Abstract
Oxytocin and vasopressin are peptide hormones secreted from the pituitary that are well known for their peripheral endocrine effects on childbirth/nursing and blood pressure/urine concentration, respectively. However, both peptides are also released in the brain, where they modulate several aspects of social behaviors. Oxytocin promotes maternal nurturing and bonding, enhances social reward, and increases the salience of social stimuli. Vasopressin modulates social communication, social investigation, territorial behavior, and aggression, predominantly in males. Both peptides facilitate social memory and pair bonding behaviors in monogamous species. Here we review the latest research delineating the neural circuitry of the brain oxytocin and vasopressin systems and summarize recent investigations into the circuit-based mechanisms modulating social behaviors. We highlight research using modern molecular genetic technologies to map, monitor activity of, or manipulate neuropeptide circuits. Species diversity in oxytocin and vasopressin effects on social behaviors are also discussed. We conclude with a discussion of the translational implications of oxytocin and vasopressin for improving social functioning in disorders with social impairments, such as autism spectrum disorder.
Keywords: autism, neuropeptides, social communication, social behavior, oxytocin, vasopressin
Social distancing and isolation during the COVID-19 pandemic have caused major disruptions to our social relationships, likely affecting our well-being, as social interactions are fundamental for physical and mental health (1). Dysfunction in social communication and behavior is also a prominent aspect of many psychopathologies including autism, schizophrenia, and social anxiety (2). Considerable insights into the biological framework regulating functional social behavior, and therefore its dysfunction, have been gained through examination of oxytocin (OXT) and arginine vasopressin (AVP) neuropeptide systems and their nonmammalian homologues.
OXT and AVP were first identified as regulators of peripheral physiology, being secreted into the bloodstream through hypothalamic terminals in posterior pituitary and median eminence to influence parturition and lactation (OXT) and water retention, blood pressure, body temperature, and the hypothalamic-pituitary-adrenal axis (AVP) (3-6). In the 1960s, work by de Wied substantially advanced the field of endocrinology by demonstrating that AVP can act in the brain to alter behavior, thereby introducing the concept of “neuropeptide” (7). Since then, OXT acting centrally has been shown to be essential for maternal behavior, mother-infant bonding, sexual behavior, and other affiliative behaviors such as pair bonding and empathy-like consoling (8-14), whereas central AVP has been shown to regulate social communication, territorial aggression, pair bonding, and mate guarding, especially in males (9, 15, 16). Some of the behavioral effects of OXT and AVP are functionally linked to their peripheral function, as OXT promotes birth and nursing but also parental nurturing, while AVP modulates urine production and also territorial behavior, which often involves urine and scent marking (17, 18). Since these peptides modulate social cognition and motivation, manipulations of the OXT and AVP systems offer promising future therapeutics for individuals with social deficits, such as in autism spectrum disorder (ASD) (19-21).
Although the neural mechanisms underlying social behavior are highly complex, recent anatomical, genetic, and molecular technologies have considerably advanced our understanding of how OXT and AVP act within the brain to regulate social behavior. Most of this recent work has been conducted in laboratory mice because of the availability of powerful genetic tools in this species, such as recombinase-expressing and conditional knockout (KO) strains that, when combined with site-specific targeting with viral vectors, allow unprecedented access to chemically and spatially defined neural systems. These advancements have allowed researchers to manipulate neurons expressing OXT and AVP and their cognate receptors within the “social behavior neural network” (SBNN), as well as in other areas, such as the hippocampus, and thereby determine how these discrete, interlinked systems alter social behavior (6, 16, 22). Since laboratory mice have limited behavioral diversity, and species differences in the distribution of OXT/AVP receptors are well documented (10), it is important to expand these intersectional tools to other species (23). For example, oxytocin and vasotocin modulate social interactions in many species including teleost fish, lizards, and birds (24-26) and genome editing approaches are now being used to examine OXT and AVP circuitry and social behavioral functions in prairie voles and medaka fish (27-30). Importantly, the disproportionate focus on OXT compared to AVP systems suggests there is a need to rebalance research so that the role of different AVP circuits in social behavior can be refined and expanded.
Consequently, this mini-review will highlight recent work on the behaviorally relevant sources of OXT and AVP, the inputs and outputs of these sources, and the receptor systems they act on to generate complex social behaviors; see (6, 16, 31-38) for reviews of foundational work that guided recent discoveries of OXT/AVP’s role in social behavior.
Neuronal Sources of Oxytocin and Vasopressin
Hypothalamus
The paraventricular (PVN) and supraoptic nuclei (SON) of the hypothalamus release OXT and AVP, both centrally and peripherally. Within these regions, each peptide is stored in large, dense core vesicles, which can be released through somatodendritic, en passant axonal release, as well as through synaptic terminals (39-41). AVP is also produced in other hypothalamic nuclei such as the suprachiasmatic nucleus, well known for regulating circadian rhythms (42), and the accessory nuclei/nucleus circularis region, a likely source of AVP regulating aggression and competitive communication (43). OXT is also produced in the medial preoptic area (MPOA), where it regulates maternal and sexual behavior (44).
Both the PVN and SON contain magnocellular OXT and AVP neurosecretory cells that project to the posterior pituitary, releasing each peptide peripherally as a hormone (45), and the PVN contains an additional population of nonneurosecretory, parvocellular cells that modulate anterior pituitary release of adrenocorticotropin (46), as well as project to other brain and spinal cord areas (47, 48); for a comprehensive review see (6, 40, 49). Some reports have shown that AVP messenger RNA (mRNA) density measurements in the SON of rats are greater in males (50), while, in mice, OXT production is greater in females (51); however, other studies have shown a similar amount of OXT and AVP in both sexes in the PVN/SON of various rodent species, nonhuman primates, and humans (49, 52).
To better understand how OXT and AVP modulate neural circuits controlling social behavior, it is important to delineate the central nervous system projections of these neurons as well as their inputs. The development of mice with Cre recombinase driven by OXT or AVP neurons as well as viruses with transgenes driven by neuropeptide promoters have considerably enhanced our understanding of OXT and AVP circuit architecture.
Oxytocin
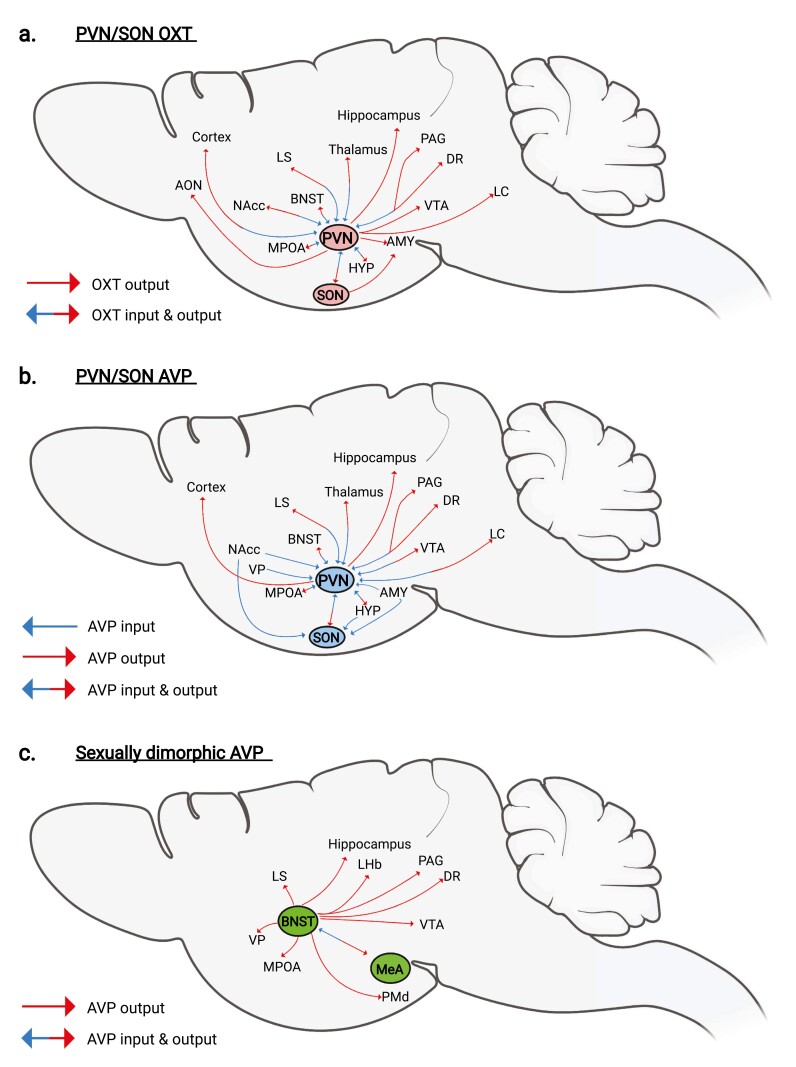
PVN OXT-expressing cells send fibers to the SON forming the neurohypophyseal tract (44), and classical retrograde tracing and modern viral tracing strategies in rats and mice have shown that the PVN OXT-expressing cells also send projections to the thalamus, cortex, amygdala, striatum, and hippocampus (Fig. 1A (53-55). The SON OXT-expressing cells have more restricted projections, mainly to the posterior pituitary, but also project to the medial and central amygdala (44). In rats and mice, several regions provide input to PVN OXT-expressing cells, including the brainstem, thalamic, hypothalamic, and cortical cell populations (56-58) (see Fig. 1A). Recently, Son et al (59) found no correlation between OXT neuronal projections and OXTR expression, consistent with paracrine signaling.
Figure 1.
Neural circuits (inputs and outputs) of oxytocin (OXT) and arginine vasopressin (AVP). Blue arrows indicate OXT and AVP neuronal inputs, while red arrows indicate OXT and AVP neuronal outputs. Combined blue and red arrows indicate regions that share both inputs and outputs. HYP refers to hypothalamic regions outside the paraventricular nucleus (PVN) and supraoptic nuclei (SON) of the hypothalamus. A, PVN and SON OXT neurons. B, PVN and SON AVP neurons. C, Sexually dimorphic AVP neurons within the bed nucleus of the stria terminalis (BNST) and medial amygdala (MeA).
