Abstract
Background:
Endoscopic bariatric therapies can help address widening management gaps in obesity. Their ability to facilitate weight loss is largely tied to influences on appetite through perturbations of gastric emptying and accommodation. As these tools gain traction in obesity therapy, their physiologic underpinnings require exploration, which may enhance efficacy, tolerance, and patient-tailored care.
Methods:
We prospectively assessed consecutive subjects with fluid-filled intragastric balloons (IGBs) (n = 18) placed between October 2016 and June 2017 or underwent endoscopic sleeve gastroplasty (ESG) (n = 23) from March 2018 to June 2018. Patients underwent physiologic appraisal at 3 months with 13C-spirulina-based gastric emptying breath test to determine time to half emptying (T50), as well as maximum tolerated volume (MTV) of a standard nutrient drink test. Changes in T50 and MTV at 3 months were compared with percent total body weight loss (%TBWL) at 3 and 6 months using best-fit linear regression.
Results:
The change in T50 at 3 months correlated with %TBWL at 3 months for IGB (P = 0.01) and ESG (P = 0.01) but with greater impact on %TBWL in IGB compared to ESG (R2 = 0.42 vs. 0.26). Change in T50 at 3 months was predictive of weight loss at 6 months for IGB (P = 0.01) but not ESG (P = 0.11). ESG was associated with greater decrease in MTV compared to IGB (340.25 ± 297.97 mL vs. 183.00 ± 217.13 mL, P = 0.08), indicting an enhanced effect on satiation through decreased gastric accommodation. Changes in MTV at 3 months did not correlate with %TBWL for either IGB (P = 0.26) or ESG (P = 0.49) but trended toward significance for predicting %TBWL at 6 months for ESG (P = 0.06) but not IGB (P = 0.19).
Conclusion:
IGB and ESG both induce weight loss but likely through distinct gastric motor function phenotypes, and gastric emptying may predict future weight loss in patients with IGB.
Keywords: Intragastric balloon, Endoscopic sleeve gastroplasty, Gastric emptying, Gastric accommodation, Obesity
Introduction
The prevalence of obesity and obesity-related illness is increasing worldwide, underscoring an urgent need for the study of the principles of obesity and development of effective strategies for combating its rise.[1] Fundamental to understanding the mechanisms of obesity is the control of appetite. Major contributing factors to the balance of caloric intake are understood through the paradigms of satiation –– the sense of feeling full during a meal that leads to meal termination, as well as satiety –– the degree of fullness that delays consumption of the next meal. Gastric motor function is known to play a role in appetite: gastric accommodation, a reduction in gastric tone and an increase in gastric compliance allow for ingestion of large volumes of solids or liquids and impact satiation; conversely, gastric emptying has been linked to satiety.[2–4]
While there are several interwoven neurohormonal signals and varied phenotypes within obesity, the prevalent gastric motor function perturbations in obese patients appear to be increased gastric accommodation (decreased satiation) and accelerated gastric emptying (decreased satiety).[5] This pattern provides a target for therapeutic intervention to restore a balance of gastric motor function to reduce caloric intake and favor weight loss. Capitalizing on gastric motor function has already been employed in the pharmacological[6] and surgical[7,8] management of obesity.
Endoscopic bariatric therapies (EBTs), given their ability to maximize weight loss and minimize risk, will play a critical role in a comprehensive, multidisciplinary approach to obesity, but less is known about their effects on gastric motor function. EBTs span transient space-occupying devices, such as intragastric balloons (IGBs) to more durable plication procedures, such as the endoscopic sleeve gastroplasty (ESG), a full-thickness suturing technique within and along the greater curvature of the stomach to create a restricted, tubular gastric lumen.[9] Given the breadth of EBTs, it is critical to understand the physiologic underpinnings of these different techniques to help illuminate important characteristics that predict optimal responders to each particular EBT. As a result, this characterization will allow us to obtain clinically significant and durable weight loss and maximize tolerability, helping personalize the optimal treatment approach for each patient.
In this study, patients who underwent IGB placement or an ESG procedure received a non-invasive physiologic appraisal with validated techniques for gastric emptying and satiation. Our aim was to determine if different EBTs brought about different changes in the gastric motor function and in turn appetite. Additionally, we investigate whether these changes correlate with degree of contemporaneous weight loss and predict the degree of future weight loss.
Methods
Ethical approval
This study was done as a collaboration between the Mayo Clinic (Rochester, MN, USA) and Madrid Sanchinarro Hospital (Madrid, Spain). This study was approved by each institution's institutional review board. Patients were enrolled after undergoing a detailed explanation of the study protocol. Informed consent was obtained from all patients.
Data collection and design
This study included consecutive patients enrolled prospectively for EBTs from a single center, Madrid Sanchinarro University Hospital, in Madrid, Spain. IGBs were placed from October 2016 through June 2017, with data collected for 6 months following the last placement. ESG was performed from March 2018 to June 2018, with data collected for 6 months following the last procedure.
