Abstract
In the present, work chemical composition and nutritional value of aerial parts of Cassia occidentalis L. was studied. The aerial parts of C. occidentalis possess favorable physicochemical properties with good nutritional value, such as high energy value, crude fibers, and vitamin levels. The X-ray fluorescence spectrophotometry data revealed that the sample is rich in minerals, especially in Fe, Ca, K, and Mn. Further, minerals such as Mg, Zn, Cu, Na, P, and S are present in good amount and depicted the nutritional value of the selected material. The plant sample is rich in phytochemicals such as flavonoids, alkaloids, lignin, tannins, and phenols. The presence of phytochemical constituents was confirmed by gas chromatography–mass spectrometry profile and high-performance thin layer chromatography fingerprinting techniques. The findings stimulate the on-farm cultivation of C. occidentalis on a large scale to relieve the iron deficiency in local community, and it can be used as a dietary supplement to treat anemia.
Keywords: Cassia occidentalis L, chemical composition, iron, minerals, phytochemicals
1. Introduction
Herbs are staging a comeback, and herbal “renaissance” is happening all over the world. In India, drugs of herbal origin have been used since ancient times in traditional systems of medicine such as Siddha and Ayurveda [1]. Indian traditional medicinal systems like Ayurveda, Unani, Siddha and modern medicine uses about 700, 600, 600 and 30 plant species, respectively [2]. Even the allopathic system of medicine has adopted a number of plant-derived drugs, which form an important segment of the modern pharmacopoeia. Some important chemical intermediates (e.g., diosgenin, solasodine, etc.) that are needed for manufacturing the modern drugs are also obtained from plants [3]. Population growth, inadequate supply of drugs, prohibitive cost of treatments, side effects of several allopathic drugs, and development of resistance to currently used drugs for infectious diseases have led to an increased emphasis on the use of plant materials as a source of medicine for a wide variety of human ailments. As part of the strategy to reduce the financial burden on the human population in developing countries, increased use of plant drugs will be recommended.
Cassia occidentalis L. (Leguminosae) is regarded as an “edible weed of agriculture” or “famine food” [4]. C. occidentalis, also known as “kasamarda,” has been mentioned in various nighandus, viz. Rajnighantu, Dhanwantari, Bhavaprakasa, Rajballaba, etc. [5]. This plant is widely consumed by local people as a coffee substitute. The seeds are brewed into a coffee-like beverage for asthma and a flower infusion is used to treat bronchitis [6]. The roots are considered as tonic, febrifuge, and diuretic; they are also used for menstrual problems, tuberculosis, anemia, and liver complaints, and as a tonic for general weakness and illness [7]. The leaves are used in gonorrhea, fevers, urinary tract disorders, and edema [8]. C. occidentalis extract is used to cure eye inflammations in Ayurveda. It is also used in Jamaican folk medicines for curing diarrhea, dysentery, constipation, fever, cancer, eczema, and venereal diseases. It is a main ingredient of Liv. 52 [9]. Herbolax, a polyherbal formulation that is commonly used in treating constipation, contains C. occidentalis as one of the ingredients [10]. A new indigenous metabolic corrective for newborns and infants, called “Bonnisan,” is also made up of C. occidentalis (0.5 mg/5 mL), which helps bring immediate relief from the discomfort caused by gastric wind [11].
C. occidentalis has been found to possess significant antibacterial, antifungal [12,13], antimalarial [14], anti-inflammatory [15], antimutagenic/anticarcinogenic [16], immunostimulant [8], laxative, analgesic, chloretic, and diuretic properties [17]. The main phytochemicals in C. occidentalis include achrosin, aloe-emodin, emodin, islandicine, kaempferol, obtusifolin, obtusin, physcion [18], anthraquinones, apigenin, aurantiobtusin, campesterol, cassiollin, chrysoobtusin, chrysophanic acid, chrysarobin, chrysophanol [19], chrysoeriol, funiculosin [20], quercetin, rhamnosides, rhein, rubrofusarin, sitosterols, tannins, and xanthorine [21]. A study of phytochemicals of C. occidentalis reveals that the nature and amount of phytochemicals vary according to the climate and soil conditions of the growing location. For example, stems, leaves, and root bark of the plant from Ivory Coast, Africa, contain no alkaloids, while a large amount of alkaloids was found in the samples collected from Ethiopia [22].
Even though C. occidentalis L. finds a large number of uses in traditional medicine as well as in ethnic food, its physicochemical properties and nutritional value remains unexplored. Hence, in the present paper, attempts were made to analyze the physicochemical properties and nutritional profile of C. occidentalis L. employing sophisticated techniques.
2. Methods
2.1. Collection of plant material
Aerial parts of C. occidentalis L. were collected from the herbal garden of STET Women’s College, Mannargudi, Tamil Nadu, India in May 2014. Identification and authentication were performed at the Department of Centre for Advanced Research in Indian System of Medicine (CARISM), SASTRA University, Thanjavur, India. The collected materials were cleaned, shade dried, and powdered. These pulverized materials were used for further analysis.
