Abstract
A new silica-based, mixed-binary chiral sorbent grafted with the macrocyclic antibiotic eremomycin and bovine serum albumin (BSA) was obtained. The sorbent-filled high-performance liquid chromatography column was capable of enantioseparation of racemic drugs, such as profens, in reversed-phase-chromatography mode. The mixed-binary eremomycin-BSA-sorbent showed better capability for profens enantioseparation as compared with a sorbent containing eremomycin only. BSA grafted onto the sorbent surface significantly reduced retention times of other proteins from the analyte solution, and free proteins (including BSA) injected as analytes were not retained on the column, and subsequently eluted with a dead volume. The drastic difference observed in the binding of profens and other proteins using the sorbent was tested for determination and enantioseparation of profens in artificial-urine solutions.
Keywords: binary chiral sorbent, bovine serum albumin, chiral stationary phase, eremomycin, profen
1. Introduction
The pharmaceutical industry in highly interested in methods capable of rapidly and accurately determining the enantiomeric composition of chiral bioactive molecules. Many therapeutic substances bearing chiral centers are clinically administered as racemic mixtures due to difficulties in stereoselective synthesis and purification [1]. Enantioseparation of drugs is necessary, because of the possibility that enantiomers of chiral therapeutic substances may exhibit significant differences in toxicity [2].
Nonsteroidal anti-inflammatory drugs, including profens, are widely applied for the treatment of pain and fever in animals and humans. Profens are chiral compounds the exhibit different pharmacological activity between the R- and S-isomers, although these drugs are often produced as racemic mixtures. The S-enantiomers exhibit anti-inflammatory activity, and drugs containing only the S-enantiomer should be consumed at a lower dose as compared with drugs containing two isomers. Recently, the R-enantiomers have attracted attention due to its anti-tumor activity. Enantioseparation and determination of profens in physiological fluids is currently an important task, and research associated with new chiral high-performance liquid chromatography (HPLC) phases to address this problem is underway.
In the field of liquid chromatography, many chiral stationary phases (CSPs) are available for enantioseparation of various chiral drugs. More than 110 CSPs developed for HPLC are now commercially available [3]. Synthesis of new chiral stationary phases and improvement of existing phases continue. Among those, protein-based and antibiotic-based CSPs have enantioselectivity for enantioseparation of a wide range of compounds.
Proteins are used as chiral selectors for CSPs, given that bovine serum albumin (BSA)-agarose conjugates were successfully applied for enantioseparation of tryptophan in 1973 [4]. currently, diverse albumins, glycoproteins, enzymes, and other substances, including ovotransferrin (conalbumin) and β-lactoglobulin, are used for this purpose, with albumins most often applied [5,6]. BSA and other proteins can separate a wide range of chiral compounds, especially pharmaceutically active compounds. For example, CSPs based on BSA were used for separations of a wide variety of acidic and neutral enantiomers, such as aromatic amino acids, N-derivatized amino acids, uncharged solutes, sulfoxides, sulfoximine derivatives [1,5–9], dansyl- and 2,4-dinitrophenylhydrazone-derivatives of amino acids [10], and enantiomers of omeprazole from human plasma [11].
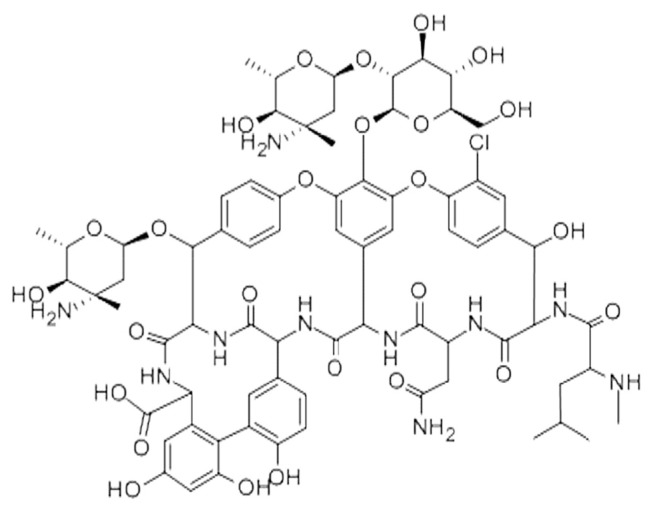
Macrocyclic antibiotics, such as ansamycins, glycopeptides, and polypeptide antibiotic thiostrepton, are also used as chiral selectors for CSPs [12]. Among these, glycopeptides are most often used for this purpose. The most important selectors from this group are vancomycin, ristocetin, teicoplanin, and the teicoplanin aglycon [13,14]. The glycopeptide antibiotic eremomycin is an efficient chiral selector for separation of optical isomers of amino acids, especially those having aromatic substituents in structures, as well as some amino acid derivatives [15–18] and profens [19]. The structure of this antibiotic is similar to the structure of vancomycin, which is widely used in commercial columns. The structure of eremomycin (Figure 1) differs from that of vancomycin in the trinucleus amino acid fragment. In this fragment, eremomycin has one chloro-substituted aromatic ring, while vancomycin has two [15,20].
