Abstract
In this study, oils from Asarum heterotropoides were extracted by traditional solvent extraction and supercritical CO2 (SC-CO2) extraction methods and their antioxidant activities along with antimicrobial and inhibitory activities against five human body odor-producing bacteria (Staphylococcus epidermidis, Propionibacterium freudenreichii, Micrococcus luteus, Corynebacterium jeikeium, and Corynebacterium xerosis) were evaluated. The oil was found to contain 15 components, among which the most abundant component was methyl eugenol (37.6%), which was identified at every condition studied in different extraction methods. The oil extracted with n-hexane and ethanol mixture exhibited a strong antioxidant activity (92% ± 2%) and the highest ABTS and 2,2-diphenyl-1-picrylhydrazyl scavenging activities (89% ± 0.2%). The highest amounts of total phenolic content and total flavonoid content were 23.1 ± 0.4 mg/g and 4.9 ± 0.1 mg/g, respectively, in the traditional method. In the SC-CO2 method performed at 200 bar/50°C using ethanol as an entrainer, the highest inhibition zone was recorded against all the aforementioned bacteria. In particular, strong antibacterial activity (38 ± 2 mm) was found against M. luteus. The minimum inhibitory concentration (MIC) for the oil against bacteria ranged from 10.1 ± 0.1 μg/mL to 46 ± 2 μg/mL. The lowest MIC was found against M. luteus. Methyl eugenol was found to be one of the major compounds working against human body odor-producing bacteria.
Keywords: Asarum heterotropoides, body odor-producing bacteria, methyl eugenol, oil, supercritical CO2
1. Introduction
Chinese people have used different herbs to treat diseases for more than two millenniums. The therapeutic effects of these drugs are attributed to the synergistic effect of their multiple pharmacologically active constituents. Asarum heterotropoides var. mandshuricum, a perennial herb endemic to China, is a traditional Chinese medicine, which is locally known as Xixin [1]. Previous studies have investigated the various components in the essential oil of this herb [2,3], and so far, 82 components have been identified. Among these, methyl eugenol was found to be the most abundant compound [3,4]. Methyl eugenol isolated from A. heterotropoides var. mandshuricum is not only abundant in this herb but also widely distributed in other aromatic plants. Methyl eugenol possesses a wide spectrum of activities against microorganisms ranging from bacteria to fungi [5]. However, some phytochemical and pharmacological studies on A. heterotropoides have reported several types of secondary metabolites, including oils, which display remarkable antioxidant, antimicrobial, antitumor, anti-inflammatory, and larvicidal properties [6–10]. Despite their medicinal importance and availability, only limited knowledge exists about the biological activities of A. heterotropoides, which is insufficient to evaluate its pharmacological effects. In addition, there is currently no information about the potential of A. heterotropoides root-derived materials to modulate human body odor-producing bacteria.
Body odor is the unpleasant smell caused by the mixing of perspiration (sweat) and bacteria on the skin. Human body odor is thought to occur due to bacterial activities on dead skin cells and secretions. The most dominant phyla responsible for producing human body odor are Actinobacteria, Firmicutes, Proteobacteria, and Bacteroidetes [11,12]. Sebaceous areas of the skin, such as the forehead and the upper back, are the least diverse habitats and are predominantly colonized by Propionibacterium spp. and Staphylococcus spp. [13]. Moist skin sites including the groin region and the axilla were commonly found to be dominated by Corynebacterium spp., and Staphylococcus spp. was reported to be intermediate in diversity compared with other skin-inhabiting organisms [14].
Oxidative stress may be induced by increasing the generation of reactive oxygen species and other free radicals. A previous study [15] reported the role of oxidative stress in the pathogenesis of skin disorders. It is known that scavengers or inhibitors of reactive oxygen species such as antioxidants may reduce hyperpigmentation [16].
In general, for extraction of organic compounds from different sources, organic solvents are used. However, conventional methods have some limitations. Because of the problems associated with traditional solvent extraction methods, there has been a growing interest in developing simpler, faster, and more efficient methods for extraction of organic compounds. In recent years, the use of supercritical (SC) fluid extraction for extracting organic compounds from different liquid and solid matrices has attracted much attention. Being eco-friendly and nontoxic, SC carbon dioxide (SC-CO2) is a promising process for the extraction and fractionation of edible lipids from different sources [17–19]. Because this extraction process uses a closed chamber, outside air cannot penetrate the vessels. Therefore, the compound recovered and the residues remaining inside experience very less oxidation compared with the traditional solvent extraction method [20].
The objective of our work was therefore to evaluate the SC fluid extraction conditions and compare its results with other solvent extraction methods for obtaining high-quality methyl eugenol from oils in A. heterotropoides. In addition, we also evaluated the antioxidant and bacteriostatic activity of these oils using five bacterial strains responsible for producing human body odor.
2. Materials and methods
2.1. Experimental materials and chemicals
Asarum heterotropoides var. mandshuricum roots were purchased from Dongeui Hanjae Company (Gyeonggido, Korea). Pure carbon dioxide (99%) used in extraction was supplied by KOSEM (Korea). Standard reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other reagents used in this study were of analytical or high-performance liquid chromatography grade.
2.2. Sample preparation
Dried roots were crushed using a mechanical blender (PN, SMKA-4000; Ansan, Korea), sieved through a sieve (710-μm mesh size), and then stored at −30°C until their further use in SC-CO2 and organic solvent extraction.
