Abstract
Over the last 20 years, it has become possible to use a precision medicine approach to the management of chronic obstructive pulmonary disease (COPD). Clinical and physiological features as well as a blood biomarker can be used to target treatments to patients most likely to benefit and avoid treatment in patients less likely to benefit. Future advances in a precision medicine approach to COPD will depend on more precise characterization of individual patients, possibly using quantitative imaging, new physiological techniques, novel biomarkers and genetic profiling. Precision medicine has led to significant improvements in the management of COPD and clinicians should use all available information to optimize the treatment of individual patients.
Keywords: Chronic obstructive pulmonary disease (COPD), Precision medicine, Biomarkers, Microbiome, Comorbidity
Introduction
Chronic obstructive pulmonary disease (COPD) is common and causes significant mortality and morbidity. The Global Burden of Disease study estimated that in 2015, 174.5 million adults worldwide had COPD,[1] but the true figure may be closer to 400 million.[2] In China, the overall prevalence of spirometry-defined COPD has been estimated as 8.6%, meaning nearly 100 million people in China have COPD.[3] COPD is the third leading cause of death globally causing around three million deaths annually.[4] In 2013, COPD was also the third leading cause of death in China accounting for nearly one million deaths each year.[5]
In the earlier stages of the disease, management focuses on improving symptoms and exercise capacity and reducing exacerbations.[6] Over the last 20 years, the management of COPD has evolved to become more holistic and it is now possible to use clinical and blood biomarkers to optimize the benefits for individual patients. The development of this precision medicine approach represents a significant step forward in COPD management.
Precision Medicine
The terms precision, personalized, and individualized medicine are often used interchangeably. Precision medicine has been defined as treatments targeted to the needs of individual patients on the basis of genetic, biomarker, phenotypic, or psychosocial characteristics that distinguish a given patient from other patients with similar clinical presentations.[7] For centuries, physicians have treated patients with different diseases differently, but generally used the same treatments for all patients with particular diseases. More recently, management was based on evidence of average treatment effects in populations of patients studied in clinical trials. Precision medicine refers to the provision of specific medical treatment for an individual according to their individual susceptibility (to respond or not to such treatment). Hence, the key to understanding the precision medicine concept is individual risk.[8] Precision medicine depends on tailoring management to individual characteristics, not only genetic or pathophysiological, but also environmental and psychosocial, as well as taking into account the preferences of each patient. It is deemed “precise” because it depends on the endotypic characterization of individual patients. It does not literally mean the creation of drugs or medical devices that are unique to a patient, but rather the ability to classify individuals according to the specific characteristics of the disease that they have including differences in their response to specific treatments.[7]
Precision medicine challenges our classification of disease. Medicine has a long history of being divided into “lumpers and splitters”; lumpers tend to group related entities together, and splitters tend to apply more precise definitions and thereby define more discrete entities. Treatment using contemporary therapy often depends on maximal specificity in the diagnosis. This has been seen to have great effects with the refinement of the classification of lung cancer and the use of targeted agents and checkpoint inhibitors.[9] However, there is a point at which splitting stops defining different diseases and moves to defining subtypes of a disease that benefit in some ways from being considered the same whilst benefit in other ways of being considered distinct. It has been suggested that clinicians should move away from diagnostic labels such as “COPD” and concentrate on identifying “treatable traits” which require precise treatment.[10] However, diagnostic labels are important to patients, helping to set expectations about prognosis and they facilitate communications between clinicians. Precision medicine requires us to characterize patients in as detailed a way as possible to identify phenotypes and underlying endotypes using the latest molecular pathological and genetic approaches, but it does not mean we have to abandon current diagnostic labels.