Figure 1.
Eremomycin structure.
Mixed chiral sorbents containing both antibiotics and proteins as selectors have not been previously described, although selectors of these two types combined in one CSP may increase a number of specific interaction points with analytes and provide greater enantioseparating ability of the sorbent. For example, Ruderisch et al [21] synthesized mixed CSPs containing modified resorcinarene and β-cyclodextrin selectors bonded to a polysiloxane for enantioselective gas chromatography. Additive effects of the two selectors on enantioselectivity of such stationary phases were observed, and the separation factor for racemates, which can be enantioseparated on only one of the two selectors of such mixed CSPs, was slightly lower, because the concentration of this selector on mixed CSPs is lower as compared with the phase with only one such selector [21].
Sizes of protein molecules are often larger than the size of the silica pores. The protein layer is formed on the silica surface only, making the pore inaccessible to the other proteins in the mobile phase, which can be used for direct analysis of physiological liquids.
Here, we prepared a silica-based CSP grafted with a peptide-antibiotic pair of eremomycin and BSA, and studied its applicability for enantioseparation of profens, including their solutions in urine.
2. Materials and methods
2.1. Materials
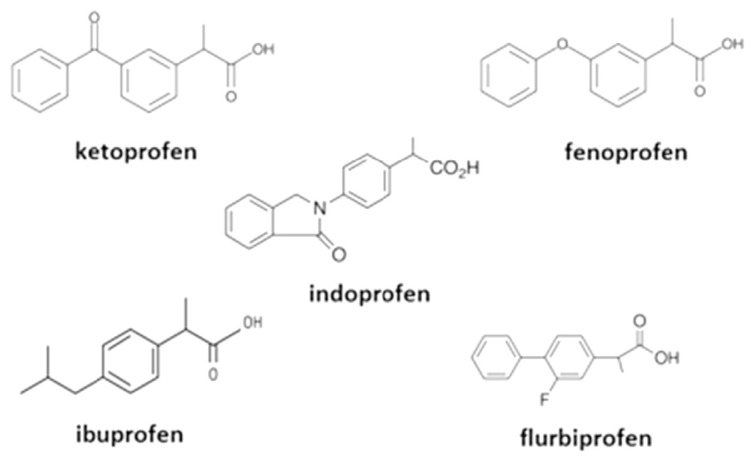
A previously prepared eremomycin-grafted silica was used [15]. BSA was purchased from Dia-M (Moscow, Russia) at a purity >99.0%. Profens was purchased from Sigma-Aldrich (St. Louis, MO, USA). HPLC-grade methanol and isopropanol were supplied by Merck (Darmstadt, Germany). Potassium dihydrogen phosphate (KH2PO4), dipotassium hydrogen phosphate trihydrate (K2HPO4*3H2O), sodium acetate (CH3COONa), acetic acid (CH3COOH), and glutaraldehyde (C5H8O2) were purchased from Chimmed (Moscow, Russia). Sodium borohydride (NaBH4) was purchased from Sigma-Aldrich. Deionized water was purified using a Milli-Q gradient system (Millipore, Molsheim, France). The profens variants used in this study are fenoprofen, flurbiprofen, ibuprofen, indoprofen, and ketoprofen (Figure 2).