2.3. SC-CO2 extraction
A laboratory-scale SC fluid extraction system was used, and the extraction was performed as follows: 180 g of root powder sample was loaded into the stainless steel extraction vessel (volume: 500 mL). A thin layer of cotton was placed at the bottom of the extraction vessel prior to loading. Before plugging the vessel with a cap, another layer of cotton was added to the top of the sample to prevent the enter of root powder into the line. CO2 was pumped at a constant pressure into the extraction vessel using a high-pressure pump (MILROYAL, Milton Roy, USA) up to the desired pressure. Ethanol as a cosolvent was pumped using a Lab Alliance Series II isocratic pump (Scientific System Inc., State College, PA, USA). The flow rate of the cosolvent was 1 mL/min. A back-pressure regulator was used to control the pressure of CO2. The extraction temperature was maintained by connecting the extraction vessel with water bath. Flow rates and accumulated gas volume passing through the apparatus were measured using a gas flow meter (Shinagawa, Tokyo, Japan). After SC-CO2 extraction, the remaining root residues and oil were stored at −30°C until further use and analysis. A. heterotropoides var. mandshuricum root powder was extracted at a temperature of 45–55°C and pressure ranging from 200 bar to 300 bar for 2 hours using a SC-CO2 apparatus. The flow rate of CO2 was kept constant at 27 g/min for all extraction conditions.
2.4. Solvent extraction
The extraction was carried out using different solvents such as ethanol, hexane, acetone, and ethanol–hexane mixture, and included the following steps: 50 g of dried root powder with 300 mL solvent was placed into the beaker and stirred for 12 hours using a magnetic stirrer at 50°C and 200 rpm. After extraction, the solution was filtered and then evaporated under reduced presser in a rotary vacuum evaporator (EYELA N-1100, Tokyo, Japan) at 45°C. The remaining residue was dried using an oven at 40°C for 6 hours, and then the residues and oil were stored at −30°C until further use and analysis.
2.5. Gas chromatography–mass spectrometry analysis
Gas chromatography–mass spectrometry (GC–MS) analysis of A. heterotropoides oil obtained by extraction with different solvents was performed using a BRUKER 450-GC series gas chromatograph, which includes an AOC-20i autosampler and TQ mass spectrometer of the Bruker 320-MS series. The capillary column type was BR-5ms (30 m × 0.25 μm ID × 0.25 μm df). For GC–MS detection, an electron ionization system was operated in the electron impact mode with 70 eV ionization energy. Helium gas (99.99%) was used as the carrier gas at a constant flow rate of 1 mL/min, and an injection volume of 2 μL was used (split ratio of 10:1). The injector temperature was maintained at 250°C, the ion-source temperature was 200°C, and the oven temperature was programmed from 110°C (isothermal temperature for 2 minutes), with an increase of 10°C/min to 200°C, then 5°C/min to 280°C, ending with a 9-minute isothermal temperature at 280°C. Mass spectra were taken at 70 eV, with a scan interval of 0.5 s and 45–450 Da fragments. The solvent delay was 0–2 minutes, and the total GC–MS running time was 36 minutes. The relative percentage amount of each component was calculated by comparing its average peak area with the total areas.
The mass spectra of GC–MS were interpreted using the database of the National Institute Standard and Technology and Wiley 275L main mass spectral library, which have more than 62,000 patterns. The spectrum of the unknown components was compared with that of known components stored in these libraries. The percentage compositions of the identified compounds were computed from the GC peak area without any correction factor and were calculated relatively.
2.6. Total phenolic content assay
The total phenolic content (TPC) of the oil was determined using the Folin–Ciocalteu colorimetric method, according to the procedures described by Li et al [21] and Wong et al [22], but with slight modifications. Approximately 1 mL of 10-time diluted (v/v) oil was mixed with 1 mL of 1:10 diluted (v/v, in deionized water) Folin–Ciocalteu reagent. After 4 minutes, 800 μL of sodium carbonate solution (7.5%, w/v) was added into the mixture. The mixture was vortexed for 5 seconds and stored at room temperature in a dark environment for 2 hours. As blank 1 mL deionized water was taken instead of 1 mL of sample. The absorbance of the mixture was measured at 765 nm against the blank using an ultraviolet (UV) spectrophotometer (UVmini-1240, Shimadzu Co., Japan). All measurements were carried out in triplicate. Gallic acid was used for calibration of standard curve.
2.7. Total flavonoid content assay
Total flavonoid content (TFC) of the oil was estimated using procedures described by Karadeniz et al [23] and Ozsoy et al [24]. In brief, 1.25 mL of deionized water was added into 0.25 mL of undiluted oil, followed by addition of 75 mL of 5% (w/v) sodium nitrite solution. The mixture was allowed to stand for 6 minutes and then 150 μL of 10% (w/v) aluminum chloride solution was added. The mixture was allowed to stand for another 5 minutes and 0.5 mL of 1M sodium hydroxide solution and 275 μL of deionized water were added. Subsequently, the mixture was vortexed for 5 seconds and its absorbance was measured at 510 nm against the blank using a UV spectrophotometer (UVmini-1240, Shimadzu Co.). All measurements were carried out in triplicate. The blank was prepared by replacing 0.25 mL of undiluted oil with 0.25 mL of deionized water. Catechin was used for calibration of the standard curve.
2.8. 2,2-Diphenyl-1-picrylhydrazyl free-radical scavenging assay
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging capacity of oil was determined based on the method described by Miliauskas et al [25], Saha et al [26], and Cai et al [27], but with some slight modifications. In brief, 3.9 mL of ethanolic DPPH (60μM) was first mixed with 0.1 mL of undiluted oil or ethanol (as control) and stored in a dark environment at room temperature for 30 minutes. Subsequently, the absorbance of oil and control was measured against ethanol (as blank) at 517 nm using a UV spectrophotometer (UVmini-1240, Shimadzu Co.). The absorbance measurements of oil and control were carried out in triplicate. The percentage of DPPH free-radical scavenging capacity was calculated using the following formula:
where As is absorbance of crude extract at 517 nm and Ac is absorbance of control at 517 nm. The samples of blank and control (0.01 mg/mL standard Trolox) were analyzed.
