Abstract
Carbonyl iron powder (CIP) has been used as a food additive or mineral supplement. However, the effects of CIP on iron deficiency anemia (IDA) and its subchronic toxicity have not been investigated. We found that oral administration of CIP at a dose of 2.96 mg/kg recovered the hemoglobin concentration of erythrocytes of IDA rats to the normal level after 8 days. The no observed adverse effect level of CIP in rats was considered to be > 200 mg/kg. The hematological and serum biochemical parameters of the rats did not differ significantly between the control and treated groups. There were no morphological changes observed in the organs including liver, kidneys, spleen, testes, stomach and intestine. Therefore, CIP might be a safe iron supplement.
Keywords: carbonyl iron powder, iron deficiency anemia, iron supplements, subchronic toxicity
1. Introduction
Iron deficiency anemia (IDA) attributable to nutritional deficiency and/or blood loss remains the most common, treatable form of anemia worldwide [1]. World Health Organization reports indicate that 2 billion people (> 30% of the world’s population) have anemia, with ~1 billion having IDA [2]. IDA is one of the most prevalent nutritional problems in the world today [3]. According to more recent reports, the global prevalence of anemia is 47% in children GED < 5 years, 30% in nonpregnant women of childbearing age, and 42% in pregnant women, while the prevalence of IDA in pregnant women in developing countries is 35–75% [4]. Iron participates in numerous biochemical reactions primarily involved in oxygen transport and storage, adenosine triphosphate production, DNA synthesis, and electron transport [5,6]. Iron deficiency may have specific effects on the central nervous system, either on neurotransmitters, or on cells or myelin, or there may be a direct effect of anemia that leads to low oxygen delivery to the brain [7]. IDA in adults can lead to a wide variety of adverse health outcomes, including diminished work capacity, impaired thermoregulation, immune dysfunction, gastrointestinal disturbances, and Helicobacter pylori infection [8–12]. IDA in children can result in neurocognitive impairment leading to psychomotor and cognitive abnormalities, which, if left unchecked, impairs learning [13,14]. IDA during pregnancy has long been associated with an increased risk of low birth weight, preterm delivery, perinatal mortality, and infant and young child mortality as well as maternal mortality [15–18].
Oral iron supplementation is a commonly used strategy to meet the increased requirements of high-risk groups, such as women of childbearing age [19]. Different generations of iron supplements have been developed, including organic and inorganic forms. Ferrous iron salts (ferrous sulfate and ferrous gluconate) are preferred because of their low cost and high bioavailability [20]. With regard to the first generation of iron supplements, ferrous sulfate is generally regarded as toxic to the gastrointestinal mucosa, resulting in poor compliance and treatment failure. Also, it has been demonstrated that ferrous sulfate can increase plasma malondialdehyde, a marker of lipid peroxidation, supporting the notion that ferrous iron may aggravate oxidative tissue damage [21]. The International Nutritional Anemia Consultative Group has reviewed the use of FeNa-EDTA as a food supplement. This compound is suggested to be the most suitable iron fortifier in developing countries where programs for the fortification of cereals, legumes, and infant complementary foods are implemented [22]. The advantage of FeNa-EDTA is that the iron present in this compound is prevented from interacting with phytates, which results in 2–3 times better absorption, compared to that obtained when other iron compounds are used in fortified diets [22]. FeNa-EDTA is reported in cereal-based and dairy foods [23,24]. However, a more recent study suggested that moderate FeNa-EDTA supplementation could improve hematological status in pregnant women with anemia [25]. Ferrous bisglycinate is a chelate that is composed of two molecules of glycine chelating to an atom of ferrous iron by covalent and coordinate covalent bonds. This particular form of chelated iron is effective in reversing IDA in adults, adolescents, and young children [26–28].
Carbonyl iron powder (CIP) is a novel material that has begun to be used as an iron supplement [29]. CIP is elemental iron with > 98% iron content, whose key physical property is its fine spherical particle size (5 μm), and it is considerably smaller than the 10–100 μm of other forms of elemental iron (e.g., reduced, electrolytic and atomized). Carbonyl iron has been approved as a mineral in dietary supplements. The US Food Chemicals Codex provides CIP particle size 95% through 325 mesh standard sieve, and with > 98% pure iron, can be added to foods [30]. Carbonyl iron was verified as a safe material for iron supplementation because its 50% lethal dose was > 5 g Fe/kg, compared with 1.3 g Fe/kg was for FeNa-EDTA, 2.8 g Fe/kg for ferrous bisglycinate, and 1.1 mg Fe/kg for FeSO4 [31,32]. Carbonyl iron was once used for iron fortification in whole-grain wheat flour in some cell models, however, studies on the use of CIP as an iron supplement (usually in higher doses, without food) in models of IDA are scarce [33].
Therefore, the objective of this study was to investigate whether CIP could be a possible iron supplement. CIP was administered to young male rats with IDA to observe its influence on hemoglobin (HGB) and other blood biochemical characteristics of rats, using ferrous sulfate as a positive control. In addition, to assess the safety of CIP when utilized for food fortification or medicinal supplementation on a daily basis, 90-day subchronic toxicity was also investigated in rats.
