Abstract
The purpose of this article is to summarize the reported antioxidant activities of a naturally abundant bioactive phenolic acid, caffeic acid (CA, 3,4-dihydroxycinnamic acid), so that new avenues for future research involving CA can be explored. CA is abundantly found in coffee, fruits, vegetables, oils, and tea. CA is among the most potential and abundantly found in nature, hydroxycinnamic acids with the potential of antioxidant behavior. Reactive oxygen species produced as a result of endogenous processes can lead to pathophysiological disturbances in the human body. Foods containing phenolic substances are a potential source for free radical scavenging; these chemicals are known as antioxidants. This review is focused on CA’s structure, availability, and potential as an antioxidant along with its mode of action. A brief overview of the literature published about the prooxidant potential of caffeic acid as well as the future perspectives of caffeic acid research is described. CA can be effectively employed as a natural antioxidant in various food products such as oils.
Keywords: anticancer, antioxidant, hydroxycinnamic acid, prooxidant, reactive oxygen species
1. Introduction
Complex metabolic processes occurring in human body result in the production of reactive oxygen species (ROS) such as hydroxyl radicals [1]. These radicals are reported to have different biological functions such as antimicrobial activity [2]. Normally, ROS production in body is balanced by scavengers (antioxidants) but in pathological condition the equilibrium is disturbed due to over-production of ROS, which subsequently reacts with intra- and extracellular species and results in ailments such as aging, cancer and necrosis [1].
Phenolics are a class of compounds isolated from plants and are stated to have free radical scavenging potential due to their stable structure after trapping the free radical. Methoxy and hydroxyl groups can affect the antioxidant potential of the molecule [3]. Hydroxycinnamic acids are aromatic acids, and caffeic acid (CA) is a member of this class. CA (Figure 1) is a phenolic acid and a catechol derivative, isolated from various plants such as Ilex paraguariensis (15 mg/100 g), Melissa officinalis (39.3 mg/100 g), Baccharis genistelloides (8 mg/100 g), and Achyrocline satureioides (4 mg/100 g) [4].
Figure 1.
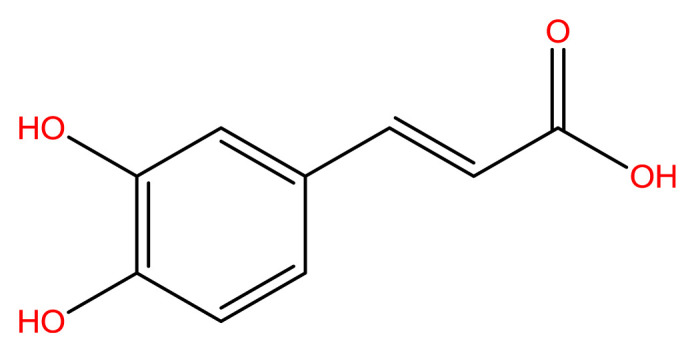
Structural formula of caffeic acid [15].
CA and its derivatives including ethyl ester and phenyl ester (CAPE) are reported for various pharmacological activities, e.g. anticancer, anti-AIDS and anti-inflammatory [5,6]. CA is described as an inhibitor for low-density lipoprotein oxidative modification which is thought to be involved in the pathogenesis of atherosclerosis [7]. Moreover, CA is also reported to react (scavenge) more efficiently with peroxyl radical as compared to the drug Trolox, which is used for oxidative stress; however, peroxyl radical involved in lipid peroxidation is a potential threat to cell membranes [8]. Increased antioxidant behavior is attributed to delocalization of the unpaired electrons in the extended conjugated side chain [9]; however, the structure stability of CA is further increased by additional hydrogen bond formation utilizing its ortho-dihydroxyl group after breaking the O-H bond [10]. Enhanced antioxidant activity of CA is also associated with the formation of o-quinone and regeneration of CA through semiquinone radical primarily generated by the reaction of antioxidant and free radical present in the medium [11].
CA derivatives have been studied and reported for their antioxidant potential: chlorogenic acid, CAPE, caffeic acid methyl ester, caffeic acid ethyl ester, caffeic acid butyl ester, caffeic acid benzyl ester, methyl caffeate (MC), and methyl dihydrocaffeate (MDC), octyl caffeate, and cichoric acid [11–13].
Recently, CA has been reported as an effective antioxidant in vitro assays such as total antioxidant activity by ferric thiocyanate process, lipid peroxidation analysis, reducing efficiency, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS+•) and 2,2-diphenyl-1-picrylhydrazyl (DPPH•) scavenging, superoxide anion scavenging analysis, and metal chelating activity against standards such as butylated hydroxyanisole and butylated hydroxytoluene [14].
This review summarizes the recent data published on the antioxidant activity of CA, along with its structural aspects and possible mode of action as an antioxidant agent.
