Abstract
Prebiotics are used to influence the growth, colonization, survival, and activity of probiotics, and enhance the innate immunity, thus improving the health status of the host. The survival, growth, and activity of probiotics are often interfered with by intrinsic factors and indigenous microbes in the gastrointestinal tract. In this study, Bulnesia sarmienti aqueous extract (BSAE) was evaluated for the growth-promoting activity of different strains of Lactobacillus acidophilus, and a simple, precise, cost-effective high-performance liquid chromatography (HPLC) method was developed and validated for the determination of active prebiotic ingredients in the extract. Different strains of L. acidophilus (probiotic) were incubated in de Man, Rogosa, and Sharpe (MRS) medium with the supplementation of BSAE in a final concentration of 0.0%, 1.0%, and 3.0% (w/v) as the sole carbon source. Growth of the probiotics was determined by measuring the pH changes and colony-forming units (CFU/mL) using the microdilution method for a period of 24 hours. The HPLC method was designed by optimizing mobile-phase composition, flow rate, column temperature, and detection wavelength. The method was validated according to the requirements of a new method, including accuracy, precision, linearity, limit of detection, limit of quantitation, and specificity. The major prebiotic active ingredients in BSAE were determined using the validated HPLC method. The rapid growth rate of different strains of L. acidophilus was observed in growth media with BSAE, whereas the decline of pH values of cultures varied in different strains of probiotics depending on the time of culture. (+)-Catechin and (−)-epicatechin were identified on the basis of their retention time, absorbance spectrum, and mass spectrometry fragmentation pattern. The developed method met the limit of all validation parameters. The prebiotic active components, (+)-catechin and (−)-epicatechin, were quantified as 1.27% and 0.71% (w/w), respectively, in crude extract, and 6.36 ± 0.06 μg/ mL and 4.47 ± 0.41 μg/mL (mean ± standard deviation), respectively, in a prebiotic capsule of BSAE by HPLC analysis. BSAE contains the active components of prebiotics and enhances the growth of L. acidophilus.
Keywords: Bulnesia sarmienti, catechin, high-performance liquid chromatography, Lactobacillus, prebiotics
1. Introduction
It has been known for more than 3 decades that the human body contains 10-fold more microbial cells (1014) than human cells [1]. Intestinal microbes are important for the improvement of a healthy, stable intestinal tract and immune system [2]. The health-promoting effects of microflora enhance immunostimulation and vitamin synthesis, and inhibit the growth of pathogenic organisms [3]. There are different genera of microbes that are being used as probiotics. In particular, lactobacilli, bifidobacter, and yeast have been developed as potential probiotics that provide nutrients to intestinal cells, increase intestinal absorption, and maintain the gut environment and immune system [4–6]. The therapeutic uses of probiotics have become a growing research interest in human infectious, inflammatory, and allergic diseases because of its potential health benefit effects [7]. The ingestion of probiotics and prebiotics, or the combination of both (synbiotic) indicates a novel new therapeutic strategy [8]. Prebiotics are nondigestible carbohydrate ingredients that selectively stimulate the growth, activity, survival, and/or colonization of probiotics in the large intestine by providing a fermentable carbon source without inducing the growth of intestinal pathogens, such as Clostridium perfringens [9,10]. Currently, prebiotics are widely used to enhance the growth of gut microflora, and induce mineral absorption, lipid metabolism, and innate immunity [11].
Prebiotic oligosaccharides can be produced in three different ways: by extraction from plant materials, microbiological synthesis or enzymatic synthesis, and enzymatic hydrolysis of polysaccharides [12]. Plants are one of the potential sources of prebiotics, and the extract of Bulnesia sarmienti could be an opportunistic agent for the management of gut microflora. B. sarmienti is a wood with aromatic incense used as traditional medicine for centuries. It is found in the area of Gran Chaco of South America. In folk medicine, its essence has been used for skin healing. The bark has also been used as a tonic for stomach ailments and for treatment of cardiovascular disorders, diabetes, and some cancers. The antitumor, antioxidant, anti-inflammatory, and antithrombosis activities of B. sarmienti extract have been previously reported [13]. However, information on the prebiotic action of B. sarmienti is limited, and of low scientific caliber. The flavonoids, (+)-catechin (trans-3,39,49,5,7-pentahydroxyflavan) and (−)-epicatechin (cis-3,39,49,5,7-pentahydroxyflavan), were reported to have prebiotic effects [14]. Interestingly, B. sarmienti extract contains large amounts of (+)-catechin and (−)-epicatechin [13,15], similar to some other fruits and plant-derived foods [16,17]. The interest in these compounds is growing rapidly because of their versatile pharmacological effects. Several analytical techniques including some high-performance liquid chromatography (HPLC) methods with different detection modes have been reported [18,19]. However, there is an exigent need for a simple, precise, rapid, and cost-effective HPLC method for the simultaneous separation and quantification of catechin and epicatechin in view of the increasing attention they receive.
Thus, the present study was designed to investigate the possible prebiotic action of B. sarmienti aqueous extract (BSAE) to influence the growth of specific gut microbes. Another aim was to develop and validate an easy, beneficial HPLC method for the determination of active prebiotic ingredients, (+)-catechin and (−)-epicatechin, in plant extracts and herbal products that would be appropriate to use in any analytical laboratory.