IGB placement
The Orbera IGB (Apollo Endosurgery, Austin, TX, USA) is a spherical structure of silicone filled with an isotonic saline serum and has a self-sealing valve that is placed and retrieved endoscopically. After diagnostic endoscopy, the Orbera was placed with the subject under monitored anesthesia sedation with similar methodology to that previously described.[10]
ESG procedure
ESG was performed with the United States Food and Drug Administration (FDA)-approved device for full-thickness endoscopic suturing system (Apollo Endosurgery, Austin, TX, USA). During the procedure, with subjects under general anesthesia, endoscopic sutures were placed in a retrograde fashion extending from the angle of the incisura to approximately 1 cm below the gastroesophageal junction leaving a small pouch in the fundus. Between six and ten sutures were used in a U-shaped pattern, performed in the following fashion: anterior wall to greater curvature to posterior wall and then back to anterior wall 1 to 2 cm proximal to the previous suture bite. The stitch was then tightened to approximate the opposing gastric walls through invagination of the gastric serosal surface, thereby creating a tubular gastric sleeve with a lumen roughly one-third the volume of the pre-procedural stomach. The suture was then secured with a cinching device and held in place by a needle and cinch T-tag at the respective ends. Post-procedure care for both IGB and ESG included monthly follow-up by a nutritionist and a psychologist, with additional emphasis on initiating an exercise program.
Nutrient drink test
Subjects were given a standardized nutrient drink test to measure satiation. Subjects consumed a liquid nutrient (Ensure, Abbott Labs [Abbott Park, IL, United States] 1 kcal/mL, 11% fat, 73% carbohydrate, 16% protein) at a rate of 30 mL/min. The time and volume to reach a maximum/unbearable level of fullness were recorded using a digital timer, at which point nutrient consumption was stopped.
Gastric emptying breath test (GEBT)
The 13C-spirulina test kit is standardized and stable at room temperature. It consists of 100 mg [13C]–S platensis, 27 g freeze-dried egg mix, six saltine crackers, and 180 mL of water, totaling 238 kcal (16.9 g carbohydrates, 14.4 g protein, and 11.2 g fat). The meal was fed to test subjects, and breath samples were collected at baseline before the meal and at 45, 90, 120, 150, 180, and 240 min by using a straw to blow into the bottom of each time designated glass screw cap test tubes to displace contained air. After recapping the tubes, the 13CO2 breath content at each time point (test tube) is determined in a centralized laboratory (AB Diagnostics, Brentwood, TN, USA) and sent back to the researchers as percent retention of the ingested solid component of the meal at each time point. These values are then used to determine the time for 50% emptying (T50). This FDA-approved GEBT was performed prior to IGB or ESG and 3 months following IGB or ESG.
Statistical analysis
Continuous variables were described by their means and standard deviations. Categorical variables were described by their frequencies. Univariate analysis was applied to scatterplot and best-fit linear regression lines for inputs of change in gastric emptying and maximum tolerated volumes (MTVs) at 3 months to dependent variables of percent total body weight loss (%TBWL) at 3 and 6 months. All reported P values were two tailed, and P values of <0.05 were considered statistically significant. Statistical analyses were conducted using JMP (SAS Institute) Software (Cary, NC, USA).
Results
Baseline characteristics
Table 1 provides baseline demographics of the two cohorts. Then, 18 patients (mean age: 41.06 ± 8.81 years, 100% female) underwent placement of the Orbera IGB (Apollo Endosurgery, Austin, TX, USA) from October 2016 through June 2017 and 23 patients (mean age: 47.69 ± 5.06 years, 87% female) underwent ESG from March 2018 to June 2018. The mean weight at baseline was greater in the ESG group than in the IGB group (113.40 ± 17.55 kg vs. 95.70 ± 13.08 kg, P < 0.01), as was mean body mass index (BMI) at baseline (41.21 ± 5.38 vs. 34.50 ± 4.46 kg/m2, P = <0.01). Baseline gastric emptying was slower in the IGB group compared with ESG (90.65 ± 15.63 min vs. 77.03 ± 19.21 min, P = 0.02). There was no significant difference in baseline MTV, which was 578.82 ± 204.51 mL for IGB and 744.45 ± 329.07 mL for ESG.
Table 1.
Baseline characteristics of subjects who underwent ESG and IGB.
