM) = 100 – (crude lipid + crude protein + ash).
Values followed by the different letters in the same column are significantly different (p < 0.05) within RB-1st or RB-2nd groups.
Contents of crude lipid, crude protein, ash, and total dietary fiber were higher in the RB-1st than in the RB-2nd (Table 1). By contrast, the total carbohydrate content of the RB-2nd (77.4–81.4%) was higher than the RB-1st (64.4–71.0%). This result could be attributed to uneven distribution of these components in rice grains. Lipid, protein, fiber, and minerals are rich in the bran and embryo, while starch is abundant in the endosperm. The DOM of RB-1st was higher in black rice than that of red rice, so a tendency could be observed that contents of crude lipid and ash in the RB-1st of the black rice were slightly lower than those of the red rice due to a dilution effect. In addition, the RB-2nd had a large amount of total carbohydrate but only 6.5–9.6% of total dietary fiber, which implies a large proportion of the RB-2nd originated from the endosperm so that there was a high amount of starch in the RB-2nd fraction. The total carbohydrate content of both RB-1st and RB-2nd were much higher than the value reported in the literature (33.5–53.5%) [17]. The contents of crude protein (9.6–12.5%) and ash (5.1–8.6%) of the RB-1st in this study were slightly lower than the reported values (11.5–17.2% and 8.0–17.7%, respectively) [17].
3.2. TP and TF in colored rice bran
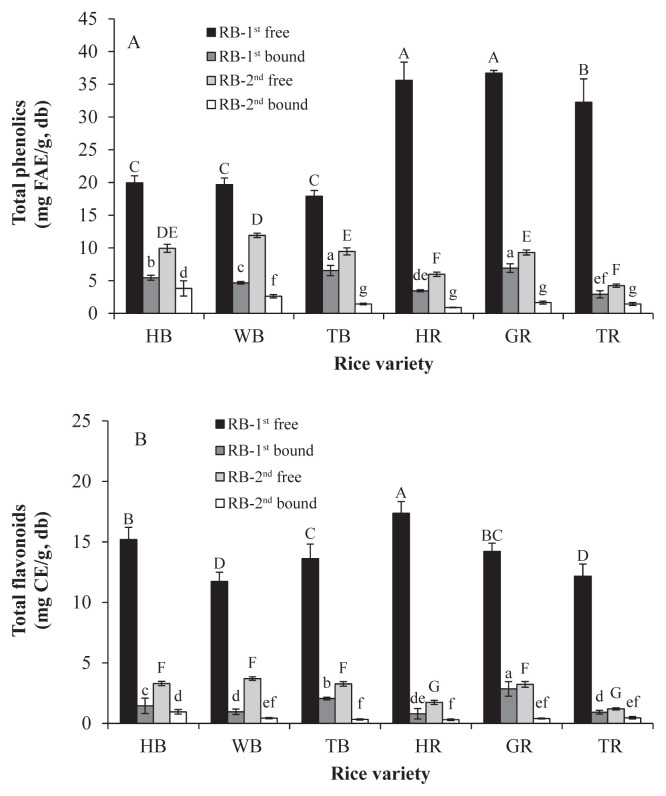
The extracts of colored rice bran were fractionated into free (CFPE) and bound (BPE) fractions, which the TP and TF were determined and shown in Figures 1A and 1B, respectively. The TP in the RB-1st ranged from 17.89 mg FAE/g DM to 36.70 mg FAE/g DM in the free fraction and from 2.92 mg FAE/g DM to 6.92 mg FAE/g DM in the bound fraction. The TP of the RB-2nd (4.24–11.92 mg FAE/g DM and 0.90–3.82 mg FAE/g DM in the free and bound fractions, respectively) was much lower than those of the corresponding RB-1st. The TF of the RB-1st were 11.72–17.37 mg CE g−1 DM in the free form and 0.79–2.85 mg CE/g DM in the bound one. Similar results were seen with the TP, the TF of the RB-2nd (1.20–3.29 mg CE/g DM and 0.30–0.95 mg CE/g DM in the free and bound fractions, respectively) were much lower than those of the RB-1st.
Figure 1.
Colored rice brain: (A) total phenolics; and (B) total flavonoids. Different capitals and small letters above the bars mean significant differences (p < 0.05) within cultivars in the free and bound fractions, respectively. CE = catechin equivalent; FAE = ferulic acid equivalent; HB =Taibalang black waxy rice; WB = black rice western Taiwan; TB = black rice Thailand; HR =Taibalang red waxy rice; GR = Guangfu red rice; TR = red rice Thailand; RB-1st = outer bran; RB-2nd = inner bran.