2. Literature search methodology
A rigorous literature search in English language was conducted, using different electronic databases including Science Direct, PLoS One, Medline, PubMed, Science Hub, and EMBASE from 1980 to 2015, using various keywords including caffeic acid and antioxidant jointly, followed by the use of caffeic acid with other keywords including inflammation, cancer, and molecular targets. The literature search was carried out to assess the bibliography of the selected articles to reveal the original research for the preparation of a comprehensive review article.
3. Results and discussion
3.1. Antioxidant activity of caffeic acid
Stability and shelf life are major issues in dietary products; these issues can be overcome by the addition of natural antioxidants such as phenolic acids [15]. CA is also among the hydroxycinnamic acids used to enhance the stability of dietary products [16,17]. However, the antioxidant potential of CA is affected by factors such as nature of oil, food processing, ingredients, and ratio of antioxidants and lipid components [17,18]. Novel dihydro-CA amides have been described for excellent free radical scavenging abilities assessed by the DPPH method, an excellent potential has been reported to protect polyunsaturated oils [11].
Addition of CA has been reported to enhance the stability of various oils by inhibition of lipid oxidation; the results are summarized in Table 1 [16,18–21].
Table 1.
Caffeic acid and/or its derivatives as antioxidants for stability enhancement of various oils.
UV radiation is responsible for biological changes such as photoaging and damages normal lymphocytes, resulting in their death. However, lymphocytes pretreated with an amount of CA (l μg/mL, 5 μg/mL, and 10 μg/mL) for 30 minutes, when irradiated with UV-B (280–320 nm), a photoprotective effect of CA has been reported in terms of decreased lipid peroxidation and reduced DNA damage showing viability of lymphocytes [22]. The maximum protection against photoaging effect has been described with pretreatment using an amount of CA (10 μg/mL) in irradiated lymphocytes [22]. The photoprotective effect of CA may be due to glutathione metabolism, which exhibits free radical scavenging. CA has also been reported to protect phospholipidic membranes [23,24] and resist against vitamin C and vitamin E depletion through the membrane [25]. CA has also been found to have photoprotective activity against UV-B radiation in both in vitro and in vivo studies [26,27]. Furthermore, CA pretreatment leads to considerable decrease in γ radiation-provoked DNA damage in cultured human lymphocytes [26]. CAPE has also been reported to have photoprotective influence against UV-B induced DNA damage [28].
Ischemia/reperfusion (I/R) injury can be caused by ROS [29]. Reported models can be used to study protective effects of antioxidants administered prior to induction of I/R injury [30,31]. CA has been described to have protective effects on I/R injury in rat’s small intestine [29] and a successful protection of mouse brain and liver mitochondria from oxidative stress, however, using CAPE and its related phenolic compounds during I/R injury showed more promising results [12]. Moreover, an excellent cytoprotective activity of CAPE and fluorinated CAPE derivatives has been published in a human umbilical vein endothelial cell model of oxidation stress [13].
A comparative in vitro study conducted to assess the antioxidant potential of CA and its conjugate SMND-309 (Figure 2) reported 92.1% and 93.4% scavenging activity of CA and SMND-309, respectively, on DPPH radical at the concentration of 20 mg/mL; therefore, a higher antioxidant effect of SMND-309 than CA can be observed [1]. In another study, 61.9% superoxide anion radical scavenging activity at 10 mg/mL has been reported for CA [14].
Figure 2.
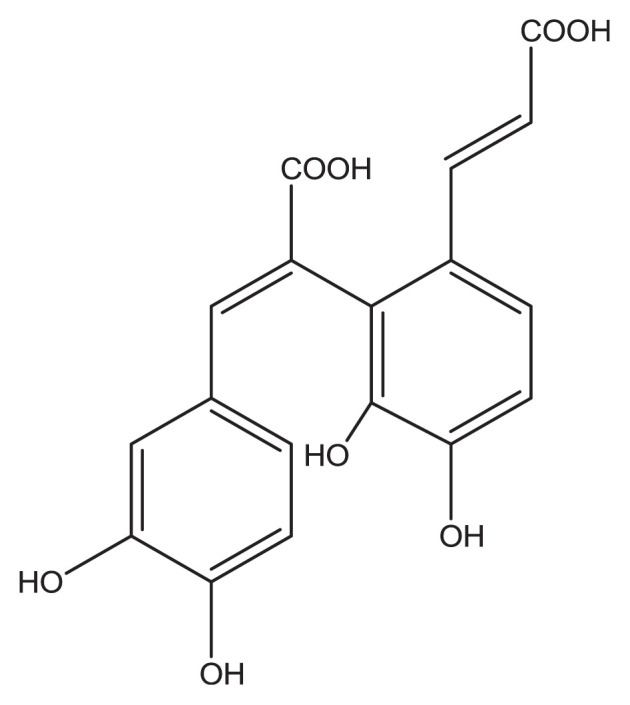
Structural formula of bis-caffeic acid (SMND-309) [1]
3.2. Synergistic effect of caffeic acid
Synergistic studies are important in therapeutic strategies; synergism in the antioxidant activity of CA in the presence of other antioxidants such as cysteinyl thiol, histidine-containing dipeptides, bovine serum albumin (BSA) and chitosan (Ch) are summarized in Tables 2 and 3. In a recent study, the synergistic effect on the antioxidant activity of two caffeates, MC and MDC, in coexistence with cysteinyl thiol was reported [32]. Coexistence of monothiol adducts with MC and of mono- and dithiol adducts with MDC exhibited synergistic antioxidant effect, which was reported to be due to the electronic features of sulfur components [33]. Recently, the enhanced antioxidant activity of CA conjugated to histidine-containing dipeptides has been described; the synergism found was possibly due to the presence of a radical scavenging moiety, imidazole in histidine. However, the highest antioxidant action has been reported for CA-Pro-His-NH2, where the existence of proline facilitates histidine for stabilizing the hydroxyl groups of CA, resulting in the synergistic antioxidant effect in both aqueous and oily systems [34]. The antioxidant potential of CA has been described in emulsions (5 mmol/kg of emulsion) of 30% sunflower oil-in-water (O/W) and 20% water-in-sunflower oil (W/O) at pH 5.4 during storage at 50°C [35]. BSA is known to possess a slight antioxidant activity (0.2%), however, a synergistically reduced rancidity by the CA-BSA blend in both W/O and O/W emulsions has been reported; CA-BSA increases the stability of both O/W and W/O emulsions by 102.9% and 50.4%, respectively; therefore, it acts more efficiently in W/O emulsions [35,36].