2. Materials and methods
2.1. Materials
CIP was purchased from International Specialty Products (Lakewood, WA, USA). Ferroids (sustained-release tablets of ferrous sulfate and vitamin complex) were produced by Medtech Xinghua Pharmaceutical Co. Ltd. (Guangzhou, China). Each tablet contained 525 mg ferrous sulfate, 500 mg vitamin C, and inactive ingredients, such as starch and dextrin. The composition of the low-iron diets used to induce the rat model of anemia were: corn starch, 640 g/kg; egg white powder, 200 g/kg; soybean oil, 40 g/kg; mineral mixture without iron, 25 g/kg; vitamin mixture, 12 g/kg; gluten, 30 g/kg; sodium chloride, 5 g/kg; calcium carbonate, 20 g/kg; DL-methionine, 3 g/kg; glucose, 25 g/kg. The formula of the mineral mixture without iron (1 kg) was: Ca(H2PO3)2, 500 g; NaCl, 104 g; K3C6H5O7·H2O, 220 g; KH2PO4, 94 g; K2SO4, 52 g; MgO, 24 g; MnCO3, 3.5 g; ZnCO3, 1.6 g; CuCO3, 300 mg; KI, 10 mg; Na2SeO3, 10 mg; KCr(SO4)2·12H2O, 55 mg; NaF, 6.35 mg; and Na2SiO3·9H2O, 145 mg. The vitamin mixture (1 kg): vitamin A (250,000 IU/g), 56.4 g; vitamin D (400,000 IU/g), 8.8 g; vitamin E (250 IU/g), 705 g; vitamin B1, 21 g; vitamin B2, 21 g; vitamin B6, 25 g; vitamin B12, 35 mg; vitamin K, 175 mg; biotin, 705 mg; folic acid, 7 g; pantothenic acid, 56 g; nicotinic acid, 106 g. The normal diet was prepared in compliance with GB14924.3-2001 for rat formula feeds issued by the Chinese National Institute of Standards for Laboratory Animals. The normal diet had other materials added, such as sesame, milk and raw eggs, thereby having more iron compared with the low-iron diet.
2.2. Animal procedures
Six-week-old male Wistar rats breeding colony (Shanghai SLAC Laboratory Animal Co. Ltd., Shanghai, China) were randomly divided into nine groups of 10. Group 1 was the control group and fed with a normal diet. Animals in Groups 2–6 were rendered anemic through low-iron diets over a period of 5 weeks, because continued depletion of iron stores leads to IDA and serious biological impairment [34]. The concentration of HGB was determined every week. When the HGB concentration was < 130 g/L, the rat model of IDA was prepared [35]. The rats in Groups 3–6 were given different iron supplements of 1.48 mg/kg, 2.96 mg/kg, and 14.8 mg/kg CIP, and Ferroids containing 14.8 mg/kg iron, respectively, by gavage. The rats in Group 2 were used as a negative control. The HGB concentration in all rat groups was determined once every 2 days thereafter during Groups 3–6 were treated with the iron supplementation. The rats of groups 7–9 received a normal diet, and Group 7 served as a control without blood collection before dissection. Groups 8 and 9 received 100 mg/kg and 200 mg/kg CIP orally, respectively. The animals were weighed individually on the first day, and once every 3 days thereafter. Also, the food intake of the rats was measured once every 3 days. Animals were killed by dislocation of cervical vertebrae at the end of 13 weeks, and all animals were fasted overnight prior to killing. All the animals were housed in stainless steel cages and maintained in a temperature- and light-controlled environment. The environmental conditions were: 12-hour light/dark cycle, a temperature range of 20–24°C, and a relative humidity of 35–60%.
All the above experiments were conducted according to the guidelines issued by the institutional Animal Care Committee and in compliance with regulations formulated by the Science and Technology Commission of Shanghai Municipality.
2.3. Hematological and serum biochemical determination
Venous blood samples from fossa orbitalis were drawn and collected in 1.5-mL Eppendorf tubes coated with Na2-EDTA for the measurement of the hematological parameters, using a Sysmex XT-1800i hematology analyzer (Sysmex Medical Electronics, Kobe, Japan), including white blood cell count, erythrocyte count, HGB, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin concentration, and platelet count. The serum biochemical parameters included alanine aminotransferase (ALT), glutamic oxaloacetic transaminase (GOT), total protein (TP), albumin (ALB), globulin (GLB), blood urea nitrogen, creatinine, blood glucose, triglyceride and total cholesterol, which were determined by Hitachi 7150 analyzer (Tokyo, Japan).
2.4. Morphological study
Complete necropsy was performed on the treated and control animals and organ weights of liver, kidneys, spleen and testes were measured at the end of the study. After fixation in 10% phosphate-buffered formalin, liver, kidneys, spleen, stomach, ileum and testes were processed in a routine manner, embedded in paraffin, and sectioned. Tissue sections were examined by light microscopy after staining with hematoxylin and eosin.
2.5. Statistical analysis
Experimental data were expressed as mean ± standard deviation of 10 animals and were analyzed using analysis of variance with the Scheffé multiple comparison method, which was applied only if significant differences were determined. Differences were considered significant at p < 0.05.
