Abstract
A high-quality method for one-pot biosynthesis of silver nanoparticles (AgNPs) using onion extracts as reductant and stabilizer is reported. The synthesized AgNPs were characterized by ultraviolet-visible spectroscopy (UV-Vis), X-ray powder diffraction (XRD), and transmission electron microscopy (TEM). UV-Vis spectroscopy results showed that the AgNP absorption band was located at a peak of 397 nm in aqueous solution. Both XRD and TEM results confirmed that the AgNPs were mainly spherical with average diameters of 6.0 nm by TEM and about 5.3–10.2 nm calculated using XRD data. The ability of AgNPs to reduce charge transfer resistance was also investigated using electrochemical impedance spectroscopy. Finally, the effect of synthesized NPs on ascorbic acid signal was investigated by square wave voltammetry. The peak current of square wave voltammograms of ascorbic acid increased linearly with its concentration in the range of 0.4–450.0μM. The detection limit for ascorbic acid was 0.1μM.
Keywords: Allium cepa L., ascorbic acid, biosynthesis, onions, silver nanoparticles, voltammetry
1. Introduction
Nanotechnology deals with biotic and abiotic materials that vary in size from 1 nm to 100 nm [1–5]. Nanoparticles (NPs) have novel properties, which depend on specific characteristics such as size, morphology, and distribution [6–12]. Nowadays, inorganic NPs and their nanocomposites are extensively used in various industries, biomedicines, and catalysis reactions [13–15]. In particular, silver NPs (AgNPs) have various important applications. Historically, silver has been known to have disinfecting effects and is used in a broad range of applications from traditional medicines to culinary items. It has been reported that AgNPs are nontoxic to humans and most effective against bacteria, viruses, and other eukaryotic microorganisms at low concentrations without any side effects [16].
AgNPs play a significant role in the field of diagnostic medicine [17], antimicrobial synthesis, and therapeutics [18,19]. Ag+ contributes to the antimicrobial ability of AgNPs under various hypothesis including Ag+ adherence, breakdown, permeabilization of cell membrane, and interaction with proteins and DNA; however, reactive oxygen species are reported to interfere with its physiological functions [20,21].
Stable AgNPs can be synthesized by chemical methods such as chemical reduction, electrochemical techniques, and photochemical reduction [22–24]. Meanwhile, the use of biosynthetic methods has attracted much attention because of their safe conditions in synthesis procedures, good distribution of synthesized NPs, use of nontoxic solvents, etc. [25].
Many biotechnological applications such as remediation of toxic metals use microorganisms such as bacteria [26] and yeast [27] for the synthesis of NPs. Nair and Pradeep [28] have synthesized NPs of gold, silver, and their alloys by treating the corresponding metal ions within cells of lactic acid bacteria present in buttermilk.
In recent years, plant-mediated biological synthesis of NPs is gaining importance due to its simplicity and eco-friendliness [29]. Moreover, plant extracts could be advantageous over microorganism synthesis because there is no need to expand the process of culturing and maintaining cell lines, and they are of low cost, fast, efficient, and generally lead to the formation of crystalline NPs with a variety of shapes and sizes [30–36].
Allium cepa L. is one of the most widely cultivated and used plants, and its bulb (onion) is used as both food and medicine. Onions (A. cepa L.) possess strong, characteristic aromas and flavors, which have made them important ingredients of various food items. Onions and onion flavors (essential oil) are important seasonings widely used in food processing. It has been shown that onion possesses various biological properties, including antibiotic, antidiabetic, antioxidant, anti-atherogenic, and anticancer effects [37].
In this study, we wish to report a green synthesis of AgNPs by reduction of silver ions using aqueous and alcoholic onion extracts for facile and fast phytosynthesis of AgNPs. The ability of AgNPs to reduce charge transfer resistance and the effect of these extracts on ascorbic acid signal using electrochemical impedance spectroscopy (EIS) and voltammetric methods is also investigated. To the best of our knowledge, only one report describing the synthesis of AgNPs using water extracts of onion can be found in the literature [38] and no further reports pertaining to the use of methanolic onion extract for green synthesis of AgNPs and its effect on ascorbic acid signal by square wave voltammetry (SWV) have been published.
2. Methods
2.1. Materials and procedure for the preparation of onion extracts
Pure silver nitrate (AgNO3), ascorbic acid, and K4[Fe(CN)6] were purchased from Merck (Tehran, Iran). NaOH was purchased from Sigma-Aldrich (Tehran, Iran). Onions (A. cepa) were purchased from a local store in Babol, and these onions were reportedly collected from Mazandaran Province (Qaemshahr, Iran). Double-distilled water was used in all experiments. A certain weight of onions (A. cepa; 10 g) was washed 10 times with double-distilled water and boiled with 100 mL water for 45 minutes and then filtered through a Whatman Number 1 filter paper. The filtrate was used as a reducing and stabilizer agent for the synthesis of AgNPs.