Figure 2.
Profens structures.
2.2. Apparatus
Experiments were carried out using a LC-20AB Prominence liquid chromatography system (Shimadzu, Japan) equipped with a vacuum degasser, a quaternary pump system, a diode-array detector SPD-M20A (Shimadzu, Japan), and a data-acquisition system running the Laboratory Solution software from the same manufacturer. The injection valve was equipped with a 20-μL sample loop.
2.3. CSP synthesis
Eremomycin was immobilized on epoxy-activated Kromasil KR100-5-SIL silica (7 μm, 100 Å) as described previously [13]. A solution of 0.2 g BSA in 25 mL 0.1 M sodium acetate buffer (pH 5.6) was added to 2 g of silica modified with eremomycin. The suspension was mixed for 2 days with ultrasonication for 15 min each over the course of 4 h. The solid phase was separated by filtration, washed with water, and 1.5 mL of glutaraldehyde in 25 mL of sodium acetate buffer was added. The suspension was stirred at room temperature for 1 day. The separated sorbent was treated with 0.16 g of NaBH4 in 25 mL of distilled water for 1 h. The sorbent was separated by filtration, washed [with water, then isopropanol:water (1:1) in sequence], and dried at room temperature for 24 h. The data from the elemental analysis of the sorbent was as follows: 1 3.8% C, 1.8% H, and 1.4% N.
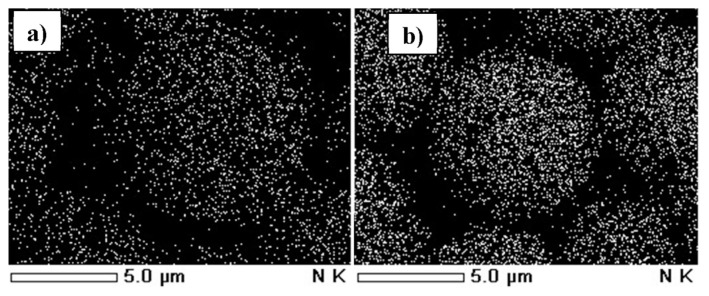
The qualitative presence of BSA on the surface of the silica with eremomycin was confirmed by scanning electron microscopy (Figure 3B). The number of nitrogen atoms and the intensity of their peaks on the spectrum increased in the final sorbent as compared to the initial silica with eremomycin only (Figure 3A).
Figure 3.
Maps of scanning electron microscopy for (A) sorbents containing eremomycin (CSP-1) and (B) sorbents containing eremomycin and BSA (CSP-2). BSA =bovine serum albumin; CSP =chiral stationary phase.
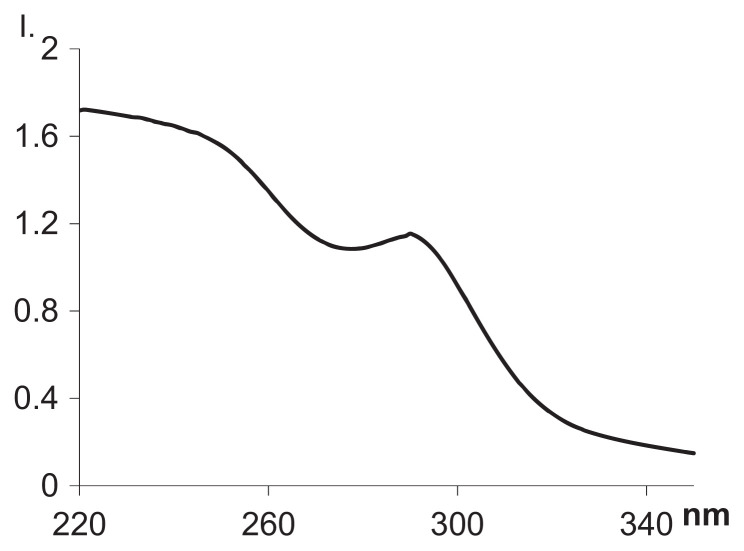
The amount of BSA immobilized on the sorbent was determined by ultraviolet spectrophotometry at 280 nm as the difference between the amount of BSA added to the reaction vessel and the amount of enzyme remaining in the supernatant after reaction and centrifugation. The absorption curve of the BSA solution is presented in Figure 4. The amount of BSA immobilized on the eremomycin-modified silica was 70 mg/g.