In the free fraction, the TP of the RB-1st of the red rice (32.26–36.70 mg FAE/g DM) was 1.8 times higher than that of the black rice (17.89–19.95 mg FAE/g DM). Shen et al [18] reported that free phenolics of black rice were higher than red rice and others reported that the free phenolics were comparable between black and red rice [19,20]. The difference of total free phenolics between black and red rice seems not absolute and it might depend on the rice varieties. The TP of the bound fraction of red rice bran was first reported in this study. In the bound fraction, the TP of both RB-1st and RB-2nd from the black rice were higher than the red ones (1.3 times and 2.0 times of the red rice, respectively). This result can be explained by their phenolic-acid profiles and will be discussed later.
The amounts of phenolics and flavnoids in the free fractions were much higher than those in the bound fractions of these six rice samples. In the free fractions, there were 73.2–91.7% and 72.2–86.9% of phenolics and 83.3–95.6% and 72.3–90.8% of flavonoids in RB-1st and RB-2nd, respectively. The results are similar to the previous studies. The brans of 12 Chinese black rice varieties (DOM ~10%) were analyzed and the percentages of phenolics and flavonoids in free form were found to be 88.2–95.6% and 96.3–97.6%, respectively [21]. Kong and Lee [22] reported that the free phenolics contributed 91.2% and 90.8% and the free flavonoids contributed 90.8% and 92.1% to the sum of the free and bound forms in bran from two Korean black rice varieties (DOM ~15%).
In this study, ferulic acid was used as a standard for quantification of TP because it is the most abundant phenolic acid in rice. The value of FAE is lower than that of gallic acid equivalent for a constant sample. The free TP in rice bran of three black rice samples in this study were in the range of the previous studies [21,22] but lower than its average. This might come from a genetic difference or the dilution effect due to the higher DOM in this study. In general, both the RB-1st and the RB-2nd of the colored rice contained more phenolic compounds than rice bran of nonpigmented rice varieties [5,21,23].
3.3. Antioxidative activity of colored rice bran
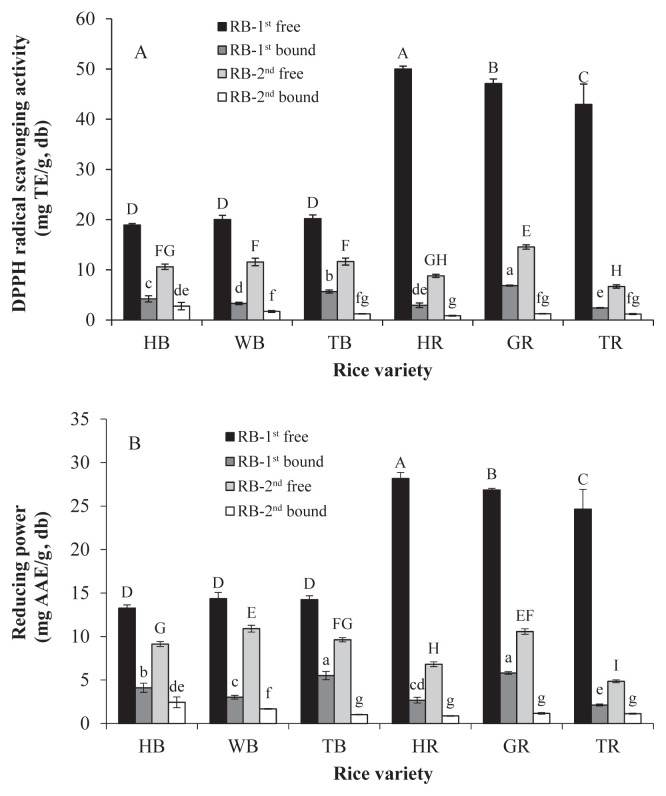
The DPPH radical scavenging activity and the reducing power of the colored rice bran are shown in Figures 2A and 2B, respectively. The DPPH radical scavenging capacities of RB-1st were 18.94–49.99 mg TE/g DM in the free fraction and 2.40–6.86 mg TE/g DM in the bound fraction. The RB-2nd had the DPPH radical scavenging capacities of 6.69–14.55 mg TE/g DM in the free fraction and 0.86–2.76 mg TE/g DM in the bound fraction. The reducing power of the RB-1st was 13.27–28.18 mg AAE/ g DM and 2.13–5.81 mg AAE/g DM in the free and bound fractions, respectively. The reducing power of the RB-2nd was 4.85–10.90 mg AAE/g DM in the free fraction and 0.87–2.44 mg AAE/g DM in the bound fraction. Both of the DPPH radical scavenging activity and the reducing power of the colored rice bran had a tendency similar to the TP regardless of bran layers, forms, and rice samples. The antioxidative activities of these colored rice bran in free fractions were much higher than that in bound fractions. The RB-1st of red rice had higher antioxidative activity than that of black rice. The antioxidative activities are highly correlated with the TP (r = 0.987 of DPPH radical scavenging activity and 0.989 of reducing power) and with the TF (r = 0.889 of DPPH radical scavenging activity and 0.905 of reducing power) at p < 0.01. These results suggest that the phenolics and flavonoids in colored rice bran would exert corresponding antioxidative activity.