2.2. Synthesis of AgNPs
A certain volume of the onion extract (10 mL) was added to a 10 mL silver nitrate solution (1 × 10−2M) and the volume was adjusted to 40 mL with deionized water. The flask was then incubated at room temperature. The measured pH was 5.42. Different conditions were used for the optimization of AgNPs synthesis in this work.
2.3. Ultraviolet-visible spectroscopy
The formation and stability of AgNPs were investigated by ultraviolet-visible (UV-Vis) spectrophotometry (Cary, UV-500, Japan). The absorption spectrum of reaction solutions as a function of reaction time, different biomaterials used, and AgNO3 dosage were recorded at same time and at wavelengths ranging from 300 nm to 600 nm.
2.4. Microscopic investigations
Transmission electron microscopy (TEM) was applied for the morphological analysis of AgNPs; 3 μL of the sample was placed on a carbon-coated copper grid and allowed to dry at room temperature. The TEM images were obtained using a Philips cm10HT version of TEM, which was operated at 100 kV.
2.5. X-ray diffraction measurements
Crystalline metallic pattern of AgNPs powder was analyzed using X-ray diffraction (XRD). To obtain a pellet of pure NPs for XRD analysis, the reaction medium was centrifuged by five cycles at 18,000 rpm for 20 minutes followed by redispersion in deionized water. The XRD patterns were recorded on an X’Pert Pro MPD, which was operated at a voltage of 40 kV and current of 40 mA with Cu-Kα radiation. The scanning was done in the region of 2θ from 20° to 80°.
2.6. Electrochemical investigation
SWV was performed using a potentiostat/galvanostat connected to a three-electrode cell and Metrohm Model 663 VA stand linked with a computer (Pentium IV, 1200 MHz) running Autolab software. The system was run on a personal computer using GPES and FRA 4.9 software. For impedance measurements, a frequency range of 100 kHz to 0.1 Hz was used. A conventional three-electrode cell assembly consisting of a platinum wire as an auxiliary electrode and an Ag/AgCl (KClsat) electrode as a reference electrode was used.
AgNPs carbon paste electrode (AgNPs/CPE) was prepared by mixing of 0.1 g of AgNPs and 0.90 g of graphite powder in the presence of a suitable amount of liquid paraffin as a binder. The mixture was then mixed well for 70 minutes until a uniformly wetted paste was obtained. A portion of the paste was filled firmly into one glass tube as described earlier to prepare AgNPs/CPE and was used as a working electrode for electrochemical investigation.
2.7. Official method
Indophenol solution was standardized by titrating it with 2.0 mL of standard ascorbic acid solution and 5 mL of HPO3 + HOAc solution to the end point (a persistent rosy pink color) [39]. The consumption of the blank was determined by titrating the indophenol solution with 7 mL of HPO3 + HOAc solution plus a given amount of water equivalent to the volume indophenol solution used in the previous standardization titration.
For sample titration, a 100 mL portion of the juice was mixed with an equal volume of HPO3 + HOAc solution before filtering. A volume of the filtrate equivalent to approximately 250 mg of ascorbic acid was then titrated with indophenol solution using the same procedure as described earlier including the titration of the blank.
3. Results and discussion
3.1. Synthesis optimization
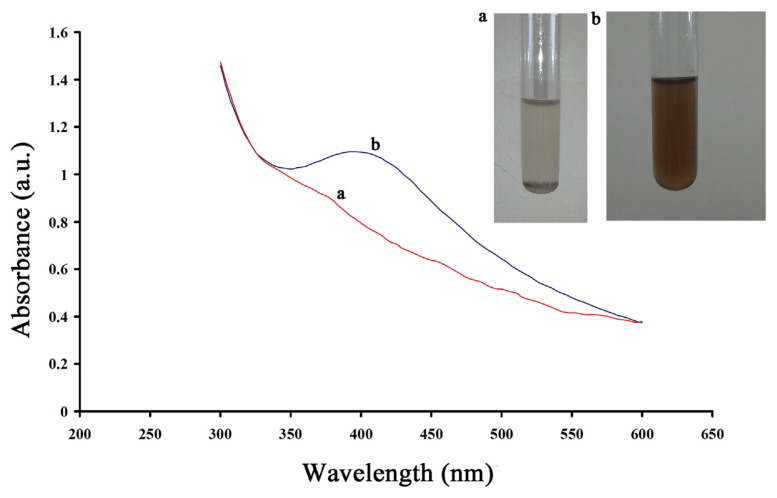
The color change from watery to brown due to the reduction of silver ion can be used as an indicator, which suggests the formation of AgNPs (Figure 1 inset). In addition, the UV-Vis spectra were used for further investigation of AgNPs synthesis in this work (Figure 1). As can be seen in Figure 1, the maximum absorbance band appears at approximately 397 nm, which is related to the synthesis of Ag0 (AgNPs). To obtain the best conditions for the synthesis of AgNPs, some factors were optimized in the synthetic procedure [40].
Figure 1.
Absorption spectrums of silver nitrate (a) and silver nanoparticles (b). Inset: silver nitrate (a) and silver nanoparticles (b) at the optimal condition (T = 35°C, pH = 10.0, and time = 18 hours).
