Abstract
Ten milkfish dumpling products purchased from retail stores in southern Taiwan were collected to determine the occurrence of biogenic amines, histamine-forming bacteria, and adulteration of pork. This study showed the high contents of aerobic plate count (APC), total coliforms (TC) and Escherichia coli in tested milkfish dumpling samples, whereas the average content of various biogenic amines in all tested samples was < 1.6 mg/100 g (< 0.05 to 1.54 mg/100 g). Three histamine-producing bacterial strains (2 isolates of Raoultella ornithinolytica and 1 isolate of Enterobacter aerogenes) isolated from tested samples produced 276.6 ppm to 561.8 ppm of histamine in trypticase soy broth supplemented with 1.0% L-histidine (TSBH). Assay of multiplex polymerase chain reaction (PCR) revealed that the adulteration rates were 50% (5/10) for pork in milkfish dumplings. In addition, milkfish dumpling stuffing was inoculated with R. ornithinolytica at 5.0 log colony forming units (CFU)/g and stored at various temperatures from 4°C to 37°C to investigate bacterial growth and formation of histamine. The histamine contents quickly increased to higher than 50 mg/100 g in samples stored at 37°C and 25°C within 24 hours and 36 hours, respectively, as well as stored at 15°C within 48 hours. Therefore, bacterial growth and histamine formation were controlled by cold storage of the samples at 4°C.
Keywords: adulteration, histamine, histamine-forming bacteria, milkfish dumpling, Raoultella ornithinolytica
1. Introduction
Histamine, one of the biogenic amines, has been known as the causative toxin of scombroid fish poisoning [1]. Scombroid fish poisoning is usually a mild illness with a variety of symptoms [1]. Scombroid fish such as tuna, mackerel, bonito, and saury that contain high levels of free histidine in their muscle are often implicated in scombroid poisoning incidents [2]. However, several species of nonscombroid fish such as mahi-mahi, bluefish, herring, and sardine have also been implicated in incidents of scombroid poisoning. In Taiwan, scombroid poisoning occurs occasionally, and the fish implicated in these outbreaks are tuna, mackerel, milkfish, swordfish and marlin [3–8].
Biogenic amines are formed mainly through the decarboxylation of specific free amino acids by exogenous decarboxylases released by the microbial species associated with seafood. Many bacterial species are known to possess histidine decarboxylase and have the ability to produce histamine [9]. Although histamine fish poisoning occurs largely due to the growth of naturally occurring bacteria, e.g., Morganella morganii, Raoultella (formerly Klebsiella) planticola, and Enterobacter aerogenes, a variety of other bacterial species capable of producing histamine have been isolated from fish [10]. Among them are the enteric bacteria, which include Proteus vulgaris, Proteus mirabilis, E. aerogenes, Enterobacter cloacae, Serratia fonticola, Serratia liquefaciens, and Citrobacter freundii [11]. In addition to the enteric bacteria, Clostridium spp., Vibrio alginolyticus, Acinetobacter lowffii, Plesiomonas shigelloides, Pseudomonas putida, Pseudomonas fluorescens, Aeromonas spp., and Photobacterium spp. have also been reported as histamine producers [11,12].
Accurate analytical methods are indispensable for the labeling of meat products. Therefore, simple and fast procedures were needed. Biomolecular techniques have been used extensively because of their high degree of specificity and applicability to heat processed products [13]. Among them, DNA hybridization [14] and polymerase chain reaction (PCR) methods [15] have been used for identification of meats and meat products. DNA hybridization generally is more complicated than PCR, which easily amplifies target regions of temple DNA in a much shorter time [16], and thus, is suitable for meat identification. Matsunaga et al [17] designed multiplex PCR primers to identify cattle, pig, chicken, sheep, goat, and horse meats. Recently, Dalmasso et al [18] developed a multiplex PCR assay to identify animal species (ruminant, poultry, fish, and pork) in feedstuffs.
A case of histamine intoxication associated with milkfish meat was reported in Tainan City in southern Taiwan in September 2014 and a high content of histamine (235 mg/100 g) was detected in suspected milkfish samples [19]. However, there was no report associated with biogenic amines, including histamine, histamine-forming bacteria, and related bacteria in commercial milkfish dumpling products. Therefore, this research was conducted to analyze 10 commonly consumed milkfish dumpling products at retail stores in southern Taiwan to obtain better understanding of the safety quality of the products. In order to avoid possible fraudulent and vague labeling of milkfish dumplings, a multiplex PCR assay was used to discriminate adulteration of ruminant, poultry, fish, and pork in milkfish dumplings. Moreover, we demonstrated that R. ornithinolytica produced significant amounts of histamine (> 50 mg/100 g) in artificially contaminated tuna dumpling stuffing stored at elevated temperatures (> 15°C) [20]. If the milkfish dumpling stuffing is contaminated with histamine formers, such as R. ornithinolytica, and stored at improper temperatures, it is important to be aware that milkfish dumplings could become a hazardous food vehicle for histamine poisoning. Currently, little information is available concerning histamine formation in contaminated milkfish dumpling stuffing. This work was undertaken to study the effect of R. ornithinolytica proliferation in milkfish dumpling stuffing on histamine formation and total volatile base nitrogen (TVBN) under the controlled storage temperatures of 4°C, 15°C, 25°C, and 37°C.
2. Materials and methods
2.1. Samples
Ten milkfish dumpling products were purchased from 10 retail stores in southern Taiwan. All samples were purchased in frozen condition, wrapped in aseptic bags, placed in ice, and transported to the laboratory for use within 8 hours.
2.2. Determination of pH value, salt content, and moisture content
Ten grams of milkfish dumpling samples were homogenized in sterile blenders with 40 mL of distilled water to make a thick slurry. The pH of this slurry was measured using a Corning 145 pH meter (Corning Glass Works, Medfield, MA, USA). Salt content in each sample was determined according to the Association of Official Analytical Chemists (AOAC) procedures [21]. Two grams of tuna dumpling sample were homogenized with 18 mL of distilled water, and then titrated with 0.1M AgNO3 using 10% w/v K2CrO4 solution as an indicator. The moisture content was conducted with the standard gravimetric method by drying 1–3 g of a test sample at 102.0 ± 2.0°C under atmospheric pressure for 2 hours. Consistency of mass was tested by additional drying until the difference in mass did not exceed 0.5 mg [21].
