Abstract
Severe asthma is “asthma which requires treatment with high dose inhaled corticosteroids (ICS) plus a second controller (and/or systemic corticosteroids) to prevent it from becoming ‘uncontrolled’ or which remains ‘uncontrolled’ despite this therapy.” The state of control was defined by symptoms, exacerbations and the degree of airflow obstruction. Therefore, for the diagnosis of severe asthma, it is important to have evidence for a diagnosis of asthma with an assessment of its severity, followed by a review of comorbidities, risk factors, triggers and an assessment of whether treatment is commensurate with severity, whether the prescribed treatments have been adhered to and whether inhaled therapy has been properly administered. Phenotyping of severe asthma has been introduced with the definition of a severe eosinophilic asthma phenotype characterized by recurrent exacerbations despite being on high dose ICS and sometimes oral corticosteroids, with a high blood eosinophil count and a raised level of nitric oxide in exhaled breath. This phenotype has been associated with a Type-2 (T2) inflammatory profile with expression of interleukin (IL)-4, IL-5, and IL-13. Molecular phenotyping has also revealed non-T2 inflammatory phenotypes such as Type-1 or Type-17 driven phenotypes. Antibody treatments targeted at the T2 targets such as anti-IL5, anti-IL5Rα, and anti-IL4Rα antibodies are now available for treating severe eosinophilic asthma, in addition to anti-immunoglobulin E antibody for severe allergic asthma. No targeted treatments are currently available for non-T2 inflammatory phenotypes. Long-term azithromycin and bronchial thermoplasty may be considered. The future lies with molecular phenotyping of the airway inflammatory process to refine asthma endotypes for precision medicine.
Keywords: Severe asthma, Biologic therapies, Eosinophils, Neutrophils, Corticosteroid insensitivity, Type 2-high inflammation
Introduction
Asthma is a global health problem. It has been defined as a heterogeneous disease characterized by airway inflammation usually presenting with a history of wheeze, shortness of breath, chest tightness, and cough that vary with time and in intensity, associated with variable airflow obstruction. It affects around 300 million people globally of all ages and ethnicities, with a death rate of ∼250,000 people each year. In China, a recent survey of a representative population of asthma found a prevalence of 4.2% of asthma in adults aged 20 or above, representing 45.7 million Chinese.[1] Asthma is associated with a high degree of morbidity, part of which is represented by those suffering from asthma despite being treated with anti-asthma medication, although many patients with asthma remain undiagnosed or are not receiving adequate medication. The recent asthma survey in China found 15.5% of asthmatics reporting at least one emergency room visit and 7.2% experiencing at least one hospital admission in the past year. It is likely that this morbidity is accounted for by inadequate treatment of asthma because only 5% to 6% of asthmatics had been treated with inhaled corticosteroids (ICS), suggesting undertreatment.[1]
It is those asthma patients who experience uncontrolled asthma despite adequate therapy who are now labelled as suffering from severe asthma that represent an important economic burden to healthcare providers. The prevalence of severe asthma has been reported to be 3.6% amongst asthmatics attending a hospital clinic in the Netherlands[2] and 4% to 6% in a Swedish cohort.[3] In China, the incidence of severe asthma amongst asthmatics ranges from 3.4% to 8.3%.[4–6] In terms of death rates from asthma, between 2011 and 2015 in high income countries, these are reported to be the highest in UK, Australia and US averaging three to five standardized deaths per million population, while those in lower income countries as high as 12 to 25 per million population in Mauritius, South Africa and Philippines (http://globalasthmareport.org/burden/mortality.php; checked on October 6, 2021). Data regarding China are sparse, but in 2004 to 2005, a standardized death rate of 24.5 per million population was reported.[7] It is to be noted that many of these asthma death rates are preventable because of inadequate diagnosis and treatment, and therefore the death rate due to uncontrolled or severe asthma would be lower than that reported. However, it is clear that the costs for uncontrolled or severe asthma are higher than those for controlled asthma. In Europe, the direct costs through hospital care, medication and indirect costs from time out of work range from 509 Euros per patient for those with controlled disease to 2281 Euros for uncontrolled disease per patient.[8] The costs of asthma in Europe are estimated to be 21 billion Euros for asthmatics aged between 16 and 54 years in 2014. In the United States, $56 billion is the estimated costs for asthma in 2015 (www.aafa.org/page/cost-of-asthma-on-society.aspx; checked on October 5, 2021).
The last 20 years of research into asthma has improved our understanding of the asthma with the recognition that far from being a single disease entity, asthma is a complex heterogenous disease that presents with several clinical phenotypes driven by a multiplicity of molecular mechanisms. This complexity of the severe asthma diathesis has become fully appreciated following the international agreement of a working definition of severe asthma that has in turn led the foundation towards an increased understanding of the underlying phenotypes and mechanisms. The clarification of the inflammatory processes such as the dominance of the Type-2 (T2) inflammation and the concomitant phenotyping into clinical and molecular phenotypes has contributed to improving the management of these patients.[9] This has subsequently led to the successful introduction of monoclonal antibody therapies directed at various targets underlying T2 inflammation, an example of precision medicine applied to severe asthma.
In this review, we will start with the agreed definition of severe asthma and the clinical approach to the diagnosis and management of patients with severe asthma. In parallel, we will describe the clinical and molecular phenotypes of severe asthma and the underlying driving mechanisms of severe asthma that underpin these phenotypes. Finally, we will review the new treatments that have been introduced for severe asthma and summarize current on-going research towards more precision medicine for severe asthma.
Defining Severe Asthma
Prior to any consensus on the definition of severe asthma, there has been several terms used by clinicians to characterize these patients such as “brittle” asthma, corticosteroid-resistant asthma, difficult-to-control asthma, difficult-to-treat asthma, difficult chronic asthma, and life-threatening asthma which qualifies the type of asthma presenting in a particular asthma patient. One of the first international definitions of the World Health Organization (WHO) Consultation on Severe Asthma defined severe asthma as “uncontrolled asthma which can result in risk of frequent severe exacerbations (or death) and/or adverse reactions to medications and/or chronic morbidity.”[10] This definition encompassed three groups of asthmatics: (1) untreated severe asthma representing patients who cannot receive treatment for their asthma because they cannot afford the treatments or do not have access to such treatments, (2) difficult-to-treat severe asthma in patients who are treated for their asthma but are not responsive to the treatments for many reasons including not being adherent to treatments or not receiving the right treatments, and (3) treatment-resistant severe asthma where control is not achieved despite the highest level of recommended treatment and asthma for which control can be maintained only with the highest level of recommended treatment.
A later definition of severe asthma from a task force of the European Respiratory Society (ERS) and American Thoracic Society (ATS)[11] focused on severe asthma being a treatment-resistant asthma after the patient has been treated effectively and all other cofounders, such as medication compliance and comorbidities have been addressed. The definition was: “When a diagnosis of asthma is confirmed and comorbidities have been addressed, severe asthma is defined as ‘asthma which requires treatment with high dose ICS plus a second controller (and/or systemic corticosteroids) to prevent it from becoming ‘uncontrolled’ despite this therapy.”[11] Uncontrolled asthma was defined according to the presence of poor symptom control, frequent severe exacerbations, serious exacerbations and presence of airflow limitation.
Diagnosing and Managing Severe Asthma
The diagnosis of severe asthma will be made in clinical practice in patients with asthma who remain uncontrolled despite receiving asthma treatments, at which stage the patient may be qualified as having “difficult-to-control asthma.” Within this cohort of difficult-to-control asthma who are referred to secondary care, a proportion will ultimately receive a diagnosis of severe asthma. This should happen when there has been exclusion of any other diagnoses that could mimic asthma, when the right treatments have been provided according to current guidelines and when the patient has been shown to be adherent to treatment. This approach will ensure that the first two types of patients under the WHO classification (untreated and difficult-to-treat asthma) have been excluded and that we have a patient with severe asthma according to the ATS-ERS definition of severe asthma.
It has been recommended that patients are evaluated within an asthma specialist service by a physician who specializes in asthma with access to the services of a whole multidisciplinary team.[12] The assessment in a multidisciplinary team environment allows the experienced clinician to ensure that the classical symptoms of asthma (fluctuating dyspnoea, wheezing, cough) are caused by asthma rather than one of the many other conditions that can cause a similar medley of clinical symptoms.
A systematic assessment of the patient with difficult-to-treat-asthma should be performed within a severe asthma service [Table 1]. First, confirmation of the diagnosis of asthma and its severity should be evaluated, a process that can take up to 3 to 6 months of evaluation in the clinic. Bronchodilator reversibility or assessing variability of airflow obstruction with a peak flow diary or, if possible, the measurement of bronchial hyperreactivity may be used to support the diagnosis of asthma. High resolution computed tomography (HRCT) of the lungs is not indicated in the routine management of asthma but it can be used to exclude alternative diagnoses or the existence of additional conditions or co-morbidities of the lung. These conditions include intra- or extra-thoracic airway obstruction, obliterative bronchiolitis, chronic obstructive pulmonary disease, congestive heart failure, hypersensitivity pneumonitis, hyper-eosinophilic syndromes, allergic bronchopulmonary aspergillosis and eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome).
Table 1.
Systematic assessment of difficult-to-treat/severe therapy resistant asthma.
| Item | Contents |
| 1 | Refer to an asthma specialized service |
| 2 | Confirmation of asthma diagnosis: Lung function variability; bronchial provocation test |
| 3 | Exclude other conditions masquerading as asthma |
| 4 | Assess severity of disease: Poor symptom control, airflow obstruction, frequent exacerbations, life-threatening severe exacerbations |
| 5 | Check inhalation technique and adherence to treatments |
| 6 | Optimization of treatment according to national guidelines |
| 7 | Assess adherence to therapy |
| 8 | Adaptation and using self-management plans |
| 9 | Identification and avoidance of trigger factors |
| 10 | Assessment and management of comorbidities |
| 11 | Phenotyping according to clinical-physiological-inflammatory parameters |
| 12 | Individualization of management plan |
Assessing the factors that may underlie symptoms or exacerbations such as poor adherence to treatments and improper inhaler technique, comorbidities, and assessment of risk factors and triggers must be made [Supplementary Table 1]. Anxiety and depression and socio-economic factors may also form the basis of uncontrolled asthma. Social and psychological or psychiatric support may also be provided. The prevalence of treatable pulmonary and non-pulmonary traits is higher in severe asthma compared to non-severe asthma.[13] Use of a systematic approach and attention to managing comorbidities can improve outcomes, quality of life, asthma control, reduce health care usage, and minimize the amount of oral corticosteroid (OCS) use.[14,15]
Addressing non-adherence to prescribed inhaled and oral therapies is important since it can exist in 30% to 70% of prescribed medication[16] in difficult-to-treat asthma, including the adherence to oral prednisolone therapy.[17,18] For oral prednisolone adherence based on prednisolonecortisol assays, the adherence varied from 33% to 45% in two specialist asthma centers.[18,19] Non-adherence has been associated with poorer asthma outcomes, from worsening symptoms scores, increase in hospitalization, exacerbations rates, and mortality.[16] Unintentional nonadherence can usually be addressed by better education, once a daily inhaler preparations, and ensuring (where possible) that if multiple inhalers in use are of the same type used in the same manner. Intentional non-adherence is a complex problem requiring further evaluation of the reasons behind it and will require other members of the asthma multidisciplinary team to be involved.[20]
Assessment of the severity of asthma based on the definition of asthma control is necessary. Severity has been defined according to either poor symptom control, or presence of frequent severe exacerbations or serious exacerbations or presence of airflow limitation.[11] Those patients that do not meet those criteria, but whose asthma control worsens when tapering corticosteroid dose, also meet the criteria of severe asthma. These criteria can predict future risks from asthma as well as side-effects of medications.