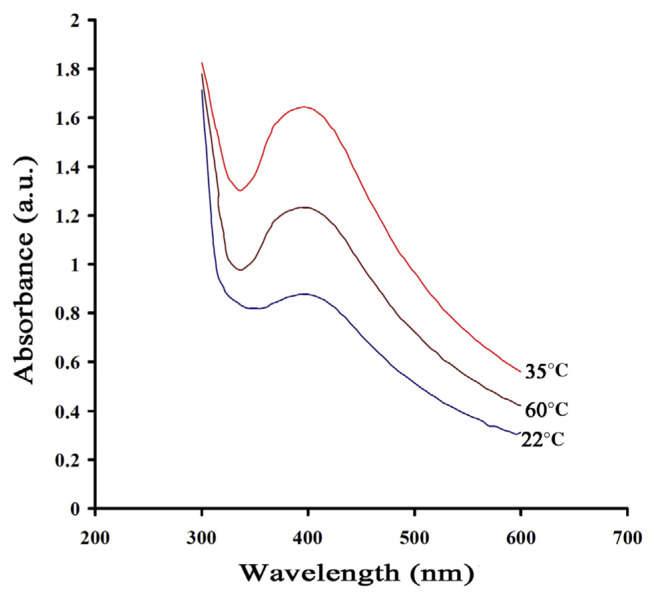
At first, the effect of temperature on the formation of AgNPs was investigated. The UV-Vis spectra of AgNPs at three different temperatures (22°C, 35°C, and 60°C) are shown in Figure 2. As can be seen, the formation rate of AgNPs was increased by increasing the temperature up to 35°C and then decreasing it.
Figure 2.
Ultraviolet-visible spectrum of silver nanoparticles at temperatures of 22°C, 35°C, and 60°C, respectively.
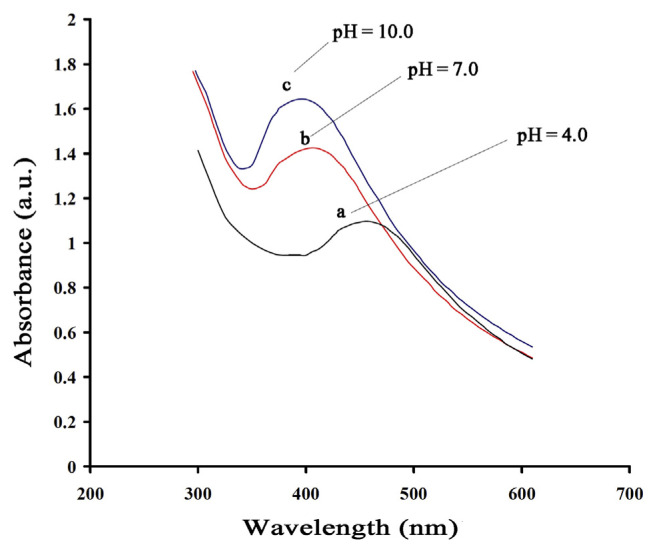
To determine the effect of pH, we studied the UV-Vis spectra of AgNPs in the pH range of 4.0–11 at 35°C. The UV-Vis spectra of AgNPs in three different conditions including acidic, neutral, and basic (pH = 4.0, 7.0, and 10.0) are shown in Figure 3. The results obtained showed that the synthesis of AgNPs can be partially completed under a neutral condition and it is completed by raising the pH value up to 10.0. Further increasing the pH leads to a decrease in the absorbance band of UV-Vis spectrum that could be related to the oxidation of Ag in the basic media. By contrast, under acidic conditions, no absorbance band for AgNPs was observed. The aggregation of AgNPs to form larger NPs was believed to be favored over the nucleation.
Figure 3.
Ultraviolet-visible spectrum of silver nanoparticles at different pH values: 4.0, 7.0, and 10.0).
