Abstract
Human society operates on large-scale cooperation. However, individual differences in cooperativeness and incentives to free ride on others' cooperation make large-scale cooperation fragile and can lead to reduced social welfare. Thus, how individual cooperation spreads through human social networks remains puzzling from ecological, evolutionary, and societal perspectives. Here, we identify oxytocin and costly punishment as biobehavioral mechanisms that facilitate the propagation of cooperation in social networks. In three laboratory experiments (n = 870 human participants: 373 males, 497 females), individuals were embedded in heterogeneous networks and made repeated decisions with feedback in games of trust (n = 342), ultimatum bargaining (n = 324), and prisoner's dilemma with punishment (n = 204). In each heterogeneous network, individuals at central positions (hub nodes) were given intranasal oxytocin (or placebo). Giving oxytocin (vs matching placebo) to central individuals increased their trust and enforcement of cooperation norms. Oxytocin-enhanced norm enforcement, but not elevated trust, explained the spreading of cooperation throughout the social network. Moreover, grounded in evolutionary game theory, we simulated computer agents that interacted in heterogeneous networks with central nodes varying in terms of cooperation and punishment levels. Simulation results confirmed that central cooperators' willingness to punish noncooperation allowed the permeation of the network and enabled the evolution of network cooperation. These results identify an oxytocin-initiated proximate mechanism explaining how individual cooperation facilitates network-wide cooperation in human society and shed light on the widespread phenomenon of heterogeneous composition and enforcement systems at all levels of life.
SIGNIFICANCE STATEMENT Human society operates on large-scale cooperation. Yet because cooperation is exploitable by free riding, how cooperation in social networks emerges remains puzzling from evolutionary and societal perspectives. Here we identify oxytocin and altruistic punishment as key factors facilitating the propagation of cooperation in human social networks. Individuals played repeated economic games in heterogeneous networks where individuals at central positions were given oxytocin or placebo. Oxytocin-enhanced cooperative norm enforcement, but not elevated trust, explained cooperation spreading throughout the social network. Evolutionary simulations confirmed that central cooperators' willingness to punish noncooperation allowed the permeation of the network and enabled the evolution of cooperation. These results identify an oxytocin-initiated proximate mechanism explaining how individual cooperation facilitates network-wide cooperation in human social networks.
Keywords: cooperation, costly punishment, heterogeneous social network, oxytocin, social evolution
Introduction
Cooperation among nonkin is a widespread phenomenon throughout the animal kingdom, human societies included (Clutton-Brock, 2009; Apicella and Silk, 2019). Remarkably, humans interact in structured social networks ranging from dozens to millions of people (Buchan et al., 2009; Hill et al., 2011). Social networks are often marked by cooperative exchange in which the benefits people extend to others exceed the costs they incur (Rand and Nowak, 2013; Hilbe et al., 2018). Yet because cooperation can be exploited by free riding—taking advantage of the cooperation of others without reciprocating—network cooperation is difficult to develop and maintain (Gracia-Lázaro et al., 2012; Maciejewski et al., 2014; Gavrilets and Richerson, 2017). While humans can prevent the breakdown of cooperation by establishing and enforcing social norms (Hauert et al., 2007; Boyd et al., 2010; Fehr and Schurtenberger, 2018), how network cooperation emerges and spreads remains poorly understood. Here, we identify a biobehavioral mechanism that increases individuals' willingness to enforce cooperation norms and facilitates the spread of cooperation in networks with varying degrees of interconnectedness.
In the field of behavioral ecology, social structure has been shown to impact key ecological and evolutionary processes (Fehl et al., 2011; Kurvers et al., 2014). Although different network structures are theoretically possible, most human social networks are characterized by small fractions of densely connected central nodes existing alongside larger fractions of sparsely connected peripheral nodes (Newman et al., 2006; Santos et al., 2006). Such heterogeneous network structures also characterized early and modern human groups and societies (Apicella et al., 2012) and resemble friendship networks and work teams, suggesting heterogeneous networks to be functional to group survival and prosperity (Santos et al., 2006, 2008; McAvoy et al., 2020). Longitudinal and naturalistic field studies have observed that influential positions are often occupied by a small proportion of the most trustworthy and cooperative individuals (Lyle and Smith, 2014; Bird and Power, 2015). Theoretical analyses and mathematical modeling suggest that strong cooperators can proliferate and establish high levels of cooperation in networks (Bowles and Gintis, 2004; Battu et al., 2018). However, empirical evidence is currently lacking about how centralized cooperative individuals enforce cooperative behaviors and its consequences for the propagation of cooperation in their social network remain elusive.
