Abstract
Cytochrome P450 BM-3, a self-sufficient P450 enzyme from Bacillus megaterium that catalyzes the subterminal hydroxylation of long-chain fatty acids, has been engineered into a catalyst for the oxidation of polycyclic aromatic hydrocarbons. The activities of a triplet mutant (A74G/F87V/L188Q) towards naphthalene, fluorene, acenaphthene, acenaphthylene, and 9-methylanthracene were 160, 53, 109, 287, and 22/min, respectively. Compared with the activities of the wild type towards these polycyclic aromatic hydrocarbons, those of the mutant were improved by up to 4 orders of magnitude. The coupling efficiencies of the mutant towards naphthalene, fluorene, acenaphthene, acenaphthylene, and 9-methylanthracene were 11, 26, 5.4, 15, and 3.2%, respectively, which were also improved several to hundreds fold. The high activities of the mutant towards polycyclic aromatic hydrocarbons indicate the potential of engineering P450 BM-3 for the biodegradation of these compounds in the environment.
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitously present in the environment and have harmful effects on humans (3, 7). Recently, the potentials of P450 enzymes to transform PAHs have been evaluated to develop novel strategies in environmental bioremediation (6, 8, 21). Hydroxylation of PAHs by P450 enables the biodegradation of PAHs. Hydroxylated PAHs are more water-soluble and are the substrates for oxidases, such as peroxidase and laccase, that decompose phenolic compounds. The oxidation of PAHs by mammalian P450 enzymes has been extensively investigated (21, 22), but because of the low stability and activity of these P450s they are less promising from the perspective of the biodegradation of PAHs in the environment. Bacterial P450s are relatively stable and highly active and can often be prepared in large amounts using recombinant expression systems. Protein engineering of bacterial P450s with activities toward PAHs could be an alternative method for this purpose (15, 16, 18).
P450cam has been engineered for the degradation of PAHs with the highest turnover, 3.0 and 4.5/min, for phenanthrene and fluoranthene, respectively (6). Cytochrome P450 BM-3 from Bacillus megaterium is another one of the best-studied bacterial P450s whose crystal structure is available (1, 4, 9, 10, 17, 20). P450 BM-3 can be largely prepared by recombinant expression in Escherichia coli (11–13) and is a stable, highly active, and self-sufficient P450 consisting of a catalytic heme domain and a diflavin reductase domain in a single polypeptide of 119 kDa (17, 20). These properties are closer to application requirements than those of other P450s. However, this P450 has a well-defined substrate specificity and catalyzes the hydroxylation of long-chain fatty acids and the epoxidation of the double bonds of long-chain unsaturated fatty acids (1, 4, 10, 17, 20).
Protein engineering of P450 BM-3 to obtain activities towards substrates other than long-chain fatty acids, such as short-chain fatty acids or indole, was reported recently (11, 19). Just before we submitted this manuscript, Carmichael and Wong (2) reported the oxidation of pyrene, phenanthrene, and fluoranthene by mutants of P450 BM-3. However, we obtained another mutant showing higher activities for the oxidation of other PAHs such as naphthalene, fluorene, acenaphthene, acenaphthylene, and 9-methylanthracene. The triplet mutant (A74G/F87V/L188Q) obtained by us does not share any mutation with the mutants reported by Carmichael and Wong except that one of the mutated positions (Phe87) is shared with some of their mutants (2). A74 and L188 in our mutation essentially constitute the entrance of the substrate access channel and have hydrophobic interactions with substrates (9), while R47 and Y51 in the mutation of Carmichael and Wong act as anchors for the carboxylate group of the substrate (2). Here, we report our results.
MATERIALS AND METHODS
Chemicals.
1,2-Dihydroxyacenaphthene was prepared by reduction of acenaphthenequinone with sodium hydrosulfite. Other chemicals were of analytical grade and are commercially available.
Randomization of specific codons of P450 BM-3.
