Abstract
The establishment of a catalytic system to enrich isoflavone aglycones in black soybean milk was investigated in this study. Beta-glucosidase, which was covalently immobilized onto cellulose beads, exhibited a significant efficiency for the conversion of 4-nitrophenyl β-d-glucuronide to p-nitrophenol over the sol–gel method. The Michaelis constant (Km) of the cellulose bead enzymatic system was determined to be 1.50 ± 0.10 mM. Operational reusability of the cellulose bead enzymatic system was justified for more than 10 batch reactions in black soy milk. Moreover, the storage stability verification indicated that the cellulose bead catalytic system was able to sustain its highest catalytic activity for 10 days. High-performance liquid chromatography results demonstrated that this enzymatic system required only 30 minutes to achieve complete isoflavone deglycosylation, and the aglycone content in the total isoflavones in black soy milk was enriched by 67% within 30 minutes by the cellulose bead enzymatic system.
Keywords: black soy milk, cellulose bead, enzyme immobilization, isoflavone deglycosylation, sol-gel, β-glucosidase
1. Introduction
Over the past decades, black soybean milk has become a popular beverage consumed in Asian countries as it is rich in isoflavone, with reports estimating its isoflavone level to be 100 mg/L [1]. Isoflavone is a subgroup of flavonoids that exhibits many potential health-enhancing benefits [2], including reduction of cardiovascular disease [3], prevention of cancer [4], protection from osteoporosis [5], and antioxidant activities [6]. Several studies have demonstrated that the isoflavone aglycone is adsorbed faster than isoflavone glycoside, which results in higher bioavailability of isoflavone aglycone [7–10]. In addition, it was revealed that the amount of isoflavones adsorbed is higher in aglycone-rich fermented soy milk than glycoside-rich nonfermented soy milk [11]. The intake of aglycone-rich black soy milk was therefore suggested to be more beneficial for the purpose of health enhancement.
In recent years, many studies have emphasized on the enrichment of isoflavone aglycones in black soy milk by fermenting it with some probiotic microorganisms [12–14] or immobilized enzyme systems [15–18]. The bioconversion of isoflavone glucosides into aglycones was achieved through the removal of the glucoside conjugates using β-glucosidase. The establishment of immobilized enzyme systems has attracted tremendous attention for isoflavone bioconversion due to the advantages of easy separation from reaction solution, reusability for reducing cost, continuous processing, and long-term stability [15–18].
In this study, a covalently immobilized enzyme system and an enzyme-entrapped system were adopted and compared for their efficiency on deglycosylation of isoflavone. Two biocompatible materials (cellulose bead and sol–gel) were used to evaluate the feasibility of black soy milk isoflavone conversion. Beta-glucosidase was immobilized on cellulose beads by covalent binding and entrapped within sol–gel by matrix entrapment, respectively. The sol–gel process is a common approach to encapsulate enzymes or proteins for various applications [19]. In this method, the formation from solution phase to gel phase provides a platform for the enzymes to be entrapped inside a matrix, in which the corresponding substrate can easily bind onto the active site of the enzyme [20]. Cellulose beads act as solid carrier onto which the enzymes attach after chemical modification. The catalytic efficacy of the two enzyme systems has been investigated by evaluating the Michaelis constant (Km) and maximal activity (Vmax). The system that possesses more efficient catalytic behavior was applied in deglycosylation of isoflavone in black soy milk and the efficiency was evaluated based on the changes in isoflavone content (genistin, daidzin, genistein, and daidzein) in black soy milk before and after deglycosylation.
2. Materials and methods
2.1. Materials
Beta-glucosidase from Aspergillus niger, cellulose acetate, tetramethyl orthosilicate, glutaraldehyde, and sodium carbonate were obtained from Sigma-Aldrich (St. Louis, MO, USA). Acetic acid, methanol, and acetonitrile were purchased from J.T. Baker (Center Valley, PA, USA). 4-Nitrophenyl β-d-glucuronide (p-NPG) purchased from Alfa Aesar (Ward Hill, USA) was used as the substrate to determine the catalytic activity of the immobilized enzyme system. The black soy milk was prepared by milling black soybeans [Glycine max (L.) Merr.] (Tainan, Number 3) with a grinder. Porous cellulose beads [21] and sol–gel [22] were prepared as previously described.
2.2. Carrier preparation and enzyme immobilization
2.2.1. Cellulose beads
Cellulose acetate (6 g) was dissolved in an organic mixture solution (50 mL; acetone:dimethyl sulfoxide = 6:4). The homogenized mixture was pipetted with a pipette syringe (size of syringe tip: Number 25, 0.5 × 25 mm) and precipitated in water. The beads were then washed with distilled water and dried overnight at room temperature to a constant weight. Cellulose beads (total volume: 40 cm3) were activated by treating them with 2.5% glutaraldehyde aqueous solution at 100°C for 30 minutes [23,24]. Activated cellulose carriers were then treated with 2.5% glutaraldehyde aqueous solution at room temperature for 60 minutes. After the beads were washed at room temperature with double-distilled water, they were incubated separately for 16 hours at 4°C with 50 mg of β-glucosidase dissolved in 50 mL of 0.1 M phosphate buffer at pH 6. The unbound enzyme was removed from the carriers using 0.1 M phosphate buffer, and the enzyme-modified carriers were stored in a fridge at 4°C until use to preserve their activity. The amount of enzymes being immobilized on the carriers was determined by monitoring the difference between the amount of β-glucosidase initially used and the amount recovered in the solution at the end of the immobilization process. The concentration of β-glucosidase was determined according to the Bradford dye-binding procedure [25]. The amount of β-glucosidase (750 U/g) immobilized on cellulose beads was estimated to be 37.54 ± 2.8 mg, which corresponds to 28.5 U of enzyme involved in the catalytic reaction.