2. Materials and methods
2.1. Chemicals and reagents
HPLC-grade methanol was purchased from Honeywell Burdick & Jackson (Muskegon, MI, USA). Deionized water was purified in a Milli-Q System (Millipore, Bedford, MA, USA). Acetic acid was obtained from the Junsei Chemical Co. Ltd. (Tokyo, Japan). (+)-Catechin and (−)-epicatechin were purchased from Sigma (St. Louis, MO, USA), and the purity was higher than 98% by HPLC analysis.
2.2. Probiotic strains and their growth conditions
The four Lactobacillus acidophilus strains—KCTC 3140, KCTC 3146, KCTC 3154, and KCTC3179—were obtained from the Korea Collection for Type Cultures (KCTC, Daejeon, South Korea). The KCTC 3140, KCTC 3146, KCTC 3154, and KCTC3179 strains of L. acidophilus were isolated from rat, pig, chicken, and human gut, respectively. All strains were cultured in de Man, Rogosa and Sharpe (MRS) broth (Difco, Detroit, MI, USA) under anaerobic and aerobic conditions at 37°C for 24 hours or 48 hours.
2.3. Preparation of BSAE
The dried bark of B. sarmienti was purchased from the Lucky Pharmaceutics Company (Daegu, South Korea). The bark was washed, sliced, and finally boiled in distilled water (10 mL water/1 g of bark) in a round bottom boiling flask at 95°C for 3 hours. The extract was cooled to room temperature, then filtered and solidified in a rotatory evaporator under vacuum. The yield of extract from the dried bark of B. sarmienti was 22.6% (w/w). The solid extract was directly suspended in distilled water and preserved at −20°C until use.
2.4. Culture of probiotic with BSAE
Optimum doses of BSAE were used in the MRS medium to determine the growth of the four probiotic strains. BSAE was supplemented in MRS medium (50 mL) at various concentrations (0.0%, 1.0%, and 3.0%, w/v) as the sole carbon source. After the media preparation, the inoculum of each probiotic [1 × 109 colony-forming units (CFU)/mL] was cultured in BSAE (prebiotic) containing MRS medium at 37°C overnight (16 hours) under either aerobic or anaerobic conditions. The growth of each strain was examined by measuring the CFUs (CFU/mL) using a broth microdilution method.
2.5. Culture fermentation
The test carbohydrate was dissolved in MRS medium and autoclaved for 15 minutes at 121°C. Each inoculum of probiotics (1 × 109 CFU/mL) was cultured in prepared MRS medium (50 mL) supplemented with carbohydrate and BSAE at different concentrations (0.0%, 1.0%, and 3.0%, w/v) as the sole carbon source for overnight (16 hours) incubation at 37°C. The growth of each strain of probiotics was monitored via pH changes in media and CFUs (CFU/mL) of the probiotics at 0 hours, 1 hour, 3 hours, 6 hours, 9 hours, 12 hours, and 24 hours. Next, a 3-mL culture from each tube was collected aseptically at all time points and centrifuged (15 minutes, 5000 rpm, at 4°C) to obtain the supernatant. The pH of the culture supernatant was measured using a digital pH meter (Orion, Model: 420A; Thermo Scientific, Waltham, MA, USA). The pH meter was standardized with pH 4 and 10 buffers prior to use. A microdilution method was used to determine the CFUs (CFU/mL) of culture at the designated time points.
2.6. Chromatographic analysis of BSAE
2.6.1. Instrumentation of chromatographic systems
The Agilent 1100 HPLC system (Agilent Technologies, Palo Alto, CA, USA) was equipped with an Agilent G1314A UV detector. An HP ODS Hypersil column (5 μm, 200 mm × 4.6 mm) was used for the separation of compounds. The temperature of the column compartment was maintained at 30°C throughout the analysis. The wavelength of detection was 280 nm and the injection volume was 20 μL. All chromatographic runs were carried out in a gradient mode with a flow rate of 1 mL/min. A mixture of HPLC-grade methanol and 0.5% (v/v) acetic acid (pH 3.50) were exploited as the mobile phase, and the ratio of eluent A (methanol) and eluent B (0.5%, v/v acetic acid, pH 3.50) was 5:95 during the first 10 minutes of each run. The proportion of “eluent A” was increased linearly to 60% (v/v) from 10 minutes to 40 minutes and then sustained for 2 minutes. Thereafter, the eluent was returned to the initial composition (eluent A: 5%, v/ v; eluent B: 95%, v/v) in 3 minutes, where it was maintained for 5 minutes in order to re-equilibrate the column prior to another injection (Table 1). The method was validated by checking the accuracy, precision, linearity, limit of detection (LOD), limit of quantitation (LOQ), and specificity prior to sample analysis. The Agilent 1946B mass selective detector (Agilent Technologies) was used with the above-mentioned HPLC system to obtain the mass spectrum of (+)-catechin and (−)-epicatechin (Figure 1). Other conditions of the system were the same as those used in UV detection. The compounds were analyzed in selected ion monitoring mode using mass/charge ratios (m/z) of 289, 435, and 449.
Table 1.