Figure 2.
Colored rice bran: (A) 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity; and (B) reducing power. Different capitals and small letters above the bars mean significant differences (p < 0.05) within cultivars in the free and bound fractions, respectively. TE = 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (trolox); AAE = ascorbic acid equivalent; HB =Taibalang black waxy rice; WB = black rice western Taiwan; TB = black rice Thailand; HR =Taibalang red waxy rice; GR = Guanggfu red rice; TR = red rice Thailand; RB-1st = outer bran; RB-2nd = inner bran.
3.4. Pigments in colored rice bran
The contents of PAs and ACNs in colored rice bran are shown in Table 2. No PA in the black rice was detected in this study. Contents of PAs of red rice ranged from 12.16 mg CE/g DM to 19.13 mg CE/g DM in the RB-1st and from 0.75 mg CE/g DM to 4.31 mg CE/g DM in the RB-2nd, respectively. The red rice cultivated in Taiwan (HR and GR) contained higher level of PAs than that from Thailand (TR).
Table 2.
Contents of proanthocyanidins and anthocyanins (ACN) in colored rice bran.
| Sample | Proanthocyanidins | Total ACN | Cy 3-glc | Pn 3-glc | Cy 3-rut |
|---|---|---|---|---|---|
|
|
|
|
|||
| mg CE/g, DM | mg Cy 3-glc E/g, DM | mg/g, DM | |||
| RB-1st | |||||
| HB | ND | 6.29 ± 0.08 ca | 2.44 ± 0.27 c (71.1)b | 0.53 ± 0.04 c (15.5) | 0.46 ± 0.04 a (13.4) |
| WB | ND | 6.70 ± 0.06 b | 3.07 ± 0.14 b (63.8) | 1.32 ± 0.03 a (27.4) | 0.42 ± 0.05 a (8.7) |
| TB | ND | 11.27 ± 0.38 a | 10.63 ± 0.66 a (88.9) | 0.81 ± 0.06 b (6.8) | 0.52 ± 0.12 a (4.3) |
| HR | 19.13 ± 0.41 a | 0.31 ± 0.01 d | 0.05 ± 0.01 d (100) | Tr | Tr |
| GR | 17.41 ± 0.63 b | 0.38 ± 0.03 d | 0.20 ± 0.04 d (100) | Tr | Tr |
| TR | 12.16 ± 0.43 c | 0.35 ± 0.02 d | 0.15 ± 0.04 d (83.3) | 0.03 ± 0.00 d (16.7) | Tr |
| RB-2nd | |||||
| HB | ND | 3.46 ± 0.11 c | 1.43 ± 0.19 c (70.8) | 0.34 ± 0.05 b (16.8) | 0.25 ± 0.04 a (12.3) |
| WB | ND | 4.92 ± 0.30 b | 2.06 ± 0.18 b (63.8) | 0.89 ± 0.07 a (27.6) | 0.28 ± 0.05 a (8.7) |
| TB | ND | 6.85 ± 0.36 a | 6.62 ± 0.39 a (89.7) | 0.40 ± 0.05 b (5.4) | 0.36 ± 0.11 a (4.9) |
| HR | 3.41 ± 0.08 b | 0.20 ± 0.01 d | 0.03 ± 0.01 d (100) | Tr | Tr |
| GR | 4.31 ± 0.77 a | 0.22 ± 0.01 d | 0.07 ± 0.01 d (100) | Tr | Tr |
| TR | 0.75 ± 0.40 c | 0.28 ± 0.01 d | 0.08 ± 0.02 d (88.9) | 0.01 ± 0.00 c (11.1) | Tr |
CE = catechin equivalent; Cy 3-glc E = cyanidin-3-glucoside equivalent; Pn-3-glc = peonidin 3-glucoside; Cy 3-rut = cyanidin 3-rutinoside; DM = dry matter; HB = Taibalang black waxy rice; WB = black rice western Taiwan; TB = black rice Thailand; HR = Taibalang red waxy rice; GR = Guangfu red rice; TR = red rice Thailand; RB-1st = outer bran; RB-2nd = inner bran; ND = not detectable; Tr = trace.