Precision Medicine and COPD
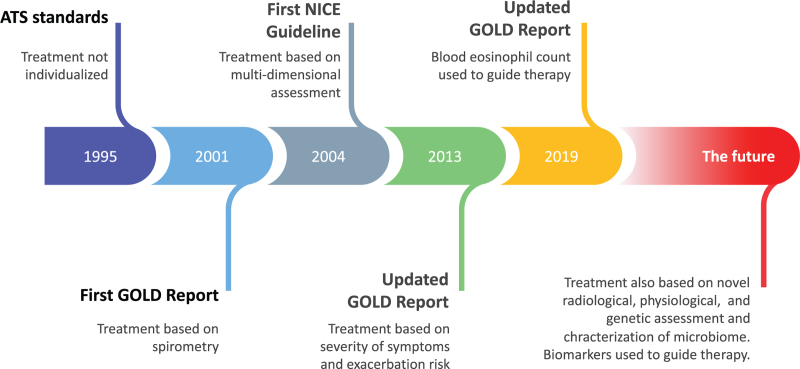
Over the last 25 years, COPD management has been based on National and International Guidelines and over time these have gradually moved toward a precision medicine approach. The American Thoracic Society standards published in 1995[11] recommended a simple stepwise approach to therapy which took no account of patient characteristics. The first Report of the Global Obstructive Lung Disease Initiative (GOLD) was a step forward in that management recommendations were based on the severity of airflow obstruction[12,13]; however, this was still a simplistic and unidimensional approach. The first recommendations that took a multidimensional approach to COPD management were those of the English National Institute for Clinical Excellence published in 2004.[14,15] Treatment recommendations were based on a holistic assessment of patients that included eight domains: smoking status, breathlessness and exercise limitation, exacerbation frequency, presence of respiratory failure, cor pulmonale, chronic productive cough, body mass index, and mental health. Recommended management varied from individual to individual to address these domains.[14]
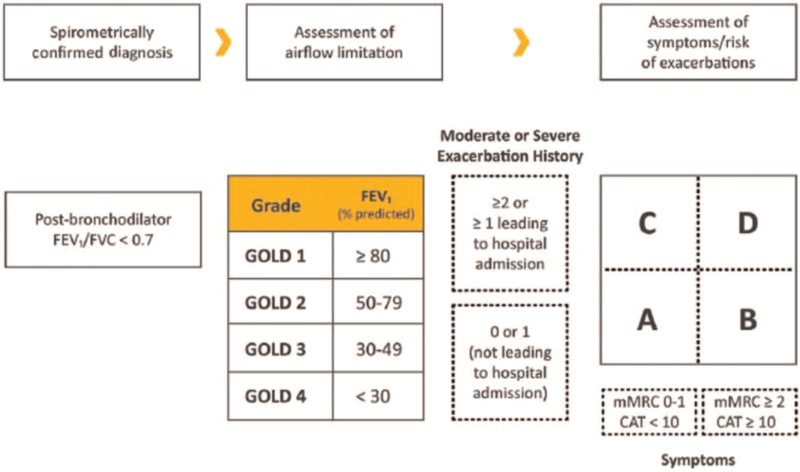
Over time the recommendations in the GOLD report have evolved to become more tailored toward individual patients. A significant step forward was the introduction in the 2013 Report of the initial assessment of patients according to the severity of their symptoms and their risk of exacerbations. Assessment also included the severity of the spirometric abnormality and the presence of comorbidities. This approach was refined in the 2017 report which has remained unchanged since then. The degree of forced expiratory volume in 1 s (FEV1) impairment, expressed as a percentage of the predicted value, is used to determine the GOLD stage (1–4). The level of symptoms, as determined by the modified Medical Research Council breathlessness score or the COPD assessment test and the risk of exacerbations, based on the number of moderate or severe exacerbations in the previous year, are used to determine the patient's GOLD group (A–D) [Figure 1]. This assessment of symptoms and risk of exacerbations is recommended as the basis for determining initial therapy.
Figure 1.
Assessment of COPD patients as recommended by the GOLD Report. CAT: Chronic obstructive pulmonary disease assessment test; COPD: Chronic obstructive pulmonary disease; FEV1: Forced expiratory volume in 1 s; FVC: Forced vital capacity; GOLD: Global Obstructive Lung Disease Initiative; mMRC: Modified Medical Research Council.
Exacerbation frequency is an important characteristic of COPD. Patients with frequent exacerbations have worse health status, lung function, and survival.[16,17] The ECLIPSE study showed that the single best predictor of exacerbations was the history of exacerbations.[18] The GOLD report uses a threshold of a history of two moderate exacerbations in the past year to identify patients at increased risk of future exacerbations. This classification has been supported by a hypothesis-free prospective cohort study of COPD patients.[19] However, there is considerable variability in exacerbation frequency over time.[20,21] Given the significance of severe (ie, hospitalized) exacerbations,[22] a history of one severe exacerbation is also considered to put patients into the high risk category.
In the 2019 report, blood eosinophil counts were also included as a factor to consider when determining initial pharmacotherapy. GOLD recommends that subsequent adjustments to pharmacotherapy are determined by characterizing patients according to their continuing symptoms, occurrence of exacerbations, and blood eosinophil counts [Figure 2].
Figure 2.
Major milestones of precision medicine approaches in guidelines on COPD management. ATS: American Thoracic Society; COPD: Chronic obstructive pulmonary disease; GOLD: Global Obstructive Lung Disease Initiative; NICE: National Institute of Clinical Excellence.