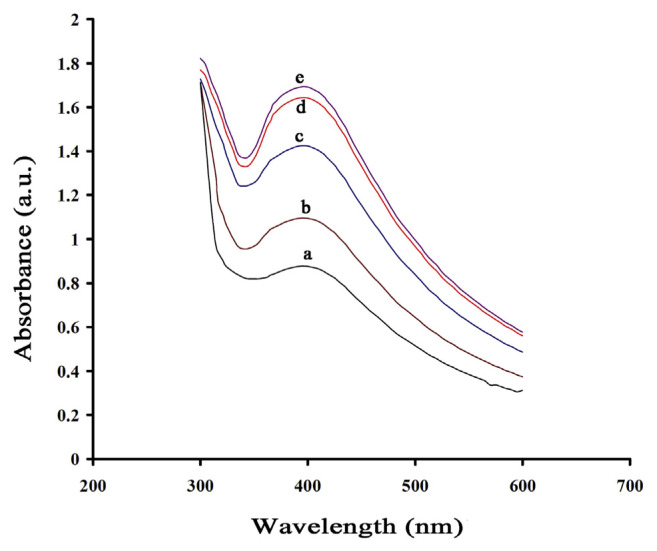
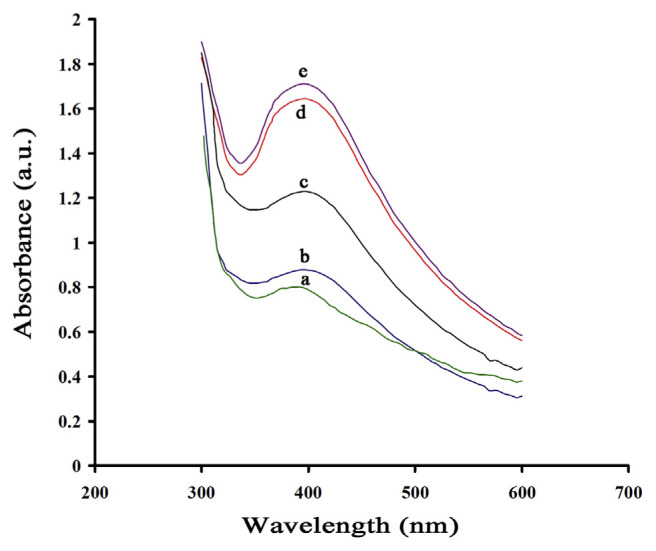
Reaction time had a great effect in the biosynthesis of AgNPs and higher yields of AgNPs were obtained by increasing the reaction time to 24 hours. Because of the instability of the AgNPs formed, an optimum duration is required, as agglomeration of AgNPs after the optimal duration results in larger particle sizes. The optimal time required for the completion of the reaction was determined to be 18 hours (Figure 4).
Figure 4.
Ultraviolet-visible spectrum of silver nanoparticles at different reaction times: (a) 3 hours; (b) 10 hours; (c) 14 hours; (d) 18 hours; and (e) 24 hours (T = 35°C and pH = 10.0).
Finally, the reaction of silver nitrate solution with different wt% of onion extract was optimized. Different concentrations of silver nitrate solutions were used to obtain the highest yield of AgNPs. In this study, we achieved a maximum yield with 5mM silver nitrate solution (Figure 5). By contrast, a 17% wt% onion extract was found to be the best to achieve the maximum yield (data not shown).
Figure 5.
Ultraviolet-visible spectrum of silver nanoparticles at different concentrations of silver nitrate solution: (a) 1.0mM; (b) 2.0mM; (c) 3.5mM; (d) 5.0mM; and (e) 6.0mM of silver nitrate (T = 35°C, pH = 10.0, and time = 18 hours).
3.2. AgNPs characterization
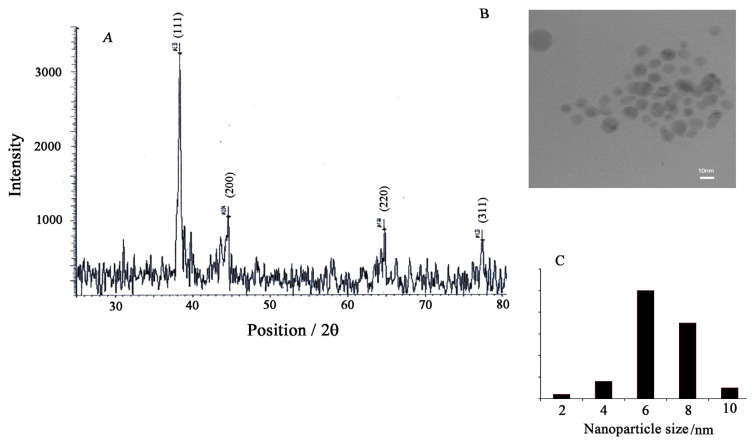
AgNPs powders were analyzed by XRD analyses. The XRD pattern of AgNPs nanopowders, in the 2θ range of 25°–80°, is shown in Figure 6A. Intense peaks of NPs (1, 1, 1), (2, 0, 0), (2, 2, 0), and (3, 1, 1) appeared, which are indexed as crystalline silver face-centered cubic phase. The mean particle size of AgNPs calculated by the Scherrer equation (D = Kλ/βcosθ) was about 5.3–10.2 nm. This value is close to particle size obtained from TEM investigation. The minor difference in particle size evaluation by the two methods is related to the aggregation and limitation in Scherrer equation for the XRD method.
Figure 6.
(A) X-ray powder diffraction patterns of silver nanoparticles synthesized by treating Allium cepa with silver nitrate aqueous solution. (B) Transmission electron microscopy micrograph of silver nanoparticle synthesized in this work. (C) Histogram of size distribution of gold nanoparticles vs. nm.
In continuation, we characterized the morphology of the as-grown nanostructures by the TEM method. The obtained micrograph of the NP is shown in Figure 6B. It is clear that AgNPs were successfully prepared in spherical shape. In addition, the histogram of size distribution of AgNPs is shown in Figure 6C. This good distribution helps to reproduce signals in the electrochemical investigation.