Values followed by different letters in the same column are significantly different (p < 0.05) within RB-1st or RB-2nd groups.
The value in the parentheses is the percentage of each anthocyanin in the sum of Cy 3-glc, Pn 3-glc, and Cy 3-rut.
The total ACNs in black rice bran in RB-1st and RB-2nd were 6.29–11.27 mg Cy 3-glc E/g DM and 3.46–6.85 mg Cy 3-glc E/g DM, respectively. Besides, the rice bran from TB contained the highest ACNs (11.27 mg Cy 3-glc E/g DM) among the three black rice samples. By contrast, red rice had only a small amount of ACNs (0.31–0.38 mg Cy 3-glc E/g DM and 0.20–0.28 mg Cy 3-glc E/g DM in their RB-1st and RB-2nd, respectively). The content of ACNs, the group of flavonoids belonging to phenolics, was higher in RB-1st than that in RB-2nd, which has similar trends to TP and TF.
Cy 3-glc, Pn 3-glc, and cyanidin 3-rutinoside, ranked by their amounts, were found in both of black and red rice brans (Table 2). The percentages of these compounds in RB-1st and in RB-2nd of all black rice samples were almost the same (values in parentheses in Table 2). Cy 3-glc was the major ACN in the black rice bran with a range of 64–90%, which was similar to that of what Laokuldilok et al [24] reported (58–91%). Pn 3-glc accounts for 5–28% of ACNs in black rice bran.
3.5. Phenolic acids profile and identification of protocatechualdehyde
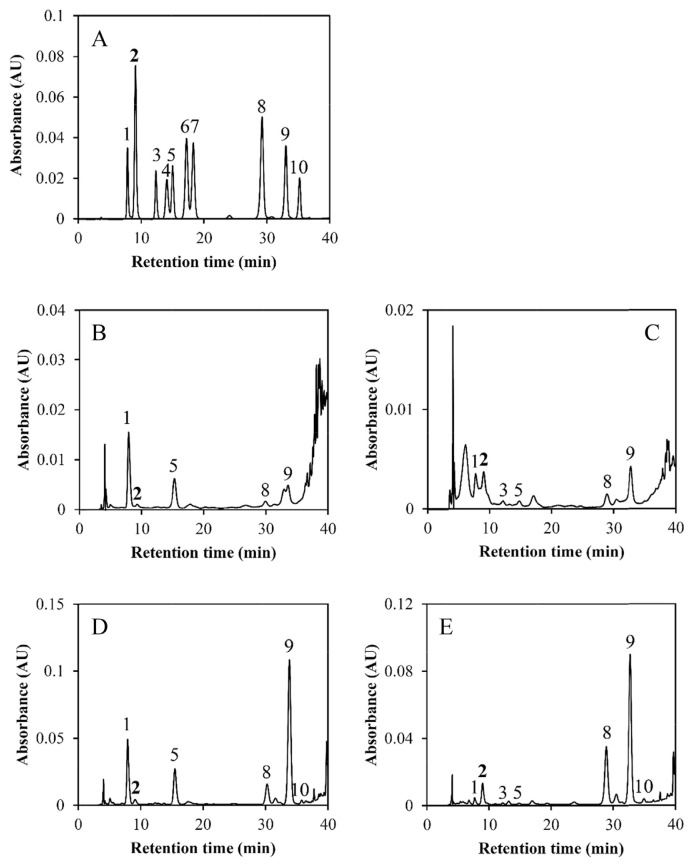
The HPLC chromatograms and the phenolic acids in the extracts of RB-1st from colored rice HB and HR are shown in Figure 3. Each phenolic acid in the extracts of both RB-1st and RB-2nd are quantified and summarized in Table 3. Higher amounts of phenolic acids were determined in the bound fractions than in the free factions of colored rice bran. The major bound phenolic acids in the RB-1st of black rice were ferulic acid (1295–1601 μg/g DM), protocatechuic acid (948–1043 μg/g DM), vanillic acid (335–652 μg/g DM), and p-coumaric acid (140–232 μg/g DM). Sinapic acid and p-hydorxybenzoic acid were the minor compounds in bound fraction in RB-1st of black rice. Ferulic acid was also the most predominant bound phenolic acid (1171–1998 μg/g DM) in RB-1st of red rice, followed by p-coumaric acid (334–574 μg/g DM). The minor amounts of the bound phenolic acids in red rice RB-1st were protocatechuic acid, sinapic acid, p-hydorxybenzoic acid, and vanillic acid. Compared with the bound phenolic acids in the colored rice bran, the amount of free phenolic acids was low. The bound phenolic acids comprised 92–97% of the TP in RB-1st and 83–95% of those in RB-2nd. The results suggest that most of the phenolic acids in colored rice bran must be hydrolyzed from biopolymers, e.g., polysaccharides and lignins, so that they can be extracted.