2.3. Microbiological analysis and isolation of histamine-forming bacteria
A 25-g portion of the milkfish dumpling sample was homogenized at high speed for 2 minutes in a sterile blender with 225 mL sterile potassium phosphate buffer (0.05 M, pH 7.0). The blender was sterilized by autoclaving for 15 minutes at 121°C before use. The homogenates were serially diluted with a sterile phosphate buffer (1:9), and 1.0 mL aliquots of the dilutant was inoculated into aerobic plate count (APC) agar (Difco, Detroit, MI, USA) containing 0.5% NaCl. Bacterial colonies were counted after the plates were incubated at 35°C for 48 hours. Bacterial numbers in the tuna dumpling samples were expressed as log10 colony forming units (CFU)/g.
To isolate histamine-forming bacteria, a 0.1 mL aliquot of the sample dilutant was spread on histamine-forming bacterium isolation agar fortified with L-histidine [22]. Following incubation of the differential agar plates for 4 days at 35°C, colonies with blue or purple color on the plates were picked and further streaked on trypticase soy agar (TSA) (Difco) to obtain pure cultures. Their ability to produce biogenic amines was determined by inoculating the isolates in trypticase soy broth (TSB) (Difco) supplemented with 1% L-histidine (TSBH) and incubated without shaking at 35°C for 24 hours. One milliliter of the culture broth was taken for quantitation of biogenic amines.
Analyses of total coliforms (TC) and Escherichia coli in these milkfish dumpling samples were conducted using the three-tube most probable number (MPN) methods [23]. Lauryl sulfate tryptose broth and brilliant green lactose bile (2%) broth were used for presumptive and confirmed tests for TC, respectively. Escherichia coli was determined by using the lauryl sulfate tryptose broth and E. coli broth. Cultures that showed positive production of gas were then confirmed by streaking on eosin methylene blue agar and indole, methyl red, Voges-Proskauer, and citrate test [23].
2.4. Identification of histamine-forming isolates
The presumptive histamine-forming isolates were identified on the basis of morphology, Gram stain, endospore stain, catalase, and oxidase reaction. The identity of histamine-forming isolates was further confirmed by amplifying and sequencing approximately 1400 bp of the 16S ribosomal DNA (rDNA) for bacteria [24,25]. Amplification of histamine-forming bacteria was performed using the universal primers UNI-L (5′-AGAGTTTGATCATGGCTCAG-3′) and UNI-R (5′-GTGTGACGGGCGGTGTGTAC-3′) [24,25]. Bacterial cells were cultured overnight in 2 mL of TSB at 35°C and then centrifuged at 5000g for 10 minutes. The cell pellet was washed and resuspended in 0.5 mL of Tris-EDTA-buffer (10 mM Tris-HCl, 1 mM EDTA; pH 8.0), and then lysed by 20% sodium dodecyl sulfate. After the solution was boiled for 20 minutes and the cellular debris was discarded following centrifugation at 13,000g for 3 minutes, total DNA in the supernatant was precipitated with 70% ethanol and used as a template DNA for PCR.
PCR amplification was performed in 20 μL reaction mixture containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 20 pmol of each primer, a 0.2 mM concentration for each of the four deoxynucleotide triphosphates, 0.5 U of Taq DNA poly-merase (Applied Biosystems, Foster City, CA, USA), and template DNA (10 ng). Amplifications were carried out for 35 cycles (94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 60 seconds) in a GeneAmp PCR 2400 Thermal Cycler (Applied Biosystems) with an initial denaturation at 94°C for 4 minutes and a final extension at 72°C for 7 minutes [24,25]. Amplicons were detected by electrophoresis on a 1.5% agarose gel staining with ethidium bromide. Amplicons were purified using a QIAquick PCR Purification Kit (Qiagen, Valencia, CA, USA) eluted in Tris-HCl buffer (10 mM, pH 8.5) prior to sequencing. The amplified DNA was directly sequenced with the ABI TaqDye Deoxy Terminator Cycle sequencing kit and ABI Model 377 automated DNA sequencer (Applied Biosystems). The sequences were analyzed with the BLAST (NCBI, Bethesda, MD, USA) for identification of histamine-forming bacteria.
2.5. Determination of TVBN
The TVBN content of the milkfish dumpling sample was measured by the method of Conway’s dish [26]. The TVBN extract of the fish sample in 6% trichloroacetic acid (TCA, Sigma, St. Louis, MO, USA) was absorbed by boric acid and then titrated with 0.02 N HCl. The TVBN content was expressed in mg/100 g fish.
2.6. Biogenic amine analysis
In total, nine biogenic amines contents were determined. Each milkfish dumpling sample was ground in a Waring Blender for 3 minutes. The ground samples (5 g) were transferred into 50 mL centrifuge tubes, and then homogenized with 20 mL of 6% TCA for 3 minutes. The homogenate was centrifuged (10,000g, 10 minutes, 4°C) and filtered through Whatman Number 2 filter paper (Whatman, Maidstone, England). The filtrate was then placed in a volumetric flask, and TCA was added to bring to a final volume of 50 mL. Samples of standard biogenic amine solutions and 1 mL aliquots of the milkfish dumpling extracts were derivatized with dansyl chloride according to the previously described method [5]. One milliliter amounts of each bacterial culture broth were also dansylated using the same procedures for milkfish dumpling extracts. The dansyl derivatives were filtrated through a 0.45 μm filter, and 20 μL aliquots were used for high performance liquid chromatography injection.
The contents of biogenic amines in the milkfish dumpling samples were determined with a high performance liquid chromatography system (Hitachi, Tokyo, Japan) consisting of a Model L-7100 pump, a Rheodyne Model 7125 syringe loading sample injector, a Model L-4000 UV-Vis detector (set at 254 nm), and a Model D-2500 Chromato-integrator. A LiChrospher 100 RP-18 reversed-phase column (5 μm, 125 mm = 4.6 mm, E. Merck, Darmstadt, Germany) was used for chromatographic separation. The gradient elution program began with 50:50 (v/v) acetonitrile:water at a flow rate of 1.0 mL/min for the 19 minutes, followed by a linear increase to 90:10 acetonitrile:water (1.0 mL/min) during the next 1.0 minute. The acetonitrile:water mix decreased to 50:50 (1.0 mL/ min) for 10 minutes.