Figure 4.
Absorption curve of BSA-sample solution. BSA =bovine serum albumin.
The prepared CSP was packed in slurry into 100 mm × 4.6 mm stainless-steel columns at 200 bars with a Knauer K-1900 pump. Isopropanol with water (1:1) was used for slurry preparation and for washing the column.
3. Results and discussion
3.1. Profens enantioseparation
To assess the effects of pH, buffer strength, and organic solvent volume of the mobile phase, chromatographic evaluation of the prepared CSP was performed in methanol-water eluents containing different buffers. The eremomycin-BSA-enriched CSP demonstrated a high enantioselectivity in separation of profens. We varied concentration of the buffer solution, pH, and organic modifier content in the mobile phase, and chromatographic parameters associated with profens enantioseparation at various buffer concentrations are shown in Table 1.
Table 1.
Chromatographic parameters of profen enantioseparation at various concentrations of buffer solution with mobile phase: MeOH/KH2PO4 (pH 4.5); 50/50 (v/v); flow rate: 0.5 mL/min.
| Profen | 0.02 M | 0.04 M | 0.10 M | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| k1′ | k2′ | Rs | α | k1′ | k2′ | Rs | α | k1′ | k2′ | Rs | α | |
| Ketoprofen | 6.5 | 9.7 | 1.74 | 1.42 | 5.1 | 7.4 | 1.88 | 1.38 | 3.6 | 5.3 | 1.75 | 1.38 |
| Ibuprofen | 3.9 | 6.0 | 1.03 | 1.42 | 2.8 | 4.2 | 1.25 | 1.36 | 1.8 | 2.7 | 0.93 | 1.31 |
| Indoprofen | 8.6 | 14.1 | 2.14 | 1.58 | 4.7 | 7.5 | 1.71 | 1.50 | 4.3 | 6.7 | 1.74 | 1.46 |
| Fenoprofen | 7.1 | 9.1 | 0.79 | 1.24 | 4.9 | 6.2 | 1.08 | 1.22 | 3.2 | 4.0 | 0.82 | 1.19 |
| Flurbiprofen | 9.8 | 17.9 | 1.83 | 1.74 | 6.4 | 11.4 | 2.05 | 1.67 | 4.2 | 7.4 | 1.59 | 1.60 |
α = selectivity; k = capacity factors; Rs = resolution.
Increased concentration of the phosphate buffer used in the mobile phase led to decreased profens retention times due to the extended interactions between the phosphate ions and the sorbent as compared to those between the analyte and the sorbent. However, using diluted-buffer solutions, the peaks associated with enantiomers became blurred. The 0.1-M buffer solution was used for all subsequent experiments.
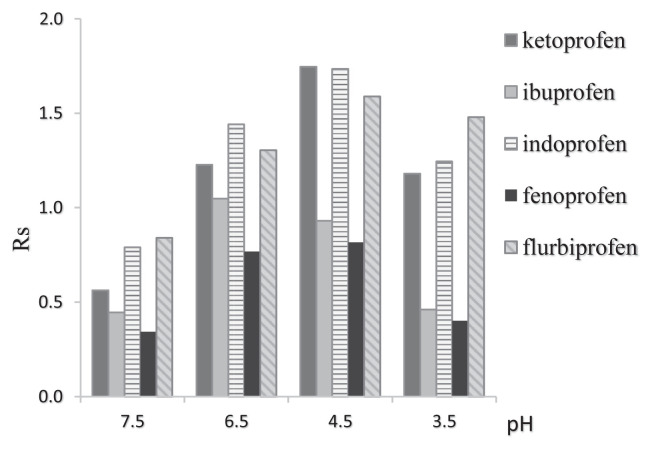
Lowering the pH of the mobile phase from 7.5 to 4.5 lead to increased retention times and peak resolutions of profens enantiomers (Figure 5). Further decreases in pH to 3.5 resulted in sharp decreases in retention times likely due to changes in the surface charge of the sorbent modified with BSA, the iso-electric point of which is 4.7 [2], with the pKa of profens ranging from 4.4 to 5.8.