Figure 3.
High performance liquid chromatography chromatograms of (A) a mixture of phenolic acid standards; extracts of (B) free fraction of outer bran (RB-1st)of Taibalang black waxy rice (HB); (C) free fraction of outer bran of Taibalang red waxy rice (HR); (D) bound fraction of outer bran of Taibalang black waxy rice; and (E) bound fraction of outer bran of Taibalang red waxy rice. Peaks: 1, protocatechuic acid; 2, protocatechualdehyde; 3, p-hydroxybenzoic acid; 4, chlorogenic acid; 5, vanillic acid; 6, caffeic acid; 7, syringic acid; 8, p-coumaric acid; 9, ferulic acid; and 10, sinapic acid.
Table 3.
Phenolic acid composition and protocatechualdehyde content of colored rice bran (μg/g, dry matter).
| Phenolic compound | RB-1st | RB-2nd | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| HB | WB | TB | HR | GR | TR | HB | WB | TB | HR | GR | TR | |
| Free | ||||||||||||
| Protocatechuic acid | 59 | 176 | 163 | 30 | 45 | 33 | 100 | 135 | 113 | 13 | 21 | 46 |
| p-Hydroxybenzoic acid | 7 | 6 | 3 | 5 | 2 | 2 | 2 | 3 | 2 | 2 | 1 | 1 |
| Vanillic acid | 719 | 86 | 45 | 6 | 8 | 6 | 43 | 68 | 32 | 4 | 6 | 8 |
| p-Coumaric acid | 12 | 10 | 9 | 11 | 10 | 10 | 7 | 8 | 7 | 8 | 7 | 7 |
| Ferulic acid | 45 | 28 | 36 | 47 | 28 | 30 | 11 | 13 | 14 | 13 | 14 | 9 |
| Sinapic acid | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Total | 302 | 306 | 256 | 98 | 93 | 81 | 162 | 228 | 167 | 40 | 49 | 70 |
| % to total phenolic acids | 8.2 | 7.9 | 7.9 | 4.6 | 3.1 | 4.7 | 12.1 | 14.1 | 17.1 | 6.2 | 4.6 | 11.5 |
| Protocatechualdehyde | 7 | 9 | 12 | 19 | 13 | 13 | 2 | 4 | 7 | 4 | 7 | 10 |
| Bound | ||||||||||||
| Protocatechuic acid | 948 | 1041 | 1043 | 95 | 164 | 28 | 26 | 69 | 44 | 20 | 11 | 20 |
| p-Hydroxybenzoic acid | 34 | 30 | 34 | 58 | 60 | 33 | 18 | 23 | 17 | 20 | 23 | 9 |
| Vanillic acid | 621 | 652 | 335 | 33 | 25 | 36 | 287 | 375 | 140 | 18 | 9 | 22 |
| p-Coumaric acid | 140 | 147 | 232 | 339 | 574 | 334 | 60 | 60 | 69 | 73 | 128 | 76 |
| Ferulic acid | 1576 | 1601 | 1295 | 1448 | 1998 | 1171 | 767 | 830 | 522 | 460 | 822 | 401 |
| Sinapic acid | 62 | 80 | 62 | 76 | 132 | 48 | 23 | 27 | 19 | 16 | 28 | 12 |
| Total | 3381 | 3551 | 3001 | 2049 | 2953 | 1650 | 1181 | 1384 | 811 | 607 | 1021 | 540 |
| % to total phenolic acids | 91.8 | 92.1 | 92.1 | 95.4 | 96.9 | 95.3 | 87.9 | 85.9 | 82.9 | 93.8 | 95.4 | 88.5 |
| Protocatechualdehyde | 40 | 26 | 44 | 95 | 188 | 118 | 17 | 14 | 16 | 40 | 38 | 21 |
HB = Taibalang black waxy rice; WB = black rice western Taiwan; TB = black rice Thailand; HR = Taibalang red waxy rice; GR = Guangfu red rice; TR = red rice Thailand; RB-1st = outer bran; RB-2nd = inner bran; ND = not detectable.
The composition of the phenolic acids of red rice was similar to the nonpigmented rice [25,26]. Besides, the black rice contained two additional phenolic acids, protocatechuic acid and vanillic acid, compared with the nonpigmented rice. It is uncertain if the protocatechuic acid and vanillic acid existed in the black rice naturally or if they were produced during extraction.
