Abstract
Myrciaria cauliflora is a functional food rich in anthocyanins, possessing antioxidative and anti-inflammatory properties. Our previous results demonstrated M. cauliflora extract (MCE) had beneficial effects in diabetic nephropathy (DN) and via the inhibition of Ras/PI3K/Akt and kidney fibrosis-related proteins. The purpose of this study was to assess the benefit of MCE in diabetes associated with kidney inflammation and glycemic regulation in streptozotocin–nicotinamide (STZ/NA)-induced diabetic mice. Compared with the untreated diabetic group, MCE significantly improved blood glucose and serum biochemical characteristic levels. Exposure to MCE increased antioxidative enzyme activity and diminished reactive oxygen synthesis. Mice receiving MCE supplementation had reduced intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), monocyte chemoattractant protein 1 (MCP-1), colony stimulating factor 1 (CSF-1), interleukin-1β (IL-1β), IL-6 and tumor necrosis factor α (TNF-α) levels compared to the untreated diabetic mice. Inflammatory and fibrotic related proteins such as collagen IV, fibronectin, Janus kinase (JAK), phosphorylated signal transducer and activator of transcription 3 (STAT3), protein kinase C beta (PKC-β), and nuclear factor kappa B (NF-κB) were also inhibited by MCE treatment in STZ/NA mice. These results suggest that MCE may be used as a hypoglycemic agent and antioxidant in Type 2 diabetic mice.
Keywords: diabetic nephropathy, inflammation, Myrciaria cauliflora extract (MCE), oxidative stress
1. Introduction
Diabetes mellitus (DM) is a chronic metabolic disorder characterized by hyperglycemia. Diabetic nephropathy (DN), a diabetic complication, is the leading cause of end-stage renal disease and is a growing global health problem [1,2]. Despite developments in pharmacological strategies to modulate diabetes, DN remains a major microvascular complication in many patients with diabetes. However, there is still insufficient understanding of the full mechanism involved in progressive diabetic renal disease.
Persistent hyperglycemia is accountable for the damages of various organs and tissues in diabetic patients. However, diabetic complications are not merely due to hyperglycemia. Previous investigations from DN patients and animals have indicated that oxidative stress and inflammation induced by hyperglycemia and dyslipidemia play significant roles in the development of vascular complications such as cardiomyopathy and nephropathy [3–7]. Hyperglycemia in diabetic patients leads to mitochondrial dysfunction, advanced glycation end processes and other factors, and generate the reactive free radicals, then triggers the fragmentation of DNA, which results in apoptotic cell death [8,9]. Hyperglycemia also causes oxidative stress, decreases the regeneration of glutathione (GSH) from oxidized GSH and reduces the availability of nicotinamide adenine dinucleotide phosphate [10,11]. These superactive radicals may also interfere with the expressions of several transcription proteins such as nuclear factor kappa B (NF-κB), protein kinases C (PKCs), and caspases [3]. On the other hand, the development of DN is associated with significant inflammatory cell infiltration and increased plasma levels of C-reactive protein and inflammatory cytokines, such as vascular cell adhesion molecule-1 (VCAM-1), tumor necrosis factor α (TNF-α), interleukin 6 (IL-6) and IL-1β [3,12,13]. Oxidative stress and inflammation are inseparably linked, as each begets and amplifies the other. Therefore, it is important to counteract diabetic pathophysiology through multitargeted therapeutic agents. Hence, therapies targeting oxidative stress and inflammation may effectively preserve normal renal function and prevent or slow the progression of DN.
Many functional foods have been identified as effective therapeutic agents for metabolic disorders such as obesity, diabetes, and its complications [14]. Polyphenol-rich foods have been recognized as important dietary sources of antioxidants for human health. They have also attracted attention as functional foods with various bioactivities, for instance, antiviral, antimicrobial, antimutagenic and anticancer activities [15–17]. Jaboticaba (Myrciaria cauliflora) is a native fruit with interesting nutritional properties from the Brazilian Atlantic Forest, and is rich in minerals, fibers, and phenolics, especially anthocyanins [18–20]. Jaboticaba is reported in popular medicine as an astringent, and is useful against diarrhea, skin irritations, gut inflammation, increasing high-density lipoprotein, and improving insulin resistance in rats in a diet-induced obesity model [19–21]. We have determined the effects of MCE intake in streptozotocin–nicotinamide (STZ/NA) mice fed a high-fat diet recently [22]. This study is to further evaluate if MCE could offer a prophylactic role against DN through inhibiting oxidative stress and inflammatory response.
2. Method
2.1. Preparation of MCE
The lyophilized fruit of M. cauliflora Berg from the Modern Garden Jabuticaba Co. Ltd, Changhua, Taiwan (100 g) were macerated and stirred with water (100 mL), and the juice was then filtered and centrifuged (10,000g, 15 min). The MCE was obtained after being filtered and concentrated under reduced pressure at 30°C and stored at −20°C before use.