Finally, treatments need to be reviewed and optimized. Combination therapy of long-acting beta-agonist (LABA) with medium/high dose ICS should be established, with addition of leukotriene modifier and long-acting muscarinic antagonist (LAMA). Use of daily oral prednisolone can be considered but with the introduction of targeted antibody therapies for severe asthma, resort to the use of OCS therapy may be reduced.
Self-management education should be provided with incorporation of a personalized written or electronic asthma action plan. A review at 3 to 6 months should determine whether the patient has severe asthma, with the diagnosis confirmed if the asthma remains uncontrolled despite optimization of therapy. If the asthma remains well-controlled, stepping-down treatment can be considered, and if asthma symptoms or exacerbations occur on stepping down high dose treatment, then severe asthma can be diagnosed.
Risk Factors and Co-morbidities of Severe Asthma
Severe asthma phenotypes have been associated with genetic factors, age of asthma onset, disease duration, exacerbations, rhinosinusitis, and inflammatory characteristics.[21–24] Early childhood-onset severe asthma is characterized by allergic sensitization and a strong family history of asthma.[25,26] On the other hand, late-onset severe asthma is often associated with female gender and reduced lung function, persistent eosinophilic inflammation, nasal polyps and sinusitis, and aspirin sensitivity.[25,27–29]
Obesity is associated with childhood and adult-onset severe asthma, but its impact may differ by age at onset and the degree of allergic inflammation.[30,31] Tobacco smoke and environmental air pollution have been considered as risk factors for more severe asthma.[32,33] Cigarette smoking, exposure to air pollution and obesity have been linked to corticosteroid insensitivity, also present in severe asthma.[34–36] Exacerbations in severe asthma are more frequent in those with comorbidities such as rhinosinusitis, gastro-oesophageal reflux disease, recurrent respiratory infections and obstructive sleep apnoea.[37] Sensitization to the fungus, Aspergillus fumigatus, has also been linked to severe asthma development in adults.[38]
Patients with severe asthma face the risks of lung function decline, recurrent exacerbations, corticosteroid-induced side effects and mortality,[39] being greater in those with uncontrolled asthma as compared to those with controlled asthma. Future risk of exacerbations can be predicted from a history of exacerbations, in addition to other factors including smoking history, poor lung function, nasal polyps, obesity, and co-morbid depression.[40–44] Loss of lung function in severe asthma has been associated with exacerbation rate, OCS usage, and age.[45] Higher mortality has been associated with poor asthma control and presence of severe airflow obstruction.[46] Severe asthma patients taking OCSs had more comorbidities that may be related to systemic corticosteroid exposure such as T2 diabetes, obesity, osteoporosis, hypertension, cataracts, and dyspeptic symptoms than those with milder asthma.[47]
HRCT scan can also be used to denote future risks. The presence of air trapping on HRCT has been associated with asthma-related hospitalizations, intensive care unit visits, duration of asthma, airway neutrophilia, and airflow obstruction.[48] Mucus plugging is another recognized feature of acute severe asthma and fatal asthma.[49] Using a quantified mucus plugging scoring system, 67% of asthmatics with forced expiratory volume in one second (FEV1) of less than 60% predicted had plugs present in four or more lung segments.[50]
Long-term OCS therapy
The co-morbidities that are associated with long-term OCS therapy need to be addressed by monitoring bone densitometry and treating reduced bone density appropriately as well as monitoring for metabolic sequelae of OCS use, such as non-alcoholic fatty liver disease. With the advent of monoclonal antibodies, or biologic therapies, the reliance on long-term OCS for the treatment of severe asthmatics has been reduced. This could occur with the early administration of such biologic therapies prior to considering the use of OCS therapy. In addition, with establishment of biologic therapies particularly antiinterleukin (IL) 5, anti-IL5Rα and anti-IL4Rα antibodies,[51–53] the dose of OCS can be reduced and sometimes discontinued.
Corticosteroid-insensitive patients continue to experience side-effects from OCS therapy.[54] Use of sputum eosinophil counts which has been recommended by the ERS-ATS guidelines may help toward adjusting to the lowest dose of OCS needed.[55] Low levels of sputum or blood eosinophil counts indicate a non-eosinophilic phenotype that is likely to be less responsive to OCS therapy, and therefore corticosteroids can be down-titrated. On the other hand, the blood eosinophil count may increase as a result of down-titration of the OCS dosage, in which case this would represent a corticosteroid-sensitive but relatively resistant eosinophilic asthma. A recent expert consensus statement relating to OCS use, tapering, adverse effects, adrenal insufficiency, and patient-physician shared decision-making has been published and will be helpful in the management of OCS therapy in severe asthma.[56]
Clinical Severe Asthma Phenotypes
Clinicians over the years have described various descriptive phenotypes such as early childhood onset severe allergic asthma, late adult-onset eosinophilic asthma, obesity-associated asthma, aspirin-associated asthma, and smoking-associated asthma. In addition, clinical phenotyping has also been done on the basis of clinical features and the presence of chronic airflow obstruction and exacerbations.[57]
Unbiased methods of clustering used in the Severe Asthma Research Program have refined these clusters with identification of phenotypes of (1) early onset atopic asthma with mild to moderate severity, (2) obese late onset non-atopic asthma female patients with frequent exacerbations, and (3) those with severe airflow obstruction and use of daily OCS therapy.[27] In the European Unbiased Biomarkers in Prediction of Respiratory Disease Outcomes (U-BIOPRED) cohort that included severe smoking and ex-smoking patients, three severe asthma phenotypes were described: (1) late-onset asthma with past or current smoking and chronic airflow obstruction, (2) non-smoking severe asthma with chronic airflow obstruction and use of OCS therapy, and (3) obese female patients with frequent exacerbations but with normal lung function.[58] Inclusion of sputum eosinophilia as a marker of eosinophilic asthma has resulted in two clusters: one of non-eosinophilic inflammation characterized by early-onset, symptom-predominant group in female obese patients, and another of eosinophilic inflammation with late-onset disease, associated with rhinosinusitis, aspirin sensitivity, and recurrent exacerbations,[25] later described as a severe eosinophilic asthma phenotype[59] [Supplementary Table 2].
Molecular Phenotypes of Severe Asthma
Definition of T2-high phenotype
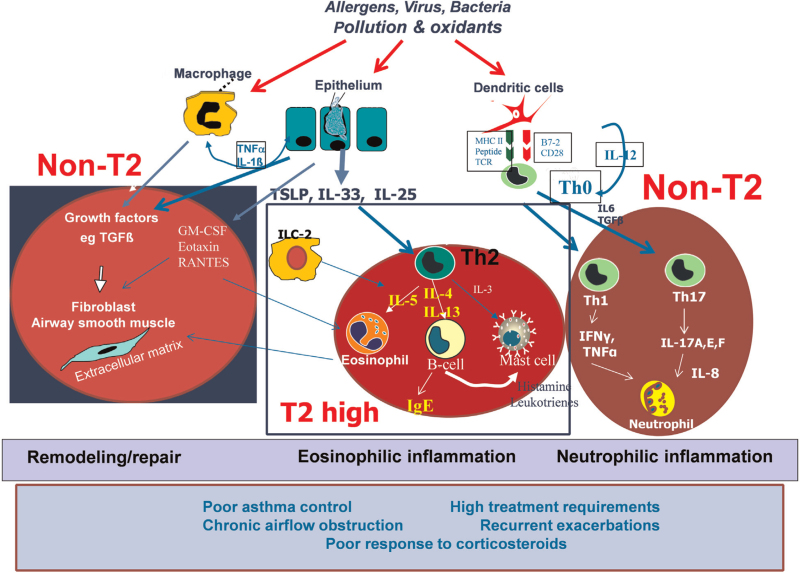
The airway epithelium responds to external stimuli such as allergens, pollutants and infectious agents (eg, viruses) to cause the recruitment and/or activation of cells that are involved in the innate and adaptive immune response (such as dendritic cells, mast cells, and innate lymphoid cells),[60] which leads to the orchestration of airway inflammation and mechanisms that drive the pathophysiology of asthma.[61] Thus, it controls the regulation of T2 cytokines through the production of the alarmins such as thymic stromal lymphopoietin (TSLP), IL-25, and IL-33, which can be induced following exposure of epithelial cells to external stimuli including environmental pollutants, viruses and allergens[62] [Figure 1]. IL-33 is a member of the IL-1 cytokine family and an inducer of chemoattractants for T-helper type 2 cells (Th2). TSLP is an IL-7-related cytokine secreted by airway epithelial cells that activates dendritic cells to release chemokines that attract and activate Th2 cells. Expression levels of both IL-33 and TSLP are increased in airway epithelium of patients with asthma, particularly in those with severe asthma.[63,64] These alarmins can be produced in response to allergens such as house dust mite and A. fumigatus in a Toll-like receptor 4/myeloid differentiation factor 88 (MyD88)-dependent manner,[65,66] as well as exposure to diesel exhaust particles[67] and to virus infections.[68] There is also increased expression of the IL-33 and TSLP receptors in sputum cells from patients with severe asthma.[69]
Figure 1.
Airway epithelium interactions with environmental factors to induce innate and adaptive immune and inflammatory responses with the release of TSLP, IL-33, and IL-25. Generation of Th2 cells and ILC2s leads to the elaboration of IL-4, IL-5, and IL-13, inducing an allergic eosinophilic inflammation with eosinophil recruitment and activation, and IgE production. Activation of Th1 and Th17 cells may contribute to neutrophilic inflammation. Airway wall remodeling and repair driven by epithelial-mesenchymal transformation and the effects of ILs on airway structural cells such as airway smooth muscle cells and epithelial cells contribute to chronic airflow obstruction and bronchial hyper-responsiveness. Targets for interleukin intervention against IL-4, IL-5, and IL-13 are shown. B7-2: Also known as cluster of differentiation 86 (CD86); GM-CSF: Granulocyte-macrophage colony-stimulating factor; IFN γ: Interferon γ; IgE: Immunoglobulin E; IL: Interleukin; ILC2: Type 2 innate lymphoid cells; MHC II: Major histocompatibility complex; RANTES: Regulated upon activation, normal T cell expressed and presumably secreted; TCR: T-cell receptor; T2: Type 2 inflammation; TGFβ: Transforming growth factor β; Th0: T helper 0; Th1: T helper 1; Th2: T helper 2; Th17: T helper 17; TNFα: Tumor necrosis factor α; TSLP: Thymic stromal lymphopoietin.
The alarmins TSLP and IL-33 direct T-helper cells towards a Th2 phenotype with the secretion of IL-4, IL-5, and IL-13, and also directly stimulate innate lymphoid T2 cells to produce IL-5 and IL-13.[62] The Th2 cytokines, including IL-3, IL-4, IL-5, IL-9, and IL-13, are expressed in bronchial submucosa of patients with the disease. IL-5 is involved in the terminal differentiation of eosinophils and in the activation of airway eosinophils. IL-13 increases the activation of inducible nitric oxide synthase (iNOS) enzyme in the epithelium, leading to an increase in exhaled nitric oxide (NO), goblet cell metaplasia and bronchial hyperresponsive-ness. IL-4 promotes immunoglobulin E (IgE) synthesis and primes the vascular endothelium for the extravasation of eosinophils.