2.7. Multiplex PCR for animal DNA analysis
The DNA extraction of milkfish dumpling meat was prepared according to the method of Dalmasso et al [18]. PCR amplification was conducted in 25 μL of 75 mM Tris-HCl (pH 8.8), 1.5 units of platinum Taq DNA polymerase (Invitrogen, Carlsbad, CA, USA), 0.2 mM dNTP mix, 2 mM MgCl2, 20 pmol, 20 pmol, 12.5 pmol, and 10 pmol of ruminant, pork, fish, and poultry primers, respectively, and 250 ng template. The sets of primers reported by Dalmasso et al [18] were ruminant primer (5′-GAAAGGACAAGAGAAATAAGG-3′ and 5′-TAGGCCCTTTTCTAGGGCA-3′), pork primer (5′-CTACATAA-GAATATCCACCACA-3′ and 5′-ACATTGTGGGATCTTCTAGGT-3′), fish primer (5′-TAAGAGGGCCGGTAAAACTC-3′ and 5′-GTGGGGTATCTAATCCCA-3′), and poultry primer (5′-TGA-GAACTACGAGCACAAAC-3′ and 5′-GGGCTATTGAGCT-CACTGTT-3′). Amplification was performed in a Thermal Cycler 2400 (Applied Biosystems, Foster City, CA, USA) with the following cycling conditions; after an initial heat denaturation step at 94°C for 10 minutes, 35 cycles were programmed as follows: 94°C for 30 seconds, 60°C for 1 minute, 72°C for 1 minute, and final extension at 72°C for 5 minutes. Amplicons were resolved by electrophoresis on 3% agarose gel (Invitrogen) run in Tris acetate EDTA buffer for 70 minutes at 110 V and stained with ethidium bromide (0.4 ng/mL) for 20 minutes [18].
2.8. Raoultella ornithinolytica strain, milkfish dumpling stuffing and storage conditions
In this study, three bacterial isolates can produce histamine in TSBH broth (Table 3). Among them, R. ornithinolytica MFS8 exhibited the highest histamine production and was used as the strain to inoculate in milkfish dumpling stuffing. The bacterium was grown on TSA slant, stored in a refrigerator (4°C), and transferred to a fresh TSA slant every month. One loop of the bacterial culture (TSA slant) was inoculated into TSB and incubated at 37°C for 18 hours. One milliliter of the enriched culture was serially diluted in 0.1% peptone water and 0.1 mL aliquots of the diluted culture were spread on APC agar (Difco) containing 0.5% NaCl. Bacterial colonies were counted after the plates were incubated at 35°C for 24 hours. The enriched culture was stored at 7°C before being used for sample inoculation. Based on the colony counts obtained, the enriched culture stored at 7°C for 24 hours was then serially diluted with 0.1% peptone water to obtain a culture suspension with the desired concentration.
Table 3.
Identification of histamine-forming bacteria isolated from milkfish dumpling samples by 16S rDNA, based on the output results from NCBI database analysis, and their production of histamine and other biogenic amines in culture broth.
| Strain | Organism identified | Percentage identity (%) | GenBank accession number | Biogenic amine production (ppm) | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| His | Cad | Spd | Spm | ||||
| MFS5 | Raoultella ornithinolytica | 99 | AB682291.1 | 276.6 | 194.0 | NDa | ND |
| MFS8 | Raoultella ornithinolytica | 99 | JF701186.1 | 561.8 | 205.5 | 0.5 | 0.1 |
| MFS9 | Enterobacter aerogenes | 99 | CP002824.1 | 404.0 | 42.0 | 27.0 | 26.0 |
ND, not detected (amine level < 0.05 ppm).
Cad = cadaverine; His = histamine; Spd = spermindine; Spm = spermine.
Fresh milkfish (the dorsal parts) that was kept in ice at the retail store was obtained from a local seafood market in Kaohsiung City, Taiwan. The purchased sample was wrapped in aseptic bags, placed in ice, and immediately transported to the laboratory for use within 4 hours. The skin of the fish dorsal parts was aseptically removed in a vertical laminar flow hood. After the fish meat was washed with absolute ethanol-acetone (1:1, v/v) and rinsed with sterile water [27], it was placed in a sterile food processor and ground to a mince. The cabbage (Brassica oleracea) was purchased from a supermarket of Kaohsiung City, washed with 50 ppm NaClO solution for 5 minutes, and rinsed with sterile water. Then, it was cut in a sterile food processor and ground to a mince. The tuna dumpling stuffing was prepared from mixing with ground milkfish meat-cabbage (1:1, w/w). Following the addition of the diluted bacterial culture to reach a concentration of 5 log CFU/g, the inoculated samples were well mixed by blending at a low speed, then aseptically dispensed in 30 g aliquots into sterile polyethylene bags, and stored at 4°C, 15°C, 25°C, and 37°C. Growth of R. ornithinolytica and contents of TVBN and histamine were monitored for test samples stored at 4°C, 15°C, 25°C, and 37°C. For samples stored at 25°C and 37°C, analyses were conducted every 12 hours. The inoculated stuffing samples that were stored at 4°C and 15°C were taken every 24 hours for analyses. The experiment was performed in triplicate in each storage temperature and sampling time.
2.9. Statistical analysis
Results were analyzed by analysis of variance and Duncan’s multiple range test. All statistical analysis was performed using the Statistical Package for Social Sciences, SPSS Version 16.0 for windows (SPSS Inc., Chicago, IL USA). Significances between means of treatments were established at a value of p < 0.05.