2.2. Physicochemical analysis
The sensory nature of dry powder of selected plant material was observed by keeping a small quantity in a Petri dish placed on a white background, and the organoleptic characters except audition were observed and tabulated. The physicochemical characters such as foreign matter; loss on drying; contents of total ash, acid-insoluble ash, water-soluble ash, and sulfated ash; and extractive values were determined according to the Ayurvedic Pharmacopoeia [23]. For the determination of foreign matter, 100 g of the sample was weighed and separated out in a thin layer. The foreign matter was detected by inspection with the unaided eye. For the determination of foreign matter, 100 g of the sample was weighed and separated out in a thin layer. The loss on drying was analyzed after drying the powdered sample in an electrical oven at 110°C until it reached a constant weight. Total ash was determined by taking ~2 g of accurately weighed sample; it was incinerated in a silica dish at a temperature not exceeding 450°C until free from carbon and weighed. The percentage of ash with reference to the air-dried drug was calculated. To determine the acid-insoluble ash, the residue of the total ash was boiled for 5 minutes with 25 mL of dilute HCl. The insoluble matter in the crucible was collected on an ash-less filter paper, washed with hot water, and ignited until a constant weight was obtained. The percentage of acid-insoluble ash was calculated with reference to the air-dried material. Water-soluble ash was determined by boiling the ash obtained from total ash for 5 minutes with 25 mL of water, and the insoluble matter was collected in an ash-less filter paper, washed with hot water, and ignited for 5 minutes at a temperature not exceeding 450°C. The difference in weight represents the water-soluble ash. For the estimation of sulfated ash, a silica crucible was heated to redness for 10 minutes, allowed to cool in a desiccators, and weighed. One gram of the substance was placed into the crucible, ignited gently at first until the substrate is thoroughly charred, and cooled; then the residue was moistened with 1 mL of sulfuric acid, heated gently until white fumes no longer evolved, and ignited at 800°C until all black particles disappeared. The crucible was allowed to cool and a few drops of sulfuric acid were added and weighed repeatedly until a constant weight was obtained in two successive measurements.
2.3. Phytochemical profile
Nearly 5 g of shade-dried, coarsely powdered material was subsequently extracted with sufficient volume of various organic solvents in the order of increasing polarity, using Soxhlet extraction apparatus for 6 hours. The extract was then concentrated and the solvent was removed completely under reduced pressure, and then the yield of the extract was calculated. Different qualitative chemical tests were performed in the aqueous extract of C. occidentalis using standard procedures to identify the major constituents, as described by Trease and Evans [24], Harborne [25], and Edeoga et al [26]. Quantification of phytochemicals such as alkaloids [27], flavonoids [28], total phenols [26], tannins [29], and lignin [30] was performed by adopting standard protocols.
2.4. Chemical composition
The carbohydrate [31], free amino acid [32], protein [33], fat [34], fiber [35], cholesterol [36], energy value [33], thiamine [37], riboflavin [38], niacin [39], vitamin E [40], and vitamin C [41] contents were estimated according to the standard procedures. Activities of enzymes such as catalase [42], lipase [43], amylase [44], acid phosphatase [45], and alkaline phosphatase [46] were also estimated. X-ray fluorescence spectrophotometry and atomic absorption spectroscopy techniques were employed to analyze the mineral composition, while phytochemical profile of selected sample was determined using gas chromatography–mass spectrometry (GC–MS) and high-performance thin layer chromatography (HPTLC) fingerprinting techniques.
3. Results and discussion
Green plants synthesize and preserve a variety of phytochemical constituents, many of which are extractable and used as raw material for various scientific investigations. Many secondary metabolites of plants are commercially important and find use in a number of pharmaceutical applications. However, a sustained supply of the source material often becomes difficult due to factors such as environmental changes, cultural practices, diverse geographical distribution, labor cost, selection of the superior plant stock, and over-exploitation by pharmaceutical industries [47]. Hence standards must be evaluated for this source material such as ash value, extractive values, chemical composition, mineral composition, GC–MS profile, and HPTLC fingerprinting. In this connection, physicochemical properties of an Indian traditional medicinal plant C. occidentalis were investigated and its nutritional value was also explored in the present study.
The data obtained in the present work revealed interesting chemical features, which were tabulated. The selected part of C. occidentalis is bitter in taste and brown in color, and has a pleasant odor (Table 1). Tests for identity, purity, and strength were also conducted for C. occidentalis. The moisture content (loss on drying) is 10.17%, which implies that the shelf life of this plant material appears to be longer. Ash content (14.27%) reveals that the plant is rich in mineral contents. Solubility in water (22.69%) is greater than that in alcohol (20.56%; Table 1). This extractive value suggests that the sample satisfies purity standards and is also rich in highly polar compounds. Among all the extracts, water extract of C. occidentalis was found to have the maximum yield (17.53%), followed by ethanol, ethyl acetate, hexane, and chloroform solvent extracts (Table 2).
Table 1.