2.2. Experimental animals
All procedures involving animals were approved by the guidelines of IACUC (Institutional Animal Care and Use Committee) of CSMU (Animal Center of Chung Shan Medical University). Five- to six-week male C57BL/6 mice were acclimatized for 7 days, then randomly divided into nondiabetic and diabetic groups, and allowed to take food and water ad libitum before any food-related treatment began. Non-obese Type 2 diabetes mice were fasted for 16 hours before receiving the chemicals. STZ dissolved in a 50 mM citric acid buffer was administered at 100 mg/kg by intraperitoneal injection twice on Days 0 and 2. NA was dissolved in saline and administered at 240 mg/kg by intraperitoneal injection 15 minutes before the administration of STZ on Day 1. The nondiabetic mice were injected with citrate buffer or saline alone. A modified high-fat diet (5 g/day per mouse) was used to induce Type 2 diabetes in the STZ/NA mice [23]. Animals were divided into five groups of 10 mice, each as follows:
Control group: Normal mice were given a control diet daily for 8 weeks.
STZ/NA group: STZ/NA treated mice were given a high-fat diet daily for 8 weeks.
STZ/NA + 0.1% MCE group: STZ/NA treated mice were given a high-fat diet daily with 0.1 % MCE for 8 weeks.
STZ/NA + 0.5% MCE group: STZ/NA treated mice were given a high-fat diet daily with 0.5 % MCE for 8 weeks.
STZ/NA + 1.0% MCE group: STZ/NA treated mice were given a high-fat diet daily with 1.0 % MCE for 8 weeks.
At the end of 8 weeks of treatment, mice were put into clean metabolic cages 1 day before sacrifice, and urine was collected for 24 hours. Then, the mice were sacrificed and the kidney, blood, and urine were isolated for analysis.
2.3. Kidney biochemical analysis
The kidney was excised, cleaned and washed with ice-cold saline (pH 7.4). It was then homogenized in Tris–HCl (0.1 M)-EDTA buffer (pH 7.4, 0.001 M) and centrifuged at 12,000g for 30 min at 4°C, and the supernatant was collected to detect the oxidative stress. The intracellular reactive oxygen species (ROS) production was measured modified by the method of LeBel and Bondy [24]. 2′,7′-Dichloro-fluorescein formation which was oxidized in the presence of ROS was measured by fluorescence spectrometer (Hitachi; Tokyo, Japan) set at an excitation wavelength of 488 nm and emission wavelength of 510 nm. o-Phthalaldehyde was used as a reagent for a fluorometric assay of GSH. After incubating the sample with o-phthalaldehyde at room temperature for 15 minutes, the solution was transferred to a quartz cuvette. Fluorescence at 420 nm was determined with the activation at 350 nm. Activity of antioxidant enzymes such as catalase (CAT), superoxide dismutase, and glutathione peroxidase was estimated by the methods reported by Ghosh et al [25].
2.4. Assay of inflammatory cytokines
Concentrations of inflammatory cytokines (IL-1β, IL-6, TNF-α) in renal tissue samples were analyzed by enzyme-linked immunosorbent assay. A SpectraMax Plus 384 Microplate Reader (Molecular Devices LLC; California, USA) was used for the measurement of optical density.
2.5. RNA isolation and real-time quantitative polymerase chain reaction (RT-PCR)
Total RNA was isolated from kidney tissues using an RNA isolation kit (Ultraspec, Biotecx Laboratories; Houston, TX, USA) according to the manufacturer’s protocol and was quantified spectrophotometrically by measuring the absorbance of an aliquot at 260 nm. The RNA samples were reverse transcribed into cDNA using a High-capacity cDNA reverse transcription kit (PE Applied Biosystems; Foster City, CA, USA). The primers used for RT-PCR were as in Table 1. RT-PCR was carried out in triplicate using the GeneAmp PCR System 2700 (Applied Biosystems). The amount of mRNA was calculated by the comparative CT method, which depends on the ratio of the amount of target genes to reference gene β-actin.
Table 1.