In the chromatograms of BPE from red rice bran, there was an obvious unknown peak at 9.1 minutes (Peak 2 in Figure 3). To identify the unknown compound, the BPE of the red rice bran was purified with SPE and further analyzed with LC-MS/MS. Based on the characteristics of the λmax (278 nm and 309 nm) and the m/z of the deprotonated parent (m/z = 137) and daughter ions (m/z = 93 or 109) analyzed with LC-MS/MS, the unknown compound was suspected to be proto-catechualdehyde (3,4-dihydroxybenzaldehyde, PCA) [27,28]. PCA standard was further analyzed with HPLC-PDA, and it was found that both of its retention time (Figure 3) and absorption spectrum were consistent with the unknown compound. Consequently, the existence of PCA in the BPE of red rice bran was confirmed. For the red rice bran, the bound PCA contents were 95–188 μg/g DM in RB-1st and 21–40 μg/g DM in RB-2nd. The black rice bran also contained a small amount of PCA (Table 3). Although PCA is a naturally occurring poly-phenol found in barley [29], it is the first time to be identified in rice bran.
The correlation analysis between the TP and the sum of phenolic acids and PCA showed there was a high correlation for the bound fraction (r = 0.894, p < 0.01) but no correlation for the free fraction (r = 0.051). This suggests that phenolic acids and PCA were the principal phenolics in the bound fraction of the colored rice bran. The major free phenolics were not phenolic acids but other compounds, such as PAs and ACNs. In addition, the sums of phenolic acids and PCA of RB-1st and RB-2nd from black rice were higher than that of the corresponding fractions from red rice, which can explain the result mentioned above that the bound TP of both RB-1st and RB-2nd from the black rice are higher than that from the red rice.
3.6. Vitamin E and γ-oryzanol
The vitamin E contents of the colored rice bran are shown in Table 4. Four tocopherols (α-, β-, γ-, and δ-tocopherol) and three tocotrienols (α-, γ-, and δ-tocotrienol) were found in the colored rice bran. The predominant vitamin E in colored rice bran was α-tocopherol (13.12–67.30 μg/g DM), γ-tocotrienol (16.91–53.09 μg/g DM), and γ-tocopherol (10.76–28.43 μg/g DM). The composition of vitamin E in the colored rice bran was similar to that of the nonpigmented rice [4,7,30]. γ-Oryzanol contents ranged from 3.59 mg/g DM to 7.72 mg/g DM in RB-1st of the colored rice (Table 4). The previous studies also reported γ-oryzanol content in nonpigmented rice bran with a range of 1.55–3.13 mg/g DM, except for one Thai variety with an unique high amount of 7.35 mg/g DM (extracted with n-hexane) [5,31]. Compared with the above studies, the RB-1st of TB had extraordinary high γ-oryzanol content (7.72 mg/g DM) even though its DOM was high (19.2%) in this study.
Table 4.
Contents of vitamin E (μg/g, dry matter) and γ-oryzanol (mg/g, dry matter) in the outer rice bran from the first pass of the polishing process of colored rice bran (RB-1st).
| Sample | HB | WB | TB | HR | GR | TR |
|---|---|---|---|---|---|---|
| Vitamin E | ||||||
| α-T | 18.37 ± 1.14 da | 30.65 ± 0.98 c | 34.56 ± 2.61 c | 40.10 ± 4.54 b | 67.30 ± 3.28 a | 13.12 ± 2.12 e |
| β-T | 0.66 ± 0.10 c | 3.41 ± 0.37 b | 5.83 ± 0.06 a | 4.07 ± 1.39 a | 4.65 ± 0.67 ab | ND |
| γ-T | 14.61 ± 0.62 c | 22.54 ± 0.20 b | 28.43 ± 1.80 a | 10.76 ± 0.76 d | 27.69 ± 0.71 a | 13.08 ± 0.93 c |
| δ-T | 2.81 ± 0.22 c | 4.28 ± 0.13 a | 3.85 ± 0.36 b | 1.99 ± 0.03 d | 4.30 ± 0.20 a | 1.29 ± 0.19 e |
| α-T3 | 3.73 ± 0.29 c | 9.99 ± 0.15 a | 20.65 ± 2.26 b | 10.94 ± 4.45 b | 11.49 ± 0.13 b | 3.57 ± 1.13 c |
| γ-T3 | 41.42 ± 3.26 c | 53.09 ± 0.76 a | 38.14 ± 2.70 c | 28.10 ± 1.39 d | 45.83 ± 3.13 b | 16.91 ± 0.76 e |
| δ-T3 | 3.98 ± 0.78 b | 6.03 ± 0.14 a | 5.83 ± 0.32 a | 3.71 ± 0.20 b | 5.66 ± 0.27 a | 2.69 ± 0.37 c |
| Total | 85.49 ± 3.24 d | 129.97 ± 1.23 b | 137.28 ± 9.75 b | 99.68 ± 9.14 c | 166.93 ± 3.65 a | 50.65 ± 5.07 e |
| γ-oryzanol | 3.95 ± 0.32 c | 4.85 ± 0.11 b | 7.72 ± 0.39 a | 3.62 ± 0.16 c | 3.59 ± 0.23 c | 3.69 ± 1.07 c |
α-T = α-tocopherol; β-T = β-tocopherol; γ-T = γ-tocopherol; δ-T = δ-tocopherol; δ-T3 = δ-tocotrienol; α-T3 = tocotrienol; β-T3 = β-tocotrienol; γ-T3 = γ-tocotrienol; δ-T3 = δ-tocotrienol; HB = Taibalang black waxy rice; WB = black rice western Taiwan; TB = black rice Thailand; HR = Taibalang red waxy rice; GR = Guangfu red rice; TR = red rice Thailand.
