Abstract
Actinidia arguta (Siebold et Zucc.) Planch. ex. Miq. is one of the most recently domesticated fruit species with increasing commercial production worldwide. It is a well-known traditional Chinese medicine and is used to reduce blood glucose and treat atopic dermatitis. In addition, it possesses antioxidant, anticancer, and antiallergic properties. In this study, we investigated the physical antifatigue and exercise performance effects of A. arguta crude alkaloids (AACA) extracted with 70% ethanol. Four groups of male Kunming mice (n = 16) were orally administered AACA at doses of 0 mg/kg/d (vehicle), 50 mg/kg/d (AACA-50), 100 mg/kg/d (AACA-100), or 200 mg/kg/d (AACA-200) for 28 days. The effect of AACA treatment on exercise performance was studied using the forelimb grip strength experiment and by the measurement of the weight-loaded swimming time. The antifatigue effect is evaluated based on fatigue-associated biochemical parameters, hepatic and muscular glycogen levels, and changes in the morphology of transverse and longitudinal sections of skeletal muscle. The results showed that AACA could elevate the endurance and grip strength in mice. The exhaustive swimming time of the AACA-50, AACA-100, and AACA-200 groups was significantly (p < 0.05) increased compared with the vehicle. The swimming time of the AACA-100 group was the longest among all groups studied. Mice in the AACA-treated groups had decreased levels of lactate, ammonia, and creatine kinase after a physical challenge compared with the vehicle group. The tissue glycogen, an important energy source during exercise, significantly increased with AACA. The morphology of transverse and longitudinal sections of skeletal muscle did not change in the vehicle group. Overall, these findings suggest that AACA possesses antifatigue effects and increases exercise performance in mice. Therefore, A. arguta may be developed as an antifatigue dietary supplement in the category of functional foods.
Keywords: Actinidia arguta crude alkaloids, antifatigue, exercise performance, mice
1. Introduction
Actinidia arguta (Siebold et Zucc.) Planch. ex. Miq., known as kiwiberry or baby kiwifruit in English, is a perennial, fast growing, and deciduous vine. It is one of the most recently domesticated fruit species with increasing commercial production worldwide [1,2]. A. arguta is a well-known traditional Chinese medicine and was recorded in the work Compendium of Materia Medica (Bencao Gangmu) by Li Shi-Zhen about 500 years ago [3]. Several studies have demonstrated the extensive bioactivities of A. arguta extracts, including its ability to reduce blood glucose [4], treat atopic dermatitis [5], and its antioxidant [6], anticancer, and antiallergic [7–9] properties.
Fatigue is defined as physical and/or mental weariness resulting in negative effects on exercise intensity, work performance, family life, and social relationships [10]. At least two mechanisms can explain the occurrence of physical fatigue: oxidative stress and energy exhaustion [11]. Exhaustive or intensive exercise can lead to the accumulation of excess reactive free radicals, resulting in tissue damage. Exhaustion theory suggests that energy source depletion and excess metabolite accumulation can lead to fatigue [12,13]. Because the available therapies for fatigue in modern medicine are very limited, potential alternatives from traditional medicine and their respective mechanisms of action are worth investigating [14]. In the past few decades, numerous studies have demonstrated that extracts from herbal medicines and foods are important resources for postponing fatigue, accelerating the elimination of fatigue-related metabolites, and improving exercise performance [15,16].
Plants produce a wide variety of secondary metabolites and >22,000 nitrogen-containing secondary metabolites have been described to date in plants [17]. Modern pharmacological studies have reported that alkaloids are the major active ingredients in Chinese herbal medicine and its derived products [18]. Alkaloids in herbal medicinal plants possess anti-inflammatory [19] and antibacterial properties [20]. However, little attention has been devoted to evaluate the function of alkaloids extracted from A. arguta.
Thus, the objective of this research was to evaluate the antifatigue activity of alkaloids extracted from A. arguta using a forced swimming test in mice. However, more clinical studies are needed to confirm the medicinal effects in light of rational bioactivity function, and further in-depth studies are needed to examine the possible mechanism. Our results suggest the use of A. arguta as an antifatigue dietary supplement, and we hope the results of this study will accelerate the use of A. arguta as an important functional food.
2. Materials and methods
2.1. Materials and reagents
Fresh “Changjiang No. 1 A. arguta fruits were purchased from the College of Food Science, Shenyang Agricultural University, Shenyang. China. “Changjiang No. 1 is a superior variety, which was bred at the Northeast wild A. arguta research base of Shenyang Agricultural University and recorded by the Seed Administration Bureau of Liaoning Province in 2011. Fruits were harvested toward the end of August, at the mature commercial harvest stage. The characteristics of the fruits at harvest were 14–16 N of firmness, and 7–8°Bx of soluble solids content. A total of 64 male Kunming mice [specific-pathogen-free (SPF) grade, weight 27 ± 2 g] were purchased from the Liaoning Longevity Biotechnology Co., Ltd (Shenyang, China). Mouse diet (SPF grade) was purchased from the Animal Experiment Center at China Medical University (Shenyang, China). All animals received humane care in compliance with the Liaoning Province Guidance on Experimental Animal Care. The animals were raised in the SPF barrier system in the Animal Experiment Center of Shenyang Agricultural University. All experimental protocols involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of Shenyang Agricultural University. The animals were acclimated to the environment and diet for 1 week prior to the experiments. During the experimental period, four mice were housed/cage with free access to diet and water. Lactic acid, ammonia, glucose, and serum creatine kinase (CK) kits were purchased from Jian Cheng Biotechnology Research Institute (Nanjing, China).
