Abstract
Non-alcoholic fatty liver disease (NAFLD) is one of the fastest-growing diseases, and its global prevalence is estimated to increase >50% by 2030. NAFLD is comorbid with metabolic syndrome, obesity, type 2 diabetes, and insulin resistance. Despite extensive research efforts, there are no pharmacologic or biological therapeutics for the treatment of NAFLD. Bile acids and sphingolipids are well-characterized signaling molecules. Over the last few decades, researchers have uncovered potential mechanisms by which bile acids and sphingolipids regulate hepatic lipid metabolism. Dysregulation of bile acid and sphingolipid metabolism has been linked to steatosis, inflammation, and fibrosis in patients with NAFLD. This clinical observation has been recapitulated in animal models, which are well-accepted by experts in the hepatology field. Recent transcriptomic and lipidomic studies also show that sphingolipids are important players in the pathogenesis of NAFLD. Moreover, the identification of bile acids as activators of sphingolipid-mediated signaling pathways established a novel theory for bile acid and sphingolipid biology. In this review, we summarize the recent advances in the understanding of bile acid and sphingolipid-mediated signaling pathways as potential contributors to NAFLD. A better understanding of the pathologic effects mediated by bile acids and sphingolipids will facilitate the development of new diagnostic and therapeutic strategies for NAFLD.
Keywords: Bile acids, Sphingolipids, Non-alcoholic fatty liver disease, Non-alcoholic steatohepatitis
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease worldwide, with 28 to 52 new cases per 1000 individuals diagnosed annually.[1–3] NAFLD's prevalence is the highest in the Middle East (31.90%), followed by South America (30.45%), Asia (27.37%), and the United States (24.13%).[1] Non-alcoholic fatty liver (NAFL) is a form of NAFLD with simple hepatic steatosis and little or no inflammation.[4] Non-alcoholic steatohepatitis (NASH) is the advanced form of NAFLD with inflammation and varying degrees of liver damage. The majority of NAFLD patients have NAFL, and only 10% to 25% of NAFLD patients develop NASH. Patients with NASH have an increased chance of developing end-stage liver disease and hepatocellular carcinoma (HCC).[5,6] In 2017, a Global Burden of Disease study conducted by the Institute of Health Metrics and Evaluation showed that 3.1% and 5.6% of liver-related deaths were due to NAFLD-associated HCC and cirrhosis, respectively.[7]
NAFLD also increases the risk and incidence of extrahepatic cancers. In 2020, clinical assessment of patient data in a retrospective cohort study of 254 patients with bladder cancer or disease between late-middle and advanced age groups (61–77 years) in Italy showed that high serum triglycerides (TGs) were significantly associated with non-muscle-invasive bladder cancer (NMBC) incidence.[8] Although NMBC-NAFLD did not meet statistical significance (P = 0.06), its near-significant positive association suggests its biological relevance. In 2021, a meta-analysis comprising data from over 182,000 middle to late-middle-aged individuals was conducted to assess cancer incidence among patients with NAFLD over 6 years, including 45,000 patients with NAFLD, and demonstrates that NAFLD diagnosis increases the risk of developing extrahepatic cancers of the gastrointestinal tract, lung, reproductive system, or urinary tract.[9] Collectively, these data show that NAFLD can have a broad and significant clinical impact on patient health. Furthermore, the inclusion of NAFLD diagnosis as a risk factor for cancer development may encourage more regular cancer screenings and improve patient outcomes across multiple medical specialties by detecting early tumor establishment.
In the past few decades, extensive studies have shown that NAFLD is a metabolic disease closely associated with obesity, type 2 diabetes, lipid metabolism, hepatic bile acid dysregulation, and, especially, insulin resistance.[10,11] Recently, a group of experts recommended renaming “NAFLD” to “MAFLD,” metabolic-associated fatty liver disease, to more accurately reflect pathogenesis and improve the clinical management of NAFLD.[12–14] Although extensive efforts have been put forth to develop pharmacotherapies, no FDA-approved pharmacologic or biologic interventions exist for NAFLD due to the incomplete understanding of the complicated pathogenesis from NAFL to NASH.[15] As a result, dietary modifications are the current gold standard for the modulation of NAFLD progression.[16] In response to the apparent clinical need for NAFLD treatment options, research into the cellular mechanisms which underlie NAFLD is essential for developing novel targeted therapeutics.
It has been well recognized that bile acids are essential signaling molecules and play critical roles in regulating hepatic lipid metabolism. Identification of the cross-talk between bile acid and sphingolipid-mediated signaling pathways opened a new direction for studying bile acids and sphingolipids in hepatic lipid metabolism and provided new opportunities to develop potential diagnostic and therapeutic tools for NALFD and other metabolic diseases.[17–20] Sphingolipids are one of the oldest discovered lipids and have been extensively studied as essential structural components of lipid rafts and important signaling molecules, which play significant roles in regulating various physiological functions.[21] In the past decade, numerous studies have shown that sphingolipid metabolism dysregulation is closely associated with multiple metabolic disorders, including NAFLD.[17–20] Furthermore, the role of bile acid-mediated signaling pathways in NAFLD has recently been extensively discussed.[22,23] In this review, we will highlight the recent advances in the sphingolipid field and discuss connections between bile acids and sphingolipid signaling with a focus on NAFLD.
NAFLD Disease Pathology
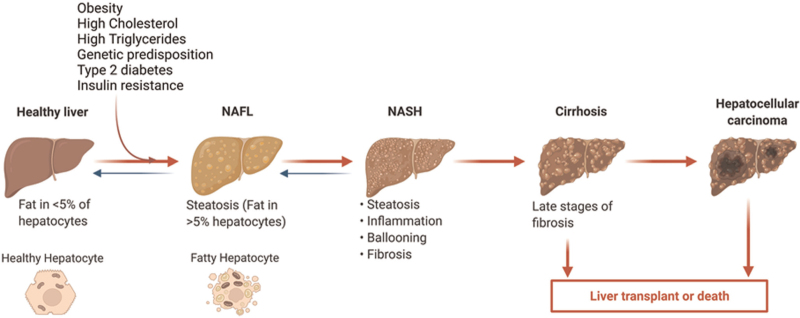
NAFLD is defined as a type of fatty liver disease not caused by alcohol consumption. There are two forms of NAFLD: NAFL and NASH. NAFL is characterized by simple fat accumulation with at least 5% steatosis but little or no inflammation and hepatocellular damage.[24] NASH is a more active form of NAFLD characterized by significant inflammation, ballooning, and fibrosis, which can progress to advanced fibrosis, cirrhosis, and HCC [Figure 1].[24] The global incidence and prevalence of NAFLD are rapidly increasing along with the obesity pandemic. It is estimated that the global NASH prevalence will increase >50% by 2030. In addition, the incidence of NASH-related HCC is projected to increase significantly and become the fastest-growing cause of liver transplantation in the United States.[25]
Figure 1.