Three sites (Phe87, Leu188, and Ala74) were randomized by site-directed mutagenesis using a Stratagene QuikChange kit (La Jolla, Calif.) as described previously (11, 13). The following PCR primers were employed for the respective sites: for Phe87, 5′-GCAGGAGACGGGTTGNNNACAAGCTGGACG-3′ and 5′-CGTCCAGCTTGTNNNCAACCCGTCTCCTGC-3′; for Leu188, 5′-GAAGCAATGAACAAGNNNCAGCGAGCAAATCCAG-3′ and 5′-CTGGATTTGCTCGCTGNNNCTTGTTCATTGCTTC-3′; and for Ala74, 5′-GCTTTGATAAAAACTTAAGTCAANNNCTTAAATTTGTACG-3′ and 5′-CGTACAAATTTAAGNNNTTGACTTAAGTTTTTATCAAAGC-3′.
Expression and purification of WT and mutant P450 BM-3.
The wild-type (WT) and mutant P450 BM-3 genes were expressed under the control of the strong, temperature-inducible PRPL-promoter of pCYTEXP1 in E. coli strain UM2, a catalase-deficient strain (14), as described previously (12). Cell disruption, purification, and determination of the concentration of the purified enzyme were carried out as described elsewhere (13).
Isolation of mutants.
Some mutants of P450 BM-3 produced pigments in the culture broth after induction of the expression of their genes in E. coli UM2. Structural analysis of the major pigment indicated that it might have a polycyclic aromatic structure. Thus, we used the pigments as indicators to screen mutants with higher activities towards PAHs. From the mutant created by randomized mutagenesis of the codon of the respective site, 100 colonies were isolated to ensure that most of the 19 possible kinds of amino acids were tested. Under these conditions, the probability of obtaining each amino acid is greater than 95%, except for tryptophan and methionine, for which the probability is 79% (12). The selected colonies were cultured in tubes containing 1 ml of Luria-Bertani medium at 30°C for 3 h, and then the temperature was shifted to 42°C for 10 h to induce the expression of P450 BM-3 mutant genes and to allow the production of pigments. After cells had been separated by centrifugation, the absorbance of the supernatants containing pigments was measured at 545 nm. The mutant that produced the largest amount of pigments among all the mutants at the same site was used as a template for the next site-specific randomized mutagenesis. All mutations described in this report have been confirmed by DNA sequencing.
Enzyme activity assay.
To measure the NADPH consumption rate, 60 μl of a 2.5 μM P450 sample was mixed with 480 μl of 0.1 M Tris-HCl buffer (pH 7.4) containing 6 μl of substrate dimethyl sulfoxide (DMSO) stock solution. The concentrations of the substrate stock solutions were 200, 20, 30, 50, and 15 mM for naphthalene, fluorene, acenaphthene, acenaphthylene, and 9-methylanthracene, respectively. After 2 min of standing, 60 μl of 3.5 mM NADPH was added to start the reaction. NADPH consumption was monitored at 340 nm with a Shimadzu MultiSpec-1500 spectrophotometer (Kyoto, Japan) at 25°C for 20 s (or 150 s for WT, because of its low activity). The NADPH concentration was calculated using εM = 6,200 M−1 cm−1 (23).
To measure the coupling efficiency (the ratio [percentage] of NADPH used for substrate oxidation to the total amount of NADPH consumed by P450), 100 μl of a 45 μM P450 sample was mixed with 4,800 μl of 0.1 M Tris-HCl buffer (pH 7.4) containing 50 μl of substrate stock solution and 80 U of catalase (9,200 U/mg; Wako Pure Chemical Industries). After 2 min of standing, 100 μl of 20.0 mM NADPH was added, followed by standing for 2 h at 25°C to allow all the NADPH to be consumed. Before extraction, an internal standard of 1-naphthalenol, at a final concentration of 15 μM, was added to a reaction mixture containing a substrate other than naphthalene. For the reaction with naphthalene, 1-fluorenol was used as the internal standard at a final concentration of 15 μM. Reaction mixtures were extracted with 3 ml of chloroform twice and then the extracts were evaporated to around 100 μl for gas-liquid chromatography (GC) analysis. The coupling efficiencies of PAHs were calculated from the amounts of products determined by GC, GC-mass spectrometry (MS), or high-performance liquid chromatography (HPLC). The hydroxylation activity was calculated by multiplying the NADPH consumption rate by the coupling efficiency.