Physicochemical properties of Cassia occidentalis.
| S. No. | Parameters | Physicochemical properties |
|---|---|---|
| 1 | Taste | Bitter |
| 2 | Color | Brown |
| 3 | Odor | Pleasant |
| 4 | Foreign matter (%) | 0.37 |
| 5 | Loss on drying (%) | 10.17 |
| 6 | Total ash (%) | 14.27 |
| 7 | Acid-insoluble ash (%) | 3.12 |
| 8 | Water solubility (%) | 0.36 |
| 9 | Sulfated ash (%) | 1.56 |
| 10 | Solubility in alcohol (%) | 20.56 |
| 11 | Solubility in water (%) | 22.69 |
Table 2.
Extractive values of Cassia occidentalis.
| S. No. | Solvent | Extractive values (%) |
|---|---|---|
| 1 | Hexane | 6.89 |
| 2 | Chloroform | 6.79 |
| 3 | Ethyl acetate | 7.74 |
| 4 | Ethanol | 9.08 |
| 5 | Water | 17.53 |
Preliminary phytochemical analysis on an aqueous extract of C. occidentalis exhibits the presence of alkaloids, carbohydrates, flavonoids, phenolic compounds, tannins, and lignins (Table 3). Flavonoids recorded a higher percentage of yield (2.45 mg/g sample) when compared with alkaloids (1.56 mg/g sample), lignin (0.34 mg/g sample), tannins (0.21 mg/g sample), and phenols (0.16 mg/g sample) in the aerial part of C. occidentalis (Table 4). Secondary metabolites play both a defensive role against herbivore, pathogen attack, and interplant competition, and an attractant role toward beneficial organisms such as pollinators or symbionts [48]. Plant secondary products also have protective actions in relation to abiotic stresses such as those associated with changes in temperature, water status, light levels, UV exposure, and mineral nutrients. Furthermore, previous work has indicated potential role of secondary products at the cellular level as plant growth regulators and modulators of gene expression, and in signal transduction [49].
Table 3.
Preliminary phytochemical screening of various extracts of Cassia occidentalis.
| Test | Reagents used | Hexane | Chloroform | Ethyl acetate | Ethanol | Water |
|---|---|---|---|---|---|---|
| Alkaloids | Dragendroff | − | − | − | − | + |
| Mayer | − | − | − | − | + | |
| Wagner | − | − | − | − | + | |
| Hager | − | − | − | − | + | |
| Reducing sugar | Fehling | — | − | − | + | − |
| Carbohydrates | Molisch | − | − | − | + | + |
| Saponins | Foam | − | − | − | − | − |
| Glycosides | Anthrone | − | − | − | − | − |
| Steroids | Liebermann–Burchard | + | + | + | + | − |
| Flavonoids | Shinado’s | − | − | − | + | + |
| Phenolic compound | Ferric chloride | + | + | − | − | + |
| Tannin | Lead acetate | − | + | + | − | + |
| Quinone | Sulfuric acid | − | − | − | − | − |
| Anthraquinone | Aqueous ammonia | − | + | − | − | − |
| Lignin | Phloroglucinol | − | + | + | + | + |
| Proteins | Millon | − | − | − | + | − |
| Amino acids | Ninhydrin | − | − | − | + | − |
Table 4.
Estimation of major phytoconstituents of Cassia occidentalis.
| S. No. | Phytoconstituents | Content (mg/g sample) |
|---|---|---|
| 1 | Flavonoid | 2.45 |
| 2 | Alkaloid | 1.56 |
| 3 | Lignin | 0.34 |
| 4 | Tannin | 0.21 |
| 5 | Phenol | 0.16 |
Flavonoids present in the plant might be responsible for its anti-inflammatory properties [50]. Alkaloids are a diverse group of secondary metabolites found to exhibit antimicrobial activity. Alkaloids are also known for decreasing blood pressure, balancing the nervous system in case of mental illness, and possessing antimalarial properties [51]. Tannins help in wound healing, act as an antiparasitic agent, and can reduce the risk of coronary heart diseases. Phenolic compounds are one of the largest and most ubiquitous groups of plant metabolites [52]. Natural antioxidants mainly come from plants in the form of phenolic compounds such as flavonoids, phenolic acids, etc. [53]. A number of studies have focused on the biological activities of phenolic compounds, which are potential antioxidants and free radical scavengers. Modern clinical studies have supported the role of steroids as anti-inflammatory and analgesic agents [54].
Nutritional value of the plant is clearly depicted in Tables 5 and 6. Energy value of the selected plant material was 34.44 kcal, and its crude fiber content was 5.69 mg/g. Intake of dietary fibers present in the selected plant can lower the serum cholesterol level, risk of coronary heart disease, hypertension, constipation, diabetes, and colon and breast cancer [55]. The recommended dietary allowances of fibers essential for children, adults, and pregnant and lactating mothers are 19–25 g/d, 21–38 g/d, and 28–29 g/d, respectively. Thus, C. occidentalis can act as a valuable source of dietary fibers in human nutrition. Other nutritional constituents found are free amino acids and carbohydrates. Total fat and cholesterol contents are as little as 0.03 mg/g. In addition to this, the plant is rich in vitamins, such as thiamine, niacin, and riboflavin, and in enzymes, especially catalase, lipase, amylase, alkaline phosphatase, and acid phosphatase (Table 5).