Table 2.
In vitro studies on synergistic effect of caffeic acid.
| Synergistic agent | Objective of study | Background of study | Antioxidant technique | Important results | Conclusion | Reference |
|---|---|---|---|---|---|---|
| Cysteinyl thiol | To study effect of cysteinyl thiol on the antioxidant activity of MC and MDC | MC and MDC can afford various thiol adducts with a recovered catechol structure. | Lipid peroxidation assay | The dependence of synergism upon the length of induction period. The mono-thiol adduct of MC and the mono-and di-thiol adducts of MDC contribute to the synergism in the antioxidant activity of both esters. | Synergistic effect of coexisting thiol on the antioxidant activity of MC and MDC | [32] |
| Histidine- containing dipeptides | To compare the antioxidant activity of histidine and CA- histidine conjugate with that of CA-β-Ala-His-NH2 | Histidine naturally exists at high concentrations in mammalian muscle tissue and nervous system. | 2,2-Diphenyl-1- picrylhydrazyl radical scavenging assay, Lipid peroxidation assay | The change in the antioxidative activity of CA by changing the position of histidine in CA-histidine conjugate. | The augmented antioxidative effect of conjugated histidine-containing dipeptides. | [34] |
| Bovine serum albumin (BSA) | To determine the influence of BSA on the total antioxidant activity of CA in model food emulsions | BSA is a minor whey protein (MW 66 kDa) with surfactant features employed for the stabilization of various food emulsions. | Total antioxidant activity | The synergistic increase in stability of the O/W and W/O emulsions containing BSA and CA by 102.9% and 50.4% respectively calculated as 2 × peroxide value + p-anisidine value, with greater synergy calculated if based on formation of headspace volatiles. | A synergistically reduced rate of development of rancidity and considerably reduced concentration of total volatiles, PV and PA for both emulsion types (O/W and W/O) by CA-BSA combination. | [35] |
MC = methyl caffeate; MDC = methyl dihydrocaffeate; O = oil; W = water.
Table 3.
In vitro antioxidant activity of chitosan (Ch)–caffeic acid (CA) grafts.
| Caffeic acid/ derivative | Grafting agent/ covalent connector | Cross-linker | Objective of study | Antioxidant technique | Important results | Conclusion | Reference |
|---|---|---|---|---|---|---|---|
| Ch-CA grafts | 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride | Not used | To investigate the antioxidant activities of different molecular weights (Mw) and grafting ratios of Ch-CA derivatives | DPPH radical scavenging assay | Maximum IC50 of radical-scavenging activity (0.064 mg/ mL) at the highest CA containing derivative. | Excellent antioxidant activity of Ch-CA grafts | [37] |
| CA grafted Ch/ CPTMS hybrid scaffolds | Potassium persulfate | CPTMS | To investigate the antioxidant activities of CA grafted Ch/CPTMS hybrid scaffolds | DPPH radical scavenging assay | Increase in the DPPH scavenging activity of CA with increase in CA contents in grafts. The reduction in the antioxidant activity of grafted CA by using potassium sulfate | Higher antioxidant property of biodegradable hybrid polymer (CA grafted Ch/CPTMS hybrid scaffolds) | [38] |
| Ch-CA-Ge films | Ammonium cerium (IV) nitrate | Ge | To graft CA to Ch for improved antioxidant activity | ABTS radical cation decolorization assay | The higher antioxidant activity of Ch-CA-Ge neutralized films than that of Ch-CA films and the Ch films. | Synergistic improvement in the antioxidant activity of Ch by making its graft with CA and then adding cross-linker, Ge. | [39] |
| CA-Ch films | Laccase | Not used | To produce synergistically active antioxidant CA-Ch films | ABTS radical cation decolorization Assay | The stronger inhibition of ABTS cation radicals by CA-Ch films as compared to the pure films of Ch. The highest radical scavenging effect of chitosan grafted to CA at pH 4.5 | The possibility and feasibility of the preparation of chitosan based novel multifunctional appliances by modifying chitosan functionality with laccase. | [40] |
ABTS = 2,2′-azinobis-(3-ethylbenzo-thiazoline-6-sulfonic acid); CPTMS = (3-chloro-propyl)tri-methoxy-silane; DPPH = 2,2-diphenyl-1-picrylhydrazyl; Ge = Genipin.
