Abstract
The competitive enzyme-linked immunosorbent assay technique was used to evaluate aflatoxin M1 (AFM1) levels in 168 samples of raw milk (135 samples and 33 samples from bulk tanks of farms and milk collection centers, respectively) and 12 samples of pasteurized milk in Fars province, Southern Iran. AFM1 was found in 55.56% of the samples with a mean concentration of 21.31 ng/L. The concentration of AFM1 in raw milk samples from farms was significantly (p < 0.05) lower than that in samples from collection centers and pasteurized milk. The concentration of AFM1 was not influenced by season, location, or type of farm. The concentrations of AFM1 in all samples were lower than the Iranian national standard limit (100 ng/L), but in 30% of raw cow milk samples they were higher than the maximum tolerance limit accepted by the European Union (50 ng/L); therefore, more effort is needed to control AFM1 levels in milk produced in Southern Iran.
Keywords: aflatoxin M1, cow milk, ELISA, Fars province, Iran
1. Introduction
The presence of aflatoxins in food and feed is of great concern worldwide because of the health issues they can cause [1]. Aflatoxins are produced mainly by two filamentous fungi, Aspergillus flavus and Aspergillus parasiticus, and rarely by A. nominus, A. tamarii, or A. pseudotamarii strains when temperatures are between 24°C and 35°C and moisture content exceeds 7% [2–4]. Among the aflatoxins (B1, B2, G1, and G2), aflatoxin B1 (AFB1) is the most prevalent and potent natural carcinogen [5]. The presence of AFB1 in feeds and the subsequent access of lactating animals to it lead these animals to metabolize it to 4-hydroxylated form in their liver and excrete it as aflatoxin M1 (AFM1) in milk, urine, and feces [6,7]. About 0.3–6.2% of AFB1 in animal feeds is converted to AFM1, and it can be found in milk 12 hours after first ingestion and decreases to an undetectable level 72 hours after last ingestion of AFB1 [8,9].
Although previously AFM1 was assigned to group 2B (agents that are possibly human carcinogens) by the International Agency for Research on Cancer [10], it was thereafter reassigned to group 1 (class of agents that are certainly human carcinogens) for demonstrated toxic and carcinogenic effects [11]. A review of the literature shows that aflatoxins are most commonly known for causing acute or chronic liver disease depending on the doses used, but they are also considered immunosuppressive, hepatotoxic, mutagenic, teratogenic, and carcinogenic [2,12].
Milk is one of the main foodstuffs in human diet especially for infants and children. Most studies indicate that processes such as pasteurization, sterilization, evaporation, concentration, or drying do not cause an appreciable change in the concentration of AFM1 in the product [7]. The AFM1 level in milk may vary according to geographic location, development level of the country, and climatic conditions; thereupon, it is important to determine its levels in produced milk in different locations to protect consumers from its harmful effects [13]. The maximum limits for AFM1 in raw milk vary in different countries depending on risk assessment and economic considerations. In the European Union (EU), the maximum level of AFM1 in liquid milk has been prescribed as 50 ng/L, whereas for United States and most of Asian countries’ regulations it is 500 ng/L, which is higher than the maximum permissible level of 100 ng/L set by the Institute of Standards and Industrial Research of Iran [14–17].
Enzyme-linked immunosorbent assay (ELISA) is the quickest and simplest method for monitoring AFM1 in milk with good sensitivity, high precision, and optimal recovery [18].
The presence of AFM1 in milk has been shown in several surveys conducted in different regions of Iran using thin layer chromatography [19,20], high-performance liquid chromatography [21–23], or ELISA [24–34], and also in different countries worldwide: Brazil [13], Portugal [35], Spain [36], Lebanon [37], Syria [38], Turkey [39–42], Pakistan [43–45], South Korea [46], Sudan [47], Egypt [48], Morocco [49,50], Thailand [51], Indonesia [52], India [53], China [54], Serbia [1,55], and Croatia [56,57]. However, no published research is available on AFM1 levels in produced raw milk in Fars province. Annually, 497,000,000 L of milk is produced in Fars province, which ranks fifth in the country and first in the southern provinces of Iran [58]. The objective of this study was to determine the level of AFM1 in produced raw milk and to investigate its geographical and seasonal difference in Fars province (south of Iran).
