Abstract
Momordica charantia L., a vegetable crop with high nutritional value, has been used as an antimutagenic, antihelminthic, anticancer, antifertility, and antidiabetic agent in traditional folk medicine. In this study, the antifungal activity of M. charantia seed extract toward Fusarium solani L. was evaluated. Results showed that M. charantia seed extract effectively inhibited the mycelial growth of F. solani, with a 50% inhibitory rate (IC50) value of 108.934 μg/mL. Further analysis with optical microscopy and fluorescence microscopy revealed that the seed extract led to deformation of cells with irregular budding, loss of integrity of cell wall, as well as disruption of the fungal cell membrane. In addition, genomic DNA was also severely affected, as small DNA fragments shorter than 50 bp appeared on agarose gel. These findings implied that M. charantia seed extract containing α-momorcharin, a typical ribosome-inactivating protein, could be an effective agent in the control of fungal pathogens, and such natural products would represent a sustainable alternative to the use of synthetic fungicides.
Keywords: alpha-momorcharin, antifungal activity, cell apoptosis, Momordica charantia, seed extract
1. Introduction
Fungal diseases have a serious effect on the growth and yield of crops, therefore, conventional fungicides have been widely used. However, this has led to numerous environmental problems. To minimize the environmental contamination caused by conventional fungicides, many alternatives have been tried, including biological control, physical treatments, and use of low toxicity chemicals such as plant extracts or natural antifungal compounds [1]. In particular, the use of active plant extracts appears to be the best option, as they exert minimal environmental impact and pose little danger to consumers [2]. Extracts of many plants possess antimicrobial and insecticidal properties both under laboratory and field tests [3]. Potentilla erecta and Salvia officinalis extracts exhibited high antifungal activities toward Phytophthora infestans [4]. The antifungal activity of Moroccan medicinal plants has also been validated against Geotrichum candidum [5]. In order to search for the best plant-derived products with antifungal activities for microbial disease control in agriculture, more efforts need to be taken [6].
Plants have evolved various defense mechanisms to combat pathogens, such as the synthesis of plant pathogenesis-related (PR) proteins [7]. Antifungal proteins and peptides, found in different plant tissues, can effectively protect plants from fungal invasion. Based on biological activities and chemical structures, antifungal proteins are classified into several types: ribosome-inactivating proteins (RIPs), defensins, lectins, chitinases, chitin-binding proteins, thaumatin-like proteins, protease inhibitors, and lipid-transfer proteins [8]. In particular, RIPs have attracted much attention because of their broad antitumor, antifungal, and antibacterial activities [9]. RIPs can greatly improve disease resistance both in traditional crops and genetically modified crops. For example, transgenic tobacco transferred a barley RIP gene has a much higher level of resistance to fungal pathogens [10]; transgenic rice with an exogenous RIP gene has enhanced resistance to sheath blight [11].
Momordica charantia L. (2x = 2n = 22), a pathogen-resistant species, is widely distributed in tropical and subtropical regions, including Asia, Africa, parts of the Amazon Basin, and South America [12]. Besides being a highly nutritious vegetable, M. charantia is also important in folk medicine, especially in China and India [13]. Medicinal properties such as antimicrobial, antitumor, immunotoxic, antiviral, anti-mutagenic, antifertility, and antidiabetic, have been clarified for M. charantia [13,14]. In our previous study, the recombinant alpha-momorcharin (α-MC), a typical RIP rich in M. charantia fruit and seed (about 10–20%; data not shown), showed significant antifungal activity toward Fusarium solani L. in vitro, with an IC50 value of 6.23μM or 181.25 μg/mL [12]. It is not clear whether the M. charantia seed extract (MSE), which contains an abundant amount of soluble α-MC, protease inhibitors, and other active ingredients, possesses antifungal activity.
The aim of this study was to determine the antifungal activity of MSE toward F. solani, a filamentous fungus causing damping-off of many crops in fields and greenhouses. In addition, its antifungal mechanism was also explored. The results showed that MSE would be an effective sustainable alternative to synthetic fungicides in control of fungal pathogens.
2. Materials and methods
2.1. Materials
Seeds of M. charantia were obtained from a local shop (Wuhan, China). F. solani (AF93239) was provided by the China Center for Type Culture Collection (Wuhan University, Wuhan, China). Protein marker and DNA marker were from Takara (Dalian, China). Anti-α-MC polyclonal rabbit antibodies were prepared as described in our previous study. Propidium iodide (PI) was bought from Invitrogen (Paisley, UK). All other reagents were of analytical grade.