Table 5.
Nutritional value and biochemical composition of Cassia occidentalis.
| S. No. | Parameters | Content |
|---|---|---|
| 1 | Energy value (kcal) | 34.44 |
| 2 | Crude fiber (mg/g) | 5.69 |
| 3 | Free amino acids (mg/g) | 1.52 |
| 4 | Carbohydrate (mg/g) | 1.38 |
| 5 | Protein (mg/g) | 0.49 |
| 6 | Total fat (mg/g) | 0.03 |
| 7 | Cholesterol (mg/g) | 0.03 |
| 8 | Thiamine (μg/g) | 6.9 |
| 9 | Niacin (μg/g) | 12.6 |
| 10 | Riboflavin (μg/g) | 71.5 |
| 11 | Catalase (μg/g) | 9.8 |
| 12 | Lipase (μg/g) | 13.6 |
| 13 | Amylase (μg/g) | 10.8 |
| 14 | Alkaline phosphatase (mg/g) | 0.41 |
| 15 | Acid phosphatase (mg/g) | 0.21 |
Table 6.
Mineral composition and heavy metal content of Cassia occidentalis.
| S. No. | Minerals | Content |
|---|---|---|
| 1 | Fe (%) | 11.036 |
| 2 | Ca (%) | 2.69 |
| 3 | Mn (%) | 2.39 |
| 4 | K (%) | 2.36 |
| 5 | Mg (%) | 1.54 |
| 6 | Zn (%) | 1.24 |
| 7 | Cu (%) | 0.74 |
| 8 | Na (%) | 0.58 |
| 9 | P (%) | 0.54 |
| 10 | S (%) | 0.29 |
| 11 | Pb (ppm) | <0.005 |
| 12 | Hg (ppm) | <0.005 |
| 13 | Cd (ppm) | <0.005 |
X-ray fluorescence spectrophotometry data suggested that the plant is rich in minerals, especially Fe, Ca, K, Mn, Mg, Zn, Cu, Na, P, and S (Table 6). From these data, it can be deduced that C. occidentalis has a high content of Fe and so can be used in the treatment of anemia. Deficiency of calcium and phosphorous leads to the classic bone symptoms associated with rickets, such as bowlegs, knock knees, curvature of the spine, and pelvic and thoracic deformities. Magnesium plays an important role in the structure and function of the human body. Iron, zinc, copper, and manganese play important roles in the improvement of the antioxidant system. The positive impact of zinc supplementation on the growth of some stunted children, and on the prevalence of selected childhood diseases such as diarrhea, suggests that zinc deficiency is likely to be a significant public health problem, especially in developing countries [56,57]. According to Food and Agricultural Organization’s (FAO’s) food balance data, it has been calculated that about 20% of the world’s population can be at a risk of zinc deficiency with an average daily intake of <70 μg/d [58]. These findings stimulate the on-farm cultivation of C. occidentalis in a large scale to relieve the iron and zinc deficiencies in local community.
The suggested concentration of lead (Pb) in plant species is 2–6 mg/L [59]. Lead has carcinogenic properties, it impairs both the respiratory and the digestive systems, and it suppresses the immune system. It is particularly harmful in children, damaging their intelligence and the nervous systems [60]. The presently investigated plant materials have chances of lead contamination from soil, water, and atmosphere, and hence a high level of Pb can be accumulated. As per the World Health Organization Guidelines, the limit for lead is 10 ppm, cadmium 0.3 ppm, and mercury 1 ppm [33]. The atomic absorption spectrophotometric investigation suggested that the selected plant material has meager quantities of Pb, Cd, and Hg, which further clarifies its use as a safe nontoxic food supplement.