2. Methods
2.1. Study area
A total of 192 milk samples were collected from three different areas in Fars province and labeled Sh, M, and S for Shiraz, Marvdasht, and Sepidan districts, respectively. Raw milk of cows from smallholder farms has was collected by milk collection centers, whereas it was transported to dairy factories directly by industrial dairy farms in Fars province. In each of these areas, raw milk was sampled from the bulk tank of three industrial dairy farms, three milk collection centers, and nine smallholder dairy farms (3 smallholder dairy farms that sold their milk to selected milk collection centers) seasonally. In each season, three pasteurized milk samples produced by dairy factories in Fars province were taken.
2.2. Milk sample preparation
Fresh milk samples (500 mL) were taken directly from storage tanks of farms or milk collection centers and pasteurized milk samples were bought from supermarkets. These samples were transported to the laboratory in ice boxes and stored in the dark at −18°C until the time of analysis. Milk samples were chilled at 10°C, of which 2 mL was centrifuged for analysis at 3500 rpm for 10 minutes at 4°C. As aflatoxins are water-soluble compounds [59], the upper creamy layers were completely discarded, and the lower phases were used for the quantitative test.
2.3. AFM1 measurement
The quantitative analysis of AFM1 was performed by competitive ELISA using an AFM1 kit (RIDASCREEN; R-Biopharm AG, Darmstadt, Germany). It had the following characteristics: detection limit, 5 ng/L; recovery rate, 95%; cross-reactivity, AFM1 100% and AFM2 30%; standard solutions, 0, 5, 10, 20, 40, 80 ng/L. The basis of the test was the antigen–antibody reaction. The wells in the microtiter strips were coated specific to AFM1 and filled with 100 μL of prepared samples or standard solutions. Antibodies were proportionally bound by shaking the plate gently and incubating at room temperature for 30 minutes in the dark. The wells were filled with 250 μL washing buffer after the complete removal of liquids. Then washing buffer was poured out, and this washing step was repeated twice. In the next step, 100 μL peroxidase conjugated AFM1 was added to the wells. Free antibodies were bound by conjugated AFM1 and any unbound enzyme conjugated AFM1 was removed by a washing step. Then, 100 μL of substrate and chromogen was added to wells and mixed gently by shaking the plate manually and incubated at room temperature for 15 minutes in the dark. Colorless chromogen was converted to blue by bound enzyme conjugate. Finally, 100 μL of 1N H2SO4 was added to wells, which led to a color change (from blue to yellow) [37]. The absorbance was measured at 450 nm in an ELISA plate reader (BioTek, Winooski, VT, USA). The absorption intensity was inversely proportional to the AFM1 concentration in the sample. A special software (RIDA SOFT Win; R-Biopharm AG) was used to draw standard curve and evaluate assays. The considered limit for positive samples was 5 ng/L AFM1.
2.4. Statistical analysis
All statistical analyses were carried out in SPSS for Windows 16.0.0 (SPSS Inc., 2007, Chicago, USA). Data were analyzed descriptively in the first step. Univariate analysis of variance was applied with AFM1 values as dependent variable and season, city, and herd type as independent variables. The means of AFM1 values was compared by using Duncan test. The relationship between contamination percentage and season or location in each type of farms was investigated using the chi-square test.
3. Results
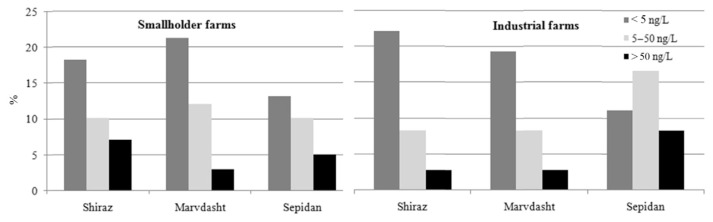
Twelve raw milk samples were missed, and only 168 samples of raw milk (135 and 33 from bulk tank of farms and milk collection centers, respectively) and 12 samples of pasteurized milk were analyzed for AFM1. An exponential correlation was obtained by plotting the percentage of absorbance (y) and concentration (x) of AFM1 (y = 96.72 –10.2x, with R2 = 0.991) on the standard curve. The detection limit was found to be 5 ng/L. The results of analysis of milk samples for AFM1 are shown in Table 1. Although 55.56% of samples were contaminated with AFM1, the concentrations were not higher than the maximum tolerance level of AFM1 in liquid milk based on the Iranian national standard (100 ng/L) and Food and Drug Administration standard (500 ng/L) [14,16]. The overall mean level of AFM1 in the samples was 21.31 ± 2.03 ng/L, and 30 (30%) of the contaminated samples had AFM1 levels higher than the maximum tolerance limit accepted by the European Union [15]. Pasteurized cow milk showed a high rate (91.67%) of contaminated samples, with a mean AFM1 level of 32.23 ng/L, but only 18.8% of the samples were higher than the permissible level of 50 ng/L as accepted by the EU. The concentration of AFM1 was not influenced by season, location, or type of farm (Table 2). The distribution of contaminated samples in different seasons or cities in smallholder and industrial farms are shown in Figures 1 and 2. The chi-square results showed no relationship between contamination percentage and season or location in smallholder farms. Unlike location, season had a significant (p < 0.05) effect on the distribution of contaminated samples, and no sample showed contamination above 50 ng/L in spring and winter in industrial farms.