2.2. Instruments and equipment
The following instruments were used in this study: TSE measurement system (TSE Systems GmbH, Bad Homburg, Germany); Synergy HT multifunctional enzyme mark instrument (Biotek Instruments, Winooski, VT, USA); high-speed refrigerated centrifuge 5805 (Eppendorf, Hamburg, Germany); Hitachi automatic biochemical analyzer 7060 (Hitachi, Tokyo, Japan); Hitachi automatic biochemical analyzer 7080 (Hitachi); and Olympus BX51 (Olympus, Japan).
2.3. Experimental design
2.3.1. Preparation of ethanol extracts of A. arguta crude alkaloids and mice feeding
Prior to the extraction of alkaloids from fruits, the peduncles were removed and dried on a lyophilizer. The dehydrated fruits were ground into powder. The dried powder was extracted three times with 70% ethanol. After filtration, the extracted ethanol was evaporated to dryness on a rotary evaporator at 60°C under reduced pressure to produce ethanol crude extract. The crude extract was dissolved in 20 mL of 2% HCl and then filtered to remove solid residues. The extraction was performed two times with the same volume of chloroform, and then the pH of the aqueous solution was adjusted to 10 by adding ammonium hydroxide. The aqueous layer was extracted three times using the same volume of chloroform during each extraction. The extracts were combined and evaporated by rotary evaporation. Ethanol extracts of A. arguta crude alkaloids (AACA) were then purified on a macroporous adsorption resin (D-101). The average purity of alkaloids was 77.6% [21].
The purity of alkaloids (%) is measured as follows: C × V/m0 × 100%, where C = concentration of alkaloid in the eluent (mg/mL); m0 = total quality of postdrying alkaloids (mg); and V = volume of eluent (mL).
The animals were raised in an SPF barrier system in the Animal Experiment Center of Shenyang Agricultural University. One week prior to the experiments, the animals were allowed to acclimatize to the environment and diet. All animals were provided with a standard laboratory diet (No. 5001; PMI Nutrition International, Brentwood, MO, USA) and distilled water. The temperature was controlled at 20–26°C with a humidity of 40–70% and a 12/12-hour day–night cycle. The animals were randomly assigned to the following four groups (16 mice/group) based on the constituents administered orally at 10 am for 28 days [0.1 mL/10 g body weight (BW)]: vehicle group, which received only water; AACA-50 group, which received water containing 50 mg/kg BW/d, AACA-100 group, which received water containing 100 mg/kg BW/d; and AACA-200 group, which received water containing 200 mg/kg BW/d. Both the vehicle and AACA groups were administered by gavage feeding [15].
2.3.2. Forelimb grip strength
To measure the forelimb grip strength, a low-force testing system was used 1 hour after the last feeding. The amount of tensile force was measured by a force transducer equipped with a metal bar (2 mm in diameter and 7.5 cm in length) for each mouse in the different groups. The mice were trained to be familiar with this procedure for 3 days before the test. No significant difference in forelimb grip strength was observed among the four groups prior to AACA administration. The maximal force recorded by the low-force system was used as the grip strength [16].
2.3.3. Swimming exercise performance test
The forced swimming test was carried out 1 hour after the last administration, as described previously but with some modifications [22]. Eight mice were used from each group for the swimming exercise, with loads of lead fish sinkers attached to the tail, equal to 5% of their BW. The swimming exercise was carried out in an acrylic plastic pool (50 cm × 50 cm × 40 cm) that was 30 cm deep with water maintained at 25 ± 1°C. Exhaustion was determined by observing loss of coordinated movements and failure to return to the surface within 7 seconds, and the swimming time was recorded immediately [23]. Times floating, struggling, and making necessary movements were considered in the swimming duration until exhaustion and possible drowning.
2.3.4. Determination of fatigue-associated biochemical parameters
The effects of AACA on serum lactate, ammonia, and glucose levels and on CK activity were evaluated after the exercise. One hour after oral administration of AACA, a 15-minute swimming exercise was performed without weight loading. Blood samples were immediately collected after the exercise. The samples were centrifuged at 1500g, 4°C for 10 minutes to separate the serum. Serum lactate, ammonia, and glucose levels and CK activity were determined using Hitachi automatic biochemical analyzer 7060 (Hitachi).
2.3.5. Determination of clinical biochemical parameters
Clinical biochemical parameters, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, lactate dehydrogenase (LDH), albumin, total bilirubin (TBIL), total protein (TP), blood urea nitrogen (BUN), creatinine, uric acid (UA), total cholesterol, and triacylglycerol, were determined using the Hitachi automatic biochemical analyzer 7080 (Hitachi).
2.3.6. Determination of tissue glycogen and visceral organ weight
Liver and muscle tissues were excised and weighed for glycogen content analysis. The weights of related visceral organs were recorded.