3. Results and discussion
3.1. Effect of CIP on concentration of HGB in erythrocytes
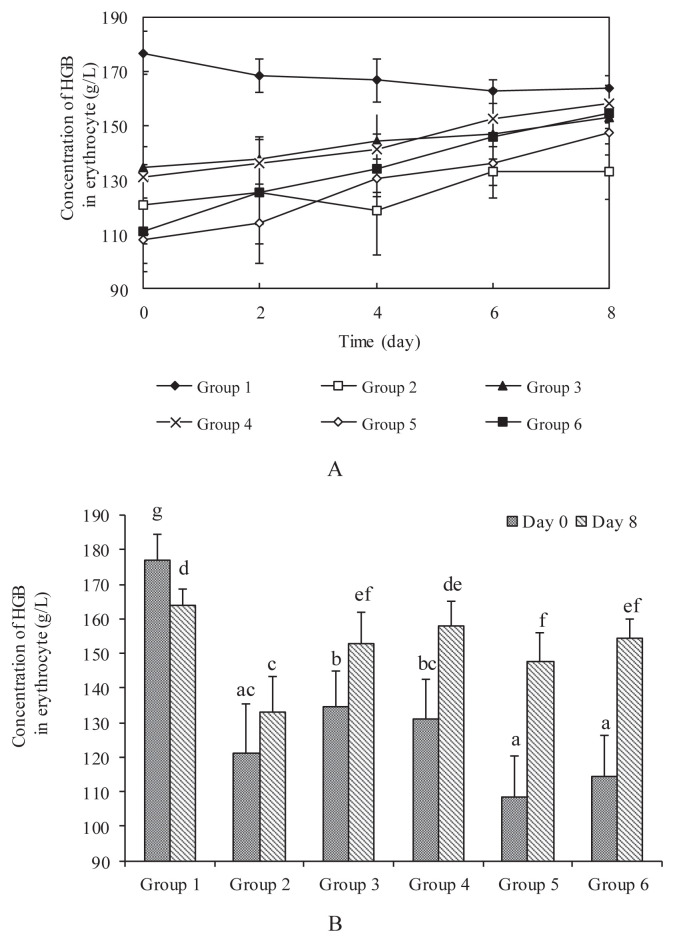
The average HGB concentration in erythrocytes of healthy rats (Group 1 controls) fed with normal feedstuff for 8 weeks was 176.8 ± 7.9 g/L, while that of rats in Groups 2–6 fed an iron-deficient diet for 8 weeks decreased to 119.3 ± 15.0 g/L. According to Yuan et al [35], rats are considered anemic when the HGB level is reduced to 25% of the original level. Groups 3–6 were fed with iron supplements, CIP at doses of 1.48 mg/kg, 2.96 mg/kg and 14.8 mg/kg and Ferroids containing 14.8 mg/kg iron, and HGB concentration was determined every 2 days. Figure 1A shows the changes in HGB concentration in erythrocytes of rats treated with different iron supplements for 8 days. The HGB concentration in Groups 3–5 fed with different doses of CIP increased to 18.1 g/L, 27.0 g/L and 39.4 g/L after 8 days, respectively, which indicated a concentration-dependent curative effect. The effect of CIP and Ferroids on HGB concentration is compared in Figure 1A. Under the condition of same dosage of Fe, 14.8 mg/kg, the average HGB concentration increased in Group 5 to 39.4 g/L and to 40.1 g/L in Group 6, indicating that CIP possessed similar bioavailability as Fe2+.
Figure 1.
Effect of different doses of oral CIP on HGB concentration in erythrocytes of male Wistar rats. (A) Time courses of HGB concentration of all experimental groups. (B) HGB concentration at Day 0 and Day 8 in all experimental groups. Group 1 was used as a control, which was fed with normal diet. Groups 2–6 were rendered anemic through their diet over a period of 8 weeks. Group 2 was used as a negative control fed with a low-iron diet throughout the experimental period. Groups 3–6 were given different iron supplements such as 1.48 mg/kg, 2.96 mg/kg and 14.8 mg/kg CIP and Ferroids containing 14.8 mg/kg Fe, respectively, by oral administration for 8 days. HGB concentration in all groups was determined once every 2 days during iron supplementation. Values are expressed as means ± standard deviation (n = 10). Means for a variable not sharing a common symbol (a–g) are significantly different (p < 0.05). CIP = carbonyl iron powder; HGB = hemoglobin.
The concentration of HGB in erythrocytes in Groups 3–6 was restored to the normal level after 8 days iron supplementation (Figure 1B). No significant difference was found between Group 1 and Group 4 (p > 0.05), which meant that a medium dose of iron supplementation (2.96 mg/kg CIP) for 8 days returned HGB level of IDA rats to normal. No significant difference was found between Group 3 and Group 5 (p > 0.05), which suggested that a high dose of iron supplementation (14.8 mg/kg CIP) did not result in a continued increase in HGB level. The above results demonstrate that a medium dose of CIP is enough for remission from IDA, which might be because of the limitation to iron absorption at high dose. When Group 5 was compared with Group 6, which was fed with Ferroids for 8 days, the average HGB concentration in erythrocytes did not differ significantly (p > 0.05). There was no significant difference between Group 3 and Group 6 (p > 0.05) or between Group 4 and Group 6 (p > 0.05). The above results show that the CIP has a similar effect on IDA as Ferroids with the same iron concentration. Therefore, CIP had at least the same bioavailability as ferrous sulfate, and this result agrees with the finding that CIP was reported to be more effective than traditional iron supplements like ferrous sulfate [31]. When some natural iron tonics, such as squid ink melanin-Fe, were administered to IDA rats at a dose of 2 mg/kg Fe for 25 days, the level of HGB approached normal. When heme iron at a dose of 2 mg/kg Fe was fed to IDA rats, 45 days were needed for HGB level to recover to normal [36,37]. All these results suggest that CIP can be used as an effective iron supplement.
3.2. Subchronic 90-day toxicity study
It is well established that iron can initiate hepatic lipid peroxidation [38]. Iron overdose results in both local and systemic effects. Large overdoses exceed the iron-binding capacity of transferrin, leaving free iron circulating in the plasma and being stored in target organs [39]. Although previous acute toxicity studies have indicated that carbonyl iron has a low toxicity level as compared with FeSO4 and FeNa-EDTA, the long-term effects based on toxicology are unknown [40]. Thus, we investigated the long-term effects of orally administered CIP at doses of 100 mg/kg and 200 mg/kg and tested for hematological, biochemical, morphological and histological data.