Table 1.
Mean ± standard error (SE), minimum (Min), and maximum (Max) aflatoxin M1 levels (ng/L) in raw and pasteurized milk samples.
| Type of milk sample | No. of samples | Mean ± SE | Contaminated samples, n (%) | Min | Max | Exceeding limit,a n (%) |
|---|---|---|---|---|---|---|
| Raw milk | ||||||
| Farm | 135 | 18.26 ± 2.29b | 64 (47.41) | 0.00 | 99.92 | 20 (31.25) |
| Collection centers | 33 | 29.82 ± 5.04a | 25 (75.26) | 0.00 | 97.68 | 8 (32.00) |
| Pasteurized milk | 12 | 32.23 ± 6.76a | 11 (91.67) | 2.04 | 90.01 | 2 (18.18) |
| Total | 180 | 21.31 ± 2.03 | 100 (55.56) | 0.00 | 99.92 | 30 (30.00) |
Means followed by different letters (a, b) are significantly different (p < 0.05).
European Union limit (50 ng/L).
Table 2.
Mean ± standard error (SE), minimum (Min), and maximum (Max) aflatoxin M1 levels (ng/L) in raw milk of farms in different seasons, cities, and farms.
| No. of samples | Mean ± SE | No of contaminated samples, n (%) | Min | Max | No of samples above limit,a n (%) | |
|---|---|---|---|---|---|---|
| Season | ||||||
| Spring | 34 | 15.55 ± 4.10 | 17 (50.00) | 0.00 | 95.04 | 5 (29.41) |
| Summer | 32 | 21.45 ± 5.68 | 11 (34.38) | 0.00 | 92.48 | 7 (63.64) |
| Fall | 34 | 21.16 ± 99.92 | 17 (50.00) | 0.00 | 99.92 | 7 (41.18) |
| Winter | 35 | 15.16 ± 78.66 | 19 (54.29) | 0.00 | 78.99 | 1 (5.26) |
| City | ||||||
| Shiraz | 47 | 19.01 ± 4.01 | 21 (44.68) | 0.00 | 92.48 | 8 (38.10) |
| Marvdasht | 47 | 15.37 ± 3.79 | 19 (40.43) | 0.00 | 99.92 | 4 (21.05) |
| Sepidan | 41 | 20.73 ± 4.13 | 24 (58.54) | 0.00 | 86.45 | 8 (33.33) |
| Type of farm | ||||||
| Smallholder | 99 | 18.42 ± 2.66 | 47 (47.47) | 0.00 | 99.92 | 15 (31.91) |
| Industrial | 36 | 17.82 ± 4.52 | 17 (47.22) | 0.00 | 86.45 | 5 (29.41) |
European Union limit (50 ng/L).
Figure 1.
Distribution of contaminated samples in different seasons.
Figure 2.
Distribution of contaminated samples in different regions.
4. Discussion
As AFM1 is a global problem, many studies have been conducted for determining the occurrence and levels of AFM1 in milk using different techniques worldwide. The results of some of the studies that used ELISA to measure AFM1 are summarized in Table 3. The mean levels of raw milk AFM1 in the present study was near to that obtained in Brazil [13], eastern part of Croatia [56], and Spain [36], but lower than that reported in other parts of Iran [25,26,28,32,33], India [53], Serbia [1], Syria [38], Turkey [42], and Lebanon [37]. These differences are probably attributable to variations in the amount of AFB1 in feedstuffs that dairy cows consume. Local weather conditions during pre-harvest and harvest stages as well as inadequate storage conditions can influence the quality of feed. AFB1 is produced by some molds that can easily grow in feeds having a moisture content between 13% and 18%, and environmental moisture between 50% and 60% [60].