2.9. ABTS free-radical scavenging assay
ABTS radical scavenging capacity assay was performed according to the procedures described by Cai et al [27], Guimarães et al [28], and Surveswaran et al [29]. The ABTS radical solution was first prepared by mixing 10 mL of 7mM ABTS solution with 10 mL of 2.45mM potassium persulfate solution in an amber bottle. Subsequently, the ABTS radical solution was allowed to stand in a dark environment at room temperature for 12–16 hours to give a dark blue solution. The ABTS radical solution was then diluted with denatured ethanol until its absorbance was equilibrated to 0.7 ± 0.02 at 734 nm before usage. Approximately 3.9 mL of ABTS radical solution was first mixed with 0.1 mL of undiluted oil or ethanol (as control) and the mixture was stored in a dark environment at room temperature for 6 minutes. Subsequently, the absorbance of oil and control was measured against ethanol (as blank) at 734 nm using a UV spectrophotometer (UVmini-1240, Shimadzu Co.). The absorbance measurements of crude and control were carried out in triplicate. The percentage of ABTS free-radical scavenging activity was calculated using the following formula:
where As is absorbance of oil at 734 nm and Ac is absorbance of control at 734 nm. The samples of blank and control (1.0 mg/ mL standard Trolox) were analyzed.
2.10. Antimicrobial screening
Five bacterial strains of skin pathogens were used in this study (Staphylococcus epidermidis KCCM 35494, Propionibacterium freudenreichii KCCM 41661, Micrococcus luteus KCCM 11211, Corynebacterium jeikeium KCCM 41661, and Corynebacterium xerosis KCCM 40941). These microbial strains were obtained from the Korean Culture Center of Microorganism (Republic of Korea). Antibacterial activities were determined using the agar diffusion method of Meillisa et al [30], but with slight modifications. McFarland Standard Number 0.5 was used in the preparation of microorganism suspension. The turbidity of bacterial suspension was adjusted according to the McFarland standard. The accurate turbidity of the bacterial suspension was confirmed by spectrophotometry at 625 nm, with the approximate cell density of each bacterial strain being 107 colony-forming units/mL. Nutrient agar (Sigma Aldrich) was used for S. epidermidis and M. luteus. Trypticase soy agar with 5% defibrinated sheep blood was used for C. jeikeium, brain–heart infusion agar (Sigma Aldrich) was used for C. xerosis, reinforced clostridial medium (Oxoid CM149) was used for P. freudenreichii, and the prepared media were sterilized at 12°C for 15 minutes. The agar was poured into sterile glass petri dishes and allowed to set, and then the bacterial suspension was spread out on the agar surface with sterile cotton. An Advantech paper disk containing A. heterotropoides oil and methyl eugenol with different dilutions or control (dimethyl sulfoxide) was then placed on the surface of agar. The plates were incubated at 37°C overnight, and antibacterial activity was obtained by measuring the diameter of the clear zone. All analyses were carried out in triplicate.
2.11. Minimum inhibitory concentration
Minimum inhibitory concentration (MIC) was determined according to the procedure described by DaSilva et al [31]. Serial dilutions of the oil were obtained using dimethyl sulfoxide. The MIC was defined as the lowest concentration of the sample at which no visible growth was observed. Visible growth (positive antibacterial activities) was established by the presence of measurable zones of inhibition after 24 hours of incubation at 37°C. The MIC was determined by subculturing the MIC dilutions onto the sterile agar plates.
2.12. Statistical analysis
All experiments were carried out in triplicate. SPSS Statistics program (version 15.0 for Windows, SPSS Inc., Chicago, IL, USA) was used for data analysis.
3. Results and discussion
3.1. Composition of oil from different extraction methods
The chemical composition of oil from A. heterotropoides is given in Tables 1 and 2. A. heterotropoides oil was characterized by the presence of 15 components. Methyl eugenol, sesamin, safrole, N-isobutyl-(2E,4Z,8Z,10E)-dodecatetraenamide, and pentadecane were the major components. The most abundant components methyl eugenol (37.6%), sesamin (22.1%), and safrole (14.7%) were found at different extraction conditions and methods (Tables 1 and 2). Some studies have reported that the contents methyl eugenol and safrole were highest in A. heterotropoides [32]. Some variations in the composition of oil were observed, and the amount of methyl eugenol was found to be the highest (37.6%) in SC-CO2 with ethanol extraction at 200 bar and 50°C (Table 1). A previous study also reported that high amounts of methyl eugenol were found in essential oils extracted using the SC-CO2 method [33,34]. The highest amount of safrole was found in oil extracted using hexane as solvent (Table 2). By contrast, the highest amount of sesamin (22.1%) was found in oil extracted using ethanol the solvent (SC-CO2) at 300 bar and 50°C (Table 1).
Table 1.
Chemical composition of Asarum heterotropoides oil, expressed as chromatographic area percentages obtained by supercritical CO2 at different extraction conditions, P (bar)/T (°C).