2.2.2. Sol–gel
As much as 26 mL of the diluted enzyme buffer solution, which contains an equal amount of enzyme attached onto cellulose beads in the immobilized enzyme system, was added into a solution containing 10 mL tetramethyl orthosilicate and 4 mL HCl (10 mM). The mixture was then vortexed for 1 minute, followed by washing (two times) with 0.1 M phosphate buffer (pH 6). The sol–gel enzymatic system obtained was aged at 4°C for more than 3 days to improve its stability [22].
2.3. Verification of enzyme immobilization
X-ray photoelectron spectroscopy, also known as electron spectroscopy for chemical analysis (ESCA), is usually used to determine the surface functional groups of various materials [26,27]. Changes in the chemical binding energy indicate the formation of new functional groups after surface modification. ESCA is commonly used in protein immobilization [28,29] and immobilization of microorganisms [30]. In this work, ESCA was used to verify successful enzyme immobilization. X-ray photoelectron spectroscopy measurements were conducted using an ESCA system (VG Microtech, MT-500, UK) with A1Kα radiation (hν = 1486.6 eV). The Thermo Scientific Avantage Data System (Thermo Fisher Scientific Inc., Hertfordshire, UK) was used to perform curve fitting and calculate the atomic concentrations.
2.4. Enzymatic activity determination
Five milligram of p-NPG, which is equal to the amount of isoflavone in 50 mL of soy milk as published previously [1], was dissolved in 0.1 M phosphate buffer (50 mL, pH 6). The β-glucosidase-immobilized system was incubated with 50 mL of p-NPG solution in a batch reactor. The reaction was stopped by adding 1 mL of 1 M sodium carbonate (Na2CO3). The absorbance of p-NPG was measured at 425 nm [31].
2.5. Morphology characterization
The surface morphologies of cellulose bead and sol–gel without and with β-glucosidase immobilization were examined by scanning electron microscopy (SEM) at an accelerating voltage of 10 kV (JSM-6510LV, JEOL, Tokyo, Japan).
2.6. Isoflavone extraction
The methods for extraction of isoflavone in black soy milk were in accordance with the procedures described previously [32]. At regular intervals, 0.75 mL of black soy milk was withdrawn and added into 0.75 mL of MeOH (100%) containing 2000 ppm benzoic acid as the internal standard. The volume ratio of black soy milk to methanol was 1:1, to achieve 50% of methanol in the mixture. The mixture was shaken for 3 minutes and then centrifuged at 8000g for 1 minute to remove the insoluble debris. The supernatant was collected and further diluted by 50% methanol. After filtering through a 0.45-μm minipore polyvinylidene fluoride filter, the filtrate was collected and analyzed by high-performance liquid chromatography (HPLC). Acetic acid (0.1%) in acetonitrile (Solvent A) and 0.1% acetic acid (0.1%) in aqueous solution (Solvent B) were used as the eluents. The gradient program of the mobile phase was performed as follows: 0 minute, A:B = 10%:90%; 25 minutes, A:B = 68%:32%; 32 minutes, A:B = 70%:30%; and 35 minutes, A:B = 90%:10%. The amount of isoflavones in black soy milk (daidzin, genistin, daidzein, and genistein) was calculated using the following equation [32]:
where AS and AIS represent the peak areas of each isoflavone species and internal standard, respectively; CIS represents the concentration of internal standard (2000 ppm); MW is the molecular weight of each isoflavone species; and VInitial and VWithdrawn represent the initial volume of black soy milk (50 mL) and the volume of black soy milk withdrawn (0.75 mL), respectively. The relative response factor (RRF) was determined to be 4.23, 7.50, 9.91, and 9.09 for daidzin, genistin, daidzein, and genistein, respectively.
2.7. HPLC analysis
Resolution of isoflavone glycosides and aglycones was measured using YMC-Pack ODS-AMC C18 column (5 μm, 250 = 4.6 mm). The column was attached to an HPLC workstation containing two LDC pumps (ConstaMetric 3200 and ConstaMetric 3500) and an LDC analytical mixer. The injection volume was 20 μL and an elution rate of 1.0 mL/min was used. Acetic acid (0.1%) in aqueous solution (Solvent A) and 0.1% acetic acid in acetonitrile (Solvent B) were used as the eluent. Detection was performed by UV absorption at 254 nm. The data were analyzed using an SISC chromatography data system (SISC Taiwan, Taipei, Taiwan).