3. Results and discussion
3.1. Chemical and microbiological quality of milkfish dumpling samples
Values of the pH, salt content, moisture, TVBN, APC, TC, and E. coli in the 10 milkfish dumpling samples from 10 retail stores are presented in Table 1. The levels of pH, salt content, moisture, TVBN, APC, TC, and E. coli in all samples ranged from 5.12 to 6.01, 0.34% to 0.56%, 57.59% to 73.82%, 4.39 mg/ 100 g to 10.97 mg/100 g, 5.52 log CFU/g to 8.11 log CFU/g, 10,000 MPN/g to 120,000 MPN/g, and < 3 MPN/g to 5000 MPN/g, respectively. Based on the Taiwanese regulatory standard of 6.47 log CFU/g of APC for uncooked frozen foods, 80% (8/10) of the milkfish dumpling samples were unacceptable. Although a Taiwanese regulatory standard of TC for uncooked frozen foods is not set, all milkfish dumpling samples contained more than the 10,000 MPN/g of TC (Table 1). Six of the 10 tuna dumpling samples (60%) contained > 100 MPN/g of E. coli, which is more than the 50 MPN/g regulatory limit in Taiwan for uncooked frozen foods (Table 1). The contents of TVBN in all of the milkfish dumpling samples were below the Taiwanese regulatory level of 15 mg/100 g (Table 1). Based on the high levels of APC, TC, and E. coli detected in the milkfish dumpling samples, those commercial milkfish dumplings could have been seriously contaminated during processing or by vegetables (such as cabbage). These results are in agreement with the previous report of Kung et al [28], in which there was high content of APC, TC, and E. coli in commercial tuna dumpling products.
Table 1.
pH, salt content, moisture, total volatile base nitrogen (TVBN), aerobic plate count (APC), total coliforms (TC), and Escherichia coli in 10 milkfish dumpling samples from retail stores in southern Taiwan.
| Sample | pH value | Salt content (%) | Moisture (%) | TVBN (mg/100 g) | APC (log CFU/g) | TC (MPN/g) | E. coli (MPN/g) |
|---|---|---|---|---|---|---|---|
| 1 | 5.38 | 0.49 | 64.80 | 4.39 | 5.52 | 10,000 | < 3 |
| 2 | 6.01 | 0.43 | 60.44 | 4.90 | 6.58 | 15,400 | 200 |
| 3 | 5.12 | 0.48 | 58.93 | 10.78 | 8.11 | 28,700 | 100 |
| 4 | 5.79 | 0.47 | 67.68 | 5.97 | 6.38 | 19,400 | < 3 |
| 5 | 5.26 | 0.51 | 68.34 | 6.35 | 7.54 | 23,200 | 5000 |
| 6 | 5.61 | 0.52 | 68.62 | 7.14 | 6.68 | 27,300 | < 3 |
| 7 | 5.48 | 0.56 | 59.83 | 10.97 | 7.13 | 120,000 | 1700 |
| 8 | 5.74 | 0.40 | 60.67 | 8.35 | 6.77 | 14,000 | < 3 |
| 9 | 5.48 | 0.54 | 57.59 | 6.53 | 7.29 | 40,400 | 2200 |
| 10 | 5.38 | 0.34 | 73.82 | 8.12 | 7.35 | 16,500 | 400 |
| Mean ± S.D. | 5.52 ± 0.27 | 0.47 ± 0.07 | 64.07 ± 5.36 | 7.35 ± 2.25 | 6.93 ± 0.72 | 31,490 ± 32,326 | 960 ± 1623 |
APC = aerobic plate count; TC = total coliforms; TVBN = total volatile base nitrogen.
3.2. Contents of biogenic amines in milkfish dumpling samples
The contents of biogenic amines in the tested milkfish dumpling products are summarized in Table 2. None of the nine tested samples contained tryptamine, 2-phenylethylamine, and agmatine (Table 2). The average content of each of the remaining six biogenic amines in all samples was < 1.6 mg/100 g. The levels of histamine in all samples ranged from 0.05 mg/100 g to 0.32 mg/100 g, lower than the 5.0 mg/100 g of histamine that is the allowable limit of the US Food and Drug Administration (USFDA) for scombroid fish and/or product (Table 2). However, strong evidence exists that biogenic amines such as putrescine, cadaverine, spermine, and spermidine in fish tissue can increase the toxic effects of histamine by inhibiting intestinal histamine-metabolizing enzymes such as diamine oxidase. Therefore, the biogenic amines can increase histamine uptake and liberate endogenous histamine in intestinal fluids [29]. Quality loss and histamine accumulation often occur after frozen tuna or mackerel are thawed and kept for a long period of time at room temperature. Since histamine is heat resistant, its toxicity remains intact in canned or cooked fish products [30].
Table 2.
Levels of biogenic amines in 10 milkfish dumpling samples from retail stores in southern Taiwan.
| Sample | Biogenic amine content (mg/100 g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Put | Cad | Try | Phe | Spd | Spe | His | Tyr | Agm | |
| 1 | ND | 0.17 | ND | ND | 0.20 | 0.09 | 0.14 | ND | ND |
| 2 | NDa | 0.10 | ND | ND | 0.16 | 0.11 | 0.04 | ND | ND |
| 3 | ND | 0.15 | ND | ND | 0.15 | 0.06 | 0.17 | ND | ND |
| 4 | ND | 0.13 | ND | ND | 0.17 | 0.08 | 0.13 | 0.12 | ND |
| 5 | 1.54 | 0.14 | ND | ND | 0.15 | 0.05 | 0.05 | 0.24 | ND |
| 6 | ND | 0.15 | ND | ND | 0.16 | 0.06 | 0.05 | ND | ND |
| 7 | ND | 0.19 | ND | ND | 0.22 | 0.09 | 0.32 | ND | ND |
| 8 | ND | 0.28 | ND | ND | 0.22 | 0.08 | 0.05 | 0.23 | ND |
| 9 | ND | 0.43 | ND | ND | 0.21 | 0.06 | 0.14 | ND | ND |
| 10 | 1.20 | 0.13 | ND | ND | ND | ND | 0.04 | 0.21 | ND |
Agm = agmatine; Cad = cadaverine; His = histamine; Phe = 2-phenylethylamine; Put = putrescine; Spd = spermindine; Spe = spermine; Try = tryptamine; Tyr = tyramine.
ND, not detected (amine level < 0.05 mg/100 g).
3.3. Isolation and identification of histamine-forming bacteria from milkfish dumpling samples
Identification of three histamine-forming bacteria isolated from the milkfish dumpling products is listed in Table 3. The identification was determined by the 16S rDNA sequences and comparative analysis with the NCBI database. Three histamine-producing bacterial strains were identified as R. ornithinolytica (two strains), and E. aerogenes (one strain). Producing histamine capability of these bacteria ranged from 276.6 ppm to 561.8 ppm in TSB supplemented with 1.0% L-histidine (TSBH). Some of them also produced different amounts of cadaverine, spermidine, and spermine through the action of their respective decarboxylase enzymes on various amino acids that also existed in TSBH (Table 3).