Table 3.
Summarized results of studies on AFM1 contamination in cow milk by ELISA in different countries.
| Country | Type of milk | No. of samples | No. of samples positive (%) | Mean (ng/L) | Range (ng/L) | No. of samples above limit (%)a | Reference |
|---|---|---|---|---|---|---|---|
| Brazil | |||||||
| MG | Raw | 129 | 129 (100.00) | 19.50 | 0.2–106 | 18 (13.95) | [13] |
| Croatia | |||||||
| Eastern part | Raw | 194 | 47 (24.23) | 20.60 | 3.7–162.3 | 13 (27.66) | [56] |
| Other parts | 143 | 12 (8.39) | 12.10 | 2.7–44.9 | 0 (0.00) | ||
| India | Liquid | 12 | 4 (33.33) | 86.00 | 28–164 | 3 (75.00) | [53] |
| Indonesia | |||||||
| Yogyakarta | Raw | 113 | 65 (57.52) | 8.53 | No report | 0 (0.00) | [52] |
| Iran | |||||||
| Ahvaz | Raw | 75 | 59 (78.67) | 60.10 | No report | 27 (45.76) | [25] |
| Ardabil | Mixb | 90 | 90 (100.00) | 37.23 | 2.9–85 | 30 (33.33) | [26] |
| Hamedan | Raw | 186 | 119 (63.98) | 43.40 | 10–410 | 14 (11.76) | [28] |
| Gilan | Raw | 90 | 56 (62.22) | No report | 2.1–131 | 28 (50.00) | [29] |
| Ilam | Raw | 54 | 34 (62.98) | 43.98 | 10.03–85.24 | 31 (57.40) | [33] |
| Traditional | 48 | 19 (39.60) | 34.21 | 8 (16.50) | |||
| Industrial Pasteurized | 52 | 10 (23.80) | 36.06 | 6 (14.28) | |||
| Mashhad | Pasteurized | 42 | 41 (97.62) | 23.00 | 6.4–71.4 | 3 (7.32) | [30] |
| Qazvin | Raw | 288 | 163 (56.60) | 90.00 | 10–250 | 113 (69.33) | |
| Sanandaj | Raw | 240 | 226 (94.17) | 12.65 | 0.01–115.9 | 10 (4.42) | |
| Pasteurized | 32 | 31 (96.88) | 12.43 | 2 (6.45) | |||
| Shiraz | Pasteurized | 624 | 624 (100.00) | No report | No report | 101 (16.19) | [24] |
| Tabriz | Pasteurized | 50 | 50 (100.00) | 50.55 | 0–259 | 22 (44.00) | [34] |
| Tehran | pasteurized | 128 | 128 (100.00) | 72.20 | 31–113 | 100 (78.00) | [31] |
| Lebanon | Raw | 38 | 28 (73.68) | 60.40 | 2.63–126 | 17 (60.71) | [37] |
| Pasteurized | 25 | 17 (68.00) | 30.60 | 3.27–84.4 | 4 (23.53) | ||
| Spain | |||||||
| Leon | Raw | 92 | 5 (5.43) | 20. 50 | 14–24.9 | 0 (0.00) | [36] |
| Serbia | Raw | 678 | 540 (79.65) | 282.00 | No report | 382 (70.74) | [1] |
| Syria | Raw | 74 | 70 (94.59) | 143.00 | 20–690 | 41 (58.57) | [38] |
| Pasteurized | 10 | 10 (100) | 492.00 | 8–765 | 8 (80.00) | ||
| Turkey | |||||||
| Ankara | Pasteurized | 85 | 75 (88.23) | No report | 5.2–127.6 | 48 (46.00) | [41] |
| Kayseri | Raw | 50 | 43 (86.00) | 8.73 | 1–30 | 0 (0.00) | [40] |
| Kayseri | Raw | 90 | 90 (100.00) | 59.9 | 5–80 | 63 (70.00) | [42] |
ELISA = enzyme-linked immunosorbent assay.