As part of the initial assessment, the GOLD Report and the World Health Organization also recommend that all patients with COPD should be screened for alpha-1 antitrypsin deficiency (AATD), especially in areas with high AATD prevalence.[23,24] Alpha-1 antitrypsin (α1-AT) is a glycoprotein protease inhibitor. Decreased α1-AT levels in lung are caused by mutations in the SERPINA1 gene and lead to an increased risk of emphysema.[25] AATD is present in only a small proportion of patients with COPD but it provides an excellent example of how precision medicine in COPD could be based on genetic abnormalities, even though identification of AATD does not currently change management significantly. Intravenous augmentation therapy with plasma-purified alpha-1 antitrypsin (AAT) was approved by the Food and Drug Administration (FDA) in 1987, but although it has been shown to decrease the loss of lung density on computed tomography (CT), it has not affected FEV1, quality of life, or exacerbations.[26,27]
In patients with severe disease, a precision medicine approach helps determine the need for oxygen therapy or ventilatory support. Arterial blood gas measurements, together with the presence or absence of clinical features of cor pulmonale and the presence or absence of erythrocytosis, determine whether long term (>15 h per day) oxygen therapy (LTOT) is indicated. LTOT increases survival in patients with severe resting hypoxemia,[28] but has no benefit in patients with resting or exercise-induced moderate desaturation.[29] The use of domiciliary non-invasive positive pressure ventilation (NPPV) is a good example of a specific intervention for a specific subgroup of patients at a specific time point. NPPV is of benefit in a very specific group of patients: those with resolution of acidosis (pH >7.30), persistent hypercapnia (PaCO2 >53 mmHg), hypoxemia (PaO2 < 55 mmHg) and >30% of sleep spent with <90% oxygen saturation 2 to 4 weeks after discharge from hospital because of an acute episode of exacerbation. In such patients, adding home NPPV to home oxygen therapy prolongs the time to readmission or death.[30]
Another example of precision medicine in COPD is the assessment of patients for bronchoscopic or surgical interventions such as lung volume reduction surgery and bronchoscopic lung volume reduction using endobronchial valves, lung volume reduction coil treatment, or vapor ablation therapy. Suitability and the selection of the optimal procedure are determined by a detailed consideration of a number of factors. These include the severity of airflow obstruction, the radiographic findings on the extent and pattern of emphysema on a high resolution CT (HRCT) scan; the extent of hyperinflation and impairment of gas transfer on lung function testing, exercise capacity, presence or absence of interlobar collateral ventilation measured by fissure integrity on HRCT or physiological assessment (endoscopic balloon occlusion and flow assessment).[31]
Biomarkers
The eosinophil count is currently the only blood biomarker the GOLD report includes to guide the choice of pharmacotherapy. Blood eosinophil counts can help clinicians estimate the likelihood of a beneficial effect of the addition of inhaled corticosteroids (ICS) to regular bronchodilator treatment on reducing exacerbation frequency. Prospective clinical trials have now shown that there is a threshold (approximately 100 eosinophils per μL) below which ICS have no benefits, but above the threshold there is a continuous relationship between blood eosinophil counts and the benefits of ICS.[32,33] Using blood eosinophil counts to guide choice of therapy and minimize adverse effects in those least likely to benefit is another example of the introduction of precision medicine for COPD.
Phenotyping the inflammatory profile of patients with COPD may allow the use of other more specific management approaches. Higher blood eosinophil counts in COPD patients are associated with increased lung eosinophil numbers and the presence of higher levels of markers of type-2 inflammation in the airways.[34–36] However, T2 inflammation in eosinophilic COPD is not identical to T2 inflammation in asthma[35] and anti-interleukin (IL)-5 therapy targeting the proliferation, differentiation, and migration of eosinophils, has shown disappointing results in patients with COPD.[37,38] However, higher blood eosinophil counts are associated with higher IL-13 expression levels suggesting pathways linking eosinophilic inflammation to airway remodeling and mucus secretion.[39] Chronic cough and sputum production are common symptoms in COPD.[40] They are associated with worse health status, and an increased risk of exacerbations and respiratory mortality.[41,42] Identifying IL-13 driven bronchitis opens the possibility of precision therapy with anti-IL-13 agents for these patients.[43]
Microbiome
The contribution of the microbiome to the symptoms and progression of COPD is still uncertain, although significant steps have been made in recent years to begin to understand its role, despite challenges such as the fact that different lung compartments have distinct microbiota, both in terms of abundance and composition. Overall, the microbiome composition is different in COPD patients compared to healthy controls and some of these differences correlate with airway inflammation.[44–47] Reduced diversity of the microbiome is found with increasing severity of COPD with a loss of part of the resident flora that is replaced by a more restricted microbiota including potential pathogens.[48,49] In a murine model, microbiota-dependent signals contribute to persistent inflammation.[50] Long-term azithromycin is recommended by GOLD to reduce exacerbation rates in patients who continue to have problems despite optimal inhaled therapy. Its mechanism of action is not completely understood but may include maintaining a selective pressure on the lung microbiome,[51] as well as actions beyond its direct antibacterial effect.[52] These may include effects on anti-inflammatory bacterial metabolites produced by the microbiome.[53] Understanding the role of the microbiome and profiling it in individual patients, as part of a precision medicine approach, may provide novel targets for therapeutic intervention.