2.3.7. Histology staining of tissues
The animals from both vehicle and AACA treatment groups were killed and their skeletal muscle tissues were collected and fixed in 10% buffered formalin, and then embedded in paraffin. They were cut transversely and longitudinally to obtain skeletal muscle cross sections. Paraffin-embedded samples were cut into 4-μm thick slices for morphological and pathological evaluations. Tissue sections were stained with hematoxylin and eosin.
2.4. Statistical analysis
The data were processed using SPSS 17.0 (IBM, Armonk, NY, USA) and expressed as mean ± standard error of the mean. Means between the groups were compared using a single-factor analysis of variance (one-way analysis of variance). The difference was considered significant for all p values < 0.05.
3. Results and discussion
3.1. Effect of BW, skeletal muscle mass, and weights of some metabolism-related organs
BW and metabolism-related organs have a certain relationship with athletic ability. In general, animals with lighter BW have low basal metabolism and small sports load. The vehicle and AACA supplementation groups did not differ in behavior during treatment. The morphological data before and after 28 days of administration of various amounts of AACA are summarized in Table 1. There was no significant difference in initial BWs among groups. The food and water intakes of mice in the AACA-treated groups were slightly greater compared with the vehicle group. In addition, the weight of the liver, muscle, kidney, and lung dose-dependently increased with AACA administrations. The relative tissue weight (%) is a measure of different tissue weights adjusted for individual BW. There were no significant differences in the relative tissue weights of the liver, skeletal muscle, kidney, and lung among the AACA groups compared with the vehicle group, although there were a few exceptions (Table 1). These data indicate that the AACA administrations did not affect the BW and metabolism-related organ weight in mice after 28 days of feeding.
Table 1.
General characteristics of the experimental mice.
| Variable | Vehicle | AACA-50 | AACA-100 | AACA-200 |
|---|---|---|---|---|
| Initial body weight (BW; g) | 27.31 ± 0.40 a | 27.62 ± 0.61 a | 27.91 ± 0.50 a | 27.54 ± 0.52 a |
| Final BW (g) | 34.60 ± 0.12 b | 35.12 ± 0.19 a,b | 36.61 ± 0.91 a | 35.91 ± 0.50 a |
| Food intake (g/d) | 6.22 ± 0.46 b | 6.49 ± 0.29 a,b | 6.87 ± 0.24 a | 6.31 ± 0.38 a,b |
| Water intake (g/d) | 7.80 ± 0.18 c | 8.03 ± 0.21 b,c | 8.48 ± 0.39 a,b | 8.64 ± 0.18 a |
| Liver (g) | 1.92 ± 0.01 c | 1.94 ± 0.02 b,c | 1.95 ± 0.02 a,b | 1.97 ± 0.02 a |
| Muscle (g) | 0.31 ± 0.01 b | 0.33 ± 0.01 b | 0.36 ± 0.01 a | 0.35 ± 0.01 a |
| Kidney (g) | 0.42 ± 0.01 a | 0.44 ± 0.01 a | 0.45 ± 0.01 a | 0.46 ± 0.01 a |
| Lung (g) | 0.31 ± 0.01 b | 0.32 ± 0.01 b | 0.34 ± 0.01 a | 0.36 ± 0.01 b |
| Relative liver weight (%) | 5.55 ± 0.02 a | 5.53 ± 0.09 a | 5.33 ± 0.02 b | 5.47 ± 0.04 a |
| Relative muscle weight (%) | 0.90 ± 0.03 c | 0.92 ± 0.06 b,c | 0.98 ± 0.01 a | 0.97 ± 0.02 a,b |
| Relative kidney weight (%) | 1.22 ± 0.03 a | 1.24 ± 0.09 a | 1.22 ± 0.01 a | 1.27 ± 0.03 a |
| Relative lung weight (%) | 0.92 ± 0.03 b | 0.93 ± 0.05 b | 0.91 ± 0.01 a | 1.01 ± 0.03 a |
Values are presented as mean ± standard error of the mean.
Values in the same row with different superscript letters (a, b, c) differ significantly (p < 0.05) by one-way analysis of variance.
AACA = Actinidia arguta crude alkaloids.
3.2. Effect of forelimb grip strength
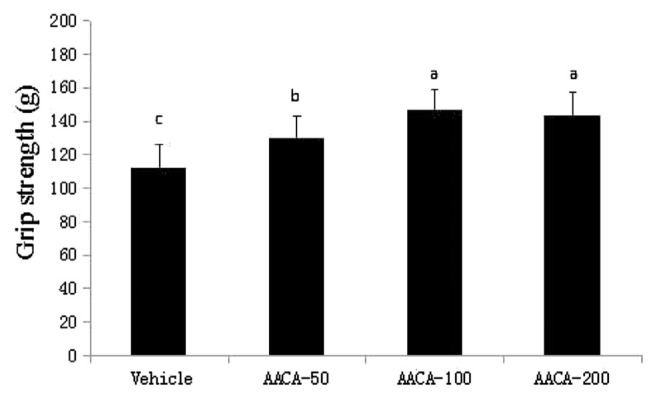
As shown in Figure 1, the grip strength of the vehicle, AACA-50, AACA-100, and AACA-200 groups was 112.3 g, 130.5 g, 146.4 g, and 143.2 g, respectively. Compared with the vehicle, these increased by 16.21%, 30.37%, and 27.52%, respectively. The results indicated that AACA can improve the forelimb grip strength of mice. Among all the study groups, the forelimb grip strength of the AACA-100 group was the highest. However, the differences between the AACA-100 and AACA-200 groups were not statistically significant (p < 0.05).