Recent work in social and behavioral neuroscience has linked cooperation in human groups to oxytocin, a neuropeptide that functions as both a hormone and a neurotransmitter (Carter, 2014; Rilling and Young, 2014). During interpersonal interactions within groups of people, elevated levels of oxytocin have been associated with reciprocity and in-group trust (van IJzendoorn and Bakermans-Kranenburg, 2012; Spengler et al., 2017), concern for fairness (Stallen et al., 2018; Liu et al., 2019), and the cooperative provision of public goods (Israel et al., 2012). Placebo-controlled pharmacological experiments have also shown that oxytocin can enhance the enforcement of cooperative norms through peer punishment. Individuals administered oxytocin, rather than matching placebo, are more likely to punish group members who free ride during the provision of public good (Aydogan et al., 2017), betray others' trust in reciprocal transactions (Daughters et al., 2017), or violate fairness norms (Stallen et al., 2018). Based on these findings, we hypothesize that oxytocin can enable central nodes in spatially heterogeneous networks to spread cooperation via two mechanisms—initiating trust and cooperation and enforcing cooperation norms through peer punishment.
To examine these possibilities, we performed three preregistered social network experiments. Human participants played repeated economic games with anonymous neighbors in heterogeneous networks, where a few male individuals (“central node”, given oxytocin or placebo) were densely connected to a larger number of less interconnected individuals (“peripheral node,” without pharmacological treatment). We revealed that locally administered oxytocin to central nodes can increase global fairness and cooperation, suggesting how a biologically prepared mechanism can help cooperation extend beyond direct interactions and spread in a social network. We ran evolutionary agent-based simulations that further confirmed the importance of norm enforcing by central agents for cooperation to spread throughout the network.
Materials and Methods
Participants
Healthy human participants (N = 870: 373 males, 497 females) were invited to participate in this study as paid volunteers. All participants had normal or corrected-to-normal vision and no history of neurologic or psychiatric disorders. Those who majored in psychology or economics, or had participated in any similar study before, were excluded from participation. The experiment involved no deception, and participants were paid $15 for showing up plus their earnings in randomly selected one-sixth of the rounds played. The experimental protocols were preregistered at OSF (Open Science Foundation; https://osf.io/hn32r), adhered to the standards set by the Declaration of Helsinki, and were approved by the local Research Ethics Committee at the State Key Laboratory of Cognitive Neuroscience and Learning, Beijing Normal University (Beijing, People's Republic of China; Agreement CNL_A_0005_001). All participants provided written informed consent after the experimental procedures had been fully explained and were informed of their right to withdraw at any time during the study.
The sample size of the current study was predetermined by power analysis using G*Power 3.1 (Faul et al., 2007). Based on the effect size reported in an earlier study on oxytocin and in-group cooperation (De Dreu et al., 2010, their Experiments 1 and 2) with the weighted averaged η2 = 0.136), 52 participants (26 per group) were sufficient to detect oxytocin effects in a between-subject design (α = 0.05, β = 0.80). We thus planned to recruit at least 54 central players (placebo, N = 27; oxytocin, N = 27), which resulted in 18 social networks (three central players per network) for each experiment. In addition, the sample size in terms of number of networks matches the number typically used in previous behavioral network studies (i.e., 6–10 networks per condition; Centola, 2010; Shirado et al., 2013; Rand et al., 2014; Li et al., 2018).
In experiment 1, 342 participants (mean age, 21.02 ± 2.34 years; 126 males, 216 females) were randomly assigned to 9 oxytocin and 10 placebo networks (18 participants per network) and played a network version of the trust game (TG). Experiment 2 recruited 324 participants (mean age, 21.14 ± 2.30 years; 165 males, 159 females) who were randomly assigned to 9 oxytocin and 9 placebo networks (18 participants per network) and played a network version of an ultimatum bargaining game (UBG). Experiment 3 recruited 288 participants (mean age, 20.65 ± 2.27 years; 116 males, 172 females) being randomly assigned to oxytocin and placebo networks (12 participants per network) and played a network version of a two-stage prisoner's dilemma game (tPDG) with costly punishment. Six tPDG networks were invalid because of platform operating problems. Another tPDG network data network was excluded because of >3 SDs of the population mean, leaving 9 oxytocin networks and 8 placebo networks (204 participants: 82 males, 122 females) for formal data analysis.