Figure 5.
Resolution of profens enantiomers at different buffer pH in the mobile phase. Mobile phase: MeOH/0.1 M KH2PO4; 50/50 (v/v); flow rate: 0.5 mL/min; λ = 220 nm.
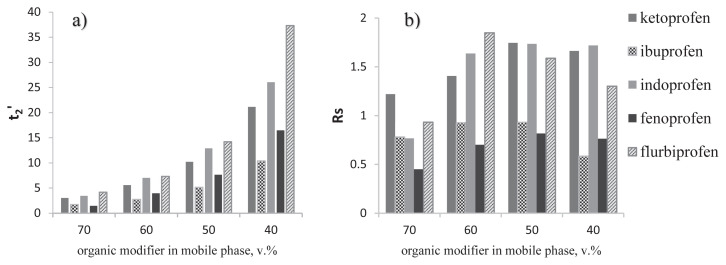
Decreased concentration of the organic modifier in the mobile phase increased the profens retention time (Figure 6A). The mobile phase contained <50% by volume of organic modifier, providing lower separation efficiency with longer analysis times. Mobile phases containing >50% by volume of organic modifier resulted in lower separation efficiency with shorter analysis times (Figures 6A and 6B). For most profens experiments, optimal peak resolutions were observed at 50% by volume of organic modifier in the mobile phase.
Figure 6.
Retention times for the (A) second profens enantiomer and (B) resolution at different organic modifier contents in the mobile phase. Mobile phase: MeOH/KH2PO4 (0.1 M, pH 4.5); flow rate: 0.5 mL/min; λ =220 nm.
3.2. Chromatographic parameters of the eremomycin-BSA-sorbent
The mixed-binary chiral eremomycin-BSA-silica is based on the eremomycin-silica sorbent. Molecules of eremomycin are small, enabling them to fix to the surface and in the pores of silica. However, BSA molecules are large and fix only on the sorbent surface. The layer of protein forms on the silica surface, while other proteins located in the mobile phase are unable to penetrate into the pores. Comparison of chromatographic parameters of initial and binary sorbents was performed to determine the effect of BSA on the sorbent surface.
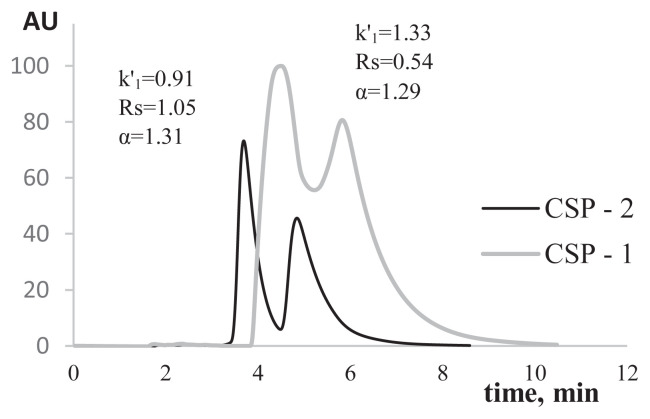
The peak resolution of profens enantiomers obtained on the synthesized sorbent was higher as compared with that of the sorbent using only eremomycin. The mobile phase containing MeOH/phosphate buffer [pH 6.5; 0.1 M; 50/50 (v/v)] showed a peak resolution increase of 0.44-fold on the sorbent with eremomycin and BSA as compared with the sorbet containing only eremomycin. Mobile phases containing MeOH/KH2PO4 [pH 4.5; 0.04 M; 60/40 (v/v)] showed a 0.44-fold increase in peak resolution. Chromatograms of ibuprofen obtained on both sorbents are shown in Figure 7.
Figure 7.
Chromatograms of ibuprofen obtained on the sorbent containing eremomycin (CSP-1) and the sorbent containing eremomycin and BSA (CSP-2). Mobile phase: MeOH/phosphate buffer solution (0.1 M, pH 6.5); 50/50 (v/v); flow rate: 0.5 mL/min; λ =220 nm.
BSA presence decreased profens retention, while the selectivity was not affected. Tentatively, BSA adsorption on the surface resulted in eremomycin-related nonspecific interactions; however, BSA introduced specific points of interaction that enhanced the resolution of peaks. Precise explanations of why these changes were observed require further studies.