In a comparative study [37], Ch with various molecular weights was mixed with CA in different ratios to prepare Ch-CA graft. Subsequently, these grafts were subjected to DPPH radical-scavenging activity assay based on reducing ability of DPPH. Half inhibition concentrations of Ch-CA samples were < 1 mg/mL but those of low-, medium- and high-molecular-weight Ch were over 10 mg/mL and radical scavenging activity appeared to be entirely dependent on the CA concentration of the polymer [37]. Moreover, the grafting between CA and Ch occurs through bond development between amine group of Ch and carboxyl group of CA; therefore, radical-scavenging activities of CA and Ch-CA were identical in the same CA concentrations. Consequently, the antioxidant activity of CA and Ch-CA directly depends upon phenol group, because carboxyl group is already engaged and thus inactivated in Ch-CA [37].
CA grafting with CSC1.0 (a Ch-based hybrid scaffold) resulted in biodegradable hybrid polymers with a higher antioxidant potential than the CSC1.0 alone [38]. Antioxidant activity has also been described for Ch, Ch cross-linked with genipin (Ch-Ge), Ch grafted with CA (Ch-CA), and Ch cross-linked with Ge and grafted with CA (Ch-CA-Ge) films [39]. The Ch-CA-Ge neutralized films exhibited 40% higher antioxidant activity than the Ch-CA films and 80% higher than the films made up of Ch alone. The improvement in antioxidant activity of Ch films made by grafting to CA, the activity was further synergistically increased by adding cross-linking agent, Ge [39]. Recently, CA-Ch films were reported to strongly inhibit the ABTS cation radicals compared to the films made up of Ch alone [40]. This improved radical scavenging effect could be due to the reproduced oxidizable hydroxyl groups in the polymeric compounds as well as their branched structure and improved stability of radicals owing to the elevated electron delocalization. The branched structure of the polymeric compounds offered the steric obstruction to the moving free radicals making their access difficult to the active sites; Ch was grafted to CA at different pH (4.5, 5.5, and 6.5) was given codes accordingly Ch4.5, Ch5.5, and Ch6.5; Ch4.5 was shown to have the highest radical scavenging effect. In Ch5.5 and Ch6.5 graft, covalent bond formation occurred between CA and Ch resulting in steric hindrance that reduced the accessibility of CA [40]. In vitro studies performed using Ch and CA grafts are summarized in Table 3.
3.3. Antioxidant and prooxidant activity of caffeic acid
Antioxidant and prooxidant effects of CA and its derivatives have been reported through a combination of mechanisms: i.e. radical scavenging activity, inhibition of lipid peroxidation and shielding against LDL oxidation [41,42]. However, CAPE was described to inhibit autoxidation of pyrogallol more efficiently than the CA and efficiently scavenge O2•− in both nonenzymatic and enzymatic reactions [42]. To evaluate antioxidant and prooxidant potential of CA in a concentration-dependent bleaching of ABTS+•, ferulic acid (a derivative of CA) was designated to show better results for ABTS+• scavenging than CA; however, in hydroxyl radical scavenging, CA is described to act as antioxidant up to dose of 5μM; after that it behaves as prooxidant [43]. CA shows a change in oxidative properties under thermal conditions; thermally decomposed products of CA are reported to act as prooxidant [44]. Studies performed to establish the antioxidant and prooxidant activity of caffeic acid are summarized in Table 4.
Table 4.