Sequences of reverse transcription-polymerase chain reaction primers.
| Gene Name | Sequence |
|---|---|
| ICAM-1 | Forward, 5′-GTGATGGCAGCCTCTTATGT-3′ |
| Reverse, 5′-GGGCTTGTCCCTTGAGTTT-3′ | |
| VCAM-1 | Forward, 5′-GATACAACCGTCTTGGTCAGCCC-3′ |
| Reverse, 5′-CAGTTGAAGGATGCGGGAGTATATG-3′ | |
| MCP-1 | Forward, 5′-CCACTCACCTGCTGCTACTCAT-3′ |
| Reverse, 5′-TGGTGATCCTCTTGTAGCTCTCC-3′ | |
| CSF-1 | Forward, 5′-GCT GTTGTTGGTCTGTCTC-3′ |
| Reverse, 5′-CATGCTCTTCATAATCCTT G-3′ | |
| TNF-α | Forward, 5′-CTACCTTGTTGCCTCCTCTTT-3′ |
| Reverse, 5′-GAGCAGAGGTTCAGTGATGTAG-3′ | |
| IL-1β | Forward, 5′-AACCTGCTGGTGTGTGACGTTC-3′ |
| Reverse, 5′-CAGCACGAGGCTTTTTTGTTGT-3′ | |
| IL-6 | Forward, 5′-ACAACCACGGCCTTCCCTACTT-3′ |
| Reverse, 5′-CACGATTTCCCAGAGAACATGTG-3′ | |
| β-Actin | Forward, 5′-AGGTATCCTGA CCCTGAAGTA-3′ |
| Reverse, 5′-CACACGCAGCTCATTGTAGA-3′ |
2.6. Immunohistochemistry for CD68 detection
Kidneys were fixed in 4% paraformaldehyde solution and embedded in paraffin. After deparaffinization and rehydration, 5-μm kidney sections were treated with 3% H2O2 for 10 minutes and with 1% bovine serum albumin in phosphate buffered saline for 30 minutes. These samples were incubated overnight at 4°C with anti-CD68 antibody (1:200) and then incubated with secondary antibody for 1 hour at room temperature. After staining the nucleus with hematoxylin for 5 minutes, the images were viewed under a Nikon Eclipse E600 microscopy system (400× amplification; Nikon Instruments Inc., Melville, NY, USA). Score of CD68 was determined by quantitative image analysis software (Pax-it, Paxcam; Villa Park, IL, USA).
2.7. Western blotting assay
Renal tissues were homogenized in lysis buffer. Proteins were collected by centrifugation at 12,000g and 4°C. The total protein samples were run on 8–12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred onto nitro-cellulose membranes and incubated in blocking buffer (5% milk and 0.5% bovine serum albumin) for 1 hour at room temperature and then incubated overnight at 4°C with the primary antibodies. After three washes with Tris-buffered saline containing 0.05% Tween 20 (TBST), membranes were incubated with secondary horseradish peroxidase-conjugated antibody for 1 hour at room temperature. Antigen–antibody complexes were then developed with an electrochemiluminescence kit (Millipore, St. Louis, Missouri, USA) and analyzed using AlphaImager Series 2200 software (Alpha Innotech; San Leandro, CA, USA).
2.8. Statistical analysis
Data was collected from 10 mice in each group, and the results are presented as means ± SD. The difference among the three groups (control, STZ/NA-induced, and MCE groups) was analyzed using one-way ANOVA. The difference between the two groups was analyzed using the Student t-test performed with Sigmaplot software (Microsoft Windows, version 12). Statistical significance was detected at the 0.05 level.
3. Results
3.1. MCE prevented kidney oxidative stress in STZ/NA mice
Oxidative stress is considered as the main pathogenesis of DN closely associated with hyperglycemia and hyperlipidemia [4]. We also examined the biochemical characteristics of STZ/NA mice and the antihyperglycemic and antihyperlipidemic effects of MCE were similar to our previous study (data not shown) [22]. To demonstrate the antioxidant effect of MCE against renal damage, oxidative damage in the kidney was assessed and showed in Table 2. The ROS production in the STZ/NA group was found to be significantly higher than in the control group. The first line of cellular defense against oxidative stress including thiol-base antioxidant system (GSH) and antioxidant enzymes such as catalase, superoxide dismutase, glutathione-S-transferase and glutathione peroxidase were measured here [26,27]. In this study, STZ/NA-induced mice were showed an obvious depletion in GSH and antioxidant enzymes in renal tissue. Excessive ROS generation decreases the activities of these enzymes in Type 2 diabetes. Importantly, treatment with MCE reduced oxidant damage in the kidneys of STZ/NA mice. The extract may have a prophylactic effect against oxidative stress and associated renal dysfunction of Type 2 diabetes.
Table 2.