Vasopressin
PVN AVP-expressing cells send projections to the thalamus, cortex, amygdala, striatum, and hippocampus (Fig. 1B). Although the number of PVN AVP neurons do not appear to differ by sex, central projections from these neurons are generally denser in female than in male mice (60). PVN/SON AVP cells receive monosynaptic inputs locally and from lateral septum (LS), bed nucleus of the stria terminalis (BNST), MPOA, ventromedial hypothalamus, and suprachiasmatic nucleus (Fig. 1B). Some differences between inputs to PVN and SON cells are apparent. For example, PVN AVP cells receive stronger input from the BNST than does the SON. Few of the inputs to PVN AVP cells are AVP immunoreactive, and may, in fact, be OXT-ergic (61, 62), suggesting functional crosstalk between OXT and AVP PVN cells. Whether release of hypothalamic OXT and AVP is synchronized across all OXT/AVP sources under various social conditions or has region-specific projections for distinct social behaviors is still unclear.
Extended Amygdala
Oxytocin
OXT neurons have been reported in the extended medial amygdala (eg, the BNST and medial amygdala proper [MeA]) in rats (63), California mice (64), and marmosets (65). Very little is known about the neuroanatomical connectivity of these neurons. However, OXT neurons in the BNST of California mice make local connections within the BNST, project to the hypothalamus, and are involved in social vigilance (66).
Vasopressin
The extended medial amygdala contains neurons that express AVP in a steroid-dependent and sexually dimorphic manner (33). Indeed, the BNST and MeA contribute to the most pronounced sex difference in AVP innervation in the brain (33, 60). For example, male rodents have 2 to 3 times as many AVP cells as females in the BNST/MeA and pronounced sex-different projections to areas involved in social behavior such as the ventral pallidum, lateral septum (LS), lateral habenula, dorsal raphe (DR), periaqueductal gray, as well as other forebrain areas (33, 60) (Fig. 1C). The sex difference in AVP expression is driven primarily by organizational effects of steroid hormones, although hormone-independent influences of sex chromosomes play a role (67). It is currently unknown which regions and neuronal cell types send monosynaptic inputs to BNST or MeA AVP.
Collectively, OXT and AVP cells in PVN receive and send projections to similar regions, including thalamus, hypothalamus, midbrain, and extended amygdala. More neuroanatomical experiments are needed to map the connectivity of OXT/AVP cells in the extended amygdala.
Oxytocin and Vasopressin Receptors
OXT has one canonical receptor type (ie, OXTR), whereas AVP has 3: V1aR, V1bR, and V2. The behavioral effects of AVP are mediated primarily by V1aR (or Avpr1a) and V1bR (16, 37, 38, 49, 68), while AVP acts on V2 to regulate fluid homeostasis in the kidney (69). Most research investigating OXT and AVP functions do so by manipulating the receptors, or receptor-expressing neurons, without regard to the source of the peptide. One of the most interesting features of the OXT/AVP system is the diversity in receptor function in relation to behavior. While there is remarkable consistency in the distribution of OXT and AVP neurons and their projections across vertebrates (33), there are striking species differences in the distributions in the distribution of OXT and AVP receptors in the brain, even between closely related species (10). Humans, chimpanzees, and other nonhuman primate species all show unique distributions of OXTR and V1aR in the brain, which may relate to species differences in primate social behaviors (70). The behavioral significance of diversity in receptor distribution, both within and between species, has been highlighted by studies of the neural basis of monogamy in prairie voles (discussed later). In addition, the distribution of OXTR messenger RNA in prairie vole brain is surprisingly widespread, suggesting that OXT may modulate essentially brain-wide circuit activity (71). In California mice, OXTR is expressed in different cell types depending on the brain region (72).
Modulation of Social Behavior
Sensory Processing of Social Cues
Olfactory processing is critical for appropriate social behavior driven by chemosignals produced by a conspecific (73). While there are no known OXT cells in the olfactory bulb (OB), PVN OXT can act early in the olfactory processing stream, via OXT receptors in the anterior olfactory nucleus. This system facilitates social recognition in rats by indirectly inhibiting the primary projection cells in the OB (74, 75), thereby reducing background “noise” and increasing signal salience. A similar process may also occur via AVP release, from inhibitory OB interneurons, in conjunction with cholinergic mechanisms (76, 77). This modulation of signal to noise may be a common neural circuit mechanism to enhance the salience of multimodal social stimuli (10, 78).
Social Motivation and Investigation
To respond adaptively to their social environment, animals must be motivated to respond to social signals. After processing social information (eg, on familiarity, sex, age, health), animals must decide how to interact: communicate, fight/compete, flee, cooperate, reproduce, and, in some species, form pair bonds and raise offspring together. OXT and AVP sources in the hypothalamus and extended amygdala play pivotal and distinct roles in social investigation, motivation, and social reward (discussed later). Further, OXT (and likely AVP) is important for long-term neural plasticity after social interactions (10).
Although constitutive KO of OXT does not alter social approach in mice (79), specific OXT systems do influence social reward, approach, and investigation. For example, Dölen et al (80), using elegant circuit manipulations, demonstrated that PVN OXT and DR serotonin projections to the nucleus accumbens (NAcc) are required for social reward in mice. Further, PVN OXT cells are responsible for the facilitatory effects of 3,4-methylendioxymethamphetamine (MDMA) on mouse prosocial behavior and social reward memory (81). PVN OXT can also facilitate social reward through its actions on brain dopamine systems (82), originating, for example, in the ventral tegmental area (VTA), and through endocannabinoid-dependent mechanisms (83). In male and female Syrian hamsters, social interaction increases the amount of c-Fos expression (a marker for neural activity) in OXT-immunoreactive neurons in the PVN and SON, while OXTRs in the VTA mediate the rewarding properties of these social interactions (84).
Similarly, in mice, social touch during early life increases both PVN OXT neuronal firing and social approach later in adulthood through interaction with substance P systems within the periaqueductal gray (58). PVN OXT cells in adults are also active during interactions with juveniles (85), and stimulation of PVN OXT terminals in the VTA increased social investigation through actions on OXTR specifically. Moreover, deletion of OXTR from dopaminergic neurons in the VTA eliminated preferences for interacting with juveniles (82). Monogamous mandarin voles raised without a father socialize less and have fewer PVN OXT cells, a behavioral difference that can be reversed by optogenetic stimulation of PVN OXT terminals within the prelimbic cortex (86). Taken together, OXT from the PVN influences social behavior via its actions on defined DR serotonin– and dopamine system–expressing populations as well as others, perhaps serving as a “gating” mechanism for the flow of information to reward systems (83, 87).
Historically OXT is known for having prosocial effects on behavior, whereas AVP is thought to play more of a role in aggression, stress, and anxiety. However, in California mice, knockdown of OXT or administration of OXTR antagonist in the anterior BNST increased social approach and decreased social vigilance, an anxiety-like behavior, which suggests that OXT may also drive social anxiety-like behavior depending on its source or context (66, 88).
AVP can have prosocial effects as well. With site-specific targeting using viral vectors, we have begun to identify which AVP sources are responsible for specific social behaviors in mice. AVP cell groups appear to play opposite roles in social investigation in males and females depending on the source. BNST AVP cell ablation and AVP knockdown both reduced male-male social investigation. These are similar to effects of BNST AVP knockdown in avian species (reviewed in [89]). PVN AVP cell ablation increased female social investigation, but does not alter investigation in males (52, 90, 91). However, PVN AVP cell stimulation in male mice caused self-grooming instead of social interactions with female mice (92). Nevertheless, there may be differential involvement of PVN and BNST AVP cells in the control of social/emotional behavior in each sex (52). There is also a sex-specific role of AVP in the regulation of social play behavior in juvenile rats, as blocking V1aR in the LS increases social play behavior in males and decreases it in females (49). The extreme sexually dimorphic nature of BNST AVP cells may explain stronger effects in males than in females. The female bias in the effects of similar manipulations of PVN AVP cells may be related to females having denser PVN AVP projections compared to males (60).
Social Recognition
Although social behavior is diverse across species, one common feature is that the animal must differentiate between conspecifics, which is critical for the establishment of relationships between animals (ie, affiliative, territorial). OXT and AVP systems have long been known to play a major role in the formation of social memory (93). For example, mice with impairments in either system show deficits in distinguishing between familiar and novel conspecifics (74, 94, 95). OXTRs in the MeA receive OXT input from the PVN that help discriminate between sex- and individual cues (53, 74, 96). Ablating OXTR expression in aromatase-positive neurons in the MeA abolishes male mouse preferences for females (96). Social memory processing also requires the hippocampus. OXT and AVP-expressing cells in PVN and SON send projections to the hippocampus, with the highest fiber density seen in the CA2 region for AVP and CA2/CA3 regions for OXT projections (97-100). OXTRs in the anterior DG-CA2/CA3 circuit facilitate social, but not object discrimination (101), and AVP projections from PVN to hippocampus influence recognition of conspecifics as well as spatial features in mice (102). For example, Smith et al (99) demonstrated that optogenetic activation of the PVN AVP cell inputs to hippocampal CA2 area enhances social memory, via a V1bR mechanism, in mice. Further, when the PVN to CA2 pathway is activated chemogenetically, nonmonogamous C57Bl6 mice form partner preferences in a species-atypical way (103). Projections from PVN to other brain regions can also alter aspects of social recognition. For example, PVN OXT input to the central amygdala mediates the ability of mice to discriminate between negative and positive emotional states of conspecifics (104).
Taken together, these studies suggest that AVP and OXT cells in the PVN help generate social preferences and influence social recognition by direct hippocampal, amygdala, and cortical synaptic release. One explanation for OXT and AVP effects on social recognition may be that these peptides act as attentional modulators, increasing the salience of social information through different circuits (10). Indeed, activated PVN OXT neurons increased not only affiliative, but also agonistic behaviors (105). For a comprehensive review on OXT and AVP’s role in social memory via the hippocampus, see (106). However, it is unlikely that just PVN projections contribute to AVP effects on social recognition as ablation of AVP cells in PVN and SON did not affect social recognition (52, 107). Extrahypothalamic AVP projections may therefore be an important regulator of social recognition. For example, BNST AVP cell ablation impairs social recognition in males, likely through its projection to the LS (94, 108, 109), and restoration of BNST—LS AVP signaling in Magel2-KO mice ameliorated social discrimination deficits (110).