Values followed by different letters in the same row are significantly different (p < 0.05).
Acknowledgments
This work was supported by the grants 98AS-3.1.4-FD-Z2(3) and 99AS-3.1.4-FD-Z2(2) from the Council of Agriculture Executive Yuan, Taiwan.
Funding Statement
This work was supported by the grants 98AS-3.1.4-FD-Z2(3) and 99AS-3.1.4-FD-Z2(2) from the Council of Agriculture Executive Yuan, Taiwan.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
REFERENCES
- 1. Teng YC. Rice industry development and its future prospects in Taiwan. Research Bulletin of Kaohsiung District Agricultural Improvement Station. 2003;14:1–23. [Google Scholar]
- 2. Gunaratne A, Wu K, Li D, Bentota A, Corke H, Cai YZ. Antioxidant activity and nutritional quality of traditional red-grained rice varieties containing proanthocyanidins. Food Chem. 2013;138:1153–61. doi: 10.1016/j.foodchem.2012.11.129. [DOI] [PubMed] [Google Scholar]
- 3. Hou Z, Qin P, Zhang Y, Cui S, Ren G. Identification of anthocyanins isolated from black rice (Oryza sativa L.) and their degradation kinetics. Food Res Int. 2013;50:691–7. [Google Scholar]
- 4. Lin PY, Lai HM. Bioactive compounds in rice during grain development. Food Chem. 2011;127:86–93. [Google Scholar]
- 5. Aguilar-Garcia C, Gavino G, Baragaño-Mosqueda M, Hevia P, Gavino V. Correlation of tocopherol, tocotrienol, γ-oryzanol, and total polyphenol content in rice bran with different antioxidant capacity assays. Food Chem. 2007;102:1228–32. [Google Scholar]
- 6. Samad N. Rice bran oil prevents neuroleptic-induced extrapyramidal symptoms in rats: Possible antioxidant mechanisms. J Food Drug Anal. 2015;23:370–5. doi: 10.1016/j.jfda.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sosulski F, Krygier K, Hogge L. Free, esterified, and insoluble-bound phenolic acids. 3. Composition of phenolic-acids in cereal and potato flours. J Agric Food Chem. 1982;30:337–40. [Google Scholar]
- 8. Dvořáková M, Guido LF, Dostálek P, Skulilová Z, Moreira MM, Barros AA. Antioxidant properties of free, soluble ester and insoluble-bound phenolic compounds in different barley varieties and corresponding malts. J I Brewing. 2008;114:27–33. [Google Scholar]
- 9. Kim HW, Lee AY, Yeo SK, Chung H, Lee JH, Hoang MH, Jia Y, Han SI, Oh SK, Lee SJ, Kim YS. Metabolic profiling and biological mechanisms of body fat reduction in mice fed the ethanolic extract of black-colored rice. Food Res Int. 2013;53:373–90. [Google Scholar]
- 10. Ling WH, Cheng QX, Ma J, Wang T. Red and black rice decrease atherosclerotic plaque formation and increase antioxidant status in rabbits. J Nutr. 2001;131:1421–6. doi: 10.1093/jn/131.5.1421. [DOI] [PubMed] [Google Scholar]
- 11. Rattanachitthawat S, Suwannalert P, Riengrojpitak S, Chaiyasut C, Pantuwatana S. Phenolic content and antioxidant activities in red unpolished Thai rice prevents oxidative stress in rats. J Med Plant Res. 2010;4:796–801. [Google Scholar]
- 12. Wang Q, Han PH, Zhang MW, Xia M, Zhu HL, Ma J, Hou MJ, Tang ZH, Ling WH. Supplementation of black rice pigment fraction improves antioxidant and anti-inflammatory status in patients with coronary heart disease. Asia Pac J Clin Nutr. 2007;16:295–301. [PubMed] [Google Scholar]
- 13.American Association of Cereal Chemists International (AACCI) Approved methods of the American Association of Cereal Chemists International. St. Paul, MN: AACCI; 2000. [Google Scholar]