GC and GC-MS analysis.
GC analysis was performed with a Shimadzu GC-17A gas chromatograph equipped with a flame ionization detector and a split injection system and fitted with a capillary column (DB-17, 30 m by 0.554 mm internal diameter; J & W Scientific, Folsom, Calif.). The column temperature was initially 100°C, and then it was raised to 280°C at the rate of 5°C/min and maintained at that temperature for 20 min. The injector and detector were operated at 280°C. Helium was used as the carrier gas at 30 kPa/cm2. GC-MS analysis was performed on a GC-MS QP5050 (Shimadzu) with a GC-17A gas chromatograph equipped with a capillary column (HR-1; 25 m by 0.25 mm internal diameter; Shinwa Kako, Kyoto, Japan) at 150°C. Helium was used as the carrier gas at 225 kPa/cm2. MS was performed in the electron impact mode at 70 eV with a source temperature of 250°C. Split injection was employed with the injection port at 250°C.
HPLC analysis.
HPLC analysis was performed to determine the amounts of 1-naphthol and 2-naphthol. The naphthalene reaction samples were extracted twice with chloroform, 6 ml in total, evaporated to dryness, and then dissolved in 5% DMSO for HPLC analysis. HPLC analysis was carried out on a C18 reverse-phase column (Cosmosil 5C AR-II; 4.6 mm internal diameter by 250 mm; Nacalai Tesque, Kyoto, Japan) and eluted at 1.0 ml/min with a methanol-water gradient containing 1% acetic acid. The methanol gradient was as follows: 0 to 4 min, 0% (vol/vol); 4 to 10 min, 0 to 28%; 10 to 50 min, 28 to 80%; 50 to 60 min, 80%. The absorbance of the eluent was monitored at 275 nm.
Determination of binding spectra.
The substrate-induced spectral shifts of P450 BM-3 enzymes were recorded with a Shimadzu MultiSpec-1500 spectrophotometer at 30°C. Naphthalene, fluorene, acenaphthene, and acenaphthylene dissolved in DMSO at concentrations of 300, 3, 15, and 15 mM, respectively, were freshly prepared for analysis. For each substrate, the substrate solution was added in small amounts to the enzyme solution containing 3.8 μM P450 in 0.1 M Tris-HCl (pH 7.4) in a cuvette, and the same amount of DMSO was added to the reference cuvette containing the enzyme solution. For each substrate for any enzyme, triplicate binding assays with 18 substrate concentrations were performed. The change in absorbance (A) was determined by subtracting A425 (trough) from the A390 (peak). The maximum heme spin-state shift of WT binding to palmitic acid was taken as a 100% heme spin-state shift. Data analysis and calculations were carried out according to methods described elsewhere (23).
RESULTS
Isolation of P450 BM-3 mutants for PAH oxidations.
Mutants of P450 BM-3 for PAH oxidations were obtained by increasing the productivity of pigments with polycyclic aromatic structure in the culture broth through three steps of site-specific randomized mutagenesis. Phe87 was selected as the first site for mutation because several mutants which have a substitution at this site with Val, Ala, or Gly were initially found to produce the pigments. Leu188 and Ala74 were selected as the second and third sites for mutation according to the results of previous work on the hydroxylation of indole and shorter-chain fatty acids (11, 12). After the first mutation, 14 colonies produced pigments after induction and the 1 producing the highest amount of pigments was selected for DNA sequencing, which revealed the substitution of Phe87 by Val (F87V). The F87V mutant was used as the template DNA for the second round of site-specific randomized mutagenesis at Leu188. Seventy-two colonies produced pigments during the cultivation and the one exhibiting the highest activity was found to contain a new substitution of Leu188 by Gln besides Phe87Val (F87V/L188Q). The A545 due to the pigment production of the culture of this double mutant was 2.3 times higher than that of F87V. This double mutant DNA was used as the template for the third round of site-specific randomized mutagenesis at Ala74. Seventy-seven colonies produced pigments and the one producing the highest amount of pigments was found to be a triple mutant containing a new substitution of Ala74 by Gly besides the Phe87Val and Leu188Gln mutations (A74G/F87V/L188Q). The A545 of the culture of this triplet mutant was 1.2 times higher than that of F87V/L188Q.