Social Communication, Territoriality, and Aggression
AVP was first shown to influence social communication by its ability to induce flank marking in Syrian hamsters in the anterior hypothalamus and MPOA (16, 111-113). AVP in these areas is also critical for the establishment of dominance relationships; for a comprehensive review on AVP effects on aggression and communication, see (114). Mice deposit urine marks to communicate social status and dominance (115). Removal of BNST AVP cells in mice increases male urine marking toward females. However, AVP knockdown in this region did not affect urine marking but did reduce other aspects of male communication (eg, vocalizations and tail rattles), suggesting that BNST AVP is involved in some aspects of male offensive/territorial signaling (91). In contrast, blockade of V1a receptors in the lateral habenula and DR (downstream BNST structures) reduced, instead of increased, male urine marking toward potential competitor males. More work is clearly needed to dissect which AVP sources and output regions are responsible for urine marking and other forms of social communication in mice.
AVP also plays a role in aggression in prairie voles and Syrian hamsters, sometimes in a sex-specific manner. For example, pharmacological studies revealed that AVP in the anterior hypothalamus facilitates intermale aggression both in prairie voles and hamsters (16, 43, 116), while the opposite effect was found in female hamsters (117). In hamsters, social isolation increases aggression both in male and females, but V1aR density is higher in males than females (114). Recently, constitutive KO of Avpr1a in Syrian hamsters paradoxically increased flank marking and aggression in both sexes, highlighting the complex role of Avpr1a in territorial behaviors and potentially highlighting the role of compensatory mechanisms and/or effects on Avpr1a KO in multiple brain regions (118). Collectively, these data suggest more work is needed using comparative and gene-editing approaches to elucidate AVP’s role in social communication and aggression.
Sexual Behavior
OXT is strongly involved both in male and female sexual behavior through central and peripheral action (38). OXT contributes to sexual arousal and sexual reward in males, while OXT influences lordosis and orgasm in females (38). In rats, PVN OXT neurons are active during sexual behavior in males and females and blocking OXT action in ventromedial hypothalamus and MPOA reduces female receptivity (38).
In mice, BNST and MeA AVP cells are active during male sexual behavior (101, 119) and knockdown of AVP within the BNST reduced the number of intromissions and ejaculations in males, but did not alter sexual behavior in females (91). Hormonal action on this cell population may help drive aspects of steroid-sensitive sexual behavior since gonadal hormones affect both male sexual behavior (120, 121) and AVP expression within the extended amygdala (33).
Parental Behavior
OXT is well known for its influence on social bonding and maternal behavior (9, 18), and its role in parental behavior highlights the putative function of OXT as an attentional modulator (122). For example, OXT from PVN increases the salience of mouse pup vocalizations, while a dam’s experience with pups adjusts the balance of excitatory and inhibitory tuning in the auditory cortex (54, 123, 124). Further, PVN OXT neurons are activated when virgin mice observe pup retrievals by another mother and optogenetic stimulation of PVN OXT efferents to auditory cortex enhances pup retrieval learning in virgin mice (124, 125). This increase in OXT release by the PVN may be driven by the posterior intralaminar complex of the thalamus inputs to the PVN (56). Other loci of PVN OXT action include the VTA and NAcc as PVN OXT projections to these areas promote paternal behaviors in mandarin voles (126). An animal’s transition to parenthood is known to affect sensory perception of infant cues. How PVN OXT modulates this change through various OXT circuits remains to be further explored. Additionally, parental/sensory deprivation or cesarean delivery can lead to disruptions of hypothalamic OXT and maternal behavior (127-130), while, in rats, enriched environments increase hypothalamic OXT in dams and their male offspring (131). Therefore, OXT, social experience, and environment heavily influence functional parental behavior.
There are far fewer studies examining the role of central AVP on parental behavior. This is not surprising given that AVP is traditionally associated with territorial behavior and anxiety. However, the seminal paper demonstrating the effect of OXT on maternal behavior also showed that AVP stimulates maternal behavior, but with a longer latency of action (132). Recent work has demonstrated that chemogenetic inhibition of PVN AVP cell activity increases nest building in male mice (while excitation decreases nest building in females), without altering other aspects of parental behavior (133). Knockdown of V1aR in the MPOA impairs maternal behavior in rats (134). Although the precise sources of AVP regulating parental behavior is undefined, AVP projections from the extended amygdala may also influence maternal behavior, as AVP in the LS facilitates paternal behavior in prairie voles (135, 136). While hypothalamic OXT plays a substantial role in rodent maternal behavior compared to AVP, the sources of AVP responsible for V1aR-mediated changes in parental behavior require further examination.
Pair Bonding and Mate Choice
Studies in monogamous prairie voles have revealed the critical roles of OXT and AVP and their receptors in pair bonding, biparental care, and mate guarding. For a detailed review of this topic, see (9). Bonding with a partner is the culmination of many of the interdependent processes discussed earlier. To pair bond, mates need to sense their partner’s social cues, be motivated to interact with them, and remember their partner’s identity. Mate guarding is a form of territoriality, while the pair bond has similarities to the maternal bond. Thus, it is not surprising that OXT and AVP are integrally involved in pair bonding in monogamous species, and mate choices more generally. Here we provide a brief overview of OXT’s and AVP’s role in pair bonding and mate choice, highlighting recent advances in the context of more classic studies.
Oxytocin and monogamy
Mating and cohabitation stimulates a partner preference in both male and female prairie voles. Blocking brain OXT receptors in either sex prevents pair bonding, potentially disrupting coordinated activity across a social salience network (137). Pharmacological studies revealed that OXT signaling in the prefrontal cortex and NAcc facilitate mating-induced pair bonding. Viral-mediated small interfering RNA knockdown of OXTR in NAcc of prairie voles inhibits partner preference (138), while overexpressing NAcc OXTR enhances partner preference formation (139). The OXT fibers in the NAcc originate from the PVN (140), and OXT is released in females during mating (141).
Robust individual differences in NAcc relate to individual variation in alloparental nurturing behavior, and resilience to early-life adversity with respect to later-life pair bonding (142, 143). This variation in OXTR density in the NAcc is determined by genetic variation in the Oxtr gene (144), which in turn predicts how early-life nurturing experience affects later-life bonding ability (143).
Recent ex vivo electrophysiological studies provide clues about how one’s social experience can alter OXTR signaling effect on NAcc activity to influence pair-bond formation and expression. In virgin female prairie voles, OXTR signaling, mimicking the first mating, decreases spontaneous activity of NAcc neurons, effectively reducing the noise in the circuit, perhaps to facilitate synaptic plasticity to make partner cues rewarding. After pair bonding, OXTR signaling increases evoked NAcc cell activity from neural inputs, perhaps amplifying the signal of the neural representation of the partner, making the partner reinforcing and motivating affiliation (140). This experience-dependent change in OXTR signaling on physiology is mediated by OXTR-endocannabinoid interactions, and blocking CB1 receptor elicits defensive rejection by the female of the partner. Interestingly, OXT stimulates the activation of NAcc activity in men in monogamous relationships when viewing images of their partner (145).
Once the pair bond is formed, withdrawal of PVN OXT signaling in NAcc mediates “grieving”-like passive coping behavior following the loss of the partner, which is mimicked in the presence of the partner by small interfering RNA knockdown of NAc OXTR (140, 146). The negative affect stimulated by the loss of OXT signaling when separated by the partner may serve to motivate partners to stay together in enduring relationships.
Prairie voles also display empathy-based consoling behavior toward their distress partner, sibling, or familiar cage mate, which is mediated by OXTR in the anterior cingulate cortex, a region involved in empathetic responses in humans (8). Oxtr KO prairie voles display altered social preferences and reduced helping behavior toward a familiar cagemate (27, 147).
Vasopressin and monogamy
AVP stimulates partner preferences as well as selective aggression, or mate guarding, in male prairie voles (15, 148). V1aR signaling in the ventral pallidum and LS promote partner preferences (149), while pair bond-induced aggression is mediated by V1aR in the lateral hypothalamus (43, 150). Viral-mediated Avpr1a receptor overexpression studies show that species differences in Avpr1a expression mediate species differences in social behavior, and variation in Avpr1a expression in the brain is related to microsatellite diversity in the gene’s 5′ flanking region (151-154). Voles display remarkable individual variation in V1aR density in the retrosplenial cortex (155), a region involved in spatial memory, and this receptor density is related to Avpr1a polymorphisms, space use in relation to nesting, and sexual fidelity in males (156). The sources of AVP regulating any of these behaviors have not been explored.
Mate choice
Medaka fish are promiscuous breeders of which females prefer to mate with familiar males that manage to maintain proximity to the female despite efforts of competing males, while males mate indiscriminately. Females with targeted Oxt or Oxtr mutations lose preference for familiar males and mate indiscriminately, while Oxt or Oxtr mutant males preferentially mate with familiar females. Thus, OXTR signaling promotes the adaptive mate preference of particular species, which in promiscuous species may be sexually dimorphic (28). In zebrafish, OXT and OXTR drive social behavior in a context-dependent manner, suggesting a broad role of OXT/OXTR in social grouping (157, 158). Like voles, both AVP and V1aR homologues in medaka fish mediate mate guarding behavior, which is related to territorial behaviors. Thus, both OXT and AVP mediate adaptive sociosexual behaviors across vertebrate taxa, although the behavior that is adaptive may differ across species.