Figure 1.
Effect of administrations of A.arguta crude alkaloids (AACA) extracts on forelimb grip strength. The forelimb grip strength was measured after the mice were fed with water (vehicle group) and 50–200 mg/kg/d A. arguta crude alkaloids (AACA-treated groups) for 28 days. Data are presented as mean ± standard error of the mean of eight mice. Values of histogram labeled with different letters (a, b, c) are significantly different (p < 0.05).
3.3. Effect of exercise performance on weight-loaded swimming exercise
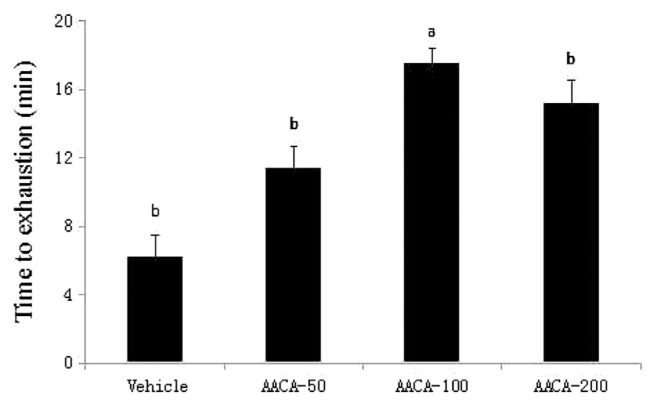
Energy metabolism during muscular activity determines the level of physiological fatigue. The main performances of fatigue are reductions on the maximum output power of the motion energy system and muscle strength [24,25]. Exercise endurance is an important variable in evaluating delayed fatigue treatment. Exercise endurance of the mice after 28 days of AACA administrations was determined using a swimming test. As shown in Figure 2, the swimming test times were 6.2 minutes, 11.4 minutes, 17.5 minutes, and 15.2 minutes in the vehicle, AACA-50, AACA-100, and AACA-200 groups, respectively. The swimming times of the AACA-50, AACA-100, and AACA-200 groups increased by 83.87%, 182.25%, and 145.16% compared with the vehicle. The swimming time of the AACA-100 group was the longest among all the groups studied. However, no obvious difference was found in swimming time between the AACA-50 and AACA-200 groups. These results indicated that AACA administrations can enhance the swimming time of mice and the best dose was 100 mg/kg/d, providing evidence that AACA possesses antifatigue activity.
Figure 2.
Effect of administrations of A.arguta crude alkaloids (AACA) extracts on swimming exercise performance. The endurance of mice was measured after 28 days of AACA administrations by an exhaustive swimming exercise with a load equivalent to 5% of the mouse’s body weight attached to its tail. Data are presented as mean ± standard error of the mean of eight mice. Values of histogram labeled with different letters (a, b) are significantly different (p < 0.05).
3.4. Effect of serum lactate, ammonia, glucose, and CK levels after acute exercise challenge
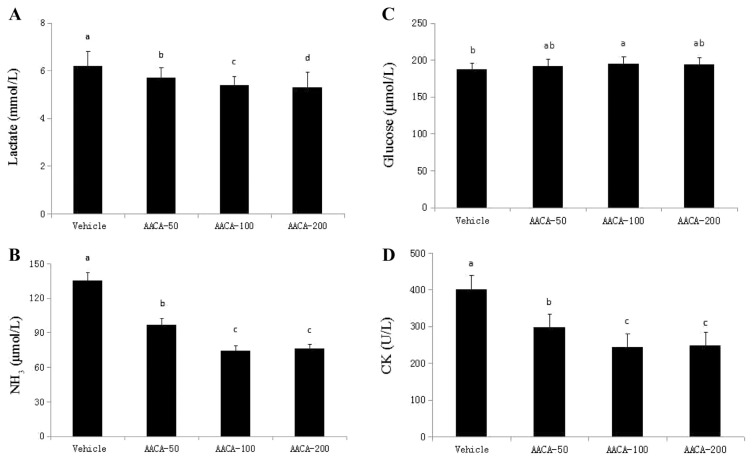
The serum lactate level is an important parameter in the fatigue system, and the normal value of lactate is approximately 2 mmol/L. As shown in Figure 3A, after a 15-minute swimming session, the lactate level of mice in all groups was above the normal value. The muscle produces a high quantity of lactate when it obtains enough energy from anaerobic glycolysis during high-intensity exercise. The serum lactate levels decreased significantly with the increasing dose of AACA compared with the vehicle by 8.1% (AACA-50), 12.9% (AACA-100), and 13.8% (AACA-200). Ammonia, a metabolite of proteins and amino acids, is released after a short period of severe exercise or maximal training. A higher level of ammonia leads to fatigue, and therefore, the serum ammonia level is closely related to fatigue and exercise performance. As shown in Figure 3B, the ammonia levels decreased significantly with AACA administrations. They were 97 μmol/L (AACA-50), 74 μmol/L (AACA-100), and 76 μmol/L (AACA-200) respectively, the AACA treated groups showed a reduction of 28.1–45.2% compared with 135 μmol/L for the vehicle. The energy supply for exercise initially came from the breakdown of glycogen and from circulating glucose released by the liver after intense exercise. Therefore, blood glucose levels are an important index for performance maintenance during exercise [26]. As shown in Figure 3C, the serum glucose levels were higher in the groups that received AACA administrations than in the vehicle. The glucose level of the AACA-100 group (197 μmol/L) was the highest among all studied groups, and it increased significantly compared with that of the vehicle (187 μmol/L). CK mainly exists in the cytoplasm and mitochondria of cells, and catalyzes the chemical reaction between phosphoinositide and high-energy phosphate bonds. High-energy phosphate bonds are the direct source of energy during muscle contraction. The CK activity in the vehicle, AACA-50, AACA-100, and AACA-200 groups was 402 U/L, 297 U/L, 243 U/L, and 247 U/L, respectively (Figure 3D). In comparison with the vehicle, the CK activity showed a significant decrease in the AACA-treated groups (CK activity reduced by 26.1%, 38.56%, and 39.6% in the AACA-50, AACA-100, and AACA-200 groups, respectively). Thus, AACA may ameliorate skeletal muscle injury induced by acute exercise challenge. The aforementioned results about four fatigue-associated biochemical indexes indicated that administrations of AACA can alleviate physical fatigue and improve exercise performance in mice.