Antioxidant effect of MCE on serum biochemical characteristics of STZ/NA mice.
| Control | STZ/NA | STZ/NA+ 1. MCE | STZ/NA+ 1. MCE | STZ/NA+ 2. MCE | |
|---|---|---|---|---|---|
| ROS production (nmol/min/mg of protein) | 35.54 ± 2.25 | 78.24 ± 4.12** | 68.27 ± 5.35*** | 45.98 ± 3.79**** | 38.79 ± 1.22**** |
| GSH (mg/g of tissue) | 23.24 ± 2.11 | 14.87 ± 1.05* | 16.58 ± 1.97 | 19.87 ± 1.78*** | 23.98 ± 2.03*** |
| CAT (U/mg of protein) | 234.22 ± 15.79 | 144.87 ± 12.30* | 155.67 ± 14.46 | 188.89 ± 12.58*** | 220.38 ± 13.69*** |
| SOD (U/mg of protein) | 145.94 ± 6.58 | 91.66 ± 5.69* | 98.94 ± 4.56 | 110.94 ± 5.65 | 139.68 ± 9.15*** |
| GST (μmol/min/mg of protein) | 0.82 ± 0.08 | 0.44 ± 0.04* | 0.53 ± 0.05 | 0.66 ± 0.03*** | 0.80 ± 0.07*** |
| GPx (nmol/min/mg of protein) | 69.39 ± 5.25 | 39.67 ± 4.31* | 43.25 ± 3.21 | 56.49 ± 5.26*** | 68.24 ± 4.92*** |
CAT = catalase; GSH = glutathione; GST = glutathione-S-transferase; GPx = glutathione peroxidase; MCE = Myrciaria cauliflora extract; ROS = reactive oxygen species; SOD = superoxide.
Data are presented as mean ± SD; n = 10 mice per group.
p < 0.05,
p < 0.001 significant difference compared to the control group determined by Student t test.
p < 0.05,
p < 0.001 significant difference compared to the STZ/NA group determined by ANOVA.
3.2. MCE reduced the inflammatory gene expression and inflammatory cell infiltration in the kidney of STZ/NA mice
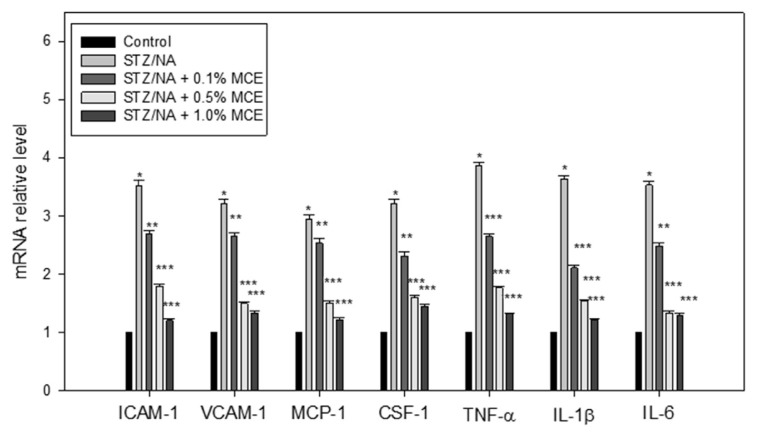
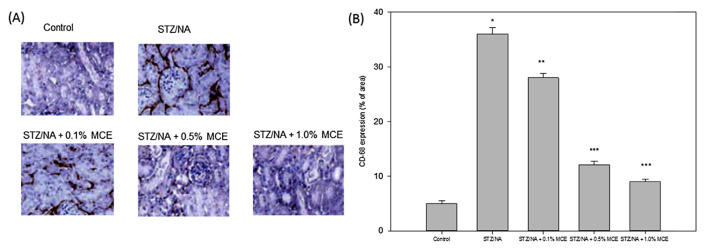
In addition to oxidative stress, hyperglycemia and hyperlipidemia in Type 2 diabetes induce renal damage associated with the severe inflammation characterized by the release of various inflammatory factors. Up-regulated ICAM-1 (Icam1), VCAM-1 (Vcam1), MCP-1 (Mcp-1), CSF-1 (Csf-1), TNF-α (Tnf-α), IL-1β (Il-1β) and IL-6 (Il-6) expression were found in STZ/NA mice. The increased mRNA level in these factors was suppressed by treatment with MCE (Fig. 1). Immunohistochemical staining for CD68 was assayed in kidney tissue samples. STZ/NA mice showed increased infiltration of CD68-positive (CD68+) macrophages in interstitial areas and no macrophage was found in the control kidney. MCE reduced renal CD68+ macrophage infiltration in STZ/NA mice (Fig. 2).
Fig. 1.
MCE prevented activation of pro-inflammatory factors in the kidneys of STZ/NA mice. The mRNA expression of ICAM-1, VCAM-1, MCP-1, CSF-1, TNFα, IL-1β, and IL-6 was analyzed using RT-PCR. * p < 0.001 versus control group; ** p < 0.05, *** p < 0.001 versus STZ/NA group.
Fig. 2.
MCE reduced the macrophage infiltration in STZ/NA mice kidney biopsies. The mice were sacrificed, and the kidney tissues in each group were collected for the detection of macrophage infiltration by CD68 staining (400×) (A) and quantified (B). The data were representative of three independent experiments. * p < 0.001 versus control group; ** p < 0.05, *** p < 0.001 versus STZ/NA group.