| Variables (%) | All (n = 41) | ESG (n = 23) | IGB (n = 18) | P |
| Age (years) | 44.78 ± 11.20 | 47.69 ± 5.06 | 41.06 ± 8.81 | 0.06 |
| Female | 38 (93) | 20 (87) | 18 (100) | 0.11 |
| BMI (kg/m2) | 38.27 ± 5.98 | 41.21 ± 5.38 | 34.50 ± 4.46 | <0.01 |
| Weight (kg) | 105.63 ± 17.92 | 113.40 ± 17.55 | 95.70 ± 13.08 | <0.01 |
| BMI at 3 months (kg/m2) | 33.17 ± 5.45 | 35.76 ± 5.06 | 29.87 ± 4.00 | <0.01 |
| TBWL at 3 months (%) | 13.32 ± 3.50 | 13.30 ± 3.15 | 13.35 ± 3.99 | 0.97 |
| BMI at 6 months (kg/m2) | 32.00 ± 6.07 | 35.34 ± 5.59 | 28.31 ± 4.21 | <0.01 |
| TBWL at 6 months (%) | 16.96 ± 6.03 | 16.17 ± 5.69 | 17.82 ± 6.43 | 0.41 |
| T50 (min) | 83.00 ± 18.81 | 77.03 ± 19.21 | 90.65 ± 15.63 | 0.02 |
| Magnitude of change in %50 (min) at 3 months (pre–post) | 38.39 ± 37.61 | 27.83 ± 24.27 | 54.60 ± 48.47 | 0.02 |
| Volume (mL) | 668.35 ± 287.65 | 744.45 ± 329.07 | 578.82 ± 204.51 | 0.08 |
| Magnitude of change in MTV (mL) at 3 months (pre–post) | 268.00 ± 272.57 | 340.25 ± 297.97 | 183.00 ± 217.13 | 0.08 |
Values were shown as mean ± standard deviation, or n (%). BMI: Body mass index; ESG: Endoscopic sleeve gastroplasty; IGBs: Intragastric balloons; MTV: Maximum tolerated volume; TBWL: Total body weight loss; T50: time for 50% emptying.
Changes in gastric motor influences of appetite from baseline
By 3 months, %TBWL was 13.35% ± 3.99% for IGB and 13.30% ± 3.15% for ESG (P = 0.97). BMI had decreased to 29.87 ± 4.00 kg/m2 for IGB and to 35.76 ± 5.06 kg/m2 for ESG. For all subjects, gastric emptying was delayed for both IGB and ESG at 3 months compared to baseline. Change in time to half gastric emptying from baseline to 3 months (ΔT503m), calculated as pre–post procedural T50, was 54.60 ± 48.47 min for IGB and 27.83 ± 24.27 min for ESG (P = 0.03). Because all patients experienced a delay in gastric emptying, change was expressed as a magnitude of change rather than a negative value. All subjects experienced a decrease in MTV at 3 months. ESG was associated with greater decrease in MTV from baseline to 3 months (ΔMTV3m), measured as pre–post procedural MTV, compared to IGB (340.25 ± 297.97 mL vs. 183.00 ± 217.13 mL, P = 0.08), indicting an enhanced effect on satiation through decreased gastric accommodation.
Correlation of changes in gastric motor function with %TBWL
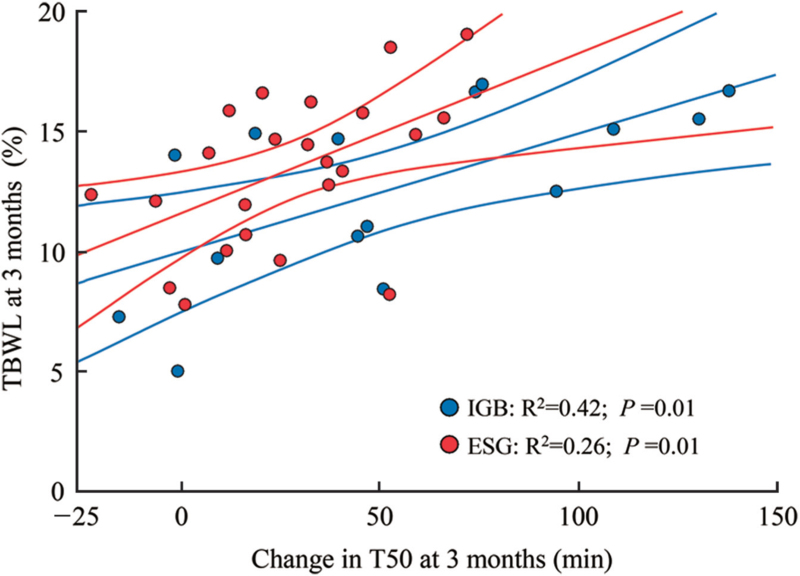
Scatterplot and best-fit linear regression lines were performed for the association between ΔT503m and %TBWL3m, respectively, for IGB and ESG [Figure 1]. This demonstrated, for both IGB and ESG, that a greater change in ΔT503m corresponded to a greater %TBWL3m, though the effect was more substantial for IGB compared to ESG (R2 = 0.42 vs. R2 = 0.26, respectively). This observation implies that changes in gastric emptying at 3 months account for about 42% of the variability in weight loss in subjects who underwent IGB compared to only about 26% of the variability in weight loss in subjects who underwent ESG.
Figure 1.
Change in T50 and %TBWL for IGB and ESG at 3 months. ESG: Endoscopic sleeve gastroplasty; IGBs: Intragastric balloons; %TBWL: Percent total body weight loss; T50: time for 50% emptying.