Figure 3.
Effect of administrations of A.arguta crude alkaloids (AACA) extracts on serum (A) lactate, (B) ammonia, (C) glucose, and (D) creatine kinase (CK) levels after an acute exercise challenge. The measurements were taken following a 15-minute forced swimming test without weight loading after 28 days of AACA administrations. Data are presented as mean ± standard error of the mean of eight mice. Values of histogram labeled with different letters (a, b, c, d) are significantly different (p < 0.05). CK = creatine kinase.
3.5. Effect of biochemical parameters
In the normal state, physiological and biochemical indexes of the internal body parts (e.g., the organs) change before the occurrence of sensory fatigue in athletes, suggesting that when these indexes accumulate to a certain degree, the body could experience sensory fatigue [27]. Adaptogens are substances that enable the normalization of physiologic responses to various stressors, enhance work performance, and increase the stress tolerance of the body [28]. To reveal the benefits following AACA feeding, we measured a number of biochemical parameters separately. As shown in Table 2, the levels of LDH, TBIL, and UA decreased in the AACA-100 and AACA-200 groups compared with the vehicle. This result was in agreement with theories that LDH, TBIL, and UA levels would be increased when the body is at the fatigue state. In general, carbohydrates and lipids are important biochemical components that provide energy within 30 minutes of performing an exercise. By contrast, protein hardly provides energy and the levels of BUN show only a small change. When exercising for longer times, however, while the body energy is improved by carbohydrates and lipids, the proteins are decomposed. Simultaneously, the value of BUN would increase significantly and the tolerance ability would become worse. Eventually, the body feels tired. Compared with the vehicle, the levels of albumin and TP had a small increase in the AACA-100 and AACA-200 groups, and the level of BUN significantly decreased in the AACA-100 and AACA-200 groups. Based on these findings, the results showed that AACA can delay physical fatigue in mice.
Table 2.
Results of biochemical analysis of the AACA groups at the end of the experiment.
| Parameter | Vehicle | AACA-50 | AACA-100 | AACA-200 |
|---|---|---|---|---|
| Aspartate aminotransferase (U/L) | 78.21 ± 2.06 a | 68.09 ± 3.24 a | 52.50 ± 2.03 a | 57.41 ± 1.85 a |
| Alanine aminotransferase (U/L) | 52.22 ± 1.95 a | 47.71 ± 1.75 a,b | 43.81 ± 1.57 b | 42.20 ± 2.63 b |
| Alkaline phosphatase (U/L) | 118 ± 13 a | 126 ± 14 a | 135 ± 13 a | 130 ± 15 a |
| Lactate dehydrogenase (U/L) | 443 ± 32 b | 450 ± 25 b | 364 ± 22 a | 379 ± 29 a |
| Albumin (g/dL) | 2.71 ± 0.12 a | 2.71 ± 0.06 a | 2.80 ± 0.06 a | 2.81 ± 0.12 a |
| Total bilirubin (μg/dL) | 56.00 ± 5.67 a | 58.81 ± 3.36 a | 53.89 ± 2.71 a | 54.93 ± 3.21 a |
| Total protein (g/dL) | 4.90 ± 0.12 a | 4.90 ± 0.06 a | 5.01 ± 0.14 a | 5.00 ± 0.15 a |
| Blood urea nitrogen (mg/dL) | 25.31 ± 1.22 a | 24.61 ± 0.85 a | 21.62 ± 1.13 b | 22.71 ± 1.01 b |
| Creatinine (mg/dL) | 0.34 ± 0.01 a | 0.31 ± 0.01 b | 0.33 ± 0.01 a | 0.33 ± 0.02 a |
| Uric acid (mg/dL) | 1.70 ± 0.12 a | 1.41 ± 0.10 b | 1.52 ± 0.12 a,b | 1.41 ± 0.12 b |
| Total cholesterol (mg/dL) | 114 ± 4 a | 120 ± 6 a,b | 126 ± 6 b | 118 ± 5 a,b |
| Triacylglycerol (mg/dL) | 83.21 ± 5.89 a | 78.39 ± 5.37 a,b | 73.60 ± 3.64 a,b | 75.59 ± 5.98 b |
Values are mean ± standard error of the mean of eight mice.