In this study, all of the histamine-forming isolates belonged to the family Enterobacteriaceae, such as R. ornithinolytica and E. aerogenes. Meanwhile, R. ornithinolytica strains MFS5 and MSF8, and E. aerogenes strain MFS9 produced 276.6 ppm, 561.8 ppm, and 404.0 ppm of histamine in TSBH, respectively (Table 3). Raoultella ornithinolytica and E. aerogenes have often been isolated from various species of scombroid fish. Raoultella ornithinolytica and E. aerogenes were the most frequently reported prolific histamine formers in tuna [31], albacore [32], and sailfish [33]. Recently, R. ornithinolytica and E. aerogenes isolated from milkfish stick, dried milkfish, and tuna dumplings were reported to be potent histamine formers capable of producing > 400 ppm of histamine in TSBH [19,28,34]. Based on the results of the higher levels of APC, E. coli, TC, and histamine-forming bacteria strains, the tested samples could be seriously contaminated during food preparation and processing.
3.4. Adulteration of ruminant, pork and poultry meats in milkfish dumpling samples
Results of the multiplex PCR assay for meat species identification of ruminant, pork, fish and poultry are shown in Table 4. The four set primers generated specific fragments of 104 bp for ruminant, 290 bp for pork, 183 bp for poultry, and 224 bp for fish. As for all milkfish dumpling meats, the fish species has always been detected and no ruminant and poultry species were detected. However, five samples (5/10, 50%) contained pork meat, whereas, only five samples (5/10, 50%) did not contain pork (Table 4). Recently, our research group revealed that adulteration rates were 88.9% and 33.3% for pork and poultry, respectively, in tuna dumpling products [28], while 80% and 4% of tuna sausage products were adulterated with pork and poultry, respectively, in Taiwan [12]. In this study, although no ruminant and poultry was adulterated in milkfish dumpling products, 50% of samples contained pork. However, minced meat and its products can be attractive targets for adulteration by substituting or partially substituting inexpensive meat or adding proteins from animal origin. Mincing removes the morphological structures of meat muscle, and it is extremely difficult to identify one type of meat from another because the adulterated components are usually very similar to the authentic product [35]. In addition, the pork adulteration leads to serious problem on religious or national relationships. In Islam, consumption of products considered non-halal, such as those containing pork, is strictly forbidden. Some Islamic countries have established strict regulations for producers and importers to stamp their products with a halal certificate to distinguish them from non-halal products [36].
Table 4.
Results of ruminant, pork, fish, and poultry meat adulteration based on multiplex polymerase chain reaction (PCR) analysis of DNA from commercial milkfish dumpling samples.
| Sample | Presence of indicated restriction fragmenta | |||
|---|---|---|---|---|
|
| ||||
| Ruminant (104 bp) | Pork (290 bp) | Poultry (183 bp) | Fish (224 bp) | |
| 1 | − | − | − | + |
| 2 | − | − | − | + |
| 3 | − | + | − | + |
| 4 | − | + | − | + |
| 5 | − | + | − | + |
| 6 | − | + | − | + |
| 7 | − | − | − | + |
| 8 | − | − | − | + |
| 9 | − | + | − | + |
| 10 | − | − | − | + |
| No. with fragment/total no. (%) | 0/10 (0) | 5/10 (50) | 0/10 (0) | 10/10 (100) |
+, present; −, absent.
3.5. Bacterial growth and histamine production by R. ornithinolytica in milkfish dumpling stuffing
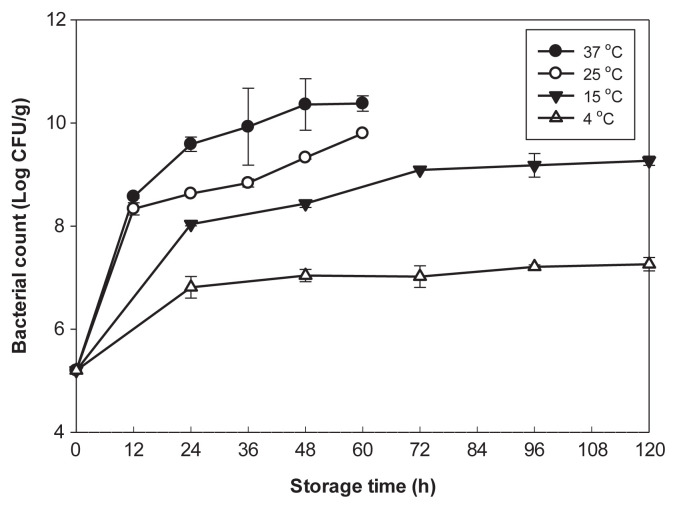
Raoultella ornithinolytica MFS8 inoculated at 5 log CFU/g grew rapidly in milkfish dumpling stuffing in 12 hours during storage at 25°C and 37°C. The bacterial number increased to 8.6 log CFU/g after 12 hours and to 10.4 log CFU/g after 48 hours at storage temperature of 37°C (Figure 1). At 25°C, R. ornithinolytica grew rapidly and reached 8.4 log CFU/g after 12 hours and 9.8 log CFU/g after 60 hours of storage. Enumeration of bacterial numbers with these samples was terminated at 60 hours for 25°C and 37°C due to the production of spoilage odors. The samples stored at 15°C supported gradual increases of the bacteria until they reached about 9.2 log CFU/g after 120 hours. However, growth of R. ornithinolytica was retarded in samples stored at 4°C up to 5 days of storage, and slowly increased to 7.2 log CFU/g after 120 hours (Figure 1). The bacterial counts stored at 37°C were significantly higher (p < 0.05) than those of samples stored at 25°C during 60 hours of storage. Bacterial populations in samples stored at 15°C were significantly higher (p < 0.05) than those of samples stored at 4°C at all times (Figure 1).
Figure 1.
Growth of Raoultella ornithinolytica in stuffing of milkfish dumpling inoculated with R. ornithinolytica MFS8 at 5.0 log colony forming units (CFU)/g during storage at 4°C, 15°C, 25°C, and 37°C. Each value represents the mean of three determinations ± standard deviation.