CIP was administered by gavage at doses of 100 mg/kg and 200 mg/kg, which were based on the 50% lethal dose (> 5 g/kg Fe) (data not shown). There were no deaths among the rats treated with CIP during the 90-day observation period, and all rats appeared to be healthy and normal. There were no abnormal signs in behavior, breathing, sensory nervous system responses, or gastrointestinal effects in the rats treated with CIP at a dose of 200 mg/kg, which was the highest dose that could be administered by gavage to rats. Accordingly, the no observed adverse effect level (NOAEL) of CIP was > 200 mg/kg. A reported NOAEL of iron sulfate was ~100 mg/kg/day in a 90-day study in mice, which showed liver and spleen effects (hemosiderosis) [41].
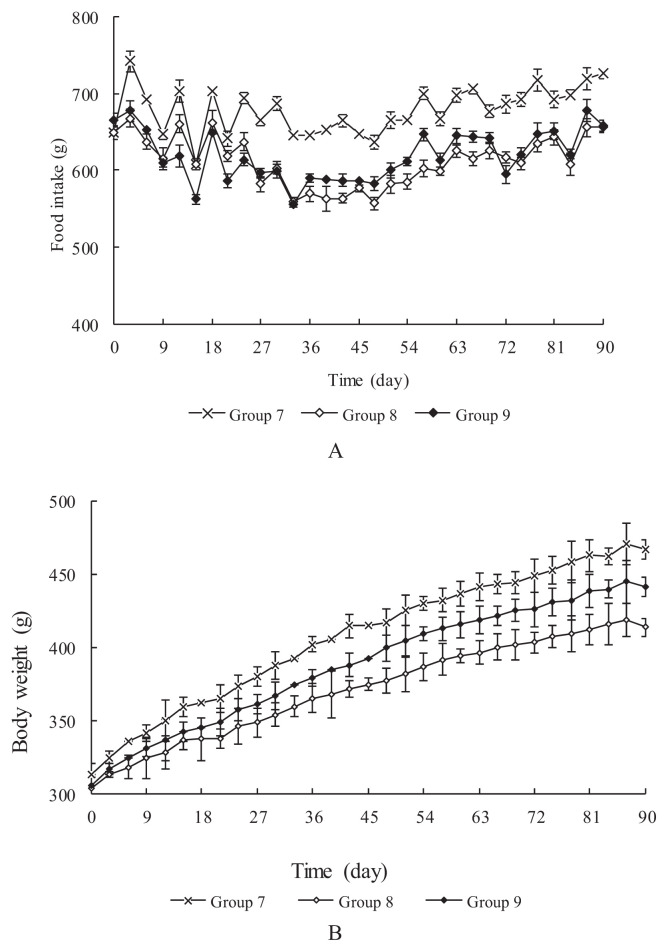
The food intake of the rats was monitored for 90 days (Figure 2A). There were some differences between control Group 7 and treated Groups 8 and 9. Such differences might have been because large doses of CIP resulted in deposition of iron in the stomach and reduced appetite, which led to lower food intake. However, there was no difference between Groups 8 and 9, even though the CIP dose differed twofold between the groups. The body weight of the rats was also monitored for 90 days (Figure 2B). Mean body weight increased slightly on the day following treatment, and the treated groups showed a lower average final weight than the control group, which coincided with the food intake curve.
Figure 2.
Mean food intake (A) and body weight (B) of male Wistar rats (10 per group) over the course of the subchronic 90-day toxicity study of dietary carbonyl iron powder.
The hematological profiles of the control and treated groups are presented in Table 1. Repeated oral administration for 90 days did not cause significant changes in hematological parameters. The serum biochemical parameters in the 90 days chronic toxicity study are summarized in Table 2. There was no difference in most serum biochemical parameters among the control and treated groups except for GOT (p < 0.05) and GLB (p < 0.05) of Group 9. Evaluation of GOT activity is a basic procedure for the diagnosis and monitoring of hepatocellular disorders or muscle damage. The increase in GOT correlates well with the extent and severity of cellular damage [42]. However, the GOT value of Group 9 was lower than that of the control group, which does not lead to the conclusion that CIP damaged liver and muscle. GLB has an immune function. Increased GLB may result from hepatocellular damage, chronic infection, or other diseases [43]. However, GLB index is insufficient for the diagnosis of liver diseases. So, to establish the extent of liver damage, comprehensive analysis of serum biochemical parameters combined with data on serum ALT is necessary.
Table 1.
Effect of oral carbonyl iron powder on hematological parameters in Wistar rats treated for 90 consecutive days.
| Parameter | Group 7 | Group 8 | Group 9 |
|---|---|---|---|
| WBC (109/L) | 12.6 ± 0.8 | 12.3 ± 0.8 | 12.2 ± 0.7 |
| RBC (1012/L) | 9.9 ± 0.4 | 9.9 ± 0.2 | 10.0 ± 0.2 |
| HGB (g/L) | 176.3 ± 2.5 | 177.7 ± 3.3 | 177.7 ± 3.1 |
| HCT (%) | 51.4 ± 1.4 | 51.4 ± 1.2 | 51.2 ± 0.8 |
| MCV (fL) | 52.4 ± 0.5 | 52.1 ± 0.6 | 52.1 ± 0.4 |
| MCHC (g/L) | 342.9 ± 3.8 | 344.6 ± 3.6 | 345.0 ± 3.5 |
| PLT (109/L) | 1021.3 ± 34.5 | 1028.2 ± 37.0 | 1037.9 ± 33.1 |
HCT = hematocrit; HGB = hemoglobin; MCHC = mean corpuscular hemoglobin concentration; MCV = mean corpuscular volume; PLT = platelet count; RBC = red blood cell count; WBC = white blood cell count.
Table 2.