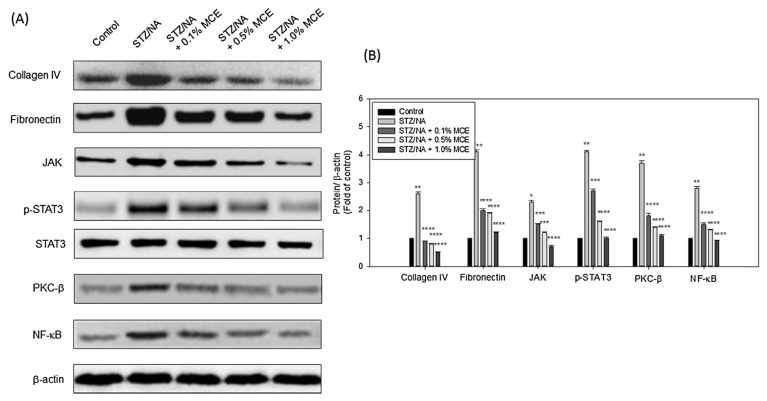
3.3. Treatment with MCE modulated the renal fibrosis of STZ/NA mice
NF-κB plays an integral role in the regulation of various inflammatory and immune responses to trigger renal fibrosis [28]. Treatment with MCE ameliorated renal fibrosis in STZ/NA-induced mice by reducing the collagen IV synthesis and fibronectin formation, as demonstrated in our previously research [22]. In this study, a similar result was shown (Fig. 3). In addition, the JAK/STAT signaling pathway constitutes one of the primary signaling pathways that regulate cytokine expression, abnormal matrix synthesis, and has increasingly been implicated in the pathophysiology of renal disease [29]. Under MCE treatment, JAK and phosphorylated STAT3 were reduced in STZ/NA-induced mice significantly. Earlier studies have reported that protein kinase C (PKC) enzymes mediated the DN by activating the expression of NF-κB under oxidative stress [30,31]. In summary, MCE also could improve the PKC/NF-κB related inflammation in STZ/NA mice.
Fig. 3.
MCE inhibited the fibrosis and inflammatory protein expression in STZ/NA mice kidney. After the mice were sacrificed, the protein expression was detected by Western blotting (A) and quantified by densitometry (B). The data were representative of three independent experiments. * p < 0.05, ** p < 0.001 versus control group; *** p < 0.05, **** p < 0.001 versus STZ/NA group.
4. Discussion
A number of diabetes-induced metabolites such as glucose, PKC, inflammation, oxidative stress and other related factors, are implicated in the pathophysiology of the DN. High blood glucose level in diabetic patients is the initial and important factor. Hyperglycemia raises the oxidative stress by aggravating glucose oxidation and mitochondrial generation of ROS. Therefore, a high blood glucose level, which causes DNA damage and contributes to accelerated apoptosis, has been considered as the key initiator of kidney damage associated with DN [32]. In the present investigation, symptoms of hyperglycemia including increased urine volume and water consumption, but decreased body weight, were ameliorated by MCE. MCE also improves the serum biochemical characteristics such as total cholesterol, triglycerides, high density lipoprotein/low density lipoprotein cholesterol, creatinine, blood urea nitrogen and insulin level, suggesting that MCE could control the blood glucose to the normal range therefore inhibit the renal injury of diabetic mice in the initial stage.
Both oxidative stress and inflammation are important mediators of the pathogenesis and progression of chronic kidney disease. Oxidative stress causes inflammation by several mechanisms, including the activation of NF-κB, and acts in a self-perpetuating cycle. Via production of reactive oxygen, nitrogen, and halogen species by activated leukocytes and resident cells, inflammation leads to oxidative stress successively [33]. In addition, among various signaling kinases, PKC seems to be another centerpiece in the pathogenesis of DN [34]. The PKC activation decreases nitric oxide production, increases expression of endothelin-1, and vascular endothelial growth factor, and then leads to endothelial dysfunction [2]. PKC-β isoforms were preferentially activated in the retina, kidney and cardiovascular tissues of diabetic rats [35,36]. Recently, a PKC-β inhibitor has been suggested by the US Food and Drug Administration for new drug applications in diabetic retinopathy [25]. Simultaneously, elevated expression of NF-κB and plasminogen activator inhibitor-1 induce tissue inflammation and thrombotic microangiopathy, therefore exacerbate the vascular injury [37]. The present results demonstrated that MCE ameliorated renal function in the STZ/NA-induced mice by decreasing renal inflammation and oxidative stress and inhibiting PKC and NF-κB expression.