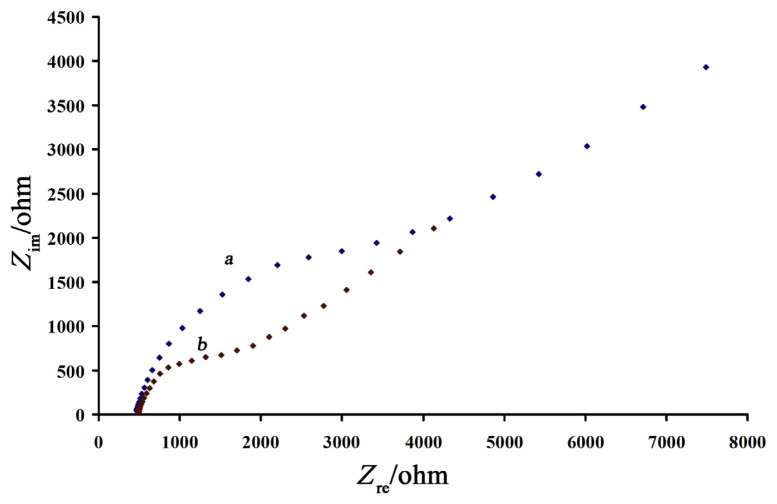
EIS was also used to investigate the electron transfer ability of AgNPs. Figure 7 presents the Nyquist diagrams of the imaginary impedance (Zim) versus the real impedance (Zre) of the EIS obtained at a surface of CPE (Figure 7, red squares) and AgNPs/CPE (Figure 7, blue squares) in the presence of 1.0mM K4[Fe(CN)6]/K3[Fe(CN)6]. The result obtained showed that at the surface of AgNPs/CPE, the semicircle diameter is lower than that of CPE, and this result suggests that the electrical conductivity of AgNPs/CPE can be comparable with CPE; besides, it also confirmed that, in the presence of AgNPs, charge transfer resistant is reduced at the surface of CPE. Therefore, these results can be applied for AgNPs as a modified sensor for electrochemical analysis systems.
Figure 7.
Impedance spectra of bare carbon paste electrode (a) and silver nanoparticles/carbon paste electrode (b) in containing 0.10 mmol/L KCl. Conditions were as follows: Edc, + 0.45 V versus Ag/AgCl; Eac, 5 mV; frequency range: 0.1–100,000 Hz.
3.3. Application of AgNPs for ascorbic acid determination
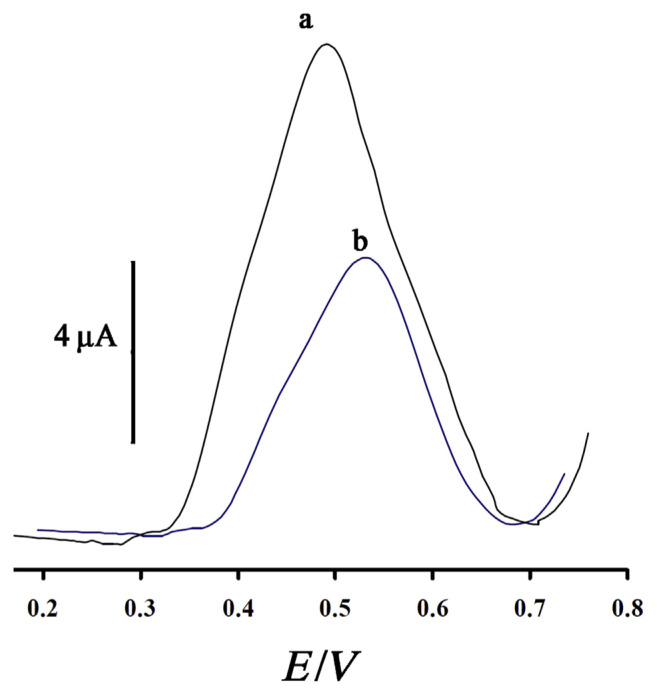
AgNPs as a conductive NP can be used for modification of electrochemical sensors for ascorbic acid determination at a trace level. Figure 8 (Curves a and b) shows the electrochemical responses of AgNPs/CPE and CPE in 50μM ascorbic acid in phosphate-buffered saline solution (pH 7.0), respectively. For AgNPs/CPE and CPE, ascorbic acid showed an oxidation peak potential (Epa) of 0.483 V and 0.553 V, respectively. However, the peak current of ascorbic acid for AgNPs/CPE was much larger than that for CPE; it was approximately 2.0 times larger than CPE by SWV. Thus, AgNPs exhibited a catalytic activity toward the oxidation of ascorbic acid.
Figure 8.
Square wave voltammograms of AgNPs/CPE (a) and CPE (b) in the presence of 50μM ascorbic acid at pH 7.0, respectively. Conditions were as follows: amplitude potential of 50 mV and frequency of 10 Hz. AgNP = silver nanoparticle; CPE = carbon paste electrode.