Effect of oral carbonyl iron powder on serum biochemical parameters in Wistar rats treated for 90 consecutive days.
| Parameter | Group 7 | Group 8 | Group 9 |
|---|---|---|---|
| ALT (U/L) | 87.3 ± 10.6 | 76.2 ± 9.7 | 90.3 ± 16.3 |
| GOT (U/L) | 149.2 ± 12.2 | 130.8 ± 16.8 | 129.7 ± 25.3 |
| TP (g/L) | 69.3 ± 2.3 | 72.8 ± 2.2 | 73.3 ± 6.3 |
| ALB (g/L) | 44 ± 1.3 | 45.5 ± 1.5 | 45.5 ± 2.9 |
| GLB (g/L) | 25.3 ± 1.4 | 27.3 ± 1.6 | 27.8 ± 3.9 |
| ALB/GLB ratio | 1.75 ± 0.1 | 1.7 ± 0.1 | 1.7 ± 0.2 |
| BUN (mmol/L) | 6.7 ± 1.4 | 6.6 ± 0.5 | 6.9 ± 0.6 |
| Cr (μmol/L) | 34.8 ± 1.9 | 33.2 ± 1.5 | 34.5 ± 4.0 |
| GLU (mmol/L) | 5.2 ± 1.1 | 5.4 ± 0.9 | 6.1 ± 0.8 |
| TG (mmol/L) | 1.4 ± 0.4 | 1.5 ± 0.5 | 1.7 ± 0.3 |
| TCH (mmol/L) | 2.0 ± 0.1 | 2.3 ± 0.3 | 2.3 ± 0.3 |
ALB = albumin; ALT = alanine aminotransferase; BUN = blood urea nitrogen; Cr = creatinine; GLB = globulin; GLU = glucose; GOT = glutamic oxaloacetic transaminase; TCH = total cholesterol; TG = triglyceride; TP = total protein.
Serum ALT concentration is one of the most commonly used indicators for assessment of general liver health and liver disease [33,44]. Serum ALT levels rise in disease states that cause hepatocellular injury, therefore, serum ALT levels can identify an ongoing liver disease process [45]. ALT levels > 5 times the upper limit of the normal suggest a potentially serious, active liver disease process, and ALT levels > 15 times the normal range indicate severe acute liver cell injury [45]. The use of many drugs is associated with elevated ALT levels, and over-the-counter medications and herbal preparations are also implicated [46]. Moreover, emerging data highlight the potential value of ALT as a measure of overall health and survival [45]. Thus, ALT level is more important for evaluating liver injury and overall health status. Accordingly, we assessed differences in ALT among treated groups and no significant differences were found (p > 0.05), which strongly demonstrated that CIP was safe for long-term administration.
Liver damage was induced in Wistar rats by administering ferrous sulfate (30 mg/kg) on the 10th day, for that ALT, ALB and TP were observed to have a surge (p < 0.01). Total cholesterol in these rats suggested an increased risk of cerebrovascular disease [47]. In rats treated with FeNa-EDTA, ALT stayed almost the same, but unfortunately ALB, white blood cell count, mean corpuscular hemoglobin concentration and other clinical chemistry values showed significant changes after 31 days or 61 days of feeding [48]. Therefore, the results of serum biochemical parameters indicated that carbonyl iron could be an effective treatment for IDA, with few side effects.
3.3. Gross pathological findings
At the end of the observation period, all rats were killed and autopsied. All major organs including liver, kidneys, spleen, and testes were examined grossly. No significant (p > 0.05) differences in organ coefficient (organ weight/body weight) among Groups 7, 8 and 9 were noted (Table 3). There were no significant (p > 0.05) differences in organ weights among these groups (Table 3). Even feeding with CIP up to 200 mg/kg had no adverse effects on organs. In rats fed with FeNa-EDTA, there was some increased risk with increased dose of CIP because there was an ~40% decrease in liver weight/body weight, resulting in possible questions regarding hepatotrophy and toxicity [31].