Translational Opportunities
Because of the effects of OXT and AVP on social behaviors, both peptide systems are potential therapeutic targets for treating disorders such as ASD, in which social functioning is compromised. OXT has been more intensively investigated in this regard, and its therapeutic potential is related to its modulation of social motivation and the salience of social stimuli (19-21). Single-cell transcriptomics studies show that PVN OXT neurons projecting within the brain that mediate social reward behaviors are disrupted in a mouse model of ASD, and those neurons are enriched for ASD-risk genes (159). Further transcriptomic work exploring the function of subtypes of OXT and AVP-expressing cells (160) would be useful in delineating relevant subcircuits driving social reward. In another ASD mouse model, OXTR signaling in VTA dopamine neurons, which mediate social reward, is disrupted, and a kinase inhibitor that restores OXTR signaling also restores disrupted ASD-relevant social behaviors (161). Prairie vole studies suggest that pharmacologically evoking OXT using melanocortin agonists may be another strategy for enhancing social functioning (162). Clinically, the efficacy of intranasally delivered OXT has been mixed, and there is a critical need for clinical trials to be designed based on our understanding of OXT’s function in the brain, such as enhancing the salience of social stimuli (20).
Both AVP and V1aR antagonists have been explored as potential ASD, schizophrenia, and drug-abuse therapies (163). Based on a subset of preclinical studies demonstrating anxiogenic and agonistic effects of AVP, the V1aR antagonist balovaptan has been tested in clinical trials in ASD individuals with mixed effects on social end points (164, 165). Similarly, male rhesus macaques in large groups that have naturally impaired social functioning have decreased AVP in cerebrospinal fluid (166). This led to investigations in pediatric ASD and control samples that also found decreased cerebrospinal fluid AVP (167, 168). Finally, intranasal AVP treatment considerably improved social abilities in children with ASD (169).
Translational opportunities for OXT and AVP may extend beyond mental health as polymorphisms in V1aR and OXTR have been linked to variations in normal as well as disordered human behavior (170). Social relationships are important for physical well-being as well (171). A recent lung cancer study found that a factor in serum samples of bonded monogamous California mice decreased oncogenic potential of tumor cells when compared to serum samples from bond-disrupted animals (172). Identifying the molecular mechanisms by which positive social relationships are transduced to improved health and reduce social stress could lead to novel approaches for improving well-being, and as OXT and AVP are inextricably involved in social relationships, they may have unforeseen translational opportunities based on their function in the brain (173).
Concluding Remarks
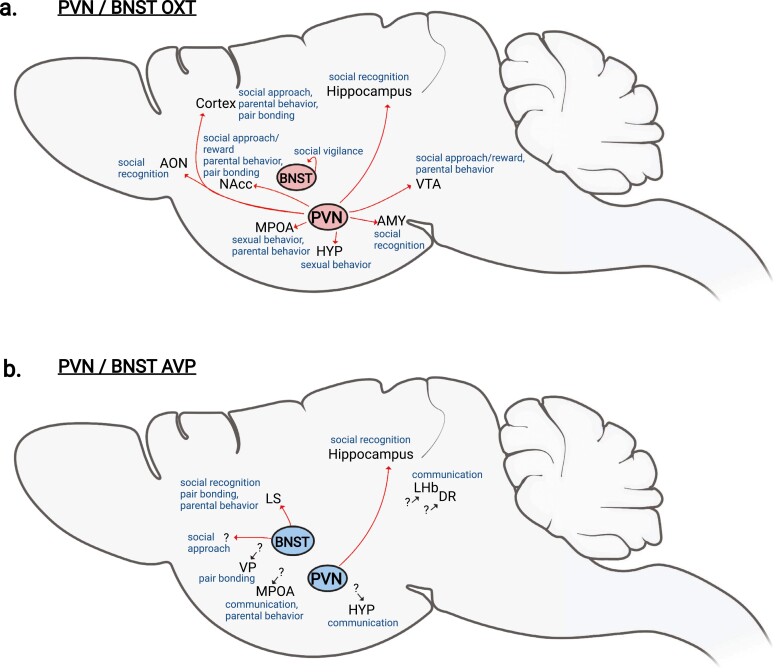
Modern molecular approaches are greatly enhancing our understanding of how and where OXT and AVP act to regulate social processes. Despite this progress, considerably more attention has been directed toward the OXT system than the AVP system in the past decade because of its perceived translational applications. However, more recent translational research suggests that both peptides have important clinical potential. Consequently, it will be important to rebalance OXT/AVP research in behavioral neuroscience. Specifically, more attention needs to be devoted to understanding the contribution of different peptide sources and their specific targets, particularly with respect to AVP (Fig. 2). Finally, the incorporation of modern molecular genetic approaches into studies of alternative model organisms with diverse social systems is essential for gaining a complete understanding of OXT and AVP function in the brain, and thereby maximizing translational opportunities (19, 174).
Figure 2.
Oxytocin (OXT) and arginine vasopressin (AVP) circuits that regulate social behavior. The paraventricular nucleus (PVN) and bed nucleus of the stria terminalis (BNST) contain OXT and AVP cell bodies that directly influence specific social behaviors: social recognition, social approach and reward, social communication, sexual behavior, parental behavior, and pair bonding and mate choice. A, PVN and BNST OXT circuits. B, PVN and BNST AVP circuits. Some contributions of AVP sources and sites of action are unknown, indicated by a short black arrow and “?”. All systems are not depicted for clarity.
Acknowledgments
We thank Dr Elliott Albers for helpful comments on the manuscript. Figures were created with BioRender.com.
Glossary
Abbreviations
- AON
accessory olfactory nucleus
- AMY
amygdala
- AVP
arginine vasopressin
- ASD
autism spectrum disorder
- BNST
bed nucleus of the stria terminalis
- DR
dorsal raphe
- HYP
hypothalamus
- KO
knockout
- LHb
lateral habenula
- LS
lateral septum
- LC
locus coeruleus
- MeA
medial amygdala
- MPOA
medial preoptic area
- NAcc
nucleus accumbens
- OB
olfactory bulb
- OXT
oxytocin
- OXTR
oxytocin receptor
- PAG
periaqueductal gray
- PVN
paraventricular nucleus of the hypothalamus
- PMd
premammillary nucleus (dorsal)
- SON
supraoptic nucleus of the hypothalamus
- VP
ventral pallidum
- VTA
ventral tegmental area
Contributor Information
Nicole Rigney, Neuroscience Institute and Center for Behavioral Neuroscience, Georgia State University, Atlanta, Georgia 30303, USA.
Geert J de Vries, Neuroscience Institute and Center for Behavioral Neuroscience, Georgia State University, Atlanta, Georgia 30303, USA; Department of Biology, Georgia State University, Atlanta, Georgia 30303, USA.
Aras Petrulis, Neuroscience Institute and Center for Behavioral Neuroscience, Georgia State University, Atlanta, Georgia 30303, USA.
Larry J Young, Center for Translational Social Neuroscience, Emory University, Atlanta, Georgia 30329, USA; Silvio O. Conte Center for Oxytocin and Social Cognition, Emory National Primate Research Center, Emory University, Atlanta, Georgia 30329, USA; Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, Atlanta, Georgia 30322, USA.
Financial Support
This work was supported by the National Institutes of Health (grant Nos. R01MH115831, P50MH100023, and R01MH112788 to L.J.Y.; R01 MH121603 and R03 MH120549 to A.P. and G.J.D.; and F31 MH125659 to N.R.).
Disclosures
The authors have nothing to disclose.
Data Availability
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.
References
- 1. Leigh-Hunt N, Bagguley D, Bash K, et al. . An overview of systematic reviews on the public health consequences of social isolation and loneliness. Public Health. 2017;152:157-171. doi: 10.1016/j.puhe.2017.07.035 [DOI] [PubMed] [Google Scholar]
- 2. Kennedy DP, Adolphs R. The social brain in psychiatric and neurological disorders. Trends Cogn Sci. 2012;16(11):559-572. doi: 10.1016/j.tics.2012.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bilezikjian LM, Vale WW. Regulation of ACTH secretion from corticotrophs: the interaction of vasopressin and CRF. Ann N Y Acad Sci. 1987;512:85-96. doi: 10.1111/j.1749-6632.1987.tb24952.x [DOI] [PubMed] [Google Scholar]
- 4. Lee HJ, Macbeth AH, Pagani JH, Young WS III. Oxytocin: the great facilitator of life. Prog Neurobiol. 2009;88(2):127-151. doi: 10.1016/j.pneurobio.2009.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stoop R. Neuromodulation by oxytocin and vasopressin. Neuron. 2012;76(1):142-159. doi: 10.1016/j.neuron.2012.09.025 [DOI] [PubMed] [Google Scholar]
- 6. Johnson ZV, Young LJ. Oxytocin and vasopressin neural networks: implications for social behavioral diversity and translational neuroscience. Neurosci Biobehav Rev. 2017;76(Pt A):87-98. doi: 10.1016/j.neubiorev.2017.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Wied D. The influence of the posterior and intermediate lobe of the pituitary and pituitary peptides on the maintenance of a conditioned avoidance response in rats. Int J Neuropharmacol. 1965;4:157-167. doi: 10.1016/0028-3908(65)90005-5 [DOI] [PubMed] [Google Scholar]
- 8. Burkett JP, Andari E, Johnson ZV, Curry DC, de Waal FBM, Young LJ. Oxytocin-dependent consolation behavior in rodents. Science. 2016;351(6271):375-378. doi: 10.1126/science.aac4785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Walum H, Young LJ. The neural mechanisms and circuitry of the pair bond. Nat Rev Neurosci. 2018;19(11):643-654. doi: 10.1038/s41583-018-0072-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Froemke RC, Young LJ. Oxytocin, neural plasticity, and social behavior. Annu Rev Neurosci. 2021;44:359-381. doi: 10.1146/annurev-neuro-102320-102847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carter CS. Oxytocin and sexual behavior. Neurosci Biobehav Rev. 1992;16(2):131-144. doi: 10.1016/s0149-7634(05)80176-9 [DOI] [PubMed] [Google Scholar]
- 12. Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci Biobehav Rev. 1995;19(2):303-314. doi: 10.1016/0149-7634(94)00070-h [DOI] [PubMed] [Google Scholar]
- 13. Kendrick KM, Da Costa AP, Broad KD, et al. . Neural control of maternal behaviour and olfactory recognition of offspring. Brain Res Bull. 1997;44(4):383-395. doi: 10.1016/s0361-9230(97)00218-9 [DOI] [PubMed] [Google Scholar]
- 14. Grinevich V, Knobloch-Bollmann HS, Eliava M, Busnelli M, Chini B. Assembling the puzzle: pathways of oxytocin signaling in the brain. Biol Psychiatry. 2016;79(3):155-164. doi: 10.1016/j.biopsych.2015.04.013 [DOI] [PubMed] [Google Scholar]
- 15. Nair HP, Young LJ. Vasopressin and pair-bond formation: genes to brain to behavior. Physiology (Bethesda). 2006;21:146-152. doi: 10.1152/physiol.00049.2005 [DOI] [PubMed] [Google Scholar]