The spectrum of NAFLD. NAFLD disease progression is linked to multiple factors, including obesity, high cholesterol, metabolic dysfunction, type 2 diabetes, and insulin resistance. The NAFL is the benign form of NAFLD and is characterized by simple steatosis with no or little inflammation. NASH is the advanced form of NAFLD associated with inflammation, ballooning, and fibrosis and has the risk of developing cirrhosis and HCC. HCC: Hepatocellular carcinoma; NAFL: Non-alcoholic fatty liver; NAFLD: Non-alcoholic fatty liver disease; NASH: Non-alcoholic steatohepatitis. The figure was created via BioRenders.com.
In the past several decades, extensive effort has been put into identifying potential mechanisms underlying NAFLD pathogenesis. It is well accepted that NAFLD is closely associated with obesity, insulin resistance, gut dysbiosis, inflammation, and disruption of bile acid and lipid metabolism.[26] In addition, genome-wide association studies have identified genetic risk mutants related to NAFLD, such as PNPLA3, TM6SF2, and SREPINA1.[27] Both clinical and pre-clinical studies indicate that dysregulation of sphingolipid metabolism is closely associated with NAFLD disease progression.[18,21] However, neither FDA-approved pharmacotherapies for NASH nor biomarkers for diagnosis or assessment of NASH disease progression and therapeutic outcomes are available.[28]
Bile Acid and Sphingolipid Metabolism
Bile acid metabolism and signaling
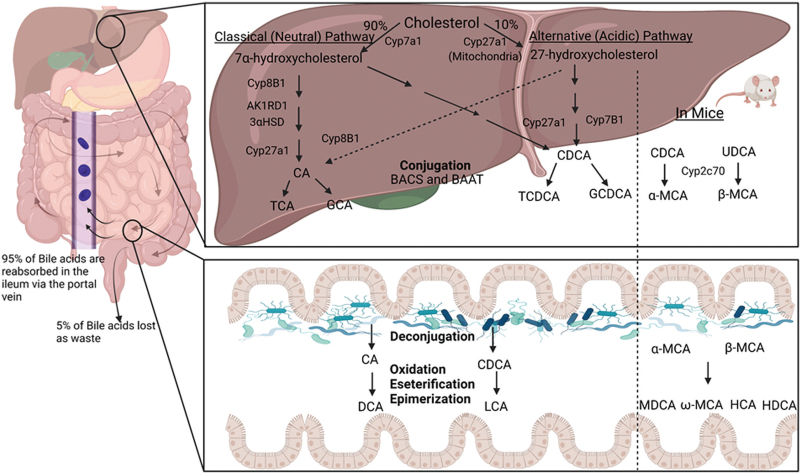
Bile acids are synthesized from free cholesterol in the hepatocytes via two well-characterized pathways, the classical or neutral pathway and the alternative or acidic pathway. The classical/neutral pathway produces ≥90% bile acid, while the alternative/acidic pathway produces the remaining ≤10%. Cholesterol 7α-hydroxylase (CYP7A1) and cholesterol 27α-hydroxylase (CYP27A1) are the rate-limiting enzymes for the classical pathway and alternative pathway, respectively. CYP7A1 is only expressed in hepatocytes, but CYP27A1 is expressed in mitochondria in multiple cell types and tissues.[23,29] Cholic acid (CA) and chenodeoxycholic acid (CDCA) are two primary bile acids in humans. In mice, CDCA is further converted into α-muricholic acid (α-MCA) via 6β-hydroxylation by a cytochrome P450 enzyme, Cyp2c70. In humans, CA and CDCA are conjugated with glycine or taurine by bile acyl-coenzyme A (CoA) synthetase and bile acid-CoA: amino acid N-acyltransferase to form conjugated bile acids (CBAs).[23] These bile salts aggregate as micelles and mix with water, cholesterol, bilirubin, and trace minerals and are stored in the gallbladder as bile.[30,31] Under normal physiological conditions, bile acids undergo enterohepatic circulation three to five times per day. Enterohepatic circulation involves the secretion of bile acids from the gallbladder to the intestinal lumen, where bile acids facilitate lipid-soluble nutrient absorption. More than 95% of bile acids are reabsorbed by enterocytes in the terminal ileum and transported to the liver via the portal vein.[23,32] About 5% of bile acids escape reabsorption and will be transformed into a variety of secondary bile acids by gut bacteria via deconjugation, oxidation, esterification, epimerization, and desulfation. CA and CDCA are transformed into deoxycholic acid and lithocholic acid (LCA) in humans. CDCA also can be transformed into ursodeoxycholic acid. In mice, α-MCA and β-MCA are converted into murideoxycholic acid. In addition, β-MCA also can be transformed into ω-MCA, hyocholic acid, and hyodeoxycholic acid. The 5% of bile acids excreted in feces are replaced through de-novo synthesis in the liver[23] [Figure 2].
Figure 2.
Bile acid synthesis and enterohepatic circulation. Bile acids are exclusively synthesized from cholesterol in the hepatocytes via two pathways, classical (neutral) pathway and alternative (acidic pathway) via multiple enzymatic reactions. Under normal physiological conditions, bile acids circulate from the liver to the bile, intestinal lumen, and return to the liver via the portal vein. More than 95% of bile acids are reabsorbed by the enterocytes in the ileum, and only 5% will be metabolized by the gut microbiome and excreted via feces. The lost bile acids will be replaced by de-novo synthesis in the liver. The composition of the bile acid pool in mice is different from that in humans. Α-MCA: α-muricholic acid; BACS: Bile acyl-CoA synthetase; BAAT: Bile acid-CoA: amino acid N-acyltransferase; β-MCA: Beta-Muricholic acid; CA: Cholic acid; CDCA: Chenodeoxycholic acid; CYP27A1: Cholesterol 27α-hydroxylase; DCA: Deoxycholic acid; GCA: Glycocholic acid; GCDCA: Glycine chenodeoxycholic acid; LCA: Lithocholic acid; MDCA: Murideoxycholic acid; TCA: Taurocholic acid; TCDCA: Taurine chenodeoxycholic acid. The figure was created via BioRenders.com.