2.2. Preparation of seed extracts
Five grams of dried seed of M. charantia was homogenized with 10 mL protein extraction buffer (25mM Na3PO4, 250mM NaCl, 10mM EDTA, 10mM thiourea, 5mM dithiothreitol, 1mM phenylmethanesulfonyl fluoride, and 1.5% polyvinylpyrrolidone, pH 7.4) at 4°C. The extract was first filtered through two layers of gauze, and then the filtrates were centrifuged at 10,000g for 30 minutes (4°C) to collect the supernatant. The concentration of total protein in the supernatant was measured with the Bradford method using bovine serum albumin as a standard [15]. Sterilized by passing through 0.22-μm-diameter Millipore Swinex filters, the MSE was adjusted to 1.0 mg/mL and kept at −20°C until further application.
2.3. Antifungal activity assay
Equal aliquots of F. solani fungal suspension were pipetted into each sterile tube containing 1 mL potato dextrose broth medium and cultured to logarithmic growth phase. Then, the F. solani suspensions were supplemented with different amounts of MSE (0–150 μg/mL) and cultured for another 24 hours. The optical density values of these fungal suspensions were measured using an Eppendorf Biophotometer Plus (Hamburg, Germany) at 595 nm. Accordingly, the growth inhibition percentage was calculated, which was defined as 100× the ratio of the A595 of the control minus the corrected A595 of the sample over the corrected A595 of the control [9].
An assay of the antifungal activity of MSE toward F. solani was carried out in potato dextrose agar (PDA) medium containing filter-sterilized protein extraction buffer (control plate) or 1 mg/mL MSE (treated plate). Briefly, each PDA plate was placed with a 20-μL fungal suspension in the center, and further incubation was done at 28 ± 1°C. These fungal colonies were observed and photographed every 12 hours for 36 hours. Three independent replicates were performed for each sample.
2.4. Morphology observation of F. solani
Fungal hyphae treated with both protein extraction buffer (control sample) and MSE (treated sample) were mounted on glass slides for lactophenol blue staining. The slides were examined and photographed with a light microscope (Olympus BH-2, Tokyo, Japan) at 100× magnification. After staining with PI, the fungal cell viability was also assessed by fluorescence microscopy (×100). Each assay was repeated in triplicate.
2.5. Detection of genomic DNA fragments
Fungal hyphae treated with protein extraction buffer or the MSE were collected through centrifugation at 12,000g for 5 minutes at 4°C. The obtained cells were lysed and processed with Apoptotic DNA Ladder kit (Bioteke, Beijing, China) according to the manufacturer’s instructions. Then, the recovered DNA was dissolved in Tris–EDTA buffer (pH 8.0) and analyzed immediately on 3% agarose gel electrophoresis.
2.6. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analysis of the seed extract
Proteins of MSE were analyzed by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the molecular weights were estimated by comparison of electrophoresis mobility with those of the standard marker proteins (ranging from 4.4 to 94.0 kDa). In addition, the SDS-PAGE gel was electroblotted by semidry protein transfer to a nitrocellulose membrane (Whatman, Dassel, Germany). The nitrocellulose membrane was first saturated with 5% (w/v) defatted milk for 1 hour at 37°C, then incubated with a 1:2000 dilution of anti-α-MC polyclonal rabbit antibodies specific for α-MC for 1 hour, and then incubated with 1:5000 dilution of alkaline phosphatase-conjugated goat antirabbit IgG secondary antibody (CWBIO, Beijing, China). Subsequently, the blotted protein was developed using 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium solution (Biosharp, Seattle, WA, USA) according to the manufacturer’s instructions.
3. Results
3.1. Antifungal activity of MSE toward mycelial growth
MSE impaired both cell integrity and viability. When F. solani were treated with a low concentration of MSE, only faint growth inhibition occurred (Table 1, group 1). When MSE concentration was increased, the growth of F. solani was inhibited gradually, indicating that MSE inhibited the fungal growth in a dose-dependent manner. In addition, the inhibitor concentration leading to 50% inhibitory rate (IC50) was calculated to be 108.934 μg/mL. Protein extraction buffer had no effect on F. solani growth, so the effect of fungal growth inhibition was attributed to MSE.