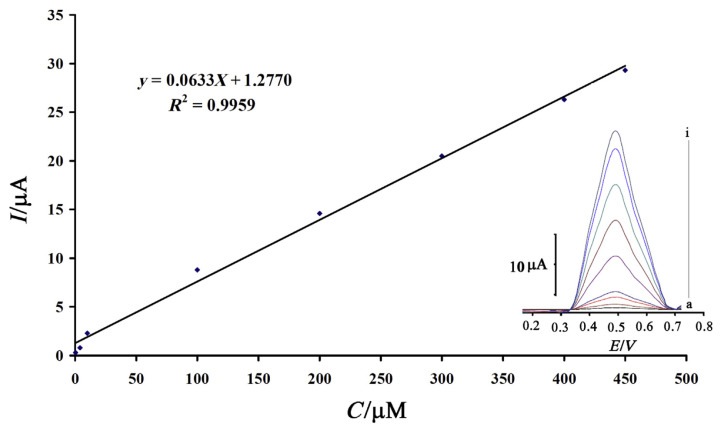
The square wave voltammograms clearly show that the plot of peak current versus ascorbic acid concentration is linear for 0.4–450.0μM of ascorbic acid, with the regression equation being Ip (μA) = (0.0639 ± 0.0033) Cascorbic acid + (1.0731 ± 0.2631) (r2 = 0.9963, n = 9), where C is micro-molar concentration of ascorbic acid and Ip is the peak current (Figure 9). The detection limit was 0.1μM ascorbic acid according to the definition of YLOD = YB + 3σ.
Figure 9.
Plots of the electrocatalytic peak current as a function of ascorbic acid concentration. The inset shows the square wave voltammograms of silver nanoparticles/carbon paste electrode in 0.1 mmol/L phosphate-buffered solution (pH 7.0) containing different concentrations of ascorbic acid: a–i correspond to 0.4 μmol/L, 5.0 μmol/L, 20.0 μmol/L, 30.0 μmol/L, 100.0 μmol/L, 200.0 μmol/L, 300.0 μmol/L, 400.0 μmol/L, and 450.0 μmol/L of ascorbic acid.
Finally, we have examined the applicability of the modified electrode for determination of ascorbic acid in natural samples. Table 1 shows the ascorbic acid content of some fresh fruit and vegetable juices determined by the proposed method and other published methods [41]. The results of statistical calculation shown in Table 1 indicate a good precision and good agreement between the repeatability of the proposed and official methods (F test) and the mean values obtained (t test).
Table 1.
Determination of ascorbic acid in real samples (n = 3).
| Sample | Vitamin C added (μmol/L) | Found (vitamin C); proposed method (μmol/L) | Found (vitamin C); published method (μmol/L) | F ex | F tab | t ex | t tab(95%) |
|---|---|---|---|---|---|---|---|
| Orange juices | — | 135.75 ± 1.31 | 136.61 ± 1.75 | 11.78 | 19.0 | 3.2 | 3.8 |
| Kiwi juices | — | 53.22 ± 0.73 | 52.87 ± 0.82 | 9.2 | 19.0 | 2.7 | 3.8 |
| Apple | — | 11.25 ± 0.45 | 12.02 ± 0.85 | 7.1 | 19.0 | 2.1 | 3.8 |
Data are presented as means ± standard deviation.
3.4. Conclusion
The high phenolic content of the water extract of onions having strong properties helps to reduce the Ag cations to AgNPs. The AgNPs synthesized using onions were characterized by EIS and XRD, and confirmed by TEM methods. UV-Vis showed peaks at a wavelength of 397 nm, thus proving the formation of spherical AgNPs. Finally, we studied the effect of AgNPs for the preparation of electrochemical sensor for determination of ascorbic acid in the dynamic range of 0.4–450.0μM.
Footnotes
Conflicts of interest
All contributing authors declare no conflicts of interest.