Characterization of Patients in the Future
Future advances in a precision medicine approach to COPD will depend on more precise characterization of individual patients. This may be based on quantitative imaging,[54] new physiological techniques,[55] better understanding of genetic signatures, and the identification of biomarkers. The blood eosinophil count is the only blood-based biomarker that has proved reliable for clinical practice. Fibrinogen has been shown to be a prognostic biomarker of exacerbations and death.[56,57] It was the most stable of 34 biomarkers assessed in the ECLIPSE cohort,[58] a critical feature for clinically useful biomarkers and has been approved by FDA as an “enrichment biomarker” for clinical trials. Fibrinogen can be measured in routine clinical practice, and it was prospectively evaluated in the IMPACT study which showed that the rate and risk of exacerbations were higher in patients with higher fibrinogen levels,[59] suggesting that fibrinogen could be used as a predictive marker in clinical practice to identify patients that are at a higher risk of experiencing exacerbations and would benefit from appropriate treatment to reduce future risk.[60]
C-reactive protein (CRP) has been studied as a biomarker to guide antibiotic therapy for exacerbations in both inpatient,[61] and community settings[62] and the GOLD report comments that antibiotic usage can be safely reduced when CRP is low. Thus, CRP could support a precision medicine approach to minimizing risk of therapy to those least likely to benefit. Procalcitonin has also been studied as a biomarker to determine the use of antibiotics in COPD exacerbations, but overall the data in hospitalized patients show no significant reduction in overall antibiotic exposure[63] and the GOLD report concludes that it is premature to base antibiotic use on procalcitonin levels. Other biomarkers in COPD have also proved disappointing, in most cases, because of poor replication of initial promising results in independent cohorts[64] and/or inability to transfer the biomarker from a discovery platform to a clinical assay.[65,66]
Comorbidities
Precision medicine in COPD must also take account of the fact that most patients with COPD have comorbidities (ie, other chronic conditions) and these must be taken into account as part of the assessment and formulation of an individual management plan. It must also take account of the fact that patients with COPD are not only physically limited, but often show great reductions in their psychological and social functioning. Comorbid depression and anxiety are present in nearly 50% of patients with COPD[67] and are associated with increased mortality, more frequent exacerbations, persistent smoking, longer and more frequent hospitalizations, increased symptom burden, worsened physical and social functioning, and decreased quality of life.[68–73] A precision medicine approach must include an assessment of these psychological factors in individuals alongside characterization of the pulmonary disease, as their treatment has the potential to improve COPD outcomes.
Palliative Interventions
Finally, a precision medicine approach to COPD must also identify the need for palliative interventions, which encompass approaches to symptom control as well as management of terminal patients close to death. Many patients continue to experience symptoms such as breathlessness despite maximal therapy and for them palliative approaches to symptom control are important. Symptoms such as fatigue and insomnia, as well as anxiety and depression may also benefit from symptom-based palliative treatments.[74] Palliative care interventions are under-used in the management of COPD[75] and patients with COPD are more likely to die with aggressive medical intervention directed toward survival, less likely to receive palliative services than patients with lung cancer and advanced directives are not implemented.[76] Precision medicine must include assessment of these individual needs and when appropriate they should be addressed in the management plan.
Conclusions
The management of COPD has undoubtedly become more precise over the last 20 years, bringing great benefits to patients. However, many challenges remain. Clinicians must consider all available biological factors and psychosocial factors at an individual patient level to improve assessment, treatment, outcomes, and cost-effectiveness.
Conflicts of interest
None.