Table 1.
Growth inhibition of Fusarium solani with different concentrations of MSE.
| Groups | Concentrations of MSE (μg/mL) | Inhibition rate (%) |
|---|---|---|
| Control | 0 | 0.00 |
| 1 | 25 | 10.22 |
| 2 | 50 | 33.44 |
| 3 | 75 | 39.744 |
| 4 | 100 | 45.856 |
| 5 | 125 | 57.216 |
| 6 | 150 | 63.136 |
MSE = Momordica charantia seed extract.
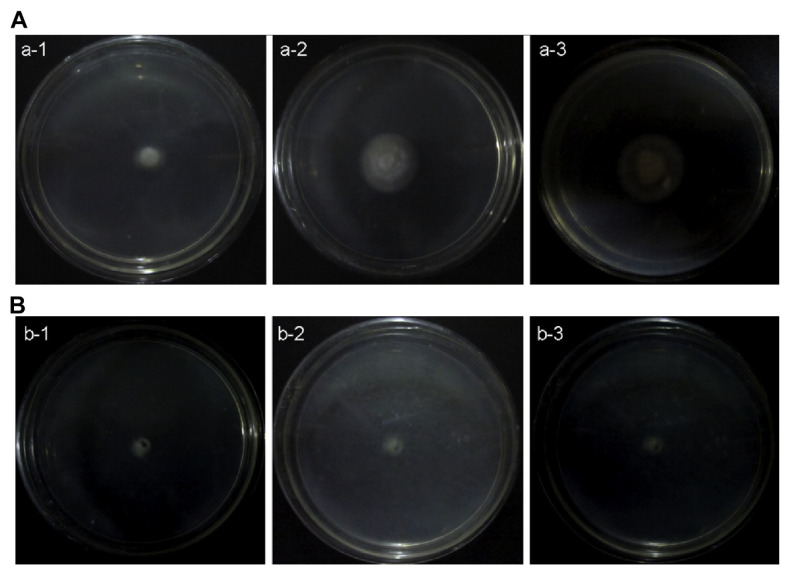
Compared with the negative control, in which F. solani hyphae grew normally and healthily (Figure 1A: a-1, a-2, a-3), the “treated plate” had almost no growth at all (Figure 1B: b-1, b-2, b-3). The diameter of the fungi plaque growing on the “control plate” had reached up to 3.8 ± 0.2 cm after a 36-hour culture (Figure 1A: a-3), but no change took place on the “treated plate.” In addition to growth inhibition, the morphology of the F. solani filament fungi had also been seriously affected by MSE (Figure 1B: b-3), which showed that MSE was highly active toward F. solani.
Figure 1.
Growth inhibition of Fusarium solani on PDA plates. (A) a-1 to a-3, F. solani was cultured on the “control plate” for 12 hours, 24 hours, and 36 hours, respectively. (B) b-1 to b-3) F. solani incubated on the “treated plate” coated with MSE for 12 hours, 24 hours, and 36 hours, respectively. MSE =Momordica charantia seed extract; PDA =potato dextrose agar.
3.2. Mechanism of antifungal activity
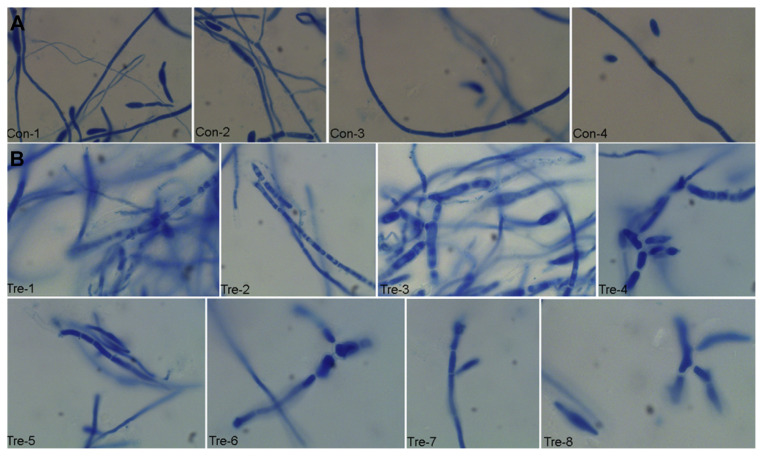
The “control sample” had homogeneous cell structure and normal mycelial apex (Figure 2A: Con-1, Con-2, Con-3, Con-4). However, the “treated sample” showed irregular shapes and heterogeneous inner structure (Figure 2B: Tre-1 to Tre-8); and the fungal tube elongation seemed to be substantially affected by MSE, as extensive septum formation was observed (Figure 2B: Tre-1 to Tre-8). Loss of symmetry, distorted apex, small swellings along the mycelia and mycelial apex, and abnormal mycelia with bursting cells were also observed in the “treated sample” (Figure 2B: Tre-1 to Tre-8).