Our data suggested that composition of mobile phases (pH and volume of organic modifier) for mixed-binary sorbents influence enantioseparation of drugs on eremomycin-sorbents. The chromatographic parameters associated with profen isomers displayed only minor changes on the mixed sorbent as compared with the sorbent containing eremomycin only. Therefore, we assume that eremomycin plays a significant role as a chiral selector in mixed-binary sorbent relative to the effect of BSA.
3.3. Effects of proteins in sample solutions
Proteins are macromolecules containing numerous amino- and carboxyl-functional groups. Therefore, when such molecules enter in chiral column as analytes, they can be adsorbed on the sorbent and change its properties, thereby rendering the column unusable. Preparation for analysis of physiological fluids that contain proteins is important, but the separation of proteins from physiological fluids is time consuming. The synthesized binary chiral sorbent contains cross-linked protein molecules in its structure that prevent the adsorption of proteins from analytes on the sorbent. This results in the proteins eluting with a dead volume.
For example, BSA aqueous solutions (0.4 mg/mL) were injected as analytes onto the column with a mixed-binary sorbent and also on an eremomycin column. BSA did not elute from the eremomycin column and eluted with a dead volume from the mixed-binary eremomycin-BSA-sorbent column.
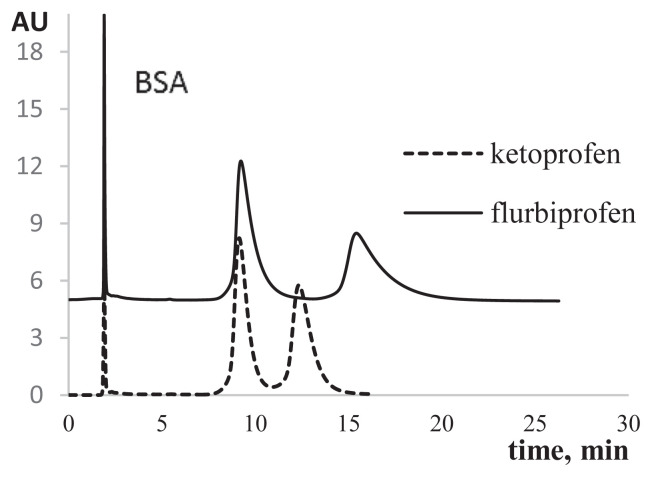
In the case of a mixed-binary sorbent, proteins in the sample solution did not significantly affect the ability of the column to enantioseparate simpler molecules. For example, chromatograms associated with ketoprofen and flurbiprofen enantioseparation in the presence of BSA in the analyte are shown in Figure 8.
Figure 8.
Profens enantioseparation on the mixed-chiral sorbent in the presence of proteins in the analyte. Mobile phase: MeOH/KH2PO4 (0.1 M, pH 4.5); 40/60 (v/v); flow rate: 0.5 mL/min.
Additionally, protein injections did not alter the properties of the mixed-chiral sorbent. Standard deviations of the capacity factor (k′), resolution (Rs), and selectivity (α) of ketoprofen enantiomers were 0.58, 0.04, and 0.004 (n = 3, p = 0.95), respectively, before and after the protein injections [mobile phase: MeOH/KH2PO4 (0.1 M; pH 4.5), 40/60 (v/v)]. The column containing the mixed-binary chiral sorbent was stable for >10 months and 400 injections. The reproducibility of the retention time and resolution of profens enantiomers did not exceed 5% on different synthesized columns of this type.