Studies showing antioxidant and pro-oxidant activity of caffeic acid (CA).
| CA/derivative | Objective of study | Background of study | Antioxidant technique | Important results | Conclusion | Reference |
|---|---|---|---|---|---|---|
| CA | To explore the possible mechanisms involved in the antioxidant and prooxidant activities of CA | Concentration- dependent antioxidant or prooxidant effects of some dietary components | DPPH radical scavenging assay, Lipid peroxidation assay | CA exhibits: 1. ROS-scavenging after 2. 2,20-azobis- amidinopropane dihydrochloride- induced damage in splenic lymphocytes and the inhibition of lipid peroxidation in mouse liver microsomes. |
Concentration- dependent antioxidant and prooxidant effects of CA | [43] |
| Decomposition products of CA | To study the CA products obtained through thermal decomposition for possible antioxidant potential | Enhanced oxidative reactions due to the presence of prooxidant molecules | DPPH radical scavenging assay | On low heating at the start of CA decomposition, an enhanced prooxidant capacity due to the development of prooxidants. On higher heating, a reduction in the prooxidant components. | Prooxidant behavior of thermal decomposition products of CA | [44] |
DPPH = 2,2-diphenyl-1-picrylhydrazyl; ROS = reactive oxygen species.
3.4. In vivo antioxidant activity of caffeic acid against chemical-induced toxicity
Toxic metals such as nickel are environmental pollutants and potential threats for humans. In a study, performed on rat liver with induced toxicity by intraperitoneally administrated Ni for 20 days (20 mg/kg body weight), CA has been reported to reduce the oxidative stress caused by Ni toxicity [45]. However, literature reveals that not only CA but also its derivatives such as CAPE can be effective against carbon tetrachloride-, cisplatin-, and cadmium-induced toxicity of liver and kidney in various animals such as mice, rabbit, and rats [46–53]. In a recently published study, CA was found effective at a dose level 250 mg/kg body weight in rats previously treated with cisplatin, which causes oxidative stress and alters activity of many antioxidant enzymes present in the small intestine of rat [54]. Furthermore, in vivo studies performed using CA and CAPE found them to be effective against various chemically induced toxicities (Table 5).
Table 5.
In vivo antioxidant activity of caffeic acid against chemical induced toxicity.
| CA or its derivative | Toxic chemical(s) | Organ studied | Reference |
|---|---|---|---|
| CA | CCl4 and alcohol | Rodent liver | [3] |
| CA | Nickel | Rat liver | [45] |
| CAPE | Cisplatin | Rabbit liver | [47] |
| CAPE | CCl4 | Rat liver | [48] |
| CAPE | CCl4 | Rat kidney | [49] |
| CAPE | CCl4 | Mice liver | [50] |
| CAPE | Cadmium | Mice liver | [52] |
| CA | Cisplatin | Rat intestine | [54] |
CA = caffeic acid; CAPE = caffeic acid phenyl ester.
4. Conclusion
CA-Ch grafts exhibit synergism in their antioxidant effect. Newer grafting agents/covalent connectors and cross-linkers should be explored to exponentially increase the antioxidant action of grafts. The antioxidant activity of CA and its derivatives should also be investigated against various toxicities induced by other metals such as arsenic, chromium, and lead. Some antioxidants such as cysteinyl thiol, histidine-containing dipeptides, bovine serum albumin and chitosan have been described to synergistically improve the antioxidant activity of CA; similarly, the antioxidant activity of CA can also be synergistically enhanced by other antioxidants. The use of antioxidants can also show signs of concentration-dependent prooxidative effect, which may lead to oxidative damage of cells, considerable health implications should be designed and adopted where CA is being consumed. CA can be effectively employed as a natural antioxidant in various food products such as oils.
Footnotes
Conflict of interests
There is no conflict of interests among authors over the contents of this article.
REFERENCES
- 1. Marques V, Farah A. Chlorogenic acids and related compounds in medicinal plants and infusions. Food Chem. 2009;113:1370–6. [Google Scholar]
- 2. Chung TW, Moon SK, Chang YC, Ko JH, Lee YC, Cho G, Kim SH, Kim JG, Kim CH. Novel and therapeutic effect of caffeic acid and caffeic acid phenyl ester on hepatocarcinoma cells: complete regression of hepatoma growth and metastasis by dual mechanism. FASEB J. 2004;18:1670–81. doi: 10.1096/fj.04-2126com. [DOI] [PubMed] [Google Scholar]