T2-high and severe eosinophilic asthma
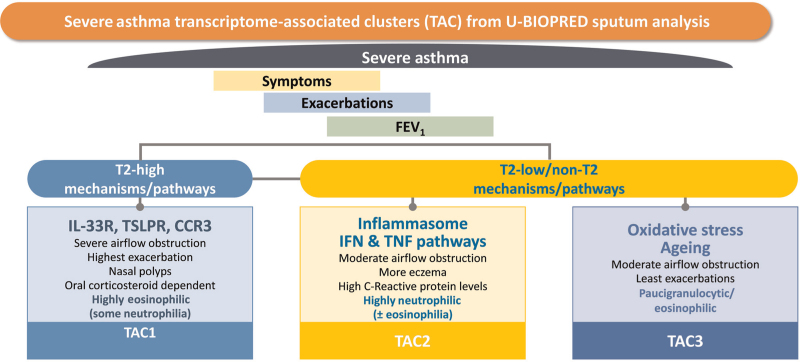
Hierarchical clustering of differentially expressed genes between eosinophilic and non-eosinophilic inflammatory profiles using sputum cell transcriptomics revealed three molecular phenotypes.[70] The first transcriptomic-associ-ated cluster (TAC1) was characterized by the immune receptors of IL-33, eotaxin (CC chemokine receptor 3), and TSLP with the highest enrichment of gene signatures for the IL-13/T2-inflammation signature being associated with sputum eosinophilia [Figure 2]. This grouped patients with severe asthma with OCS dependency, frequent exacerbations, and severe airflow obstruction, characteristics of the severe eosinophilic asthma phenotype[59] [Supplementary Table 2].
Figure 2.
Three transcriptomic-associated clusters (TAC) obtained from hierarchical clustering of differentially expressed genes obtained by comparing the transcriptome of sputum cells obtained from eosinophil-high and eosinophil-low sputum from patients with severe asthma. Each of the lower boxes represent the genes or class of genes that characterize each TAC signature, the salient clinical features and the sputum granulocytic inflammation. CCR3: CC chemokine receptor 3; FEV1: Forced expiratory volume in one second; IFN: Interferon; IL-33R: Interleukin 33 receptor; TNF: Tumor necrosis factor; T2: Type 2 inflammation; TSLPR: Thymic stromal lymphopoietin receptor; U-BIOPRED: Unbiased Biomarkers in Prediction of Respiratory Disease Outcomes.
Using a different approach to determine the enrichment of an IL-13/T2-high gene expression signature in bronchial epithelial cells of patients with asthma, a T2-high phenotype was found in up to 37% of patients with severe asthma.[71] The T2-high patients were more symptomatic despite treatment with ICS and most often on OCS therapy, characterized by higher levels of nitric oxide in exhaled breath and of blood and sputum eosinophils. This, therefore, defined the molecular signature of the severe eosinophilic asthma. Using a composite measure of IL-4, IL-5, and IL-13 gene expression in induced sputum cells from severe asthma patients, 70% of patients on high dose ICS had a T2-high phenotype.[72]
The “T2-high” phenotype[70] is likely to be driven by activated eosinophils through the production of cytotoxic proteins such as major basic protein, eosinophil peroxidase, eosinophil cationic protein and eosinophil derived neurotoxin, which cause damage to epithelial and other lung structural cells.[73] Release of proinflammatory cytokines and chemokines and of lipid eicosanoid products may contribute to bronchial hyperresponsiveness and airway wall remodeling. The success of anti-IL5 and anti-IL5Rα antibody treatments in reducing exacerbations in severe eosinophilic asthma has firmed up this phenotype as an endotype while the therapeutic effects of anti-IgE antibody support the severe allergic asthma phenotype.
Non-T2 pathways and neutrophilic asthma
The second cluster, TAC2, derived from the U-BIOPRED sputum transcriptomic analysis was characterized by inflammasome-associated genes, interferon-α and tumor necrosis factor-α-associated genes, with sputum neutrophilia, high serum C-reactive protein levels and a higher prevalence of eczema, defining patients with neutrophilic asthma[69] [Figure 2]. The third phenotype (TAC3) was characterized by metabolic pathway genes, ubiquitination and mitochondrial function which are normally reduced in asthma and with paucigranulocytic inflammation and little airflow obstruction. The non-T2 pathways that could drive asthma pathobiology include type 1 innate lymphoid cells, inflammasome, neutrophil and IL-17 activation, oxidative phosphorylation, and interferon-gamma (IFNγ) activation.[74] Th1 CD4+ T cells produce IFNγ which plays a role in controlling intracellular infections and in autoimmunity. Th1 cells expressing IFNγ are increased in bronchoalveolar lavage fluid, sputum cells and bronchial biopsies from patients with asthma.[75–77] Bronchoalveolar lavage fluid from severe asthmatic patients showed greater Th1 cells and neutrophil numbers accompanied by higher IFNγ levels than in non-severe asthmatics.[78]
Th17 cells are CD4+ T cells that express IL-17A, IL-17E, IL-17F, and IL-22 mediating neutrophil activation via IL-8 production.[79,80] Th17-associated cytokines IL-17A and IL-17F have been localized in the airways of patients with severe asthma.[81] Th17 cells have been implicated as a cause of neutrophilia in severe asthma.[82] An “IL-17-high” asthma phenotype has been characterized by bronchial epithelial dysfunction and upregulated antimicrobial and inflammatory response.[83] An IL-6 transsignaling signature developed in U-BIOPRED also indicated an IL-6 phenotype that drives non-T2 airway inflammation and epithelial dysfunction.[84]
Sputum neutrophilia has been associated with severe asthma, corticosteroid insensitivity, and chronic airflow obstruction[85–88] and is also observed during acute exacerbations.[89,90] Sputum neutrophilic inflammation associated with inflammasome activation supports the presence of a neutrophilic phenotype of asthma.[70] In neutrophilic asthma, an elevated gene expression of nucleotide-binding domain and leucine-rich, repeat-containing family, pyrin domain containing 3, caspase-1 and IL-1β in sputum cells has been reported.[69,91] Increased airway neutrophilia may be the result of infection, exposure to air pollutants, or treatment with corticosteroids, particularly OCSs.[92]
Non-T2 pathways: airway wall remodeling
Airway wall remodeling is characterized by an increase in airway smooth muscle mass, subepithelial fibrosis and increased numbers of mucous glands and goblet cells. The epithelium in severe asthma is thicker than in mild-moderate asthma, with increased proliferation, apoptosis and release of pro-inflammatory factors.[93] Airway smooth muscle mass increase is associated with airflow obstruction and bronchial hyper-responsiveness, with an enhanced proliferation rate.[85,94,95] Fibrocytes, which can differentiate into myofibroblasts, are increased in blood and in airway smooth muscle bundles in asthma patients with fixed airway obstruction and/or severe disease.[96–98] Subepithelial thickening of the bronchial reticular layer is a feature of asthma of all severities.[22,85,99] Patients with severe asthma have increased expression of transforming growth factor-β isoforms and collagen deposition, compared to those with mild asthma, in association with eosinophilia.[100,101] In severe eosinophilic asthma, the increased subbasement membrane thickness was associated with matrix metallopeptidase 10 and MET protooncogene, receptor tyrosine kinase (MET) which are the most differentially expressed proteins that drive airway wall remodeling.[102]
HRCT scan studies in asthmatic subjects may reveal abnormal radiologic findings, such as bronchial wall thickening, bronchial wall dilatation, bronchiectasis, mosaic lung attenuation, mucus plugging, prominent centrilobular opacities, emphysema, and atelectasis.[103,104] Mosaic lung attenuation represents involvement of small airways (<2 mm) and acinar air spaces. Its delineation can be improved by using a expiratory phase computerized tomography (CT).[105] HRCT scan abnormalities such as bronchial wall thickening (62%), bronchial enlargement (40%), and emphysema (8%) have been shown to be present in 80% of severe asthma subjects.[106]
Non-T2 pathways: corticosteroid insensitivity
Severe asthma may be considered as having poor therapeutic response to corticosteroid therapy.[107,108] The defining feature of severe asthma is need for high dose ICS to gain disease control or despite this, the disease is poorly controlled (as previously described in this review), with only 62% of moderate to severe asthma gaining control with ICS and LABAs inhalers.[109] Corticosteroid insensitivity has been defined clinically by <15% improvement of peak expiratory flow rates from baseline after receiving 7 to 12 days of 30 mg of prednisolone.[110] Half of severe asthma patients in the U-BIOPRED cohort were on OCS therapy, and had a higher prevalence of nasal polyps, uncontrolled asthma, and a higher number of exacerbations compared with those not on OCS maintenance,[111] associated with elevated fractional exhaled nitric oxide (FeNO) and sputum eosinophils, evidence of corticosteroid insensitivity. After treatment with intramuscular triamcinolone, most patients showed little response in terms of lung function (FEV1), blood eosinophil count or levels of FeNO, accompanied by little change in the expression of IL-4, IL-5, and IL-13 genes in sputum cells, indicating a degree of corticosteroid insensitivity in this group.[112]
Cigarette smoking, bacterial infections, obesity, and deficiency of vitamin D have been associated with corticosteroid insensitivity.[107,108] Impaired nuclear translocation of the glucocorticoid receptor, activation of mitogen-activated protein kinase pathways, activation of transcription factors by IFNγ and increased oxidative stress resulting in reduction in histone deacetylase expression and activity are implicated molecular mechanisms.[108] The observations of overexpression of Haemophilus parain fluenzae (H. parainfluenzae) in bronchoalveolar lavage fluid and culture of H. parainfluenzae with macrophages inducing corticosteroid resistance in these cells link steroid resistance to Haemophilus infection.[113] Th17 cells may be induced by bacterial infections, and have been implicated in corticosteroid insensitivity.[114,115] The anti-IL5α antibody, mepolizumab,[51] anti-IL-5Rα antibody, benralizumab,[52] and anti-IL4Ra antibody, dupilumab[53] reduced maintenance dose of OCS by 50% in OCS-dependent patients with severe eosinophilic asthma, implicating a role for these T2 cytokines in corticosteroid insensitivity. Therefore, both T2 and non-T2 pathways may underlie corticosteroid insensitivity in severe asthma.
Phenotyping and Biomarkers for Severe Asthma
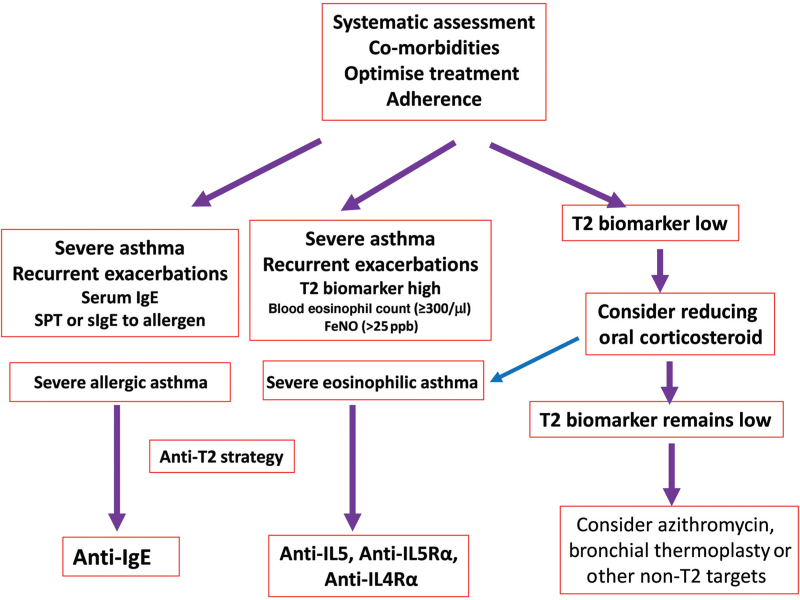
After the diagnosis of severe asthma has been ascertained, phenotyping in terms of whether the patient falls within the category of severe eosinophilic asthma should be determined [Figure 3]. This is often referred to as a T2 high phenotype asthma because the severe eosinophilic asthma has been linked to T2 inflammation as measured in the bronchial epithelium by the expression of genes involved in T2 inflammation.[71] Using this measure of T2 inflammation, a blood eosinophil count of 115 cells/mL was found to have the highest sensitivity and specificity for detecting T2 inflammation with a receptor operating curve discrimination of 68.9%.[71] The reason why this phenotype should be ascertained is that there are biologic treatments that target specific components of the type-2 inflammation (IL-5, IL-4, and IL-13) and provide clinical benefits when given to severe eosinophilic asthma patients.