Figure 2.
Morphology observations of Fusarium solani hyphae under light microscope (×100). (A) Hyphae treated just with the protein extraction buffer. (B) The mycelia incubated with Momordica charantia seed extract (MSE).
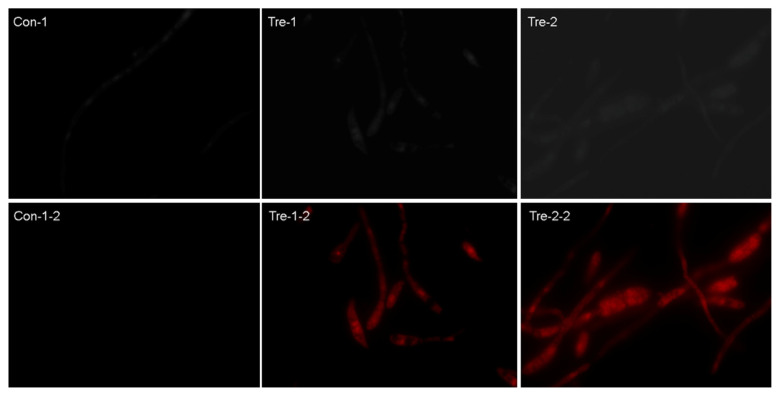
PI, membrane-impermeant and generally excluded from viable cells, is commonly used to distinguish dead cells from live cells. Hyphae treated with MSE were more sensitive to PI stain than the control hyphae (Figure 3). Different from the “control sample” (Figure 3: Con-1-2), a large increase in the number of fluorescent cells was observed in MSE-treated fungal cells (Figure 3: Tre-1-2 and Tre-2-2). Moreover, the “treated sample” had an enlarged and deformed nucleus, as well as masses of densely stained granular material localized in cytoplasm (Figure 3: Tre-1-2 and Tre-2-2). MSE could induce cell membrane permeabilization, destruction of cell inner structures, and correspondingly apoptosis-mediated cell death.
Figure 3.
Microscopic observation of hyphae stained with propidium iodide (PI). “-1” and “-2” =the hyphae stained with PI was observed under the light microscope (×100); “-1-2” and “-2-2” =the PI stained hyphae was observed under the fluorescence microscopy (×100); “Con-” =the hyphae treated with protein extraction buffer, the healthy hyphae; “Tre-” =the seed extract treated hyphae, the unhealthy hyphae.