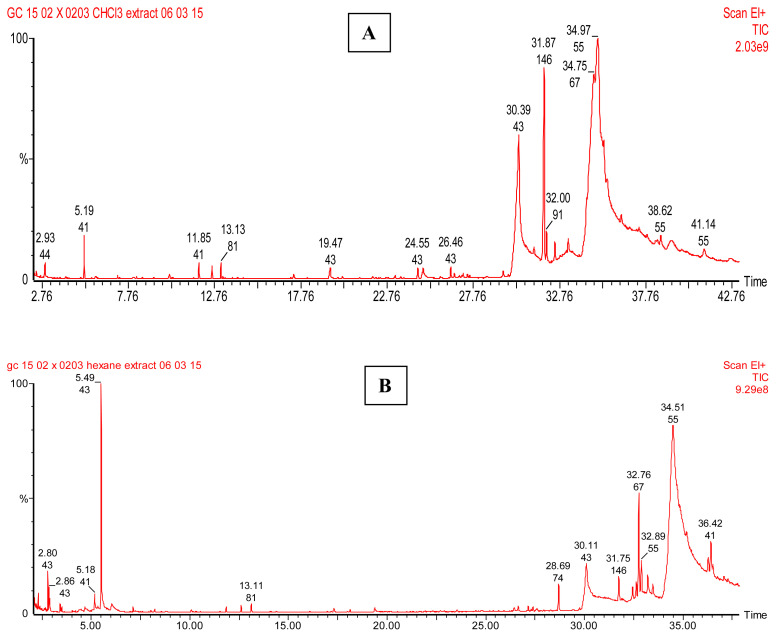
GC–MS analysis of the hexane extract of C. occidentalis revealed the presence of volatile phytochemical compounds such as nonanoic acid, dodecanoic acid, tetradecanoic acid, n-hexadecanoic acid, 10-octadecenoic acid methyl ester, 9,12-octadecadienoic acid, and oleic acid 3-hydroxypropyl ester (Figure 1A and Table 7). GC–MS of the chloroform extract revealed the presence of phytochemical compounds such as hexanoic acid, octanoic acid, n-decanoic acid, 9-oxononanoic acid, dodecanoic acid, 1,6-anhydro-à-d-galactofuranose, 3-ethyl-2-hydroxy-2-cyclopenten-1-one, tetradecanoic acid, n-hexadecanoic acid, and phytol (Figure 1B and Table 8). Phytoconstituents such as dodecanoic acid, tetradecanoic acid, and n-hexadecanoic acid were commonly found in both hexane and chloroform extracts of C. occidentalis. These compounds were also detected in the GC–MS analysis of surface of clay tea pots [61].
Figure 1.
GC–MS chromatogram of (A) chloroform and (B) hexane extracts of Cassia occidentalis. GC–MS = gas chromatography–mass spectrometry.
Table 7.
GC–MS profile of hexane extract of Cassia occidentalis.
| S. No. | Peak name | Retention time (min) | Peak area | % Peak area |
|---|---|---|---|---|
| 1 | Nonanoic acid | 10.06 | 502,244 | 0.1584 |
| 2 | Dodecanoic acid | 19.38 | 1,149,147 | 0.3625 |
| 3 | Tetradecanoic acid | 24.79 | 515,242 | 0.1625 |
| 4 | n-Hexadecanoic acid | 30.11 | 25,483,818 | 8.0380 |
| 5 | 10-Octadecenoic acid methyl ester | 32.89 | 13,129,060 | 4.1411 |
| 6 | 9,12-Octadecadienoic acid | 34.51 | 171,295,296 | 54.0291 |
| 7 | Oleic acid 3-hydroxypropyl ester | 36.52 | 2,453,627 | 0.7739 |
GC–MS = gas chromatography–mass spectrometry.
Table 8.
GC–MS profile of chloroform extract of Cassia occidentalis.
| S. No. | Peak name | Retention time (min) | Peak area | % Peak area |
|---|---|---|---|---|
| 1 | Hexanoic acid | 5.86 | 1,692,489 | 0.3081 |
| 2 | Octanoic acid | 10.14 | 1,974,734 | 0.3595 |
| 3 | n-Decanoic acid | 14.43 | 373,347 | 0.0680 |
| 4 | 9-Oxononanoic acid | 17.35 | 2,940,956 | 0.5354 |
| 5 | Dodecanoic acid | 19.47 | 7,191,673 | 1.3092 |
| 6 | 1,6-Anhydro-à-d-galactofuranose | 21.67 | 58,710 | 0.0107 |
| 7 | 3-ethyl-2-hydroxy-2-cyclopenten-1-one | 22.36 | 482,644 | 0.0879 |
| 8 | Tetradecanoic acid | 24.85 | 10,046,852 | 1.8289 |
| 9 | n-Hexadecanoic acid | 30.39 | 152,382,432 | 27.7395 |
| 10 | Phytol | 33.27 | 5,594,367 | 1.0184 |
GC–MS = gas chromatography–mass spectrometry.
HPTLC fingerprinting of the plant is presented in Figure 2 with Rf values under 254 nm and 366 nm, which confirms the presence of different types of phytochemical compounds in the aqueous extract of the aerial part of C. occidentalis (Table 9). Four bands of dark green color were observed with Rf values of 0.10, 0.21, 0.80, and 0.86 at 254 nm, while 12 bands of red (Rf values 0.14 and 0.80), pink (Rf value 0.19), green (Rf values 0.32 and 0.46), fluorescence blue (Rf values 0.65 and 0.74), yellow (Rf values 0.77 and 0.88), brown (Rf values 0.83 and 0.92), and fluorescence green (Rf value 0.97) colors were noted at 366 nm. The presence of such bands of different colors and Rf values indicated the occurrence of different types of phytochemicals in the extract of C. occidentalis.
Figure 2.
HPTLC fingerprinting profile of Cassia occidentalis. HPTLC = high-performance thin layer chromatography.
Table 9.