Bile acids are not only detergents but are also important signaling molecules. In the past 2 decades, numerous studies have shown that bile acid-mediated activation of the nuclear receptor, farnesoid X receptor (FXR), and G protein-coupled receptors (GPCRs), Takeda G-protein-coupled receptor 5 (TGR5) and sphingosine-1 phosphate receptor 2 (S1PR2), play important roles in the progression of NAFLD and other metabolic diseases via modulating hepatic lipid metabolism, immune responses, and the gut microbiome.[22,23,33]
Sphingolipid metabolism and signaling pathways
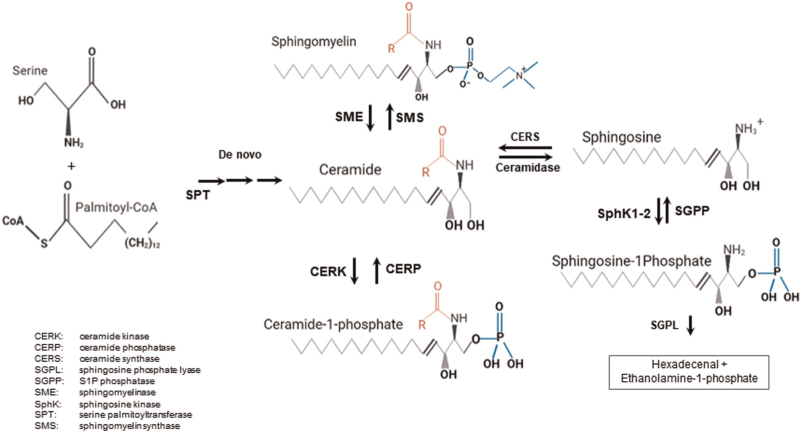
Sphingolipids are essential structural components of all eukaryotic membranes. The synthetic pathways of sphingolipids have been well characterized. More than 40 enzymes are involved in de-novo synthesis, catabolism, recycling, and interconversion of these lipids in reversible reactions.[21] Ceramide is the core molecule of the complex sphingolipids. In canonical biosynthesis, ceramide is predominately produced in the endoplasmic reticulum (ER). The initial step of the de-novo synthesis of ceramide occurs through the condensation of palmitoyl-CoA and L-serine to form 3-keto-dihydro sphinganine (3-KDS), which is facilitated by the enzyme serine palmitoyltransferase (SPT).[34] 3-ketoreductase reduces 3-KDS to form dihydrosphingosine, an intermediate possessing a sphingosine base. In the next step, a fatty acyl group is added to dihydrosphingosine by dihydroceramide synthase to generate dihydroceramide. Finally, the enzyme dihydroceramide desaturase 1 (DES1) inserts a double bond into the sphingosine base of dihydroceramide to form ceramide. Ceramide can then be further transformed into ceramide-1 phosphate, acyl ceramide, sphingomyelin, glycoceramides, or sphingosine.[21,35] Like sphingosine-1 phosphate (S1P), ceramide is also an important bioactive molecule. So far, over 200 ceramides and 28 ceramide synthases (CerS) have been discovered in mammals.[36] Each ceramide is generated from combinations of sphingosine bases and fatty acids and performs unique functions.[37,38] Ceramide production is tightly controlled by ORMDL family proteins, which sense ceramide levels and inhibit SPT. Since sphingosine can only be formed from ceramide, ORMDL proteins regulate sphingolipid levels.[20]
To generate S1P, ceramide is transported by ceramide transfer protein to the Golgi apparatus, where polar head groups are added.[34] Ceramide possesses a sphingolipid core, which, when N-deacetylated by ceramidase, is broken down to sphingosine and a free fatty acid (FFA).[39] Sphingosine is then phosphorylated by sphingosine kinases (SphKs) to form S1P. Two isoforms of SphK have been identified and characterized. SphK1 is predominantly localized in the cytosol. SphK2 is mainly in the nucleus and mitochondria.[39,40] Sphingosine can be converted back to ceramide via CerS, which is typically localized near the ER but is also found in the nuclear membrane and mitochondria.[21] Tissue concentrations of S1P remain low since most synthesized S1P is secreted or dephosphorylated by S1P phosphatase or catabolized by S1P lyase[21] [Figure 3]. The intracellular S1P is transported to the extracellular space by a specific transporter in the cell membrane, either spinster homolog 2 or ATP binding cassette transporters.
Figure 3.
Synthetic pathways of sphingolipids. The ceramide is the core molecule of sphingolipids. It can be formed from an amino acid, serine, and palmitoyl-CoA, by de-novo synthesis or from sphingomyelin via sphingomyelinase or from sphingosine by ceramidase. Ceramide can be converted to C1P by ceramide kinase. Sphingosine can be phosphorylated by sphingosine kinase to form S1P, which can be dephosphorylated by SGPP or further metabolized by SGPL to hexadecental + ethanolamine-1 phosphate. C1P: Ceramide-1 phosphate; SGPP: S1P phosphatase; SGPL: Sphingosine phosphate lyase; S1P: Sphingosine-1 phosphate.
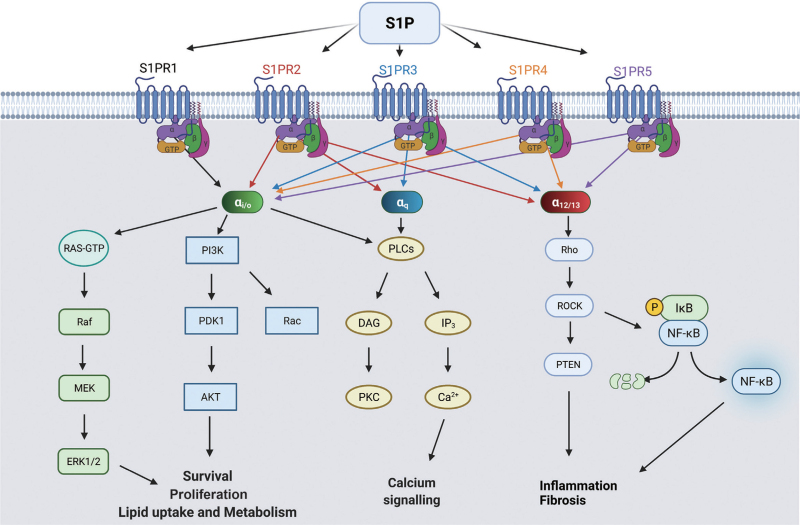
Sphingolipids are not only important structural components of lipid membrane but also important signaling molecules. S1P is the most well-characterized sphingolipid as a signaling molecule. It can directly act as an intracellular signaling molecule or extracellularly via five GPCRs, S1PR1-5, which are differentially expressed in multiple tissues and cells and can activate various signaling pathways via coupling with different G-proteins [Figure 4].
Figure 4.