Mobile-phase gradient.
| Time(min) | % A (methanol, v/v) | % B (0.5% acetic acid, v/v) |
|---|---|---|
| 0 | 5 | 95 |
| 10 | 5 | 95 |
| 40 | 60 | 40 |
| 42 | 60 | 40 |
| 45 | 5 | 95 |
| 50 | 5 | 95 |
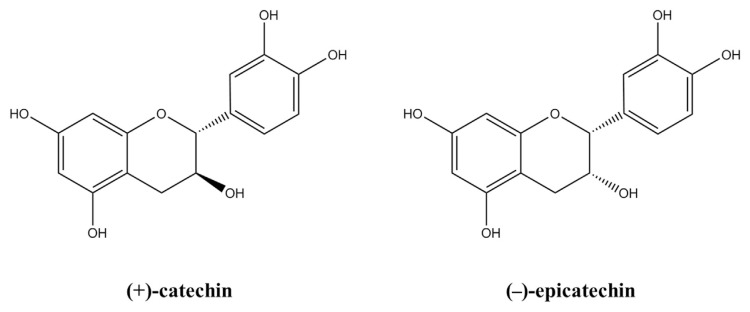
Figure 1.
Chemical structures of (+)-catechin and (−)-epicatechin [18].
2.6.2. Preparation of standard solution
Parent stock solutions (1 mg/mL) of (+)-catechin and (−)-epicatechin were separately prepared by dissolving in 50% (v/v) methanol in clear glass vials. A series of working standards were prepared using the appropriate dilutions to obtain concentrations of 1.56 μg/mL, 3.12 μg/mL, 6.25 μg/mL, 12.50 μg/mL, and 25.00 μg/mL in 0.5% (v/v) acetic acid. These working standards were used in the determination of accuracy, precision, and linearity. All stock solutions and working standard solutions were immediately stored at 4°C. (+)-Catechin and (−)-epicatechin in a concentration of 6.25 μg/mL were also freshly prepared in a similar manner and used as control in the quantitation of those compounds in the plant extract.
2.6.3. Preparation of sample solution
Ten capsules of BSAE were cut into small pieces and put into a 50-mL conical tube, into which 10 mL methanol was added. After vortex mixing for 15 minutes and subsequent sonication treatment for 1 minute, the mixture was centrifuged at 1200g for 20 minutes. The supernatant was transferred to a clean tube and diluted by 100 times with 0.5% (v/v) acetic acid. Thereafter, the supernatant was passed through a 0.45-μm filter and injected into the HPLC system. In the validation of the analytical method, the extract samples were preanalyzed, and the extract having the known amount of (+)-catechin and (−)-epicatechin was then spiked with extra 1.56 μg/mL, 6.25 μg/mL, and 25.00 μg/mL of (+)-catechin and (−)-epicatechin. The mixtures were analyzed to check the recovery of the added (+)-catechin and (−)-epicatechin at different levels in the plant material. In a sample analysis, the dried extract and capsule samples were prepared, similar to the procedure mentioned above deprived of spiking with extra (+)-catechin and (−)-epicatechin.
2.6.4. Validation of analytical method
The accuracy of an analytical method is the extent to which test results were generated by the method and the true value of analytes. The precision of a method is the extent to which the individual test results of multiple injections of a series of standards agree. The working standards having 1.56 μg/mL, 6.25 μg/mL, and 25.00 μg/mL of (+)-catechin and (−)-epicatechin were injected six times into the HPLC system for the determination of accuracy and precision of the analytical method. The linearity of an analytical method is its ability to elicit test results that are directly proportional to the concentration of analytes in samples within a given range. Linearity is determined by a series of three injections of five standards—1.56 μg/mL, 3.12 μg/mL, 6.25 μg/mL, 12.50 μg/mL, and 25.00 μg/mL of (+)-catechin and (−)-epicatechin. The linear regression analysis was carried out by plotting the peak areas (y-axis) of each compound against the respective concentrations (x-axis) of (+)-catechin and (−)-epicatechin. The linearity for the relationship between peak area and concentration was demonstrated by a correlation coefficient (r2) > 0.99. Recovery is expressed as the amount of analyte found in a spiked sample as a percentage to the theoretical amount thought to be present in the medium. The absolute recovery rates of (+)-catechin and (−)-epicatechin from BSAE and capsules of BSAE were evaluated in three different concentrations with triplicate analysis. The lower LOD is defined as the lowest concentration of analyte in a sample that can be detected, but not necessarily quantitated, under the stated experimental conditions. It can be calculated from the standard deviation (SD) of the response and the slope associated with the calibration curve according to the equation: LOD = (SD × 3.3)/ slope. The lower LOQ is also similarly defined, but as the lowest concentration of analyte in a sample that can be quantifiable under the stated experimental conditions. It can also be calculated from the SD of the response and the slope associated with the calibration curve, but according to the following equation: LOQ = (SD × 10)/slope. The specificity of the method was ascertained by analyzing the standard compounds and the extract. The peaks for (+)-catechin and (−)-epicatechin in the sample were confirmed by comparing the retention times of the sample peak with that of the standard. The peak purity of those compounds was assessed by comparing the spectra at two levels: peak start (S) and peak end (E) positions.
2.7. Data analysis
All data were calculated as means ±SD, and multiple group comparisons were performed using one-way analysis of variance, followed by the Duncan’s multiple range tests for comparisons. Values not sharing the same letter were significantly different (p < 0.05). The statistical analysis was performed using the SAS 9.4 software (SAS Institute, Cary, NC, USA).