- 16. Albers HE. Species, sex and individual differences in the vasotocin/vasopressin system: relationship to neurochemical signaling in the social behavior neural network. Front Neuroendocrinol. 2015;36:49-71. doi: 10.1016/j.yfrne.2014.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arakawa H, Higuchi Y. Exocrine scent marking: coordinative role of arginine vasopressin in the systemic regulation of social signaling behaviors. Neurosci Biobehav Rev. 2022;136:104597. doi: 10.1016/j.neubiorev.2022.104597 [DOI] [PubMed] [Google Scholar]
- 18. Rilling JK, Young LJ. The biology of mammalian parenting and its effect on offspring social development. Science. 2014;345(6198):771-776. doi: 10.1126/science.1252723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ford CL, Young LJ. Translational opportunities for circuit-based social neuroscience: advancing 21st century psychiatry. Curr Opin Neurobiol. 2021;68:1-8. doi: 10.1016/j.conb.2020.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ford CL, Young LJ. Refining oxytocin therapy for autism: context is key. Nat Rev Neurol. 2022;18(2):67-68. doi: 10.1038/s41582-021-00602-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DeMayo MM, Young LJ, Hickie IB, Song YJC, Guastella AJ. Circuits for social learning: a unified model and application to autism spectrum disorder. Neurosci Biobehav Rev. 2019;107:388-398. doi: 10.1016/j.neubiorev.2019.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kelly AA. consideration of brain networks modulating social behavior. Horm Behav. 2022;141:105138. doi: 10.1016/j.yhbeh.2022.105138 [DOI] [PubMed] [Google Scholar]
- 23. Boender AJ, Young LJ. Oxytocin, vasopressin and social behavior in the age of genome editing: a comparative perspective. Horm Behav. 2020;124:104780. doi: 10.1016/j.yhbeh.2020.104780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Silva AC, Pandolfi M. Vasotocinergic control of agonistic behavior told by neotropical fishes. Gen Comp Endocrinol. 2019;273:67-72. doi: 10.1016/j.ygcen.2018.04.025 [DOI] [PubMed] [Google Scholar]
- 25. Campos SM, Belkasim SS. Chemical communication in lizards and a potential role for vasotocin in modulating social interactions. Integr Comp Biol. 2021;61(1):205-220. doi: 10.1093/icb/icab044 [DOI] [PubMed] [Google Scholar]
- 26. Goodson JL, Schrock SE, Klatt JD, Kabelik D, Kingsbury MA. Mesotocin and nonapeptide receptors promote estrildid flocking behavior. Science. 2009;325(5942):862-866. doi: 10.1126/science.1174929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Horie K, Inoue K, Suzuki S, et al. . Oxytocin receptor knockout prairie voles generated by CRISPR/Cas9 editing show reduced preference for social novelty and exaggerated repetitive behaviors. Horm Behav. 2019;111:60-69. doi: 10.1016/j.yhbeh.2018.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yokoi S, Naruse K, Kamei Y, et al. . Sexually dimorphic role of oxytocin in medaka mate choice. Proc Natl Acad Sci U S A. 2020;117(9):4802-4808. doi: 10.1073/pnas.1921446117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yokoi S, Okuyama T, Kamei Y, et al. . An essential role of the arginine vasotocin system in mate-guarding behaviors in triadic relationships of medaka fish (Oryzias latipes). PLoS Genet. 2015;11(2):e1005009. doi: 10.1371/journal.pgen.1005009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Horie K, Inoue K, Nishimori K, Young LJ. Investigation of Oxtr-expressing neurons projecting to nucleus accumbens using Oxtr-ires-Cre knock-in prairie voles (Microtus ochrogaster). Neuroscience. 2020;448:312-324. doi: 10.1016/j.neuroscience.2020.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res Rev. 2001;35(3):246-265. doi: 10.1016/s0165-0173(01)00043-1 [DOI] [PubMed] [Google Scholar]
- 32. Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25(3-4):150-176. doi: 10.1016/j.yfrne.2004.05.001 [DOI] [PubMed] [Google Scholar]
- 33. De Vries GJ, Panzica GC. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: different mechanisms, similar endpoints. Neuroscience. 2006;138(3):947-955. doi: 10.1016/j.neuroscience.2005.07.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Vries GJ. Sex differences in vasopressin and oxytocin innervation of the brain. Prog Brain Res. 2008;170:17-27. doi: 10.1016/S0079-6123(08)00402-0 [DOI] [PubMed] [Google Scholar]
- 35. Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322(5903):900-904. doi: 10.1126/science.1158668 [DOI] [PubMed] [Google Scholar]
- 36. Bredewold R, Veenema AH. Sex differences in the regulation of social and anxiety-related behaviors: insights from vasopressin and oxytocin brain systems. Curr Opin Neurobiol. 2018;49:132-140. doi: 10.1016/j.conb.2018.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Smith CJW, DiBenedictis BT, Veenema AH. Comparing vasopressin and oxytocin fiber and receptor density patterns in the social behavior neural network: implications for cross-system signaling. Front Neuroendocrinol. 2019;53:100737. doi: 10.1016/j.yfrne.2019.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jurek B, Neumann ID. The oxytocin receptor: from intracellular signaling to behavior. Physiol Rev. 2018;98(3):1805-1908. doi: 10.1152/physrev.00031.2017 [DOI] [PubMed] [Google Scholar]
- 39. Grinevich V, Ludwig M. The multiple faces of the oxytocin and vasopressin systems in the brain. J Neuroendocrinol. 2021;33(11):e13004. [DOI] [PubMed] [Google Scholar]
- 40. Grinevich V, Neumann ID. Brain oxytocin: how puzzle stones from animal studies translate into psychiatry. Mol Psychiatry. 2021;26(1):265-279. doi: 10.1038/s41380-020-0802-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Oti T, Satoh K, Uta D, et al. . Oxytocin influences male sexual activity via non-synaptic axonal release in the spinal cord. Curr Biol. 2021;31(1):103-114.e5. doi: 10.1016/j.cub.2020.09.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rohr KE, Telega A, Savaglio A, Evans JA. Vasopressin regulates daily rhythms and circadian clock circuits in a manner influenced by sex. Horm Behav. 2021;127:104888. doi: 10.1016/j.yhbeh.2020.104888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gobrogge KL, Liu Y, Young LJ, Wang Z. Anterior hypothalamic vasopressin regulates pair-bonding and drug-induced aggression in a monogamous rodent. Proc Natl Acad Sci U S A. 2009;106(45):19144-19149. doi: 10.1073/pnas.0908620106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jirikowski GF. Diversity of central oxytocinergic projections. Cell Tissue Res. 2019;375(1):41-48. doi: 10.1007/s00441-018-2960-5 [DOI] [PubMed] [Google Scholar]
- 45. Scott Young W III, Gainer H. Transgenesis and the study of expression, cellular targeting and function of oxytocin, vasopressin and their receptors. Neuroendocrinology. 2003;78(4):185-203. doi: 10.1159/000073702 [DOI] [PubMed] [Google Scholar]
- 46. Gillies GE, Linton EA, Lowry PJ. Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature. 1982;299(5881):355-357. doi: 10.1038/299355a0 [DOI] [PubMed] [Google Scholar]
- 47. Castel M, Morris JF. The neurophysin-containing innervation of the forebrain of the mouse. Neuroscience. 1988;24(3):937-966. doi: 10.1016/0306-4522(88)90078-4 [DOI] [PubMed] [Google Scholar]
- 48. Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7(2):126-136. doi: 10.1038/nrn1845 [DOI] [PubMed] [Google Scholar]
- 49. Dumais KM, Veenema AH. Vasopressin and oxytocin receptor systems in the brain: sex differences and sex-specific regulation of social behavior. Front Neuroendocrinol. 2016;40:1-23. doi: 10.1016/j.yfrne.2015.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Taylor PV, Veenema AH, Paul MJ, Bredewold R, Isaacs S, de Vries GJ. Sexually dimorphic effects of a prenatal immune challenge on social play and vasopressin expression in juvenile rats. Biol Sex Differ. 2012;3(1):15. doi: 10.1186/2042-6410-3-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Häussler HU, Jirikowski GF, Caldwell JD. Sex differences among oxytocin-immunoreactive neuronal systems in the mouse hypothalamus. J Chem Neuroanat. 1990;3(4):271-276. [PubMed] [Google Scholar]
- 52. Rigney N, Whylings J, de Vries GJ, Petrulis A. Sex differences in the control of social investigation and anxiety by vasopressin cells of the paraventricular nucleus of the hypothalamus. Neuroendocrinology. 2021;111(6):521-535. doi: 10.1159/000509421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Knobloch HS, Charlet A, Hoffmann LC, et al. . Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73(3):553-566. doi: 10.1016/j.neuron.2011.11.030 [DOI] [PubMed] [Google Scholar]
- 54. Mitre M, Marlin BJ, Schiavo JK, et al. . A distributed network for social cognition enriched for oxytocin receptors. J Neurosci. 2016;36(8):2517-2535. doi: 10.1523/JNEUROSCI.2409-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang B, Qiu L, Xiao W, et al. . Reconstruction of the hypothalamo-neurohypophysial system and functional dissection of magnocellular oxytocin neurons in the brain. Neuron. 2021;109(2):331-346.e7. doi: 10.1016/j.neuron.2020.10.032 [DOI] [PubMed] [Google Scholar]
- 56. Dobolyi A, Cservenák M, Young LJ. Thalamic integration of social stimuli regulating parental behavior and the oxytocin system. Front Neuroendocrinol. 2018;51:102-115. doi: 10.1016/j.yfrne.2018.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tang Y, Benusiglio D, Lefevre A, et al. . Social touch promotes interfemale communication via activation of parvocellular oxytocin neurons. Nat Neurosci. 2020;23(9):1125-1137. doi: 10.1038/s41593-020-0674-y [DOI] [PubMed] [Google Scholar]
- 58. Yu H, Miao W, Ji E, et al. . Social touch-like tactile stimulation activates a tachykinin 1-oxytocin pathway to promote social interactions. Neuron. 2022;110(6):1051-1067.e7. doi: 10.1016/j.neuron.2021.12.022 [DOI] [PubMed] [Google Scholar]