European Union limit (50 ng/L).
Raw, pasteurized, and sterilized.
The contamination rate of AFM1 in pasteurized milk in different parts of Iran was reported to be very high [24,27,30,31,34]. In Iran, pasteurization plants usually receive milk either directly from industrial farms or indirectly via milk collection centers without testing of milk for contamination with AFM1. The maximum levels of AFM1 in all samples were lower than the Iranian national standard limit (100 ng/L), which could be attributed to the activities of the Iran Veterinary Organization on testing and monitoring of raw milk in different locations of the country.
The concentration of AFM1 in raw milk from farms was significantly (p < 0.05) lower than that in milk samples from collection centers and in pasteurized milk. The raw milk produced in smallholder farms is collected and pooled with other milk during cooling in milk collection centers and then transported to pasteurization plants in Iran. No testing for contamination with AFM1 is done prior to receiving raw milk in milk collection centers. This leads to mixing of raw milk with different AFM1 levels, and subsequently elevating the level of contamination in transported milk to pasteurization plants. Many authors in Iran [19,20,26,32,33], Croatia [57], Serbia [1], and Turkey [39] reported higher AFM1 levels during cold seasons as compared to hot seasons, because stored feeds with a higher probability of containing AFB1 (e.g., dry hay, corn, concentrates, and silages) are used in much greater amounts for cow feeding during cold season, and this results in increased AFM1 content in milk. These differences are presumably attributable to variation in feeding systems. Dairy cows have been fed indoors without a grazing period during the year in Fars province. In conclusion, the results indicate that milk produced in Fars province is safe for human consumption according to the defined maximum tolerance level of AFM1 issued by the Iranian national standard. AFM1 concentrations exceeded 50 ng/L (maximum tolerance level of AFM1 in the EU) in 30% of samples; therefore, a more sustained effort is needed to control AFM1 level in milk produced in Fars province. The most effective way of controlling AFM1 is to monitor feed for AFB1. AFB1 can be controlled in animal feedstuffs by improving the production practices and using appropriate storage conditions. Dairy companies and milk collection centers should be required by relevant government organizations to test received milk for AFM1. The potential health risks of AFM1 may be reduced by enhancing the awareness of farmers, dairy producers, and consumers regarding the toxicity potential of aflatoxins.
Footnotes
Conflicts of interest
The author declare that he has no conflicts of interest.
REFERENCES
- 1. Tomašević I, Petrović J, Jovetić M, Raičević S, Milojević M, Miočinović J. Two year survey on the occurrence and seasonal variation of aflatoxin M1 in milk and milk products in Serbia. Food Control. 2015;56:64–70. [Google Scholar]
- 2. Williams JH, Phillips TD, Jolly PE, Stiles JK, Jolly CM, Aggarwal D. Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. Am J Clin Nutr. 2004;80:1106–22. doi: 10.1093/ajcn/80.5.1106. [DOI] [PubMed] [Google Scholar]
- 3. Goto T, Peterson SW, Ito Y, Wicklaw DT. Mycotoxin producing ability of A. tamarii. Mycotoxins. 1997;44:17–20. [Google Scholar]
- 4. Ito Y, Peterson SW, Wicklaw DT, Goto T. Aspergillus pseudotamarii, a new aflatoxin producing sp Mycol Res. 2001;105:233–9. [Google Scholar]
- 5. Gourama H, Bullerman LB. Aspergillus flavus and Aspergillus parasiticus: aflatoxigenic fungi of concern in foods and feeds: a review. J Food Prot. 1995;58:1395–409. doi: 10.4315/0362-028X-58.12.1395. [DOI] [PubMed] [Google Scholar]