S1P Receptor signaling. S1P interacts with five different GPCRs named S1PR1-5. S1PR1 couples with G alpha subunit Gαi/o. S1PR2 and S1PR3 couple with Gαi/o, Gαq, and Gα12/13, while S1PR4 and S1PR5 couple with Gαi/o and Gα12/13. Gαi/o activates the RAS-Raf-MEK-ERK and PI3K, (PDK1, Akt pathways, leading to transcription of survival, proliferation, and lipid uptake and metabolism genes. Gαq activates phosphoinositide-specific PLC, which generates IP3 and DAG, resulting in calcium influx and protein kinase C activation, respectively. Gα12/13 activates the Rho, ROCK, PTEN pathway. PTEN antagonizes PI3K, halting cell proliferation. ROCK activates NF-κB, which translocates to the nucleus and transcribes pro-inflammatory genes, leading to inflammation and fibrosis. Akt: Protein kinase B; DAG: Diacylglycerol; ERK: Extracellular-signal-regulated kinase; GPCRs: G protein-coupled receptors; IP3: Inositol trisphosphate; MEK: MAPK/ERK kinase; NF-κB: Nuclear factor kappa B; PDK1: 3-phosphoinositide-dependent kinase-1; PI3K: Phosphoinositide-3-kinase; PLC: Phospholipase C; PTEN: Phosphate and tensin homolog; Raf: Rapidly Accelerated Fibrosarcoma; ROCK: Rho kinase S1P: Sphingosine-1 phosphate; S1PR2: Sphingosine-1 phosphate receptor 2. The figure was created via BioRenders.com.
Bile Acids and Sphingolipids in NAFLD
The liver is physiologically equipped to optimize energy usage necessary for survival under primitive conditions.[41] As such, the liver restricts circulating lipid levels by serving as a reservoir for short-term lipid storage.[42] In metabolic associated NAFLD, adipocyte hypertrophy and insulin resistance induce lipolysis and increase serum FFA, promoting lipid uptake into hepatocytes and other organ tissue, driving steatosis.[43,44] Although not completely understood, the onset of inflammation that drives the progression from NAFL to NASH is attributed to a mosaic of factors, including gut dysbiosis, genetics, and diet.[45] Pro-inflammatory conditions accelerate the progression from NAFL to NASH, characterized by hepatocyte injury (ballooning) and hepatic inflammation in the presence of steatosis.[45] As NAFLD progresses, the liver expands in size due to the excessive uptake of fats. With repeated insult due to chronic inflammation, resident cell populations may be gradually replaced with non-functional, fibrotic tissue.[46] In NAFLD, hepatic stellate cells (HSCs) differentiate to a myofibroblast-like phenotype that enables the excessive production of extracellular matrix (ECM) components.[46] ECM is a driver of progressive fibrosis.[47] reactive oxygen species (ROS), cytokines, and chemokines, especially transforming growth factor-β (TGFβ) and tissue inhibitor of matrix metalloproteinase 1 (TIMP1), inhibit matrix metalloproteinases that degrade ECM, promoting fibrogenesis.[5,40] In the recent decade, extensive studies from animal models and human patients have found that NAFLD disease progression is closely associated with bile acid dysregulation and sphingolipid homeostasis.[48–60]
Mechanisms linking bile acid metabolism to the development of NAFLD
Consistently, clinical studies have demonstrated that primary and secondary CBAs are tightly associated with NAFLD progression and NASH severity.[30,48,49,51,52] A recent study reported that elevated hepatic taurochenodeoxycholic acid (TCDCA) and taurocholic acid (TCA) in NAFLD patients was associated with symptom onset, disease progression, or death.[51] A similar study demonstrated that serum CBA elevations are not observed in NAFL and occur in both non-obese and obese patient cohorts.[52] Although NAFLD is not correlated to cholestasis, increases in primary CBAs, especially TCA, and a decrease in secondary bile acids, have been observed.[48] In a similar study where the blood chemistries of 102 NAFLD and 50 healthy patients were monitored, NAFLD patients exhibited highly significant elevations in plasma total, primary, and secondary bile acids. Changes in bile acid composition were significantly correlated with steatosis. Increased primary CBAs, TCA, taurodeoxycholic acid, TCDCA, glycocholic acid (GCA), and glycodeoxycholic acid, were associated with hepatic inflammation. Interestingly, GCA and glycine chenodeoxycholic acid (GCDCA) correlated with fibrosis severity and point mutations in the tribbles homolog 1 gene, which functions as a negative regulator of retinoic acid receptors.[49] These patient data strongly suggest that dysregulation of bile acid homeostasis represents a key mechanism underlying inflammation and fibrosis in NAFLD. Importantly, patient genetic profiling is an essential consideration for data stratification in future studies and may provide opportunities for novel pharmacogenomic therapies.
Our previous studies showed that bile acids play a crucial role in hepatic lipid metabolism through activation of S1PR2. In hepatocytes, the primary CBAs, such as TCA, GCA, GCDCA, and TCDCA, can activate extracellular-signal-regulated kinase (ERK)1/2 and protein kinase B (AKT) pathways via S1PR2.[53–55] Both S1PR2 and SphK2 knockout (S1PR2−/− and SphK2−/−) mice were prone to develop fatty liver upon high-fat diet feeding.[56] By using the chronic bile fistula rat model and S1PR2−/− and SphK2−/− mouse models, we further identified that TCA-induced activation of S1PR2 induced expression and activation of SphK2, which results in the generation of nuclear S1P.[56] Nuclear S1P has been identified as a potent inhibitor of histone deacetylases (HDACs).[57] HDACs have been recently recognized as important pathogenetic players in NAFLD.[58] However, due to the broad regulator effect on gene transcription, targeting HDACs alone may not be feasible as the treatment for NAFLD.[58]
Since the identification of FXR as the first bile acid nuclear receptor and TGR5 as the first bile acid-activated GPCR, extensive studies have been done to examine the therapeutic potential for NAFLD by modulating FXR and TGR5 activity. The role of FXR and TGR5 in NAFLD has been recently reviewed.[23] Several bile acid-like drugs have been evaluated in clinical trials. In 2020, obeticholic acid, an FXR agonist, was evaluated for the treatment of NASH associated fibrosis. Due to incomplete review, uncertain long-term outcomes, and the possible risk of liver failure, it did not progress past phase III trials despite fibrosis improvement.[59]
A recent study reported that Sevelamer, a bile acid sequestrant, reversed liver injury and prevented NASH development in a high-fat and high-sucrose diet-induced mouse NAFLD model.[60] Sevelamer is a non-absorbable polymer that not only binds bile acids in the intestine to form non-absorbable complexes and reduces bile acid uptake by intestinal epithelial cells but also modulates the gut microbiome and bile acid metabolism.[60] The role of bile acids and gut microbiome in NAFLD has also been reviewed recently.[22]
Mechanisms linking sphingolipid metabolism to the development of NAFLD
Sphingolipids are important signaling molecules and have been extensively studied in various human diseases, including metabolic diseases and NAFLD.[61,62] Ceramide and S1P are the two most well-characterized sphingolipids. Lipid profiling studies have shown that increased ceramide is closely correlated with the pathogenesis of metabolic diseases, such as obesity and insulin resistance in animal models.[20] The metabolomics and lipidomics analysis of human patient samples also indicate the association of ceramide levels in the serum and tissue with obesity, NAFL, and NASH.[20] The key enzymes involved in sphingolipid metabolism have been studied as therapeutic targets for metabolic diseases. Inhibition of SPT with Myriocin, a potent SPT inhibitor, or deletion of Sptlc1 or Sptlc2, has been studied in animal models for modulating insulin sensitivity, hepatic steatosis, and adipocyte function.[20] In addition, the DES1 inhibitor, fenretinide, has been reported to resolve insulin resistance and hepatic steatosis in obese mice.[63] However, another study reported that fenretinide accelerated atherosclerosis in apoE-deficient mice.[64] More studies are needed to delineate the potential mechanisms underlying the differential effects of the DES1 inhibitor.