| Components | Concentration (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| SC-CO2 extraction | SC-CO2 with ethanol extraction | |||||||||
|
|
|
|||||||||
| Conditions (bar/°C) | ||||||||||
|
| ||||||||||
| 300/50 | 250/50 | 200/50 | 200/45 | 200/55 | 300/50 | 250/50 | 200/50 | 200/45 | 200/55 | |
| Eucarvone | 0.7 | 0.8 | 0.8 | 0.8 | 0.9 | 0.6 | 0.7 | 0.6 | 0.7 | 0.8 |
| 3,5-Dimethoxytoluene | 5.6 | 6.5 | 12.8 | 5.5 | 7.1 | 3.6 | 4.5 | 4.7 | 6.2 | 4.8 |
| Safrole | 9.1 | 9.8 | 11.3 | 8.6 | 11.2 | 8.6 | 9.4 | 9.2 | 9.2 | 10.9 |
| Methyl eugenol | 35.4 | 36.9 | 36.8 | 35.8 | 36.5 | 35.8 | 37.6 | 37.6 | 36.1 | 37.2 |
| Myristicin | 1 | 1.2 | 0.7 | 1.2 | 1.9 | 1 | 1.5 | 1.3 | 1 | 1.1 |
| Pentadecane | 4.2 | 3.8 | 4 | 5.3 | 7.1 | 5.1 | 5.6 | 5.1 | 4.6 | 4.3 |
| Trans-isocroweacin | 5.5 | 5.4 | 7.3 | 2.5 | 4.6 | 3 | 3.5 | 3.7 | 5.1 | 5.8 |
| 3,4-Benzocyclodec-3-ene-1,5-diyn-7-one | 3 | 2.8 | 2.3 | 3.4 | 2.6 | 2.8 | 3.1 | 3.5 | 2.6 | 2.9 |
| 4,6-Dimethoxy-5-methyl phthalide | 2.3 | 2.3 | 1.7 | 2.5 | 2.2 | 2 | 2.5 | 2.7 | 2.1 | 2.3 |
| Linoleic acid | 3.2 | 2.5 | 1.8 | 4 | 1.4 | 2 | 2 | 1.9 | 1.6 | 3.9 |
| N-Isobutyl-(2E,4Z,8Z,10E)-dodecatetraenamide | 8.6 | 6.3 | 6.2 | 8.8 | 5.2 | 9.6 | 8.7 | 9 | 7.9 | 11.3 |
| N-Isobutyl-(2E,4Z,8Z,10E)-dodecatetraenamide | 3.5 | 3.1 | 2 | 3.8 | 2.3 | 3.7 | 4.1 | 4.1 | 4.1 | 3.7 |
| Sesamin | 18 | 18.7 | 12.4 | 17.8 | 17 | 22.1 | 17. | 16.7 | 19.1 | 11.1 |
SC = supercritical.
Table 2.
Chemical composition of Asarum heterotropoides oil obtained by solvent extraction with different organic solvents.
| Components | Concentration (%) | |||
|---|---|---|---|---|
|
| ||||
| Ethanol | Hexane | Ethanol + hexane | Acetone | |
| Eucarvone | 1.3 | 1.6 | 1.1 | 1.6 |
| 3,5-Dimethoxytoluene | 10.2 | 11.2 | 9.2 | 11.3 |
| Safrole | 13.4 | 14.7 | 12.9 | 14.7 |
| Methyl eugenol | 33.3 | 33.3 | 34 | 33.2 |
| Myristicin | — | 0.9 | — | 0.9 |
| Pentadecane | 5 | 7.8 | 5.2 | 7.8 |
| Trans-isocroweacin | 5.9 | 4.7 | 5.4 | 4.7 |
| 3,4-Benzocyclodec-3-ene-1,5-diyn-7-one | 1.9 | 2.3 | 2.3 | 2.3 |
| 4,6-Dimethoxy-5-methyl phthalide | 1.7 | 1.8 | 2.2 | 1.9 |
| Linoleic acid | 3 | 3 | 3.3 | 3 |
| N-Isobutyl-(2E,4Z,8Z,10E)-dodecatetraenamide | 6.5 | 6.3 | 6.4 | 4.7 |
| N-Isobutyl-(2E,4Z,8Z,10E)-dodecatetraenamide | 3 | 3 | 2.6 | 3 |
| Sesamin | 14.2 | 9.6 | 15.5 | 11.1 |
3.2. Antioxidant activity
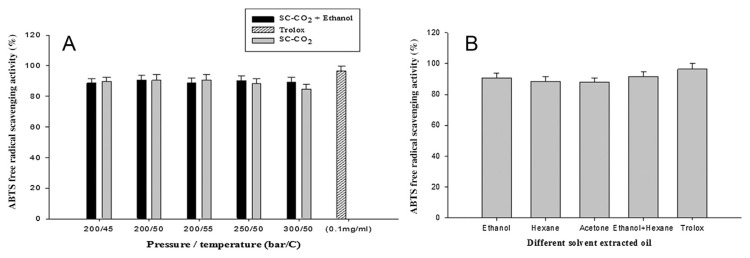
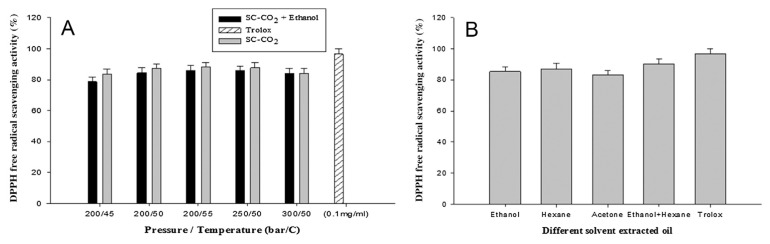
The DPPH and ABTS methods were used to evaluate the antioxidant activity of A. heterotropoides oil using different extraction methods (only SC-CO2, SC-CO2 with ethanol, and traditional solvent). The DPPH radical was thought to be reduced to the corresponding hydrazine when it reacted with the donating hydrogen substances. The ABTS radical scavenging assay is considered an excellent tool for investigating the antioxidant activity of hydrogen-donating antioxidants and chain-breaking antioxidants [35]. A. heterotropoides oil exhibited a good antioxidant activity, and the results are shown in Figures 1 and 2. In the SC-CO2 extraction method, the ethanol-extracted oil showed the highest ABTS radical scavenging activity (91% ± 2%) at 200 bar and 50°C (Figure 1A). By contrast, in the traditional solvent method, the highest result (92% ± 2%) was obtained for the ethanol–hexane-extracted oil (Figure 1B). In SC-CO2 extraction method highest DPPH activity (89% ± 2%) was found only at 200 bar and 55°C (Figure 2A) and in traditional method (89.04% ± 0.2%) was found in ethanol with hexane extraction (Figure 2B). However, the activity showed slight variations due to changes in condition in both extraction methods. The higher antioxidant capacity of the oil of A. heterotropoides could be the synergistic property attributed to the presence of phenolic and flavonoid components. In addition, methyl eugenol and eugenol may well be the main contributors to the antioxidant activity. The antioxidant activity of eugenol has been reported several times [36–38].