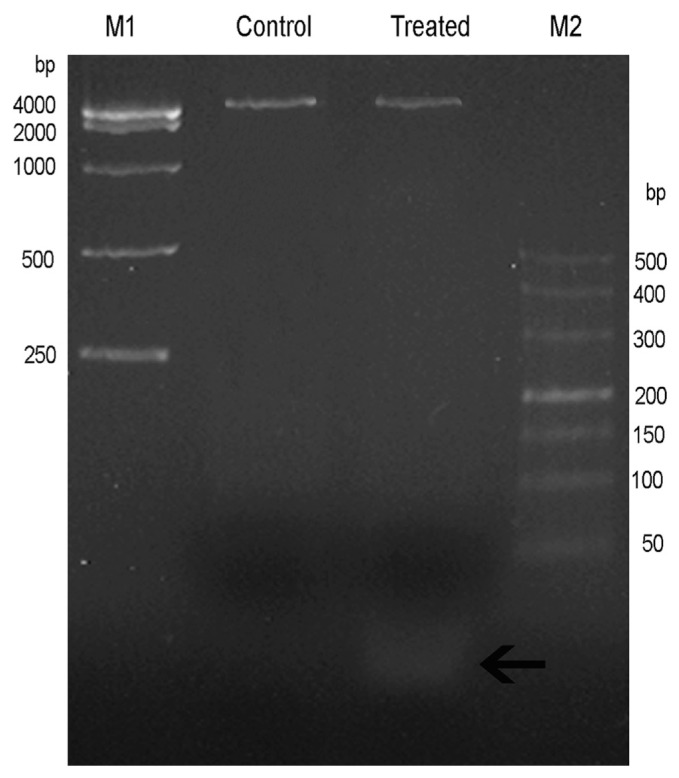
PI staining and subsequent fluorescence microscopy observation showed that the nucleus of F. solani had been markedly affected by MSE. Therefore, a detailed analysis needs to be carried out to characterize whether F. solani genomic DNA was also affected, for example, the DNA ladder phenomenon. The genomic DNA of both the treated sample and control sample was isolated by the Apoptotic DNA Ladder kit. In addition, the right amount of RNase A was added into the incubation buffer prior to lysis extraction and subsequent extraction process. Results from 3% agarose gel showed that a major DNA band bigger than 4000 bp appeared on the top of the gel (Figure 4). Moreover, extra fragments shorter than 50 bp existed in the “Treated” lane (Figure 4), which might be caused by MSE. As MSE-treated F. solani (with damaged genomic DNA) grew unhealthily and irregularly, we speculated that MSE affected the integrity of fungi DNA, which correspondingly restrained the fungi growth. Although F. solani hyphae treated with MSE exhibited the characteristics of apoptosis, no typical DNA ladder was observed. Triplicate assays were carried out, and the results remained the same.
Figure 4.
Characterization of Fusarium solani genomic DNA on 3% agarose gel. Control =genomic DNA from the protein extraction buffer treated hyphae; M1 and M2 =two different DNA markers; MSE =Momordica charantia seed extract; Treated =genomic DNA of MSE-treated hyphae, the black arrow indicating the short fragments.
3.3. Characterization of antifungal protein in MSE
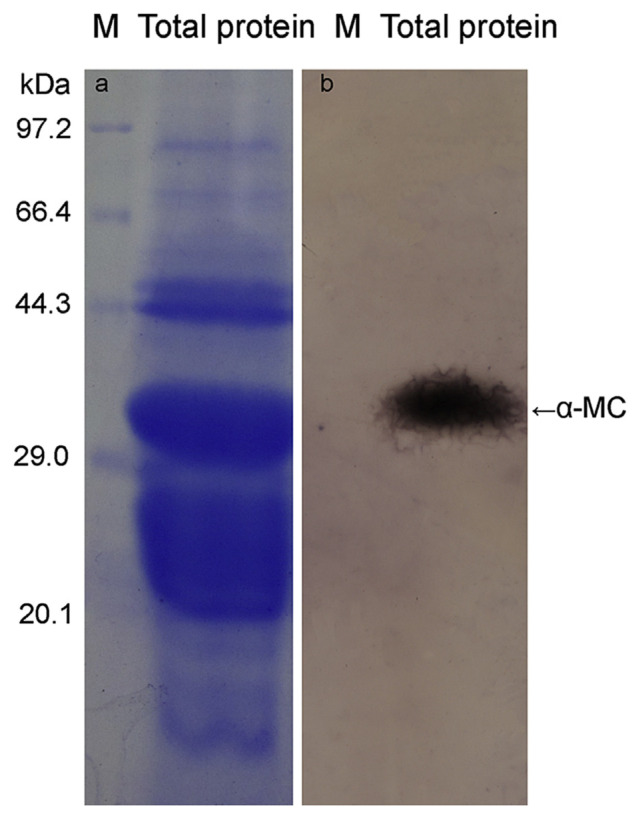
The sodium phosphate buffer (pH 7.0) was used to isolate MSE. In total, 5 g of dried seed yielded 8.53 mL mixtures, and the protein concentration was considered to be 191.5722 mg/mL. Through characterization on 12% SDS-PAGE, proteins in the mixture covered a broad range of 10.0–97.2 kDa, and the major portion concentrated between 18.0 and 45.2 kDa (Figure 5A). A main protein band appeared around 29–31.5 kDa, similar to the previously isolated α-MC, a highly active antifungal protein. Western blotting results showed that the special protein spot was really α-MC (Figure 5B). As a seed storage protein, α-MC may contribute significantly to the antifungal activity of seed extract. However, other antifungal proteins and peptides found in the MSE need to be further identified.