It has been well characterized that the genetic risk variant of patatin-like phospholipase domain-containing protein 3 (PNPLA3)I148M has a significant impact on NAFLD disease severity in both pediatric and adult patients. It also has been reported that NASH patients carrying the PNPLA3 mutant are predisposed to HCC development.[65] A recent study using the well-characterized western diet and sugar water (WDSW)-induced NASH mouse model showed that hepatocyte-specific overexpression of human PNPLA3I148M accelerated NASH progression with increased steatosis, inflammation, fibrosis, and HCC.[66] Interestingly, the transcriptomic and metabolomic analysis showed that the ceramide level was markedly upregulated in PNPLA3I148M mice upon WDSW feeding, which was accompanied by significant changes in sphingolipid metabolism and signaling pathways.[66] The key genes involved in ceramide synthesis, sphingomyelin and salvage pathways were markedly increased, such as Sptlc1/2, Kdsr, Smpd1/2, and Cers.[66] This study further indicated the potential role of sphingolipids in NAFLD pathogenesis.
Inflammation and fibrosis are two major driving forces of NAFL to NASH-HCC progression. S1P is the most well-characterized sphingolipids and has been extensively studied for its role in inflammation.[67] S1P can directly activate intracellular signaling pathways or via five GPCRs to regulate various physiological and pathological processes.[67] It has been shown that S1P-mediated activation of S1PR1/S1PR3 activates HSCs and promotes HSC migration and differentiation into myofibroblasts, enabling excessive ECM protein deposition.[68] Activation of hepatic S1PR1 led to nuclear factor kappa B activation and increased chemokine and cytokine production in a diet-induced NASH mouse model.[69] A previous study identified the activation of the GP130-STAT3 signaling pathway in human NAFLD patients.[70]
In the recent study, multiple inflammatory pathways were activated, including activation of the Janus kinase-STAT3 in the PNPLA3I148M NASH mouse model.[66] Additionally, in-vitro studies further showed that the culture of human HSCs, LX2 cells, with the conditioned media from HepG2 hepatocytes, which express PNPLA3I148M, significantly induced TGF-β1 and procollagen I and III expression.[66] These studies suggest that sphingolipid-mediated signaling in hepatocytes activates the fibrogenic response in NAFLD.
Sphingolipid interactions are further nuanced by signal location and cell type. The isoforms Sphk1 and Sphk2 have divergent roles in NAFLD pathology; this is likely due to the subcellular localization of each enzyme. SphK1 is predominantly localized in the cytosol. SphK2 is mainly in the nucleus and mitochondria.[39,40] S1P generated by Sphk1 is involved in pro-inflammatory signaling through S1PR1-3. After synthesis, S1P is exported from the cell and interacts with S1PR1-5. Each GPCR mediates pathways linked to Ras family small GTPases, Rac and Rho, and protein kinases, including p38/MAPK, PI3K/AKT, JNK, and phospholipase Ca2+/inositol triphosphate/diacylglycerol.[20,55,63] Consistent with this, global deletion of SphK1 in HFD-fed mice was protective against insulin resistance and systemic inflammation.[71] However, the roles of SphK1 can vary depending on cell type. Recent work by Anderson et al[44] demonstrated that in-vivo adipocyte-specific deletion of SphK1 developed a NASH phenotype, which was further exacerbated by HFD feeding. These mice incurred weight gain, a two-fold increase in hepatocyte TG accumulation, and upregulation of lipogenic genes (Fasn and Dgat2) and pro-inflammatory cytokine genes TNFα and MCP1. There was no evidence of a compensatory effect in the SphK2 isoform despite SphK1 deletion, suggesting that these effects were isolated to the role of SphK1.[44] Although mechanisms clarifying how adipocyte SphK1 deletion increases hepatic lipid accumulation are not fully understood, these contributions highlight the interconnectivity between adipocytes SphK1 and liver function. These data are especially important to consider in future metabolic and NASH studies.
Summary and Future Perspectives
Despite NAFLD being one of the fastest growing diseases, continued research is needed to elucidate the biological mechanisms underlying NAFLD to develop novel therapeutic interventions.[24] Multiple recent reviews have summarized the potential therapeutic and diagnostic value in targeting components of bile acid and sphingolipid metabolism.[15,23,72–75] Therapeutics which target different components of bile acid metabolism can have unanticipated effects on glucose homeostasis or lipid metabolism, but adverse side effects are mostly isolated to gastointestinal dysregulation.[76] However, bile acid pathways appear to be a sensible pharmacologic target. Bile acids tightly correlate with NAFLD severity and BA-driven mechanisms regulate hepatic lipid uptake, inflammation, and fibrosis.[30]
Future studies may benefit from considering subtle structural differences in bile acids and sphingolipids, as well as the localization of these signaling molecules. In addition, the role of sphingolipids in the cross-talk of gut and liver has not been well studied, especially the impact of sphingolipids on gut microbiomes and intestinal barrier function. Furthermore, incorporating personalized analysis of specific genetic risk factors and underlying enterohepatic circulation abnormalities may improve diagnosis and treatment for NAFLD.