Figure 1.
ABTS free-radical scavenging activity: (A) SC-CO2-extracted oil; (B) traditional solvent-extracted oil. SC-CO2 = supercritical carbon dioxide.
Figure 2.
2,2-Diphenyl-1-picrylhydrazyl (DPPH) free-radical scavenging activity: (A) SC-CO2-extracted oil; (B) traditional solvent-extracted oil. Values are presented as means ± standard deviation (n =3). SC-CO2 = supercritical carbon dioxide.
3.3. Total phenolic and flavonoid contents
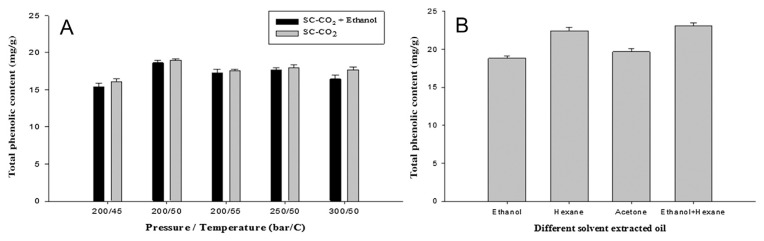
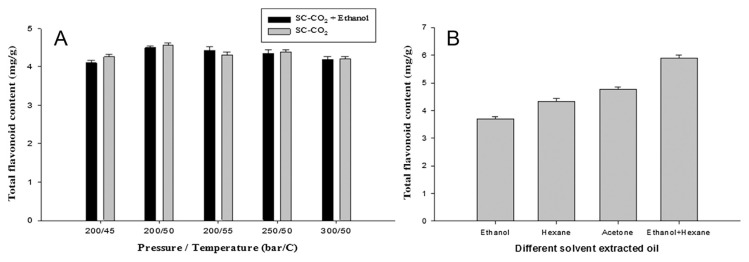
Phenolic and flavonoid compounds are important antioxidants in many plants. Thus, their contents were determined in A. heterotropoides oil extracted by the SC-CO2 and traditional solvent extraction methods and the variations at different conditions were evaluated. It was observed that the contents of phenolic and flavonoid compounds of traditional solvent-extracted oil were slightly higher than those of SC-CO2-extracted oil, which might be responsible for the stronger antioxidant activity of the former. In traditional solvent extraction using ethanol or hexane as the solvent, the highest amounts of TPC and TFC were 23.1 ± 0.4 mg/g and TFC 4.9 ± 0.1 mg/g, respectively (Figures 3B and 4B). By contrast, in SC-CO2 extraction, the highest amounts of TPC and TFC were 19 ± 0.2 mg/g and 4.6 ± 0.1 mg/g, respectively, at 200 bar and 50°C (Figures 3A and 4A).
Figure 3.
Total phenolic content: (A) SC-CO2-extracted oil; (B) traditional solvent-extracted oil. Values are presented as means ± standard deviation (n =3). SC-CO2 = supercritical carbon dioxide.
Figure 4.
Total flavonoid content: (A) SC-CO2-extracted oil; (B) traditional solvent-extracted oil. Values are presented as means ± standard deviation (n =3). SC-CO2 = supercritical carbon dioxide.
3.4. Antimicrobial activity
The antimicrobial activity of the oil was initially tested by measuring the diameter of the inhibition zone using the agar diffusion method (see Tables 3 and 4). It is obvious that the oils showed significant activity against the tested microorganisms with inhibition zone ranging from 15 ± 1.2 mm to 38 ± 1.3 mm in the traditional extraction method (Table 4). By contrast, in SC-CO2 extraction, the inhibition zone ranged from 10 ± 0.4 mm to 38 ± 2 mm (Table 3). However, the extracted oils differ significantly in their activity against the tested microorganisms. In SC-CO2 extraction with ethanol at 200 bar and 50°C, the highest inhibition zone was observed against all five bacteria. Under this condition, the inhibition zone was 38 ± 2 mm, 31 ± 1.5 mm, 25 ± 1.5 mm, 23 ± 1.3 mm, and 21 ± 1.1 mm against M. luteus, C. jeikeium, P. freudenreichii, S. epidermidis, and C. xerosis, respectively (Table 3). In this study, better activity was found against M. luteus. The traditional solvent extraction method showed the best result for the ethanol–hexane-extracted oil, and a trend similar to that of the SC-CO2-extracted oil was observed. Although there was a synergetic effect of active compounds on these five bacteria, oils with high amounts of methyl eugenol exhibited the highest activity on bacteria. In a previous study, methyl eugenol of basil oil exhibited the highest activity on S. epidermidis, M. luteus, and C. xerosis [39].
Table 3.
Antimicrobial activity (inhibition zones diameter and MIC) of the Asarum heterotropoides oil extracted by supercritical CO2.