Anthocyanins are members of the flavonoid group of phytochemicals with an unsaturated C ring and a hydroxyl at position 3. Due to the glycosylated structure of polyhydroxy and polymethoxy derivatives of 2-phenylbenzopyrylium, acylated or nonacylated with aliphatic acids, phytochemicals rich in anthocyanins are a rich source of potential therapeutic agents such as antioxidants. Indeed, many studies have proved that anthocyanins serve as antioxidants and have anti-inflammatory properties in metabolic diseases. Experimental evidence and clinical perspectives reveal that anthocyanins could prevent or reverse obesity- and Type 2 DM-related pathologies through a considerable number of mechanisms including promotion of antioxidant and anti-inflammatory activities, improvement of insulin resistance, and hypolipidemic and hypoglycemic actions [38]. Other researches also showed that anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via AMPK activation in white adipose tissue, skeletal muscle and the liver in Type 2 diabetic mice [39,40]. Our previous study proved the main anthocyanins of MCE were delphinidin and cyanidin. MCE ameliorated the structural and functional abnormalities of the diabetic kidney in mice and might be associated with inhibition of Ras regulated renal fibrosis. Here, we further verified that MCE attenuated diabetes-induced renal damage via inhibition of oxidative stress and inflammatory response. Although MCE could reduce inflammatory cytokines, decrease macrophage infiltration, and inhibit JAK, phosphorylated STAT3, PKC-β and NF-κB in STZ/NA mice, the detailed mechanism needs to be clarified further. With these benefits, MCE may be used to treat Type 2 diabetes and its associated renal injury. Our results offer a potent clinical trial of M. cauliflora extract for the development of an oral hypoglycemic agent.
Acknowledgments
This investigation was supported by the Chung Shan Medical University and Chung Shan Medical University Hospital (CSH-2012-C-021).
Funding Statement
This investigation was supported by the Chung Shan Medical University and Chung Shan Medical University Hospital (CSH-2012-C-021).
Footnotes
Conflicts of interest
The authors declare that they have no competing interests.
REFERENCES
- 1. Bukhari SA, Shamshari WA, Ur-Rahman M, Zia-Ul-Haq M, Jaafar HZ. Computer aided screening of secreted frizzled-related protein 4 (SFRP4): a potential control for diabetes mellitus. Molecules. 2014;19:10129–36. doi: 10.3390/molecules190710129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kanwar YS, Wada J, Sun L, Xie P, Wallner EI, Chen S, Chugh S, Danesh FR. Diabetic nephropathy: mechanisms of renal disease progression. Exp Biol Med (Maywood) 2008;233:4–11. doi: 10.3181/0705-MR-134. [DOI] [PubMed] [Google Scholar]
- 3. Bhattacharya S, Manna P, Gachhui R, Sil PC. D-saccharic acid 1,4-lactone protects diabetic rat kidney by ameliorating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via NF-kappaB and PKC signaling. Toxicol Appl Pharmacol. 2013;267:16–29. doi: 10.1016/j.taap.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 4. Dewanjee S, Das AK, Sahu R, Gangopadhyay M. Antidiabetic activity of Diospyros peregrina fruit: effect on hyperglycemia, hyperlipidemia and augmented oxidative stress in experimental type 2 diabetes. Food Chem Toxicol. 2009;47:2679–85. doi: 10.1016/j.fct.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 5. Sassy-Prigent C, Heudes D, Mandet C, Belair MF, Michel O, Perdereau B, Bariety J, Bruneval P. Early glomerular macrophage recruitment in streptozotocin-induced diabetic rats. Diabetes. 2000;49:466–75. doi: 10.2337/diabetes.49.3.466. [DOI] [PubMed] [Google Scholar]
- 6. Okada S, Shikata K, Matsuda M, Ogawa D, Usui H, Kido Y, Nagase R, Wada J, Shikata Y, Makino H. Intercellular adhesion molecule-1-deficient mice are resistant against renal injury after induction of diabetes. Diabetes. 2003;52:2586–93. doi: 10.2337/diabetes.52.10.2586. [DOI] [PubMed] [Google Scholar]