PAH oxidation activity.
The WT showed quite low NADPH consumption rates and coupling efficiencies for all of the PAHs (Tables 1 and 2). The first substitution of Phe87Val improved the NADPH consumption rates from several- to nearly 50-fold (Table 1) and the coupling efficiencies towards these PAHs several- to 200-fold (Table 2). In total, the first substitution improved the enzyme activities towards these PAHs by 2 to 3 orders of magnitude (Table 3). The second substitution of Leu188Gln also significantly improved the enzyme activities towards all the three-ring PAHs by several- to more than 30-fold. The third substitution of Ala74Gly again greatly enhanced the enzyme activities towards all the PAHs, mainly by increasing the NADPH consumption rates. At last, the activities of the triplet mutant towards all these PAHs were 2 to 4 orders of magnitude higher than those of the WT.
TABLE 1.
NADPH consumption activities of WT P450 BM-3 and mutants towards PAHs
| Substrate | Activitya
|
|||
|---|---|---|---|---|
| WT | F87V | F87V/L188Q | A74G/F87V/ L188Q | |
| Naphthalene | 17.2 ± 0.9 | 767 ± 18 | 1,040 ± 10 | 1,420 ± 8 |
| Fluorene | 3.6 ± 0.6 | 49.2 ± 3.7 | 96.1 ± 2.1 | 203 ± 4 |
| Acenaphthene | 16.2 ± 1.3 | 94.2 ± 7.9 | 1,520 ± 10 | 2,020 ± 20 |
| Acenaphthylene | 18.0 ± 2.3 | 482 ± 7 | 945 ± 8 | 1,620 ± 10 |
| 9-Methylanthracene | 9.3 ± 0.9 | 124 ± 9 | 384 ± 7 | 683 ± 10 |
NADPH consumption activities were measured in units of nanomoles of NADPH consumed per nanomole of P450 per minute. Three independent experiments were done for each measurement.
TABLE 2.
Coupling efficiencies of WT P450 BM-3 and mutants on oxidation of unnatural substrates, PAHs
| Substrate | Coupling efficiency (%)
|
|||
|---|---|---|---|---|
| WT | F87V | F87V/L188Q | A74G/F87V/ L188Q | |
| Naphthalene | 4.38 ± 0.27 | 14.5 ± 0.8 | 11.0 ± 0.2 | 11.3 ± 0.5 |
| Fluorene | 0.39 ± 0.10 | 5.63 ± 0.15 | 23.6 ± 1.0 | 26.0 ± 0.4 |
| Acenaphthene | 0.017 ± 0.01 | 3.43 ± 0.14 | 5.00 ± 0.11 | 5.42 ± 0.08 |
| Acenaphthylene | 0.68 ± 0.12 | 14.8 ± 0.4 | 15.8 ± 0.1 | 15.2 ± 0.1 |
| 9-Methylanthracene | 0.021 ± 0.01 | 0.58 ± 0.22 | 2.41 ± 0.06 | 3.20 ± 0.22 |
Coupling efficiency is the ratio of NADPH used for substrate oxidation to the total amount of consumed NADPH by P450. Three independent experiments were done for each measurement.
TABLE 3.
Oxidation of PAHs by WT P450 BM-3 and mutants
| Substrate | Substrate oxidation ratea
|
|||
|---|---|---|---|---|
| WT | F87V | F87V/L188Q | A74G/F87V/L188Q | |
| Naphthalene | 0.76 | 111 | 115 | 160 |
| Fluorene | 0.014 | 2.8 | 22.7 | 52.8 |
| Acenaphthene | 0.0028 | 2.2 | 76.2 | 109 |
| Acenaphthylene | 0.12 | 71.3 | 149 | 287 |
| 9-Methylanthracene | 0.0019 | 0.71 | 9.2 | 21.8 |
Product oxidation rates were measured in units of nanomoles of product produced per nanomole of P450 per minute. Three independent experiments were done for each measurement.