- 59. Son S, Manjila SB, Newmaster KT, et al. . Whole-brain wiring diagram of oxytocin system in adult mice. J Neurosci. 2022;42(25):5021-5033. doi: 10.1523/JNEUROSCI.0307-22.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rood BD, Stott RT, You S, Smith CJW, Woodbury ME, De Vries GJ. Site of origin of and sex differences in the vasopressin innervation of the mouse (Mus musculus) brain. J Comp Neurol. 2013;521(10):2321-2358. doi: 10.1002/cne.23288 [DOI] [PubMed] [Google Scholar]
- 61. Wei HH, Yuan XS, Chen ZK, et al. . Presynaptic inputs to vasopressin neurons in the hypothalamic supraoptic nucleus and paraventricular nucleus in mice. Exp Neurol. 2021;343:113784. doi: 10.1016/j.expneurol.2021.113784 [DOI] [PubMed] [Google Scholar]
- 62. Kim A, Madara JC, Wu C, Andermann ML, Lowell BB. Neural basis for regulation of vasopressin secretion by anticipated disturbances in osmolality. Elife 2021;10:e66609. doi: 10.7554/eLife.66609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. DiBenedictis BT, Nussbaum ER, Cheung HK, Veenema AH. Quantitative mapping reveals age and sex differences in vasopressin, but not oxytocin, immunoreactivity in the rat social behavior neural network. J Comp Neurol. 2017;525(11):2549-2570. doi: 10.1002/cne.24216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Steinman MQ, Duque-Wilckens N, Greenberg GD, et al. . Sex-specific effects of stress on oxytocin neurons correspond with responses to intranasal oxytocin. Biol Psychiatry. 2016;80(5):406-414. doi: 10.1016/j.biopsych.2015.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wang Z, Moody K, Newman JD, Insel TR. Vasopressin and oxytocin immunoreactive neurons and fibers in the forebrain of male and female common marmosets (Callithrix jacchus). Synapse. 1997;27(1):14-25. doi: [DOI] [PubMed] [Google Scholar]
- 66. Duque-Wilckens N, Torres LY, Yokoyama S, et al. . Extrahypothalamic oxytocin neurons drive stress-induced social vigilance and avoidance. Proc Natl Acad Sci U S A. 2020;117(42):26406-26413. doi: 10.1073/pnas.2011890117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. De Vries GJ, Rissman EF, Simerly RB, et al. . A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. 2002;22(20):9005-9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. French JA, Taylor JH, Mustoe AC, Cavanaugh J. Neuropeptide diversity and the regulation of social behavior in New World primates. Front Neuroendocrinol. 2016;42:18-39. doi: 10.1016/j.yfrne.2016.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Juul KV, Bichet DG, Nielsen S, Nørgaard JP. The physiological and pathophysiological functions of renal and extrarenal vasopressin V2 receptors. Am J Physiol Renal Physiol. 2014;306(9):F931-F940. doi: 10.1152/ajprenal.00604.2013 [DOI] [PubMed] [Google Scholar]
- 70. Rogers Flattery CN, Coppeto DJ, Inoue K, Rilling JK, Preuss TM, Young LJ. Distribution of brain oxytocin and vasopressin V1a receptors in chimpanzees (Pan troglodytes): comparison with humans and other primate species. Brain Struct Funct. 2022;227(5):1907-1919. doi: 10.1007/s00429-021-02369-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Inoue K, Ford CL, Horie K, Young LJ. Oxytocin receptors are widely distributed in prairie vole (Microtus ochrogaster) brain: relation to social behavior, genetic polymorphisms, and the dopamine system. J Comp Neurol. Published online June 28, 2022. doi: 10.1002/cne.25382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Luo PX, Zakharenkov HC, Torres LY, et al. . Oxytocin receptor behavioral effects and cell types in the bed nucleus of the stria terminalis. Horm Behav. 2022;143:105203. doi: 10.1016/j.yhbeh.2022.105203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wyatt TD. Pheromones and Animal Behaviour. Cambridge University Press; 2003. [Google Scholar]
- 74. Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21(20):8278-8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Oettl LL, Ravi N, Schneider M, et al. . Oxytocin enhances social recognition by modulating cortical control of early olfactory processing. Neuron. 2016;90(3):609-621. doi: 10.1016/j.neuron.2016.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tobin VA, Hashimoto H, Wacker DW, et al. . An intrinsic vasopressin system in the olfactory bulb is involved in social recognition. Nature. 2010;464(7287):413-417. doi: 10.1038/nature08826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Suyama H, Egger V, Lukas M. Top-down acetylcholine signaling via olfactory bulb vasopressin cells contributes to social discrimination in rats. Commun Biol. 2021;4(1):603. doi: 10.1038/s42003-021-02129-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Namba T, Taniguchi M, Murata Y, et al. . Activation of arginine vasopressin receptor 1a facilitates the induction of long-term potentiation in the accessory olfactory bulb of male mice. Neurosci Lett. 2016;634:107-113. doi: 10.1016/j.neulet.2016.09.056 [DOI] [PubMed] [Google Scholar]
- 79. Crawley JN, Chen T, Puri A, et al. . Social approach behaviors in oxytocin knockout mice: comparison of two independent lines tested in different laboratory environments. Neuropeptides. 2007;41(3):145-163. doi: 10.1016/j.npep.2007.02.002 [DOI] [PubMed] [Google Scholar]
- 80. Dölen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501(7466):179-184. doi: 10.1038/nature12518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nardou R, Lewis EM, Rothhaas R, et al. . Oxytocin-dependent reopening of a social reward learning critical period with MDMA. Nature. 2019;569(7754):116-120. doi: 10.1038/s41586-019-1075-9 [DOI] [PubMed] [Google Scholar]
- 82. Hung LW, Neuner S, Polepalli JS, et al. . Gating of social reward by oxytocin in the ventral tegmental area. Science. 2017;357(6358):1406-1411. doi: 10.1126/science.aan4994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Xiao L, Priest MF, Kozorovitskiy Y. Oxytocin functions as a spatiotemporal filter for excitatory synaptic inputs to VTA dopamine neurons. Elife. 2018;7:e33892. doi: 10.7554/eLife.33892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Borland JM, Aiani LM, Norvelle A, et al. . Sex-dependent regulation of social reward by oxytocin receptors in the ventral tegmental area. Neuropsychopharmacology. 2019;44(4):785-792. doi: 10.1038/s41386-018-0262-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Resendez SL, Namboodiri VMK, Otis JM, et al. . Social stimuli induce activation of oxytocin neurons within the paraventricular nucleus of the hypothalamus to promote social behavior in male mice. J Neurosci. 2020;40(11):2282-2295. doi: 10.1523/JNEUROSCI.1515-18.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. He Z, Young L, Ma XM, et al. . Increased anxiety and decreased sociability induced by paternal deprivation involve the PVN-PrL OTergic pathway. Elife. 2019;8:e44026. doi: 10.7554/eLife.44026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Peris J, MacFadyen K, Smith JA, de Kloet AD, Wang L, Krause EG. Oxytocin receptors are expressed on dopamine and glutamate neurons in the mouse ventral tegmental area that project to nucleus accumbens and other mesolimbic targets. J Comp Neurol. 2017;525(5):1094-1108. doi: 10.1002/cne.24116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Duque-Wilckens N, Steinman MQ, Busnelli M, et al. . Oxytocin receptors in the anteromedial bed nucleus of the stria terminalis promote stress-induced social avoidance in female California mice. Biol Psychiatry. 2018;83(3):203-213. doi: 10.1016/j.biopsych.2017.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kelly AM, Goodson JL. Social functions of individual vasopressin-oxytocin cell groups in vertebrates: what do we really know? Front Neuroendocrinol. 2014;35(4):512-529. doi: 10.1016/j.yfrne.2014.04.005 [DOI] [PubMed] [Google Scholar]
- 90. Rigney N, Whylings J, Mieda M, de Vries G, Petrulis A. Sexually dimorphic vasopressin cells modulate social investigation and communication in sex-specific ways. eNeuro. 2019;6(1):ENEURO.0415-18.2019. doi: 10.1523/ENEURO.0415-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rigney N, Zbib A, Vries GJ, Petrulis A. Knockdown of sexually differentiated vasopressin expression in the bed nucleus of the stria terminalis reduces social and sexual behavior in male, but not female, mice. J Neuroendocrinol. Published online December 22, 2021. doi: 10.1111/jne.13083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Islam MT, Maejima T, Matsui A, Mieda M. Paraventricular hypothalamic vasopressin neurons induce self-grooming in mice. Mol Brain. 2022;15(1):47. doi: 10.1186/s13041-022-00932-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Aspesi D, Choleris E. Neuroendocrine underpinning of social recognition in males and females. J Neuroendocrinol. 2021;34(2):e13070. doi: 10.1111/jne.13070 [DOI] [PubMed] [Google Scholar]
- 94. Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron. 2005;47(4):503-513. doi: 10.1016/j.neuron.2005.06.031 [DOI] [PubMed] [Google Scholar]
- 95. Wersinger SR, Temple JL, Caldwell HK, Young WS III. Inactivation of the oxytocin and the vasopressin (Avp) 1b receptor genes, but not the Avp 1a receptor gene, differentially impairs the Bruce effect in laboratory mice (Mus musculus). Endocrinology. 2008;149(1):116-121. doi: 10.1210/en.2007-1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Yao S, Bergan J, Lanjuin A, Dulac C. Oxytocin signaling in the medial amygdala is required for sex discrimination of social cues. Elife. 2017;6:e31373. doi: 10.7554/eLife.31373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zhang L, Hernández VS. Synaptic innervation to rat hippocampus by vasopressin-immuno-positive fibres from the hypothalamic supraoptic and paraventricular nuclei. Neuroscience. 2013;228:139-162. doi: 10.1016/j.neuroscience.2012.10.010 [DOI] [PubMed] [Google Scholar]