- 3. Kashiwada Y, Nishizawa M, Yamagishi T, Tanaka T, Nonaka G, Cosentino LM, Snider JV, Lee K. Anti-AIDS agents, 18. Sodium and potassium salts of caffeic acid tetramers from Arnebia euchroma as anti-HIV agents. J Nat Prod. 1995;58:392–400. doi: 10.1021/np50117a007. [DOI] [PubMed] [Google Scholar]
- 4. Nardini M, D’Aquino M, Tomassi G, Gentili V, Di Felice M, Scaccini C. Inhibition of human low-density lipoprotein oxidation by caffeic acid and other hydroxycinnamic acid derivatives. Free Radical Biol Med. 1995;19:541–52. doi: 10.1016/0891-5849(95)00052-y. [DOI] [PubMed] [Google Scholar]
- 5. Laranjinha JA, Almeida LM, Madeira VM. Reactivity of dietary phenolic acids with peroxyl radicals: antioxidant activity upon low density lipoprotein peroxidation. Biochem Pharmacol. 1994;48:487–94. doi: 10.1016/0006-2952(94)90278-x. [DOI] [PubMed] [Google Scholar]
- 6. Fesen MR, Pommier Y, Leteurtre F, Hiroguchi S, Yung J, Kohn KW. Inhibition of HIV-1 integrase by flavones, caffeic acid phenethyl ester (CAPE) and related compounds. Biochem Pharmacol. 1994;48:595–608. doi: 10.1016/0006-2952(94)90291-7. [DOI] [PubMed] [Google Scholar]
- 7. Wang F, Yang J. A comparative study of caffeic acid and a novel caffeic acid conjugate SMND-309 on antioxidant properties in vitro. LWT Food Sci Technol. 2012;46:239–44. [Google Scholar]
- 8. Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 9. Janbaz KH, Saeed SA, Gilani AH. Studies on the protective effects of caffeic acid and quercetin on chemical-induced hepatotoxicity in rodents. Phytomed. 2004;11:424–30. doi: 10.1016/j.phymed.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 10. Meyer AS, Donovan JL, Pearson DA, Waterhouse AL, Frankel EN. Fruit hydroxycinnamic acids inhibit human low-density lipoprotein oxidation in vitro. J Agr Food Chem. 1998;46:1783–7. [Google Scholar]
- 11. Aladedunye F, Catel Y, Przybylski R. Novel caffeic acid amide antioxidants: Synthesis, radical scavenging activity and performance under storage and frying conditions. Food Chem. 2012;130:945–52. doi: 10.1021/jf102287h. [DOI] [PubMed] [Google Scholar]
- 12. Feng Y, Lu YW, Xu PH, Long Y, Wu WM, Li W, Wang R. Caffeic acid phenethyl ester and its related compounds limit the functional alterations of the isolated mouse brain and liver mitochondria submitted to in vitro anoxia–reoxygenation: relationship to their antioxidant activities. Biochim Biophys Acta. 2008;1780:659–72. doi: 10.1016/j.bbagen.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 13. Wang X, Stavchansky S, Kerwin SM, Bowman PD. Structure–activity relationships in the cytoprotective effect of caffeic acid phenethyl ester (CAPE) and fluorinated derivatives: effects on heme oxygenase-1 induction and antioxidant activities. Eur J Pharmacol. 2010;635:16–22. doi: 10.1016/j.ejphar.2010.02.034. [DOI] [PubMed] [Google Scholar]
- 14. Gülçin I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid) Toxicol. 2006;217:213–20. doi: 10.1016/j.tox.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 15. Siddharth J, Sharma MP. Modeling and analysis of oxidation and thermal stability of biodiesel. Int J Energy Sci. 2011;1:93–8. [Google Scholar]
- 16. Sun WD, Thakorlal J, Zhou J. Effects of added phenolics on the storage stability of avocado and coconut oils. Int J Food SciTechnol. 2011;46:1575–85. [Google Scholar]
- 17. Damasceno SS, Santos NA, Santos IMG, Souza AL, Souza AG, Queiroz N. Caffeic and ferulic acids: an investigation of the effect of antioxidants on the stability of soybean biodiesel during storage. Fuel. 2013;107:641–6. [Google Scholar]
- 18. Kowalski R. Changes of linoleic acid concentration during heating of some plant-origin oils with polyphenol addition. J Food Quality. 2010;33:269–82. [Google Scholar]
- 19. Marinova EM, Toneva A, Yanishlieva N. Comparison of the antioxidative properties of caffeic and chlorogenic acids. Food Chem. 2009;114:1498–502. [Google Scholar]
- 20. Réblová Z. Effect of temperature on the antioxidant activity of phenolic acids. Czech J Food Sci. 2012;30:171–7. [Google Scholar]
- 21. Luo M, Zhang RY, Zheng Z, Wang JL, Ji JB. Impact of some natural derivatives on the oxidative stability of soybean oil based biodesel. J Brazil Chem Soc. 2012;23:241–6. [Google Scholar]