3. Results and discussion
3.1. Effect of BSAE on growth of L. acidophilus strains
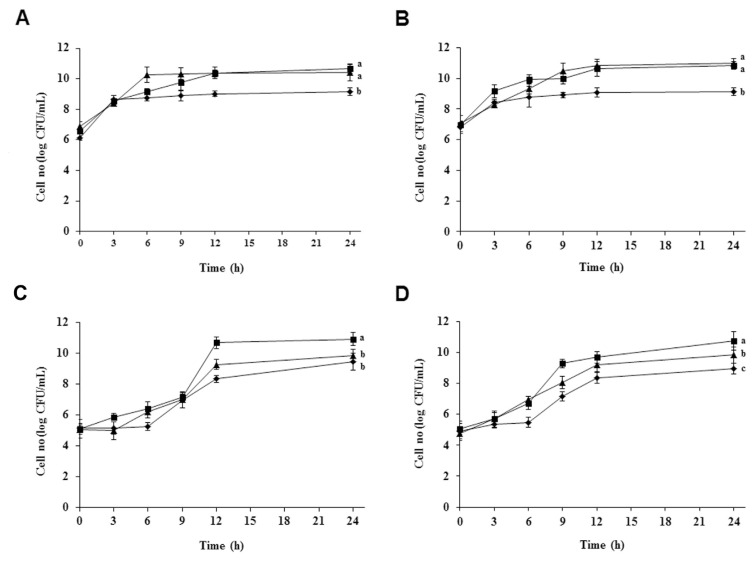
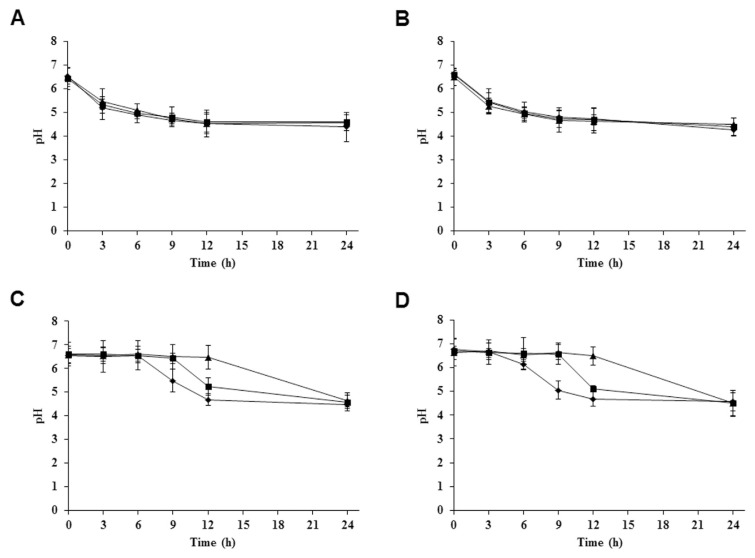
Within the lactic acid bacteria, the subgroup of the Lactobacillus complex is of particular interest because many members occupy important ecologic niches in the gastrointestinal tracts of humans and animals, and L. acidophilus is probably the most well-known species of this genus [20]. There are four strains of L. acidophilus—KCTC 3140, KCTC 3146, KCTC 3154, and KCTC 3179, which were isolated from rat, pig, chicken, and human intestines, respectively. The different strains were cultured in MRS broth containing 0.0%, 1.0%, and 3.0% (w/v) BSAE. The growth of four tested strains of L. acidophilus probiotic was determined at 0 hours, 3 hours, 6 hours, 9 hours, 12 hours, and 24 hours. Among the four strains, the rapid growth rate of L. acidophilus KCTC 3140 and L. acidophilus KCTC 3146 was found in MRS broth containing 3.0% (w/v) BSAE (Figures 2A and 2B), whereas the growth rate of L. acidophilus KCTC 3154 and KCTC 3179 was observed to be faster in MRS broth containing 1.0% (w/v) BSAE (Figures 2C and 2D). During the growth period of L. acidophilus KCTC 3140 and KCTC 3146, there was no difference in pH values between 0.0%, 1.0%, and 3.0% (w/v) of BSAE in MRS broth (Figures 3A and 3B). Meanwhile, the pH of the growth medium of L. acidophilus KCTC 3154 and KCTC 3179 was decreased at 1.0% and 3.0% (w/v) concentration of BSAE after 9 hours (Figures 3C and 3D). The decrease in pH over time results from the breakdown of lactose to form lactic acid. The lack of differences in pH of the treatments compared with the control broth may be caused by the buffering capacity of the components of growth media [21]. The prebiotic action of the said extract may be attributable to the existence of two prebiotic active compounds, (+)-catechin and (−)-epicatechin. Catechin and epicatechin improve gut health; they also influence the growth of specific large intestinal microflora through their prebiotic actions, as well as a number of probiotics increased by daily ingestion of prebiotics [14,22].
Figure 2.