| Microorganism | SC-CO 2 extraction (bar/°C) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 200/45 | 200/50 | 200/55 | 250/50 | 300/50 | ||||||
|
|
|
|
|
|
||||||
| DIZ | MIC | DIZ | MIC | DIZ | MIC | DIZ | MIC | DIZ | MIC | |
| Staphylococcus epidermidis | 20 ± 1.3 | 21 ± 0.1 | 22 ± 1.1 | 17.3 ± 0.6 | 22 ± 1.3 | 17.8 ± 1.3 | 20 ± 1.1 | 19.3 ± 1.6 | 15 ± 0.2 | 19.3 ± 1.6 |
| Micrococcus luteus | 30 ± 1.4 | 16 ± 0.4 | 38 ± 0.5 | 10.5 ± 0.1 | 38 ± 1.2 | 11.5 ± 3 | 30 ± 1.2 | 20 ± 0.6 | 28 ± 1.5 | 19.7 ± 0.6 |
| Corynebacterium jeikeium | 20 ± 1.2 | 14 ± 1 | 32 ± 1.2 | 20 ± 1.2 | 32 ± 0.5 | 21 ± 0.6 | 19 ± 1.4 | 35.5 ± 1.2 | 16 ± 1.2 | 35.5 ± 1.4 |
| Corynebacterium xerosis | 18 ± 0.5 | 23.2 ± 0.1 | 20 ± 1.2 | 19 ± 0.0 | 17 ± 1.2 | 19.8 ± 0.1 | 18 ± 1.2 | 26.1 ± 0.0 | 10 ± 0.4 | 26.1 ± 0.0 |
| Propionibacterium freudenreichii | 20 ± 1.3 | 35.1 ± 1.2 | 25 ± 1.3 | 30.5 ± 0.8 | 25 ± 1.1 | 30.1 ± 0.1 | 18 ± 0.5 | 46 ± 2 | 16 ± 1.2 | 46 ± 2 |
| SC-CO 2 with ethanol extraction | ||||||||||
| S. epidermidis | 18 ± 0.2 | 17.3 ± 0.1 | 23 ± 1.3 | 20.2 ± 0.2 | 21 ± 1.3 | 20 ± 0.1 | 18 ± 1.2 | 25.5 ± 0.7 | 16 ± 1.2 | 22.1 ± 0.0 |
| M. luteus | 32 ± 1.2 | 17.1 ± 0.2 | 38 ± 2 | 10.1 ± 0.1 | 30 ± 1.3 | 13 ± 0.1 | 32 ± 1.1 | 14.5 ± 0.8 | 30 ± 1.2 | 15.6 ± 1.7 |
| C. jeikeium | 24 ± 1.14 | 20.5 ± 0.2 | 31 ± 1.5 | 11.5 ± 0.1 | 22 ± 1.2 | 15.5 ± 0.0 | 22 ± 1.2 | 14.8 ± 0.7 | 20 ± 1.3 | 17.7 ± 0.6 |
| C. xerosis | 15 ± 1.5 | 24 ± 0.4 | 21 ± 1.1 | 22.8 ± 0.2 | 18 ± 0.6 | 21.8 ± 0.1 | 16 ± 0.4 | 23.6 ± 1.3 | 14 ± 0.3 | 21 ± 0.2 |
| P. freudenreichii | 22 ± 1.5 | 36.5 ± 1.4 | 25 ± 1.5 | 15.2 ± 1.1 | 23 ± 1.3 | 16.2 ± 0.3 | 21 ± .5 | 17.7 ± 0.3 | 20 ± 1.1 | 18.5 ± 0.1 |
Values are presented as mean ± standard deviation. Negative control dimethyl sulfoxide was not inhibited by microorganisms and its data are not included in the table.
DIZ = diameter of inhibition zones (mm) including disk diameter (8 mm); MIC = minimum inhibitory concentration (μg/mL); SC = supercritical.
Table 4.
Antimicrobial activity (inhibition zones diameter and MIC) of the Asarum heterotropoides oil extracted by the traditional solvent method.
| Microorganism name | Solvent extraction | Standard | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Ethanol | Hexane | Acetone | Ethanol + hexane | Methyl eugenol | ||||||
|
|
|
|
|
|
||||||
| DIZ | MIC | DIZ | MIC | DIZ | MIC | DIZ | MIC | DIZ | MIC | |
| Staphylococcus epidermidis | 16 ± 0.3 | 27.8 ± 0.1 | 20 ± 1.2 | 20.2 ± 2.7 | 18 ± 0.6 | 22.8 ± 1.1 | 26 ± 1.2 | 16.6 ± 0.6 | 18 ± 1.0 | 0.3 ± 1.1 |
| Micrococcus luteus | 20 ± 1.2 | 20 ± 0.00 | 32 ± 1.5 | 17.2 ± 0.0 | 30 ± 1.5 | 18.8 ± 0.0 | 38 ± 1.3 | 12.6 ± 1.0 | 20 ± 2.1 | 0.3 ± 1.0 |
| Corynebacterium jeikeium | 18 ± 1.3 | 28.1 ± 0.7 | 24 ± 1.3 | 20 ± 0.0 | 20 ± 1.2 | 21 ± 2.1 | 35 ± 1.3 | 15 ± 0.1 | 19 ± 0.7 | 0.3 ± 0.2 |
| Corynebacterium xerosis | 15 ± 1.2 | 27 ± 0.1 | 18 ± 1.1 | 24.8 ± 0.3 | 16 ± 1.1 | 26 ± 1.0 | 24 ± 1.3 | 18.5 ± 1.8 | ND | ND |
| Propionibacterium freudenreichii | 17 ± 1.2 | 20.8 ± 0.4 | 21 ± 1.2 | 20 ± 0.0 | 20 ± 1.2 | 19 ± 0.2 | 28 ± 1.4 | 15.1 ± 1.4 | 16 ± 1.6 | 2.6 ± 1.3 |
Values are presented as mean ± standard deviation. Negative control dimethyl sulfoxide was not inhibited by microorganisms and its data are not included in the table.
DIZ = diameter of inhibition zones (mm) including disk diameter (8 mm); MIC = minimum inhibitory concentration (μg/mL); ND = not detected; SC = supercritical.
The MIC for the oil against bacteria ranged from 10.1 ± 0.1 μg/mL to 46 ± 2 μg/mL (Tables 3 and 4). Relatively lowest MIC was found against M. luteus at 200 bar and 50°C in SC-CO2 with ethanol-extracted oil (10.1 ± 0.1 μg/mL). For S. epidermidis, C. jeikeium, C. xerosis, and P. freudenreichii, lowest MICs were 16.6 ± 0.6 μg/mL, 11.5 ± 0.1 μg/mL, 18.5 ± 1.8 μg/mL, and 15.1 ± 1.4 μg/mL, respectively. It was observed that most of the minimum MICs were observed at the same condition, and in this condition the content of methyl eugenol was high. Therefore, it can be assumed that methyl eugenol is the key component responsible for inhibiting the human body odor-producing bacteria.