Table 3.
Effect of oral carbonyl iron powder on relative organ weight in Wistar rats treated for 90 consecutive days.
| Parameter | Group 7 | Group 8 | Group 9 |
|---|---|---|---|
| Liver weight (g) | 11.39 ± 1.00 | 10.74 ± 2.11 | 10.90 ± 1.25 |
| Liver weight/body weight (×10−2) | 2.42 ± 0.24 | 2.41 ± 0.23 | 2.59 ± 0.28 |
| Kidney weight (g) | 1.32 ± 0.12 | 1.21 ± 0.24 | 1.18 ± 0.13 |
| Kidney weight/body weight (×10−3) | 2.80 ± 0.31 | 2.71 ± 0.34 | 2.82 ± 0.18 |
| Spleen weight (g) | 0.69 ± 0.07 | 0.65 ± 0.10 | 0.58 ± 0.07 |
| Spleen weight/body weight (×10−3) | 1.46 ± 0.13 | 1.45 ± 0.2 | 1.38 ± 0.19 |
| Testis weight (g) | 1.76 ± 0.13 | 1.78 ± 0.12 | 1.71 ± 0.19 |
| Testis weight/body weight (×10−3) | 3.72 ± 0.28 | 3.99 ± 0.33 | 4.06 ± 0.34 |
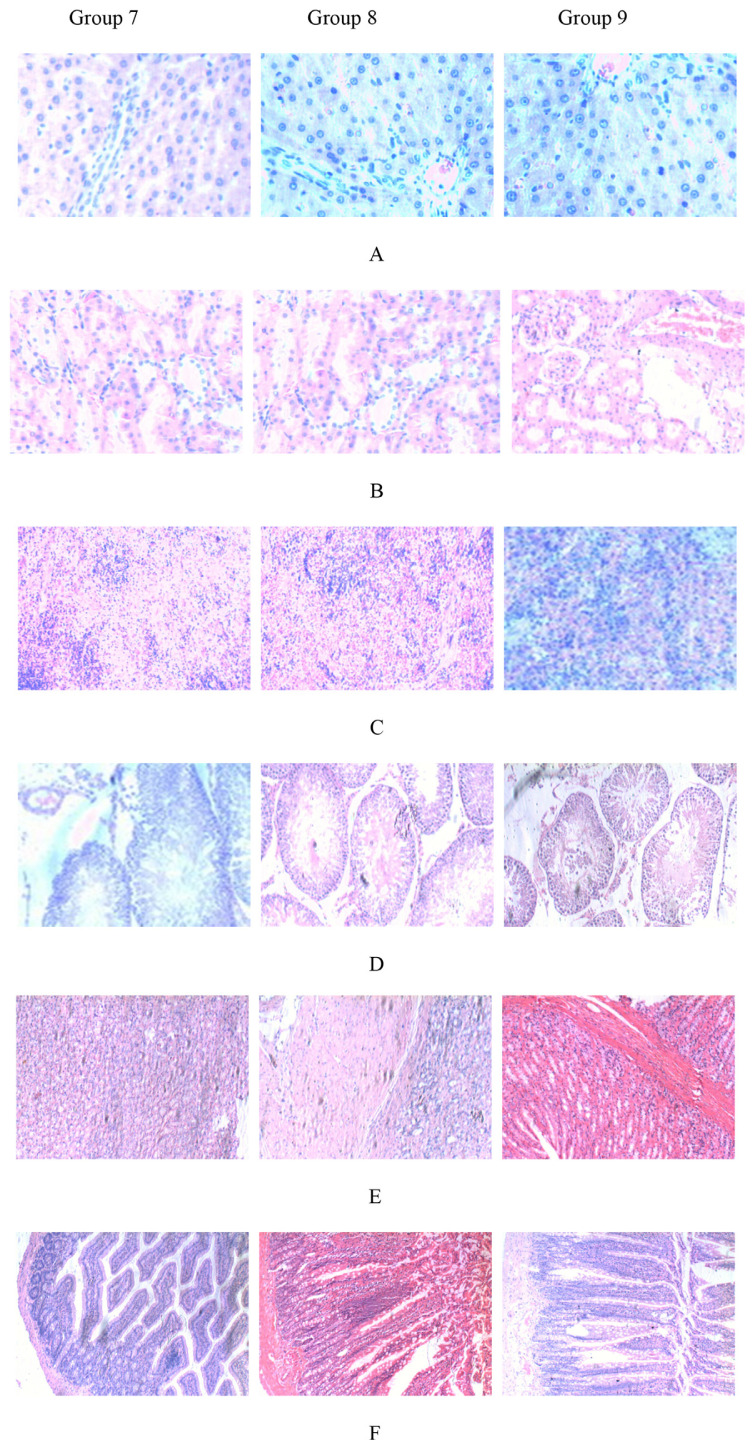
Small pieces of liver, kidneys, spleen, testes, stomach and intestine were fixed in 10% neutral-buffered formalin, and cut into sections that were observed under a light microscope. Histological observations of liver, kidneys, spleen, stomach, intestine and testes showed no treatment-related differences compared with the control group, regardless of the dose of CIP used (Figure 3). The texture of tissue is clear and without typical pathological changes like inflammatory infiltration, of treated groups compared with the control group. Several studies have examined that different iron fortifiers such as ferrous bisglycinate, ferrous fumarate, FeNa-EDTA, and ferrous sulfate had varing degrees of toxic effects on certian organs [4,31,32]. Oral ferrous fumarate and ferrous sulfate therapy may both reinforce intestinal inflammation under microscopic evaluation, showing crypt loss, inflammatory infiltration, and crypt abscesses [44]. CIP did not cause significant organ lesions.
Figure 3.
Paraffin sections of organs of male Wistar rats: (A) liver, (B) kidney, (C) spleen, (D) testis, (E) stomach, and (F) intestine.
3.4. Conclusions
CIP is a newly developed food additive or iron supplement that has therapeutic potential for IDA, with potent bioavailability and low toxicity based on administration and subchronic toxicity experiments in Wistar rats. After 8 days of administration of CIP, the HGB concentration of IDA rats returned to normal. The results obtained from the subchronic toxicity experiment showed no significant change in hematological, biochemical, morphological and histological features. The NOAEL of CIP was > 200 mg/kg. Thus, CIP has the potential to be used as an iron supplement.
Acknowledgments
This work was supported by “Open Funding Project of the State Key Laboratory of Bioreactor Engineering and “National Major Science and Technology Projects of China (No. 2012ZX09304009)”.
Funding Statement
This work was supported by “Open Funding Project of the State Key Laboratory of Bioreactor Engineering and “National Major Science and Technology Projects of China (No. 2012ZX09304009)”.