Growth curves of four Lactobacillus acidophilus strains. (A) L. acidophilus KCTC 3140 isolated from rat gut, (B) L. acidophilus KCTC 3146 isolated from pig gut, (C) L. acidophilus KCTC 3154 isolated from chicken gut, and (D) L. acidophilus KCTC 3179 isolated from human gut cultured in MRS broth in the absence of BSAE (◆), and in the presence of 1.0% BSAE (■), and 3.0% BSAE (▲) (w/v). Culture sample was taken at designated times and spread on MRS plates to count surviving cells. Results are presented from three independent analyses. Values not sharing the same letter are significantly different (p < 0.05). BSAE = Bulnesia sarmienti aqueous extract; CFU = colony forming units; MRS = de Man, Rogosa, and Sharpe.
Figure 3.
Observation of pH changes in culture media using pH meter. pH tolerance of four Lactobacillus acidophilus strains—(A) L. acidophilus KCTC 3140 isolated from rat gut, (B) L. acidophilus KCTC 3146 isolated from pig gut, (C) L. acidophilus KCTC 3154 isolated from chicken gut, and (D) L. acidophilus KCTC 3179 isolated from human gut—was determined in culture media during growth in MRS broth in the absence of BSAE (◆), and in the presence of 1.0% BSAE (■) and 3.0% BSAE (▲) (w/ v). Results are presented from three independent analyses. Values not sharing the same letter are significantly different (p < 0.05). BSAE = Bulnesia sarmienti aqueous extract; MRS = de Man, Rogosa, and Sharpe.
3.2. Development of method
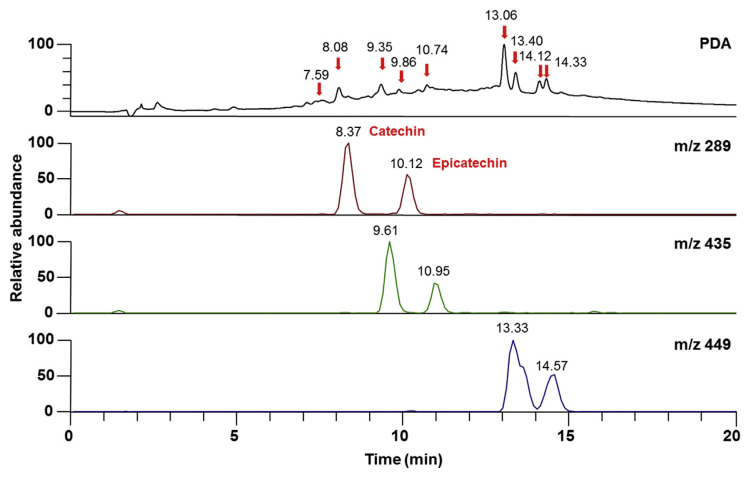
In the current study, we developed the HPLC method in order to identify and quantify (+)-catechin and (−)-epicatechin simultaneously from an extract. Generally, it requires several trials to select a suitable mobile phase and to develop a method, because of the complexity of the chemical composition of plant extracts and the affinities of the components toward various solvents. The proportions of the organic and aqueous phases were adjusted to obtain a rapid and simple assay method with a reasonable runtime, suitable retention time, and sharp peak. The instruments under the optimized conditions gave well-resolved symmetric band for (+)-catechin and (−)-epicatechin from the pure compounds as well as from the plant extract. In HPLC chromatogram (Figure 4), peaks of pure compounds of (+)-catechin and (−)-epicatechin were observed at the retention times between 7 and 12 minutes. When the extract solutions were injected six times, the retention times of flavonoids present in extract were found to be the same with some other unidentified peaks.
Figure 4.
Representative PDA chromatogram and selected ion mass chromatograms of catechin and epicatechin with UV detection at 280 nm. PDA = photodiode array.
3.3. Validation of method
3.3.1. Accuracy and precision
Accuracy and precision were determined from quality control standards in three different concentrations by calculating the percentage of accuracy and percentage of relative standard deviation (%RSD), respectively, for each set of test samples. Table 2 shows the accuracy, intraday precision (repeatability), and interday precision (reproducibility) of this assay method. The accuracy of the analysis method obtained for (+)-catechin was 132.87 ± 0.05%, 97.71 ± 0.17%, and 102.64 ± 0.09% from the injection of 1.56 μg/mL, 6.25 μg/mL, and 25.00 μg/mL of pure compound, respectively. In the case of (−)-epicatechin, the method showed 94.81 ± 0.24%, 105.96 ± 0.07%, and 97.25 ± 0.08% accuracy for 1.56 μg/mL, 6.25 μg/mL, and 25.00 μg/mL of pure (−)-epicatechin, respectively. The intra-and interday precision of this assay method was within the limits for all tested concentrations according to the guidelines for analytical method development and validation [23,24]. The %RSD value is within the limits, indicating that the assay method is validated depending on the precision.
Table 2.