Analysis of products from PAHs.
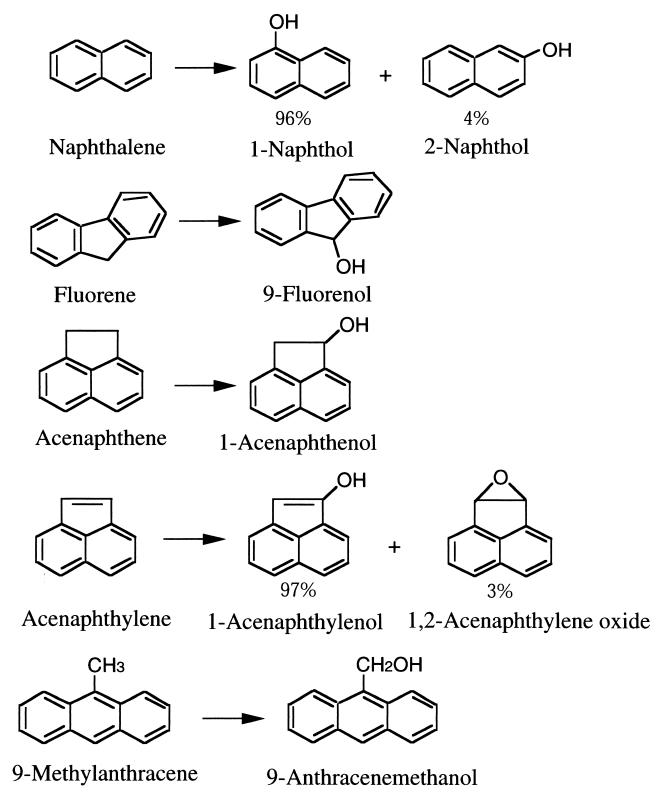
The products derived from PAHs by the WT and all mutants of P450 BM-3 are summarized in Fig. 1. The WT and all mutants catalyzed the oxidation of naphthalene to a mixture of 96% 1-naphthol (retention time [RT], 36.2 min) and 4% 2-naphthol (RT, 34.8 min), as confirmed by coelution with authentic samples on HPLC. In our experiments, to correctly evaluate the reaction selectivity, the further oxidation of 1-naphthol or 2-naphthol was limited to a negligible level by using NADPH at a low concentration (0.4 mM).
FIG. 1.
Proposed reactions catalyzed by the P450 BM-3 mutants.
The oxidation of fluorene and 9-methylanthracene by all enzymes produced only one product for each substrate, 9-fluorenol (RT, 33.5 min) and 9-anthracenemethanol (RT, 48.4 min), respectively, as confirmed by coelution with authentic samples on GC and by the results of GC-MS. There was no evidence of other products or further oxidation of the products to other compounds.
The oxidation of acenaphthene by all mutants produced one product (RT, 30.4 min) which was identified as 1-acenaphthenol by coelution with an authentic sample on GC and by the results of GC-MS. When a high concentration of NADPH (4 mM) was used, a further oxidized compound was detected which was identified as 1,2-dihydroxyacenaphthene (RT, 34.7 min) by coelution with a chemically synthesized sample on GC and by the results of GC-MS.