Funding
This study was supported by Department of Veteran Affairs Merit Award (No. I01BX004033), Research Career Scientist Award (No. IK6BX0094477), and National Institutes of Health Grant (Nos. R01 DK104893, R01DK-057543, and R21 AA026629-01).
Conflicts of interest
None.
Footnotes
How to cite this article: Jackson KG, Way GW, Zhou H. Bile acids and sphingolipids in non-alcoholic fatty liver disease. Chin Med J 2022;135:1163–1171. doi: 10.1097/CM9.0000000000002156
Contributor Information
Kaitlyn G. Jackson, Email: jacksonkg@vcu.edu.
Grayson W. Way, Email: waygw@vcu.edu.
Huiping Zhou, Email: huiping.zhou@vcuhealth.org.
References
- 1.Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2019; 69:2672–2682. doi: 10.1002/hep.30251. [DOI] [PubMed] [Google Scholar]
- 2.Cheemerla S, Balakrishnan M. Global epidemiology of chronic liver disease. Clin Liver Dis 2021; 17:365–370. doi: 10.1002/cld.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xian YX, Weng JP, Xu F. MAFLD vs. NAFLD: shared features and potential changes in epidemiology, pathophysiology, diagnosis, and pharmacotherapy. Chin Med J 2020; 134:8–19. doi: 10.1097/CM9.0000000000001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rohrbach T, Maceyka M, Spiegel S. Sphingosine kinase and sphingosine-1-phosphate in liver pathobiology. Crit Rev Biochem Mol Biol 2017; 52:543–553. doi: 10.1080/10409238.2017.1337706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kisseleva T, Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol 2021; 18:151–166. doi: 10.1038/s41575-020-00372-7. [DOI] [PubMed] [Google Scholar]
- 6.Rohrbach TD, Asgharpour A, Maczis MA, Montefusco D, Cowart LA, Bedossa P, et al. FTY720/fingolimod decreases hepatic steatosis and expression of fatty acid synthase in diet-induced nonalcoholic fatty liver disease in mice. J Lipid Res 2019; 60:1311–1322. doi: 10.1194/jlr.m093799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paik JM, Golabi P, Younossi Y, Mishra A, Younossi ZM. Changes in the global burden of chronic liver diseases from 2012 to 2017: the growing impact of NAFLD. Hepatology 2020; 72:1605–1616. doi: 10.1002/hep.31173. [DOI] [PubMed] [Google Scholar]
- 8.Tarantino G, Crocetto F, Di Vito C, Creta M, Martino R, Pandolfo SD, et al. Association of NAFLD and insulin resistance with non metastatic bladder cancer patients: a cross-sectional retrospective study. J Clin Med 2021; 10:346.doi: 10.3390/jcm10020346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mantovani A, Petracca G, Beatrice G, Csermely A, Tilg H, Byrne CD, et al. Non-alcoholic fatty liver disease and increased risk of incident extrahepatic cancers: a meta-analysis of observational cohort studies. Gut 2022; 71:778–788. doi: 10.1136/gutjnl-2021-324191. [DOI] [PubMed] [Google Scholar]
- 10.Neuschwander-Tetri BA. Therapeutic landscape for NAFLD in 2020. Gastroenterology 2020; 158:1984–1998.e3. doi: 10.1053/j.gastro.2020.01.051. [DOI] [PubMed] [Google Scholar]
- 11.Tilg H, Adolph TE, Moschen AR. Multiple parallel hits hypothesis in NAFLD - revisited after a decade. Hepatology 2021; 73:833–842. doi: 10.1002/hep.31518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng KI, Sun DQ, Jin Y, Zhu PW, Zheng MH. Clinical utility of the MAFLD definition. J Hepatol 2021; 74:989–991. doi: 10.1016/j.jhep.2020.12.016. [DOI] [PubMed] [Google Scholar]
- 13.Eslam M, Sanyal AJ, George J. International Consensus Panel. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 2020; 158:1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 14.Zheng KI, Fan JG, Shi JP, Wong VW, Eslam M, George J, Zheng MH. From NAFLD to MAFLD: a “redefining” moment for fatty liver disease. Chin Med J 2020; 133:2271–2273. doi: 10.1097/CM9.0000000000000981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferguson D, Finck BN. Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat Rev Endocrinol 2021; 17:484–495. doi: 10.1038/s41574-021-00507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houttu V, Csader S, Nieuwdorp M, Holleboom AG, Schwab U. Dietary interventions in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis. Front Nutr 2021; 8:716783.doi: 10.3389/fnut.2021.716783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iqbal J, Walsh MT, Hammad SM, Hussain MM. Sphingolipids and lipoproteins in health and metabolic disorders. Trends Endocrinol Metab 2017; 28:506–518. doi: 10.1016/j.tem.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nikolova-Karakashian M. Sphingolipids at the crossroads of NAFLD and senescence. Adv Cancer Res 2018; 140:155–190. doi: 10.1016/bs.acr.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Simon J, Ouro A, Ala-Ibanibo L, Presa N, Delgado TC, Martínez-Chantar ML. Sphingolipids in non-alcoholic fatty liver disease and hepatocellular carcinoma: ceramide turnover. Int J Mol Sci 2019; 21:40.doi: 10.3390/ijms21010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green CD, Maceyka M, Cowart LA, Spiegel S. Sphingolipids in metabolic disease: the good, the bad, and the unknown. Cell Metab 2021; 33:1293–1306. doi: 10.1016/j.cmet.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hannun YA, Obeid LM. Sphingolipids and their metabolism in physiology and disease. Nat Rev Mol Cell Biol 2018; 19:175–191. doi: 10.1038/nrm.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hylemon PB, Su L, Zheng PC, Bajaj JS, Zhou H. Bile acids, gut microbiome and the road to fatty liver disease. Compr Physiol 2021; 12:2719–2730. doi: 10.1002/cphy.c210024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xue R, Su L, Lai S, Wang Y, Zhao D, Fan J, et al. Bile acid receptors and the gut-liver axis in nonalcoholic fatty liver disease. Cells 2021; 10:2806.doi: 10.3390/cells10112806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brunt EM, Kleiner DE, Carpenter DH, Rinella M, Harrison SA, Loomba R, et al. NAFLD: reporting histologic findings in clinical practice. Hepatology 2021; 73:2028–2038. doi: 10.1002/hep.31599. [DOI] [PubMed] [Google Scholar]