The central players in all networks received oxytocin or placebo and were male participants matched on social value orientation (p values > 0.25), inequality aversion (p values > 0.21), prosocial personality scores (p values > 0.11), and age (p values > 0.05). We recruited only male central players to avoid potential confounding factors associated with sex differences in oxytocin effects (Fischer-Shofty et al., 2013; Rilling et al., 2014), consistent with previous studies examining oxytocin effects on social cognition and cooperation (De Dreu et al., 2010; Ma et al., 2016; Liu et al., 2019; Zhang et al., 2019). All the peripheral players in the oxytocin and placebo networks were matched on age (p values > 0.45), gender (p values > 0.05), and inequality aversion scores (p values > 0.18).
Procedure
All experiments in this study followed a randomized double-blind, placebo-controlled, between-session design. For each network, three male participants were randomly assigned as the central nodes, completed social preference measures on the day before the experiment, and were invited to a behavioral testing room for oxytocin administration 50 min before the network experiments. Upon arrival, participants completed mood measurements using the positive and negative affect scale before the oxytocin or placebo administration (which was measured again at the end of the experiment to monitor mood change. There was no mood difference between participants in oxytocin and placebo networks before the treatment administration and at the end of the experiment (positive affect, p values > 0.36; negative affect, p values > 0.26; state anxiety, p values > 0.15 in all experiments). Comparisons of mood changes revealed no significant difference in central players administered placebo or oxytocin (positive affect, p values > 0.51; negative affect, p values > 0.30; state anxiety, p values > 0.93 in all experiments). Then, a single dose of 24 IU oxytocin or placebo (containing the active ingredients, except for the neuropeptide) was intranasally self-administered with nasal sprays to central players under experimenters' supervision. The procedure for the oxytocin/placebo administration was similar to that in previous work, which showed significant oxytocin effects on social decision-making and/or prosociality (Liu et al., 2019; Zhang et al., 2019). The spray was administered to each participant three times with each administration of one inhalation of 4 IU into each nostril. Thirty-five minutes later, central players in the UBG or TG networks took part in individual versions of UBG or TG, with a hypothetical player in one round without feedback, to test the effects of oxytocin on individual-level decision-making. The individual version of the tPDG was not performed by central players, as we found that the cooperation and punishment propensity in a one-shot nonfeedback tPDG was less informative regarding behaviors in a repeated setting with feedback.
Then, all the central and peripheral players were invited into the network testing room at the same time and randomly assigned to individual cubicles for the experiment. All participants were presented with the rules and settings of the network decision-making game and underwent five practice rounds to become familiarized with the rules and decision-making interface (Extended Data Fig. 1-1, detailed experimental instructions for experiments 1–3). The experimental platform was programmed in PHP, MySQL, and JavaScript. Participants were not informed of the number of rounds, the size of the network, or the number of neighbors. Participants made decisions in a self-paced manner, and once all participants had made decisions, all participants of the network entered the next round at the same time.
Experimental design
Trust game network.
Each TG network contained 9 investors and 9 trustees, with all investors being connected to their trustee neighbors, and vice versa. Each TG network contained three central investors (each connected to a larger number of neighbors: six trustees), six peripheral investors (each connected to a smaller number of neighbors: three trustees), and nine trustees (each connected to two central and two peripheral investors; Fig. 1a).
Figure 1.