Figure 5.
SDS-PAGE analysis (a) and Western blotting test of MSE with alpha-momorcharia antibody (b).
4. Discussion
Plant disease caused by pathogenic fungi severely affects crop growth and reduces crop yields worldwide, especially in developing countries. Besides yield losses and food decay, fungi also pose a serious risk for consumers because of dangerous secondary metabolites [16]. Therefore, the control of pathogenic fungi is urgent and important. For a long time, synthetic fungicides have been used widely to fight against pathogenic fungi, which further led to the selective proliferation of fungicide-resistant isolates. Therefore, the search for a natural substitute with antifungal activity has been encouraged.
Crude plant tissue extracts, generally a mixture of active and nonactive compounds, provide many advantages as antimicrobial agents [17,18]. The first is their natural origin, which seems much safer for consumers and the environment; second, they are at low risk for resistance development of pathogenic fungi; third, they represent a rich source of potential bioactive compounds [18]. As various modes of action exist in tissue extract, it is difficult for pathogens to develop resistance to such a mixture of components. Therefore, the development of management strategies to replace or supplement synthetic fungicides with natural bioactive products is desirable. In particular, the antifungal proteins collected from the Cucurbitaceae family, pathogen-resistant species in the natural environment, are most promising because of their specific pathogen-related activities [19].
To date, many types of antimicrobial components have been isolated and characterized, including proteins, peptides, phenolics, and essential oils [20]. In particular, RIPs, a type of N-glycosidases cleaving the N-glycosidic bond of adenine-4324 in the GAGA hairpin of the sarcin/rincin loop of the 28S rRNA, play an important role in plant–pathogen interaction [21]. For example, Zea mays b-32 efficiently inhibits the growth of Fusarium verticillioides [22]. Many mechanisms have been speculated upon, such as N-glycosidase activity, DNase-like activity, and oxidative stress-inducing stimulus [23].
Active proteins in the MSE mixture may permeate cellular membrane, lead to the instability of the membrane, and accordingly affect macromolecular synthesis. At the same time, functions of organelles can also be affected. For example, protein synthesis on ribosomes may be arrested by RIPs present in the seed extract, as fungal ribosomes are more sensitive to RIP than that of mammalian and plant [24]. In this assay, genomic DNA degradation of the MSE-treated F. solani hyphae was observed. As no smear bands appeared on the gel (effect of DNase), the short DNA fragments might be generated by N-glycosidase activity and DNase-like activity of α-MC. Compared with the IC50 value of recombinant α-MC toward F. solani (181.25 μg/mL), the MSE had an even smaller IC50 value (108.934 μg/mL), inferring that MSE could be more effective as potential fungicide in the control of fungal pathogens. Other antifungal proteins present in MSE might collaborate with RIP α-MC and enhance the overall antifungal activity.
Fungicides have been considered to act directly against fungi, as well as induce a defense response in plant. The same mode of action has also been presumed for plant extracts against fungal pathogens [6,17,25–28]. Genes encoding PR proteins, such as RIP, could be activated during the pathogen attack process, which are induced through the action of signaling compounds such as salicylic acid, jasmonic acid, or ethylene [6]. The plant extracts may also act in synergy with elicitors or other defense proteins, which can have an additive effect when used together.
Plant extracts have been used for the treatment of plant diseases caused by pathogenic microbes since the 1990s. MSE has proved to be a highly active antifungal agent in this study, which implies that MSE may be effective in the control of fungal pathogens, and such natural products would represent a sustainable alternative to the use of synthetic fungicides. We also believe that extracts of plants belonging to the Cucurbitaceae family, including M. charantia, may be considered as natural and nontoxic biofungicides against pathogens.
Acknowledgments
This work was supported by the General Research Fund of Huanggang Normal University (No. 2014022203), funds from Hubei Collaborative Innovation Center for the Characteristic Resources Exploitation of Dabie Mountains (2015TD07), and research grants from the Doctor Fund of Huanggang Normal University (No. 2013030903).