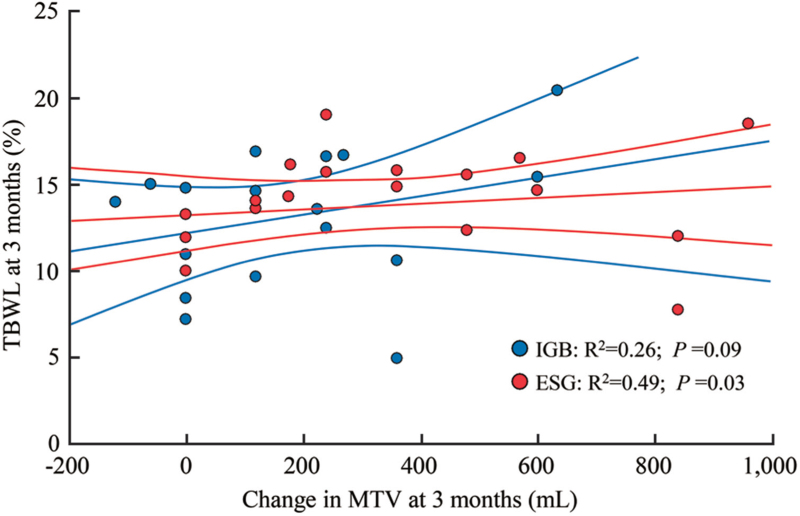
Scatterplot and best-fit linear regression lines for the association between ΔMTV3m and %TBWL3m, respectively, for IGB and ESG showed a significant relationship between ΔMTV3m and %TBWL3m for ESG (P = 0.03) but not for IGB (P = 0.09) [Figure 2].
Figure 2.
Change in MTV and %TBWL for IGB and ESG at 3 months. ESG: Endoscopic sleeve gastroplasty; IGBs: Intragastric balloons; %TBWL: Percent total body weight loss; MTV: Maximum tolerated volume.
Changes in gastric motor function and prediction of weight loss
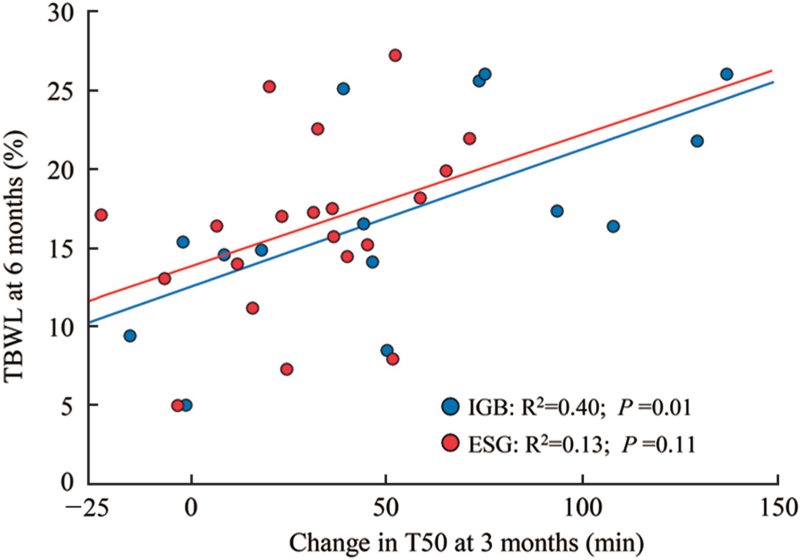
Percent TBWL at 6 months (%TBWL6m) was similar for IGB and ESG (17.82 ± 6.43% vs. 16.17 ± 5.69%, respectively, P = 0.41). To determine if the degree of delay in gastric emptying at 3 months could predict weight loss at 6 months, scatterplot and best-fit linear regression lines were performed for the association between ΔT503m and %TBWL6m, respectively, for IGB and ESG [Figure 3]. ΔT503m was predictive of %TBWL6m for IGB (R2 = 0.40, P = 0.01) but not for ESG (P = 0.11).
Figure 3.
Change in T50 and %TBWL for IGB and ESG at 6 months. ESG: Endoscopic sleeve gastroplasty; IGBs: Intragastric balloons; %TBWL: Percent total body weight loss; T50: time for 50% emptying.
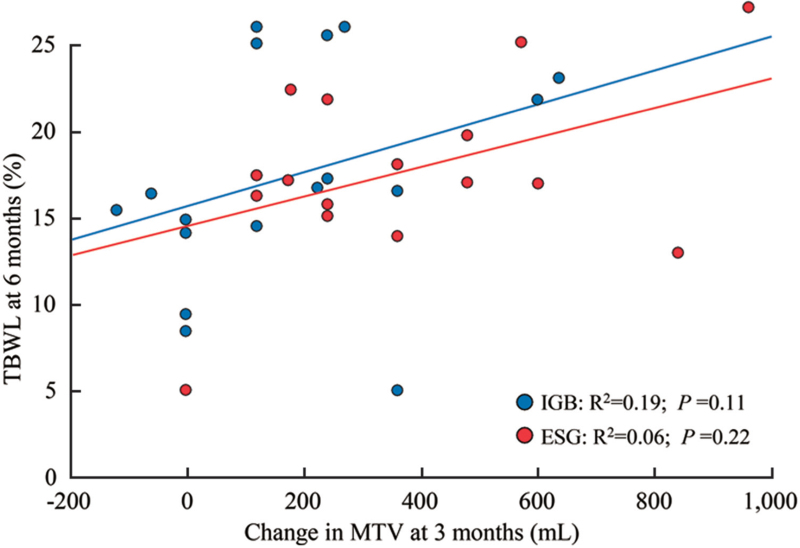
Predictive ability of ΔMTV3m for weight loss at 6 months was not observed. Scatterplot and best-fit linear regression lines for the association between ΔMTV3m and %TBWL6m showed no statistically significant relationship for IGB (P = 0.11) or ESG (P = 0.22) [Figure 4].