- 98. Cui Z, Gerfen CR, Young WS III. Hypothalamic and other connections with dorsal CA2 area of the mouse hippocampus. J Comp Neurol. 2013;521(8):1844-1866. doi: 10.1002/cne.23263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Smith AS, Williams Avram SK, Cymerblit-Sabba A, Song J, Young WS. Targeted activation of the hippocampal CA2 area strongly enhances social memory. Mol Psychiatry. 2016;21(8):1137-1144. doi: 10.1038/mp.2015.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Tirko NN, Eyring KW, Carcea I, et al. . Oxytocin transforms firing mode of CA2 hippocampal neurons. Neuron. 2018;100(3):593-608.e3. doi: 10.1016/j.neuron.2018.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Raam T, McAvoy KM, Besnard A, Veenema AH, Sahay A. Hippocampal oxytocin receptors are necessary for discrimination of social stimuli. Nat Commun. 2017;8(1):2001. doi: 10.1038/s41467-017-02173-0 Erratum in: Nat Commun. 2018;9(1):552. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5722862/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Boyle L, Posani L, Irfan S, Siegelbaum SA, Fusi S. The geometry of hippocampal CA2 representations enables abstract coding of social familiarity and identity. bioRXiv. doi: 10.1101/2022.01.24.477361. [DOI] [Google Scholar]
- 103. Cymerblit-Sabba A, Smith AS, Williams Avram SK, et al. . Inducing partner preference in mice by chemogenetic stimulation of CA2 hippocampal subfield. Front Mol Neurosci. 2020;13:61. doi: 10.3389/fnmol.2020.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ferretti V, Maltese F, Contarini G, et al. . Oxytocin signaling in the central amygdala modulates emotion discrimination in mice. Curr Biol. 2019;29(12):1938-1953.e6. doi: 10.1016/j.cub.2019.04.070 [DOI] [PubMed] [Google Scholar]
- 105. Anpilov S, Shemesh Y, Eren N, et al. . Wireless optogenetic stimulation of oxytocin neurons in a semi-natural setup dynamically elevates both pro-social and agonistic behaviors. Neuron. 2020;107(4):644-655.e7. doi: 10.1016/j.neuron.2020.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Cilz NI, Cymerblit-Sabba A, Young WS. Oxytocin and vasopressin in the rodent hippocampus. Genes Brain Behav. 2019;18(1):e12535. doi: 10.1111/gbb.12535 [DOI] [PubMed] [Google Scholar]
- 107. Watanabe J, Takayanagi Y, Yoshida M, et al. . Conditional ablation of vasopressin-synthesizing neurons in transgenic rats. J Neuroendocrinol. 2021;33(12):e13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Whylings J, Rigney N, Peters NV, de Vries GJ, Petrulis A. Sexually dimorphic role of BNST vasopressin cells in sickness and social behavior in male and female mice. Brain Behav Immun. 2020;83:68-77. doi: 10.1016/j.bbi.2019.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Veenema AH, Bredewold R, De Vries GJ. Vasopressin regulates social recognition in juvenile and adult rats of both sexes, but in sex- and age-specific ways. Horm Behav. 2012;61(1):50-56. doi: 10.1016/j.yhbeh.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Borie AM, Dromard Y, Guillon G, et al. . Correction of vasopressin deficit in the lateral septum ameliorates social deficits of mouse autism model. J Clin Invest. 2021;131(2):e144450. doi: 10.1172/JCI144450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ferris CF, Albers HE, Wesolowski SM, Goldman BD, Luman SE. Vasopressin injected into the hypothalamus triggers a stereotypic behavior in golden hamsters. Science. 1984;224(4648):521-523. doi: 10.1126/science.6538700 [DOI] [PubMed] [Google Scholar]
- 112. Albers HE, Hennessey AC, Whitman DC. Vasopressin and the regulation of hamster social behavior. Ann N Y Acad Sci. 1992;652:227-242. doi: 10.1111/j.1749-6632.1992.tb34358.x [DOI] [PubMed] [Google Scholar]
- 113. Ferris CF, Albers HE, Wesolowski SM, Goldman BD, Luman SE. Vasopressin injected into the hypothalamus triggers a stereotypic behavior in golden hamsters. Science. 1984;224(4648):521-523. doi: 10.1126/science.6538700 [DOI] [PubMed] [Google Scholar]
- 114. Terranova JI, Ferris CF, Albers HE. Sex differences in the regulation of offensive aggression and dominance by arginine-vasopressin. Front Endocrinol. 2017;8:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hurst JL, Beynon RJ. Scent wars: the chemobiology of competitive signalling in mice. Bioessays. 2004;26(12):1288-1298. doi: 10.1002/bies.20147 [DOI] [PubMed] [Google Scholar]
- 116. Ferris CF, Melloni RH Jr, Koppel G, Perry KW, Fuller RW, Delville Y. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J Neurosci. 1997;17(11):4331-4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Gutzler SJ, Karom M, Erwin WD, Albers HE. Arginine-vasopressin and the regulation of aggression in female Syrian hamsters (Mesocricetus auratus). Eur J Neurosci. 2010;31(9):1655-1663. doi: 10.1111/j.1460-9568.2010.07190.x [DOI] [PubMed] [Google Scholar]
- 118. Taylor JH, Walton JC, McCann KE, et al. . CRISPR-Cas9 editing of the arginine-vasopressin V1a receptor produces paradoxical changes in social behavior in Syrian hamsters. Proc Natl Acad Sci U S A. 2022;119(19):e2121037119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ho JM, Murray JH, Demas GE, Goodson JL. Vasopressin cell groups exhibit strongly divergent responses to copulation and male-male interactions in mice. Horm Behav. 2010;58(3):368-377. doi: 10.1016/j.yhbeh.2010.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Baum MJ, Vreeburg JT. Copulation in castrated male rats following combined treatment with estradiol and dihydrotestosterone. Science. 1973;182(4109):283-285. doi: 10.1126/science.182.4109.283 [DOI] [PubMed] [Google Scholar]
- 121. de Vries GJ, Buijs RM, Sluiter AA. Gonadal hormone actions on the morphology of the vasopressinergic innervation of the adult rat brain. Brain Res. 1984;298(1):141-145. doi: 10.1016/0006-8993(84)91157-0 [DOI] [PubMed] [Google Scholar]
- 122. Valtcheva S, Froemke RC. Neuromodulation of maternal circuits by oxytocin. Cell Tissue Res. 2019;375(1):57-68. doi: 10.1007/s00441-018-2883-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Marlin BJ, Mitre M, D’amour JA, Chao MV, Froemke RC. Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature. 2015;520(7548):499-504. doi: 10.1038/nature14402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Schiavo JK, Valtcheva S, Bair-Marshall CJ, Song SC, Martin KA, Froemke RC. Innate and plastic mechanisms for maternal behaviour in auditory cortex. Nature. 2020;587(7834):426-431. doi: 10.1038/s41586-020-2807-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Carcea I, Caraballo NL, Marlin BJ, et al. . Oxytocin neurons enable social transmission of maternal behaviour. Nature. 2021;596(7873):553-557. doi: 10.1038/s41586-021-03814-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. He Z, Zhang L, Hou W, et al. . Paraventricular nucleus oxytocin subsystems promote active paternal behaviors in mandarin voles. J Neurosci. 2021;41(31):6699-6713. doi: 10.1523/JNEUROSCI.2864-20.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Zheng JJ, Li SJ, Zhang XD, et al. . Oxytocin mediates early experience-dependent cross-modal plasticity in the sensory cortices. Nat Neurosci. 2014;17(3):391-399. doi: 10.1038/nn.3634 [DOI] [PubMed] [Google Scholar]
- 128. Feng T, An S, Kinden R, Zhang X, Jia R, Tai F. Alteration in oxytocin levels induced by early social environment affects maternal behavior and estrogen receptor alpha in mandarin voles (Microtus mandarinus). Behav Brain Res. 2019;365:36-47. doi: 10.1016/j.bbr.2019.02.038 [DOI] [PubMed] [Google Scholar]
- 129. Li T, Jia SW, Hou D, et al. . Intranasal oxytocin restores maternal behavior and oxytocin neuronal activity in the supraoptic nucleus in rat dams with cesarean delivery. Neuroscience. 2021;468:235-246. doi: 10.1016/j.neuroscience.2021.06.020 [DOI] [PubMed] [Google Scholar]
- 130. Baracz SJ, Everett NA, Robinson KJ, Campbell GR, Cornish JL. Maternal separation changes maternal care, anxiety‐like behaviour and expression of paraventricular oxytocin and corticotrophin‐releasing factor immunoreactivity in lactating rats. J Neuroendocrinol. 2020;32(6):e12861. doi: 10.1111/jne.12861 [DOI] [PubMed] [Google Scholar]
- 131. Cutuli D, Berretta E, Caporali P, et al. . Effects of pre-reproductive maternal enrichment on maternal care, offspring’s play behavior and oxytocinergic neurons. Neuropharmacology. 2019;145(Pt A):99-113. doi: 10.1016/j.neuropharm.2018.02.015 [DOI] [PubMed] [Google Scholar]
- 132. Pedersen CA, Ascher JA, Monroe YL, Prange AJ Jr. Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216(4546):648-650. doi: 10.1126/science.7071605 [DOI] [PubMed] [Google Scholar]
- 133. Bendesky A, Kwon YM, Lassance JM, et al. . The genetic basis of parental care evolution in monogamous mice. Nature. 2017;544(7651):434-439. doi: 10.1038/nature22074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Bayerl DS, Bosch OJ. Brain vasopressin signaling modulates aspects of maternal behavior in lactating rats. Genes Brain Behav. 2019;18(1):e12517. doi: 10.1111/gbb.12517 [DOI] [PubMed] [Google Scholar]