3.4. Profens enantioseparation from urine samples
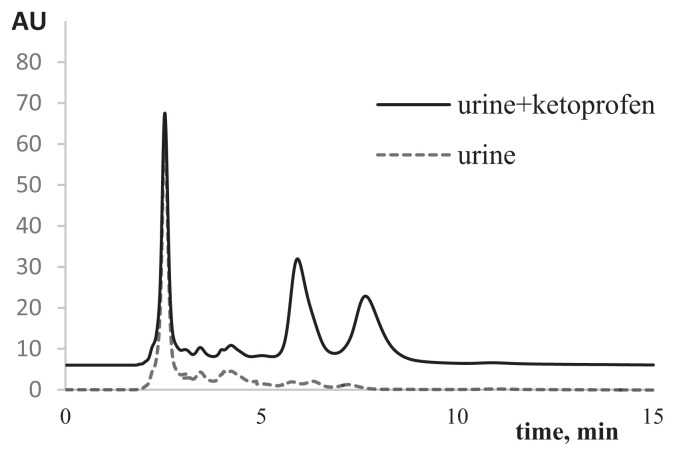
A series of calibrated solutions consisting of ketoprofen in urine:water (1:4) mixtures were prepared, and the solutions were analyzed chromatographically using columns containing mixed-chiral sorbents. Chromatograms of used urine:-water (1:4) mixtures and ketoprofen in such mixtures are shown in Figure 9. The minimal detectable concentrations for each ketoprofen enantiomer was determined as 8.5 μg/mL (assumed signal-to-noise ratio: 3:1).
Figure 9.
Chromatograms of urine and the test sample of ketoprofen in urine. Mobile phase: MeOH/KH2PO4 (0.1 M, pH 4.5); 50/50 (v/v); flow rate: 0.5 mL/min; λ =254 nm.
In the artificially prepared test solution of ketoprofen in urine (0.067 mg/mL), the protein content was successfully measured with high accuracy (0.07±0.01 mg/mL; n = 3; p = 0.95) using the calibration curve obtained above. The mixed-binary eremomycin-BSA-sorbent permitted enantioseparation of all profens compounds in the urine:water (1:4) mixtures.
4. Conclusions
Here, we prepared a mixed-binary chiral sorbent on silica modified with the antibiotic eremomycin and BSA, and enantioseparation of five profens compounds by reversed-phase chromatography was conducted using a column containing the mixed sorbent. The mixed sorbent provided a higher resolution of enantiomers as compared with results obtained using a sorbent with only eremomycin as the chiral selector. The presence of BSA on the surface of the mixed sorbent prevented protein retention by the column without affecting enantioseparation. This allowed the successful measurement of ketoprofen from modeled urine solutions with the minimal detection concentrations for each enantiomer (8.5 mg/mL).
Acknowledgments
This work was supported by a grant (No15-03-05979a) from the Russian Foundation for basic research, Russia.
Funding Statement
This work was supported by a grant (No 15-03-05979a) from the Russian Foundation for basic research, Russia.
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
REFERENCES
- 1. Millot MC. Review: separation of drug enantiomers by liquid chromatography and capillary electrophoresis, using immobilized proteins as chiral selectors. J Chromatogr B. 2003;797:131–59. doi: 10.1016/j.jchromb.2003.08.035. [DOI] [PubMed] [Google Scholar]
- 2.Aboul-Enein HY, Abou-Basha LI. The impact of stereochemistry on drug development and use. New York: Wiley; 1997. [Google Scholar]
- 3. Tang M, Zhuang J, Liu W. Development of chiral stationary phases for high-performance liquid chromatographic separation. Trends Anal Chem. 2012;39:180–94. [Google Scholar]
- 4. Stewart KK, Doherty RF. Resolution of DL-tryptophan by affinity chromatography on bovine-serum albumin-agarose columns. Proc Natl Acad Sci USA. 1973;70:2850–2. doi: 10.1073/pnas.70.10.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haginaka J. Protein-based chiral stationary phases for high-performance liquid chromatography enantioseparations. J Chromatogr A. 2001;906:253–73. doi: 10.1016/s0021-9673(00)00504-5. [DOI] [PubMed] [Google Scholar]
- 6. Zheng Y, Wang X, Ji Y. Monoliths with proteins as chiral selectors for enantiomer separation. Talanta. 2012;91:7–17. doi: 10.1016/j.talanta.2012.01.039. [DOI] [PubMed] [Google Scholar]
- 7.Allenmark S. Chromatographic enantioseparation Methods and applications. 2nd ed. Chapter 7. New York: Ellis Horwood; 1991. [Google Scholar]
