Abstract
Plant tissue culture technique is widely used in the conservation and utilization of rare and endangered medicinal plants and it is crucial for tissue culture stocks to obtain the ability to produce similar bioactive components as their wild correspondences. In this paper, a headspace gas chromatography–mass spectrometry method combined with chemometric methods was applied to analyze and evaluate the volatile compounds in tissue-cultured and wild Dendrobium huoshanense Cheng and Tang, Dendrobium officinale Kimura et Migo and Dendrobium moniliforme (Linn.) Sw. In total, 63 volatile compounds were separated, with 53 being identified from the three Dendrobium spp. samples. Different provenances of Dendrobiums had characteristic chemicals and showed remarkable quantity discrepancy of common compositions. The similarity evaluation disclosed that the accumulation of volatile compounds in Dendrobium samples might be affected by their provenance. Principal component analysis showed that the first three components explained 85.9% of data variance, demonstrating a good discrimination between samples. Gas chromatography–mass spectrometry techniques, combined with chemometrics, might be an effective strategy for identifying the species and their provenance, especially in the assessment of tissue-cultured Dendrobium quality for use in raw herbal medicines.
Keywords: Dendrobium huoshanense, Dendrobium moniliforme, Dendrobium officinale, gas chromatography-mass spectrometry, principal component analysis
1. Introduction
Dendrobium, a precious traditional Chinese medicine, has been used in the preparation of herbal medicines in China for more than 2000 years. Sections of the stems of Dendrobiums have long been used to cure throat inflammation, nourish the stomach, promote secretion of saliva or as a tonic to promote the production of body fluid and improve the quality of life [1]. Seventy-four species of Dendrobium and two varieties are found in China [2]. The slow growth rate and excessive harvesting had left some of them critically endangered, especially Dendrobium huoshanense [3].
Plant tissue culture technique is widely used in the conservation and utilization of rare and endangered medicinal plants due to its remarkable ability of quickly increasing their biomass [4,5]. In the traditional product region, tissue-cultured dendrobiums have already become the major resource of pharmaceutical Dendrobiums. It is vital for tissue culture stocks to obtain the ability to produce similar bioactive components as their wild correspondences besides keeping genetic information and morphologies homoplastic between different provenances. Therefore, establishing a fast, quality identification method to evaluate the chemical similarity of the wild and tissue-cultured Dendrobium is a critical step for assurance of quality and safety in the traditional Chinese medicine industry.
The volatile components of herbal medicines contain a significant number of compounds and are used as markers for authenticity. The variations of volatile components in plants might be caused by differences in species, habitats, variety, cultivation patterns, or the extraction and analysis methods applied for composition determination [6,7]. Accordingly, it might be practical to establish a gas chromatography–mass spectrometry (GC–MS) fingerprint method based on the analysis of the volatile components to evaluate the similarity between tissue-cultured medicinal plants and their wild correspondences.
In this paper, we aimed to apply the headspace GC–MS technique coupled with a series of chemometric methods to fingerprint the volatile compounds from the stems of tissue-cultured and wild D. huoshanense, Dendrobium officinale, and Dendrobium moniliforme. To our knowledge, no documents have ever mentioned the discrimination and similarity evaluation of the tissue-cultured and wild Dendrobium plants by GC–MS method. Therefore, our study might be beneficial for developing a rapid, feasible and economical tool based on GC–MS for the identification and quality evaluation between different provenances of Dendrobium species, and provide new insights into the utilization and conservation of rare and endangered medicinal plants by tissue culture techniques.
2. Methods
2.1. In vitro callus growth protocol, plant materials, and chemicals
The in vitro plantlets of the three tissue-cultured dendrobiums were regenerated via protocorm-like bodies in the laboratories of West Anhui Biotechnology Research Center of Natural Medicine and Traditional Chinese Medicine and were then transplanted in the cultivated base in Huoshan count, Anhui Province, China. The current season’s vegetative stems of tissue-cultured and wild D. huoshanense, D. officinale, and D. moniliforme were collected in October 2013, from Huoshan County, Anhui Province, China. All the plant materials were identified by Professor Nai-Fu Chen, Anhui Biotechnology Research Center of Plant Cell Engineering, Anhui Province, China. The voucher specimens were deposited at the Herbarium, College of Biotechnology and Pharmaceutical Engineering, West Anhui University, Anhui Province, China (Table 1).
Table 1.
List of Dendrobium samples.
| Samples | Abbreviation | Source | Voucher No. |
|---|---|---|---|
| Wild Dendrobium huoshanense | W-DHS | Huoshan, Anhui, China | 201310HS0101Y |
| Tissue-cultured D. huoshanense | TC-DHS | Huoshan, Anhui, China | 201310HS0101T |
| Wild Dendrobium officinale | W-DO | Huoshan, Anhui, China | 201310HS0201Y |
| Tissue-cultured D. officinale | TC-DO | Huoshan, Anhui, China | 201310HS0201T |
| Wild Dendrobium moniliforme | W-DM | Huoshan, Anhui, China | 201310HS0301Y |
| Tissue-cultured D. moniliforme | TC-DM | Huoshan, Anhui, China | 201310HS0301T |
The authentic chemicals and alkane standard solutions of C8–C20 (mixture no. 115321-01-4PAK) were purchased from ANPEL Laboratory Technologies Inc. (Shanghai, China).
The fresh collected Dendrobium samples were washed thoroughly in tap water and then freeze-dried by a Micro-Modulyo lyophilizer (Thermo Fisher Scientific, West Palm Beach, FL, USA), powdered in a blender and then every 2.0 g of dried sample was performed for headspace/GC–MS analysis.