Funding Statement
This work was supported by the General Research Fund of Huanggang Normal University (No. 2014022203), funds from Hubei Collaborative Innovation Center for the Characteristic Resources Exploitation of Dabie Mountains (2015TD07), and research grants from the Doctor Fund of Huanggang Normal University (No. 2013030903).
Footnotes
Conflicts of interest
All contributing authors declare no conflicts of interest.
REFERENCES
- 1. Sayago JE, Ordoñez RM, Kovacevich LN, Torres S, María II. Antifungal activity of extracts of extremophile plants from the Argentine Puna to control citrus postharvest pathogens and green mold. Postharvest Biol Technol. 2012;67:19–24. [Google Scholar]
- 2. Varma J, Dubey NK. Prospectives of botanical and microbial products as pesticides of tomorrow. Curr Sci. 1999;76:172–9. [Google Scholar]
- 3. Ashraf A, Sarfraz RA, Rashid MA, Shahid M. Antioxidant, antimicrobial, antitumor, and cytotoxic activities of an important medicinal plant (Euphorbia royleana) from Pakistan. J Food Drug Anal. 2015;23:109–15. doi: 10.1016/j.jfda.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaeser P, Steiner U. Antifungal activity of plant extracts against potato late blight (Phytophthora infestans) In: Lyr H, Russell PE, Dehne HW, Sisler HD, editors. Modern fungicides and antifungal compounds: II. Friedrichroda, Thuringia, Germany. 1999. pp. 491–9. [Google Scholar]
- 5. Talibi I, Askarne L, Boubaker H, Boudyach EH, Msanda F, Saadi B, Ben Aoumar AA. Antifungal activity of Moroccan medicinal plants against citrus sour rot agent Geotrichum candidum. Lett Appl Microbiol. 2012;55:155–61. doi: 10.1111/j.1472-765X.2012.03273.x. [DOI] [PubMed] [Google Scholar]
- 6. Švecová E, Proietti S, Caruso C, Colla G, Crinò P. Antifungal activity of Vitex agnus-castus extract against Pythium ultimum in tomato. Crop Prot. 2013;43:223–30. [Google Scholar]
- 7. Van Loon LC, Rep M, Pieterse CMJ. Significance of inducible defense-related proteins in infected plants. Annu Rev Phytopathol. 2006;44:135–62. doi: 10.1146/annurev.phyto.44.070505.143425. [DOI] [PubMed] [Google Scholar]
- 8. Wong JH, Ip DCW, Ng TB, Chan YS, Fang F, Pan WL. A defensin-like peptide from Phaseolus vulgaris cv. ‘King Pole Bean’. Food Chem. 2012;135:408–14. doi: 10.1016/j.foodchem.2012.04.119. [DOI] [PubMed] [Google Scholar]
- 9. Wang S, Zhang YB, Liu HG, He Y, Yan JJ, Wu ZH, Yi D. Molecular cloning and functional analysis of a recombinant ribosome-inactivating protein (alpha-momorcharin) from Momordica charantia. Appl Microbiol Biotechnol. 2012;96:939–50. doi: 10.1007/s00253-012-3886-6. [DOI] [PubMed] [Google Scholar]
- 10. Logemann J, Jach G, Tommerup H, Mundy J, Schell J. Expression of a barley ribosome-inactivating protein leads to increased fungal protection in transgenic tobacco plants. Nat Biotechnol. 1992;10:305–8. [Google Scholar]
- 11. Kim JK, Jang IC, Wu R, Zuo WN, Boston RS, Lee YH, Ahn IP, Nahm BH. Co-expression of a modified maize ribosome-inactivating protein and a rice basic chitinase gene in transgenic rice plants confers enhanced resistance to sheath blight. Transgenic Res. 2003;12:475–84. doi: 10.1023/a:1024276127001. [DOI] [PubMed] [Google Scholar]
- 12. Wang SZ, Pan L, Hu K, Chen CY, Ding Y. Development and characterization of polymorphic microsatellite markers in Momordica charantia (Cucurbitaceae) Am J Bot. 2010;97:e75–8. doi: 10.3732/ajb.1000153. [DOI] [PubMed] [Google Scholar]
- 13. Krawinkel MB, Keding GB. Bitter gourd (Momordica charantia): a dietary approach to hyperglycemia. Nutr Rev. 2006;64:331–7. doi: 10.1301/nr.2006.jul.331-337. [DOI] [PubMed] [Google Scholar]
- 14. Nerurkar P, Ray RB. Bitter melon: antagonist to cancer. Pharm Res. 2010;27:1049–53. doi: 10.1007/s11095-010-0057-2. [DOI] [PubMed] [Google Scholar]