3.5. Conclusion
This study has shown that it is feasible to use A. heterotropoides oil as a natural powerful antimicrobial ingredient against human body odor-producing bacteria in modern medicine as well as in cosmetics. SC-CO2 extraction will be a good method to extract such oils, as it allows to obtain high amounts of methyl eugenol. It was also observed that the high amounts of methyl eugenol show strong activity against human body odor-producing bacteria.
Acknowledgments
This research was financially supported by the Ministry of Trade, Industry and Energy and Korea Institute for Advancement of Technology through the Promoting Regional Specialized Industry.
Funding Statement
This research was financially supported by the Ministry of Trade, Industry and Energy and Korea Institute for Advancement of Technology through the Promoting Regional Specialized Industry.
Footnotes
Conflicts of interest
All contributing authors declare no conflicts of interest.
REFERENCES
- 1.Huang SM, Kelly LM, Gilbert MG. Aristolochiaceae. In: Wu CY, Raven PH, editors. Flora of China. Beijing, China and St. Louis, MO: Science Press Inc., and Missouri Botanical Garden Press; 2003. pp. 246–69. [Google Scholar]
- 2. Zeng HY, Jin YZ, Bao LT, Wang P. Analysis on fingerprint chromatogram of the volatile oils obtained from Asarum heterotropoides with different methods. J Test Meas Tech. 2004;18:232–6. [Google Scholar]
- 3. Zhang F, Fu SP, Xu Q, Xiao HB, Cai SQ, Liang XM. Study on GC fingerprint of the constituents in Herba Asari. Zhongguo Zhong Yao Za Zhi. 2004;29:411–3. [Article in Chinese] [PubMed] [Google Scholar]
- 4. Kosuge T, Yokota M, Nukaya H, Gotoh Y, Nagasawa M. Studies on antitussive principles of Asiasari radix. Chem Pharm Bull (Tokyo) 1978;26:2284–5. [Google Scholar]
- 5. Kivanç M. Antimicrobial activity of “Cörtük” (Echinophora sibthorpiana Guss.) spice, its essential oil and methyl-eugenol. Nahrung. 1988;32:369–73. doi: 10.1002/food.19880320631. [DOI] [PubMed] [Google Scholar]
- 6. Hashimoto K, Yanagisawa T, Okui Y, Maruno M, Fujita T. Studies on anti-allergic components in the roots of Asiasarum sieboldi. Planta Med. 1994;60:124–7. doi: 10.1055/s-2006-959432. [DOI] [PubMed] [Google Scholar]
- 7. Takasaki M, Konoshima T, Yasuda I, Hamano T, Tokuda H. Inhibitory effects of shouseiryu-to on two-stage carcinogenesis. II1. Anti-tumor-promoting activities of lignans from Asiasarum heterotropoides var. mandshuricum. Biol Pharm Bull. 1997;20:776–80. doi: 10.1248/bpb.20.776. [DOI] [PubMed] [Google Scholar]
- 8. Dan Y, Liu HY, Gao WW, Chen SL. Activities of essential oils from Asarum heterotropoides var. mandshuricum against five phytopathogens. Crop Prot. 2010;29:295–9. [Google Scholar]
- 9. Perumalsamy H, Chang KS, Park C, Ahn Y-J. Larvicidal activity of Asarum heterotropoides root constituents against insecticide-susceptible and -resistant Culex pipiens pallens and Aedes aegypti and Ochlerotatus togoi. J Agric Food Chem. 2010;58:10001–6. doi: 10.1021/jf102193k. [DOI] [PubMed] [Google Scholar]
- 10. Huang J, Wang HQ, Zhang C, Li GY, Lin RC, Wang JH. A new tetrahydrofuran-type lignan with anti-inflammatory activity from Asarum heterotropoides Fr. Schmidt var. mandshuricum. J Asian Nat Prod Res. 2014;16:387–92. doi: 10.1080/10286020.2013.820713. [DOI] [PubMed] [Google Scholar]
- 11. Grice EA, Kong HH, Renaud G, Young AC, Bouffard GG, Blakesley RW, Wolfsberg TG, Turner ML, Segre JA. NISC Comparative Sequencing Program. A diversity profile of the human skin microbiota. Genome Res. 2008;18:1043–50. doi: 10.1101/gr.075549.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science. 2009;326:1694–7. doi: 10.1126/science.1177486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, Bouffard GG, Blakesley RW, Murray PR, Green ED, Turner ML, Segre JA. NISC Comparative Sequencing Program. Topographical and temporal diversity of the human skin microbiome. Science. 2009;324:1190–2. doi: 10.1126/science.1171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fredrich E, Barzantny H, Brune I, Tauch A. Daily battle against body odor: towards the activity of the axillary microbiota. Trends Microbiol. 2003;21:305–12. doi: 10.1016/j.tim.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 15. Yamakoshi J, Otsuka F, Sano A, Tokutake S, Saito M, Kikuchi M, Kubota Y. Lightening effect on ultraviolet-induced pigmentation of guinea pig skin by oral administration of a proanthocyanidin-rich extract from grape seeds. Pigment Cell Res. 2003;16:629–38. doi: 10.1046/j.1600-0749.2003.00093.x. [DOI] [PubMed] [Google Scholar]
- 16. Ma W, Wlaschek M, Tantcheva-Poor I, Schneider LA, Naderi L, Razi-Wolf Z, Schuller J, Scharffetter-Kochanek K. Chronological ageing and photoageing of the fibroblasts and the dermal connective tissue. Clin Exp Dermatol. 2001;26:592–9. doi: 10.1046/j.1365-2230.2001.00905.x. [DOI] [PubMed] [Google Scholar]