- 6. Fallah AA. Aflatoxin M1 contamination in dairy products marketed in Iran during winter and summer. Food Control. 2010;21:1478–81. [Google Scholar]
- 7. Prandini A, Tansini G, Sigolo S, Filippi L, Laporta M, Piva G. On the occurrence of aflatoxin M1 in milk and dairy products. Food Chem Toxicol. 2009;47:984–91. doi: 10.1016/j.fct.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 8. Creppy EE. Update of survey, regulation and toxic effect of mycotoxins in Europe. Toxicol Lett. 2002;127:19–28. doi: 10.1016/s0378-4274(01)00479-9. [DOI] [PubMed] [Google Scholar]
- 9.Van Egmond HP. Introduction. In: Van Egmond HP, editor. Mycotoxins in dairy products. London: Elsevier Applied Science; 1989. pp. 1–10. [Google Scholar]
- 10.IARC. IARC monographs on the evaluation of carcinogenic risks to humans 56. Lyon, France: International Agency for Research on Cancer, Scientific Publication; 1993. Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins; pp. 19–23. [Google Scholar]
- 11.International Agency for Research on Cancer (IARC) IARC monograph on the evaluation of carcinogenic risk to humans. 82. Lyon, France: IARC Scientific Publication; 2002. Some traditional herbal medicines, some mycotoxins, naphthalene and styrene; pp. 171–5. [PMC free article] [PubMed] [Google Scholar]
- 12. Flores-Flores ME, Lizarraga E, López de Cerain A, González-Peñas E. Presence of mycotoxins in animal milk: a review. Food Control. 2015;53:163–76. [Google Scholar]
- 13. Picinin LCA, Cerqueira MMOP, Vargas EA, Lana ÂMQ, Toaldo IM, Bordignon-Luiz MT. Influence of climate conditions on aflatoxin M1 contamination in raw milk from Minas Gerais State, Brazil. Food Control. 2013;31:419–24. [Google Scholar]
- 14.Institute of Standard and Industrial Research of Iran (ISIRI) Food and feed—mycotoxins—maximum tolerated level. ISIRI; 2010. pp. 1–10. [Google Scholar]
- 15.Europian Commission (EC) Commission regulation (EU) No 165/2010 of 26 February 2010 amending regulation (Europian Commission) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards aflatoxins. L50. Official Journal of the European Union; 2010. p. 4. [Google Scholar]
- 16.United States Food and Drug Administration (FDA); Administration FaD, editor. FDA/ORA Compliance Policy Guides. 2007. CPG Sec. 527, 400 Whole Milk, Lowfat Milk, Skim Milk — Aflatoxin M1. [Google Scholar]
- 17. Anukul N, Vangnai K, Mahakarnchanakul W. Significance of regulation limits in mycotoxin contamination in Asia and risk management programs at the national level. J Food Drug Anal. 2013;21:227–41. [Google Scholar]
- 18. Rosi P, Borsari A, Lasi G, Lodi S, Galanti A, Fava A, Girotti S, Ferri E. Aflatoxin M1 in milk: reliability of the immunoenzymatic assay. Int Dairy J. 2007;17:429–35. [Google Scholar]
- 19. Kamkar A. A study on the occurrence of aflatoxin M1 in raw milk produced in Sarab city of Iran. Food Control. 2005;16:593–9. [Google Scholar]
- 20. Fallah AA, Rahnama M, Jafari T, Saei-Dehkordi SS. Seasonal variation of aflatoxin M1 contamination in industrial and traditional Iranian dairy products. Food Control. 2011;22:1653–6. [Google Scholar]
- 21. Behfar A, Nazari Khorasgani Z, Alemzadeh Z, Goudarzi M, Ebrahimi R, Tarhani N. Determination of aflatoxin M1 levels in produced pasteurized milk in Ahvaz City by using HPLC. Jundishapur J Nat Pharm Prod. 2012;7:80–4. [PMC free article] [PubMed] [Google Scholar]
- 22. Ganjeizadeh Rohani F, Aminaee MM, Kianfar M. Survey of aflatoxin M1 in cow’s milk for human consumption in Kerman Province of Iran. Food Addit Contam Part B Surveill. 2011;4:191–4. doi: 10.1080/19393210.2011.599866. [DOI] [PubMed] [Google Scholar]