Values in the same row with different superscripts letters (a, b) differ significantly (p < 0.05) by one-way analysis of variance.
AACA = Actinidia arguta crude alkaloids.
3.6. Effect of hepatic and muscular glycogen levels
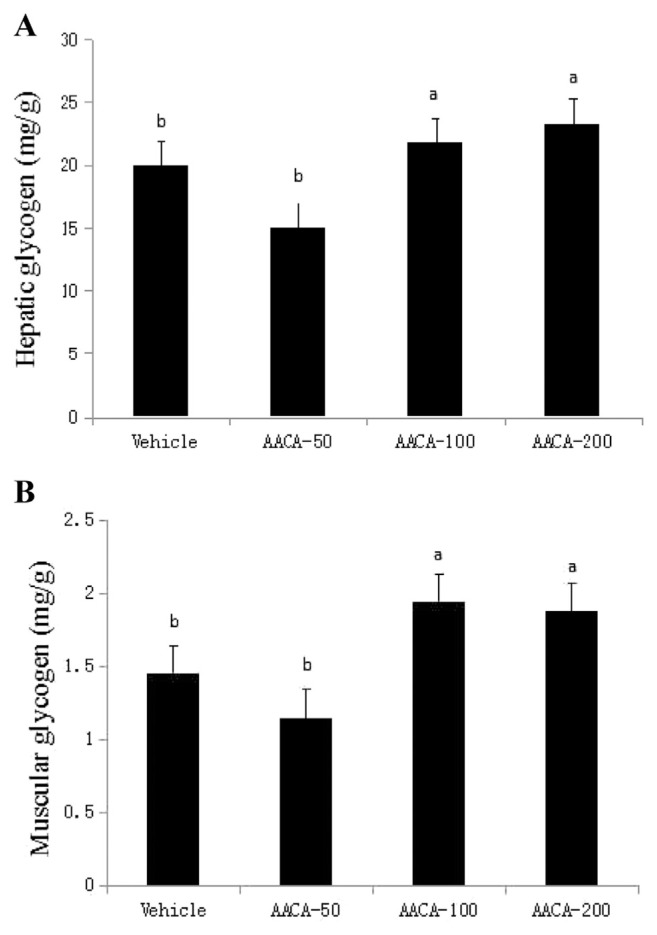
Being the main source of adenosine triphosphate, glycogen is the main energy source of the body. The body’s movement, endurance, and levels of glycogen are directly related; in most cases, depletion of glycogen is usually accompanied by sheer exhaustion. Muscle glycogen is the main energy source of the body when performing strenuous exercises for a long time. While performing exhaustive exercise, excessive consumption of muscle glycogen can trigger a lower glucose concentration in the blood, which can promote lactate accumulation in large amounts, as a result of which athletes experience fatigue. When the body energy consumption is large to such a degree that the glucose concentration begins to get low, liver glycogen, the important energy storage material, will be decomposed by glucagon to glucose. Hence, it drives the body’s glucose concentration to rise in order to balance the glucose concentration. As shown in Figure 4A, hepatic glycogen levels in the vehicle, AACA-50, AACA-100, and AACA-200 groups were 19.91 mg/g, 15.01 mg/g, 21.08 mg/g, and 23.28 mg/g, respectively. Glycogen content was significantly enhanced in the liver tissues of the AACA-100 and AACA-200 groups compared with the vehicle. Muscular glycogen levels in the vehicle, AACA-50, AACA-100, and AACA-200 groups were 1.44 mg/g, 1.42 mg/g, 1.93 mg/g, and 1.87 mg/g, respectively (Figure 4B). A significant increase in muscle glycogen content was observed in the AACA-100 and AACA-200 groups compared with the vehicle. The experimental results showed that there were significant differences in glycogen content between the AACA-100 or AACA-200 and vehicle groups, but not between the AACA-50 and vehicle groups. This suggests that feeding with AACA (100 mg/kg/d or 200 mg/kg/d) may increase the hepatic and muscular glycogen content, and prevent fatigue further.
Figure 4.
Effect of administrations of A.arguta crude alkaloids (AACA) extracts on (A) hepatic and (B) muscular glycogen levels at the end of the experiments. The measurements were taken after 28 days of AACA administrations. Data are presented as mean ± standard error of the mean of eight mice. Values of histogram labeled with different letters (a, b) are significantly different (p < 0.05).