Figure 3.
Management algorithm for severe asthma. After confirming the diagnosis of severe asthma, Type-2 (T2) biomarkers which are currently based on blood eosinophil count, fractional exhaled nitric oxide (FeNO) and total serum immunoglobulin E (IgE) and presence of allergies can be used to diagnose severe eosinophilic or severe allergic asthma. Those with high-T2 biomarker and regular exacerbations of asthma can be offered biologic anti-T2 treatments. Those with low T2-biomarker will need to have a reduction in oral corticosteroid dosage (OCS) in case the steroids are suppressing the level of T2-biomarkers. Trial of long-term azithromycin or bronchial thermoplasty may be considered. IL: Interleukin; IL4Rα: Interleukin 4 receptor alpha; IL5Rα: Interleukin 5 receptor alpha; ppb: Part per billion; SPT: Skin prick tests; sIgE: Specific immunoglobulin E.
Blood eosinophil count has been used to identify T2-high status, derived from the clinical trials of these biologic therapies. A level of 300 cells/mL blood eosinophil count has been used as the cut-off point although a level of > 150 cells/mL has also been used, with most studies showing that the higher the cut-off point used, the greater the beneficial effects of the biologic therapies (anti-IL5, anti-IL5Rα, and anti-IL4Ra antibodies) in terms of reduction in exacerbation rates. For anti-IgE therapy, a blood eosinophil count > 260/μL is predictive of this response in severe allergic patients.[116]
Sputum eosinophils are the most sensitive and specific noninvasive biomarker for eosinophilic airway inflamma-tion,[117] but is difficult to obtain reliably and measure easily. A differential cell count of > 2% to 3% indicates an underlying eosinophilic inflammatory process and is diagnostic of eosinophilic airway disease.[117]
FeNO is another biomarker of T2 inflammation of the airways, indicating the activity of T2 cytokines IL-4 and IL-13, as these cytokines upregulate the expression of epithelial iNOS.[118] Recent evidence has confirmed the utility of using FeNO as an indicator of T2 inflammation, with its use as a tool for precision medicine in the management of severe asthma during periods of stability and instability.[119] The severe asthma ATS/ERS and Global Initiative for Asthma (GINA) guidelines have indicated that a FeNO > 20 parts per billion (ppb) is enough to indicate T2-high signal and to identify those who are likely to respond to current anti-T2 biologic therapies.[120]
Therapeutic Approaches for Severe Asthma
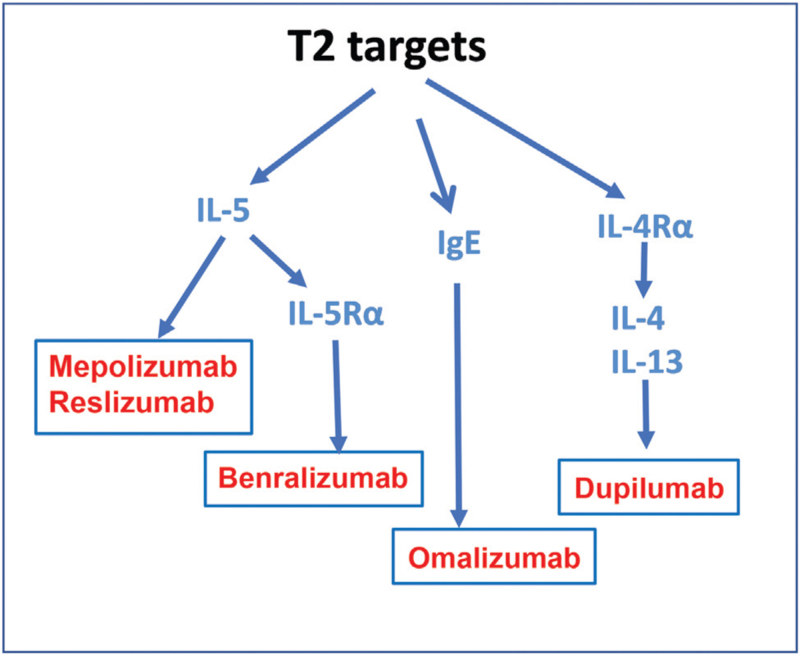
Once the patient with severe asthma has been phenotyped, consideration of therapeutic approaches remains the final important aim. Use of currently available controller treatments need to be ascertained irrespective of the phenotype of the asthma. Consideration needs to be given regarding new biologic therapies, particularly if the patient fulfils the criteria of a severe allergic or severe eosinophilic asthma, both being under the umbrella of T2-high inflammation[121] [Figure 3]. The list of currently available anti-T2 biologic therapies is shown in Figure 4. This represents the start of precision medicine for severe asthma.[9,122] There are currently no specific therapies for the various T2-low phenotypes, including neutrophilic asthma, airway wall remodeling and corticosteroid insensitivity. However, long-term azithromycin and bronchial thermoplasty may be considered. Currently, anti-TSLP and anti-IL-33 antibody therapies are being developed which might target both T2-high and T2-low phenotypes.
Figure 4.
Therapeutic antibodies targeting type 2 cytokines, IL-4, IL-5, and IL-13, and anti-IgE. IgE: Immunoglobulin E; IL: Interleukin; IL4Rα: Interleukin 4 receptor alpha; IL5Rα: Interleukin 5 receptor alpha; T2: Type 2 inflammation.
Current Controller Treatments
Patients with severe asthma are by definition usually at steps 4 and 5 of the therapeutic recommendations of the GINA guidelines (https://ginasthma.org/wp-content/uploads/2021/05/GINA-Main-Report-2021-V2-WMS.pdf). Controller therapies using a combination of ICS and LABA remain the backbone of treatment, with the possibility of using the maximum dose of ICS, although the step-up of medium to high-dose ICS may only provide a small improvement in control. Nevertheless, a trial of high dose ICS is recommended with the proviso that if there is little benefit, maintenance ICS dose should revert to the medium dose. The more recent recommendation from GINA is on the use of the maintenance and reliever therapy approach using the single inhaler of formoterol and an ICS, for which there is support for its efficacy in reducing severe exacerbations.[123] Triple therapy of LABA-LAMA-ICS is now recommended for the treatment of severe asthma, with previous studies showing that the LAMA, tiotropium bromide, added to ICS-LABA inhalation improved lung function and reduced exacerbations compared to ICS-LABA alone.[124]
Biologic antibody therapies
Omalizumab
Omalizumab, the first approved biologic for asthma, is a recombinant humanized monoclonal antibody that binds to circulating free IgE, thereby preventing IgE binding to its receptor (FceR1) expressed on the surface of basophils and mast cells and therefore the release of mediators from these cells.[125] Omalizumab is indicated for severe allergic asthma in whom appropriate pharmacotherapy and allergy avoidance have not been helpful and in those with a total serum IgE from 30 to 700 IU/mL (with the possibility of including those with serum IgE levels up to 1300 IU/mL) and raised specific IgE to at least one aeroallergen.[11] A meta-analysis that included 3000 patients with moderate to severe asthma reported a decreased risk of exacerbation while ICS doses were successfully reduced during omalizumab treatment.[126] In a study of severe allergic asthma, omalizumab led to a 25% reduction in exacerbations while improving quality of life scores and asthma symptom scores.[127]
Treatment response should be evaluated at 4 months, using a combination of quality of life, exacerbation frequency and health care use. In a post hoc analysis of the EXACT study of omalizumab in subjects with moderate-to-severe persistent asthma, a high blood eosinophil count > 260 cells/mL and a FeNO > 19.5 ppb were found to predict those patients likely to benefit most with a reduction in exacerbation frequency.[117] However, in a prospective real-world study (PROSPERO study), these biomarkers had minimal predictive value for omalizumab treatment outcomes, particularly for exacerbation reduction,[128] questioning the need for using these biomarkers to predict responders.
Anti-IL-5/anti-IL-5Rα antibodies: mepolizumab, reslizumab, and benralizumab
Mepolizumab, reslizumab, and benralizumab block the IL-5 pathway which is the key cytokine for eosinophil maturation, survival and transition of this granulocyte from the bone marrow into the systemic circulation. Mepolizumab and reslizumab are immunoglobulin G (IgG) 4 humanized monoclonal antibodies that bind IL-5, thus preventing it from binding to the IL-5Rα on eosinophils.[121] On the other hand, benralizumab is an IgG1 humanized monoclonal antibody that targets the α subunit of the IL-5 receptor. It induces apoptosis of eosinophils and basophils through antibody-dependent cell mediated cytotoxicity.
These three monoclonal antibodies (mAbs) have been approved for use in patients with severe eosinophilic asthma that demonstrate a blood eosinophil count of ≥300 cells/μL and experience frequent exacerbations. They reduce the risk of exacerbations by 40% to 50% with modest effects on lung function,[129–132] which have been replicated in real-world practice.[133–135] Mepolizumab and benralizumab also reduced the maintenance dose of OCS in OCS-dependent patients with severe eosinophilic asthma by 50% while reducing the frequency of exacerbations.[51,52]
The 2019 GINA guidelines for severe asthma recommend the use of an anti-IL-5 and anti-IL-5Rα antibody for patients who remain uncontrolled with exacerbations in the past year despite step 4 or 5 therapy and who have a blood eosinophil count > 300 cells/μL (https://ginasthma.org/wp-content/uploads/2021/08/SA-Pocket-guide-v3.0-SCREEN-WMS.pdf). The ERS-ATS recommendation on the other hand suggests a blood eosinophil count cut-off point ≥150 cells/μL.[119]
Anti-IL4Rα antibody: dupilumab
Dupilumab is a fully humanized monoclonal antibody directed at the α-subunit of the IL-4 receptor leading to the blockade of the effect of the T2-cytokines, IL-4, and IL-13,[121] involved in airway recruitment of eosinophils, B cell class switch to IgE production, goblet cell hyperplasia and mucus production, airway remodeling through airway smooth muscle proliferation and collagen deposition and airway epithelial cell expression of iNOS. Dupilumab decreased exacerbations in moderate to severe uncontrolled asthma, with greater improvements in those with higher blood eosinophil levels, reaching 67% in those patients with blood eosinophils ≥300 cells/ μL.[136,137] The greatest treatment benefit in terms of reduction in exacerbation rates and improvement in FEV1 as compared with placebo was observed in patients with elevated T2 biomarkers (baseline blood eosinophil count of ≥150 cells/μL and baseline FeNO of > 25 ppb).[128] It is also effective in reducing OCS dose in those with severe OCS-dependent asthma while reducing exacerbation risk.[53] Dupilumab therapy is also associated with improvements in lung function, asthma control and quality of life.
While the GINA severe asthma guidelines suggest using dupilumab for patients with severe eosinophilic asthma with exacerbations in the past year with either a blood eosinophil count of ≥150 cells/μL or a FeNO>25 ppb (https://ginasthma.org/wp-content/uploads/2021/08/SA-Pocket-guide-v3.0-SCREEN-WMS.pdf; checked on October 5, 2021), the ERS-ATS guidelines advise its use in severe eosinophilic asthma including corticosteroid-dependent asthma regardless of blood eosinophil counts.[119] However, those with higher blood eosinophil counts and higher FeNO are expected to respond better.