HPTLC fingerprinting profile of Cassia occidentalis.
| S. No. | Rf values | Color of the spot | ||
|---|---|---|---|---|
|
|
|
|||
| 254 nm | 366 nm | 254 nm | 366 nm | |
| 1 | 0.10 | 0.14 | Dark green | Red |
| 2 | 0.21 | 0.19 | Dark green | Pink |
| 3 | 0.80 | 0.23 | Dark green | Red |
| 4 | 0.86 | 0.32 | Dark green | Green |
| 5 | — | 0.46 | — | Green |
| 6 | — | 0.65 | — | Fluorescence blue |
| 7 | — | 0.74 | — | Fluorescence blue |
| 8 | — | 0.77 | — | Yellow |
| 9 | — | 0.83 | — | Brown |
| 10 | — | 0.88 | — | Yellow |
| 11 | — | 0.92 | — | Brown |
| 12 | — | 0.97 | — | Fluorescence green |
HPTLC = high-performance thin layer chromatography.
3.1. Conclusion
Plant samples are safe and effective in treating various ailments without serious side effects. However, the lacuna existing in the herbal industry is the lack of standardization. Hence, attempts were made in the present work to determine the physical properties and chemical composition of an Indian medicinal plant C. occidentalis L., and also to evaluate its nutritional potential so that it can be used as a herbal supplement for treating various diseases and disorders. The findings of the present study revealed that the selected plant has high levels of energy value, crude fibers, vitamins, and minerals, especially iron and zinc, with antioxidant enzymes. Hence, C. occidentalis can be used as a safe, nutritious, and medicinally active food supplement. The sample has many favorable physicochemical characteristics such as high ash content, water solubility, and extract yield. Physicochemical properties determined in the present study will be useful in the identification and authentication of this plant material and can be used as quality control parameters. The presence of certain phytochemical constituents was confirmed by qualitative, quantitative, GC–MS, and HPTLC analyses.
Acknowledgments
The authors are thankful to the management and administrative authorities of SASTRA University for their support and encouragement in this research project.
Funding Statement
The authors are thankful to the management and administrative authorities of SASTRA University for their support and encouragement in this research project.
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
REFERENCES
- 1. Ballabh B, Chaurasia OP. Traditional medicinal plants of cold desert Ladakh—used in treatment of cold, cough and fever. J Ethnopharmacol. 2007;112:341–5. doi: 10.1016/j.jep.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 2. Pandey MM, Rastogi S, Rawat AKS. Indian herbal drug for general healthcare: an overview. IJAM. 2008;6:3. [Google Scholar]
- 3. Patwardhan B, Warude D, Pushpangadan P, Bhatt N. Ayurveda and traditional Chinese medicine: a comparative overview. J Evid Based Complementary Altern Med. 2005;2:465–73. doi: 10.1093/ecam/neh140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Humphry C, Clegg MS, Keen C, Grivetti LE. Food diversity & drought survival—the Hausa example. Int J Food Sci Nutr. 1993;44:1–16. [Google Scholar]
- 5. Reeta M, Sharma R. Kasamarda (Senna occidentalis Linn.): Ayurvedic approach. JPSI. 2013;2:25–7. [Google Scholar]
- 6. Babitha S. A stimulatory effect of Cassia occidentalis on melanoblast differentiation and migration. Arch Dermatol Res. 2011;303:211–6. doi: 10.1007/s00403-011-1127-y. [DOI] [PubMed] [Google Scholar]
- 7. Arya V. Antioxidant activity of organic and aqueous leaf extracts of Cassia occidentalis L. in relation to their phenolic content. Nat Prod Res. 2011;25:1473–9. doi: 10.1080/14786419.2010.545351. [DOI] [PubMed] [Google Scholar]
- 8. Yadav J. Cassia occidentalis L.: a review on its ethnobotany, phytochemical and pharmacological profile. Fitoterapia. 2010;81:223–30. doi: 10.1016/j.fitote.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 9. Kolhapure SA, Mitra WS. Meta-analysis of 50 phase III clinical trials in evaluation of efficacy and safety of Liv. 52 in infective hepatitis. Med Update. 2004;12:51–61. [Google Scholar]
- 10. Reddy K, Kulkarni KL. A clinical trial of Herbolax in constipation during post-operative period. Antiseptic. 2001;7:252–3. [Google Scholar]
- 11. Bonnisan DJ. A metabolic corrective in gastrointestinal disorders of newborn. Probe. 1973;2:73–8. [Google Scholar]
- 12. Hussain HSN, Deeni YY. Plants in Kano ethnomedicine: screening for antimicrobial activity and alkaloids. Ind J Pharmacol. 1991;29:51–6. [Google Scholar]