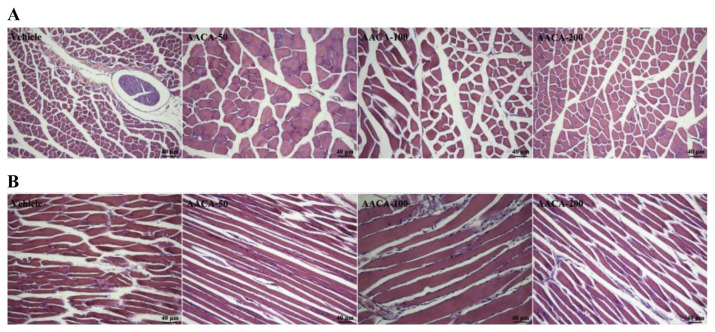
3.7. Effect of AACA on muscular tissues
We also examined whether AACA treatments could cause any negative effect on skeletal muscle tissues of healthy mice. We examined plasma aminotransferase levels (AST and ALT), CK activities (Table 2 and Figure 3D), and muscular morphology in AACA-treated mice (Figures 5A and 5B), and found no indication of a deleterious effect associated with AACA treatment.
Figure 5.
Effect of A.arguta crude alkaloids (AACA) treatments on the morphology of (A) transverse and (B) longitudinal sections of skeletal muscle. Mice were pretreated with vehicle, 50 mg/kg, 100 mg/kg, and 200 mg/kg of AACA for 28 days. All mice were killed and the morphology of skeletal muscle was examined at the end of the experiments. Specimens were photographed using a light microscope (hematoxylin and eosin stain; magnification: 100×; scale bar: 40 μm).
4. Conclusion
Our study results showed that AACA could significantly increase the swimming time in the weight-loaded swimming test (11.3 minutes) and forelimb grip strength (34.1 g) in mice in the AACA-100 group compared with the vehicle. Further, AACA elevates exercise performance by increasing levels of glucose, albumin, and TP and decreasing levels of lactate, ammonia, CK, UA, and BUN. The reserves of hepatic (3.37 mg/g) and muscular (0.49 mg/g) glycogen increased after exercise, which could provide energy for body movement and improve exercise performance. Our study results suggest that alkaloids extracted from A. arguta could be used as a novel antifatigue and exercise performance agent with physiological benefits when taken at optimized and reasonable doses. Because A. arguta alkaloids have antifatigue effects, A. arguta will be an important index in future breeding experiments and can be used as an important functional food in the future.
Acknowledgments
The research was supported by the Ministry of Agriculture of the National Public Welfare Industry Project of China (Project No. 200903013).
Funding Statement
The research was supported by the Ministry of Agriculture of the National Public Welfare Industry Project of China (Project No. 200903013).
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
REFERENCES
- 1. Wang YH, Xu FX, Feng XQ, MacArthur RL. Modulation of Actinidia arguta fruit ripening by three ethylene biosynthesis inhibitors. Food Chem. 2015;173:405–13. doi: 10.1016/j.foodchem.2014.10.044. [DOI] [PubMed] [Google Scholar]
- 2. Latocha P, Krupa T, Jankowski P, Radzanowska J. Changes in postharvest physicochemical and sensory characteristics of hardy kiwifruits (Actinidia arguta and its hybrid) after cold storage under normal versus controlled atmosphere. Postharvest Biol Technol. 2014;88:21–33. [Google Scholar]
- 3.Xuan L. Doctor thesis. Shenyang, China: Shenyang Agricultural University; 2013. Preliminary structure identification, antioxidant activity and immunity activity of the polysaccharides from Actinidia arguta. [Google Scholar]
- 4. Liu YJ, Liu JF, Tian XY, Wang XD, Wang LX, Ren DM. Polysaccharide of Actinidia arguta and activity of blood glucose and lipid of decline. Shi Pin Yu Sheng Wu Ji Shu Xue Bao. 2012;31:86–9. [In Chinese, English abstract] [Google Scholar]
- 5. Kim JY, Lee IK, Son MW, Kim KH. Effects of orally administered Actinidia arguta (hardy Kiwi) fruit extract on 2-chloro-1,3,5-trinitrobenzene-induced atopic dermatitis-like skin lesions in NC/Nga mice. J Med Food. 2009;12:1004–15. doi: 10.1089/jmf.2009.0080. [DOI] [PubMed] [Google Scholar]
- 6. Lee J, Sowndhararajan K, Kim M, Kim J, Kim D, Kim S, Kim GY, Kim S, Jhoo JW. Antioxidant, inhibition of α-glucosidase and suppression of nitric oxide production in LPS-induced murine macrophages by different fractions of Actinidia arguta stem. Saudi J Biol Sci. 2014;21:532–8. doi: 10.1016/j.sjbs.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Webby RF. A flavonol triglycoside from Actinidia arguta var. giraldii. Phytochemistry. 1991;30:2443–4. doi: 10.1016/0031-9422(91)83680-j. [DOI] [PubMed] [Google Scholar]
- 8. Matich AJ, Young H, Allen JM, Wang MY, Fielder S, McNeilage MA, MacRae EA. Actinidia arguta: volatile compounds in fruit and flowers. Phytochemistry. 2003;63:285–301. doi: 10.1016/s0031-9422(03)00142-0. [DOI] [PubMed] [Google Scholar]