Footnotes
How to cite this article: Halpin DM. Precision medicine in chronic obstructive pulmonary disease. Chin Med J 2022;135:1156–1162. doi: 10.1097/CM9.0000000000002042
References
- 1.GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med 2017; 5:691–706. doi: 10.1016/S2213-2600(17)30293-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adeloye D, Chua S, Lee C, Basquill C, Papana A, Theodoratou E, et al. Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J Glob Health 2015; 5:020415.doi: 10.7189/jogh.05-020415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang C, Xu J, Yang L, Xu Y, Zhang X, Bai C, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet 2018; 391:1706–1717. doi: 10.1016/S0140-6736(18)30841-9. [DOI] [PubMed] [Google Scholar]
- 4.Halpin D, Celli BR, Criner GJ, Frith P, López Varela MV, Salvi S, et al. It is time for the world to take COPD seriously: a statement from the GOLD board of directors. Eur Respir J 2019; 54:1900914.doi: 10.1183/13993003.00914-2019. [DOI] [PubMed] [Google Scholar]
- 5.Zhou M, Wang H, Zhu J, Chen W, Wang L, Liu S, et al. Cause-specific mortality for 240 causes in China during 1990–2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet 2016; 387:251–272. doi: 10.1016/S0140-6736(15)00551-6. [DOI] [PubMed] [Google Scholar]
- 6.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2021 Report. Available from: http://www.goldcopd.org/. [Accessed on September 9, 2021]. [Google Scholar]
- 7.Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease. Washington (DC): National Academies Press (US); 2011. [PubMed] [Google Scholar]
- 8.Jameson JL, Longo DL. Precision medicine--personalized, problematic, and promising. N Engl J Med 2015; 372:2229–2234. doi: 10.1056/NEJMsb1503104. [DOI] [PubMed] [Google Scholar]
- 9.Vargas AJ, Harris CC. Biomarker development in the precision medicine era: lung cancer as a case study. Nat Rev Cancer 2016; 16:525–537. doi: 10.1038/nrc.2016.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agusti A, Bel E, Thomas M, Vogelmeier C, Brusselle G, Holgate S, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J 2016; 47:410–419. doi: 10.1183/13993003.01359-2015. [DOI] [PubMed] [Google Scholar]
- 11.Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. American Thoracic Society. Am J Respir Crit Care Med 1995; 152:S77–S121. [PubMed] [Google Scholar]
- 12.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 2001; 163:1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 13.Fabbri LM, Hurd SS. GOLD Scientific Committee. Global strategy for the diagnosis, management and prevention of COPD: 2003 update. Eur Respir J 2003; 22:1–2. doi: 10.1183/09031936.03.00063703. [DOI] [PubMed] [Google Scholar]
- 14.Halpin D. NICE guidance for COPD. Thorax 2004; 59:181–182. doi: 10.1136/thx.2004.021113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Collaborating Centre for Chronic Conditions. Chronic obstructive pulmonary disease. National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax 2004; 59: (Suppl 1): 1–232. [PMC free article] [PubMed] [Google Scholar]
- 16.Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005; 60:925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soler-Cataluña JJ, Rodriguez-Roisin R. Frequent chronic obstructive pulmonary disease exacerbators: how much real, how much fictitious. COPD 2010; 7:276–284. doi: 10.3109/15412555.2010.496817. [DOI] [PubMed] [Google Scholar]
- 18.Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal-Singer R, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363:1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 19.Le Rouzic O, Roche N, Cortot AB, Tillie-Leblond I, Masure F, Perez T, et al. Defining the “frequent exacerbator” phenotype in COPD: a hypothesis-free approach. Chest 2018; 153:1106–1115. doi: 10.1016/j.chest.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Han MK, Quibrera PM, Carretta EE, Barr RG, Bleecker ER, Bowler RP, et al. Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med 2017; 5:619–626. doi: 10.1016/S2213-2600(17)30207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reilev M, Lykkegaard J, Halling A, Vestbo J, Søndergaard J, Pottegård A. Stability of the frequent COPD exacerbator in the general population: a Danish nationwide register-based study. NPJ Prim Care Respir Med 2017; 27:25.doi: 10.1038/s41533-017-0029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Aymerich J, Serra Pons I, Mannino DM, Maas AK, Miller DP, Davis KJ. Lung function impairment, COPD hospitalisations and subsequent mortality. Thorax 2011; 66:585–590. doi: 10.1136/thx.2010.152876. [DOI] [PubMed] [Google Scholar]
- 23.Alpha 1-antitrypsin deficiency: memorandum from a WHO meeting. Bull World Health Organ 1997; 75:397–415. [PMC free article] [PubMed] [Google Scholar]