The indications for using these expensive biologic therapies are also dependent on the regulations set by the health care system of each country, which could be determined not only by being medically suitable for such therapies according to biomarker status, but also by other factors such as cost-benefit issues and affordability.
Other Therapies for Severe Asthma
Bronchial thermoplasty
Bronchial thermoplasty is a bronchoscopic procedure whereby intraluminal thermal energy is applied to the airway wall, with the aim of ablating the airway smooth muscle, although epithelial cells and nerves are also affected.[138] In patients with severe asthma, there is a modest effect in improving asthma-related quality of life scores and bringing some reduction in asthma exacerbations.[11] A sustained reduction in severe exacerbations over a 3-year follow-up period after bronchial thermoplasty in severe asthma with no improvement in pre- or post-bronchodilator FEV1 has been reported.[139] There is limited information on the identity of patients with severe asthma who will benefit most from bronchial thermoplasty. It has been suggested that bronchial thermoplasty should be considered for patients with severe asthma associated with non-T2 inflammation and non-eosinophilic inflammation. This procedure in patients with severe asthma resulted in 11.8% of 152 procedures associated with emergency respiratory readmission and in 48.1% having a post-procedural stay in hospital.[140] A 10-year review of clinical data in patients that have undergone bronchial thermoplasty reported an increased rate of bronchiectasis.[141]
The Asthma guidelines of the Expert Panel Working Group of the National Heart Lung, and Blood Institute in the US have conditionally recommended against bronchial thermoplasty in individuals aged 18 years and older with persistent asthma.[142] It advised for the conduct of registered clinical trials and long-term registry studies of bronchial thermoplasty to fully assess the clinical benefits and harms.
Long-term macrolide therapy
An abnormal microbiome has been associated with severe asthma.[143] The use of antibiotics in the treatment of severe asthma is therefore of interest with a potential two-pronged mode of action using macrolide antibiotics as an antineutrophilic and an anti-bacterial agent. In a placebo-controlled study of patients with symptomatic asthma on ICS and LABA, azithromycin administered three times per week reduced the number of exacerbations (1.86 per patient year in the control group versus 1.07 in the active group) together with an improvement in asthma and quality of life.[144] It was not clear whether severe exacerbations or hospitalizations were reduced. Patients with eosinophilic asthma benefited as well as patients with non-eosinophilic asthma. Azithromycin reduced airway H. influenzae load, with no changes in total or pathogenic bacterial loads. But there is emergence of antimicrobial resistance. It is not known whether this therapy does improve outcomes among patients with severe asthma. The ERS-ATS severe asthma guidelines suggest a trial of macrolide treatment to reduce asthma exacerbations in adult asthma subjects on GINA/National Asthma Education and Prevention Program (NAEPP) step 5 therapy that remain persistently symptomatic or uncontrolled, but they suggest against the use of chronic macrolide treatment in children and adolescents with severe uncontrolled asthma.[119]
Future directions
The introduction of these T2 targeted biologic antibody treatments for severe asthma remains a big advance in the treatment of asthma. One particular area of therapeutic advance has been in reducing the need for OCS therapy in severe asthma by these biologic therapies. The current development of other biologics such as the anti-TSLP and the anti-IL-33 antibody represents further development as they blocks the effect of these alarmins, TSLP and IL-33, produced by epithelial cells interacting with environmental factors such as allergens, pollutants, and infectious agents. Patients with severe, uncontrolled asthma who received tezepelumab, a monoclonal antibody to TSLP, had fewer exacerbations and better lung function, asthma control, and health-related quality of life than those who received placebo irrespective of the blood eosinophil counts.[145] Thus, these may be targeting not only T2-mechanisms but also non-T2 mechanisms. Once these non-T2 pathways that may be driving severe asthma are defined, specific therapies can be developed to treat patients with non-T2 phenotype. The failure of some non-T2 targeted therapies such as anti-IL-17 or anti-tumor necrosis factor antibody treatments may have resulted from inadequate selection of asthma phenotype. Further advances will result from the application of precision medicine to severe asthma, which is bringing the right treatment to the right patient.[9] The introduction of these efficacious treatments raises the issue as to whether these may represent disease modifying treatments, particularly if introduced earlier at the onset of severe asthma. Therefore, the best time to introduce these treatments for severe asthma with respect to the available treatments needs to be determined. The future looks very promising for severe asthma.
Funding
SG, JY and KFC are supported by the Sanming Project of Medicine in Shenzhen “Integrated Airways Disease Research Programme” (No. SZSM201612096). PD, HA-W, PB, and KFC are supported by UK Research Innovation projects (No. MRC MR/T010371/1; EPSRC: EP/T003189/1).
Conflicts of interest
KFC has received honoraria for participating in Advisory Board meetings of GSK, AstraZeneca, Roche, Novartis, Merck, Boehringer Ingelheim, TEVA and Shionogi regarding treatments for asthma, chronic obstructive pulmonary disease and chronic cough and has also been renumerated for speaking engagements. PHP has received honoraria for participating in Advisory Board meetings of GSK and AstraZeneca, regarding treatments for asthma and has also been remunerated for speaking engagements. Other authors have no conflicts of interest to declare.
Supplementary Material
Footnotes
How to cite this article: Chung KF, Dixey P, Abubakar-Waziri H, Bhavsar P, Patel PH, Guo S, Ji Y. Characteristics, phenotypes, mechanisms and management of severe asthma. Chin MedJ 2022;135:1141–1155. doi: 10.1097/CM9.0000000000001990
Supplemental digital content is available for this article.
References
- 1.Huang K, Yang T, Xu J, Yang L, Zhao J, Zhang X, et al. Prevalence, risk factors, and management of asthma in China: a national cross-sectional study. Lancet 2019; 394:407–418. doi: 10.1016/s0140-6736(19)31147-x. [DOI] [PubMed] [Google Scholar]
- 2.Hekking PP, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol 2015; 135:896–902. doi: 10.1016/j.jaci.2014.08.042. [DOI] [PubMed] [Google Scholar]
- 3.Backman H, Jansson SA, Stridsman C, Eriksson B, Hedman L, Eklund BM, et al. Severe asthma - a population study perspective. Clin Exp Allergy 2019; 49:819–828. doi: 10.1111/cea.13378. [DOI] [PubMed] [Google Scholar]
- 4.Su N, Lin JT, Wang WY, Chen P, Zhou X, Wan HY, et al. A crosssection study of severe asthma in eight provinces of China (in Chinese). Zhonghua Nei Ke Za Zhi 2016; 55:917–921. doi: 10.3760/cma.j. issn.0578-1426.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Wang WY, Lin JT, Zhou X, Chen P, Wan HY, Yin KS, et al. A survey on clinical characteristics and risk factors of severe asthma in China (in Chinese). Zhonghua Yi Xue Za Zhi 2020; 100:1106–1111. doi: 10.3760/cma.j.cn112137-20191117-02497. [DOI] [PubMed] [Google Scholar]
- 6.Wang G, Wang F, Gibson PG, Guo M, Zhang WJ, Gao P, et al. Severe and uncontrolled asthma in China: a cross-sectional survey from the Australasian Severe Asthma Network. J Thorac Dis 2017; 9:1333–1344. doi: 10.21037/jtd.2017.04.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li XY, Hu N, Huang ZJ, Jiang Y, Wu F. Mortality and death cause proportion of respiratory disease in China, 2004-2005 (in Chinese). Zhonghua Yu Fang Yi Xue Za Zhi 2010; 44:298–302. doi: 10.3760/cma.j. issn.0253-9624.2010.04.005. [PubMed] [Google Scholar]
- 8.Accordini S, Corsico AG, Braggion M, Gerbase MW, Gislason D, Gulsvik A, et al. The cost of persistent asthma in Europe: an international population-based study in adults. Int Arch Allergy Immunol 2013; 160:93–101. doi: 10.1159/000338998. [DOI] [PubMed] [Google Scholar]
- 9.Chung KF, Adcock IM. Precision medicine for the discovery of treatable mechanisms in severe asthma. Allergy 2019; 74:1649–1659. doi: 10.1111/all.13771. [DOI] [PubMed] [Google Scholar]
- 10.Bousquet J, Mantzouranis E, Cruz AA, Ait-Khaled N, Baena-Cagnani CE, Bleecker ER, et al. Uniform definition of asthma severity, control, and exacerbations: document presented for the World Health Organization Consultation on Severe Asthma. J Allergy Clin Immunol 2010; 126:926–938. doi: 10.1016/j.jaci.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 11.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43:343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 12.Chung KF. Diagnosis and management of severe asthma. Semin Respir Crit Care Med 2018; 39:91–99. doi: 10.1055/s-0037-1607391. [DOI] [PubMed] [Google Scholar]
- 13.Simpson AJ, Hekking PP, Shaw DE, Fleming LJ, Roberts G, Riley JH, et al. Treatable traits in the European U-BIOPRED adult asthma cohorts. Allergy 2019; 74:406–411. doi: 10.1111/all.13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tay TR, Lee J, Radhakrishna N, Hore-Lacy F, Stirling R, Hoy R, et al. A structured approach to specialist-referred difficult asthma patients improves control of comorbidities and enhances asthma outcomes. J Allergy Clin Immunol Pract 2017; 5:956–964.e3. doi: 10.1016/j.jaip.2016.12.030. [DOI] [PubMed] [Google Scholar]
- 15.Gibeon D, Heaney LG, Brightling CE, Niven R, Mansur AH, Chaudhuri R, et al. Dedicated severe asthma services improve health-care use and quality of life. Chest 2015; 148:870–876. doi: 10.1378/chest.14-3056. [DOI] [PubMed] [Google Scholar]
- 16.Lindsay JT, Heaney LG. Non-adherence in difficult asthma and advances in detection. Expert Rev Respir Med 2013; 7:607–614. doi: 10.1586/17476348.2013.842129. [DOI] [PubMed] [Google Scholar]
- 17.Robinson DS, Campbell DA, Durham SR, Pfeffer J, Barnes PJ, Chung KF. Asthma and Allergy Research Group of the National Heart and Lung Institute. Systematic assessment of difficult-to-treat asthma. Eur Respir J 2003; 22:478–483. doi: 10.1183/09031936.03.00017003. [DOI] [PubMed] [Google Scholar]
- 18.Gamble J, Stevenson M, McClean E, Heaney LG. The prevalence of nonadherence in difficult asthma. Am J Respir Crit Care Med 2009; 180:817–822. doi: 10.1164/rccm.200902-0166OC. [DOI] [PubMed] [Google Scholar]