2.2. Equipment and conditions
GC–MS analysis was performed with Trace 1300 gas chromatograph coupled to ISQ mass spectrometer (Thermo Fisher Scientific, West Palm Beach, FL, USA) series equipment including a TriPLUS RSH autosampler. The volatile compounds were separated on a TG-5 MS capillary column (30 m × 0.25 mm, 0.25 μm film thickness). Total program time was 39 minutes and the column oven temperature program was: 50°C (maintained for 1 minute) to 60°C at 1°C/min, to 200°C at 5°C/min. The carrier gas was Helium, 1.0 mL/min, split ratio 5:1, injector temperature 250°C. The samples were heated in the agitator oven for 10 minutes with constant incubation mode at 140°C. The injection volume was 2.5μL. The MS transfer line and ion source were at 280°C and 250°C, respectively. The MS mode was electron impact. The mass range scanned was 40–350 atomic mass units.
2.3. Identification of the separated compounds
The identification of the separated compounds was carried out by three different methods: (1) retention indices [8] of the compounds to be identified compared the retention index values detected by the same type of capillary column in the National Institute of Technology and Standards mass spectra libraries; (2) retention times of authentic standards in the same equipment and conditions; and (3) mass spectra, with indexes of relative match above 800 (US National Institute of Technology and Standards mass spectra libraries and also authentic chemicals). Compounds were marked as tentatively identified when identification was only based on mass spectral data.
All peaks found in at least two of the three total ion chromatograms (TIC) were taken into account when calculating the total area of peaks (100%) and the relative areas of the volatile compounds.
2.4. Quantitative analysis of the volatile compounds
According to the resolved chromatogram and mass spectra, the quantitative analysis of each component can be directly calculated by the overall volume integration [6,9–12]. They are proportional to the content of the peak as integration based on TIC when calculating the total area of peaks (100%) and the relative areas of the volatile compounds. The peak area in TIC was chosen as the analytical signal for the relative content. The precision and repeatability of the determination were established using six individual weighed powder samples. Relative standard deviations of peak areas of selected components were calculated.
2.5. Statistical analysis
The similarities of the fingerprint in the samples were evaluated by the correlation coefficient (rcor) calculated by included cosine angle using SPSS software Version 16.0 (CAMO Software AS, Woodbridge, NJ, USA). For the characterization of the investigated Dendrobium samples, the obtained GC–MS profiles were subjected to principal component analysis (PCA) with MetaboAnalyst 3.0 [13,14].
3. Results and discussion
3.1. Fingerprinting of volatile components by GC–MS analysis
After resolution, according to the GC–MS analysis of the six Dendrobium samples, a total of 63 compounds were separated and 52 were identified.
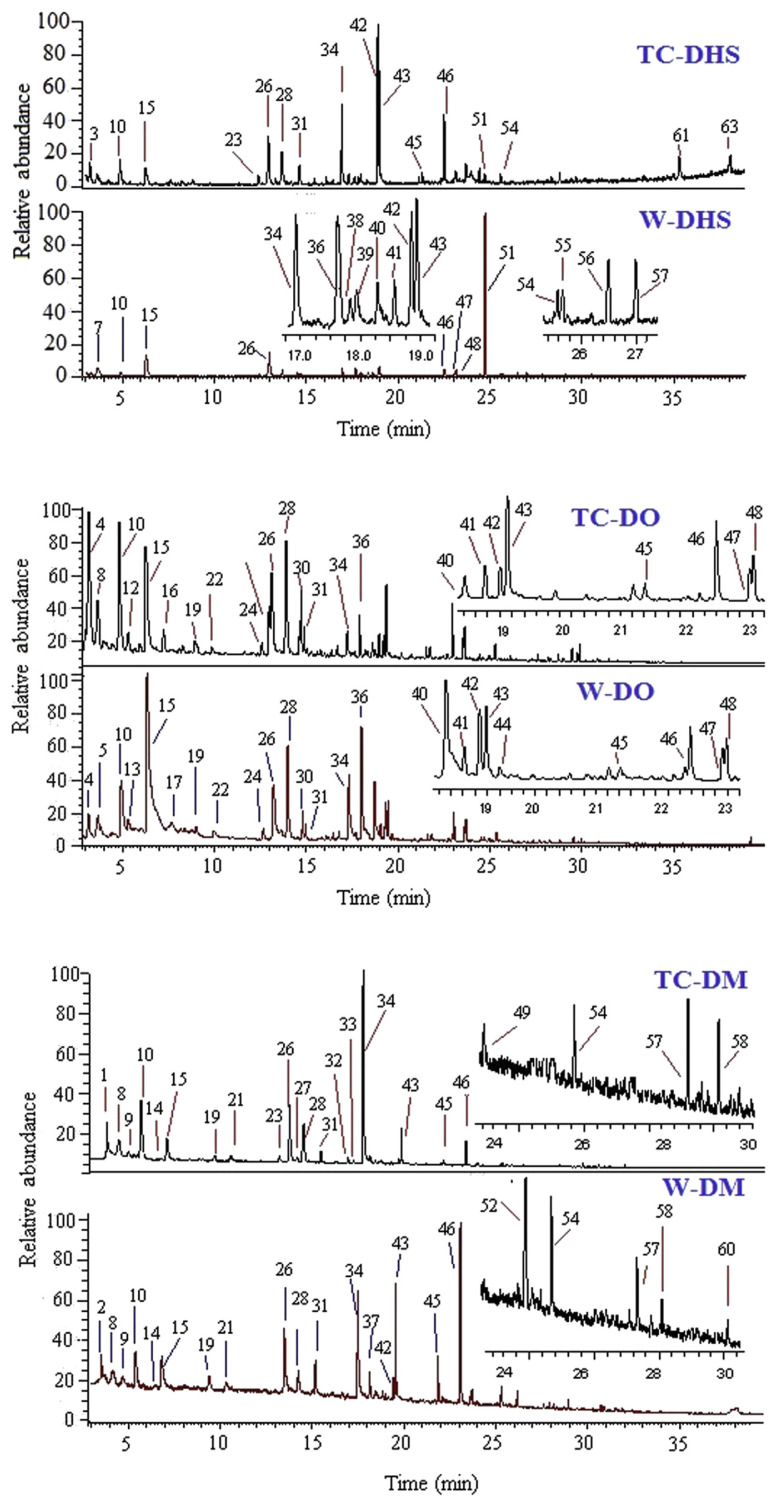
The characteristic GC–MS chromatograms of tissue-cultured D. huoshanense (TC-DHS) and wild D. huoshanense (W-DHS), tissue-cultured D. officinale (TC-DO) and wild D. officinale (W-DO), tissue-cultured D. moniliforme (TC-DM) and wild D. moniliforme (W-DM) are presented in Figure 1, showing the specific volatile compound profiles from different Dendrobium samples, isolated using the headspace technique and analyzed by GC–MS. The main compounds were normal chain aliphatic alcohols and aldehydes, esters, aromatic compounds and terpene, the most abundant components were 1-decanol (in W-DHS, 41.4 ± 3.87%), furfural (in W-DO, 20.84 ± 1.75%), and 1-dodecene (in TC-DHS, 18.56 ± 2.35%; Table 2). The relative proportion of the three main compounds (expressed as a percentage of total peak area) accounted for over 30% of all volatile compounds found in the Dendrobium samples.