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
REFERENCES
- 1. Brugnara C. Iron deficiency and erythropoiesis: new diagnostic approaches. Clin Chem. 2003;49:1573–8. doi: 10.1373/49.10.1573. [DOI] [PubMed] [Google Scholar]
- 2. Severance S, Hamza I. Trafficking of heme and porphyrins in metazoa. Chem Rev. 2009;109:4596–616. doi: 10.1021/cr9001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ramakrishnan U. Prevalence of micronutrient malnutrition worldwide. Nutr Rev. 2002;60:S46–52. doi: 10.1301/00296640260130731. [DOI] [PubMed] [Google Scholar]
- 4. Patil SS, Khanwelkar CC, Patil SK, Thorat VM, Jadhav SA, Sontakke AV. Comparison of efficacy, tolerability, and cost of newer with conventional oral iron preparation. Al Ameen J Med Sci. 2013;6:29–33. [Google Scholar]
- 5. Clark SF. Iron deficiency anemia. Nutr Clin Pract. 2008;23:128–41. doi: 10.1177/0884533608314536. [DOI] [PubMed] [Google Scholar]
- 6. Huang CY, Wu CH, Yang JI, Li YH, Kuo JM. Evaluation of iron-binding activity of collagen peptides prepared from the scales of four cultivated fishes in Taiwan. J Food Drug Anal. 2015;23:671–8. doi: 10.1016/j.jfda.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gordon N. Iron deficiency and the intellect. Brain Dev. 2003;25:3–8. doi: 10.1016/s0387-7604(02)00148-1. [DOI] [PubMed] [Google Scholar]
- 8. Beard JL. Iron biology in immune function, muscle metabolism and neuronal functioning. J Nutr. 2001;131(Suppl):S568–79. doi: 10.1093/jn/131.2.568S. [DOI] [PubMed] [Google Scholar]
- 9. Franceschi F, Gasbarrini A. Helicobacter pylori and extragastric diseases. Best Pract Res Clin Gastroenterol. 2007;21:325–34. doi: 10.1016/j.bpg.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 10. Haas JD, Brownlie T. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr. 2001;131(Suppl):S676–88. doi: 10.1093/jn/131.2.676S. [DOI] [PubMed] [Google Scholar]
- 11. Hershko C, Patz J, Ronson A. The anemia of achylia gastrica revisited. Blood Cells Mol Dis. 2007;39:178–83. doi: 10.1016/j.bcmd.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 12. Oppenheimer SJ. Iron and its relation to immunity and infectious disease. J Nutr. 2001;131(Suppl):S616–33. doi: 10.1093/jn/131.2.616S. [DOI] [PubMed] [Google Scholar]
- 13. Beard J. Iron deficiency alters brain development and functioning. J Nutr. 2003;133(Suppl):S1468–72. doi: 10.1093/jn/133.5.1468S. [DOI] [PubMed] [Google Scholar]
- 14. Lozoff B, Corapci F, Burden MJ, Kaciroti N, Angulo-Barroso R, Sazawal S, Black M. Preschool-aged children with iron deficiency anemia show altered affect and behavior. J Nutr. 2007;137:683–9. doi: 10.1093/jn/137.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brabin BJ, Hakimi M, Pelletier D. An analysis of anemia and pregnancy-related maternal mortality. J Nutr. 2001;131(Suppl):S604–14. doi: 10.1093/jn/131.2.604S. [DOI] [PubMed] [Google Scholar]
- 16. Cook JD. Defining optimal body iron. Proc Nutr Soc. 1999;58:489–95. doi: 10.1017/s0029665199000634. [DOI] [PubMed] [Google Scholar]
- 17. Lozoff B, Kaciroti N, Walter T. Iron deficiency in infancy: applying a physiologic framework for prediction. Am J Clin Nutr. 2006;84:1412–21. doi: 10.1093/ajcn/84.6.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rasmussen KM. Is there a causal relationship between iron deficiency or iron-deficiency anemia and weight at birth length of gestation and perinatal mortality? J Nutr. 2001;131(Suppl):S590–601. doi: 10.1093/jn/131.2.590S. [DOI] [PubMed] [Google Scholar]
- 19. Baltussen R, Knai C, Sharan M. Iron fortification and iron supplementation are cost-effective interventions to reduce iron deficiency in four subregions of the world. J Nutr. 2004;134:2678–84. doi: 10.1093/jn/134.10.2678. [DOI] [PubMed] [Google Scholar]
- 20. Cook JD. Diagnosis and management of iron-deficiency anaemia. Best Pract Res Clin Haematol. 2005;18:319–32. doi: 10.1016/j.beha.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 21. Erichsen K, Ulvik RJ, Grimstad T, Berstad A, Berge RK, Hausken T. Effects of ferrous sulphate and non-ionic iron-polymaltose complex on markers of oxidative tissue damage in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2005;22:831–8. doi: 10.1111/j.1365-2036.2005.02652.x. [DOI] [PubMed] [Google Scholar]
- 22.IVACG Secretariat. A report of the international nutritional anemia consultative group: iron EDTA for food fortification. New York: The Nutrition Foundation; 1993. pp. 10–2. [Google Scholar]
- 23. Drago SR, Valencia ME. Mineral dialyzability in milk and fermented dairy products fortified with FeNaEDTA. J Agric Food Chem. 2008;56:2553–7. doi: 10.1021/jf073009u. [DOI] [PubMed] [Google Scholar]
- 24. Wagner CC, Baran EJ. Vibrational spectra of two Fe(III)/EDTA complexes useful for iron supplementation. Spectrochim Acta A Mol Biomol Spectrosc. 2010;75:807–10. doi: 10.1016/j.saa.2009.11.059. [DOI] [PubMed] [Google Scholar]
- 25. Han XX, Sun YY, Ma AG, Yang F, Zhang FZ, Jiang DC, Li Y. Moderate NaFeEDTA and ferrous sulfate supplementation can improve both hematologic status and oxidative stress in anemic pregnant women. Asia Pac J Clin Nutr. 2011;20:514–20. [PubMed] [Google Scholar]