- 13. Saganuwan AS, Gulumbe ML. Evaluation of in vitro antimicrobial activities and phytochemical constituents of C. occidentalis. Animal Res Int. 2006;3:566–9. [Google Scholar]
- 14. Tona L, Mesia K, Ngimbi NP, Chrimwami B, Ahoka O, Cimanga K. In vivo antimalarial activity of Cassia occidentalis, Morinda morindoides and Phyllanthus niruri. Ann Trop Med Parasitol. 2001;95:47–57. [PubMed] [Google Scholar]
- 15. Sadique J, Chandra T, Thenmozhi V, Elango V. Biochemical modes of action of Cassia occidentalis and Cardiospermum halicacabum in inflammation. J Ethnopharmacol. 1987;19:201–12. doi: 10.1016/0378-8741(87)90042-0. [DOI] [PubMed] [Google Scholar]
- 16. Sharma N, Trikha P, Athar M, Raisuddin S. Protective effect of Cassia occidentalis extract on chemical-induced chromosomal aberrations in mice. Drug Chem Toxicol. 1999;22:643–53. doi: 10.3109/01480549908993173. [DOI] [PubMed] [Google Scholar]
- 17. Cowans MM. Plant materials as antimicrobial agents. Med Res Rev. 1999;12:564–82. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alves AC. Pharmacological study of the root of Cassia occidentalis. Anals Fac Farm Porto. 1965;24:65–119. [Google Scholar]
- 19. Anton R, Duqenois P. L’emplois des Cassia dans les pays tropicaux et subtropicaux examine d’apresquelquesuns des constutiants chimiques decesplantes medicinales. Plantes Med Phytother. 1968;2:255–68. [in French] [Google Scholar]
- 20. Kudav NA, Kulkarni AB. Chemical investigation on Cassia occidentalis II. Isolation of islandicin, helminthosporin, xanthorin and NMR spectral studies of cassiollin and its derivatives. Ind J Chem. 1974;12:1042–4. [Google Scholar]
- 21. Chukwujekwu JC, Coombes PH, Mulholland DA, Staden J. Emodin, an antibacterial anthraquinone from the roots of Cassia occidentalis. S Afr J Bot. 2006;72:295–307. [Google Scholar]
- 22. Smolenski IJ, Silinis H, Farnsworth NR. Alkaloid screening VI. Lloydia. 1975;38:225–56. [PubMed] [Google Scholar]
- 23.Government of India. The Ayurvedic pharmacopoeia of India. Vol. 1. New Delhi: Department of Indian system of medicine and Homeopathy, Ministry of Health and Family Welfare, Government of India; 2001. pp. 142–3. [Google Scholar]
- 24.Trease GE, Evans WC. Pharmacognosy. 11th ed. London: Brailliar Tiridel Can Macmillan Publishers; 1989. [Google Scholar]
- 25.Harborne JB. Phytochemical methods. 3rd ed. London: Chapman and Hall; 1998. pp. 1–302. [Google Scholar]
- 26. Edeoga HO, Okwu DE, Mbaebie BO. Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol. 2005;4:685–8. [Google Scholar]
- 27.Harborne JB. Phytochemical methods. 1st ed. London: Chapman and Hall; 1973. pp. 49–188. [Google Scholar]
- 28. Boham BA, Kocipai AC. Flavonoids and condensed tannins from leaves of Hawaiian Vaccinium vaticulatum and V. calycinium. Pacific Sci. 1994;48:458–63. [Google Scholar]
- 29. Van-Burden TP, Robinson WC. Formation of complexes between protein and tannic acid. J Agric Food Chem. 1969;17:772–7. [Google Scholar]
- 30. Czerkawski JW. The determination of lignin. Br J Nutr. 1967;21:325–32. doi: 10.1079/bjn19670034. [DOI] [PubMed] [Google Scholar]
- 31. Southgate DAT. Determination of carbohydrates in foods I. – Available carbohydrate. J Sci Food Agric. 1969;20:326–30. doi: 10.1002/jsfa.2740200602. [DOI] [PubMed] [Google Scholar]
- 32. Zhang Z. A novel colorimetric determination of free amino acids content in tea infusions with 2,4-dinitrofluorobenzene. J Food Comp Anal. 2009;22:137–41. [Google Scholar]
- 33. Rajashree R, Divya G, Sushma P, Kanchan I, Ashwagandha A, Shatavari A. Formulations as herbal medicines and nutraceuticals. Res J Pharm Sci. 2012;1:10–5. [Google Scholar]
- 34. Eller F, King JW. Determination of fat content in foods by analytical SFE. Semin Food Anal. 1996;1:14–6. [Google Scholar]
- 35. Van Soest PJ, McQueen RW. The chemistry and estimation of fibre. Proc Nutr Soc. 1973;32:123–30. doi: 10.1079/pns19730029. [DOI] [PubMed] [Google Scholar]
- 36. Valsta LM, Lemstrom A, Ovaskainen ML, Lampi AM, Toivo J, Korhonen T, Piironen V. Estimation of plant sterol and cholesterol intake in Finland: quality of new values and their effect on intake. Brit J Nutr. 2004;92:671–8. doi: 10.1079/bjn20041234. [DOI] [PubMed] [Google Scholar]
- 37. Chaikelis AS. An adaptation of the standard photofluorometric analysis to the estimation of thiamine in plants. Biol Rev City Coll NY. 1947;9:22–5. [PubMed] [Google Scholar]
- 38. Weisberg SM. Estimation of riboflavin. Ind Eng Chem Anal Ed. 1937;9:523–4. [Google Scholar]
- 39. Okwu DE, Josiah C. Evaluation of the chemical composition of two Nigerian medicinal plants. Afr J Biotechnol. 2006;5:357–61. [Google Scholar]