- 17. Esquivel MM, Bandarra NM, Fontan I, Bernardo-Gil MG, Batista I, Nunes ML, Empis JA. Supercritical carbon dioxide extraction of sardine Sardina pilchardus oil. Lebenson Wiss Technol. 1997;30:715–20. [Google Scholar]
- 18. Davarnejad R, Kassim KM, Zainal A, Sata SA. Extraction of fish oil by fractionation through supercritical carbon dioxide. J Chem Eng. 2008;53:2128–32. [Google Scholar]
- 19. Rubio-Rodriguez N, De Diego SM, Beltran S, Jaime I, Sanz MT, Rovira J. Supercritical fluid extraction of the omega-3 rich oil contained in hake (Merluccius capensis–Merluccius paradoxus) by-products: Study of the influence of process parameters on the extraction yield and oil quality. J Supercrit Fluids. 2008;47:215–26. [Google Scholar]
- 20. Uddin MS, Kishimura H, Chun BS. Isolation and characterization of lecithin from squid (Todarodes pacificus) viscera deoiled by supercritical carbon dioxide extraction. J Food Sci. 2011;10:217–22. doi: 10.1111/j.1750-3841.2010.02039.x. [DOI] [PubMed] [Google Scholar]
- 21. Li HB, Wong CC, Cheng KW, Chen F. Antioxidant properties in vitro and total phenolic contents in methanol extracts from medicinal plants. Lebenson Wiss Technol. 2008;41:385–90. [Google Scholar]
- 22. Wong SP, Leong LP, Koh JHW. Antioxidant activities of aqueous extracts of selected plants. Food Chem. 2006;99:775–83. [Google Scholar]
- 23. Karadeniz F, Burdurlu HS, Koca N, Soyer Y. Antioxidant activity of selected fruits and vegetables grown in Turkey. Turk J Agric For. 2005;29:297–3. [Google Scholar]
- 24. Ozsoy N, Can A, Yanardag R, Akev N. Antioxidant activity of Smilax excelsa L. leaf extracts. Food Chem. 2007;110:571–83. [Google Scholar]
- 25. Miliauskas G, Venskutonis PR, Beek TA. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004;85:231–7. [Google Scholar]
- 26. Saha K, Lajis NH, Israf DA, Hamzah AS, Khozirah S, Khamis S, Syahida A. Evaluation of antioxidant and nitric oxide inhibitory activities of selected Malaysian medicinal plants. J Ethnopharmacol. 2004;92:263–7. doi: 10.1016/j.jep.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 27. Cai YZ, Sun M, Xing J, Luo Q, Corke H. Structure-radical scavenging activity relationships of phenolic compounds from traditional Chinese medical plants. Life Sci. 2006;78:2872–88. doi: 10.1016/j.lfs.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 28. Guimarães CM, Gião MS, Martinez SS, Pintado AI, Pintado ME, Bento LS, Malcata FX. Antioxidant activity of sugar molasses, including protective effect against DNA oxidative damage. J Food Sci. 2007;72:39–43. doi: 10.1111/j.1750-3841.2006.00231.x. [DOI] [PubMed] [Google Scholar]
- 29. Surveswaran S, Cai YZ, Corke H, Sun M. Systematic evaluation of natural phenolic antioxidants from 133 Indian medicinal plants. Food Chem. 2007;102:938–53. [Google Scholar]
- 30. Meillisa A, Siahaan EA, Park JN, Woo HC, Chun BS. Effect of subcritical water hydrolysate in the brown seaweed Saccharina japonica as a potential antibacterial agent on foodborne pathogens. J Appl Phycol. 2013;25:763–9. [Google Scholar]
- 31. DaSilva JKR, Silva JRA, Nascimento SB, da Luz SF, Meireles EN, Alves CN, Ramos AR, Maia JG. Antifungal activity and computational study of constituents from Piper divaricatum essential oil against Fusarium infection in black pepper. Molecules. 2014;19:17926–42. doi: 10.3390/molecules191117926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jadhav VM, Thorat RM, Kadam VJ, Gholve SB. Kesharaja: hair vitalizing herbs. Int J Pharmtech Res. 2009;1:456–67. [Google Scholar]
- 33. Caredda A, Marongiu B, Porcedda S, Soro C. Supercritical carbon dioxide extraction and characterization of Laurus nobilis essential oil. J Agric Food Chem. 2002;50:1492–6. doi: 10.1021/jf0108563. [DOI] [PubMed] [Google Scholar]
- 34. Ivanović J, Mišić D, Ristić M, Pešić O, Žižović I. Supercritical CO2 extract and essential oil of bay (Laurus nobilis L.) chemical composition and antibacterial activity. J Serb Chem Soc. 2010;75:395–4. [Google Scholar]
- 35. Leong LP, Shui G. An investigation of antioxidant capacity of fruits in Singapore markets. Food Chem. 2002;76:69–75. [Google Scholar]
- 36. Lee SJ, Umano K, Shibamoto T, Lee KG. Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. J Food Chem. 2005;91:131–7. [Google Scholar]
- 37. Ito M, Murakami K, Yoshino M. Antioxidant action of eugenol compounds: role of metal ion in the inhibition of lipid peroxidation. Food Chem Toxicol. 2005;43:461–6. doi: 10.1016/j.fct.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 38. Dorman HJD, Surai P, Deans SG. In vitro antioxidant activity of a number of plant essential oils and phytoconstituents. J Essent Oil Res. 2000;12:241–8. [Google Scholar]
- 39. Koba K, Poutouli PW, Raynaud C, Chaumont JP, Sanda K. Chemical composition and antimicrobial properties of different basil essential oils chemotypes from Togo. Bangladesh J Pharmacol. 2009;4:1–8. [Google Scholar]