- 22. Prasad NR, Jeyanthimala K, Ramachandran S. Caffeic acid modulates ultraviolet radiation-B induced oxidative damage in human blood lymphocytes. J Photochem Photobiol B. 2009;95:196–203. doi: 10.1016/j.jphotobiol.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 23. Saija A, Tomaino A, Trombetta D, Giacchi M, De Pasquale A, Bonina F. Influence of different penetration enhancers on in vitro skin permeation and in vivo photoprotective effect of flavonoids. Int J Pharm. 1998;175:85–94. [Google Scholar]
- 24. Saija A, Tomaino A, Trombetta D, De Pasquale A, Uccella N, Barbuzzi T, Paolino D, Bonina F. In vitro and in vivo evaluation of caffeic and ferulic acids as topical photoprotective agents. Int J Pharm. 2000;199:39–47. doi: 10.1016/s0378-5173(00)00358-6. [DOI] [PubMed] [Google Scholar]
- 25. Pamela M. The effects of stress and aging on glutathione metabolism. Ageing Res Rev. 2005;4:288–314. doi: 10.1016/j.arr.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 26. Devipriya N, Sudheer AR, Menon VP. Caffeic acid protects human peripheral blood lymphocytes against gamma radiation-induced cellular damage. J Biochem Mol Toxicol. 2008;22:175–86. doi: 10.1002/jbt.20228. [DOI] [PubMed] [Google Scholar]
- 27. Hung CY, Yen GC. Antioxidant activity of phenolic compounds isolated from Mesona procumbens Hemsl. J Agr Food Chem. 2002;50:2993–7. doi: 10.1021/jf011454y. [DOI] [PubMed] [Google Scholar]
- 28. Lee KJ, Choi YP, Hwang YP, Chung YC, Jeong HG. Protective effect of caffeic acid phenyl ester on tert-butyl hydroperoxide induced oxidative hepatotoxicity and DNA damage. J Food Chem Toxicol. 2008;46:2445–50. doi: 10.1016/j.fct.2008.03.032. [DOI] [PubMed] [Google Scholar]
- 29. Sato Y, Itagaki S, Kurokawa T, Ogura J, Kobayashi M, Hirano T, Sugawara M, Iseki K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int J Pharm. 2011;403:136–8. doi: 10.1016/j.ijpharm.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 30. Guneli E, Cavdar Z, Islekel H, Sarioglu S, Erbayraktar S, Kiray M, Sokmen S, Yilmaz O, Gokmen N. Erythropoietin protects the intestine against ischemia/ reperfusion injury in rats. Mol Med. 2007;13:509–17. doi: 10.2119/2007-00032.Guneli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guven A, Tunc T, Topal T, Kul M, Korkmaz A, Gundogdu G, Onguru O, Ozturk H. Alpha-lipoic acid and ebselen prevent ischemia/reperfusion injury in the rat intestine. Surg Today. 2008;38:1029–35. doi: 10.1007/s00595-007-3752-9. [DOI] [PubMed] [Google Scholar]
- 32. Fujimoto A, Inai M, Masuda T. Chemical evidence for the synergistic effect of a cysteinyl thiol on the antioxidant activity of caffeic and dihydrocaffeic esters. Food Chem. 2013;138:1483–92. doi: 10.1016/j.foodchem.2012.11.073. [DOI] [PubMed] [Google Scholar]
- 33. Masuda T, Yamada K, Akiyama J, Someya T, Odaka Y, Takeda Y, Tori M, Nakashima K, Maekawa T, Sone Y. Antioxidation mechanism studies of caffeic acid: identification of antioxidation products of methyl caffeate from lipid oxidation. J Agr Food Chem. 2008;56:5947–52. doi: 10.1021/jf800781b. [DOI] [PubMed] [Google Scholar]
- 34. Seo HS, Kwak SY, Lee YS. Antioxidative activities of histidine containing caffeic acid-dipeptides. Bioorg Med Chem Lett. 2010;20:4266–72. doi: 10.1016/j.bmcl.2010.04.135. [DOI] [PubMed] [Google Scholar]
- 35. Conde E, Gordon MH, Moure A, Dominguez H. Effects of caffeic acid and bovine serum albumin in reducing the rate of development of rancidity in oil-in-water and water-in-oil emulsions. Food Chem. 2011;129:1652–9. [Google Scholar]
- 36. Almajano MP, Gordon M. Synergistic effect of BSA on antioxidant activities in model food emulsions. J Am Oil Chem Soc. 2004;81:275–80. [Google Scholar]
- 37. Aytekin AO, Morimura S, Kida K. Synthesis of chitosan-caffeic acid derivatives and evaluation of their antioxidant activities. J Biosci Bioeng. 2011;111:212–6. doi: 10.1016/j.jbiosc.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 38. Shiu JC, Ho MH, Yu SH, Chao AC, Su YR, Chen WJ, Chiang ZC, Yang WP. Preparation and characterization of caffeic acid grafted chitosan/CPTMS hybrid scaffolds. Carbohydrate Polymers. 2010;79:724–30. [Google Scholar]
- 39. Nunes C, Maricato É, Cunha Â, Nunes A, da Silva JAL, Coimbra MA. Chitosan–caffeic acid–genipin films presenting enhanced antioxidant activity and stability in acidic media. Carbohydrate Polymers. 2013;91:236–43. doi: 10.1016/j.carbpol.2012.08.033. [DOI] [PubMed] [Google Scholar]