Figure 1.
Total ion chromatograms of headspace GC–MS analysis of volatile compounds from different provenances of tissue-cultured and wild Dendrobium varieties. The numbering refers to Table 2. TC-DHS = tissue-cultured Dendrobium huoshanense; TC-DM = tissue-cultured Dendrobium moniliforme; TC-DO = tissue-cultured Dendrobium officinale; W-DHS = wild D. huoshanense; W-DM = wild D. moniliforme; W-DO = wild D. officinale.
Table 2.
Mean relative concentrations (expressed as % from total peak areas) and standard deviations of volatile compounds from different provenances of Dendrobiums analyzed by HS-GC/MS technique.
| No | Chemical | RM | RI | TC-DHS | W-DHS | TC-DO | W-DO | TC-DM | W-DM |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Cyclohexenea,b,c | 874 | 704 | — | — | — | — | 6.15 ± 1.25 | — |
| 2 | Methyl isobutyl ketonea,b | 832 | 721 | — | — | 0.24 ± 0.07 | — | — | 6.38 ± 0.87 |
| 3 | 3-hydroxy-2-butanonea,b,c | 842 | 733 | 3.53 ± 0.21 | — | — | — | — | 2.17 ± 0.11 |
| 4 | N,N-dimethyl-propanamidea | 803 | 741 | — | 1.09 ± 0.08 | 12.64 ± 1.19 | 0.14 ± 0.08 | — | — |
| 5 | Not identified | — | 752 | — | — | — | 1.98 ± 0.17 | — | — |
| 6 | 3-methyl-1-butanola,b,c | 819 | 762 | — | — | — | — | — | 0.86 ± 0.08 |
| 7 | 1-pentanola,b,c | 915 | 775 | — | 5.17 ± 0.55 | — | — | — | — |
| 8 | Not identified | — | 779 | — | — | 4.41 ± 0.23 | — | 4.64 ± 0.77 | 2.04 ± 0.24 |
| 9 | 1-octenea,b | 821 | 785 | — | — | 0.61 ± 0.05 | — | 1.33 ± 0.27 | 0.69 ± 0.07 |
| 10 | Hexanala,b,c | 914 | 790 | 4.84 ± 0.15 | 2.01 ± 0.07 | 10.39 ± 0.62 | 8.59 ± 0.33 | 12.95 ± 2.56 | 5.14 ± 0.88 |
| 11 | (S)-2-hydroxypropanoic acida | 807 | 801 | — | — | — | 1.21 ± 0.25 | — | — |
| 12 | Cyclopentanola,b,c | 896 | 803 | — | — | 1.4 ± 0.09 | — | — | — |
| 13 | 2,3-butanediola,b,c | 878 | 806 | — | — | — | 0.41 ± 0.11 | — | — |
| 14 | 4-methyloctanoic acida | 952 | 820 | — | 0.42 ± 0.04 | 0.57 ± 0.06 | 0.79 ± 0.14 | 0.16 ± 0.07 | 0.32 ± 0.05 |
| 15 | Furfurala,b | 878 | 833 | 2.48 ± 0.41 | 10.16 ± 1.18 | 11.75 ± 0.61 | 20.84 ± 1.75 | 4.68 ± 0.85 | 4.95 ± 0.66 |
| 16 | 3-methyl-1-pentanola,b | 887 | 846 | — | — | 1.66 ± 0.11 | — | — | — |
| 17 | Not identified | — | 857 | — | — | 0.13 ± 0.02 | 1.94 ± 0.22 | — | — |
| 18 | 2-heptanonea,b | 875 | 873 | — | 0.15 ± 0.03 | 0.46 ± 0.05 | 0.24 ± 0.06 | 0.26 ± 0.09 | 0.13 ± 0.02 |
| 19 | 2-ethyl-heptanoic acida,b | 842 | 877 | — | 0.26 ± 0.04 | 0.9 ± 0.04 | 0.71 ± 0.08 | 1.12 ± 0.07 | 1.71 ± 0.15 |
| 20 | 2-hexen-1-ol, (E)-a,b | 921 | 881 | — | — | 0.53 ± 0.05 | — | — | — |
| 21 | Not identified | — | 895 | — | — | — | — | 1.21 ± 0.18 | 1.06 ± 0.09 |
| 22 | Not identified | — | 899 | — | — | 0.49 ± 0.08 | 0.63 ± 0.07 | — | — |
| 23 | 11-hexadecen-1-ol, (E)-a | 809 | 961 | 0.92 ± 0.06 | 0.56 ± 0.04 | — | — | 1.08 ± 0.11 | — |
| 24 | Benzaldehydea,b | 933 | 982 | — | — | 1.03 ± 0.11 | 1.28 ± 0.22 | ||
| 25 | 4,4-dimethyl-2-cyclohexen-1-onea | 815 | 992 | — | — | 2.89 ± 0.78 | — | — | — |
| 26 | Benzeneacetaldehydea,b | 903 | 1003 | 8.05 ± 1.33 | 9.44 ± 0.87 | 6.64 ± 1.12 | 6.5 ± 0.75 | 11.01 ± 1.03 | 9.78 ± 0.94 |
| 27 | Cis-9-tetradecen-1-ola | 824 | 1014 | 0.34 ± 0.06 | — | — | — | 0.59 ± 0.08 | — |
| 28 | Eucalyptola,b | 847 | 1032 | 6.14 ± 1.47 | 2.39 ± 0.54 | 10.31 ± 1.96 | 10.79 ± 1.32 | 7.98 ± 0.85 | 3.02 ± |
| 29 | Not identified | — | — | — | — | 0.88 ± 0.21 | — | — | — |