The oxidation of acenaphthylene by all mutants produced one peak on GC (RT, 30.6 min), but the peak was separated into a major peak (RT, 1.7 min) and a minor one (RT, 1.6 min) on GC-MS. The major one was 1-acenaphthylenol and the minor one was 1,2-acenaphthylene oxide, as suggested by the results of GC-MS analysis. GC-MS data, EI-MZ m/z (M+-x intensity [percentage]), for the major product (1-acenaphthylenol) were as follows: 168(M+, 85), 140(M+−28, 100), 139(M+−29, 90), 113(M+−55, 12), 89(M+−79, 13), 84(M+−84, 16), 70(M+−98, 69), 63(M+−105, 29), 50(M+−118, 6). Values for the minor product (1,2-acenaphthylene oxide) were 168(M+, 78), 152(M+−16, 46), 140(M+−28, 83), 139(M+−29, 90), 113(M+−55, 11), 89(M+−79, 11), 84(M+−84, 22), 69(M+−99, 56), 63(M+−105, 33), 50(M+−118, 10). The peak area of the minor product was 3.1% of that of the total products. When a high concentration of NADPH (2 mM) was used, two further oxidized products were detected which were identified as 1,2-dihydroxyacenaphthene (RT, 34.7 min) and acenaphthenequinone (RT, 36.7 min) by coelution with a chemically synthesized sample and the authentic compound, respectively, on GC and by the results of GC-MS.
Analysis of substrate binding.
Only the mutations described here did not cause a heme spin-state shift, i.e., the hemes in these mutant enzymes without substrate binding are all in a low spin-state. Substrate binding to P450 usually triggers a heme spin-state shift from low to high, and it can be monitored as the changes of P450 absorbance spectra. The heme spin-state shift and dissociation constant assay results are given in Table 4. The binding of 9-methylanthracene was not examined because of its strong absorbance in the spectral range of analysis. The first substitution of Phe87Val significantly improved the heme spin-state shift for all four PAHs but had nearly no effect on the dissociation constant for all four substrates. The further two substitutions had no significant effect on either the heme spin-state shift or the dissociation constant for all four substrates.
TABLE 4.
Heme spin-state shifts of WT P450 BM-3 and mutants on the binding of PAHs, and dissociation constants
| Substrate | Heme spin-state shift (%)
|
Kd (μM)
|
||||||
|---|---|---|---|---|---|---|---|---|
| WT | F87V | F87V/L188Q | A74G/F87V/L188Q | WT | F87V | F87V/L188Q | A74G/F87V/L188Q | |
| Naphthalene | 14.5 ± 2.8 | 72.5 ± 2.9 | 67.8 ± 3.0 | 68.4 ± 3.4 | 143 ± 10 | 150 ± 8 | 162 ± 9 | 141 ± 7 |
| Fluorene | 18.8 ± 1.9 | 22.0 ± 3.7 | 14.5 ± 2.7 | 25.9 ± 4.7 | 7.2 ± 1.4 | 6.8 ± 2.1 | 6.4 ± 1.9 | 12.1 ± 2.2 |
| Acenaphthene | 14.1 ± 2.4 | 37.1 ± 2.2 | 37.3 ± 4.1 | 36.5 ± 4.0 | 6.8 ± 1.1 | 24.4 ± 2.9 | 22.1 ± 2.2 | 23.7 ± 3.1 |
| Acenaphthylene | 14.9 ± 2.9 | 44.5 ± 4.1 | 40.8 ± 3.7 | 36.4 ± 3.6 | 24.7 ± 4.9 | 30.5 ± 6.8 | 17.9 ± 1.2 | 30.1 ± 2.5 |
DISCUSSION
Phe87 plays a critical role in the control of PAH binding to P450 BM-3. The aromatic ring of Phe87 extends into the heme pocket and is positioned above the porphyrin plane (9), which may prevent PAH binding close to the active site. Replacement of Phe87 with Val does not significantly affect the affinity of P450 BM-3 for these PAHs, but it largely increases the PAH-induced heme spin-state shift (Table 4) and coupling efficiencies of NADPH utilization (Table 2). These results suggest that the PAHs bind close to the heme with better positioning in the active site with respect to the activated oxygen intermediate that is generated during the catalytic cycle.
The PAH oxidation rates of the triplet mutant were 3 to 4 orders of magnitude faster than those of the WT. Compared with the activities of most mammalian P450s with turnover numbers of around 1.0/min (5), the activities of the triplet mutant towards these substrates are quite high. Coupling efficiency is an important factor for determining the substrate oxidation rate. The highest couplings obtained on engineering of P450cam towards phenanthrene and fluoranthene were only 1.3 and 3.1%, respectively (6). The coupling efficiencies of the triplet mutant towards these three-ring PAHs were improved 1 to 2 orders of magnitude from that of the WT, and the values themselves are also significantly high (Table 2).