- 19.Robinson DS, Campbell DA, Durham SR, Pfeffer J, Barnes PJ, Chung KF, et al. Systematic assessment of difficult-to-treat asthma. Eur Respir J 2003; 22:478–483. doi: 10.1183/09031936.03.00017003. [DOI] [PubMed] [Google Scholar]
- 20.Horne R. Compliance, adherence, and concordance: implications for asthma treatment. Chest 2006; 130:65S–72S. doi: 10.1378/chest.130.1_suppl.65S. [DOI] [PubMed] [Google Scholar]
- 21.Fitzpatrick AM, Teague WG, Meyers DA, Peters SP, Li X, Li H, et al. Heterogeneity of severe asthma in childhood: confirmation by cluster analysis of children in the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. J Allergy Clin Immunol 2011; 127:382–389.e1-13. doi: 10.1016/j.jaci.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wenzel SE, Schwartz LB, Langmack EL, Halliday JL, Trudeau JB, Gibbs RL, et al. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med 1999; 160:1001–1008. doi: 10.1164/ajrccm.160.3.9812110. [DOI] [PubMed] [Google Scholar]
- 23.Wenzel SE, Balzar S, Ampleford E, Hawkins GA, Busse WW, Calhoun WJ, et al. IL4R alpha mutations are associated with asthma exacerbations and mast cell/IgE expression. Am J Respir Crit Care Med 2007; 175:570–576. doi: 10.1164/rccm.200607-909OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haselkorn T, Fish JE, Zeiger RS, Szefler SJ, Miller DP, Chipps BE, et al. Consistently very poorly controlled asthma, as defined by the impairment domain of the Expert Panel Report 3 guidelines, increases risk for future severe asthma exacerbations in The Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens (TENOR) study. J Allergy Clin Immunol 2009; 124:895–902.e1-4. doi: 10.1016/j.jaci.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 25.Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, et al. Cluster analysis and clinical asthma phenotypes. AmJ Respir Crit Care Med 2008; 178:218–224. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol 2004; 113:101–108. doi: 10.1016/j.jaci.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 27.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med 2010; 181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ten Brinke A, Zwinderman AH, Sterk PJ, Rabe KF, Bel EH. Factors associated with persistent airflow limitation in severe asthma. Am J Respir Crit Care Med 2001; 164:744–748. doi: 10.1164/ajrccm.164.5.2011026. [DOI] [PubMed] [Google Scholar]
- 29.ten Brinke A, van Dissel JT, Sterk PJ, Zwinderman AH, Rabe KF, Bel EH. Persistent airflow limitation in adult-onset nonatopic asthma is associated with serologic evidence of Chlamydia pneumoniae infection. J Allergy Clin Immunol 2001; 107:449–454. doi: 10.1067/mai.2001.113047. [DOI] [PubMed] [Google Scholar]
- 30.Holguin F, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Erzurum SC, et al. Obesity and asthma: an association modified by age of asthma onset. J Allergy Clin Immunol 2011; 127:1486–1493e2. doi: 10.1016/j.jaci.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dixon AE, Pratley RE, Forgione PM, Kaminsky DA, Whittaker-Leclair LA, Griffes LA, et al. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J Allergy Clin Immunol 2011; 128:508–515.e1-2. doi: 10.1016/j.jaci.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Comhair SA, Gaston BM, Ricci KS, Hammel J, Dweik RA, Teague WG, et al. Detrimental effects of environmental tobacco smoke in relation to asthma severity. PloS One 2011; 6:e18574.doi: 10.1371/journal.pone.0018574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaudhuri R, Livingston E, McMahon AD, Lafferty J, Fraser I, Spears M, et al. Effects of smoking cessation on lung function and airway inflammation in smokers with asthma. Am J Respir Crit Care Med 2006; 174:127–133. doi: 10.1164/rccm.200510-1589OC. [DOI] [PubMed] [Google Scholar]
- 34.Peters-Golden M, Swern A, Bird SS, Hustad CM, Grant E, Edelman JM. Influence of body mass index on the response to asthma controller agents. Eur Respir J 2006; 27:495–503. doi: 10.1183/09031936.06.00077205. [DOI] [PubMed] [Google Scholar]
- 35.Lazarus SC, Chinchilli VM, Rollings NJ, Boushey HA, Cherniack R, Craig TJ, et al. Smoking affects response to inhaled corticosteroids or leukotriene receptor antagonists in asthma. Am J Respir Crit Care Med 2007; 175:783–790. doi: 10.1164/rccm.200511-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rider CF, Carlsten C. Air pollution and resistance to inhaled glucocorticoids: evidence, mechanisms and gaps to fill. Pharmacol Ther 2019; 194:1–21. doi: 10.1016/j.pharmthera.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 37.ten Brinke A, Sterk PJ, Masclee AA, Spinhoven P, Schmidt JT, Zwinderman AH, et al. Risk factors of frequent exacerbations in difficult-to-treat asthma. Eur Respir J 2005; 26:812–818. doi: 10.1183/09031936.05.00037905. [DOI] [PubMed] [Google Scholar]
- 38.Denning DW, O’Driscoll BR, Hogaboam CM, Bowyer P, Niven RM. The link between fungi and severe asthma: a summary of the evidence. Eur Respir J 2006; 27:615–626. doi: 10.1183/09031936.06.00074705. [DOI] [PubMed] [Google Scholar]
- 39.Song WJ, Lee JH, Kang Y, Joung WJ, Chung KF. Future risks in patients with severe asthma. Allergy Asthma Immunol Res 2019; 11:763–778. doi: 10.4168/aair.2019.11.6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McDonald VM, Hiles SA, Godbout K, Harvey ES, Marks GB, Hew M, et al. Treatable traits can be identified in a severe asthma registry and predict future exacerbations. Physical activity associates with disease characteristics of severe asthma, bronchiectasis and COPD. Respirology 2019; 24:37–47. doi: 10.1111/resp.13389 10.1111/resp.13428. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka A, Uno T, Sato H, Jinno M, Hirai K, Miyata Y, et al. Predicting future risk of exacerbations in Japanese patients with adult asthma: a prospective 1-year follow up study. Allergol Int 2017; 66:568–573. doi: 10.1016/j.alit.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 42.Boer S, Sont JK, Loijmans RJB, Snoeck-Stroband JB, Ter Riet G, Schermer TRJ, et al. Development and validation of personalized prediction to estimate future risk of severe exacerbations and uncontrolled asthma in patients with asthma, using clinical parameters and early treatment response. J Allergy Clin Immunol Pract 2019; 7:175–182.e5. doi: 10.1016/j.jaip.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 43.Loymans RJ, Honkoop PJ, Termeer EH, Snoeck-Stroband JB, Assendelft WJ, Schermer TR, et al. Identifying patients at risk for severe exacerbations of asthma: development and external validation of a multivariable prediction model. Thorax 2016;71:838–846. doi: 10.1136/thoraxjnl-2015-208138. [DOI] [PubMed] [Google Scholar]
- 44.Bloom CI, Palmer T, Feary J, Quint JK, Cullinan P. Exacerbation patterns in adults with asthma in England. A population-based study. Am J Respir Crit Care Med 2019; 199:446–453. doi: 10.1164/rccm.201808-1516OC. [DOI] [PubMed] [Google Scholar]
- 45.Matsunaga K, Akamatsu K, Miyatake A, Ichinose M. Natural history and risk factors of obstructive changes over a 10-year period in severe asthma. Respir Med 2013; 107:355–360. doi: 10.1016/j.rmed.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 46.Fernandes AG, Souza-Machado C, Coelho RC, Franco PA, Esquivel RM, Souza-Machado A, et al. Risk factors for death in patients with severe asthma. J Bras Pneumol 2014; 40:364–372. doi: 10.1590/s1806-37132014000400003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sweeney J, Patterson CC, Menzies-Gow A, Niven RM, Mansur AH, Bucknall C, et al. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the Optimum Patient Care Research Database and the British Thoracic Difficult Asthma Registry. Thorax 2016;71:339–346. doi: 10.1136/thoraxjnl-2015-207630. [DOI] [PubMed] [Google Scholar]
- 48.Busacker A, Newell JD, Jr, Keefe T, Hoffman EA, Granroth JC, Castro M, et al. A multivariate analysis of risk factors for the airtrapping asthmatic phenotype as measured by quantitative CT analysis. Chest 2009; 135:48–56. doi: 10.1378/chest.08-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dunnill MS. The pathology of asthma, with special reference to changes in the bronchial mucosa. J Clin Pathol 1960; 13:27–33. doi: 10.1136/jcp.13.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dunican EM, Elicker BM, Gierada DS, Nagle SK, Schiebler ML, Newell JD, et al. Mucus plugs in patients with asthma linked to eosinophilia and airflow obstruction. J Clin Invest 2018; 128:997–1009. doi: 10.1172/JCI95693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, et al. Oral glucocorticoid-sparing effect of mepoli-zumab in eosinophilic asthma. N Engl J Med 2014; 371:1189–1197. doi: 10.1056/NEJMoa1403291. [DOI] [PubMed] [Google Scholar]
- 52.Nair P, Wenzel S, Rabe KF, Bourdin A, Lugogo NL, Kuna P, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med 2017; 376:2448–2458. doi: 10.1056/NEJMoa1703501. [DOI] [PubMed] [Google Scholar]
- 53.Rabe KF, Nair P, Brusselle G, Maspero JF, Castro M, Sher L, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med 2018; 378:2475–2485. doi: 10.1056/NEJMoa1804093. [DOI] [PubMed] [Google Scholar]
- 54.Gibeon D, Batuwita K, Osmond M, Heaney LG, Brightling CE, Niven R, et al. Obesity-associated severe asthma represents a distinct clinical phenotype: analysis of the British Thoracic Society Difficult Asthma Registry Patient cohort according to BMI. Chest 2013; 143:406–414. doi: 10.1378/chest.12-0872. [DOI] [PubMed] [Google Scholar]
- 55.Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet 2002; 360:1715–1721. doi: 10.1016/S0140-6736(02)11679-5. [DOI] [PubMed] [Google Scholar]
- 56.Suehs CM, Menzies-Gow A, Price D, Bleecker ER, Canonica GW, Gurnell M, et al. Expert consensus on the tapering of oral corticosteroids for the treatment of asthma. A Delphi study. Am J Respir Crit Care Med 2021; 203:871–881. doi: 10.1164/rccm.202007-2721OC. [DOI] [PubMed] [Google Scholar]
- 57.Chung KF. Asthma phenotyping: a necessity for improved therapeutic precision and new targeted therapies. J Intern Med 2016; 279:192–204. doi: 10.1111/joim.12382. [DOI] [PubMed] [Google Scholar]
- 58.Lefaudeux D, De Meulder B, Loza MJ, Peffer N, Rowe A, Baribaud F, et al. U-BIOPRED clinical adult asthma clusters linked to a subset of sputum omics. J Allergy Clin Immunol 2017; 139:1797–1807. doi: 10.1016/j.jaci.2016.08.048. [DOI] [PubMed] [Google Scholar]
- 59.Buhl R, Humbert M, Bjermer L, Chanez P, Heaney LG, Pavord I, et al. Severe eosinophilic asthma: a roadmap to consensus. Eur Respir J 2017; 49:1700634.doi: 10.1183/13993003.00634-2017. [DOI] [PubMed] [Google Scholar]
- 60.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med 2012; 18:684–692. doi: 10.1038/nm.2737. [DOI] [PubMed] [Google Scholar]