| 30 | 1-octanola,b | 839 | 1051 | — | 1 ± 0.11 | 3.8 ± 0.96 | 2.29 ± 0.77 | — | 0.18 ± 0.03 |
| 31 | Cyclopentadecanola | 804 | 1061 | 2.28 ± 0.22 | 0.7 ± 0.05 | 1.3 ± 0.23 | 1.18 ± 0.21 | 1.6 ± 0.08 | 3.44 ± 0.39 |
| 32 | Acetophenonea,b | 856 | 1069 | 0.48 ± 0.08 | 0.16 ± 0.06 | 0.38 ± 0.11 | 0.46 ± 0.08 | 0.77 ± 0.09 | 0.49 ± 0.07 |
| 33 | 1-octanola,b,c | 897 | 1083 | — | — | 0.69 ± 0.12 | 0.64 ± 0.07 | 0.46 ± 0.06 | — |
| 34 | Terpinolenea,b,c | 868 | 1097 | 11.78 ± 2.13 | 2.65 ± 0.39 | 1.46 ± 0.32 | 5.62 ± 0.99 | 28.18 ± | 11.22 ± 1.22 |
| 35 | Nonanala,b | 884 | 1108 | 0.71 ± 0.09 | — | 0.22 ± 0.08 | 0.23 ± 0.07 | 0.61 ± 0.04 | — |
| 36 | Linaloola,b | 981 | 1114 | 0.52 ± 0.07 | 3.22 ± 0.58 | 2.07 ± 0.55 | 9.46 ± 0.82 | — | — |
| 37 | Nonanala,b,c | 947 | 1128 | — | — | — | — | 0.24 ± 0.03 | 2.37 ± 0.15 |
| 38 | 1-acetyl-2-methyl-1-cyclopentenea | 936 | 1135 | — | 0.3 ± 0.05 | 0.33 ± 0.04 | 0.26 ± 0.07 | 0.48 ± 0.08 | 0.41 ± 0.07 |
| 39 | Undecanala | 892 | 1151 | 0.88 ± 0.06 | 0.6 ± 0.04 | — | — | — | — |
| 40 | 3-ethyl-phenola,b | 845 | 1165 | — | 0.69 ± 0.09 | 0.73 ± 0.11 | 5.33 ± 0.88 | — | 0.54 ± 0.09 |
| 41 | Not identified | — | 1171 | — | 0.75 ± 0.08 | 0.99 ± 021 | 0.89 ± 0.09 | — | — |
| 42 | 1-dodecenea,b | 848 | 1175 | 18.56 ± 2.35 | 2.08 ± 0.79 | 0.88 ± 0.16 | 2.51 ± 0.44 | 0.48 ± 0.08 | 1.68 ± 0.11 |
| 43 | Octanoic acida,b | 854 | 1185 | 14.07 ± 1.15 | 2.5 ± 0.57 | 3.07 ± 0.22 | 2.81 ± 0.45 | 4.22 ± 0.77 | 9.98 ± 0.65 |
| 44 | A,4-dimethy l-3-cyclohexene-1-acetaldehydea | 817 | 1184 | — | — | — | 0.34 ± 0.05 | — | — |
| 45 | Glycolophenonea | 832 | 1193 | 0.69 ± 0.05 | 0.12 ± 0.05 | 0.47 ± 0.13 | 0.44 ± 0.08 | 0.74 ± 0.08 | 3.52 ± 0.54 |
| 46 | Octanoic acid ethyl estera,b | 899 | 1203 | 7.85 ± 1.87 | 1.99 ± 0.15 | 2.47 ± 0.22 | 2.01 ± 023 | 2.61 ± 0.21 | 14.24 ± 2.13 |
| 47 | 4-terpinenola,b | 821 | 1220 | — | 1.08 ± 0.13 | 0.81 ± 0.09 | 1.02 ± 0.08 | — | 0.46 ± 0.05 |
| 48 | Not identified | — | 1234 | 0.75 ± 0.11 | 1.41 ± 0.21 | 1.27 ± 0.22 | 1.49 ± 0.17 | 0.44 ± 0.07 | 1.07 ± 0.05 |
| 49 | Isolongifolene epoxidea | 809 | 1253 | — | — | — | — | 0.12 ± 0.04 | — |
| 50 | 2,3,5-trimethyl-phenola,b,c | 884 | 1266 | 2.13 ± 0.09 | — | — | — | — | — |
| 51 | 1-decanola,b | 875 | 1285 | 0.69 ± 0.07 | 41.4 ± 3.87 | — | — | — | — |
| 52 | 9-tetradecen-1-ol, (E)-a | 855 | 1295 | — | — | 0.65 ± 0.07 | — | — | 1.98 ± 0.21 |
| 53 | 3,4-dihydro-5,7-dimethyl-1(2H)-Naphthalenonea | 801 | 1311 | — | — | — | 0.76 ± 0.08 | — | — |
| 54 | 2-naphthaleneethanola | 832 | 1329 | 0.5 ± 0.1 | 0.26 ± 0.04 | 0.17 ± 0.03 | — | 0.21 ± 0.04 | 1.15 ± 0.14 |
| 55 | 1-hexadecen-1-ol, (Z)-a | 896 | 1347 | — | 0.81 ± 0.08 | — | — | — | — |
| 56 | 1-undecanola,b | 854 | 1368 | — | 0.68 ± 0.11 | — | — | — | — |
| 57 | Not identified | — | 1396 | — | 0.1 ± 0.02 | 0.15 ± 0.04 | 0.19 ± 0.05 | 0.13 ± 0.03 | 0.34 ± 0.07 |
| 58 | Dodecanala,b,c | 875 | 1426 | 0.25 ± 0.06 | 0.15 ± 0.03 | 0.18 ± 0.05 | — | 0.09 ± 0.02 | 0.73 ± 0.09 |
| 59 | Not identified | — | 1488 | — | — | 0.58 ± 0.07 | 0.21 ± 0.04 | — | — |
| 60 | 2-naphthalenola,b | 855 | 1524 | — | 0.14 ± 0.03 | 0.11 ± 0.05 | — | — | 0.24 ± 0.07 |
| 61 | N-tridecanola,b,c | 891 | 1591 | 4.42 ± 0.24 | — | — | — | — | — |
| 62 | Not identified | — | 1675 | — | — | — | — | — | 0.63 ± 0.09 |
| 63 | Pentadecanoic acida,b | 826 | 1889 | 2.39 ± 0.31 | — | — | — | — | — |