- 14. Kitts DD, Wijewickreme AN, Hu C. Antioxidant properties of North American ginseng extract. Mol Cell Biol. 2000;203:1–10. doi: 10.1023/a:1007078414639. [DOI] [PubMed] [Google Scholar]
- 15. Yen GC, Chen HY. Antioxidant activity of various tea extracts relation to their antimutagenicity. J Agric Food Chem. 1995;43:27–32. [Google Scholar]
- 16. Saunders RM. Rice bran: Composition and potential food uses. Food Rev Int. 1985;1:465–95. [Google Scholar]
- 17.Barber S, de Barber CB. Rice bran: Chemistry and technology. In: Luh BS, editor. Rice: production and utilization. Westport, CT: AVI Pub. Co; 1980. pp. 790–862. [Google Scholar]
- 18. Shen Y, Jin L, Xiao P, Lu Y, Bao J. Total phenolics, flavonoids, antioxidant capacity in rice grain and their relations to grain color, size and weight. J Cereal Sci. 2009;49:106–11. [Google Scholar]
- 19. Finocchiaro F, Ferrari B, Gianinetti A. A study of biodiversity of flavonoid content in the rice caryopsis evidencing simultaneous accumulation of anthocyanins and proanthocyanidins in a black-grained genotype. J Cereal Sci. 2010;51:28–34. [Google Scholar]
- 20. Sompong R, Siebenhandl-Ehn S, Linsberger-Martin G, Berghofer E. Physicochemical and antioxidative properties of red and black rice varieties from Thailand, China, and Sri Lanka. Food Chem. 2011;124:132–40. [Google Scholar]
- 21. Zhang MW, Zhang RF, Zhang FX, Liu RH. Phenolic profiles and antioxidant activity of black rice bran of different commercially available varieties. J Agric Food Chem. 2010;58:7580–7. doi: 10.1021/jf1007665. [DOI] [PubMed] [Google Scholar]
- 22. Kong S, Lee J. Antioxidants in milling fractions of black rice cultivars. Food Chem. 2010;120:278–81. [Google Scholar]
- 23. Butsat S, Siriamornpun S. Antioxidant capacities and phenolic compounds of the husk, bran and endosperm of Thai rice. Food Chem. 2010;119:606–13. [Google Scholar]
- 24. Laokuldilok T, Shoemaker CF, Jongkaewwattana S, Tulyathan V. Antioxidants and antioxidant activity of several pigmented rice brans. J Agric Food Chem. 2010;5:193–9. doi: 10.1021/jf103649q. [DOI] [PubMed] [Google Scholar]
- 25. Tian S, Nakamura K, Cui T, Kayahara H. High-performance liquid chromatographic determination of phenolic compounds in rice. J Chromatogr A. 2005;1063:121–8. doi: 10.1016/j.chroma.2004.11.075. [DOI] [PubMed] [Google Scholar]
- 26. Zhou ZK, Robards K, Helliwell S, Blanchard C. The distribution of phenolic acids in rice. Food Chem. 2004;87:401–6. [Google Scholar]
- 27.Linstrom PJ, Mallard WG. NIST Standard Reference database Number 69. National Institute of Standards and Technology; Jun, 2005. [Accessed 10 January 2010]. NIST Chemistry WebBook. Available at: http://webbook.nist.gov/chemistry/ [Google Scholar]
- 28. Watanabe M, Ohshita Y, Tsushida T. Antioxidant compounds from buckwheat (Fagopyrum esculentum Moench) hulls. J Agric Food Chem. 1997;45:1039–44. [Google Scholar]
- 29. Etoh H, Murakamik K, Yogoh T, Ishikawa H, Fukuyama Y, Tanaka H. Antioxidative compounds in barley tea. Biosci Biotechnol Biochem. 2004;68:2616–8. doi: 10.1271/bbb.68.2616. [DOI] [PubMed] [Google Scholar]
- 30. Jang S, Xu Z. Lipophilic and hydrophilic antioxidants and their antioxidant activities in purple rice bran. J Agric Food Chem. 2009;57:858–62. doi: 10.1021/jf803113c. [DOI] [PubMed] [Google Scholar]
- 31. Imsanguan P, Roaysubtawee A, Borirak R, Pongamphai S, Douglas S, Douglas PL. Extraction of α-tocopherol and γ-oryzanol from rice bran. LWT-Food Sci Technol. 2008;41:1417–24. [Google Scholar]