The activities of P450 BM-3 towards pyrene, phenanthrene, and fluoranthene were also increased by the combination of the three mutations (R47L/Y51F/A264G) (2). However, there is no common mutation between this mutant and our triplet mutant (F87V/L188G/A74G). Therefore, it should be possible, perhaps with the help of X-ray structure analysis data, to further increase the activity towards PAHs by combining additional mutation(s). Such work and further study on application of the P450 BM-3 mutant for the biodegradation of PAHs by whole cells coexpressing an NADPH regeneration enzyme are in progress.
ACKNOWLEDGMENTS
We thank T. Sakaki, Division of Food Science and Biotechnology, Graduate School of Agriculture, Kyoto University, for useful discussions.
Q.-S. Li is a postdoctoral fellow supported by the Japan Society for Promotion of Science (JSPS). This work was supported in part by a grant-in-aid of JSPS for foreign researchers (no. P99115 to Q.S.L.) and by a grant from the Research for the Future Program (JSPS-RFTF 97I00302 to S.S.).
REFERENCES
- 1.Boddupalli S S, Estabrook R W, Peterson J A. Fatty acid monooxygenation by cytochrome P450BM-3. J Biol Chem. 1990;265:4233–4239. [PubMed] [Google Scholar]
- 2.Carmichael A B, Wong L-L. Protein engineering of Bacillus megaterium CYP102: the oxidation of polycyclic aromatic hydrocarbons. Eur J Biochem. 2001;268:3117–3125. doi: 10.1046/j.1432-1327.2001.02212.x. [DOI] [PubMed] [Google Scholar]
- 3.Cerniglia C E. Biodegradation of PAHs. Biodegradation. 1992;3:351–368. [Google Scholar]
- 4.Graham-Lorence S E, Truan G, Peterson J A, Falck J R, Wei S, Helvig C, Capdevila J H. An active site substitution, F87V, converts cytochrome P450BM-3 into a regio- and stereoselective (14S, 15R)-arachidonic acid epoxygenase. J Biol Chem. 1997;272:1127–1135. doi: 10.1074/jbc.272.2.1127. [DOI] [PubMed] [Google Scholar]
- 5.Guengerich F P. Reactions and significance of cytochrome P-450 enzymes. J Biol Chem. 1991;266:10019–10022. [PubMed] [Google Scholar]
- 6.Harford-Cross C F, Carmichael A B, Allan F K, England P A, Rouch D A, Wong L-L. Protein engineering of cytochrome P450cam (CYP101) for the oxidation of polycyclic aromatic hydrocarbons. Protein Eng. 2000;13:121–128. doi: 10.1093/protein/13.2.121. [DOI] [PubMed] [Google Scholar]
- 7.Johnson A C, Larsen P F, Gadbois D F, Houmason A W. The distribution of polycyclic aromatic hydrocarbons in the surficial sediments of Penobscot Bay (Maine, USA) in relation to possible sources and to other sites worldwide. Mar Environ Res. 1985;15:1–16. [Google Scholar]
- 8.Joo H, Lin Z, Arnold F H. Laboratory evolution of peroxide-mediated cytochrome P450 hydroxylation. Nature. 1999;339:670–673. doi: 10.1038/21395. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Poulos T L. The structure of the cytochrome p450BM-3 haem domain complexed with the fatty acid structure, palmitoleic acid. Nat Struct Biol. 1997;4:140–146. doi: 10.1038/nsb0297-140. [DOI] [PubMed] [Google Scholar]
- 10.Li H, Poulos T L. Fatty acid metabolism, conformational change, and electron transfer in cytochrome P-450(BM-3) Biochim Biophys Acta. 1999;1441:141–149. doi: 10.1016/s1388-1981(99)00161-4. [DOI] [PubMed] [Google Scholar]