- 24.Miravitlles M, Dirksen A, Ferrarotti I, Koblizek V, Lange P, Mahadeva R, et al. European Respiratory Society statement: diagnosis and treatment of pulmonary disease in (1-antitrypsin deficiency. Eur Respir J 2017; 50:1700610.doi: 10.1183/13993003.00610-2017. [DOI] [PubMed] [Google Scholar]
- 25.Strnad P, McElvaney NG, Lomas DA. Alpha1-antitrypsin deficiency. N Engl J Med 2020; 382:1443–1455. doi: 10.1056/NEJMra1910234. [DOI] [PubMed] [Google Scholar]
- 26.McElvaney NG, Burdon J, Holmes M, Glanville A, Wark PA, Thompson PJ, et al. Long-term efficacy and safety of (1 proteinase inhibitor treatment for emphysema caused by severe (1 antitrypsin deficiency: an open-label extension trial (RAPID-OLE). Lancet Respir Med 2017; 5:51–60. doi: 10.1016/S2213-2600(16)30430-1. [DOI] [PubMed] [Google Scholar]
- 27.Chapman KR, Burdon JG, Piitulainen E, Sandhaus RA, Seersholm N, Stocks JM, et al. Intravenous augmentation treatment and lung density in severe (1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-controlled trial. Lancet 2015; 386:360–368. doi: 10.1016/S0140-6736(15)60860-1. [DOI] [PubMed] [Google Scholar]
- 28.Cranston JM, Crockett AJ, Moss JR, Alpers JH. Domiciliary oxygen for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2005; 2005:CD001744.doi: 10.1002/14651858.CD001744.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long-Term Oxygen Treatment Trial Research Group, Albert RK, Au DH, Blackford AL, Casaburi R, Cooper JA, Jr, et al. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med 2016; 375:1617–1627. doi: 10.1056/NEJMoa1604344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy PB, Rehal S, Arbane G, Bourke S, Calverley P, Crook AM, et al. Effect of home noninvasive ventilation with oxygen therapy vs oxygen therapy alone on hospital readmission or death after an acute COPD exacerbation: a randomized clinical trial. JAMA 2017; 317:2177–2186. doi: 10.1001/jama.2017.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herth F, Slebos DJ, Criner GJ, Valipour A, Sciurba F, Shah PL. Endoscopic lung volume reduction: an expert panel recommendation - update 2019. Respiration 2019; 97:548–557. doi: 10.1159/000496122. [DOI] [PubMed] [Google Scholar]
- 32.Pascoe S, Barnes N, Brusselle G, Compton C, Criner GJ, Dransfield MT, et al. Blood eosinophils and treatment response with triple and dual combination therapy in chronic obstructive pulmonary disease: analysis of the IMPACT trial. Lancet Respir Med 2019; 7:745–756. doi: 10.1016/S2213-2600(19)30190-0. [DOI] [PubMed] [Google Scholar]
- 33.Rabe KF, Martinez FJ, Ferguson GT, Wang C, Singh D, Wedzicha JA, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med 2020; 383:35–48. doi: 10.1056/NEJMoa1916046. [DOI] [PubMed] [Google Scholar]
- 34.Christenson SA, Steiling K, van den Berge M, Hijazi K, Hiemstra PS, Postma DS, et al. Asthma-COPD overlap. Clinical relevance of genomic signatures of type 2 inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015; 191:758–766. doi: 10.1164/rccm.201408-1458OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Higham A, Beech A, Wolosianka S, Jackson N, Long G, Kolsum U, et al. Type 2 inflammation in eosinophilic chronic obstructive pulmonary disease. Allergy 2021; 76:1861–1864. doi: 10.1111/all.14661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.George L, Taylor AR, Esteve-Codina A, Soler Artigas M, Thun GA, Bates S, et al. Blood eosinophil count and airway epithelial transcriptome relationships in COPD versus asthma. Allergy 2020; 75:370–380. doi: 10.1111/all.14016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pavord ID, Chanez P, Criner GJ, Kerstjens H, Korn S, Lugogo N, et al. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N Engl J Med 2017; 377:1613–1629. doi: 10.1056/NEJMoa1708208. [DOI] [PubMed] [Google Scholar]
- 38.Criner GJ, Celli BR, Brightling CE, Agusti A, Papi A, Singh D, et al. Benralizumab for the prevention of COPD exacerbations. N Engl J Med 2019; 381:1023–1034. doi: 10.1056/NEJMoa1905248. [DOI] [PubMed] [Google Scholar]
- 39.Atherton HC, Jones G, Danahay H. IL-13-induced changes in the goblet cell density of human bronchial epithelial cell cultures: MAP kinase and phosphatidylinositol 3-kinase regulation. Am J Physiol Lung Cell Mol Physiol 2003; 285:L730–739. doi: 10.1152/ajplung.00089.2003. [DOI] [PubMed] [Google Scholar]
- 40.Kim V, Criner GJ. Chronic bronchitis and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013; 187:228–237. doi: 10.1164/rccm.201210-1843CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heijdra YF, Pinto-Plata VM, Kenney LA, Rassulo J, Celli BR. Cough and phlegm are important predictors of health status in smokers without COPD. Chest 2002; 121:1427–1433. doi: 10.1378/chest.121.5.1427. [DOI] [PubMed] [Google Scholar]