— = not detected in our experimental conditions; HS-GC/MS = headspace gas chromatography–mass spectrometry; RI = retention indices; RM = relative match; TC-DHS = tissue-cultured Dendrobium huoshanense; TC-DM = tissue-cultured Dendrobium moniliforme; TC-DO = tissue-cultured Dendrobium officinale; W-DHS = wild D. huoshanense; W-DM = wild D. moniliforme; W-DO = wild D. officinale.
Tentatively identified based on the National Institute of Technology and Standards library.
Identified based on the RI in literature.
Identified based on the authentic chemicals.
3.2. Semiquantitative GC–MS analysis
Semiquantitative GC–MS analysis, a common quantity method when standard chemicals are difficult to obtain [6,8,15], was applied to evaluate the contents of the separated compounds in the Dendrobium samples by calculating the overall volume integration in TIC. The peak area in TIC was chosen as the analytical signal for the relative content because it is proportional to the content of the peak as integration based on TIC when calculating the total area of peaks (100%) and the relative areas of the volatile compounds. The separated components are listed in Table 2 and expressed as percentages of total peak area (%). As can be observed, each Dendrobium had its own major compounds. In TC-DHS, the contents of 1-dodecene (42), octanoic acid (43), terpinolene (34), and benzaldehyde (26) were 16.33–20.79%, 12.97–15.18%, 9.65–13.91%, and 6.72–9.38%, respectively; W-DHS showed a distinctive characteristic of high content of 1-decanol (51), at over 45%, while only 0.69 ± 0.07% of 51 was detected in TC-DHS and no compound 51 was detected in D. officinale and D. moniliforme in our experimental conditions. The most abundant volatile compounds in TC-DO were propanamide, N,N-dimethyl-(4) (11.45–13.83%), Furfural (15) (11.14–12.36%), hexanal (10) (9.77–11.01%) and eucalyptol (28) (8.35–12.27%). W-DO had almost the same major compounds as those in its tissue-cultured correspondence. In TC-DM, the major compounds were benzeneacetaldehyde (34), hexanal (10), benzaldehyde (26), and cis-9-tetradecen-1-ol (28), while they were dodecanal (46), terpinolene (34), octanoic acid (43), benzaldehyde (26), and methyl isobutyl ketone (2) in W-DM.
Comparing volatile compounds profiles from the six investigated Dendrobium stocks, each sample had its characteristic volatile compounds. For example, the characteristic chemicals of TC-DHS were 2,3,5-trimethyl-phenol (50), n-tridecanol (61), and pentadecanoic acid (63) in contrast to W-DHS and the other two Dendrobium species. Similarly, the characteristic chemicals of TC-DO were 3-methyl-1-pentanol (16), 2-hexen-1-ol, (E)- (20), and 4,4-dimethyl-2-cyclohexen-1-one (25); TC-DM’s characteristic chemicals were cyclohexene (1) and isolongifolene epoxide (49). The contents of some common volatile compounds varied remarkably between different provenances of the three dendrobiums. For example, TC-DHS had over 21% of 1-dodecene (42), about 10–40 times the contents in the other five Dendrobium samples. About 22% of furfural (15) was detected in W-DO, obviously higher than that in other samples. The content of terpinolene (34, 28.18 ± 1.2%) was more than twice that in W-DM. From the GC–MS spectra profiles, the characteristic chemicals and the contents of common volatile compounds into consideration, the six Dendrobium samples could be discriminated approximately.