- 40. Jayashree V, Solimabi W, Kamat SY. Distribution of tocopherol (vitamin E) in marine algae from Goa, West coast of India. Indian J Mar Sci. 1985;14:228–9. [Google Scholar]
- 41. Sarkiyayi S, Ikioda H. Estimation of thiamin and ascorbic acid contents in fresh and dried Hibiscus sabdarriffa (Roselle) and Lactuca sativa (Tettuce) Adv J Food Sci Technol. 2010;2:47–9. [Google Scholar]
- 42. Sinha AK. Assay of catalase. Anal Biochem. 1972;2:389–94. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 43. Arzoglou PL, Tavridou A, Lessinger JM, Tzimas G, Ferard G. Spectrophotometric determination of lipase activity in the presence of increased triolein concentration. Ann Biol Clin. 1992;50:155–60. [PubMed] [Google Scholar]
- 44. Huggins C, Russel PS. Colorimetric determination of amylase. Ann Surg. 1948;128:668–78. doi: 10.1097/00000658-194810000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kolari KK, Sarjala T. Acid phosphatase activity and phosphorus nutrition in Scots pine needles. Tree Physiol. 1995;15:747–52. doi: 10.1093/treephys/15.11.747. [DOI] [PubMed] [Google Scholar]
- 46. Olusegun OF. Characterization of alkaline phosphatase from the seeds of Dacryode sedulis. Int J Eng Sci. 2013;2:31–6. [Google Scholar]
- 47. Singh PS, Salwan C, Mann AS. Evaluation of anti-diabetic activity of leaves of Cassia occidentalis. Int J Res Pharm Chem. 2011;1:904–13. [Google Scholar]
- 48.Wink M, Schimmer O. Modes of action of defensive secondary metabolites. In: Wink M, editor. Functions of plant secondary metabolites and their exploitation in biotechnology. Boca Raton, FL: CRC Press; 1999. pp. 17–112. [Google Scholar]
- 49.Kaufman PB, Cseke LJ, Warber S, Duke JA, Brielmann HL. Natural products from plants. Boca Raton, FL: CRC Press; 1999. [Google Scholar]
- 50. Kunle OF, Egharevba HO. Preliminary studies on Vernonia ambigua: phytochemistry and antimicrobial screening of the whole plant. Ethnobot Leaf. 2009;13:1216–21. [Google Scholar]
- 51. Ronan B, Ademir JSJ, Alaide BO. Plant-derived antimalarial agents: new leads and efficient phytomedicine. Part II. Non-alkaloid natural products—a review. Molecules. 2009;14:3037–72. doi: 10.3390/molecules14083037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Singh R, Singh S. Evaluation of antioxidant potential of ethyl acetate extract/fractions of Acacia auricliformis A. Cunn. Food Chem Toxicol. 2007;45:1216–23. doi: 10.1016/j.fct.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 53. Ali SS, Kasoju N, Singh AL, Sharanabasava H, Sahuand A, Bora U. Indian medicinal herbs as source of antioxidants. Food Res Int. 2008;41:S1–15. [Google Scholar]
- 54. Singh AP. Short review: distribution of steroid like compounds in plant flora. Pharmacogn Mag. 2006;2:87–9. [Google Scholar]
- 55. Ishida H, Suzuno H, Sugiyama N, Innami S, Todokoro T, Maekawa A. Nutritional evaluation of chemical component of leaves, stalks and stems of sweet potatoes (Ipomoea batataspoir) Food Chem. 2000;68:359–67. [Google Scholar]
- 56. Osendarp SJ, West CE, Black RE. The need for maternal zinc supplementation in developing countries: an unresolved issue. J Nutr. 2003;133:817–27. doi: 10.1093/jn/133.3.817S. [DOI] [PubMed] [Google Scholar]
- 57. Hussain J, Khan AL, Rehman N, Hamayun M, Shah T, Nisar M, Bano T, Shinwari ZK, Lee IJ. Proximate and nutrient analysis of selected vegetable species: a case study of Karak region of Pakistan. Afr J Biotechnol. 2009;8:2725–9. [Google Scholar]
- 58. Holt C, Brown KH. International Zinc Nutrition Consultative Group (IZINCG) assessment of the risk of zinc deficiency in populations and options for its control. Food Nut Bull. 2004;25:94–103. [PubMed] [Google Scholar]
- 59. Zakir S, Sarwar M, Allen J, Khan MN, Butt MS. Variation in physicochemical characteristics of some cultivars of sweet potato. Pak J Bot. 2006;38:283–91. [Google Scholar]
- 60. Zhong WS, Ren T, Zhao LJ. Determination of Pb (lead), Cd (cadmium), Cr (chromium), Cu (copper), and Ni (nickel) in Chinese tea with high-resolution continuum source graphite furnace atomic absorption spectrometry. J Food Drug Anal. 2016;24:46–55. doi: 10.1016/j.jfda.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chung TY, Kuo PC, Liao ZH, Shih YE, Yang ML, Cheng ML, Wu CC, Tzen JTC. Analysis of lipophilic compounds of tea coated on the surface of clay teapots. J Food Drug Anal. 2015;23:71–81. doi: 10.1016/j.jfda.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]