Intra- and interday precisions and percent accuracies of catechin and epicatechin.
| Intraday | Interday | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Precision mean ± SD | %RSD | Accuracy (%)mean ± SD | Precision mean ± SD | %RSD | Accuracy (%)mean ± SD | |
| Catechin (μg/mL) | ||||||
| 1.56 | 2.08 ± 0.02a | 0.80 | 132.87 ± 0.05 | 1.93 ± 0.16a | 8.23 | 123.65 ± 0.22 |
| 6.25 | 6.11 ± 0.08b | 1.25 | 97.71 ± 0.17 | 6.04 ± 0.55b | 9.20 | 96.56 ± 0.67 |
| 25 | 25.66 ± 0.05c | 0.18 | 102.64 ± 0.09 | 25.15 ± 0.35c | 1.41 | 100.60 ± 0.42 |
| Epicatechin (μg/mL) | ||||||
| 1.56 | 1.48 ± 0.17a | 11.39 | 94.81 ± 0.24 | 1.48 ± 0.18a | 11.89 | 94.81 ± 0.26 |
| 6.25 | 6.62 ± 0.03b | 0.52 | 105.96 ± 0.07 | 6.62 ± 0.08b | 1.23 | 105.92 ± 0.11 |
| 25 | 24.31 ± 0.05c | 0.20 | 97.25 ± 0.08 | 24.55 ± 0.50c | 2.05 | 98.21 ± 0.64 |
Results are presented from three independent analyses.
Values not sharing the same letter are significantly different (p <0.05). Percent accuracies were determined from the individual precision values.
RSD = relative standard deviation; SD = standard deviation.
3.3.2. Linearity
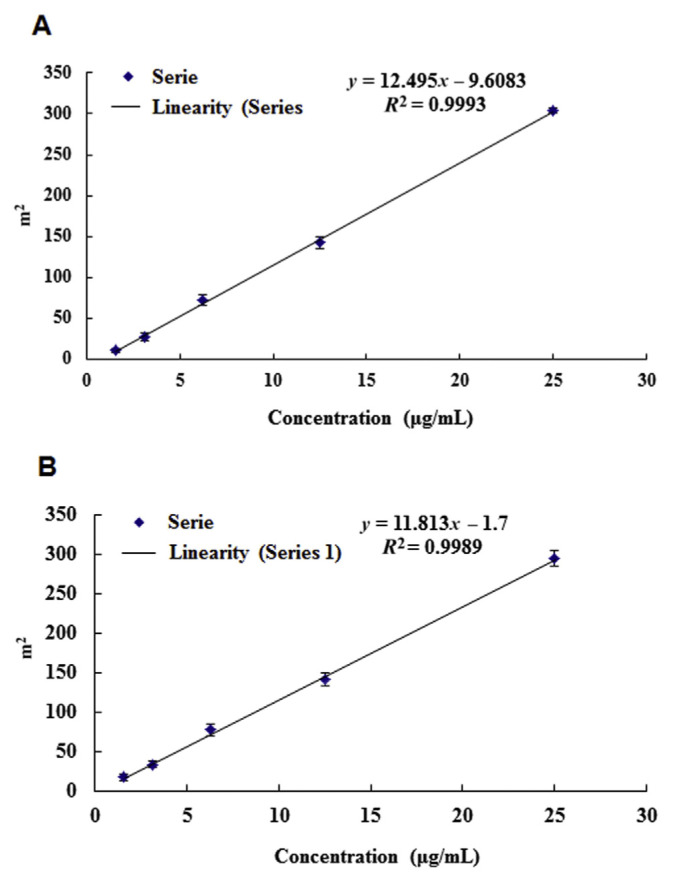
Linearity is the ability of a method to produce test results that are directly proportional to the concentrations of the analyte within a given range. Linear responses were established from the concentration–response curve of each compound (catechin and epicatechin) on the basis of five standards covering the concentration range of 1.56–25.00 μg/mL. The acceptance criterion for linearity is that the correlation coefficient (r2) should not be <0.990 for the least squares method of the analysis of the line [25]. The correlation coefficients of (+)-catechin and (−)-epicatechin were 0.9993 and 0.9989, respectively (Figure 5). This result demonstrates the linearity of this method over a wide dynamic range.
Figure 5.
Standard calibration curves for the detection of compounds. (A) Catechin. (B) Epicatechin. Results are presented from three replicate injections.
3.3.3. Percent recovery of (+)-catechin and (−)-epicatechin in extract
The recovery rates of (+)-catechin and (−)-epicatechin, shown in Table 3, were determined at three different concentrations with triplicate injections by comparing the mean of peak areas. The mean recoveries of (+)-catechin in extract were 67.54–90.98% (w/v) when the extract was spiked with pure (+)-catechin. The mean recoveries of (−)-epicatechin were 58.84–90.75% (w/v) in extract spiked with (−)-epicatechin (Table 3). The recovery percentages of both compounds are lower, which could be attributable to the interference of the sample matrix or the slowness of extraction of those compounds from the extract matrix. Moreover, the low recovery is justified in this case, as the expected recovery is dependent on the percentage of analyte in the matrix [24], indicating that the analytical method is validated.
Table 3.
Percent recovery of catechin and epicatechin.
| Catechin (μg/mL) | Epicatechin (μg/mL) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| 1.56 | 6.25 | 25 | 1.56 | 6.25 | 25 | |
| Recovery (%, w/v) | 67.54 ± 2.30 | 69.56 ± 1.26 | 90.98 ± 0.58 | 58.84 ± 4.13 | 91.36 ± 0.98 | 90.75 ± 0.42 |
Results are presented as mean ± standard deviation of three replicate injections.