The GC–MS analysis reflected the low boiling point chemical constitutes of Dendrobium (the volatile metabolites), which are usually influenced by not only the genetic materials but also the growing conditions [16–21]. Our experiments revealed that the accumulation of the volatile metabolites in Dendrobiums might be affected by their provenances to a much greater extent than we had expected. For medicinal plants, the volatile compounds might originate in three possible ways: the metabolism of the plants themselves, the metabolism of the endophytic fungi in the medicine plants, or the defensive substance of the endophytes and/or the host plants. For the three investigated tissue-cultured Dendrobiums, removing the endophytic fungi in the sterile stage of test-tube seeding might cause the absence of the endophyte-originated metabolites in the plants, although more experimental data are still needed. Keeping the chemicals similar between the tissue-cultured medicinal plants and their wild correspondences is a challenge and bottleneck in the conservation and utilization of the endangered Dendrobium species, especially D. huoshanense, using tissue culture technology.
3.3. Similarity analysis of GC–MS fingerprint of different provenances of dendrobiums
The similarity evaluation based on chromatographic finger-print analysis of different samples is a common technique [22]. To evaluate further the similarity of different provenances of Dendrobiums, the correlation coefficient calculated by included cosine angle was used as a similarity measure in the present study to appraise the similarity of the six Dendrobium samples. As shown in Table 3, different provenances of Dendrobium samples exhibited rather low similarity with the correlation coefficients 0.7–0.8, indicating that the three investigated Dendrobiums had some specific volatile compounds between different provenances, although they obtained the similar genetic information and cultivated on the same conditions. Furthermore, the correlation coefficients of different species were obviously lower than those between different origins consisted with the same genetic information between different origins while much more differences among different species. Li et al [23] had reported that the genetic distance between D. moniliforme and D. huoshanense was smaller than that between D. huoshanense and D. tosaense, and D. huoshanense should be the synonym of D. moniliforme. Our study showed that the coefficients of TC-DHS and W-DM were much higher than those of DHS and DO, which might provide further evidence for Li et al’s suggestion [23].
Table 3.
The correlation coefficients based on chromatographic fingerprint analysis of the six Dendrobium samples.
| TC-DHS | W-DHS | TC-DO | W-DO | TC-DM | W-DM | |
|---|---|---|---|---|---|---|
| TC-DHS | 1.000 | 0.726 | 0.394 | 0.438 | 0.596 | 0.720 |
| W-DHS | 1.000 | 0.247 | 0.295 | 0.485 | 0.558 | |
| TC-DO | 1.000 | 0.765 | 0.471 | 0.473 | ||
| W-DO | 1.000 | 0.533 | 0.501 | |||
| TC-DM | 1.000 | 0.790 | ||||
| W-DM | 1.000 |
TC-DHS = tissue-cultured Dendrobium huoshanense; TC-DM = tissue-cultured Dendrobium moniliforme; TC-DO = tissue-cultured Dendrobium officinale; W-DHS = wild D. huoshanense; W-DM = wild D. moniliforme; W-DO = wild D. officinale.
3.4. PCA
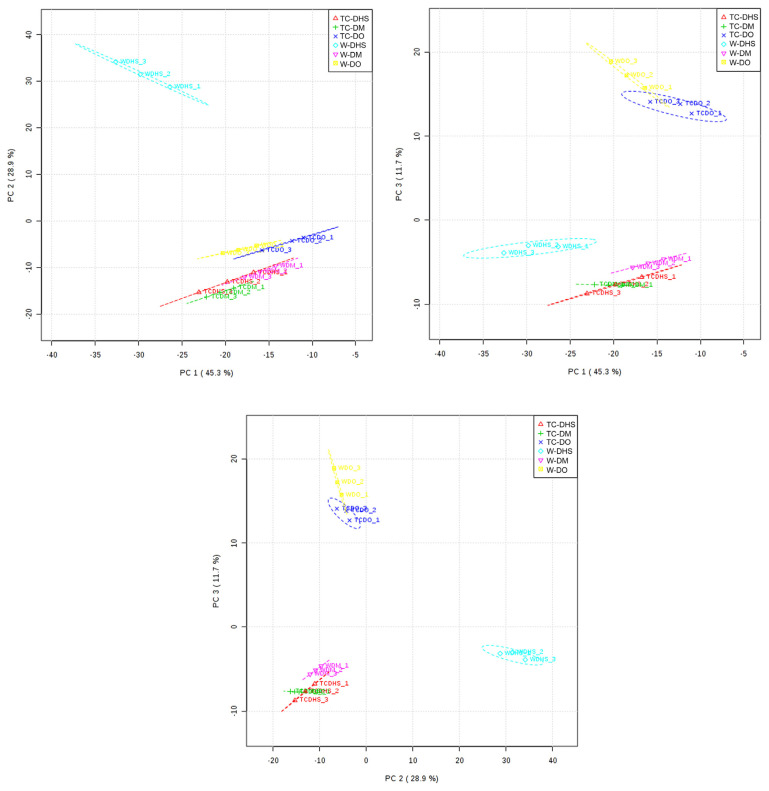
Similarity analysis embodies the characteristics of GC–MS fingerprint with integrity and fuzzy [24]. However, this analytical method cannot clearly show the relationships among nonadjacent objects [24,25]. In order to discriminate the investigated Dendrobiums more clearly, PCA of the data was performed by taking into consideration the volatile profiles of both wild and tissue-cultured Dendrobium samples.