- 15. Sharma N, Park SW, Vepachedu R, Barbieri L, Clani M, Stirpe F, Savary BJ, Vivanco JM. Isolation and characterization of an RIP (ribosome-inactivating protein)-like protein from tobacco with dual enzymatic activity. Plant Physiol. 2004;134:171–81. doi: 10.1104/pp.103.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen YJ, Dai GH. Antifungal activity of plant extracts against Colletotrichum lagenarium, the causal agent of anthracnose in cucumber. J Sci Food Agric. 2012;92:1937–43. doi: 10.1002/jsfa.5565. [DOI] [PubMed] [Google Scholar]
- 17. Kosanić M, Ranković B, Rančić A, Stanojković T. Evaluation of metal concentration and antioxidant, antimicrobial, and anticancer potentials of two edible mushrooms Lactarius deliciosus and Macrolepiota procera. J Food Drug Anal. 2016:1–6. doi: 10.1016/j.jfda.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tripathi P, Dubey N, Shukla A. Use of some essential oils as post-harvest botanical fungicides in the management of grey mould of grapes caused by Botrytis cinerea. World J Microbiol Biotechnol. 2008;24:39–46. [Google Scholar]
- 19. MacGibbon DB, Mann JD. Inhibition of animal and pathogenic fungal proteases by phloem exudate from pumpkin fruits (Cucurbitaceae) J Sci Food Agric. 1986;37:515–22. [Google Scholar]
- 20. Braca A, Siciliano T, Arrigo MD, Germanò MP. Chemical composition and antimicrobial activity of Momordica charantia seed essential oil. Fitoterapia. 2008;79:123–5. doi: 10.1016/j.fitote.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 21. Wang RR, Au KY, Zheng HY, Gao LM, Zhang X, Luo RH, Law SK, Mak AN, Wong KB, Zhang MX, Pang W, Zhang GH, Shaw PC, Zheng YT. The Recombinant maize Ribosome-inactivating protein transiently reduces viral load in SHIV89.6 infected Chinese rhesus macaques. Toxins. 2015;7:156–69. doi: 10.3390/toxins7010156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lanzanova C, Giuffrida MG, Motto M, Baro C, Donn G, Hartings H, Lupotto E, Careri M, Elviri L, Balconi C. The Zea mays b-32 ribosome-inactivating protein efficiently inhibits growth of Fusarium verticillioides on leaf pieces in vitro. Eur J Plant Pathol. 2009;124:471–82. [Google Scholar]
- 23. Bhaskar A, Deb U, Kumar O, Rao PVL. Abrin induced oxidative stress mediated DNA damage in human leukemic cells and its reversal by N-acetylcysteine. Toxicol In Vitro. 2008;22:1902–8. doi: 10.1016/j.tiv.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 24. Hartley MR, Chaddock JA, Bonness MS. The structure and function of ribosome-inactivating proteins. Trends Plant Sci. 1966;1:252. [Google Scholar]
- 25. Vechet L, Burketova L, Sindelarova M. A comparative study of the efficiency of several sources of induced resistance to powdery mildew (Blumeria graminis f. sp. tritici) in wheat under field conditions. Crop Prot. 2009;28:151–4. [Google Scholar]
- 26. Kagale S, Marimuthu T, Kagale J, Thayumanavan B, Samiyappan R. Induction of systemic resistance in rice by leaf extracts of Zizyphus jujuba and Ipomoea carnea against Rhizoctonia solani. Plant Signal Behav. 2011;6:919–23. doi: 10.4161/psb.6.7.15304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kalidindi N, Nandeep R, Swetha S, Kalidindi B. Antifungal and antioxidant activities of organic and aqueous extracts of Annona squamosa linn. leaves. J Food Drug Anal. 2015;23:795–802. doi: 10.1016/j.jfda.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stefanović OD, Tešić JD, Čomić LR. Melilotus albus and dorycnium herbaceum extracts as source of phenolic compounds and their antimicrobial, antibiofilm, and antioxidant potentials. J Food Drug Anal. 2015;23:417–24. doi: 10.1016/j.jfda.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]