2.8. Statistical analysis
The data expressed in various studies were plotted using Sigma Plot 11.0 (Systat Software, Inc., Chicago, IL, USA) and expressed as standard error (±). Each value represents the mean of three independent experiments, with average standard deviation less than 5%.
3. Results
3.1. Chemical modification of β-glucosidase attached onto cellulose bead
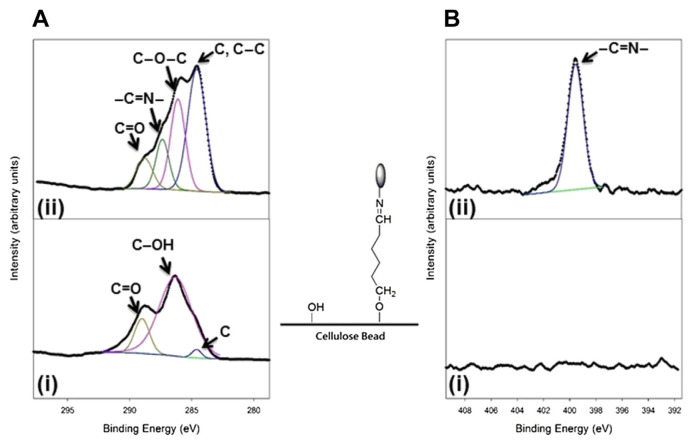
To testify the chemical modification of β-glucosidase attached onto cellulose beads, the elemental compositions and chemical structure characteristics obtained using ESCA were evaluated and used as indicators. The ESCA spectra in Figure 1 (cellulose beads without and with enzyme immobilization) illustrated the specific functional groups of unmodified and modified cellulose beads. The spectral features in the C 1s ESCA spectrum (Panel i in Figure 1A) observed at 284.6 eV, 286.2 eV, and 288.8 eV represented the carbon (C), hydroxyl (C–OH), and carbonyl (C==O) groups, indicating that the surface of cellulose beads mainly contains C–OH groups. In the N 1s ESCA spectrum (Panel i in Figure 1B), no apparent peak was observed, which indicates that there is no nitrogen atom on the surface of cellulose beads. After chemical modification, the specific peaks observed at 286.1 eV (C–O–C) and 287 eV (–C==N–) in the C 1s ESCA spectrum mainly resulted from covalent binding between cellulose bead and β-glucosidase using glutaraldehyde as a cross-linking reagent (Panel ii in Figure 1A). A significant increase of a new peak at 399.8 eV (–C==N–) can be observed in the N 1s ESCA spectrum (Panel ii in Figure 1B), which confirms the successful immobilization onto cellulose beads through the amino acid of β-glucosidase. The results demonstrate that the abundant −OH groups on the surface of cellulose beads were cross-linked with glutaraldehyde through covalent binding. Amino acids on β-glucosidase subsequently reacted with glutaraldehyde via –C==N–bonds, which led to an attachment between enzymes and cellulose beads.
Figure 1.
The observed (A) C 1s and (B) N 1s electron spectroscopy for chemical analysis (ESCA) spectra used for examining enzyme immobilization: (i) clean cellulose beads and (ii) cellulose beads with enzyme immobilization. The characteristic functional groups responsible for the corresponding ESCA signals are indicated by arrows. The atomic compositions of cellulose beads without and with enzyme immobilization were determined as follows: (a) clean cellulose beads: C, 66.16 ± 0.1; O, 33.83 ± 0.1 and (b) cellulose beads with enzyme immobilization: C, 60.06 ± 0.2; O, 35.49 ± 0.1; N, 4.47 ± 0.1.
The relative atomic percentage among C, O, and N obtained from the ESCA spectra for cellulose beads without and with enzyme immobilization was further investigated (Figure 1). Prior to enzyme immobilization, the C/N ratio of cellulose beads was 66.16/0, which indicates the presence of 66.16% of carbon and 0% of nitrogen on the surface of clean cellulose beads. After enzyme immobilization through chemical modification, the relative content of C decreases to 60.04, whereas the relative content of N increases to 4.47, which confirms the covalent binding of beta-glucosidase onto cellulose beads through the amino groups.