- 11.Li Q S, Schwaneberg U, Fischer F, Schmid R D. Directed evolution of the fatty acid hydroxylase P450BM-3 into an indole-hydroxylating catalyst. Chem Eur J. 2000;6:1531–1535. doi: 10.1002/(sici)1521-3765(20000502)6:9<1531::aid-chem1531>3.3.co;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Li Q S, Schwaneberg U, Fischer M, Schmitt J, Pleiss J, Lutz-Wahl S, Schmid R D. Rational evolution of a medium chain-specific cytochrome P450 BM-3 variant. Biochim Biophys Acta. 2001;1545:114–121. doi: 10.1016/s0167-4838(00)00268-5. [DOI] [PubMed] [Google Scholar]
- 13.Li Q S, Ogawa J, Shimizu S. Critical role of the residue size at position 87 in H2O2-dependent substrate hydroxylation activity and H2O2 inactivation of cytochrome P450 BM-3. Biochem Biophys Res Commun. 2001;280:1258–1261. doi: 10.1006/bbrc.2001.4261. [DOI] [PubMed] [Google Scholar]
- 14.Loewen P C, Triggs B L, George C S, Hrabarchuk B E. Genetic mapping of katG, a locus that affects synthesis of the bifunctional catalase-peroxidase hydroperoxidase I in Escherichia coli. J Bacteriol. 1985;162:661–667. doi: 10.1128/jb.162.2.661-667.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mansuy D. The great diversity of reactions catalyzed by cytochromes P450. Comp Biochem Physiol C. 1998;121:5–14. doi: 10.1016/s0742-8413(98)10026-9. [DOI] [PubMed] [Google Scholar]
- 16.Miles C S, Ost T W B, Noble M A, Munro A W, Chapman S K. Protein engineering of cytochromes P450. Biochim Biophys Acta. 2000;1543:383–407. doi: 10.1016/s0167-4838(00)00236-3. [DOI] [PubMed] [Google Scholar]
- 17.Narhi L O, Fulco A J. Characterization of a catalytically self-sufficient 119,000-Dalton cytochrome P450 monoxygenase induced by barbiturates in Bacillus megaterium. J Biol Chem. 1986;261:7160–7169. [PubMed] [Google Scholar]
- 18.Ortiz de Montellano P R, editor. Cytochrome P450: structure, mechanism and biochemistry. 2nd ed. New York, N.Y: Plenum Press; 1995. [Google Scholar]
- 19.Ost T W B, Miles C S, Murdoch J, Cheung Y-F, Reid G A, Chapman S K, Munro A W. Rational redesign of the substrate binding site of flavocytochrome P450 BM-3. FEBS Lett. 2000;486:173–177. doi: 10.1016/s0014-5793(00)02267-5. [DOI] [PubMed] [Google Scholar]
- 20.Ruettinger R T, Wen L P, Fulco A J. Coding nucleotide, 5′ regulatory, and deduced amino acid sequences of P-450BM-3, a single peptide cytochrome P-450: NADPH-P-450 reductase from Bacillus megaterium. J Biol Chem. 1989;264:10987–10995. [PubMed] [Google Scholar]
- 21.Shou M, Gonzalez F J, Gelboin H V. Stereoselective epoxidation and hydration at the K-region of polycyclic aromatic hydrocarbons by cDNA-expressed cytochromes P450 1A1, 1A2, and epoxide hydrolase. Biochemistry. 1996;35:15807–15813. doi: 10.1021/bi962042z. [DOI] [PubMed] [Google Scholar]
- 22.Sono M, Roach M R, Coulter E D, Dawson J H. Heme-containing oxygenases. Chem Rev. 1996;96:2841–2887. doi: 10.1021/cr9500500. [DOI] [PubMed] [Google Scholar]
- 23.Truan G, Peterson J A. Thr268 in substrate binding and catalysis in P4350 BM-3. Arch Biochem Biophys. 1998;349:53–64. doi: 10.1006/abbi.1997.0400. [DOI] [PubMed] [Google Scholar]