Figure 4.
Change in MTV and %TBWL for IGB and ESG at 6 months. ESG: Endoscopic sleeve gastroplasty; IGBs: Intragastric balloons; %TBWL: Percent total body weight loss; MTV: Maximum tolerated volume.
Discussion
In this study, we have observed that both IGB and ESG are effective weight loss strategies and show a statistically similar %TBWL at 3 and 6 months, consistent with prior studies that have used these weight loss strategies[11,12]; however, here, we have presented novel data revealing distinct physiologic underpinnings of gastric motor functions and their effects on and ability to predict weight loss in different forms of EBT. Our study demonstrates that magnitude of delays in gastric emptying at 3 months correlates with %TBWL at 3 months for both the IGB and ESG and predicts %TBWL at 6 months only for the IGB. Additionally, ESG resulted in a greater decrease in MTV compared to IGB at 3 months that was predictive of weight loss at 3 months but was not predictive of weight loss at 6 months.
The role of gastric emptying has been explored in prior IGB studies. Mion et al[13] showed that while gastric emptying decreased following IGB, this did not correlate with weight loss. The latter finding has since been contradicted by two subsequent studies. Su et al[14] showed gastric emptying was delayed (using both solid and liquid meal nuclear scintigraphy) at 3 months in ten subjects with obesity undergoing IGB and further demonstrated that the degree of delay in T50 correlated to weight loss at 3 months using Spearman correlation. Gomez et al[15] demonstrated that gastric emptying (measured by nuclear scintigraphy 2 and 4 months after IGB placement in 15 subjects with obesity) was delayed in subjects with a fluid-filled IGB compared to controls. When dichotomized by change in gastric emptying (<50% delay vs. >50% delay in emptying), those with >50% delay in gastric emptying at 2 months saw greater %TBWL at 6 and 12 months compared to those who had <50% change in gastric emptying at 2 months, laying the groundwork for a predictive role of delay in gastric emptying for future weight loss. A meta-analysis of these aforementioned three studies revealed fluid-filled IGBs delayed T50 by a mean of 116.4 min (95% confidence interval, 29.4–203.4 min), with a demonstrable association on meta-regression between delay in gastric emptying and weight loss.[16]
Here, we have confirmed the findings of Su et al that fluid filled IGBs delay gastric emptying and that this degree of gastric emptying at 3 months correlates with %TBWL at 3 months. We have further provided evidence that change in gastric emptying at 3 months can predict weight loss at 6 months for IGB. Additionally, we observed that change in MTV at 3 months was not associated with weight loss at 3 months or 6 months and therefore suggests that gastric accommodation plays less of a role in weight loss enacted by IGB placement. The role of delayed gastric emptying from IGB may explain why most IGBs are associated with a substantive incidence of upper gastrointestinal symptoms, especially abdominal pain and nausea, and may also explain why early device removal, as recent meta-analysis of 68 studies of IGB revealed, is around 7.5%.[17]
For the ESG, this study helps fill knowledge gaps in the mechanisms of action, as physiologic appraisal of ESG is limited in the published literature. Abu Dayyeh et al[18] demonstrated in obese patients who underwent ESG that gastric emptying (by nuclear scintigraphy) was delayed from baseline by approximately 90 min 3 months following the procedure. Here, we have demonstrated that there is a statistically significant delay in ΔT503m and that there is a statistically significant correlation with ΔT503m and %TBWL3m (P = 0.01). Such an increase in T50 is important given robust meta-analysis data that showed surgical sleeve gastrectomy reduced T50, likely due to high pressure system created by the surgical stapler and surgical removal of the fundus, which is involved in gastric accommodation and ghrelin production. Overall, this finding suggests that the two procedures may have different mechanistic effects to induce weight loss.[16] However, the association between the change in ΔT503m and %TBWL at 6 months was non-significant (P = 0.11), suggesting other mechanisms are responsible for the weight loss beyond 3 months. Indeed, even at 3 months, the significant correlation between ΔMTV3m and %TBWL3m for ESG suggests that change in gastric accommodation likely accounts for the difference in variance explained by gastric emptying between the IGB and the ESG (R2 = 0.26 for ESG vs. R2 = 0.42 for IGB). All together this suggests that while the IGB induces weight loss through changes in gastric emptying, the ESG induces weight loss through changes in gastric emptying and gastric accommodation, in addition to other factors not measured in our study, such as alteration in gut-central nervous system hormonal and enteric nervous system signaling.