- 25.Murag S, Ahmed A, Kim D. Recent epidemiology of nonalcoholic fatty liver disease. Gut Liver 2021; 15:206–216. doi: 10.5009/gnl20127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2021; 18:223–238. doi: 10.1038/s41575-020-00381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krawczyk M, Liebe R, Lammert F. Toward genetic prediction of nonalcoholic fatty liver disease trajectories: PNPLA3 and beyond. Gastroenterology 2020; 158:1865–1880.e1. doi: 10.1053/j.gastro.2020.01.053. [DOI] [PubMed] [Google Scholar]
- 28.Masoodi M, Gastaldelli A, Hyötyläinen T, Arretxe E, Alonso C, Gaggini M, et al. Metabolomics and lipidomics in NAFLD: biomarkers and non-invasive diagnostic tests. Nat Rev Gastroenterol Hepatol 2021; 18:835–856. doi: 10.1038/s41575-021-00502-9. [DOI] [PubMed] [Google Scholar]
- 29.Chiang JYL, Ferrell JM. Bile acid receptors FXR and TGR5 signaling in fatty liver diseases and therapy. Am J Physiol Gastrointest Liver Physiol 2020; 318:G554–G573. doi: 10.1152/ajpgi.00223.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gottlieb A, Canbay A. Why bile acids are so important in non-alcoholic fatty liver disease (NAFLD) progression. Cells 2019; 8:1358.doi: 10.3390/cells8111358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hofmann AF. The continuing importance of bile acids in liver and intestinal disease. Arch Intern Med 1999; 159:2647–2658. doi: 10.1001/archinte.159.22.2647. [DOI] [PubMed] [Google Scholar]
- 32.Rehfeld JF. Cholecystokinin-from local gut hormone to ubiquitous messenger. Front Endocrinol (Lausanne) 2017; 8:47.doi: 10.3389/fendo.2017.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keitel V, Stindt J, Häussinger D. Bile acid-activated receptors: GPBAR1 (TGR5) and other G protein-coupled receptors. Handb Exp Pharmacol 2019; 256:19–49. doi: 10.1007/164_2019_230. [DOI] [PubMed] [Google Scholar]
- 34.Huang X, Withers BR, Dickson RC. Sphingolipids and lifespan regulation. Biochim Biophys Acta 2014; 1841:657–664. doi: 10.1016/j.bbalip.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saied EM, Arenz C. Stereoselective synthesis of novel sphingoid bases utilized for exploring the secrets of sphinx. Int J Mol Sci 2021; 22:8171.doi: 10.3390/ijms22158171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hannun YA, Obeid LM. Many ceramides. J Biol Chem 2011; 286:27855–27862. doi: 10.1074/jbc.r111.254359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hajduch E, Lachkar F, Ferré P, Foufelle F. Roles of ceramides in non-alcoholic fatty liver disease. J Clin Med 2021; 10:792.doi: 10.3390/jcm10040792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albeituni S, Stiban J. Roles of ceramides and other sphingolipids in immune cell function and inflammation. Adv Exp Med boil 2019; 1161:169–191. doi: 10.1007/978-3-030-21735-8_15. [DOI] [PubMed] [Google Scholar]
- 39.Rosen H, Gonzalez-Cabrera PJ, Sanna MG, Brown S. Sphingosine 1-phosphate receptor signaling. Annu Rev Biochem 2009; 78:743–768. doi: 10.1146/annurev.biochem.78.072407.103733. [DOI] [PubMed] [Google Scholar]
- 40.González-Fernández B, Sánchez DI, González-Gallego J, Tuñón MJ. Sphingosine 1-phosphate signaling as a target in hepatic fibrosis therapy. Front Pharmacol 2017; 8:579.doi: 10.3389/fphar.2017.00579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cahill GF, Jr. Starvation in man. Clin Endocrinol Metab 1976; 5:397–415. doi: 10.1016/s0300-595x(76)80028-x. [DOI] [PubMed] [Google Scholar]
- 42.Azzu V, Vacca M, Virtue S, Allison M, Vidal-Puig A. Adipose tissue-liver cross talk in the control of whole-body metabolism: implications in nonalcoholic fatty liver disease. Gastroenterology 2020; 158:1899–1912. doi: 10.1053/j.gastro.2019.12.054. [DOI] [PubMed] [Google Scholar]
- 43.Sunny NE, Bril F, Cusi K. Mitochondrial adaptation in nonalcoholic fatty liver disease: novel mechanisms and treatment strategies. Trends Endocrinol Metab 2017; 28:250–260. doi: 10.1016/j.tem.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 44.Anderson AK, Lambert JM, Montefusco DJ, Tran BN, Roddy P, Holland WL, et al. Depletion of adipocyte sphingosine kinase 1 leads to cell hypertrophy, impaired lipolysis, and nonalcoholic fatty liver disease. J Lipid Res 2020; 61:1328–1340. doi: 10.1194/jlr.ra120000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tilg H, Adolph TE, Moschen AR. Multiple parallel hits hypothesis in nonalcoholic fatty liver disease: revisited after a decade. Hepatology 2021; 73:833–842. doi: 10.1002/hep.31518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Younossi ZM, Loomba R, Anstee QM, Rinella ME, Bugianesi E, Marchesini G, et al. Diagnostic modalities for nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and associated fibrosis. Hepatology 2018; 68:349–360. doi: 10.1002/hep.29721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herrera J, Henke CA, Bitterman PB. Extracellular matrix as a driver of progressive fibrosis. J Clin Invest 2018; 128:45–53. doi: 10.1172/jci93557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Puri P, Daita K, Joyce A, Mirshahi F, Santhekadur PK, Cazanave S, et al. The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids. Hepatology 2018; 67:534–548. doi: 10.1002/hep.29359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nimer N, Choucair I, Wang Z, Nemet I, Li L, Gukasyan J, et al. Bile acids profile, histopathological indices and genetic variants for non-alcoholic fatty liver disease progression. Metabolism 2021; 116:154457.doi: 10.1016/j.metabol.2020.154457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y, Tai YL, Zhao D, Zhang Y, Yan J, Kakiyama G, et al. Berberine prevents disease progression of nonalcoholic steatohepatitis through modulating multiple pathways. Cells 2021; 10:210.doi: 10.3390/cells10020210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wegermann K, Howe C, Henao R, Wang Y, Guy CD, Abdelmalek MF, et al. Serum bile acid, vitamin E, and serotonin metabolites are associated with future liver-related events in nonalcoholic fatty liver disease. Hepatol Commun 2021; 5:608–617. doi: 10.1002/hep4.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jung Y, Koo BK, Jang SY, Kim D, Lee H, Lee DH, et al. Association between circulating bile acid alterations and nonalcoholic steatohepatitis independent of obesity and diabetes mellitus. Liver Int 2021; 41:2892–2902. doi: 10.1111/liv.15030. [DOI] [PubMed] [Google Scholar]