3.3.4. LOD and LOQ
According to International Conference on Harmonization guideline [26], there are several approaches to determine the lower LOD and lower LOQ. Visual evaluation, signal/noise ratio, and the use of SD of the response and the slope of the calibration curve are the generally used methods. The LOD and LOQ in the present study were determined based on the last approach. The LOD was 0.15 μg/mL for (+)-catechin and 0.28 μg/mL for (−)-epicatechin since the peak areas for these concentrations were distinguishable from the response given by the blank sample. The LOQ of (+)-catechin was 0.45 μg/mL, and that of (−)-epicatechin was 0.84 μg/mL. Therefore, both catechin and epicatechin can be reliably quantified at concentrations higher than 1 μg/mL.
3.3.5. Specificity
Specificity of the analytical method ensures that the signals measured come from the desired compounds, and there is no interference from diluents, extract materials, and mobile phase. Photodiodearraydetectionalsosupportedthespecificity of the method and provided evidence for the homogeneity of the peaks of analytes. Peaks obtained from recovery experiments were checked for uniformity using UV spectra taken from different points of the peak of interest. These spectra were superimposed whenever overlaid, showing that there was no other coeluting peaks, in every instance for each of the analytes. The data obtained in the validation study proved that the proposed method is validated and can be used for the determination and quantification of catechin and epicatechin.
3.4. Quantification of catechin and epicatechin in different samples
The amounts of catechin and epicatechin in BSAE were determined to be 1.27% (w/w) and 0.71% (w/w), respectively, using the HPLC analysis. The amounts of these compounds in diluted supernatant of BSAE capsules were determined as shown in Table 4. The average amounts of catechin and epicatechin were 6.36 μg/mL and 4.47 μg/mL, respectively, in the supernatant prepared from the encapsulated extract. Therefore, the amounts of catechin and epicatechin in a capsule were 636 μg/mL and 447 μg, respectively, which were calculated from the amount in each microliter, multiplied by the total dilution factor.
Table 4.
Determination of the amount of catechin and epicatechin from the diluted supernatant of Bulnesis sarmienti capsules.
| Area | Amount (μg/mL) | |
|---|---|---|
| Catechin | 70.18 ± 0.81 | 6.36 ± 0.06 |
| Epicatechin | 51.08 ± 4.79 | 4.47 ± 0.41 |
Results are presented as mean ± standard deviation of three replicate injections.
3.5. HPLC-mass spectroscopic separation
The photodiode array detection of BSAE revealed catechin and epicatechin with some unidentified compounds. In HPLC-mass spectrometry (MS) analysis, the dominant isomer yielded a base peak at m/z 289, secondary ion at m/z 435, and strong ion at m/z 449, and were assigned for (+)-catechin and (−)-epicatechin (Figure 1). The chromatograms of HPLC-MS analysis of this extract are shown in Figure 4.
4. Conclusion
The aim of method validation was to confirm that the present method is suitable for its intended purpose. After optimization of the HPLC conditions, the described method was extensively validated in terms of accuracy, precision, linearity, LOD, LOQ, and specificity. This proposed method has proven to be simple, precise, rapid, and reliable. The validated method provides a good resolution in response to these compounds. It may be possible to perform quantitative analysis of plant extracts or herbal medicines having catechin and epicatechin within a short analysis time by using this single procedure. BSAE enhance the growth of the four L. acidophilus strains in vitro. The rapid growth of four L. acidophilus strains was influenced by the prebiotic activities of catechin and epicatechin present in BSAE. The findings suggest that BSAE may promote the survival, colonization, and activity of the above probiotic strains in the gastrointestinal tract, thereby resulting in the improvement of their beneficial effects in health. Further studies are necessary to investigate the above selected prebiotics in vivo in which the survival, persistence, and colonization of these probiotics are assessed thoroughly.
Acknowledgments
This research was supported in part by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Technology Development Program funded by Ministry of Agriculture, Food and Rural Affairs and in part by the project titled “Development of feed additives with immune enhancement in fishes using lactic acid bacteria separated from the eel and plant extracts” funded by the Ministry of Oceans and Fisheries, South Korea.
Funding Statement
Funded by the Ministry of Oceans and Fisheries, South Korea.
Footnotes
Conflicts of interests
All authors declare no conflicts of interest.