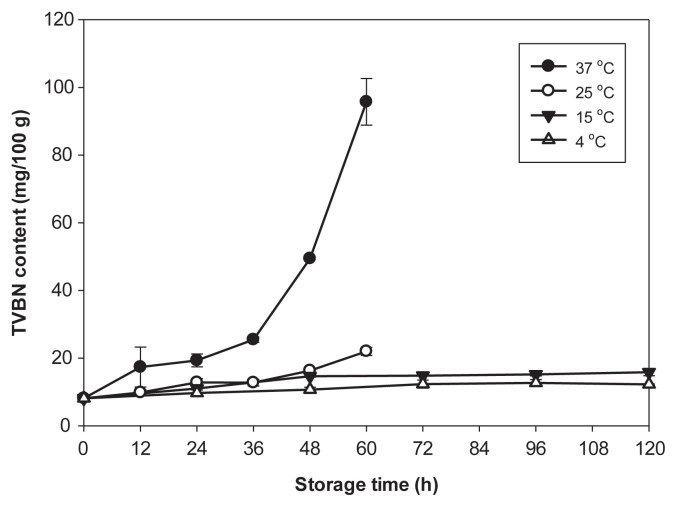
TVBN, including trimethylamine, dimethylamine, and ammonia, is one of the most widely used indicators for fish quality and spoilage [37]. The levels of TVBN increased rapidly in samples during storage at 37°C (Figure 2). TVBN in samples increased to 50 mg/100 g for 48 hours and to 96 mg/100 g for 60 hours after storage at 37°C. The TVBN levels all exceeded the decomposition limit level of 30 mg/100 g for fish quality determination. All samples stored at 25°C also had levels of TVBN < 25 mg/100 g during storage time, reaching 23.0 mg/ 100 g in 60 hours. When stored at 4°C and 15°C, the TVBN levels only slightly increased, reaching about 14 mg/100 g and 18 mg/100 g after 120 hours, respectively. Although the TVBN levels of the samples between 4°C, 15°C, and 25°C were not significantly different (p > 0.05) before 48 hours of storage, those of the samples at 37°C were significantly higher (p < 0.05) than those of samples stored at other temperatures during storage time (Figure 2). The increase of TVBN is related to the formation of volatile basic components, such as ammonia, trimethylamine, and others, by enzyme autolysis and bacterial spoilage. Therefore, the elevated temperature (37°C) can increase autolytic enzyme activity and bacterial proliferation.
Figure 2.
Formation of total volatile base nitrogen (TVBN) in stuffing of milkfish dumpling inoculated with Raoultella ornithinolytica MFS8 at 5.0 log colony forming units (CFU)/g during storage at 4°C, 15°C, 25°C, and 37°C. Each value represents the mean of three determinations ± standard deviation.
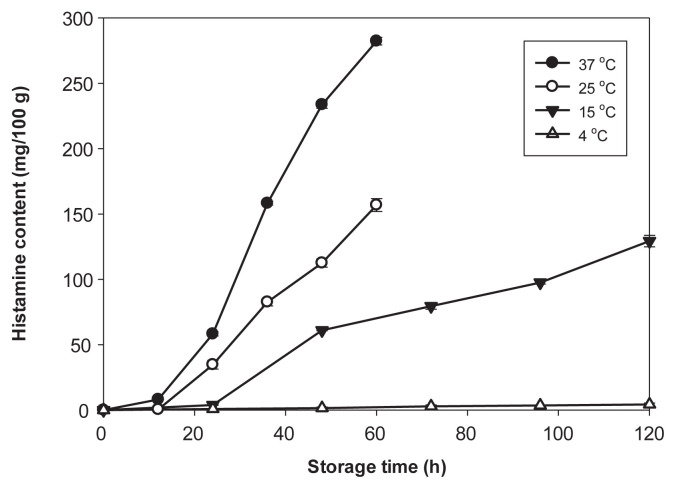
Formation of histamine in samples was significantly faster in samples stored at 25°C and 37°C than at 15°C and 4°C (p < 0.05) (Figure 3). Histamine contents increased to 35 mg/100 g and 60 mg/100 g after 24 hours of storage at 25°C and 37°C, respectively (p < 0.05). After 36 hours of storage, histamine contents increased rapidly to 80 mg/100 g at 25°C and 160 mg/ 100 g at 37°C, respectively (Figure 3). When the samples were stored at 15°C, a low level of histamine (5.3 mg/100 g) was detected in samples after 24 hours. Thereafter, the stuffing samples continued to accumulate histamine to 60 mg/100 g at 48 hours, while the highest level (130 mg/100 g) was detected in samples after 120 hours at 15°C. Histamine production in samples stored at 4°C for 120 hours was negligible (< 5.0 mg/ 100 g). According to the statistical analysis, the histamine contents of samples at 37°C for the same storage time were significantly higher than those of other storage temperatures (p < 0.05). Therefore, the optimal temperature for histamine production by R. ornithinolytica in spiked samples was 37°C. The highest levels of histamine were detected after growth of R. ornithinolytica had reached the late logarithmic phase in the samples stored at temperatures above 15°C. This corresponded to an early observation that maximum histidine decarboxylase activity was observed during the late logarithmic phase of bacterial growth [38]. Similarly, we previously demonstrated that high histamine contents were produced in milkfish muscle by E. aerogenes and in tuna dumpling stuffing by R. ornithinolytica during the late logarithmic phase of growth [20,39]. However, Kim et al [40] reported that the highest level of histamine was detected in contaminated fish muscle after M. morganii had reached the stationary phase of growth. The difference between the observations could be due to the use of different histamine producers and fish species in those studies.
Figure 3.
Change of histamine in stuffing of milkfish dumpling inoculated with Raoultella ornithinolytica MFS8 at 5.0 log colony forming units (CFU)/g during storage at 4°C, 15°C, 25°C, and 37°C. Each value represents the mean of three determinations ± standard deviation.