Prior work by Abu Dayyeh et al[18] demonstrated in four patients who underwent ESG that there was a decrease in MTV from baseline to 3 months after ESG, leading to post-procedural termination of a meal at 11.5 ± 2.3 min compared to 35.2 ± 9.9 min at baseline (P = 0.01). This change is physiologically plausible, as satiation is linked to accommodation. Accommodation is a vagally mediated response that leads to gastric relaxation principally at the gastric fundus and proximal body, and ESG is a procedure that brings the suture line up toward the fundus, theoretically restricting gastric reservoir volume. Looking to other gastric plication procedures can be instructive in understanding this principle. One such procedure, the primary obesity surgical endoluminal (POSE) involves endoluminal placement of full-thickness tissue plications in the fundus and distal body, with the hypothetical consequence of mechanical restriction of the stomach to accommodate a meal and delaying gastric emptying.[19] In a study of 18 patients who underwent POSE, MTV was reduced at both 2 and 6 months post-procedurally from baseline, though admittedly POSE has a greater focus on fundic restriction.[20]
The ability to predict weight loss at 6 months from ΔT503m for subjects who underwent IGB was an important finding in this study. Clinical success with an eye toward durable weight loss from EBTs has been defined as a % excess weight loss of at least 25% by the Preservation and Incorporation of Valuable Endoscopic Innovation Guidelines.[21,22] To that end, it becomes crucial to identify those patients who will be unlikely to respond to a particular EBT so that either a different EBT can be pursued or an additional therapy, such as adjunctive pharmacologic agents, can be added to augment weight loss. While larger prospective studies are needed to provide a more granular and robust appraisal of degree of physiologic change in gastric motor function needed to achieve clinical success, our study reveals that an easily administered office-based gastric emptying breath test may have a role in identifying those who will and, more crucially, will not respond sufficiently to IGB. While we found no statistically significant method of predicting weight loss at 6 months for the ESG from either ΔT503m or ΔMTV3m, we suspect that larger prospective trials may identify a role for accommodative tests in predicting response to ESG.
Limitations to this study include the relatively small sample size and differences in baseline characteristics between the two groups, such as patient sex. Further limitations include notable baseline characteristics that were both over-represented in this study, as well as those statistically more represented in one group. First, the IGB arm contained only females and the ESG contained 87% female participants. Prior work has demonstrated sex-based differences in gastric motor function, with women having smaller MTV than men and greater delays in gastric emptying compared to men for both liquids and solids.[23,24] This limits the external and internal validity of our study as men, having a faster gastric emptying at baseline, may experience a greater change in gastric emptying from either IGB or ESG. Sex-based differences in response to and tolerability of EBTs have not yet been explored and may play a role in further studies to maximize a patient-centered approach to EBT. Second, the internal validity of our study is hampered by a greater baseline BMI in the ESG group (a product of subjects’ being selected for more intensive therapy with a higher class of obesity), as it has been demonstrated that obesity has varying effects on gastric motor function but with a trend toward increased gastric accommodation (decreased satiation) and accelerated gastric emptying (decreased satiety).[5] Our study was primarily focused on understanding the physiologic underpinnings inducing weight loss with both ESG and IGB. As the most robust weight loss with EBTs is seen within the first 6 months, we utilized this timeframe to study the physiologic changes associated with IGB and ESG. However, future studies assessing the use of these EBTs with longer duration of follow-up should repeat these procedures at 6 months or 1 year to provide further information on the durability of these physiologic changes.
While the mechanism of action for weight loss in IGB and ESG appears to incorporate delay in gastric emptying, more so for IGB, and a potential role of satiation in ESG, the perturbations in gastric motor function do not completely elucidate the variations in weight loss based on the linear regression models presented here. Other studies have explored the influence of appetite regulating hormones like ghrelin and leptin during the course of IGB placement without an overwhelmingly compelling narrative to explain weight loss.[13,25,26] Less is known about the hormonal changes seen in ESG. Abu Dayyeh et al[18] saw no observable statistical differences in levels of glucagon-like peptide-1 and peptide YY and fasting and post-prandial ghrelin from baseline in four subjects 3 months after ESG, though Graus-Morales et al[27] did demonstrate a significant decrease in leptin at 12 months assessment after ESG. There is still much to be learned about the physiologic mechanisms of weight loss in EBTs.
The IGB and ESG are both effective and safe EBTs. However, the physiologic mechanisms by which both procedures promote weight loss are still an area of active research. The findings from this study suggest that changes in gastric emptying and satiety play a role in weight loss for both the IGB and ESG, and changes in MTV and satiation may play an additional role following the ESG.