3.2. Morphology of cellulose bead and sol–gel
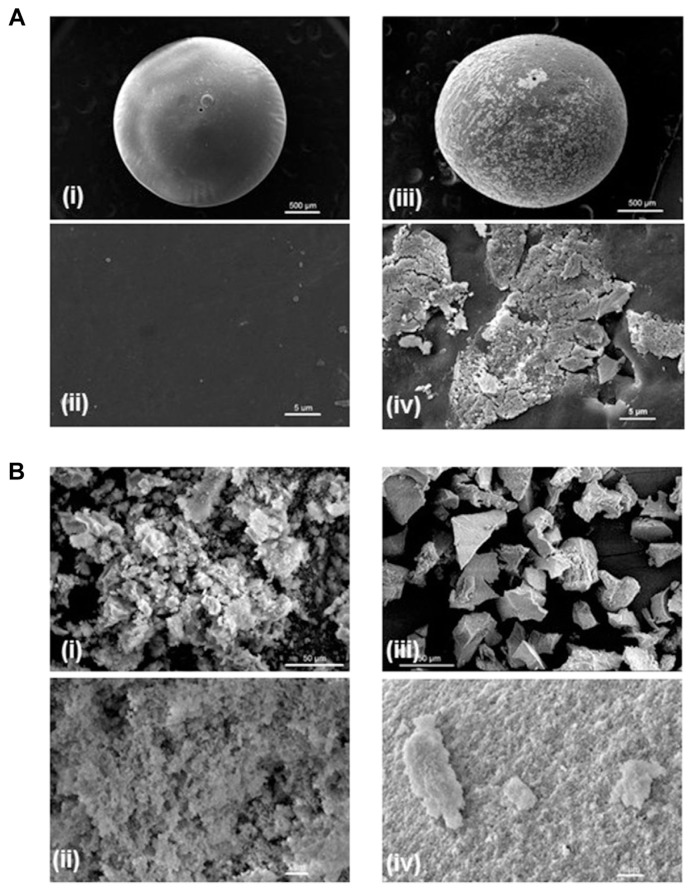
The SEM images of cellulose beads with and without enzyme immobilization are presented in Figure 2. The diameter of cellulose bead was approximately 1 mm. Prior to the enzyme attachment, the surface of the cellulose bead was smooth (Panels i and ii in Figure 2A). After enzyme immobilization through covalent binding, the surface of cellulose bead was covered by some granulated areas, which represent the regions of enzyme attachment (Panels iii and iv in Figure 2A). This provided an evidence that β-glucosidase was successfully immobilized onto the cellulose beads. Figure 2B shows the images of sol–gel with and without enzyme immobilization. Sol–gel exhibits a porous structure prior to the enzyme entrapment (Panels i and ii in Figure 2B); nevertheless, it became compact after enzyme immobilization (Panels iii and iv in Figure 2B). Beta-glucosidase was successfully enclosed within sol–gel to fill up the pores, which accounts for the compact phenomenon.
Figure 2.
Scanning electron microscopy images of surface morphologies of (A) cellulose beads and (B) sol–gel (b) without (Panels i and ii) and with (Panels iii and iv) β-glucosidase immobilization.
3.3. Comparison of efficiency between the two beta-glucosidase-immobilized systems
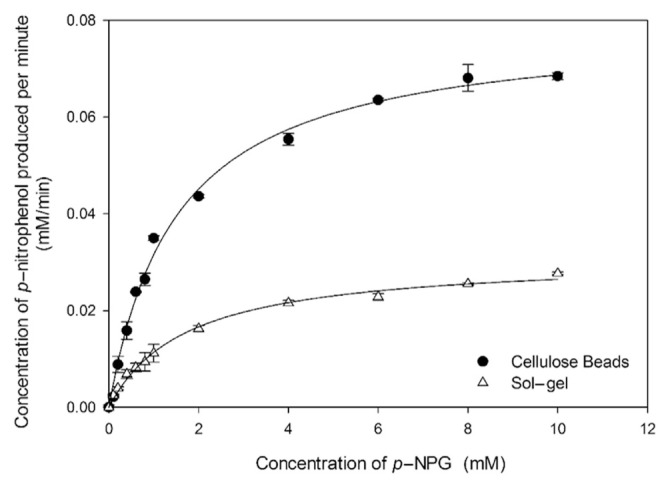
Figure 3 presents a comparison of the efficiency between the cellulose bead enzymatic system and the sol–gel enzymatic system with an increase in the p-NPG concentration. The Michaelis constant (Km) reflects the effective characteristics of the enzyme and the affinity between enzyme and substrate, which was determined using the Michaelis–Menten equation
Figure 3.
Catalytic behaviors of the immobilized β-glucosidase system using cellulose beads and sol–gel as enzyme carriers. The Michaelis constant (Km) was determined by the fitting curve. All the experiments were performed in triplicates.
where [S] represents various concentrations of p-NPG. The maximal activity (Vmax) indicates the intrinsic characteristic of the enzyme, at which all active sites of the enzyme are all bound to the substrate.
The Km and Vmax values of the two enzymatic systems calculated from the Michaelis–Menten equation are summarized in Table 1. The values of Km for the cellulose bead system and the sol–gel system have been estimated to be 1.50 ± 0.10 mM and 1.73 ± 0.13 mM, respectively. As shown in Figure 3, significant differences in Km determination between the cellulose bead and sol–gel systems occurred with the substrate concentration of 2 mM (p < 0.05). The lower Km of sol–gel implies that the affinity between beta-glucosidase and p-NPG of the sol–gel system was smaller than that of the cellulose beads enzymatic system. It may be explained that the diffusion of p-NPG into the active site of enzymes entrapped in sol–gel required more time compared with the cellulose beads system, where p-NPG was able to contact enzymes directly as enzymes were immobilized on the surface of cellulose beads. Moreover, the cellulose bead system had two times better Vmax value than the sol–gel system, indicating that the cellulose bead system displays higher velocity when saturated with p-NPG. The cellulose bead system exhibits lower Km and higher Vmax compared with the sol–gel system, which directly proves the more effective catalytic activity of the cellulose bead enzymatic system. The results obtained were as expected; in the case of sol–gel entrapment immobilization, it might require longer time for the substrate to access the active site of the enzyme. Beta-glucosidase immobilized through covalent binding was directly anchored on the surface of cellulose beads, which facilitates the reaction between enzyme and substrate to enhance the catalytic efficiency. To achieve effective deglycosylation of isoflavones in soy milk, cellulose beads were used as β-glucosidase immobilizer in the following study.