The USFDA has indicated that fish containing histamine at levels > 50 mg/100 g (500 ppm) should be considered a potential hazard for human health [41]. It is important to realize that the presence of histamine in a fish does not change the color or smell of that fish, because histamine is a colorless and odorless compound. A fish with no obvious sign of spoilage may contain a high level of histamine and be consumed. When the TVBN contents had not reached yet the decomposition index level of 30 mg/100 g, the histamine contents had increased to > 50 mg/100 g in samples stored at 37°C, 25°C, and 15°C for 24 hours, 36 hours, and 48 hours, respectively (Figures 2 and 3). Therefore, use of the TVBN value as an indicator to predict histamine contents and risk of histamine fish poisoning should be avoided.
4. Conclusion
This study showed that most milkfish dumpling products sold in southern Taiwan contained APC and E. coli levels greater than Taiwanese regulatory limit of 6.47 log CFU/g and 50 MPN/g, respectively. The histamine contents in all tested milkfish dumpling products were < the 5 mg/100g USFDA guideline. The R. ornithinolytica (two strains) and E. aerogenes (one strain) isolates were proven to be prolific histamine-formers with ability to produce > 276 ppm histamine in TSBH medium. All dumpling samples contained fish meat, but half of them were adulterated with pork. In addition, we demonstrated that the improperly prepared milkfish dumpling stuffing with R. ornithinolytica contamination could lead to production of hazardous levels of histamine over time when stored at temperatures > 15°C. At this short storage time in a refrigerator, even the contaminated milkfish dumpling stuffing with histamine formers, such as R. ornithinolytica, will not produce hazardous levels of histamine to cause foodborne poisoning.
Acknowledgments
The study was supported by the National Science Council, Taiwan, R.O.C. (Contract Number MOST 104-2320-B-022-001).
Funding Statement
The study was supported by the National Science Council, Taiwan, R.O.C. (Contract Number MOST 104-2320-B-022-001).
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
REFERENCES
- 1. Taylor SL. Histamine food poisoning: toxicology and clinical aspects. Crit Rev Toxicol. 1986;17:91–117. doi: 10.3109/10408448609023767. [DOI] [PubMed] [Google Scholar]
- 2. Chen KT, Malison MD. Outbreak of scombroid fish poisoning, Taiwan. Am J Public Health. 1987;77:1335–6. doi: 10.2105/ajph.77.10.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tsai YH, Kung HF, Lee TM, Chen HC, Chou SS, Wei CI, Hwang DF. Determination of histamine in canned mackerel implicated in a food born poisoning. Food Control. 2005;16:579–85. [Google Scholar]
- 4. Chen HC, Kung HF, Chen WC, Lin WF, Hwang DF, Lee YC, Tsai YH. Determination of histamine and histamine-forming bacteria in tuna dumpling implicated in a food-borne poisoning. Food Chem. 2008;106:612–8. [Google Scholar]
- 5. Chen HC, Huang YR, Hsu HH, Lin CS, Chen WC, Lin CM, Tsai YH. Determination of histamine and biogenic amines in fish cubes (Tetrapturus angustirostris) implicated in a food borne poisoning. Food Control. 2010;21:13–8. [Google Scholar]
- 6. Chang SC, Kung HF, Chen HC, Lin CS, Tsai YH. Determination of histamine and bacterial isolation in swordfish fillets (Xiphias gladius) implicated in a food borne poisoning. Food Control. 2008;19:16–21. [Google Scholar]
- 7. Chen HC, Lee YC, Lin CM, Hwang DF, Tsai YH. Determination of histamine and bacterial isolation in marlin fillets (Makaira nigricans) implicated in a foodborne poisoning. J Food Saf. 2010;30:699–710. [Google Scholar]
- 8. Tsai YH, Kung HF, Chen HC, Chang SC, Hsu HH, Wei CI. Determination of histamine and histamine-forming bacteria in dried milkfish (Chanos chanos) implicated in a food-born poisoning. Food Chem. 2007;105:1289–96. [Google Scholar]
- 9.An H, Ben-Gigirey B. Scombrotoxin poisoning. In: Millar I, Gray D, Strachan N, editors. Microbiology of seafoods. London: Chapman & Hall Ltd; 1998. pp. 68–9. [Google Scholar]
- 10.Stratton JE, Taylor SL. Scombroid poisoning. In: Kvenberg JE, editor. Microbiology of marine food products. 2nd ed. New York: AVI Publishing; 1991. pp. 331–51. [Google Scholar]
- 11. Kim SH, Barros-Velazquez J, Ben-Gigirey B, Eun JB, Jun SH, Wei CI, An H. Identification of the main bacteria contributing to histamine formation in seafood to ensure product safety. Food Sci Biotechnol. 2003;12:451–60. [Google Scholar]
- 12. Kung HF, Tsai YH, Chang SS, Hong TY. Biogenic amine content, histamine-forming bacteria, and adulteration of pork in tuna sausage products. J Food Prot. 2012;75:1814–22. doi: 10.4315/0362-028X.JFP-12-061. [DOI] [PubMed] [Google Scholar]
- 13. Momcilovic D, Rasooly A. Detection and analysis of animal materials in food and feed. J Food Prot. 2000;63:1602–9. doi: 10.4315/0362-028x-63.11.1602. [DOI] [PubMed] [Google Scholar]
- 14. Chikuni K, Ozutsumi K, Koishikawa T, Kato S. Species identification of cooked meats by DNA hybridization assay. Meat Sci. 1990;27:119–28. doi: 10.1016/0309-1740(90)90060-J. [DOI] [PubMed] [Google Scholar]
- 15. Fei S, Okayama T, Yamanoue M, Nishikawa I, Mannen H, Tsuji S. Species identification of meats and meat products by PCR. Anim Sci Tech. 1996;67:900–5. [Google Scholar]
- 16. Saiki RK, Schart S, Faloona F, Mullis KB, Horn GT, Erlich HA, Arnheim N. Enzymatic amplification of β-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985;230:1350–4. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- 17. Matsunaga T, Chikuni K, Tanabe R, Muroya S, Shibata K, Yamada J, Shinmura Y. A quick and simple method for the identification of meat species and meat products by PCR assay. Meat Sci. 1999;51:143–8. doi: 10.1016/s0309-1740(98)00112-0. [DOI] [PubMed] [Google Scholar]