- 61.Gon Y, Hashimoto S. Role of airway epithelial barrier dysfunction in pathogenesis of asthma. Allergol Int 2018; 67:12–17. doi: 10.1016/j.alit.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 62.Liu C, Zhang X, Xiang Y, Qu X, Liu H, Liu C, et al. Role of epithelial chemokines in the pathogenesis of airway inflammation in asthma (Review). Mol Med Rep 2018; 17:6935–6941. doi: 10.3892/mmr.2018.8739. [DOI] [PubMed] [Google Scholar]
- 63.Prefontaine D, Nadigel J, Chouiali F, Audusseau S, Semlali A, Chakir J, et al. Increased IL-33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol 2010; 125:752–754. doi: 10.1016/j.jaci.2009.12.935. [DOI] [PubMed] [Google Scholar]
- 64.Ying S, O’Connor B, Ratoff J, Meng Q, Fang C, Cousins D, et al. Expression and cellular provenance of thymic stromal lympho-poietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol 2008; 181:2790–2798. doi: 10.4049/jimmunol.181.4.2790. [DOI] [PubMed] [Google Scholar]
- 65.Cayrol C, Duval A, Schmitt P, Roga S, Camus M, Stella A, et al. Environmental allergens induce allergic inflammation through proteolytic maturation of IL-33. Nat Immunol 2018; 19:375–385. doi: 10.1038/s41590-018-0067-5. [DOI] [PubMed] [Google Scholar]
- 66.Willart MA, Deswarte K, Pouliot P, Braun H, Beyaert R, Lambrecht BN, et al. Interleukin-1a controls allergic sensitization to inhaled house dust mite via the epithelial release of GM-CSF and IL-33. J Exp Med 2012; 209:1505–1517. doi: 10.1084/jem.20112691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weng CM, Wang CH, Lee MJ, He JR, Huang HY, Chao MW, et al. Aryl hydrocarbon receptor activation by diesel exhaust particles mediates epithelium-derived cytokines expression in severe allergic asthma. Allergy 2018; 73:2192–2204. doi: 10.1111/all.13462. [DOI] [PubMed] [Google Scholar]
- 68.Altman MC, Lai Y, Nolin JD, Long S, Chen CC, Piliponsky AM, et al. Airway epithelium-shifted mast cell infiltration regulates asthmatic inflammation via IL-33 signaling. J Clin Invest 2019; 129:4979–4991. doi: 10.1172/jci126402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rossios C, Pavlidis S, Hoda U, Kuo CH, Wiegman C, Russell K, et al. Sputum transcriptomics reveal upregulation of IL-1 receptor family members in patients with severe asthma. J Allergy Clin Immunol 2018; 141:560–570. doi: 10.1016/j.jaci.2017.02.045. [DOI] [PubMed] [Google Scholar]
- 70.Kuo CS, Pavlidis S, Loza M, Baribaud F, Rowe A, Pandis I, et al. T-helper cell type 2 (Th2) and non-Th2 molecular phenotypes of asthma using sputum transcriptomics in U-BIOPRED. Eur Respir J 2017; 49:1602135.doi: 10.1183/13993003.02135-2016. [DOI] [PubMed] [Google Scholar]
- 71.Pavlidis S, Takahashi K, Ng Kee Kwong F, Xie J, Hoda U, Sun K, et al. T2-high” in severe asthma related to blood eosinophil, exhaled nitric oxide and serum periostin. Eur Respir J 2019; 53:1800938.doi: 10.1183/13993003.00938-2018. [DOI] [PubMed] [Google Scholar]
- 72.Peters MC, Mekonnen ZK, Yuan S, Bhakta NR, Woodruff PG, Fahy JV. Measures of gene expression in sputum cells can identify TH2-high and TH2-low subtypes of asthma. J Allergy Clin Immunol 2014; 133:388–394. doi: 10.1016/j.jaci.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Venge P, Dahl R, Fredens K, Peterson CG. Epithelial injury by human eosinophils. Am Rev Respir Dis 1988; 138:S54–S57. doi: 10.1164/ajrccm/138.6_Pt_2.S54. [DOI] [PubMed] [Google Scholar]
- 74.Kuo CS, Pavlidis S, Loza M, Baribaud F, Rowe A, Pandis I, et al. A transcriptome-driven analysis of epithelial brushings and bronchial biopsies to define asthma phenotypes in U-BIOPRED. Am J Respir Crit Care Med 2017; 195:443–455. doi: 10.1164/rccm.201512-2452OC. [DOI] [PubMed] [Google Scholar]
- 75.Krug N, Madden J, Redington AE, Lackie P, Djukanovic R, Schauer U, et al. T-cell cytokine profile evaluated at the single cell level in BAL and blood in allergic asthma. Am J Respir Cell Mol Biol 1996; 14:319–326. doi: 10.1165/ajrcmb.14.4.8600935. [DOI] [PubMed] [Google Scholar]
- 76.Boniface S, Koscher V, Mamessier E, El Biaze M, Dupuy P, Lorec AM, et al. Assessment of T lymphocyte cytokine production in induced sputum from asthmatics: a flow cytometry study. Clin Exp Allergy 2003; 33:1238–1243. doi: 10.1046/j.1365-2222.2003.01762.x. [DOI] [PubMed] [Google Scholar]
- 77.Cho SH, Stanciu LA, Holgate ST, Johnston SL. Increased interleukin-4, interleukin-5, and interferon-gamma in airway CD4+ and CD8+ T cells in atopic asthma. Am J Respir Crit Care Med 2005; 171:224–230. doi: 10.1164/rccm.200310-1416OC. [DOI] [PubMed] [Google Scholar]
- 78.Raundhal M, Morse C, Khare A, Oriss TB, Milosevic J, Trudeau J, et al. High IFN-g and low SLPI mark severe asthma in mice and humans. J Clin Invest 2015; 125:3037–3050. doi: 10.1172/jci80911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roussel L, Houle F, Chan C, Yao Y, Berube J, Olivenstein R, et al. IL-17 promotes p38 MAPK-dependent endothelial activation enhancing neutrophil recruitment to sites of inflammation. J Immunol 2010; 184:4531–4537. doi: 10.4049/jimmu-nol.0903162. [DOI] [PubMed] [Google Scholar]
- 80.Halwani R, Al-Muhsen S, Hamid Q. T helper 17 cells in airway diseases: from laboratory bench to bedside. Chest 2013; 143:494–501. doi: 10.1378/chest.12-0598. [DOI] [PubMed] [Google Scholar]
- 81.Doe C, Bafadhel M, Siddiqui S, Desai D, Mistry V, Rugman P, et al. Expression of the T helper 17-associated cytokines IL-17A and IL-17F in asthma and COPD. Chest 2010; 138:1140–1147. doi: 10.1378/chest.09-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Al-Ramli W, Prefontaine D, Chouiali F, Martin JG, Olivenstein R, Lemiere C, et al. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol 2009; 123:1185–1187. doi: 10.1016/j.jaci.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 83.Östling J, van Geest M, Schofield JPR, Jevnikar Z, Wilson S, Ward J, et al. IL-17-high asthma with features of a psoriasis immunophenotype. J Allergy Clin Immunol 2019; 144:1198–1213. doi: 10.1016/j.jaci.2019.03.027. [DOI] [PubMed] [Google Scholar]
- 84.Jevnikar Z, Östling J, Ax E, Calvén J, Thörn K, Israelsson E, et al. Epithelial IL-6 trans-signaling defines a new asthma phenotype with increased airway inflammation. J Allergy Clin Immunol 2019; 143:577–590. doi: 10.1016/j.jaci.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 85.Macedo P, Hew M, Torrego A, Jouneau S, Oates T, Durham A, et al. Inflammatory biomarkers in airways of patients with severe asthma compared with non-severe asthma. Clin Exp Allergy 2009; 39:1668–1676. doi: 10.1111/j.1365-2222.2009.03319.x. [DOI] [PubMed] [Google Scholar]
- 86.Jatakanon A, Uasuf C, Maziak W, Lim S, Chung KF, Barnes PJ. Neutrophilic inflammation in severe persistent asthma. Am J Respir Crit Care Med 1999; 160:1532–1539. doi: 10.1164/ajrccm.160.5.9806170. [DOI] [PubMed] [Google Scholar]
- 87.Pavord ID, Brightling CE, Woltmann G, Wardlaw AJ. Non-eosinophilic corticosteroid unresponsive asthma. Lancet 1999; 353:2213–2214. doi: 10.1016/S0140-6736(99)01813-9. [DOI] [PubMed] [Google Scholar]
- 88.Moore WC, Hastie AT, Li X, Li H, Busse WW, Jarjour NN, et al. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol 2014; 133:1557–1563.e5. doi: 10.1016/j.jaci.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fahy JV, Kim KW, Liu J, Boushey HA. Prominent neutrophilic inflammation in sputum from subjects with asthma exacerbation. J Allergy Clin Immunol 1995; 95:843–852. doi: 10.1016/s0091-6749(95)70128-1. [DOI] [PubMed] [Google Scholar]
- 90.Qiu R, Xie J, Chung KF, Li N, Yang Z, He M, et al. Asthma phenotypes defined from parameters obtained during recovery from a hospital-treated exacerbation. J Allergy Clin Immunol Pract 2018; 6:1960–1967. doi: 10.1016/j.jaip.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 91.Simpson JL, Phipps S, Baines KJ, Oreo KM, Gunawardhana L, Gibson PG. Elevated expression of the NLRP3 inflammasome in neutrophilic asthma. Eur Respir J 2014; 43:1067–1076. doi: 10.1183/09031936.00105013. [DOI] [PubMed] [Google Scholar]
- 92.Nguyen LT, Lim S, Oates T, Chung KF. Increase in airway neutrophils after oral but not inhaled corticosteroid therapy in mild asthma. Respir Med 2005; 99:200–207. doi: 10.1016/j. rmed.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 93.Cohen L, EX, Tarsi J, Ramkumar T, Horiuchi TK, Cochran R, et al. Epithelial cell proliferation contributes to airway remodeling in severe asthma. Am J Respir Crit Care Med 2007; 176:138–145. doi: 10.1164/rccm.200607-1062OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.James AL, Bai TR, Mauad T, Abramson MJ, Dolhnikoff M, McKay KO, et al. Airway smooth muscle thickness in asthma is related to severity but not duration of asthma. Eur Respir J 2009; 34:1040–1045. doi: 10.1183/09031936.00181608. [DOI] [PubMed] [Google Scholar]
- 95.Kaminska M, Foley S, Maghni K, Storness-Bliss C, Coxson H, Ghezzo H, et al. Airway remodeling in subjects with severe asthma with or without chronic persistent airflow obstruction. J Allergy Clin Immunol 2009; 124:45–51.e1-4. doi: 10.1016/j. jaci.2009.03.049. [DOI] [PubMed] [Google Scholar]
- 96.Saunders R, Siddiqui S, Kaur D, Doe C, Sutcliffe A, Hollins F, et al. Fibrocyte localization to the airway smooth muscle is a feature of asthma. J Allergy Clin Immunol 2009; 123:376–384. doi: 10.1016/j.jaci.2008.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang CH, Huang CD, Lin HC, Lee KY, Lin SM, Liu CY, et al. Increased circulating fibrocytes in asthma with chronic airflow obstruction. Am J Respir Crit Care Med 2008; 178:583–591. doi: 10.1164/rccm.200710-1557OC. [DOI] [PubMed] [Google Scholar]
- 98.Lo CY, Michaeloudes C, Bhavsar PK, Huang CD, Wang CH, Kuo HP, et al. Increased phenotypic differentiation and reduced corticosteroid sensitivity of fibrocytes in severe asthma. J Allergy Clin Immunol 2015; 135:1186–1195.e1-6. doi: 10.1016/j.jaci.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 99.Bourdin A, Neveu D, Vachier I, Paganin F, Godard P, Chanez P. Specificity of basement membrane thickening in severe asthma. J Allergy Clin Immunol 2007; 119:1367–1374. doi: 10.1016/j. jaci.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 100.Balzar S, Chu HW, Silkoff P, Cundall M, Trudeau JB, Strand M, et al. Increased TGF-beta2 in severe asthma with eosinophilia. J Allergy Clin Immunol 2005; 115:110–117. doi: 10.1016/j. jaci.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 101.Xie S, Sukkar MB, Issa R, Khorasani NM, Chung KF. Mechanisms of induction of airway smooth muscle hyperplasia by transforming growth factor-beta. AmJ Physiol Lung Cell Mol Physiol 2007; 293:L245–L253. doi: 10.1152/ajplung.00068.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kuo CS, Pavlidis S, Zhu J, Loza M, Baribaud F, Rowe A, et al. Contribution of airway eosinophils in airway wall remodeling in asthma: role of MMP-10 and MET. Allergy 2019; 74:1102–1112. doi: 10.1111/all.13727. [DOI] [PubMed] [Google Scholar]