Table 1.
Kinetic parameters characterizing the sol–gel and the cellulose bead enzymatic systems.
| Sol–gel | Cellulose bead | |
|---|---|---|
| Km (mM) | 1.73 ± 0.14a | 1.50 ± 0.10b |
| Vmax (mM/min) | 0.03 ± 0.001a | 0.08 ± 0.002b |
All experiments were performed in triplicates. Values (in the same row) not marked by the same letter are significantly different (p < 0.05; n = 3).
3.4. Hydrolysis of isoflavone glycosides in black soy milk to isoflavone aglycones by the cellulose bead enzymatic system
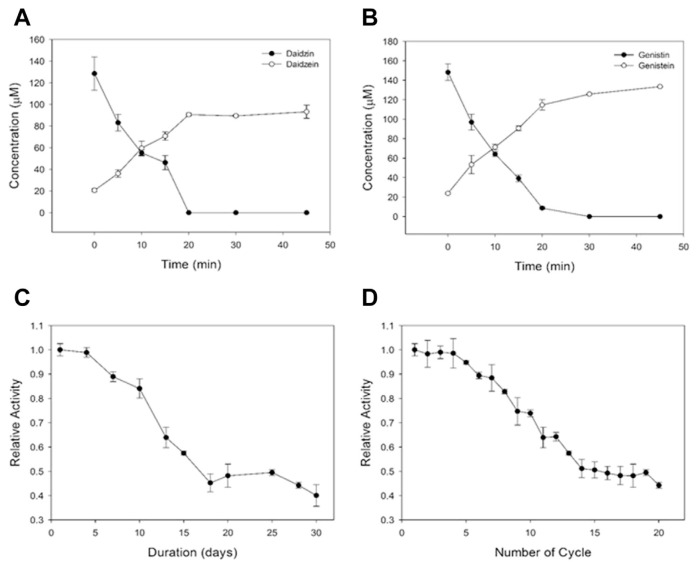
Fifty milliliter of black soy milk was used as the substrate for the catalytic conversion from isoflavone glycosides to isoflavone aglycones. The isoflavone content for different experimental conditions are presented in Table 2. Following 10 minutes of treatment, the total amount of isoflavone glycosides reduced significantly from 276 μM (daidzin, 128 μM; genistin, 148 μM) to 119 μM (daidzin, 55 μM; genistin, 64 μM). Meanwhile, the total amount of aglycones increased from 45 μM (daidzein, 21 μM; genistein, 24 μM) to 131 μM (daidzein, 60 μM; genistein, 71 μM), which reveals that the immobilization of enzyme on cellulose beads was feasible during the hydrolysis of isoflavone in black soy milk. A complete deglycosylation of both daidzin and genistin by the cellulose bead enzymatic system was achieved in 30 minutes (Figures 4A and 4B). Enrichment of the two isoflavone aglycones (by 67%) in black soy milk was accomplished within 30 minutes by the cellulose bead enzymatic system (Table 2).
Table 2.
Isoflavone contents of black soy milk treated at various reaction times by the cellulose bead enzymatic system.
| Reaction time | Glycoside (μM) | Aglycone (μM) | Aglycosylation rate (%)a | Aglycone (%) in the total isoflavonesb | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||
| Daidzin | Genistin | Daidzein | Genistein | Daidzin | Genistin | ||
| Nontreated | 128 ± 15c | 148 ± 9c | 21 ± 2c | 24 ± 1c | — | — | 13.8 ± 0.36c |
| 5 min | 83 ± 8d | 97 ± 8d | 36 ± 3d | 62 ± 9d | 34.2 ± 2.55c | 75.2 ± 1.88c | 30.7 ± 0.98d |
| 10 min | 55 ± 3e | 64 ± 2e | 60 ± 7e | 71 ± 3e | 53.1 ± 1.89d | 56.5 ± 2.13d | 40.7 ± 1.74e |
| 15 min | 46 ± 7e | 39 ± 3f | 71 ± 4e | 91 ± 3f | 60.9 ± 3.25e | 61.3 ± 1.45d | 50.2 ± 2.74f |
| 20 min | 0f | 9 ± 1g | 91 ± 1f | 115 ± 5g | 54.4 ± 1.77d | 65.2 ± 0.87d | 63.9 ± 1.32g |
| 30 min | 0f | 0h | 89 ± 1f | 126 ± 13g | 53.5 ± 1.99d | 68.8 ± 1.34be | 67.0 ± 1.97h |
| 45 min | 0f | 0h | 93 ± 6f | 133 ± 1g | 56.5 ± 0.61d | 74.0 ± 1.35e | 70.6 ± 1.83h |
All experiments were performed in triplicates. Values (in the same column) not marked by the same letter are significantly different (p < 0.05; n = 3).