- 135. Wang Z, Ferris CF, De Vries GJ. Role of septal vasopressin innervation in paternal behavior in prairie voles (Microtus ochrogaster). Proc Natl Acad Sci U S A. 1994;91(1):400-404. doi: 10.1073/pnas.91.1.400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Bosch OJ, Neumann ID. Brain vasopressin is an important regulator of maternal behavior independent of dams’ trait anxiety. Proc Natl Acad Sci U S A. 2008;105(44):17139-17144. doi: 10.1073/pnas.0807412105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Johnson ZV, Walum H, Jamal YA, et al. . Central oxytocin receptors mediate mating-induced partner preferences and enhance correlated activation across forebrain nuclei in male prairie voles. Horm Behav. 2016;79:8-17. doi: 10.1016/j.yhbeh.2015.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Keebaugh AC, Barrett CE, Laprairie JL, Jenkins JJ, Young LJ. RNAi knockdown of oxytocin receptor in the nucleus accumbens inhibits social attachment and parental care in monogamous female prairie voles. Soc Neurosci. 2015;10(5):561-570. doi: 10.1080/17470919.2015.1040893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Keebaugh AC, Young LJ. Increasing oxytocin receptor expression in the nucleus accumbens of pre-pubertal female prairie voles enhances alloparental responsiveness and partner preference formation as adults. Horm Behav. 2011;60(5):498-504. doi: 10.1016/j.yhbeh.2011.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Borie AM, Agezo S, Lunsford P, et al. . Social experience alters oxytocinergic modulation in the nucleus accumbens of female prairie voles. Curr Biol. 2022;32(5):1026-1037.e4. doi: 10.1016/j.cub.2022.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Ross HE, Cole CD, Smith Y, et al. . Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009;162(4):892-903. doi: 10.1016/j.neuroscience.2009.05.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Barrett CE, Arambula SE, Young LJ. The oxytocin system promotes resilience to the effects of neonatal isolation on adult social attachment in female prairie voles. Transl Psychiatry. 2015;5:e606. doi: 10.1038/tp.2015.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Ahern TH, Olsen S, Tudino R, Beery AK. Natural variation in the oxytocin receptor gene and rearing interact to influence reproductive and nonreproductive social behavior and receptor binding. Psychoneuroendocrinology. 2021;128:105209. doi: 10.1016/j.psyneuen.2021.105209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. King LB, Walum H, Inoue K, Eyrich NW, Young LJ. Variation in the oxytocin receptor gene predicts brain region-specific expression and social attachment. Biol Psychiatry. 2016;80(2):160-169. doi: 10.1016/j.biopsych.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Scheele D, Wille A, Kendrick KM, et al. . Oxytocin enhances brain reward system responses in men viewing the face of their female partner. Proc Natl Acad Sci U S A. 2013;110(50):20308-20313. doi: 10.1073/pnas.1314190110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Pohl TT, Young LJ, Bosch OJ. Lost connections: oxytocin and the neural, physiological, and behavioral consequences of disrupted relationships. Int J Psychophysiol. 2019;136:54-63. doi: 10.1016/j.ijpsycho.2017.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kitano K, Yamagishi A, Horie K, Nishimori K, Sato N. Helping behavior in prairie voles: a model of empathy and the importance of oxytocin. iScience. 2022;25(4):103991. doi: 10.1016/j.isci.2022.103991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Donaldson ZR, Spiegel L, Young LJ. Central vasopressin V1a receptor activation is independently necessary for both partner preference formation and expression in socially monogamous male prairie voles. Behav Neurosci. 2010;124(1):159-163. doi: 10.1037/a0018094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7(10):1048-1054. doi: 10.1038/nn1327 [DOI] [PubMed] [Google Scholar]
- 150. Barrett CE, Keebaugh AC, Ahern TH, Bass CE, Terwilliger EF, Young LJ. Variation in vasopressin receptor (Avpr1a) expression creates diversity in behaviors related to monogamy in prairie voles. Horm Behav. 2013;63(3):518-526. doi: 10.1016/j.yhbeh.2013.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Donaldson ZR, Young LJ. The relative contribution of proximal 5′ flanking sequence and microsatellite variation on brain vasopressin 1a receptor (Avpr1a) gene expression and behavior. PLoS Genet. 2013;9(8):e1003729. doi: 10.1371/journal.pgen.1003729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Lim MM, Wang Z, Olazábal DE, Ren X, Terwilliger EF, Young LJ. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004;429(6993):754-757. doi: 10.1038/nature02539 [DOI] [PubMed] [Google Scholar]
- 153. Hammock EAD, Young LJ. Microsatellite instability generates diversity in brain and sociobehavioral traits. Science. 2005;308(5728):1630-1634. doi: 10.1126/science.1111427 [DOI] [PubMed] [Google Scholar]
- 154. Young LJ, Nilsen R, Waymire KG, MacGregor GR, Insel TR. Increased affiliative response to vasopressin in mice expressing the V1a receptor from a monogamous vole. Nature. 1999;400(6746):766-768. doi: 10.1038/23475 [DOI] [PubMed] [Google Scholar]
- 155. Phelps SM, Young LJ. Extraordinary diversity in vasopressin (V1a) receptor distributions among wild prairie voles (Microtus ochrogaster): patterns of variation and covariation. J Comp Neurol. 2003;466(4):564-576. doi: 10.1002/cne.10902 [DOI] [PubMed] [Google Scholar]
- 156. Okhovat M, Berrio A, Wallace G, Ophir AG, Phelps SM. Sexual fidelity trade-offs promote regulatory variation in the prairie vole brain. Science. 2015;350(6266):1371-1374. doi: 10.1126/science.aac5791 [DOI] [PubMed] [Google Scholar]
- 157. Gemmer A, Mirkes K, Anneser L, et al. . Oxytocin receptors influence the development and maintenance of social behavior in zebrafish (Danio rerio). Sci Rep. 2022;12(1):4322. doi: 10.1038/s41598-022-07990-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Nunes AR, Gliksberg M, Varela SAM, et al. . Developmental effects of oxytocin neurons on social affiliation and processing of social information. J Neurosci. 2021;41(42):8742-8760. doi: 10.1523/JNEUROSCI.2939-20.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Lewis EM, Stein-O’Brien GL, Patino AV, et al. . Parallel social information processing circuits are differentially impacted in autism. Neuron. 2020;108(4):659-675.e6. doi: 10.1016/j.neuron.2020.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Romanov RA, Zeisel A, Bakker J, et al. . Molecular interrogation of hypothalamic organization reveals distinct dopamine neuronal subtypes. Nat Neurosci. 2017;20(2):176-188. doi: 10.1038/nn.4462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Hörnberg H, Pérez-Garci E, Schreiner D, et al. . Rescue of oxytocin response and social behaviour in a mouse model of autism. Nature. 2020;584(7820):252-256. doi: 10.1038/s41586-020-2563-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Modi ME, Inoue K, Barrett CE, et al. . Melanocortin receptor agonists facilitate oxytocin-dependent partner preference formation in the prairie vole. Neuropsychopharmacology. 2015;40(8):1856-1865. doi: 10.1038/npp.2015.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Rae M, Lemos Duarte M, Gomes I, Camarini R, Devi LA. Oxytocin and vasopressin: signalling, behavioural modulation and potential therapeutic effects. Br J Pharmacol. 2022;179(8):1544-1564. doi: 10.1111/bph.15481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Jacob S, Veenstra-VanderWeele J, Murphy D, et al. . Efficacy and safety of balovaptan for socialisation and communication difficulties in autistic adults in North America and Europe: a phase 3, randomised, placebo-controlled trial. Lancet Psychiatry. 2022;9(3):199-210. doi: 10.1016/S2215-0366(21)00429-6 [DOI] [PubMed] [Google Scholar]
- 165. Bolognani F, Del Valle Rubido M, Squassante L, et al. . A phase 2 clinical trial of a vasopressin V1a receptor antagonist shows improved adaptive behaviors in men with autism spectrum disorder. Sci Transl Med. 2019;11(491):eaat7838. doi: 10.1126/scitranslmed.aat7838 [DOI] [PubMed] [Google Scholar]
- 166. Parker KJ, Garner JP, Oztan O, et al. . Arginine vasopressin in cerebrospinal fluid is a marker of sociality in nonhuman primates. Sci Transl Med. 2018;10(439):eaam9100. doi: 10.1126/scitranslmed.aam9100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Oztan O, Garner JP, Constantino JN, Parker KJ. Neonatal CSF vasopressin concentration predicts later medical record diagnoses of autism spectrum disorder. Proc Natl Acad Sci U S A. 2020;117(19):10609-10613. doi: 10.1073/pnas.1919050117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Oztan O, Garner JP, Partap S, et al. . Cerebrospinal fluid vasopressin and symptom severity in children with autism. Ann Neurol. 2018;84(4):611-615. doi: 10.1002/ana.25314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Parker KJ, Oztan O, Libove RA, et al. . A randomized placebo-controlled pilot trial shows that intranasal vasopressin improves social deficits in children with autism. Sci Transl Med. 2019;11(491):eaau7356. doi: 10.1126/scitranslmed.aau7356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Aspé-Sánchez M, Moreno M, Rivera MI, Rossi A, Ewer J. Oxytocin and vasopressin receptor gene polymorphisms: role in social and psychiatric traits. Front Neurosci. 2015;9:510. doi: 10.3389/fnins.2015.00510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Bosch OJ, Young LJ. Oxytocin and social relationships: from attachment to bond disruption. Curr Top Behav Neurosci. 2018;35:97-117. doi: 10.1007/7854_2017_10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Naderi A, Soltanmaohammadi E, Kaza V, Barlow S, Chatzistamou I, Kiaris H. Persistent effects of pair bonding in lung cancer cell growth in monogamous Peromyscus californicus. Elife. 2021;10:e64711. doi: 10.7554/eLife.64711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Ford CL, Young LJ. Harnessing the healing power of love. Trends Mol Med. 2021;27(9):833-834. doi: 10.1016/j.molmed.2021.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Johnson ZV, Young LJ. Evolutionary diversity as a catalyst for biological discovery. Integr Zool. 2018;13(6):616-633. doi: 10.1111/1749-4877.12339 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.