- 7. Chow F, Ozols E, Nikolic-Paterson DJ, Atkins RC, Tesch GH. Macrophages in mouse type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney Int. 2004;65:116–28. doi: 10.1111/j.1523-1755.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- 8. Maritim AC, Sanders RA, Watkins JB., III Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 9. Dewanjee S, Gangopadhyay M, Sahu R, Karmakar S. Cadmium induced pathophysiology: prophylactic role of edible jute (Corchorus olitorius) leaves with special emphasis on oxidative stress and mitochondrial involvement. Food Chem Toxicol. 2013;60:188–98. doi: 10.1016/j.fct.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 10. Vander Jagt DL, Hassebrook RK, Hunsaker LA, Brown WM, Royer RE. Metabolism of the 2-oxoaldehyde methylglyoxal by aldose reductase and by glyoxalase-I: roles for glutathione in both enzymes and implications for diabetic complications. Chem Biol Interact. 2001;130–132:549–62. doi: 10.1016/s0009-2797(00)00298-2. [DOI] [PubMed] [Google Scholar]
- 11. Ayalasomayajula SP, Kompella UB. Subconjunctivally administered celecoxib-PLGA microparticles sustain retinal drug levels and alleviate diabetes-induced oxidative stress in a rat model. Eur J Pharmacol. 2005;511:191–8. doi: 10.1016/j.ejphar.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 12. Rivero A, Mora C, Muros M, Garcia J, Herrera H, Navarro-Gonzalez JF. Pathogenic perspectives for the role of inflammation in diabetic nephropathy. Clin Sci (Lond) 2009;116:479–92. doi: 10.1042/CS20080394. [DOI] [PubMed] [Google Scholar]
- 13. Navarro-Gonzalez JF, Mora-Fernandez C, Muros de Fuentes M, Garcia-Perez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol. 2011;7:327–40. doi: 10.1038/nrneph.2011.51. [DOI] [PubMed] [Google Scholar]
- 14. Jones PJ, Varady KA. Are functional foods redefining nutritional requirements? Appl Physiol Nutr Metab. 2008;33:118–23. doi: 10.1139/H07-134. [DOI] [PubMed] [Google Scholar]
- 15. Nakayama M, Suzuki K, Toda M, Okubo S, Hara Y, Shimamura T. Inhibition of the infectivity of influenza virus by tea polyphenols. Antiviral Res. 1993;21:289–99. doi: 10.1016/0166-3542(93)90008-7. [DOI] [PubMed] [Google Scholar]
- 16. Hsieh TC, Wu JM. Differential effects on growth, cell cycle arrest, and induction of apoptosis by resveratrol in human prostate cancer cell lines. Exp Cell Res. 1999;249:109–15. doi: 10.1006/excr.1999.4471. [DOI] [PubMed] [Google Scholar]
- 17. Pal S, Choudhuri T, Chattopadhyay S, Bhattacharya A, Datta GK, Das T, Sa G. Mechanisms of curcumin-induced apoptosis of Ehrlich’s ascites carcinoma cells. Biochem Biophys Res Commun. 2001;288:658–65. doi: 10.1006/bbrc.2001.5823. [DOI] [PubMed] [Google Scholar]
- 18. Dugo P, Mondello L, Errante G, Zappia G, Dugo G. Identification of anthocyanins in berries by narrow-bore high-performance liquid chromatography with electrospray ionization detection. J Agric Food Chem. 2001;49:3987–92. doi: 10.1021/jf001495e. [DOI] [PubMed] [Google Scholar]
- 19. Boari Lima Ade J, Duarte Correa A, Carvalho Alves AP, Patto Abreu CM, Dantas-Barros AM. Chemical characterization of the jabuticaba fruits (Myrciaria cauliflora Berg) and their fractions. Arch Latinoam Nutr. 2008;58:416–21. [PubMed] [Google Scholar]
- 20. Leite-Legattia AV, Batistaa ÂG, Romanelli N, Draganoa V, Marquesa AC, Maltaa LG, Ricciob MF, Eberlinb MN, Machadoc ART, Carvalho-Silvac LB, Ruiz ALTG, Carvalho JE, Pastore GM, Maróstica MR., Junior Jaboticaba peel: Antioxidant compounds, antiproliferative and antimutagenic activities. Food Res Int. 2012;49:596–603. [Google Scholar]
- 21. Reynertson KA, Wallace AM, Adachi S, Gil RR, Yang H, Basile MJ, D’Armiento J, Weinstein IB, Kennelly EJ. Bioactive depsides and anthocyanins from jaboticaba (Myrciaria cauliflora) J Nat Prod. 2006;69:1228–30. doi: 10.1021/np0600999. [DOI] [PubMed] [Google Scholar]
- 22. Wu C-C, Hung C-N, Shin Y-C, Wang C-J, Huang H-P. Myrciaria cauliflora extracts attenuate diabetic nephropathy involving the Ras signaling pathway in streptozotocin/nicotinamide mice on a high fat diet. J Food Drug Anal. 2016;24:136–46. doi: 10.1016/j.jfda.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Raska CS, Parker CE, Huang C, Han J, Glish GL, Pope M, Borchers CH. Pseudo-MS3 in a MALDI orthogonal quadrupole-time of flight mass spectrometer. J Am Soc Mass Spectrom. 2002;13:1034–41. doi: 10.1016/S1044-0305(02)00433-6. [DOI] [PubMed] [Google Scholar]