- 42.Lahousse L, Seys L, Joos GF, Franco OH, Stricker BH, Brusselle GG. Epidemiology and impact of chronic bronchitis in chronic obstructive pulmonary disease. Eur Respir J 2017; 50:1602470.doi: 10.1183/13993003.02470-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fieldes M, Bourguignon C, Assou S, Nasri A, Fort A, Vachier I, et al. Targeted therapy in eosinophilic chronic obstructive pulmonary disease. ERJ Open Res 2021; 7:00437–2020. doi: 10.1183/23120541.00437-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barker BL, Haldar K, Patel H, Pavord ID, Barer MR, Brightling CE, et al. Association between pathogens detected using quantitative polymerase chain reaction with airway inflammation in COPD at stable state and exacerbations. Chest 2015; 147:46–55. doi: 10.1378/chest.14-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Einarsson GG, Comer DM, McIlreavey L, Parkhill J, Ennis M, Tunney MM, et al. Community dynamics and the lower airway microbiota in stable chronic obstructive pulmonary disease, smokers and healthy non-smokers. Thorax 2016; 71:795–803. doi: 10.1136/thoraxjnl-2015-207235. [DOI] [PubMed] [Google Scholar]
- 46.Erb-Downward JR, Thompson DL, Han MK, Freeman CM, McCloskey L, Schmidt LA, et al. Analysis of the lung microbiome in the “healthy” smoker and in COPD. PLoS One 2011; 6:e16384.doi: 10.1371/journal.pone.0016384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ditz B, Christenson S, Rossen J, Brightling C, Kerstjens H, van den Berge M, et al. Sputum microbiome profiling in COPD: beyond singular pathogen detection. Thorax 2020; 75:338–344. doi: 10.1136/thoraxjnl-2019-214168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garcia-Nuñez M, Millares L, Pomares X, Ferrari R, Pérez-Brocal V, Gallego M, et al. Severity-related changes of bronchial microbiome in chronic obstructive pulmonary disease. J Clin Microbiol 2014; 52:4217–4223. doi: 10.1128/JCM.01967-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Galiana A, Aguirre E, Rodriguez JC, Mira A, Santibañez M, Candela I, et al. Sputum microbiota in moderate versus severe patients with COPD. Eur Respir J 2014; 43:1787–1790. doi: 10.1183/09031936.00191513. [DOI] [PubMed] [Google Scholar]
- 50.Yadava K, Pattaroni C, Sichelstiel AK, Trompette A, Gollwitzer ES, Salami O, et al. Microbiota promotes chronic pulmonary inflammation by enhancing IL-17A and autoantibodies. Am J Respir Crit Care Med 2016; 193:975–987. doi: 10.1164/rccm.201504-0779OC. [DOI] [PubMed] [Google Scholar]
- 51.Dickson RP, Martinez FJ, Huffnagle GB. The role of the microbiome in exacerbations of chronic lung diseases. Lancet 2014; 384:691–702. doi: 10.1016/S0140-6736(14)61136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blasi F, Mantero M, Aliberti S. Antibiotics as immunomodulant agents in COPD. Curr Opin Pharmacol 2012; 12:293–299. doi: 10.1016/j.coph.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 53.Segal LN, Clemente JC, Wu BG, Wikoff WR, Gao Z, Li Y, et al. Randomised, double-blind, placebo-controlled trial with azithromycin selects for anti-inflammatory microbial metabolites in the emphysematous lung. Thorax 2017; 72:13–22. doi: 10.1136/thoraxjnl-2016-208599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lynch DA, Austin JH, Hogg JC, Grenier PA, Kauczor HU, Bankier AA, et al. CT-definable subtypes of chronic obstructive pulmonary disease: a statement of the Fleischner Society. Radiology 2015; 277:192–205. doi: 10.1148/radiol.2015141579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Milne S, Hammans C, Watson S, Farah CS, Thamrin C, King GG. Bronchodilator responses in respiratory impedance, hyperinflation and gas trapping in COPD. COPD 2018; 15:341–349. doi: 10.1080/15412555.2018.1458217. [DOI] [PubMed] [Google Scholar]
- 56.Groenewegen KH, Postma DS, Hop WC, Wielders PL, Schlösser NJ, Wouters EF, et al. Increased systemic inflammation is a risk factor for COPD exacerbations. Chest 2008; 133:350–357. doi: 10.1378/chest.07-1342. [DOI] [PubMed] [Google Scholar]