REFERENCES
- 1. Granqvist C, Buhrman R, Wyns J, Sievers A. Far-infrared absorption in ultrafine Al particles. Phys Rev Lett. 1976;37:625–9. [Google Scholar]
- 2. Baker C, Pradhan A, Pakstis L, Pochan DJ, Shah SI. Synthesis and antibacterial properties of silver nanoparticles. J Nanosci Nanotechnol. 2005;5:244–9. doi: 10.1166/jnn.2005.034. [DOI] [PubMed] [Google Scholar]
- 3. Karimi-Maleh H, Tahernejad-Javazmi F, Ensafi AA, Moradi R, Mallakpour S, Beitollahi H. A high sensitive biosensor based on FePt/CNTs nanocomposite/N-(4-hydroxyphenyl)-3,5-dinitrobenzamide modified carbon paste electrode for simultaneous determination of glutathione and piroxicam. Biosens Bioelectron. 2014;60:1–7. doi: 10.1016/j.bios.2014.03.055. [DOI] [PubMed] [Google Scholar]
- 4. Karimi-Maleh H, Biparva P, Hatami M. A novel modified carbon paste electrode based on NiO/CNTs nanocomposite and (9,10-dihydro-9,10-ethanoanthracene-11,12-dicarboximido)-4-ethylbenzene-1,2-diol as a mediator for simultaneous determination of cysteamine, nicotinamide adenine dinucleotide and folic acid. Biosens Bioelectron. 2013;48:270–5. doi: 10.1016/j.bios.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 5. Elyasi M, Khalilzadeh MA, Karimi-Maleh H. High sensitive voltammetric sensor based on Pt/CNTs nanocomposite modified ionic liquid carbon paste electrode for determination of Sudan I in food samples. Food Chem. 2013;141:4311–7. doi: 10.1016/j.foodchem.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 6. Najafi M, Khalilzadeh MA, Karimi-Maleh H. A new strategy for determination of bisphenol A in the presence of Sudan I using a ZnO/CNTs/ionic liquid paste electrode in food samples. Food Chem. 2014;158:125–31. doi: 10.1016/j.foodchem.2014.02.082. [DOI] [PubMed] [Google Scholar]
- 7. Eren T, Atar N, Yola ML, Karimi-Maleh H. A sensitive molecularly imprinted polymer based quartz crystal microbalance nanosensor for selective determination of lovastatin in red yeast rice. Food Chem. 2015;185:430–6. doi: 10.1016/j.foodchem.2015.03.153. [DOI] [PubMed] [Google Scholar]
- 8. Karimi-Maleh H, Tahernejad-Javazmi T, Atar N, Yola ML, Gupta VK, Ensafi AA. A novel DNA biosensor based on a pencil graphite electrode modified with polypyrrole/functionalized multiwalled carbon nanotubes for determination of 6-mercaptopurine anticancer drug. Ind Eng Chem Res. 2015;54:3634–9. [Google Scholar]
- 9. Karimi-Maleh H, Rostami S, Gupta VK, Fouladgar M. Evaluation of ZnO nanoparticle ionic liquid composite as a voltammetric sensing of isoprenaline in the presence of aspirin for liquid phase determination. J Mol Liq. 2015;201:102–7. [Google Scholar]
- 10. Karimi-Maleh H, Tahernejad-Javazmi F, Daryanavard M, Hadadzadeh H, Ensafi AA, Abbasghorbani M. Electrocatalytic and simultaneous determination of ascorbic acid, nicotinamide adenine dinucleotide and folic acid at ruthenium(II) complex-ZnO/CNTs nanocomposite modified carbon paste electrode. Electroanalysis. 2014;26:962–70. [Google Scholar]
- 11. Moradi R, Sebt SA, Karimi-Maleh H, Sadeghi R, Karimi F, Bahari A, Arabi H. Synthesis and application of FePt/CNTs nanocomposite as a sensor and novel amide ligand as a mediator for simultaneous determination of glutathione, nicotinamide adenine dinucleotide and tryptophan. Phys Chem Chem Phys. 2013;15:5888–97. doi: 10.1039/c3cp00033h. [DOI] [PubMed] [Google Scholar]
- 12. Roodbari Shahmiri R, Bahari A, Karimi-Maleh H, Hosseinzadeh R, Mirnia N. Ethynylferrocene–NiO/MWCNT nanocomposite modified carbon paste electrode as a novel voltammetric sensor for simultaneous determination of glutathione and acetaminophen. Sens Actuators B Chem. 2013;177:70–7. [Google Scholar]
- 13. Chen Q, Shen X, Gao H. One-step synthesis of silver-poly(4-vinylpyridine) hybrid micro gels by irradiation and surfactant-free emulsion polymerization, the photoluminescence characteristics. Colloids Surf A Physicochem Eng Asp. 2006;275:45–9. [Google Scholar]
- 14. Friedrich KA, Henglein F, Stimming U, Unkauf W. Investigation of Pt particles on gold substrates by IR spectroscopy particle structure and catalytic activity. Colloids Surf A Physicochem Eng Asp. 1998;134:193–206. [Google Scholar]
- 15. Dimitrov DS. Interactions of antibody-conjugated nanoparticles with biological surfaces. Colloids Surf A Physicochem Eng Asp. 2006;282:8–10. [Google Scholar]
- 16. Jiang HS, Li M, Chang FY, Li W, Yin LY. Physiological analysis of silver nanoparticles and AgNO3 toxicity to Spirodela polyrhiza. Environ Toxicol Chem. 2012;31:1880–6. doi: 10.1002/etc.1899. [DOI] [PubMed] [Google Scholar]