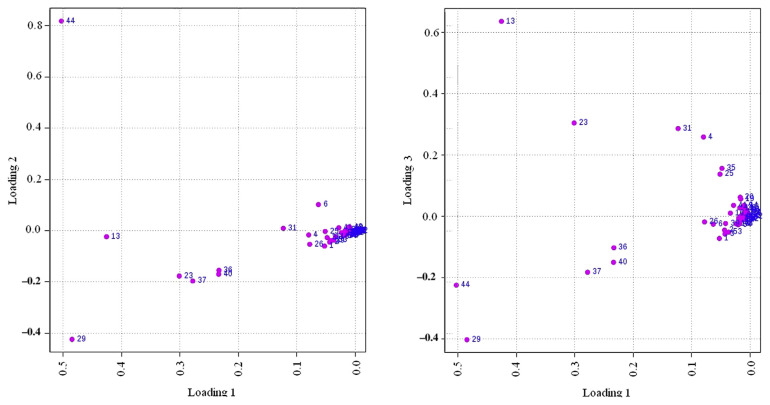
The PCA (Figures 2 and 3) results of the first three principal components explained 85.9% of the variance of the data, showing a good discrimination between the samples. The score plot and the loading plot gave us valuable information regarding the correlation between Dendrobiums and volatile compositions to reveal the correlation between variables (volatile compounds and Dendrobium species and provenance). Thus, based on their volatile profiles, TC-DHS, TC-DO, and TC-DM could be discriminated easily from their wild correspondences W-DHS, W-DO and W-DM (Figure 2). Also, the volatile profile of TC-DHS was similar to the volatile profile of D. moniliforme samples. The loading plot of the three first components (Figure 3) showed a strong relationship between five major compounds 44, 29, 15, 23, and 37 (nonanal) in Dendrobiums.
Figure 2.
Two-dimensional score-plots of principal components. TC-DHS = tissue-cultured Dendrobium huoshanense; TC-DM = tissue-cultured Dendrobium moniliforme; TC-DO = tissue-cultured Dendrobium officinale; W-DHS = wild D. huoshanense; W-DM = wild D. moniliforme; W-DO = wild D. officinale.
Figure 3.
The correlation loadings biplots for tissue-cultured and wild Dendrobium varieties. The numbering refers to Table 2.
4. Conclusions
The use of GC–MS combined with chemometrics to analyze the volatile compounds of Dendrobiums can effectively classify and identify the three investigated Dendrobium species of different provenances and suggest reasons for their varying chemical compositions. The results obtained in this study can provide a comprehensive evaluation for the quality of Dendrobiums of different provenances and an optimization evaluation method for medicinal herb quality control.
By using PCA as well as similarity evaluation based on the GC–MS data, it was revealed that the qualitative compositions and the contents of the volatile compounds in Dendrobiums were generally characteristic and specific between tissue-cultured and wild stocks; this suggests that the chemical constituents in tissue-cultured Dendrobiums are quite different from those in their corresponding wild stocks although they have similar genetic information and homoplastic morphologies and were cultivated in the same environmental conditions. In addition, further investigations, such as whether the variation in chemicals between tissue-cultured and wild Dendrobiums would increase or decrease their pharmacodynamic action, are critically needed for the conservation and utilization of rare and endangered Dendrobiums.
Acknowledgments
This work was supported by: National Natural Science Foundation of China (NSFC No. 81274021, NSFC No.81573536, No.81171797); Anhui Provincal Natural Science Foundation (1608085MH221); the Provincial Level Nature Science Foundation of Anhui Education Department (KJ2016A886, KJ2015ZD43); Project funded by China Postdoctoral Science Foundation (2014M551791); and the Research-based Learning Program of West Anhui University (AH201510376024, AH201510376023).
Funding Statement
This work was supported by: National Natural Science Foundation of China (NSFC No. 81274021, NSFC No.81573536, No.81171797); Anhui Provincal Natural Science Foundation (1608085MH221); the Provincial Level Nature Science Foundation of Anhui Education Department (KJ2016A886, KJ2015ZD43); Project funded by China Postdoctoral Science Foundation (2014M551791); and the Research-based Learning Program of West Anhui University (AH201510376024, AH201510376023).
Footnotes
Conflicts of interest
All authors have no conflicts of interest to declare.