Conflicts of interest
Barham Abu Dayyeh reports consultant roles with Boston Scientific, Medtronic, USGI, Metamodix, and has had speaking roles with Olympus, Johnson and Johnson (speaker), and Endogastric solutions (speaker). He has grant and research support from Medtronic, Apollo Endosurgery, USGI, Cairn Diagnostics, Aspire, and Spatz medical. Andrew C. Storm reports consultant roles with ACS: GI Dynamics, ERBE, Endo-TAGSS, Olympus, Enterasense and Apollo Endosurgery. He reports research support from Boston Scientific. Gontrand Lopez Nava is a consultant for Apollo Endosurgery, USGI Medical, and Nitinotes. Andres Acosta reports grants from National Institutes of Health- National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (NIH-NIDDK), American Neurogastroenterology and Motility Society, and Mayo Clinic Center for Individualized Medicine. He reports personal fees from Rhythm Pharmaceuticals, General Mills, Gila Therapeutics; is a stockholder of Gila Therapeutics and Phenomix Sciences; and patent PCT/US62/589915 licensed to Phenomix Sciences. All other authors have no disclosures.
Footnotes
How to cite this article: Rapaka B, Maselli DB, Lopez-Nava G, Bautista-Castaño I, Matar R, Jaruvongvanich V, Vargas EJ, Storm AC, Acosta A, Abu Dayyeh BK. Effects on physiologic measures of appetite from intragastric balloon and endoscopic sleeve gastroplasty: results of a prospective study. Chin Med J 2022;135:1234–1241. doi: 10.1097/CM9.0000000000002097
References
- 1.Camilleri M, Staiano A. Insights on obesity in children and adults: individualizing management. Trends Endocrinol Metab 2019; 30:724–734. doi: 10.1016/j.tem.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halawi H, Camilleri M, Acosta A, Vazquez-Roque M, Oduyebo I, Burton D, et al. Relationship of gastric emptying or accommodation with satiation, satiety, and postprandial symptoms in health. Am J Physiol Gastrointest Liver Physiol 2017; 313:G442–G447. doi: 10.1152/ajpgi.00190.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acosta A, Camilleri M, Shin A, Vazquez-Roque MI, Iturrino J, Burton D, et al. Quantitative gastrointestinal and psychological traits associated with obesity and response to weight-loss therapy. Gastroenterology 2015; 148:537.e4–546.e4. doi: 10.1053/j.gastro.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delgado-Aros S, Camilleri M, Castillo EJ, Cremonini F, Stephens D, Ferber I, et al. Effect of gastric volume or emptying on meal-related symptoms after liquid nutrients in obesity: a pharmacologic study. Clin Gastroenterol Hepatol 2005; 3:997–1006. doi: 10.1016/s1542-3565(05)00285-5. [DOI] [PubMed] [Google Scholar]
- 5.Acosta A, Camilleri M. Gastrointestinal morbidity in obesity. Ann N Y Acad Sci 2014; 1311:42–56. doi: 10.1111/nyas.12385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halawi H, Khemani D, Eckert D, O’Neill, Kadouh H, Grothe K, et al. Effects of liraglutide on weight, satiation, and gastric functions in obesity: a randomised, placebo-controlled pilot trial. Lancet Gastroenterol Hepatol 2017; 2:890–899. doi: 10.1016/S2468-1253(17)30285-6. [DOI] [PubMed] [Google Scholar]
- 7.Burgerhart JS, van Rutte PWJ, Edelbroek MAL, Wyndaele DNJ, Smulders JF, van de Meeberg PC, et al. Association between postprandial symptoms and gastric emptying after sleeve gastrectomy. Obesity Surg 2015; 25:209–214. doi: 10.1007/s11695-014-1410-z. [DOI] [PubMed] [Google Scholar]
- 8.Deden LN, Cooiman MI, Aarts EO, Janssen IMC, Gotthardt M, Hendrickx BW, et al. Gastric pouch emptying of solid food in patients with successful and unsuccessful weight loss after Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis 2017; 13:1840–1846. doi: 10.1016/j.soard.2017.07.031. [DOI] [PubMed] [Google Scholar]
- 9.Abu Dayyeh BK, Rajan E, Gostout CJ. Endoscopic sleeve gastroplasty: a potential endoscopic alternative to surgical sleeve gastrectomy for treatment of obesity. Gastrointest Endosc 2013; 78:530–535. doi: 10.1016/j.gie.2013.04.197. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Nava G, Rubio MA, Prados S, Pastor G, Rodriguez Cruz M, Companioni E, et al. BioEnterics® intragastric balloon (BIB®). Single ambulatory center Spanish experience with 714 consecutive patients treated with one or two consecutive balloons. Obes Surg 2011; 21:5–9. doi: 10.1007/s11695-010-0093-3. [DOI] [PubMed] [Google Scholar]
- 11.Courcoulas A, Abu Dayyeh BK, Eaton L, Robinson J, Woodman G, Fusco M, et al. Intragastric balloon as an adjunct to lifestyle intervention: a randomized controlled trial. Int J Obes 2017; 41:427–433. doi: 10.1038/ijo.2016.229. [DOI] [PubMed] [Google Scholar]