- 53.Studer E, Zhou X, Zhao R, Wang Y, Takabe K, Nagahashi M, et al. Conjugated bile acids activate the sphingosine-1-phosphate receptor 2 in primary rodent hepatocytes. Hepatology 2012; 55:267–276. doi: 10.1002/hep.24681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cao R, Cronk ZX, Zha W, Sun L, Wang X, Fang Y, et al. Bile acids regulate hepatic gluconeogenic genes and farnesoid X receptor via G(alpha)i-protein-coupled receptors and the AKT pathway. J Lipid Res 2010; 51:2234–2244. doi: 10.1194/jlr.M004929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dent P, Fang Y, Gupta S, Studer E, Mitchell C, Spiegel S, et al. Conjugated bile acids promote ERK1/2 and AKT activation via a pertussis toxin-sensitive mechanism in murine and human hepatocytes. Hepatology 2005; 42:1291–1299. doi: 10.1002/hep.20942. [DOI] [PubMed] [Google Scholar]
- 56.Nagahashi M, Takabe K, Liu R, Peng K, Wang X, Wang Y, et al. Conjugated bile acid-activated S1P receptor 2 is a key regulator of sphingosine kinase 2 and hepatic gene expression. Hepatology 2015; 61:1216–1226. doi: 10.1002/hep.27592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 2009; 325:1254–1257. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fu S, Yu M, Tan Y, Liu D. Role of histone deacetylase on nonalcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol 2021; 15:353–361. doi: 10.1080/17474124.2021.1854089. [DOI] [PubMed] [Google Scholar]
- 59.Intercept Receives Complete Response Letter from FDA for Obeticholic Acid for the Treatment of Fibrosis Due to NASH. Intercept pharmaceuticals, 2020. Available from: https://ir.interceptpharma.com/news-releases/news-release-details/intercept-receives-complete-response-letter-fda-obeticholic-acid. [Accessed on February 11, 2022]. [Google Scholar]
- 60.Takahashi S, Luo Y, Ranjit S, Xie C, Libby AE, Orlicky DJ, et al. Bile acid sequestration reverses liver injury and prevents progression of nonalcoholic steatohepatitis in Western diet-fed mice. J Biol Chem 2020; 295:4733–4747. doi: 10.1074/jbc.ra119.011913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Borodzicz S, Czarzasta K, Kuch M, Cudnoch-Jedrzejewska A. Sphingolipids in cardiovascular diseases and metabolic disorders. Lipids Health Dis 2015; 14:55.doi: 10.1186/s12944-015-0053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Régnier M, Polizzi A, Guillou H, Loiseau N. Sphingolipid metabolism in non-alcoholic fatty liver diseases. Biochimie 2019; 159:9–22. doi: 10.1016/j.biochi.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 63.Koh IU, Jun HS, Choi JS, Lim JH, Kim WH, Yoon JB, et al. Fenretinide ameliorates insulin resistance and fatty liver in obese mice. Biol Pharm Bull 2012; 35:369–375. doi: 10.1248/bpb.35.369. [DOI] [PubMed] [Google Scholar]
- 64.Busnelli M, Manzini S, Bonacina F, Soldati S, Barbieri SS, Amadio P, et al. Fenretinide treatment accelerates atherosclerosis development in apoE-deficient mice in spite of beneficial metabolic effects. Br J Pharmacol 2020; 177:328–345. doi: 10.1111/bph.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shao X, Uojima H, Arai T, Ogawa Y, Setsu T, Atsukawa M, et al. The risk of cirrhosis and its complications based on PNPLA3 rs738409 G allele frequency. Dig Dis 2021; Online ahead of print. doi: 10.1159/000521062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Banini BA, Kumar DP, Cazanave S, Seneshaw M, Mirshahi F, Santhekadur PK, et al. Identification of a metabolic, transcriptomic, and molecular signature of patatin-like phospholipase domain containing 3-mediated acceleration of steatohepatitis. Hepatology 2021; 73:1290–1306. doi: 10.1002/hep.31609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Obinata H, Hla T. Sphingosine 1-phosphate and inflammation. Int Immunol 2019; 31:617–625. doi: 10.1093/intimm/dxz037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kolios G, Valatas V, Kouroumalis E. Role of kupffer cells in the pathogenesis of liver disease. World J Gastroenterol 2006; 12:7413–7420. doi: 10.3748/wjg.v12.i46.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Geng T, Sutter A, Harland MD, Law BA, Ross JS, Lewin D, et al. SphK1 mediates hepatic inflammation in a mouse model of NASH induced by high saturated fat feeding and initiates proinflammatory signaling in hepatocytes. J Lipid Res 2015; 56:2359–2371. doi: 10.1194/jlr.M063511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Min HK, Mirshahi F, Verdianelli A, Pacana T, Patel V, Park CG, et al. Activation of the GP130-STAT3 axis and its potential implications in nonalcoholic fatty liver disease. Am J Physiol Gastrointest Liver Physiol 2015; 308:G794–G803. doi: 10.1152/ajpgi.00390.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang J, Badeanlou L, Bielawski J, Ciaraldi TP, Samad F. Sphingosine kinase 1 regulates adipose proinflammatory responses and insulin resistance. Am J Physiol Endocrinol Metab 2014; 306:E756–E768. doi: 10.1152/ajpendo.00549.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wei S, Ma X, Zhao Y. Mechanism of hydrophobic bile acid-induced hepatocyte injury and drug discovery. Front Pharmacol 2020; 11:1084.doi: 10.3389/fphar.2020.01084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fang Y, Hegazy L, Finck BN, Elgendy B. Recent advances in the medicinal chemistry of farnesoid X receptor. J Med Chem 2021; 64:17545–17571. doi: 10.1021/acs.jmedchem.1c01017. [DOI] [PubMed] [Google Scholar]
- 74.Castillo-Castro C, Martagón-Rosado AJ, Ortiz-Lopez R, Garrido-Treviño LF, Villegas-Albo M, Bosques-Padilla FJ. Promising diagnostic biomarkers of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: from clinical proteomics to microbiome. World J Hepatol 2021; 13:1494–1511. doi: 10.4254/wjh.v13.i11.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Negi CK, Babica P, Bajard L, Bienertova-Vasku J, Tarantino G. Insights into the molecular targets and emerging pharmacotherapeutic interventions for nonalcoholic fatty liver disease. Metabolism 2022; 126:154925.doi: 10.1016/j.metabol.2021.154925. [DOI] [PubMed] [Google Scholar]
- 76.Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall HU, Kipnes M, et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 2013; 145:574–582. doi: 10.1053/j.gastro.2013.05.042. [DOI] [PubMed] [Google Scholar]