Aglycosylation rate (%) = (increased molarity of isoflavone aglycones/decreased molarity of the corresponding glycosylated isoflavone) × 100%.
Aglycone (%) in the total isoflavones = (the amount of aglycones/the total amount of initial isoflavones) × 100.
Figure 4.
(A) Conversion of daidzin to daidzein in black soy milk by the cellulose bead enzymatic system. (B) Conversion of genistin to genistein in black soy milk by the cellulose bead enzymatic system. (C) Storage stability of the cellulose bead enzymatic system in black soy milk recorded from 1 day to 30 days. (D) Operational reusability of the cellulose bead enzymatic system in black soy milk. All the experiments were performed in triplicates.
3.5. Storage stability and reusability of the cellulose bead enzymatic system
The storage stability of the cellulose bead enzymatic system was investigated in black soy milk for 30 days (Figure 4C). This examination is worthwhile because black soy milk is considered to be a complicated environment containing plenty of proteins or compounds, which may cause enzyme degradation. It was found that the cellulose bead enzymatic system retained 80% of its original activity after 10 days. A dramatic decrease of relative activity (0.45) was observed after 15 days. By contrast, the catalytic capability retained 70% of its original activity for 10 batch reactions. The results demonstrate that the storage stability and reusability of the β-glucosidase immobilized system were 10 days and 10 batch cycles, respectively.
4. Discussion
Numerous studies have reported that the utilization of β-glucosidase isolated from microorganisms acts as a potential approach for deglycosylation of isoflavone glycosides [33–35]; nevertheless, suspended enzymes without any support can only perform single catalysis owing to the difficulty in retrieving enzymes from products. Lately, several research topics were dedicated to the establishment of β-glucosidase immobilized system [15–18]. Our previous study utilizing glass microspheres as a solid carrier for β-glucosidase immobilization reveals an efficient method to achieve complete isoflavone deglycosylation in 30 minutes [15]. In addition, we evaluated the fact that β-glucosidase immobilized on spent coffee grounds was capable of catalyzing isoflavone glycosides in black soy milk [16]. Chang et al [17] used chitosan–carbon bead as an enzyme immobilization support to hydrolyze isoflavone glycoside in an aqueous–organic two-phase system. Ethyl acetate was used as the organic phase due to its good solubility. The hydrolysis completion time for daidzin and genistin was estimated to be 40 minutes and 60 minutes, respectively. Chitosan bead was also selected as an enzyme carrier to immobilize soybean β-glucosidase for enhancement of aglycones in commercial soy milk [18]. The results demonstrated that there was a 24% increase in aglycones content after 60 minutes of catalytic reaction.
In this work, we reported a method to bind β-glucosidase onto cellulose beads for the enrichment of isoflavone aglycones in black soy milk. The reaction time for complete deglycosylation in this study was only 30 minutes, which is shorter in comparison with other studies utilizing chitosan beads as the enzyme carrier. Moreover, with respect to their application in a packed or fluidized bed reactor, cellulose beads (approximately 1 mm) have more suitable size than glass microspheres (approximately 10 μm). By contrast, good reusability and long-term storage stability increase the values of enzyme-immobilized systems in the industrial application. It has been reported that the reusability and storage stability of β-glucosidase immobilized onto chitosan–carbon composite were 7 reuses and 60 days, respectively [17]. Our previous results demonstrated that the glass microsphere enzymatic system can catalyze 40 reactions and be stored stably for 40 days [15]. Furthermore, the spent coffee ground enzymatic system was confirmed to have 30 batch reactions reusability and stability of 20 days [16]. However, substrates used to verify reusability and storage stability in the aforementioned cases were glycosides dissolved in an aqueous–organic system [17] and p-NPG dissolved in phosphate buffer solution [15,16], respectively. In this study, black soy milk, which contains large amounts of proteins, polysaccharides, and other sugar components, was directly used as the substrate to examine reusability and stability of cellulose bead enzymatic system. The capacity of 10 reuses and the long-term storage (stable for 10 days) of black soy milk make this catalytic system a remarkable one for the scale-up preparation of aglycones-rich products.
5. Conclusion
In summary, the immobilization of β-glucosidase on cellulose beads is capable of catalyzing the isoflavone hydrolysis in black soy milk. The capacity of 10 consecutive uses and 10-day stability in black soy milk offer an economic approach to prepare the aglycone-rich black soy milk in place of fermentation in industrial production. Moreover, for large-scale preparations of aglycone-rich black soy milk, determination of optimal condition of the cellulose beads enzymatic system in a fluidized bed reactor will be the next challenge.
Acknowledgements
This study was financially supported by the Ministry of Science and Technology, Taiwan, R.O.C. (104-2221-E-002-125-MY3) and Ministry of Health and Welfare, Taiwan, R.O.C. (MOHW104-FDA-F-113-000364).