REFERENCES
- 1. Gerritsen J, Smidt H, Rijkers GT, de Vos WM. Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr. 2011;6:209–40. doi: 10.1007/s12263-011-0229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chang CLT, Chung CY, Kuo CH, Kuo TF, Yang CW, Yang WC. Beneficial effect of Bidens pilosa on body weight gain, food conversion ratio, gut bacteria and coccidiosis in chickens. Plos One. 2016;11:e0146141. doi: 10.1371/journal.pone.0146141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saulnier DM, Kolida S, Gibson GR. Microbiology of the human intestinal tract and approaches for its dietary modulation. Curr Pharm Des. 2009;15:1403–14. doi: 10.2174/138161209788168128. [DOI] [PubMed] [Google Scholar]
- 4. Ouwehand AC, Salminen S, Isolauri E. Probiotics: an overview of beneficial effects. Antonie van Leeuwenoek. 2002;82:279–89. [PubMed] [Google Scholar]
- 5.Ballongue J. Bifidobacteria and probiotic action. In: Salimen S, von Wright A, Ouwehand AC, editors. Lactic acid bacteria: microbiology and functional aspects. 3rd ed. New York: Marcel Dekker; 2004. pp. 67–124. [Google Scholar]
- 6. Bauer E, Williams BA, Smidt H, Verstegen MWA, Mosenthin R. Influence of the gastrointestinal microbiota on development of the immune system in young animals. Curr Issues Intest Microbiol. 2006;7:35–51. [PubMed] [Google Scholar]
- 7. Kechagia M, Basoulis D, Konstantopoulou S, Dimitriadi D, Gyftopoulou K, Skarmoutsou N, Fakiri EM. Health benefits of probiotics: a review. ISRN Nutr. 2013 doi: 10.5402/2013/481651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Geier MS, Butler RN, Howarth GS. Probiotics, prebiotics and synbiotics: a role in chemoprevention for colorectal cancer. Cancer Biol Ther. 2006;5:1265–9. doi: 10.4161/cbt.5.10.3296. [DOI] [PubMed] [Google Scholar]
- 9. Teitelbaum JE, Walker WA. Nutritional impact of pre- and probiotics as protective gastrointestinal organisms. Annu Rev Nutr. 2002;22:107–38. doi: 10.1146/annurev.nutr.22.110901.145412. [DOI] [PubMed] [Google Scholar]
- 10. Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004;17:259–75. doi: 10.1079/NRR200479. [DOI] [PubMed] [Google Scholar]
- 11. Tzounis X, Rodiguez-Mateos A, Vulevic J, Gibson GR, Kwik-Uribe C, Spencer JP. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am J Clin Nutr. 2011;93:62–72. doi: 10.3945/ajcn.110.000075. [DOI] [PubMed] [Google Scholar]
- 12. Díez-Municio M, Herrero M, Olano A, Moreno FJ. Synthesis of novel bioactive lactose-derived oligosaccharides by microbial glycoside hydrolases. Microb Biotechnol. 2014;7:315–31. doi: 10.1111/1751-7915.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kamruzzaman SM, Endale M, Oh WJ, Park SC, Kim KS, Hong JH, Kwak YS, Yun BS, Rhee MH. Inhibitory effects of Bulnesia sarmienti aqueous extract on agonist-induced platelet activation and thrombus formation involves miogen-activated protein kinases. J Ethnopharmacol. 2010;130:614–20. doi: 10.1016/j.jep.2010.05.049. [DOI] [PubMed] [Google Scholar]
- 14. Tzounis X, Vulevic J, Kuhnle GG, George T, Leonczak J, Gibson GR, Kwik-Uribe C, Spencer JP. Flavanol monomer-induced changes to the human faecal microflora. Br J of Nutr. 2008;99:782–7. doi: 10.1017/S0007114507853384. [DOI] [PubMed] [Google Scholar]
- 15. Mollah ML, Kim JO, Lee GD, Park CH, Hong JH, Kim HY, Kim KS. Growth inhibitory effects of a Bulnesia sarmienti aqueous extract on A549 cells in vitro and S180 cells in vivo. Immunopharmacol Immunotoxicol. 2009;31:492–8. doi: 10.1080/08923970902810432. [DOI] [PubMed] [Google Scholar]
- 16.Macheix JJ, Fleuriet A, Billot J. Fruit phenolics. Vol. 324. Boca Raton, FL: CRC Press; 1990. p. 370. [Google Scholar]
- 17.Shahidi F, Naczk M. Food phenolics: sources, chemistry, effects and application. Vol. 111. Lancaster: Technomic Publishing Company Inc; 1995. p. 125. [Google Scholar]
- 18. Donovan JL, Luthria DL, Stremple P, Waterhouse AL. Analysis of (+)-catechin, (−)-epicatechin and their 3′- and 4′-O-methylated analogs. A comparison of sensitive methods. J Chromatogr B Biomed Sci Appl. 1999;726:277–83. doi: 10.1016/s0378-4347(99)00019-5. [DOI] [PubMed] [Google Scholar]
- 19. Dubey N, Dubey N, Mehta R, Saluja A. A selective high performance liquid chromatographic method for estimation of catechin in ayurvedic taila preparations. Asian J Res Chem. 2009;2:66–9. [Google Scholar]
- 20. Jafarei P, Ebrahimi MT. Lactobacillus acidophilus cell structure and application. Afr J Microbiol Res. 2011;5:4033–42. [Google Scholar]
- 21. Olson DW, Kayanush A. Effect of prebiotics on Lactobacillus acidophilus growth and resulting pH changes in skim milk and a model peptone system. J Microb Biochem Technol. 2012;4:121–5. [Google Scholar]
- 22. Roberfroid M. Prebiotics: the concept revisited. J Nutr. 2007;137:830S–7S. doi: 10.1093/jn/137.3.830S. [DOI] [PubMed] [Google Scholar]
- 23.Guidance for Industry: Bioanalytical Method Validation. Maryland, USA: Food and Drug Administration; 2013. [Google Scholar]
- 24.Standard Format and Guidance for AOAC Standard Method Performance Requirement. Maryland, USA: AOAC International; 2011. [Google Scholar]
- 25.International Conference on Harmonization (ICH) “Validation of Analytical Procedures—PA/PH/OMCL (05) 47 DEF,” elaborated by OMCL Network/EDQM of the. Council of Europe; 2005. [Google Scholar]
- 26.ICH-Q2B Validation of Analytical Procedures: Methodology of Technical Requirements for Registration of Pharmaceuticals for Human Use. Geneva, Switzerland: International Conference on Harmonization; 1996. [Google Scholar]