- 23. Tajkarimi M, Aliabadi-Sh F, Salah Nejad A, Poursoltani H, Motallebi AA, Mahdavi H. Aflatoxin M1 contamination in winter and summer milk in 14 states in Iran. Food Control. 2008;19:1033–6. [Google Scholar]
- 24. Alborzi S, Pourabbas B, Rashidi M, Astaneh B. Aflatoxin M1 contamination in pasteurized milk in Shiraz (south of Iran) Food Control. 2006;17:582–4. [Google Scholar]
- 25. Rahimi E, Bonyadian M, Rafei M, Kazemeini HR. Occurrence of aflatoxin M1 in raw milk of five dairy species in Ahvaz, Iran. Food Chem Toxicol. 2010;48:129–31. doi: 10.1016/j.fct.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 26. Nemati M, Mehran MA, Hamed PK, Masoud A. A survey on the occurrence of aflatoxin M1 in milk samples in Ardabil, Iran. Food Control. 2010;21:1022–4. [Google Scholar]
- 27. Mohammadian B, Khezri M, Ghasemipour N, Mafakheri S, Poorghafour Langroudi P. Aflatoxin M1 contamination of raw and pasteurized milk produced in Sanandaj, Iran. Arch Razi Inst. 2010;65:99–104. [Google Scholar]
- 28. Ghiasian SA, Maghsood AH, Neyestani TR, Mirhendi SH. Occurrence of aflatoxin M1 during the summer and winter seasons in Hamedan, Iran. J Food Safety. 2007;27:188–98. [Google Scholar]
- 29. Laleh rokhi M, Kazemi Darsanaki R, Mohammadi M, Kolavani MH, Issazadeh K, Azizollahi Aliabadi M. Determination of aflatoxin M1 levels in raw milk samples in Gilan, Iran. Adv Stud Biol. 2013;5:151–6. [Google Scholar]
- 30. Mohamadi Sani A, Khezri M, Moradnia H. Determination of aflatoxin M1 in milk by ELISA technique in Mashad (Northeast of Iran) ISRN Toxicol [Internet] 2012:1–4. doi: 10.5402/2012/121926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oveisi M-R, Jannat B, Sadeghi N, Hajimahmoodi M, Nikzad A. Presence of aflatoxin M1 in milk and infant milk products in Tehran, Iran. Food Control. 2007;18:1216–8. [Google Scholar]
- 32. Mahmoudi R, Norian R. Aflatoxin B1 and M1 contamination in cow feeds and milk from Iran. Food Agric Immunol. 2015;26:131–7. [Google Scholar]
- 33. Vagef R, Mahmoudi R. Occurrence of Aflatoxin M1 in raw and pasteurized milk produced in west region of Iran (during summer and winter) Int Food Res J. 2013;20:1421–5. [Google Scholar]
- 34. Movassagh Ghazani MH. Aflatoxin M1 contamination in pasteurized milk in Tabriz (northwest of Iran) Food Chem Toxicol. 2009;7:1624–5. doi: 10.1016/j.fct.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 35. Martins ML, Martins HM. Aflatoxin M1 in raw and ultra high temperature treated milk commercialized in Portugal. Food Addit Contam Part A. 2000;17:871–4. doi: 10.1080/026520300420457. [DOI] [PubMed] [Google Scholar]
- 36. Velasco MLR, Delso MMC, Escudero DO. ELISA and HPLC determination of the occurrence of aflatoxin M1 in raw cow’s milk. Food Addit Contam. 2003;20:276–80. doi: 10.1080/0265203021000045208. [DOI] [PubMed] [Google Scholar]
- 37. Assem E, Mohamad A, Oula EA. A survey on the occurrence of aflatoxin M1 in raw and processed milk samples marketed in Lebanon. Food Control. 2011;22:1856–8. [Google Scholar]
- 38. Ghanem I, Orfi M. Aflatoxin M1 in raw, pasteurized and powdered milk available in the Syrian market. Food Control. 2009;20:603–5. [Google Scholar]
- 39. Golge O. A survey on the occurrence of aflatoxin M1 in raw milk produced in Adana province of Turkey. Food Control. 2014;45:150–5. [Google Scholar]
- 40. Ertas N, Gonulalan Z, Yildirim Y, Karadal F. A survey of concentration of aflatoxin M1 in dairy products marketed in Turkey. Food Control. 2011;22:1956–9. [Google Scholar]
- 41. Celik TH, Sarimehmetoglu B, Kuplulu O. Aflatoxin M1 contamination in pasteurised milk. Veterinarski Arhiv. 2005;75:57–65. [Google Scholar]