Funding Statement
This study was financially supported by the Ministry of Science and Technology, Taiwan, R.O.C. (104-2221-E-002-125-MY3) and Ministry of Health and Welfare, Taiwan, R.O.C. (MOHW104-FDA-F-113-000364).
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
REFERENCES
- 1. Murphy PA, Song T, Buseman G, Barua K, Beecher GR, Trainer D, Holden J. Isoflavones in retail and institutional soy foods. J Agric Food Chem. 1999;47:2697–704. doi: 10.1021/jf981144o. [DOI] [PubMed] [Google Scholar]
- 2. Chen KI, Erh MH, Su NW, Liu WH, Chou CC, Cheng KC. Soyfoods and soybean products: from traditional use to modern applications. Appl Microbiol Biotechnol. 2012;96:9–22. doi: 10.1007/s00253-012-4330-7. [DOI] [PubMed] [Google Scholar]
- 3. Lichtenstein AH. Soy protein, isoflavones and cardiovascular disease risk. J Nutr. 1998;128:1589–92. doi: 10.1093/jn/128.10.1589. [DOI] [PubMed] [Google Scholar]
- 4. Magee PJ, Rowland IR. Phyto-oestrogens, their mechanism of action: Current evidence for a role in breast and prostate cancer. Br J Nutr. 2004;91:513–31. doi: 10.1079/BJN20031075. [DOI] [PubMed] [Google Scholar]
- 5. Atkinson C, Compston JE, Day NE, Dowsett M, Bingham SA. The effects of phytoestrogen isoflavones on bone density in women: A double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2004;79:326–33. doi: 10.1093/ajcn/79.2.326. [DOI] [PubMed] [Google Scholar]
- 6. Takahashi R, Ohmori R, Kiyose C, Momiyama Y, Ohsuzu F, Kondo K. Antioxidant activities of black and yellow soybeans against low density lipoprotein oxidation. J Agric Food Chem. 2005;53:4578–82. doi: 10.1021/jf048062m. [DOI] [PubMed] [Google Scholar]
- 7. Okabe Y, Shimazu T, Tanimoto H. Higher bioavailability of isoflavones after a single ingestion of aglycone-rich fermented soybeans compared with glucoside-rich non-fermented soybeans in Japanese postmenopausal women. J Sci Food Agric. 2011;91:658–63. doi: 10.1002/jsfa.4228. [DOI] [PubMed] [Google Scholar]
- 8. Zheng Y, Hu J, Murphy PA, Alekel DL, Franke WD, Hendrich S. Rapid gut transit time and slow fecal isoflavone disappearance phenotype are associated with greater genistein bioavailability in women. J Nutr. 2003;133:3110–6. doi: 10.1093/jn/133.10.3110. [DOI] [PubMed] [Google Scholar]
- 9. Khaodhiar L, Ricciotti HA, Li L, Pan W, Schickel M, Zhou J, Blackburn GL. Daidzein-rich isoflavone aglycones are potentially effective in reducing hot flashes in menopausal women. Menopause. 2008;15:125–32. [PMC free article] [PubMed] [Google Scholar]
- 10. Izumi T, Piskula MK, Osawa S, Obata A, Tobe K, Saito M, Kataoka S, Kubota Y, Kikuchi M. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J Nutr. 2000;130:1695–9. doi: 10.1093/jn/130.7.1695. [DOI] [PubMed] [Google Scholar]
- 11. Kano M, Takayanagi T, Harada K, Sawada S, Ishikawa F. Bioavailability of isoflavones after ingestion of soy beverages in healthy adults. J Nutr. 2006;136:2291–6. doi: 10.1093/jn/136.9.2291. [DOI] [PubMed] [Google Scholar]
- 12. Tsangalis D, Ashton JF, Stojanovska L, Wilcox G, Shah NP. Development of an isoflavone aglycone-enriched soymilk using soy germ, soy protein isolate and bifidobacteria. Food Res Int. 2004;37:301–12. [Google Scholar]
- 13. Cheng KC, Lin JT, Wu JY, Liu WH. Isoflavone conversion of black soybean by immobilized Rhizopus spp. Food Biotechnol. 2010;24:312–31. [Google Scholar]
- 14. Cheng KC, Wu JY, Lin JT, Liu WH. Enhancements of isoflavone aglycones, total phenolic content, and antioxidant activity of black soybean by solid-state fermentation with Rhizopus spp. Eur Food Res Technol. 2013;236:1107–13. [Google Scholar]
- 15. Chen KI, Lo YC, Su NW, Chou CC, Cheng KC. Enrichment of two isoflavone aglycones in black soymilk by immobilized β-glucosidase on solid carriers. J Agric Food Chem. 2012;60:12540–6. doi: 10.1021/jf304405t. [DOI] [PubMed] [Google Scholar]
- 16. Chen KI, Lo YC, Liu CW, Yu RC, Chou CC, Cheng KC. Enrichment of two isoflavone aglycones in black soymilk by using spent coffee grounds as an immobiliser for β-glucosidase. Food Chem. 2013;139:79–85. doi: 10.1016/j.foodchem.2013.01.093. [DOI] [PubMed] [Google Scholar]