- 24. Kobayashi Y, Nakanishi Y, Taniguchi H, Sekine S, Igaki H, Tachimori Y, Kato H, Matsubara H, Okazumi S, Shimoda T. Histological diversity in basaloid squamous cell carcinoma of the esophagus. Dis Esophagus. 2009;22:231–8. doi: 10.1111/j.1442-2050.2008.00864.x. [DOI] [PubMed] [Google Scholar]
- 25. Budhiraja S, Singh J. Protein kinase C beta inhibitors: a new therapeutic target for diabetic nephropathy and vascular complications. Fundam Clin Pharmacol. 2008;22:231–40. doi: 10.1111/j.1472-8206.2008.00583.x. [DOI] [PubMed] [Google Scholar]
- 26. Das AK, Sahu R, Dua TK, Bag S, Gangopadhyay M, Sinha MK, Dewanjee S. Arsenic-induced myocardial injury: protective role of Corchorus olitorius leaves. Food Chem Toxicol. 2010;48:1210–7. doi: 10.1016/j.fct.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 27. Dutta M, Ghosh D, Ghosh AK, Bose G, Chattopadhyay A, Rudra S, Dey M, Bandyopadhyay A, Pattari SK, Mallick S, Bandyopadhyay D. High fat diet aggravates arsenic induced oxidative stress in rat heart and liver. Food Chem Toxicol. 2014;66:262–77. doi: 10.1016/j.fct.2014.01.050. [DOI] [PubMed] [Google Scholar]
- 28. Manucha W. Mitochondria and oxidative stress participation in renal inflammatory process. Medicina (B Aires) 2014;74:254–8. [PubMed] [Google Scholar]
- 29. Matsui F, Meldrum KK. The role of the Janus kinase family/signal transducer and activator of transcription signaling pathway in fibrotic renal disease. J Surg Res. 2012;178:339–45. doi: 10.1016/j.jss.2012.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010;106:1319–31. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu H, Yang H, Wang D, Liu Y, Liu X, Li Y, Xie L, Wang G. Insulin regulates P-glycoprotein in rat brain microvessel endothelial cells via an insulin receptor-mediated PKC/NF-kappaB pathway but not a PI3K/Akt pathway. Eur J Pharmacol. 2009;602:277–82. doi: 10.1016/j.ejphar.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 32. Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–70. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ruiz S, Pergola PE, Zager RA, Vaziri ND. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013;83:1029–41. doi: 10.1038/ki.2012.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li J, Gobe G. Protein kinase C activation and its role in kidney disease. Nephrology (Carlton) 2006;11:428–34. doi: 10.1111/j.1440-1797.2006.00673.x. [DOI] [PubMed] [Google Scholar]
- 35. Kang N, Alexander G, Park JK, Maasch C, Buchwalow I, Luft FC, Haller H. Differential expression of protein kinase C isoforms in streptozotocin-induced diabetic rats. Kidney Int. 1999;56:1737–50. doi: 10.1046/j.1523-1755.1999.00725.x. [DOI] [PubMed] [Google Scholar]
- 36. Ishii H, Jirousek MR, Koya D, Takagi C, Xia P, Clermont A, Bursell SE, Kern TS, Ballas LM, Heath WF, Stramm LE, Feener EP, King GL. Amelioration of vascular dysfunctions in diabetic rats by an oral PKC beta inhibitor. Science. 1996;272:728–31. doi: 10.1126/science.272.5262.728. [DOI] [PubMed] [Google Scholar]
- 37. Wolf G. New insights into the pathophysiology of diabetic nephropathy: from haemodynamics to molecular pathology. Eur J Clin Invest. 2004;34:785–96. doi: 10.1111/j.1365-2362.2004.01429.x. [DOI] [PubMed] [Google Scholar]
- 38. Guo H, Ling W. The update of anthocyanins on obesity and type 2 diabetes: experimental evidence and clinical perspectives. Rev Endocr Metab Disord. 2015;16:1–13. doi: 10.1007/s11154-014-9302-z. [DOI] [PubMed] [Google Scholar]
- 39. Takikawa M, Inoue S, Horio F, Tsuda T. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J Nutr. 2010;140:527–33. doi: 10.3945/jn.109.118216. [DOI] [PubMed] [Google Scholar]
- 40. Kurimoto Y, Shibayama Y, Inoue S, Soga M, Takikawa M, Ito C, Nanba F, Yoshida T, Yamashita Y, Ashida H, Tsuda T. Black soybean seed coat extract ameliorates hyperglycemia and insulin sensitivity via the activation of AMP-activated protein kinase in diabetic mice. J Agric Food Chem. 2013;61:5558–64. doi: 10.1021/jf401190y. [DOI] [PubMed] [Google Scholar]