- 8.Wainer IW. Drug stereochemistry: analytical methods and pharmacology. 2nd ed. Chapter 6. New York: Marcel Dekker; 1993. [Google Scholar]
- 9. Allenmark S. Optical resolution by liquid chromatography on immobilized bovine serum albumin. J Liq Chromatogr. 1986;9:425–42. [Google Scholar]
- 10. Allenmark S, Andersson S. Direct liquid chromatographic separation of enantiomers on immobilized protein stationary phases, V. Optical resolution of N-(2,4-dinitrophenyl)- and dansyl- D,L-amino acids. J Chromatogr A. 1986;351:231–8. doi: 10.1016/s0021-9673(00)94608-9. [DOI] [PubMed] [Google Scholar]
- 11. Cairns AM, Chiou RH-Y, Rogers JD, Demetriades JL. Enantioselective high-performance liquid chromatographic determination of omeprazole in human plasma. J Chromatogr B. 1995;666:323–8. doi: 10.1016/0378-4347(94)00587-u. [DOI] [PubMed] [Google Scholar]
- 12. Ward TJ, Farris AB., III Chiral separations using the macrocyclic antibiotics: a review. J Chromatogr A. 2001;906:73–89. doi: 10.1016/s0021-9673(00)00941-9. [DOI] [PubMed] [Google Scholar]
- 13. ScribaGerhard KE. Chiralrecognition mechanisms inanalytical separation sciences. Chromatographia. 2012;75:815–38. [Google Scholar]
- 14. Ribeiro AR, Maia AS, Cass QB, Tiritan ME. Enantioseparation of chiral pharmaceuticals in biomedical and environmental analyses by liquid chromatography: an overview. J Chromatogr B. 2014;968:8–21. doi: 10.1016/j.jchromb.2014.02.049. [DOI] [PubMed] [Google Scholar]
- 15. Staroverov SM, Kuznetsov MA, Nesterenko PN, Vasiarov GG, Katrukha GS, Fedorova GB. New chiral stationary phase with macrocyclic glycopeptide antibiotic eremomycin chemically bonded to silica. J Chromatogr A. 2006;1108:263–7. doi: 10.1016/j.chroma.2006.01.073. [DOI] [PubMed] [Google Scholar]
- 16. Petrusevska K, Kuznetsov MA, Gedicke K, Meshko V, Staroverov SM, Seidel–Morgenstern A. Chromatographic enantioseparation of amino acids using a new chiral stationary phase based on a macrocyclic glycopeptide antibiotic. J Sep Sci. 2006;29:1447–57. doi: 10.1002/jssc.200600036. [DOI] [PubMed] [Google Scholar]
- 17.Berthod A, Qiu HX, Staroverov SM, Kuznestov MA, Armstrong DW. Chiral recognition with macrocyclic glycopeptides: mechanisms and applications. In: Berthod A, editor. Chiral recognition in separation methods. Berlin: 2010. pp. 203–22. [Google Scholar]
- 18. Zhang L, Gedicke K, Kuznestov MA, Staroverov SM, Seidel–Morgenstern A. Application of an eremomycin-chiral stationary phase for the separation of DL-methionine using simulated moving bed technology. J Chromatogr A. 2007;1162:90–6. doi: 10.1016/j.chroma.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 19. Kuznetsov MA, Nesterenko PN, Vasiarov GG, Staroverov SM. Sorbents with immobilized glycopeptide antibiotics for separating optical isomers by high-performance liquid chromatography. Appl Biochem Microbiol. 2006;42(6):536–44. [PubMed] [Google Scholar]
- 20. Gausel GF, Brazhnikova MG, Lomakina NN, Berdnikova TF, Fedorova GB, Tokareva NL, Borisova VN, Batta GY. Eremomycin – new glycopeptide antibiotic: chemical properties and structure. J Antibiot. 1989;42(12):1790–9. doi: 10.7164/antibiotics.42.1790. [DOI] [PubMed] [Google Scholar]
- 21. Ruderisch A, Pfeiffer J, Schurig V. Mixed chiral stationary phase containing modified resorcinarene and β-cyclodextrin selectors bonded to a polysiloxane for enantioselective gas chromatography. J Chromatogr A. 2003;994:127–35. doi: 10.1016/s0021-9673(03)00423-0. [DOI] [PubMed] [Google Scholar]