- 42. Buldu HM, Kov AN, Uraz G. Aflatoxin M1 contamination in cow’s milk in Kayseri (central Turkey) Turk J Vet Anim Sci. 2011;35:87–91. [Google Scholar]
- 43. Hussain I, Anwar J. A study on contamination of aflatoxin M1 in raw milk in the Punjab province of Pakistan. Food Control. 2008;19:393–5. [Google Scholar]
- 44. Asi RM, Iqbal SZ, Ariño A, Hussain A. Effect of seasonal variations and lactation times on aflatoxin M1 contamination in milk of different species from Punjab, Pakistan. Food Control. 2012;25:34–8. [Google Scholar]
- 45. Hussain I, Anwar J, Asi MR, Munawar MA, Kashif M. Aflatoxin M1 contamination in milk from five dairy species in Pakistan. Food Control. 2010;21:122–4. [Google Scholar]
- 46. Lee JE, Kwak B-M, Ahn J-H, Jeon T-H. Occurrence of aflatoxin M1 in raw milk in South Korea using an immunoaffinity column and liquid chromatography. Food Control. 2009;20:136–8. [Google Scholar]
- 47. Elzupir AO, Elhussein AM. Determination of aflatoxin M1 in dairy cattle milk in Khartoum State, Sudan. Food Control. 2010;21:945–6. [Google Scholar]
- 48. Motawee MM, Bauer J, McMahon DJ. Survey of aflatoxin M1 in cow, goat, buffalo and camel milks in Ismailia-Egypt. Bull Environ ContamToxicol. 2009;83:766–9. doi: 10.1007/s00128-009-9840-3. [DOI] [PubMed] [Google Scholar]
- 49. El Marnissi B, Belkhou R, Morgavi DP, Bennani L, Boudra H. Occurrence of aflatoxin M1 in raw milk collected from traditional dairies in Morocco. Food Chem Toxicol. 2012;50:2819–21. doi: 10.1016/j.fct.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 50. Zinedine A, González-Osnaya L, Soriano JM, Moltó JC, Idrissi L, Mañes J. Presence of aflatoxin M1 in pasteurized milk from Morocco. Int J Food Microbiol. 2007;114:25–9. doi: 10.1016/j.ijfoodmicro.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 51. Ruangwises S, Ruangwises N. Occurrence of aflatoxin M1 in pasteurized milk of the school milk project in Thailand. J Food Protect. 2009;72:1761–3. doi: 10.4315/0362-028x-72.8.1761. [DOI] [PubMed] [Google Scholar]
- 52. Nuryono N, Agus A, Wedhastri S, Maryudani YB, Sigit Setyabudi FMC, Böhm J, Razzazi-Fazeli E. A limited survey of aflatoxin M1 in milk from Indonesia by ELISA. Food Control. 2009;20:721–4. [Google Scholar]
- 53. Rastogi S, Dwivedi PD, Khanna SK, Das M. Detection of Aflatoxin M1 contamination in milk and infant milk products from Indian markets by ELISA. Food Control. 2004;15:287–90. [Google Scholar]
- 54. Xiong JL, Wang YM, Ma MR, Liu JX. Seasonal variation of aflatoxin M1 in raw milk from the Yangtze River Delta region of China. Food Control. 2013;34:703–6. [Google Scholar]
- 55. Škrbić B, Živančev J, Antić I, Godula M. Levels of aflatoxin M1 in different types of milk collected in Serbia: assessment of human and animal exposure. Food Control. 2014;40:113–9. [Google Scholar]
- 56. Bilandžić N, Božić Đ, Đokić M, Sedak M, Kolanović BS, Varenina I, Cvetnić Z. . Assessment of aflatoxin M1 contamination in the milk of four dairy species in Croatia. Food Control. 2014;43:18–21. [Google Scholar]
- 57. Bilandžić N, Varenina I, Kolanović BS, Božić Đ, Đokić M, Sedak M, Tanković S, Potocnjak D, Cvetnić Z. Monitoring of aflatoxin M1 in raw milk during four seasons in Croatia. Food Control. 2015;54:331–7. [Google Scholar]
- 58.Agriculture Statistics of Iran. The yearbook of agriculture statistics of Iran. Tehran, Iran: Bureau of Statistics and Information Technology, The Ministry of Jihad-E-Agriculture; 2013. [Google Scholar]
- 59.Deshpande SS. Fungal toxins. In: Deshpande SS, editor. Handbook of food toxicology. New York: Marcel Decker; 2002. pp. 387–456. [Google Scholar]
- 60. Unusan N. Occurrence of aflatoxin M1 in UHT milk in Turkey. Food Chem Toxicol. 2006;44:1897–900. doi: 10.1016/j.fct.2006.06.010. [DOI] [PubMed] [Google Scholar]