- 103.Paganin F, Seneterre E, Chanez P, Daures JP, Bruel JM, Michel FB, et al. Computed tomography of the lungs in asthma: influence of disease severity and etiology. Am J Respir Crit Care Med 1996; 153:110–114. doi: 10.1164/ajrccm.153.1.8542102. [DOI] [PubMed] [Google Scholar]
- 104.Webb WR. High-resolution computed tomography of obstructive lung disease. Radiol Clin North Am 1994; 32:745–757. [PubMed] [Google Scholar]
- 105.Muller NL, Staples CA, Miller RR, Abboud RT. “Density mask”. An objective method to quantitate emphysema using computed tomography. Chest 1988; 94:782–787. doi: 10.1378/chest.94.4.782. [DOI] [PubMed] [Google Scholar]
- 106.Gupta S, Siddiqui S, Haldar P, Raj JV, Entwisle JJ, Wardlaw AJ, et al. Qualitative analysis of high-resolution CT scans in severe asthma. Chest 2009; 136:1521–1528. doi: 10.1378/chest.09-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hew M, Chung KF. Corticosteroid insensitivity in severe asthma: significance, mechanisms and aetiology. Intern Med J 2010; 40:323–334. doi: 10.1111/j.1445-5994.2010.02192.x. [DOI] [PubMed] [Google Scholar]
- 108.Bhavsar P, Harmer G, Adcock IM, Chung KF. Corticosteroid responsiveness and resistance. In: Chung KF, Israel E, Gibson PG, eds. Severe Asthma [ERS Monograph]. Sheffield: European Respiratory Society; 2019: 211–230. [Google Scholar]
- 109.Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA, et al. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med 2004; 170:836–844. doi: 10.1164/rccm.200401-033OC. [DOI] [PubMed] [Google Scholar]
- 110.Colice GL, Stampone P, Leung DY, Szefler SJ. Oral corticosteroids in poorly controlled asthma. J Allergy Clin Immunol 2005; 115:200–201. doi: 10.1016/j.jaci.2004.07.065. [DOI] [PubMed] [Google Scholar]
- 111.Shaw DE, Sousa AR, Fowler SJ, Fleming LJ, Roberts G, Corfield J, et al. Clinical and inflammatory characteristics of the European U-BIOPRED adult severe asthma cohort. Eur Respir J 2015; 46:1308–1321. doi: 10.1183/13993003.00779-2015. [DOI] [PubMed] [Google Scholar]
- 112.Peters MC, Kerr S, Dunican EM, Woodruff PG, Fajt ML, Levy BD, et al. Refractory airway type 2 inflammation in a large subgroup of asthmatic patients treated with inhaled corticosteroids. J Allergy Clin Immunol 2019; 143:104–113.e14. doi: 10.1016/j. jaci.2017.12.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Goleva E, Jackson LP, Harris JK, Robertson CE, Sutherland ER, Hall CF, et al. The effects of airway microbiome on corticosteroid responsiveness in asthma. Am J Respir Crit Care Med 2013; 188:1193–1201. doi: 10.1164/rccm.201304-0775OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol 2008; 181:4089–4097. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ramesh R, Kozhaya L, McKevitt K, Djuretic IM, Carlson TJ, Quintero MA, et al. Pro-inflammatory human Th17 cells selectively express P-glycoprotein and are refractory to glucocorticoids. J Exp Med 2014; 211:89–104. doi: 10.1084/jem.20130301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hanania NA, Wenzel S, Rosen K, Hsieh HJ, Mosesova S, Choy DF, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med 2013; 187:804–811. doi: 10.1164/rccm.201208-1414OC. [DOI] [PubMed] [Google Scholar]
- 117.Svenningsen S, Fowler SJ, Nair P. Clinical biomarkers and noninvasive assessment of severe asthma. In: Chung KF, Israel E, Gibson PG, eds. Severe Asthma [ERS Monograph]. Sheffield: European Respiratory Society; 2019: 93–112. [Google Scholar]
- 118.Berry MA, Shaw DE, Green RH, Brightling CE, Wardlaw AJ, Pavord ID. The use of exhaled nitric oxide concentration to identify eosinophilic airway inflammation: an observational study in adults with asthma. Clin Exp Allergy 2005; 35:1175–1179. doi: 10.1111/j.1365-2222.2005.02314.x. [DOI] [PubMed] [Google Scholar]
- 119.Chung KF. Increasing utility of FeNO as a biomarker of type-2 inflammation in severe asthma. Lancet Respir Med 2021; 9:1083–1084. doi: 10.1016/S2213-2600(21)00170-3. [DOI] [PubMed] [Google Scholar]
- 120.Holguin F, Cardet JC, Chung KF, Diver S, Ferreira DS, Fitzpatrick A, et al. Management of severe asthma: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J 2020; 55:1900588.doi: 10.1183/13993003.00588-2019. [DOI] [PubMed] [Google Scholar]
- 121.Chung KF. Targeting the interleukin pathway in the treatment of asthma. Lancet 2015; 386:1086–1096. doi: 10.1016/s0140-6736 (15)00157-9. [DOI] [PubMed] [Google Scholar]
- 122.Chung KF. New treatments for severe treatment-resistant asthma: targeting the right patient. Lancet Respir Med 2013; 1:639–652. doi: 10.1016/S2213-2600(13)70128-0. [DOI] [PubMed] [Google Scholar]
- 123.Kew KM, Karner C, Mindus SM, Ferrara G. Combination formoterol and budesonide as maintenance and reliever therapy versus combination inhaler maintenance for chronic asthma in adults and children. Cochrane Database Syst Rev 2013; 12:CD009019.doi: 10.1002/14651858.CD009019.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kerstjens HA, Engel M, Dahl R, Paggiaro P, Beck E, Vandewalker M, et al. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med 2012; 367:1198–1207. doi: 10.1056/NEJMoa1208606. [DOI] [PubMed] [Google Scholar]
- 125.Peters MC, Wenzel SE. Intersection of biology and therapeutics: type 2 targeted therapeutics for adult asthma. Lancet 2020; 395:371–383. doi: 10.1016/S0140-6736(19)33005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rodrigo GJ, Neffen H, Castro-Rodriguez JA. Efficacy and safety of subcutaneous omalizumab vs placebo as add-on therapy to corticosteroids for children and adults with asthma: a systematic review. Chest 2011; 139:28–35. doi: 10.1378/chest.10-1194. [DOI] [PubMed] [Google Scholar]
- 127.Hanania NA, Alpan O, Hamilos DL, Condemi JJ, Reyes-Rivera I, Zhu J, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med 2011; 154:573–582. doi: 10.7326/0003-4819-154-9-201105030-00002. [DOI] [PubMed] [Google Scholar]
- 128.Casale TB, Luskin AT, Busse W, Zeiger RS, Trzaskoma B, Yang M, et al. Omalizumab effectiveness by biomarker status in patients with asthma: evidence from PROSPERO, a prospective real-world study. J Allergy Clin Immunol Pract 2019; 7:156e1–164e1. doi: 10.1016/j.jaip.2018.04.043. [DOI] [PubMed] [Google Scholar]
- 129.Corren J, Weinstein S, Janka L, Zangrilli J, Garin M. Phase 3 study of reslizumab in patients with poorly controlled asthma: effects across a broad range of eosinophil counts. Chest 2016; 150:799–810. doi: 10.1016/j.chest.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 130.Bleecker ER, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016; 388:2115–2127. doi: 10.1016/S0140-6736(16)31324-1. [DOI] [PubMed] [Google Scholar]
- 131.FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016; 388:2128–2141. doi: 10.1016/S0140-6736(16)31322-8. [DOI] [PubMed] [Google Scholar]
- 132.Chupp GL, Bradford ES, Albers FC, Bratton DJ, Wang-Jairaj J, Nelsen LM, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med 2017; 5:390–400. doi: 10.1016/s2213-2600 (17)30125-x. [DOI] [PubMed] [Google Scholar]
- 133.Harrison T, Canonica GW, Chupp G, Lee J, Schleich F, Welte T, et al. Real-world mepolizumab in the prospective severe asthma REALITI-A study: initial analysis. Eur Respir J 2020; 56:2000151.doi: 10.1183/13993003.00151-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kavanagh JE, Hearn AP, Dhariwal J, d’Ancona G, Douiri A, Roxas C, et al. Real-world effectiveness of benralizumab in severe eosinophilic asthma. Chest 2021; 159:496–506. doi: 10.1016/j. chest.2020.08.2083. [DOI] [PubMed] [Google Scholar]
- 135.Wechsler ME, Peters SP, Hill TD, Ariely R, DePietro MR, Driessen MT, et al. Clinical outcomes and health-care resource use associated with reslizumab treatment in adults with severe eosinophilic asthma in real-world practice. Chest 2021; 159:1734–1746. doi: 10.1016/j.chest.2020.11.060. [DOI] [PubMed] [Google Scholar]
- 136.Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med 2018; 378:2486–2496. doi: 10.1056/NEJMoa1804092. [DOI] [PubMed] [Google Scholar]
- 137.Wenzel S, Castro M, Corren J, Maspero J, Wang L, Zhang B, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting beta2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet 2016; 388:31–44. doi: 10.1016/s0140-6736(16) 30307-5. [DOI] [PubMed] [Google Scholar]
- 138.Pretolani M, Bergqvist A, Thabut G, Dombret MC, Knapp D, Hamidi F, et al. Effectiveness of bronchial thermoplasty in patients with severe refractory asthma: clinical and histopathologic correlations. J Allergy Clin Immunol 2017; 139:1176–1185. doi: 10.1016/j.jaci.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 139.Chupp G, Laviolette M, Cohn L, McEvoy C, Bansal S, Shifren A, et al. Long-term outcomes of bronchial thermoplasty in subjects with severe asthma: a comparison of 3-year follow-up results from two prospective multicentre studies. Eur Respir J 2017; 50:1700017.doi: 10.1183/13993003.00017-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Burn J, Sims AJ, Keltie K, Patrick H, Welham SA, Heaney LG, et al. Procedural and short-term safety of bronchial thermoplasty in clinical practice: evidence from a national registry and Hospital Episode Statistics. J Asthma 2017; 54:872–879. doi: 10.1080/02770903.2016.1263652. [DOI] [PubMed] [Google Scholar]
- 141.Chaudhuri R, Rubin A, Sumino K, Lapa ESJR, Niven R, Siddiqui S, et al. Safety and effectiveness of bronchial thermoplasty after 10 years in patients with persistent asthma (BT10+): a follow-up of three randomised controlled trials. Lancet Respir Med 2021; 9:457–466. doi: 10.1016/S2213-2600(20)30408-2. [DOI] [PubMed] [Google Scholar]
- 142.Cloutier MM, Baptist AP, Blake KV, Brooks EG, Bryant-Stephens T, DiMango E, et al. 2020 focused updates to the asthma management guidelines: a report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J Allergy Clin Immunol 2020; 146:1217–1270. doi: 10.1016/j.jaci.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chung KF. Airway microbial dysbiosis in asthmatic patients: a target for prevention and treatment? J Allergy Clin Immunol 2017; 139:1071–1081. doi: 10.1016/j.jaci.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 144.Gibson PG, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet 2017; 390:659–668. doi: 10.1016/s0140-6736(17) 31281-3. [DOI] [PubMed] [Google Scholar]
- 145.Menzies-Gow A, Corren J, Bourdin A, Chupp G, Israel E, Wechsler ME, et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med 2021; 384:1800–1809. doi: 10.1056/NEJMoa2034975. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.