REFERENCES
- 1. Fan YJ, He XJ, Zhou SD, Luo A, He T, Chun Z. Composition analysis and antioxidant activity of polysaccharide from Dendrobium denneanum. Int J Biol Macromol. 2009;45:169–73. doi: 10.1016/j.ijbiomac.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 2.Bao XS, Shun QS, Chen LZ. The medicinal plants of Dendrobium (SHI-HU) in China. Shanghai: Shanghai Medial University Press; 2001. [Google Scholar]
- 3. Yang L, Wang ZT, Xu LS. Simultaneous determination of phenols (bibenzyl, phenanthrene, and fluorenone) in Dendrobium species by high-performance liquid chromatography with diode array detection. J Chromatogr A. 2006;1104:230–7. doi: 10.1016/j.chroma.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 4. Gago J, Pérez-Tornero O, Landín M, Burgos L, Gallego PP. Improving knowledge of plant tissue culture and media formulation by neurofuzzy logic: a practical case of data mining using apricot databases. J Plant Physiol. 2011;168:1858–65. doi: 10.1016/j.jplph.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 5. Gonda S, Kiss-Szikszai A, Szűcs Z, Máthé C, Vasas G. Effects of N source concentration and ratio on phenylethanoid glycoside pattern in tissue cultures of Plantago lanceolata L.: a metabolomics driven full-factorial experiment with LC-ESI–MS3. Phytochem. 2014;106:44–54. doi: 10.1016/j.phytochem.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 6. Socaci SA, Socaciu C, Maria T, Loan V, Pintea A. In-tube extraction and GC–MS analysis of volatile components from wild and cultivated sea buckthorn (Hippophae rhamnoides L. ssp. Carpatica) berry varieties and juice. Phytochem Anal. 2013;24:319–28. doi: 10.1002/pca.2413. [DOI] [PubMed] [Google Scholar]
- 7. Figueiredo AC, Barroso JG, Pedro LG, Scheffer JJC. Factors affecting secondary metabolite production in plants: volatile components and essential oils. Flavour Frag J. 2008;23:213–26. [Google Scholar]
- 8. Wang YM, Yi LZ, Liang YZ, Li H, Yuan D, Gao H, Zeng M. Comparative analysis of essential oil components in Pericarpium citri Reticulatae Viride and Pericarpium citri Reticulatae by GC–MS combined with chemometric resolution method. J Pharm Biomed Anal. 2008;46:66–74. doi: 10.1016/j.jpba.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 9. Yeh TS, Lin TC, Chen CC, Wen HM. Analysis of free and bound formaldehyde in squid and squid products by gas chromatography–mass spectrometry. J Food Drug Anal. 2013;21:190–7. [Google Scholar]
- 10. Chen HC, Peng LW, Sheu MJ, Lin LY, Chiang HM, Wu CT, Wu CS, Chen YC. Effects of hot water treatment on the essential oils of calamondin. J Food Drug Anal. 2013;21:363–8. [Google Scholar]
- 11. Tao NP, Wu R, Zhou PG, Gu SQ, Wu W. Characterization of odor-active compounds in cooked meat of farmed obscure puffer (Takifugu obscurus) using gas chromatography–mass spectrometry–olfactometry. J Food Drug Anal. 2014;22:431–8. doi: 10.1016/j.jfda.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sung W. Volatile constituents detected in smoke condensates from the combination of the smoking ingredients sucrose, black tea leaves, and bread flour. J Food Drug Anal. 2013;21:292–300. [Google Scholar]
- 13. Xia JG, Mandal R, Sinelnikov I, David D, Wishart DS. MetaboAnalyst 2.0—a comprehensive server for metabolomic data analysis. Nucl Acids Res. 2012;40:127–33. doi: 10.1093/nar/gks374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xia JG, Psychogios N, Young N, Wishart DS. MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucl Acids Res. 2009;37:652–60. doi: 10.1093/nar/gkp356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tan HS, Hu DD, Song JZ, Xu Y, Cai SF, Chen QL, Meng QW, Li SL, Chen SL, Mao Q, Xu HX. Distinguishing Radix Angelica sinensis from different regions by HS SFME/GC–MS. Food Chem. 2015;186:200–6. doi: 10.1016/j.foodchem.2014.05.152. [DOI] [PubMed] [Google Scholar]
- 16. Chen ND, Chen H, Li J, Sang MM, Ding S, Yu H. Discrimination and similarity evaluation of tissue-cultured and wild Dendrobium species using Fourier transform infrared spectroscopy. J Mol Struct. 2015;1086:255–65. [Google Scholar]
- 17. Chen ND, Chen NF, Li J, Cao CY, Wang JM. Rapid authentication of different ages of tissue-cultured and wild Dendrobium huoshanense as well as wild Dendrobium henanense using FTIR and 2D-COS. J Mol Struct. 2015;1101:101–8. [Google Scholar]
- 18. Yen HF, Hsieh CT, Hsieh TJ, Chang FR, Wang CK. In vitro anti-diabetic effect and chemical component analysis of 29 essential oils products. J Food Drug Anal. 2015;23:124–9. doi: 10.1016/j.jfda.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu S, Xu T, Huang D. Chemical compositions of the volatile extracts from seeds of Dendranthema nankingense and Borago officinalis. J Food Drug Anal. 2015;23:253–9. doi: 10.1016/j.jfda.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Javadi N, Abas F, Mediani A, Abd Hamid A, Khatib A, Simoh S, Shaari K. Effect of storage time on metabolite profile and alpha-glucosidase inhibitory activity of Cosmos caudatus leaves—GCMS based metabolomics approach. J Food Drug Anal. 2015;23:433–41. doi: 10.1016/j.jfda.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen YZ, Kao SY, Jian HC, Yu YM, Li JY, Wang WH, Tsai CW. Determination of cholesterol and four phytosterols in foods without derivatization by gas chromatography–tandem mass spectrometry. J Food Drug Anal. 2015;23:636–44. doi: 10.1016/j.jfda.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gan F, Ye R. New approach on similarity analysis of chromatographic fingerprint of herbal medicine. J Chromatogr A. 2006;1104:100–5. doi: 10.1016/j.chroma.2005.11.099. [DOI] [PubMed] [Google Scholar]
- 23. Li GL, Zhang JX, Zeng SJ, Wu KL, Duan J. Systematic position of Dendrobium huoshanense inferred from ITS, Nad intron2 and psbA-trnH DNA sequences. Guangdong Agric Sci. 2013;13:145–7. [Google Scholar]
- 24. Liu J, Chen XF, Yang WY, Liu WG, Jiang T. Study on establishment of RP-HPLC and GC–MS fingerprints for wild germplasm resource of Ophiopogon japonicus in Sichuan and hierarchical clustering analysis. J Chin Med Mater. 2010;35:2726–30. [in Chinese, English abstract] [PubMed] [Google Scholar]
- 25. Zhang Y, Wang ZZ. Comparative analysis of essential oil components of three Phlomis species in Qinling Mountains of China. J Pharmaceut Biomed. 2008;47:213–7. doi: 10.1016/j.jpba.2007.12.027. [DOI] [PubMed] [Google Scholar]