- 12.Fuller NR, Pearson S, Lau NS, Wlodarczyk J, Halstead MB, Tee HP, et al. An intragastric balloon in the treatment of obese individuals with metabolic syndrome: a randomized controlled study. Obesity (Silver Spring) 2013; 21:1561–1570. doi: 10.1002/oby.20414. [DOI] [PubMed] [Google Scholar]
- 13.Mion F, Napoleon B, Roman S, Malvoisin E, Trepo F, Pujol B, et al. Effects of intragastric balloon on gastric emptying and plasma ghrelin levels in non-morbid obese patients. Obes Surg 2005; 15:510–516. doi: 10.1381/0960892053723411. [DOI] [PubMed] [Google Scholar]
- 14.Su HJ, Kao CH, Chen WC, Chang TT, Lin CY. Effect of intragastric balloon on gastric emptying time in humans for weight control. Clin Nucl Med 2013; 38:863–868. doi: 10.1097/RLU.0000000000000224. [DOI] [PubMed] [Google Scholar]
- 15.Gomez V, Woodman G, Abu Dayyeh BK. Delayed gastric emptying as a proposed mechanism of action during intragastric balloon therapy: results of a prospective study. Obesity (Silver Spring) 2016; 24:1849–1853. doi: 10.1002/oby.21555. [DOI] [PubMed] [Google Scholar]
- 16.Vargas EJ, Bazerbachi F, Calderon G, Prokop LJ, Gomez V, Hassan Murad M, et al. Changes in time of gastric emptying after surgical and endoscopic bariatrics and weight loss: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2020; 18:57.e5–68.e5. doi: 10.1016/j.cgh.2019.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bazerbachi F, Vargas EJ, Abu Dayyeh BK. Endoscopic bariatric therapy: a guide to the intragastric balloon. Am J Gastroenterol 2019; 114:1421–1431. doi: 10.14309/ajg.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 18.Abu Dayyeh BK, Acosta A, Camilleri M, Mundi MS, Rajan E, Topazian MD, et al. Endoscopic sleeve gastroplasty alters gastric physiology and induces loss of body weight in obese individuals. Clin Gastroenterol Hepatol 2017; 15:37.e1–43.e1. doi: 10.1016/j.cgh.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 19.Espinós JC, Turró R, Mata A, Espinós JC, Turró R, Mata A, et al. Early experience with the Incisionless Operating Platform™ (IOP) for the treatment of obesity: the Primary Obesity Surgery Endolumenal (POSE) procedure. Obes Surg 2013; 23:1375–1383. doi: 10.1007/s11695-013-0937-8. [DOI] [PubMed] [Google Scholar]
- 20.Espinos JC, Turro R, Moragas G, Bronstone A, Buchwald JN, Mearin F, et al. Gastrointestinal physiological changes and their relationship to weight loss following the POSE procedure. Obes Surg 2016; 26:1081–1089. doi: 10.1007/s11695-015-1863-8. [DOI] [PubMed] [Google Scholar]
- 21.Ginsberg GG, Chand B, Cote GA, Dallal RM, Edmundowicz SA, et al. ASGE/ASMBS Task Force on Endoscopic Bariatric Therapy. A pathway to endoscopic bariatric therapies. Gastrointest Endosc 2011; 74:943–953. doi: 10.1016/j.gie.2011.08.053. [DOI] [PubMed] [Google Scholar]
- 22.Abu Dayyeh BK, Kumar N, Edmundowicz SA, Jonnalagadda S, Larsen M, et al. ASGE Bariatric Endoscopy Task Force and ASGE Technology Committee. ASGE Bariatric Endoscopy Task Force systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting endoscopic bariatric therapies. Gastrointest Endosc 2015; 82:425–438. doi: 10.1016/j.gie.2015.03.1964. [DOI] [PubMed] [Google Scholar]
- 23.Monrroy H, Pribic T, Galan C, Nieto A, Amigo N, Accarino A, et al. Meal enjoyment and tolerance in women and men. Nutrients 2019; 11:119.doi: 10.3390/nu11010119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Datz FL, Christian PE, Moore J. Gender-related differences in gastric emptying. J Nucl Med 1987; 28:1204–1207. [PubMed] [Google Scholar]
- 25.Konopko-Zubrzycka M, Baniukiewicz A, Wroblewski E, Kowalska I, Zarzycki W, Górska M, et al. The effect of intragastric balloon on plasma ghrelin, leptin, and adiponectin levels in patients with morbid obesity. J Clin Endocrinol Metab 2009; 94:1644–1649. doi: 10.1210/jc.2008-1083. [DOI] [PubMed] [Google Scholar]
- 26.Martinez-Brocca MA, Belda O, Parejo J, Jimenez L, del Valle A, Pereira JL, et al. Intragastric balloon-induced satiety is not mediated by modification in fasting or postprandial plasma ghrelin levels in morbid obesity. Obes Surg 2007; 17:649–657. doi: 10.1007/s11695-007-9109-z. [DOI] [PubMed] [Google Scholar]
- 27.Graus Morales J, Crespo Perez L, Marques A, Arribas BM, Arribas RB, Ramo E, et al. Modified endoscopic gastroplasty for the treatment of obesity. Surg Endosc 2018; 32:3936–3942. doi: 10.1007/s00464-018-6133-0. [DOI] [PubMed] [Google Scholar]