- 9. Ravipati AS, Zhang L, Koyyalamudi SR, Jeong SC, Reddy N, Bartlett J, Smith PT, Shanmugam K, Münch G, Wu MJ, Satyanarayanan M, Vysetti B. Antioxidant and anti-inflammatory activities of selected Chinese medicinal plants and their relation with antioxidant content. BMC Complement Altern Med. 2012;12:173–87. doi: 10.1186/1472-6882-12-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mehta RK, Agnew MJ. Influence of mental workload on muscle endurance, fatigue, and recovery during intermittent static work. Eur J Appl Physiol. 2012;112:2891–902. doi: 10.1007/s00421-011-2264-x. [DOI] [PubMed] [Google Scholar]
- 11. Coombes JS, Rowell B, Dodd SL, Demirel HA, Naito H, Shanely RA, Powers SK. Effects of vitamin E deficiency on fatigue and muscle contractile properties. Eur J Appl Physiol. 2002;87:272–7. doi: 10.1007/s00421-002-0631-3. [DOI] [PubMed] [Google Scholar]
- 12. Nybo L. CNS fatigue and prolonged exercise: effect of glucose supplementation. Med Sci Sports Exerc. 2003;35:589–94. doi: 10.1249/01.MSS.0000058433.85789.66. [DOI] [PubMed] [Google Scholar]
- 13. You LJ, Zhao MM, Regenstein JM, Ren JY. In vitro antioxidant activity and in vivo anti-fatigue effect of loach (Misgurnus anguillicaudatus) peptides prepared by papain digestion. Food Chem. 2011;124:188–94. doi: 10.1021/jf2016368. [DOI] [PubMed] [Google Scholar]
- 14. Tharakan B, Dhanasekaran M, Manyam BV. Antioxidant and DNA protecting properties of anti-fatigue herb Trichopus zeylanicus. Phytother Res. 2005;19:669–73. doi: 10.1002/ptr.1725. [DOI] [PubMed] [Google Scholar]
- 15. Yeh TS, Chuang HL, Huang WC, Chen YM, Huang CC, Hsu MC. Astragalus membranaceus improves exercise performance and ameliorates exercise-induced fatigue in trained mice. Molecules. 2014;19:2793–807. doi: 10.3390/molecules19032793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee BR, Lee JH, An HJ. Effects of Taraxacum officinale on fatigue and immunological parameters in mice. Molecules. 2012;17:13253–65. doi: 10.3390/molecules171113253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wink M. Alkaloids: properties and determination. In: Caballero B, Finglas PM, Toldrá F, editors. Encyclopedia of food and health. Amsterdam, The Netherlands: Elsevier; 2016. pp. 97–105. [Google Scholar]
- 18. Liu YN, Song X, Yan RQ, Li TX, Chai X, Qi AD, Wang YF, Jiang ZZ. Development and validation of a UPLC-DAD-MS method for characterization and quantification of alkaloids in Menispermi Rhizoma and its preparations. J Food Drug Anal. 2013;21:206–18. [Google Scholar]
- 19. Gao C, Huang XX, Bai M, Wu J, Li JY, Liu QB, Li LZ, Song SJ. Anti-inflammatory sesquiterpene pyridine alkaloids from Tripterygium wilfordii. Fitoterapia. 2015;105:49–54. doi: 10.1016/j.fitote.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 20. Liu L, Chen YY, Qin XJ, Wang B, Jin Q, Liu YP, Luo XD. Antibacterial monoterpenoid indole alkaloids from Alstonia scholaris cultivated in temperate zone. Fitoterapia. 2015;105:160–4. doi: 10.1016/j.fitote.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 21.Liu YY, Liu CJ. International Conference on Materials. Environment and Biological Engineering (MEBE 2015) Amsterdam, The Netherlands: Atlantis Press; 2015. Extraction process optimization of total alkaloid from Actinidia arguta; pp. 131–4. [Google Scholar]
- 22. Jung K, Kim IH, Han D. Effect of medicinal plant extracts on forced swimming capacity in mice. J Ethnopharmacol. 2004;93:75–81. doi: 10.1016/j.jep.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 23. Kim KM, Kawada T, Ishihara K, Inoue K, Fushiki T. Increase in swimming endurance capacity of mice by capsaicin-induced adrenal catecholamine secretion. Biosci Biotechnol Biochem. 1997;61:1718–23. doi: 10.1271/bbb.61.1718. [DOI] [PubMed] [Google Scholar]
- 24. Tang KJ, Nie RX, Jing LJ, Chen QS. Anti-athletic fatigue activity of saponins (Ginsenosides) from American ginseng (Panax quinquefolium L.) Afr J Pharm Pharmacol. 2009;3:301–6. [Google Scholar]
- 25. Zhang HL, Li J, Li G, Wang DM, Zhu LP, Yang DP. Structural characterization and anti-fatigue activity of polysaccharides from the roots of Morinda officinalis. Int J Biol Macromol. 2009;44:257–61. doi: 10.1016/j.ijbiomac.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 26. Suh SH, Paik IY, Jacobs K. Regulation of blood glucose homeostasis during prolonged exercise. Mol Cells. 2007;23:272–9. [PubMed] [Google Scholar]
- 27. Grace MH, Yousef GG, Kurmukov AG, Raskin I, Lila MA. Phytochemical characterization of an adaptogenic preparation from Rhodiola heterodonta. Nat Prod Commun. 2009;4:1053–8. [PMC free article] [PubMed] [Google Scholar]
- 28. Chiang HM, Chen HC, Wu CS, Wu PY, Wen KC. Rhodiola plants: chemistry and biological activity. J Food Drug Anal. 2015;23:359–69. doi: 10.1016/j.jfda.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]