- 18. Dalmasso A, Fontanella E, Piatti P, Civera T, Rosati S, Bottero MT. A multiplex PCR assay for the identification of animal species in feedstuffs. Mol Cell Probes. 2004;18:81–7. doi: 10.1016/j.mcp.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 19. Lee YC, Kung HF, Wu CH, Hsu HM, Chen HC, Huang TC, Tsai YH. Determination of histamine in milkfish stick implicated in a foodborne poisoning. J Food Drug Anal. 2016;24:63–71. doi: 10.1016/j.jfda.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee YC, Kung HF, Lin CS, Hwang CC, Lin CM, Tsai YH. Histamine production by Enterobacter aerogenes in tuna dumpling stuffing at various storage temperatures. Food Chem. 2012;131:405–12. [Google Scholar]
- 21.AOAC. Official Methods of Analysis of AOAC International. 16th ed. Gaithersburg, MD: AOAC International; 1995. [Google Scholar]
- 22. Niven CF, Jeffreg MB, Corlett DA. Differential plating medium for quantitative detection of histamine-producing bacteria. Appl Environ Microbiol. 1981;41:321–2. doi: 10.1128/aem.41.1.321-322.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Food and Drug Administration. Bacteriological Analytical Manual. Gaithersburg, MD: AOAC International; 1998. [Google Scholar]
- 24. Kuhnert P, Capaul S, Nicolet J, Frey J. Phylogenetic positions of Clostridium chauvoei and Clostridium septicum based on 16S rRNA gene sequences. Int J Syst Bacteriol. 1996;46:1174–6. doi: 10.1099/00207713-46-4-1174. [DOI] [PubMed] [Google Scholar]
- 25. Kuhnert P, Heyberger-Meyer B, Nicolet J, Frey J. Characterization of PaxA and its operon: a cohemolytic RTX toxin determinant from pathogenic Pasteurella aerogenes. Infect Immun. 2000;68:6–12. doi: 10.1128/iai.68.1.6-12.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cobb BF, Aoaniz I, Thompson CA. Biochemical and microbial studies on shrimp: volatile nitrogen and amino nitrogen analysis. J Food Sci. 1973;38:431–5. [Google Scholar]
- 27. Lin CS, Kung HF, Lin CM, Tsai HC, Tsai YH. Histamine production by Raoultella ornithinolytica in mahi-mahi meat at various storage temperature. J Food Drug Anal. 2016;24:305–10. doi: 10.1016/j.jfda.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kung HF, Lee YC, Huang YR, Lin WF, Lin CM, Chen WC, Tsai YH. Biogenic amines content, histamine-forming bacteria, and adulteration of pork and poultry in tuna dumpling products. Food Control. 2010;21:977–82. [Google Scholar]
- 29. Flick GJ, Oria MP, Douglas L. Potential hazards in cold-smoked fish: Biogenic amines. J Food Sci. 2001;66(Suppl):1088–99. [Google Scholar]
- 30. Lopez-Sabater EI, Rodriguez-Jerez JJ, Roig-Sagues AX, Mora-Ventura MAT. Bacteriological quality of tuna fish (Thunnus thynnus) destined for canning: Effect of tuna handling on presence of histidine decarboxylase bacteria and histamine level. J Food Prot. 1994;57:318–23. doi: 10.4315/0362-028X-57.4.318. [DOI] [PubMed] [Google Scholar]
- 31. Lopez-Sabater EI, Rodriguez-Jerez JJ, Hernandez-Herrero M, Roig-Sagues AX, Mora-Ventura MAT. Sensory quality and histamine formation during controlled decomposition of tuna (Thunnus thynnus) J Food Prot. 1996;59:167–74. doi: 10.4315/0362-028X-59.2.167. [DOI] [PubMed] [Google Scholar]
- 32. Kim SH, Field KG, Morrissey MT, Price RJ, Wei CI, An H. Source and identification of histamine-producing bacteria from fresh and temperature-abused albacore. J Food Prot. 2001;64:1035–44. doi: 10.4315/0362-028x-64.7.1035. [DOI] [PubMed] [Google Scholar]
- 33. Tsai YH, Kung HF, Lee TM, Lin GT, Hwang DF. Histamine related hygienic qualities and bacteria found in popular commercial scombroid fish fillets in Taiwan. J Food Prot. 2004;67:407–12. doi: 10.4315/0362-028x-67.2.407. [DOI] [PubMed] [Google Scholar]
- 34. Hsu HH, Chuang TC, Lin HC, Huang YR, Lin CM, Kung HF, Tsai YH. Histamine content and histamine-forming bacteria in dried milkfish (Chanos chanos) products. Food Chem. 2009;114:933–8. [Google Scholar]
- 35. Hargin KD. Authenticity issues in meat and meat products. Meat Sci. 1996;43:277–89. doi: 10.1016/0309-1740(96)00072-1. [DOI] [PubMed] [Google Scholar]
- 36. Hermanto S, Fatimah W. Differentiation of bovine and porcine gelatin based on spectroscopic and electrophoretic analysis. J Food Pharm Sci. 2013;1:68–72. [Google Scholar]
- 37. Gill TA. Objective analysis of seafood quality. Food Rev Int. 1990;6:681–714. [Google Scholar]
- 38. Behling AR, Taylor SL. Bacterial histamine production as a function of temperature and time of incubation. J Food Sci. 1982;47:131–4. 137. [Google Scholar]
- 39. Tsai YH, Chang SC, Kung HF, Wei CI, Hwang DF. Histamine production by Enterobacter aerogenes in sailfish and milkfish at various storage temperatures. J Food Prot. 2005;68:1690–5. doi: 10.4315/0362-028x-68.8.1690. [DOI] [PubMed] [Google Scholar]
- 40. Kim SH, Price RJ, Morrissey MT, Field KG, Wei CI, An H. Histamine production by Morganella morganii in mackerel, albacore, mahi-mahi, and salmon at various storage temperatures. J Food Sci. 2002;67:1522–8. [Google Scholar]
- 41.USFDA (US Food and Drug Administration) Fish and fishery products hazards and controls guide. 3rd ed. Washington, D.C: Department of Health and Human Services, Public Health Service, Food and Drug Administration, Center for Food Safety and Applied Nutrition, Office of Seafood; 2001. Chapter 7 Scombrotoxin (histamine) formation; pp. 73–93. [Google Scholar]