- 17. Chang J, Lee YS, Fang SJ, Park DJ, Choi YL. Hydrolysis of isoflavone glycoside by immobilization of β-glucosidase on a chitosan-carbon in two-phase system. Int J Biol Macromol. 2013;61:465–70. doi: 10.1016/j.ijbiomac.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 18. Grade LC, Moreira AA, Varea GD, Mandarino JMG, da Silva JB, Ida EI, Ribeiro MLL. Soybean beta-glucosidase immobilisated on chitosan beads and its application in soy drink increase the aglycones. Braz Arch Biol Technol. 2014;57:766–73. [Google Scholar]
- 19. Avnir D, Braun S, Lev O, Ottolenghi M. Enzymes and other proteins entrapped in sol-gel materials. Chem Mater. 1994;6:1605–14. [Google Scholar]
- 20. Hench LL, West JK. The sol-gel process. Chem Rev. 1990;90:33–72. [Google Scholar]
- 21. Chen LF, Tsao GT. Physical characteristics of porous cellulose beads as supporting material for immobilized enzymes. Biotechnol Bioeng. 1976;18:1507–16. doi: 10.1002/bit.260181103. [DOI] [PubMed] [Google Scholar]
- 22. Figueira JA, Dias FF, Sato HH, Fernandes P. Screening of supports for the immobilization of β-glucosidase. Enzyme Res. 2011;2011:642460. doi: 10.4061/2011/642460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bai YX, Li YF. Preparation and characterization of crosslinked porous cellulose beads. Carbohydr Polym. 2006;64:402–7. [Google Scholar]
- 24. Chen LF, Tsao GT. Chemical procedures for enzyme immobilization on porous cellulose beads. Biotechnol Bioeng. 1977;19:1463–73. doi: 10.1002/bit.260191005. [DOI] [PubMed] [Google Scholar]
- 25. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 26. Östenson M, Järund H, Toriz G, Gatenholm P. Determination of surface functional groups in lignocellulosic materials by chemical derivatization and ESCA analysis. Cellulose. 2006;13:157–70. [Google Scholar]
- 27. Kuzmenko V, Kalogeropoulos T, Thunberg J, Johannesson S, Hägg D, Enoksson P, Gatenholm P. Enhanced growth of neural networks on conductive cellulose-derived nanofibrous scaffolds. Mater Sci Eng C Mater Biol Appl. 2016;58:14–23. doi: 10.1016/j.msec.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 28. Lin S-P, Pan C-Y, Tseng K-C, Lin M-C, Chen C-D, Tsai C-C, Yu S-H, Sun Y-C, Lin T-W, Chen Y-T. A reversible surface functionalized nanowire transistor to study protein–protein interactions. Nano Today. 2009;4:235–43. [Google Scholar]
- 29. Khoobi M, Motevalizadeh SF, Asadgol Z, Forootanfar H, Shafiee A, Faramarzi MA. Polyethyleneimine-modified super paramagnetic Fe3O4 nanoparticles for lipase immobilization: Characterization and application. Mater Chem Phys. 2015;149–150:77–86. [Google Scholar]
- 30. Huang PJ, Chang KL, Hsieh JF, Chen ST. Catalysis of rice straw hydrolysis by the combination of immobilized cellulase from Aspergillus niger on β-cyclodextrin-Fe3O4 nanoparticles and ionic liquid. Biomed Res Int. 2015;2015:409103. doi: 10.1155/2015/409103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Singh RK, Zhang YW, Nguyen NP, Jeya M, Lee JK. Covalent immobilization of β-1,4-glucosidase from Agaricus arvensis onto functionalized silicon oxide nanoparticles. Appl Microbiol Biotechnol. 2011;89:337–44. doi: 10.1007/s00253-010-2768-z. [DOI] [PubMed] [Google Scholar]
- 32. Chiou TY, Lin YH, Su NW, Lee MH. Beta-glucosidase isolated from soybean okara shows specificity toward glucosyl isoflavones. J Agric Food Chem. 2010;58:8872–8. doi: 10.1021/jf101848x. [DOI] [PubMed] [Google Scholar]
- 33. Yeom SJ, Kim BN, Kim YS, Oh DK. Hydrolysis of isoflavone glycosides by a thermostable β-glucosidase from Pyrococcus furiosus. J Agric Food Chem. 2012;60:1535–41. doi: 10.1021/jf204432g. [DOI] [PubMed] [Google Scholar]
- 34. Li G, Jiang Y, Fan XJ, Liu YH. Molecular cloning and characterization of a novel β-glucosidase with high hydrolyzing ability for soybean isoflavone glycosides and glucose-tolerance from soil metagenomic library. Bioresour Technol. 2012;123:15–22. doi: 10.1016/j.biortech.2012.07.083. [DOI] [PubMed] [Google Scholar]
- 35. Song X, Xue Y, Wang Q, Wu X. Comparison of three thermostable β-glucosidases for application in the hydrolysis of soybean isoflavone glycosides. J Agric Food Chem. 2011;59:1954–61. doi: 10.1021/jf1046915. [DOI] [PubMed] [Google Scholar]