- 17. Schultz S, Smith DR, Mock JJ, Schultz DA. Single-target molecule detection with nonbleaching multicolor optical immunolabels. Proc Natl Acad Sci U S A. 2000;97:996–1001. doi: 10.1073/pnas.97.3.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009;27:76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 19. Elechiguerra JL, Burt JL, Morones JR, Camacho-Bragado A, Gao X, Lara HH, Yacaman MJ. Interaction of silver nanoparticles with HIV-1. J Nanobiotechnol. 2005;3:6–15. doi: 10.1186/1477-3155-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qin Y. Silver-containing alginate fibers and dressings. Int Wound J. 2005;2:172–6. doi: 10.1111/j.1742-4801.2005.00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hermans MH. Silver-containing dressings and the need for evidence. Am J Nurs. 2006;106:60–8. doi: 10.1097/00000446-200612000-00025. [DOI] [PubMed] [Google Scholar]
- 22. Frattini A, Pellegri N, Nicastro D, de Sanctis O. Effect of amine groups in the synthesis of Ag nanoparticles using aminosilanes. Mater Chem Phys. 2005;94:148–52. [Google Scholar]
- 23. Khan Z, Al-Thabaiti SA, Obaid AY, Al-Youbi AO. Preparation and characterization of silver nanoparticles by chemical reduction method. Colloids Surf B Biointerfaces. 2011;82:513–7. doi: 10.1016/j.colsurfb.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 24. Chen W, Cai W, Zhang L, Wang G, Zhang L. Sonochemical processes and formation of gold nanoparticles within pores of mesoporous silica. J Colloid Interface Sci. 2001;238:291–5. doi: 10.1006/jcis.2001.7525. [DOI] [PubMed] [Google Scholar]
- 25. Baghizadeh A, Ranjbar S, Gupta VK, Asif M, Pourseyedi S, Karimi MJ, Mohammadinejad R. Green synthesis of silver nanoparticles using seed extract of Calendula officinalis in liquid phase. J Mol Liq. 2015;207:159–63. [Google Scholar]
- 26. Stephen JR, Maenaughton SJ. Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: a novel biological approach to nanoparticle synthesis Curr Opin Biotechnol 1999. 10 230 3 10361068 [Google Scholar]
- 27. Mehra RK, Winge DR. Metal ion resistance in fungi: molecular mechanisms and their regulated expression. J Cell Biochem. 1991;45:30–40. doi: 10.1002/jcb.240450109. [DOI] [PubMed] [Google Scholar]
- 28. Nair B, Pradeep T. Preparation of gold nanoparticles from Mirabilis jalapa flowers. Cryst Growth Des. 2002;2:293–8. [Google Scholar]
- 29. Shankar SS, Rai A, Ahmad A, Sastry M. Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using neem (Azadirachta indica) leaf broth. J Colloid Interface Sci. 2004;275:496–502. doi: 10.1016/j.jcis.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 30. Mittal AK, Chisti Y, Banerjee UC. Synthesis of metallic nanoparticles using plant extracts. Biotechnol Adv. 2013;31:346–56. doi: 10.1016/j.biotechadv.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 31. Haverkamp RG, Marshall, Van Agterveld D. Pick your carats: nanoparticles of gold–silver–copper alloy produced in vivo. J Nanopart Res. 2007;9:697–700. [Google Scholar]
- 32. Iravani S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011;13:2638–40. [Google Scholar]
- 33. Sastry M, Ahmad A, Islam Khan M, Kumar R. Biosynthesis of metal nanoparticles using fungi and actinomycete. Curr Sci. 2003;85:162–70. [Google Scholar]
- 34. Mandal D, Bolander ME, Mukhopadhyay D, Sarkar G, Mukherjee P. The use of microorganisms for the formation of metal nanoparticles and their application. Appl Microbiol Biotechnol. 2006;69:485–92. doi: 10.1007/s00253-005-0179-3. [DOI] [PubMed] [Google Scholar]
- 35. Zhang YX, Zheng J, Gao G, Kong YF, Zhi X, Wang K, Zhang XQ, Cui DX. Biosynthesis of gold nanoparticles using chloroplasts. Int J Nanomedicine. 2011;6:2899–906. doi: 10.2147/IJN.S24785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Khalil MMH, Ismail EH, El-Magdoub F. Biosynthesis of Au nanoparticles using olive leaf extract. Arab J Chem. 2012;5:431–7. [Google Scholar]
- 37. Corea G, Fattorusso E, Lanzotti V, Capasso R, Izzo AA. Antispasmodic saponins from bulbs of red onion, Allium cepa L var. tropea. J Agric Food Chem. 2005;53:935–40. doi: 10.1021/jf048404o. [DOI] [PubMed] [Google Scholar]
- 38. Saxena A, Tripathi RM, Singh RP. Biological synthesis of silver nanoparticles by using onion (Allium cepa) extract and their antibacterial activity. Dig J Nanomater Biostruct. 2010;5:427–32. [Google Scholar]
- 39.Horwitz W. AOAC Official analytical chemists. 16th ed. Washington, DC: AOAC; 1995. p. 16. [Google Scholar]
- 40. Paramelle D, Sadovoy A, Gorelik S, Free P, Hobley J, Fernig DG. A rapid method to estimate the concentration of citrate capped silver nanoparticles from UV-visible light spectra. Analyst. 2014;139:4855–61. doi: 10.1039/c4an00978a. [DOI] [PubMed] [Google Scholar]
- 41. Baghizadeh A, Karimi-Maleh H, Khoshnama Z, Hassankhani A, Abbasghorbani M. A voltammetric sensor for simultaneous determination of vitamin C and vitamin B6 in food samples using ZrO2 nanoparticle/ionic liquids carbon paste electrode. Food Anal Method. 2015;8:549–57. [Google Scholar]