- 40. Boźič M, Gorgieva S, Kokol V. Laccase-mediated functionalization of chitosan by caffeic and gallic acids for modulating antioxidant and antimicrobial properties. Carbohydrate Polymers. 2012;87:2388–98. doi: 10.1016/j.carbpol.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 41. Cheng JC, Dai F, Zhou B, Yang L, Liu ZL. Antioxidant activity of hydroxycinnamic acid derivatives in human low density lipoprotein: mechanism and structure–activity relationship. Food Chem. 2007;104:132–9. [Google Scholar]
- 42. Wu WM, Lu L, Long Y, Wang T, Liu L, Chen Q, Wang R. Free radical scavenging and antioxidative activities of caffeic acid phenethyl ester (CAPE) and its related compounds in solution and membranes: a structure–activity insight. Food Chem. 2007;105:107–15. [Google Scholar]
- 43. Maurya DK, Devasagayam TPA. Antioxidant and prooxidant nature of hydroxycinnamic acid derivatives ferulic and caffeic acids. Food Chem Toxicol. 2010;48:3369–73. doi: 10.1016/j.fct.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 44. Andueza S, Manzocco L, Paz de Peña M, Cid C, Nicoli C. Caffeic acid decomposition products: antioxidants or pro-oxidants? Food Res Int. 2009;42:51–5. [Google Scholar]
- 45. Pari L, Prasath A. Efficacy of caffeic acid in preventing nickel induced oxidative damage in liver of rats. Chem Biol Interact. 2008;173:77–83. doi: 10.1016/j.cbi.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 46. Russo A, Longo R, Vanella A. Antioxidant activity of propolis: role of caffeic acid phenethyl ester and galangin. Fitoterapia. 2002;73(Suppl 1):S21–9. doi: 10.1016/s0367-326x(02)00187-9. [DOI] [PubMed] [Google Scholar]
- 47. Kart A, Cigremis Y, Karaman M, Ozen H. Caffeic acid phenethyl ester (CAPE) ameliorates cisplatin-induced hepatotoxicity in rabbit. Exp Toxicol Pathol. 2010;62:45–52. doi: 10.1016/j.etp.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 48. Kus I, Colakoglu N, Pekmez H, Seckin D, Ogeturk M, Sarsilmaz M. Protective effects of caffeic acid phenethyl ester (CAPE) on carbon tetrachloride-induced hepatotoxicity in rats. Acta Histochemica. 2004;106:289–97. doi: 10.1016/j.acthis.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 49. Ogeturk M, Kus I, Colakoglu N, Zararsiz I, Ilhan N, Sarsilmaz M. Caffeic acid phenethyl ester protects kidneys against carbon tetrachloride toxicity in rats. J Ethnopharmacol. 2005;97:273–80. doi: 10.1016/j.jep.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 50. Lee KJ, Choi JH, Khanal T, Hwang YP, Chung YC, Jeong HG. Protective effect of caffeic acid phenethyl ester against carbon tetrachloride-induced hepatotoxicity in mice. Toxicology. 2008;248:18–24. doi: 10.1016/j.tox.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 51. Iraz M, Ozerol E, Gulec M, Tasdemir S, Idiz N, Fadillioglu E, Naziroglu M, Akyol O. Protective effect of caffeic acid phenethyl ester (CAPE) administration on cisplatin-induced oxidative damage to liver in rat. Cell Biochem Funct. 2006;24:357–61. doi: 10.1002/cbf.1232. [DOI] [PubMed] [Google Scholar]
- 52. Chen F, Gong P. Caffeic Acid Phenethyl Ester Protect Mice Hepatic Damage Against Cadmium Exposure. Procedia Environ Sci. 2011;8:633–6. [Google Scholar]
- 53. Ali BH, Al Moundhri MS. Agents ameliorating or augmenting the nephrotoxicity of cisplatin and other platinum compounds: a review of some recent research. Food Chem Toxicol. 2006;44:1173–83. doi: 10.1016/j.fct.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 54. Arivarasu NA, Shubha P, Riaz M. Oral administration of caffeic acid ameliorates the effect of cisplatin on brush border membrane enzymes and antioxidant system in rat intestine. Exp Toxicol Pathol. 2013;65:21–5. doi: 10.1016/j.etp.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 55. Chen JH, Ho CT. Antioxidant activities of caffeic acid and its related hydroxycinnamic acid compounds. J Agr Food Chem. 1997;45:2374–8. [Google Scholar]
- 56. Milić BL, Djilas SM, Čanadanović-Brunet JM. Antioxidative activity of phenolic compounds on the metal-ion breakdown of lipid peroxidation system. Food Chem. 1998;61:443–7. [Google Scholar]
- 57. Sánchez-Alonso I, Careche M, Moreno P, González MJ, Medina I. Testing caffeic acid as a natural antioxidant in functional fish-fibre restructured products. LWT Food Sci Technol. 2011;44:1149–55. [Google Scholar]
- 58. Medina I, Gallardo JM, González MJ, Lois S, Hedges N. Effect of molecular structure of phenolic families as hydroxycinnamic acids and catechins on their antioxidant effectiveness in minced fish muscle. J Agr Food Chem. 2007;55:3889–95. doi: 10.1021/jf063498i. [DOI] [PubMed] [Google Scholar]