- 57.Mannino DM, Tal-Singer R, Lomas DA, Vestbo J, Graham Barr R, Tetzlaff K, et al. Plasma fibrinogen as a biomarker for mortality and hospitalized exacerbations in people with COPD. Chronic Obstr Pulm Dis 2015; 2:23–34. doi: 10.15326/jcopdf.2.1.2014.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dickens JA, Miller BE, Edwards LD, Silverman EK, Lomas DA, Tal-Singer R, et al. COPD association and repeatability of blood biomarkers in the ECLIPSE cohort. Respir Res 2011; 12:146.doi: 10.1186/1465-9921-12-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singh D, Criner GJ, Dransfield MT, Halpin D, Han MK, Lange P, et al. InforMing the Pathway of COPD Treatment (IMPACT) trial: fibrinogen levels predict risk of moderate or severe exacerbations. Respir Res 2021; 22:130.doi: 10.1186/s12931-021-01706-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duvoix A, Dickens J, Haq I, Mannino D, Miller B, Tal-Singer R, et al. Blood fibrinogen as a biomarker of chronic obstructive pulmonary disease. Thorax 2013; 68:670–676. doi: 10.1136/thoraxjnl-2012-201871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prins HJ, Duijkers R, van der Valk P, Schoorl M, Daniels J, van der Werf TS, et al. CRP-guided antibiotic treatment in acute exacerbations of COPD in hospital admissions. Eur Respir J 2019; 53:1802014. doi: 10.1183/13993003.02014-2018. [DOI] [PubMed] [Google Scholar]
- 62.Butler CC, Gillespie D, White P, Bates J, Lowe R, Thomas-Jones E, et al. C-reactive protein testing to guide antibiotic prescribing for COPD exacerbations. N Engl J Med 2019; 381:111–120. doi: 10.1056/NEJMoa1803185. [DOI] [PubMed] [Google Scholar]
- 63.Chen K, Pleasants KA, Pleasants RA, Beiko T, Washburn RG, Yu Z, et al. Procalcitonin for antibiotic prescription in chronic obstructive pulmonary disease exacerbations: systematic review, meta-analysis, and clinical perspective. Pulm Ther 2020; 6:201–214. doi: 10.1007/s41030-020-00123-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keene JD, Jacobson S, Kechris K, Kinney GL, Foreman MG, Doerschuk CM, et al. Biomarkers predictive of exacerbations in the SPIROMICS and COPDGene cohorts. Am J Respir Crit Care Med 2017; 195:473–481. doi: 10.1164/rccm.201607-1330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hollander Z, DeMarco ML, Sadatsafavi M, McManus BM, Ng RT, Sin DD. Biomarker development in COPD: moving from P values to products to impact patient care. Chest 2017; 151:455–467. doi: 10.1016/j.chest.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 66.Stockley RA, Halpin D, Celli BR, Singh D. Chronic obstructive pulmonary disease biomarkers and their interpretation. Am J Respir Crit Care Med 2019; 199:1195–1204. doi: 10.1164/rccm.201810-1860SO. [DOI] [PubMed] [Google Scholar]
- 67.Yohannes AM, Willgoss TG, Baldwin RC, Connolly MJ. Depression and anxiety in chronic heart failure and chronic obstructive pulmonary disease: prevalence, relevance, clinical implications and management principles. Int J Geriatr Psychiatry 2010; 25:1209–1221. doi: 10.1002/gps.2463. [DOI] [PubMed] [Google Scholar]
- 68.de Voogd JN, Wempe JB, Koëter GH, Postema K, van Sonderen E, Ranchor AV, et al. Depressive symptoms as predictors of mortality in patients with COPD. Chest 2009; 135:619–625. doi: 10.1378/chest.08-0078. [DOI] [PubMed] [Google Scholar]
- 69.Ng TP, Niti M, Tan WC, Cao Z, Ong KC, Eng P. Depressive symptoms and chronic obstructive pulmonary disease: effect on mortality, hospital readmission, symptom burden, functional status, and quality of life. Arch Intern Med 2007; 167:60–67. doi: 10.1001/archinte.167.1.60. [DOI] [PubMed] [Google Scholar]
- 70.Eisner MD, Blanc PD, Yelin EH, Katz PP, Sanchez G, Iribarren C, et al. Influence of anxiety on health outcomes in COPD. Thorax 2010; 65:229–234. doi: 10.1136/thx.2009.126201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu W, Collet JP, Shapiro S, Lin Y, Yang T, Platt RW, et al. Independent effect of depression and anxiety on chronic obstructive pulmonary disease exacerbations and hospitalizations. Am J Respir Crit Care Med 2008; 178:913–920. doi: 10.1164/rccm.200804-619OC. [DOI] [PubMed] [Google Scholar]
- 72.Quint JK, Baghai-Ravary R, Donaldson GC, Wedzicha JA. Relationship between depression and exacerbations in COPD. Eur Respir J 2008; 32:53–60. doi: 10.1183/09031936.00120107. [DOI] [PubMed] [Google Scholar]
- 73.Halpin D, Hyland M, Blake S, Seamark C, Pinnuck M, Ward D, et al. Understanding fear and anxiety in patients at the time of an exacerbation of chronic obstructive pulmonary disease: a qualitative study. JRSM Open 2015; 6:2054270415614543.doi: 10.1177/2054270415614543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Halpin DM, Seamark DA, Seamark CJ. Palliative and end-of-life care for patients with respiratory disease. Eur Respir Monograph 2009; 43:327–353. [Google Scholar]
- 75.Halpin DMG. Palliative care for chronic obstructive pulmonary disease. Signs of progress, but still a long way to go. Am J Respir Crit Care Med 2018; 198:1356–1358. doi: 10.1164/rccm.201805-0955ED. [DOI] [PubMed] [Google Scholar]
- 76.Claessens MT, Lynn J, Zhong Z, Desbiens NA, Phillips RS, Wu AW, et al. Dying with lung cancer or chronic obstructive pulmonary disease: insights from SUPPORT. Study to understand prognoses and preferences for outcomes and risks of treatments. J Am Geriatr Soc 2000; 48:S146–S153. doi: 10.1111/j.1532-5415.2000.tb03124.x. [DOI] [PubMed] [Google Scholar]