- 26. Pineda O, Ashmead HD, Perez JM, Ponce-Lemus C. Effectiveness of iron amino acid chelate on the treatment of iron deficiency anemia in adolescents. J Appl Nutr. 1994;46:2–13. [Google Scholar]
- 27. Iost C, Name JJ, Jeppsen RB, Ashmead HD. Repleating hemoglobin in iron deficiency anemia in young children through liquid milk fortification with bioavailable iron amino acid chelate. J Am Coll Nutr. 1998;17:187–94. doi: 10.1080/07315724.1998.10718745. [DOI] [PubMed] [Google Scholar]
- 28. Bovell-Benjamin AC, Viteri FE, Allen LH. Iron absorption from ferrous bisglycinate and ferric trisglycinate in whole maize is regulated by iron status. Am J Clin Nutr. 2000;71:1563–9. doi: 10.1093/ajcn/71.6.1563. [DOI] [PubMed] [Google Scholar]
- 29. Gordeuk VR, Brittenham GM, Bravo J, Hughes MA, Keating LJ. Prevention of iron deficiency with carbonyl iron in female blood donors. Transfusion. 1990;30:239–45. doi: 10.1046/j.1537-2995.1990.30390194345.x. [DOI] [PubMed] [Google Scholar]
- 30. Hurrell R, Bothwell T, Cook JD, Dary O, Davidsson L, Fairweather-Tait S, Hallberg L, Lynch S, Rosado J, Walter T, Whittaker P. The usefulness of elemental iron for cereal flour fortification: a sustain task force report. Nutr Rev. 2002;60:391–406. doi: 10.1301/002966402320964061. [DOI] [PubMed] [Google Scholar]
- 31. Whittaker P, Ali SF, Imam SZ, Dunkel VC. Acute toxicity of carbonyl iron and sodium iron EDTA compared with ferrous sulfate in young rats. Toxicol Pharmacol. 2002;36:280–6. doi: 10.1006/rtph.2002.1577. [DOI] [PubMed] [Google Scholar]
- 32. Jeppsen RB, Borzelleca JF. Safety evaluation of ferrous bisglycinate chelate. Food Chem Toxicol. 1999;37:723–31. doi: 10.1016/s0278-6915(99)00052-6. [DOI] [PubMed] [Google Scholar]
- 33. Kloots W, den Kamp DO, Abrahamse L. In vitro iron availability from iron-fortified whole-grain wheat flour. J Agric Food Chem. 2004;52:8132–6. doi: 10.1021/jf040010+. [DOI] [PubMed] [Google Scholar]
- 34. Swanson CA. Iron intake and regulation: implications for iron deficiency and iron overload. Alcohol. 2003;30:99–102. doi: 10.1016/s0741-8329(03)00103-4. [DOI] [PubMed] [Google Scholar]
- 35. Yuan L, Geng LN, Ge L, Yu P, Duan XL, Chen J, Chang YZ. Effect of iron liposomes on anemia of inflammation. Int J Pharm. 2013;454:82–9. doi: 10.1016/j.ijpharm.2013.06.078. [DOI] [PubMed] [Google Scholar]
- 36. Lei M, Xue CH, Wang YM, Li ZJ, Xue Y, Wang JF. Effect of squid ink melanin-Fe on iron deficiency anemia remission. J Food Sci. 2008;73:H207–11. doi: 10.1111/j.1750-3841.2008.00930.x. [DOI] [PubMed] [Google Scholar]
- 37. Tang N, Zhu YT, Zhuang H. Antioxidant and anti-anemia activity of heme iron obtained from bovine hemoglobin. Food Sci Biotechnol. 2015;24:635–42. [Google Scholar]
- 38. Knutson MD, Walter PB, Ames BN, Viteri FE. Both iron deficiency and daily iron supplements increase lipid peroxidation in rats. J Nutr. 2000;130:621–8. doi: 10.1093/jn/130.3.621. [DOI] [PubMed] [Google Scholar]
- 39. Craven CM, Alexander J, Eldridge M, Kushner JP, Bernstein S, Kaplan J. Tissue distribution and clearance kinetics of non-transferrin-bound iron in the hypotransferrinemic mouse: a rodent model for hemochromatosis. PNAS. 1987;84:3457–61. doi: 10.1073/pnas.84.10.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, Vianello L, Zanuso F, Mozzi F, Milani S, Conte D, Colombo M, Sirchia G. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1–9. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 41. European Food Safety Authority (EFSA) Conclusion on peer review of the pesticide risk assessment of the active substance iron sulfate. EFSA J. 2012;10:2521. [Google Scholar]
- 42. Lin JD, Lin PY, Chen LM, Fang WH, Lin LP, Loh CH. Serum glutamic-oxaloacetic transaminase (GOT) and glutamic-pyruvic transaminase (GPT) levels in children and adolescents with intellectual disabilities. Res Dev Disabil. 2010;31:172–7. doi: 10.1016/j.ridd.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 43. O’Connell TX, Horita TJ, Kasravi B. Understanding and interpreting serum protein electrophoresis. Am Fam Physician. 2005;71:105–12. [PubMed] [Google Scholar]
- 44. Erichsen K, Milde AM, Arslan G, Helgeland L, Gudbrandsen OA, Ulvik RJ, Berge RK, Hausken T, Berstad A. Low-dose oral ferrous fumarate aggravated intestinal inflammation in rats with DSS-induced colitis. Inflamm Bowel Dis. 2005;11:744–8. doi: 10.1097/01.mib.0000174374.83601.86. [DOI] [PubMed] [Google Scholar]
- 45. Kim WR, Flamm SL, Di Bisceglie AM, Bodenheimer HC. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008;47:1363–70. doi: 10.1002/hep.22109. [DOI] [PubMed] [Google Scholar]
- 46. Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123:1367–84. doi: 10.1053/gast.2002.36061. [DOI] [PubMed] [Google Scholar]
- 47. Lindenstrøm E, Boysen G, Nyboe J. Influence of total cholesterol high density lipoprotein cholesterol and triglycerides on risk of cerebrovascular disease: the Copenhagen City Heart Study. BMJ. 1994;309:11–5. doi: 10.1136/bmj.309.6946.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Appel MJ, Kuper CF, Woutersen RA. Disposition accumulation and toxicity of iron fed as iron (II) sulfate or as sodium iron EDTA in rats. Food Chem Toxicol. 2001;39:261–9. doi: 10.1016/s0278-6915(00)00137-x. [DOI] [PubMed] [Google Scholar]