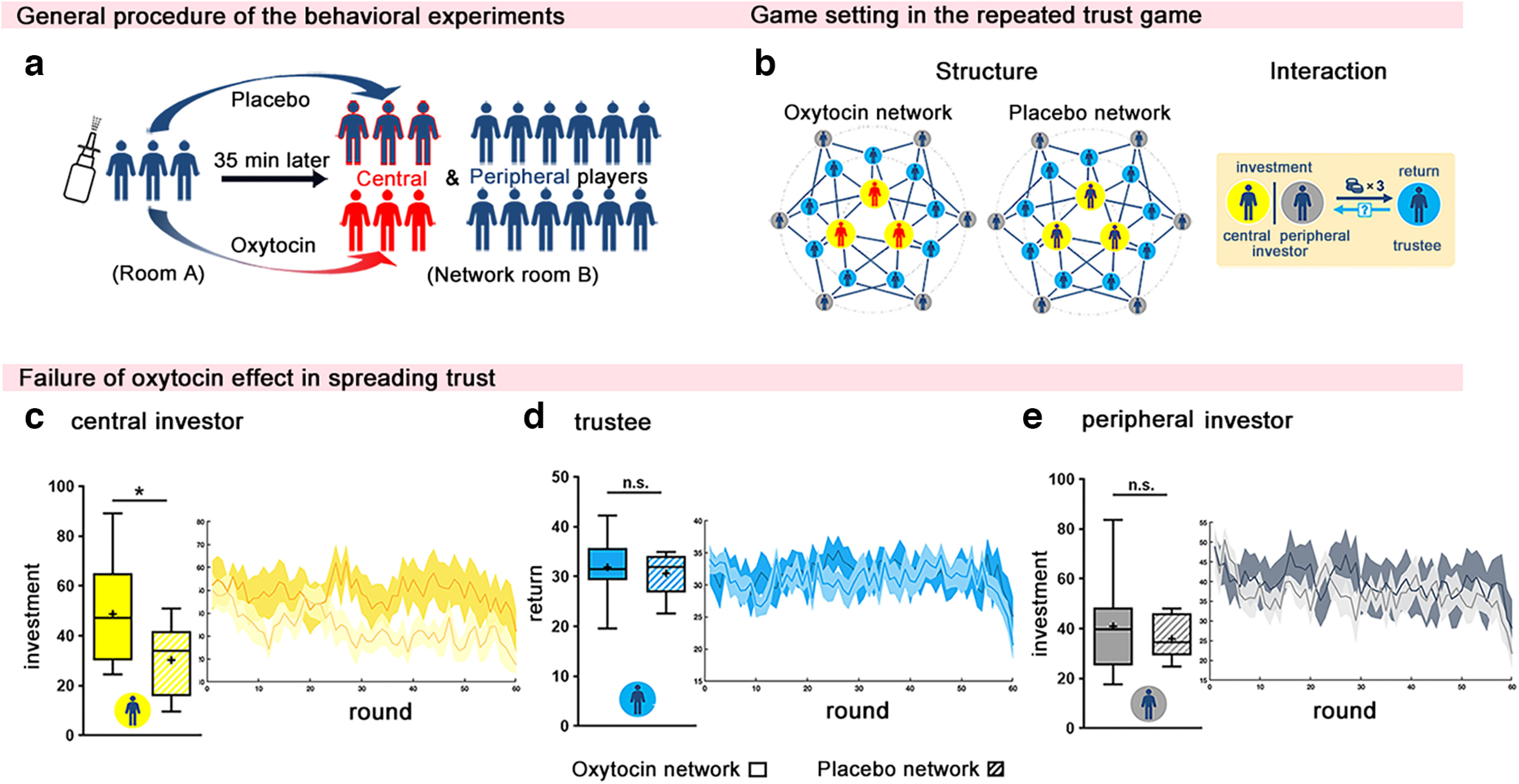
The results for the effects of oxytocin on trust in a repeated trust game network. a, Participant assignment before the experiment. For each network, three participants were randomly assigned as the central nodes and were invited into behavioral testing room A for oxytocin/placebo administration 35 min before the network experiments. Then, all the central and other peripheral players were invited into network testing room B at the same time and randomly assigned to individual cubicles for the experiment. b, Network structure and rules. Each TG network consisted of 18 participants, with 9 trustees (blue-filled circle) and 9 investors (yellow-filled circles indicate central investors, and gray-filled circles indicate peripheral investors). In each TG round, an investor made a single investment t (0 ≤ t ≤ 100) in all of his or her neighboring trustees. Trustees received the tripled amount, 3t, and had to decide on one single return rate r (0 ≤ r ≤ 1) that would apply to all of his or her neighboring investors. c–e, The administration of oxytocin enhanced the investment of central investors (c), but influenced neither the return rate of trustees (d) nor the investment of peripheral investors (e). The test statistics shown in the figure are based on two-tailed independent-sample t tests comparing oxytocin and placebo networks with *p < 0.05, n.s., not significant. Data are plotted as box plots for each condition, with horizontal lines indicating median values, fixation indicating mean values, boxes indicating 25% and 75% quartiles, and whiskers indicating the 2.5–97.5% percentile range. The round-by-round dynamics of decisions over time are presented on the right side of each box plot with each solid line representing the mean value of each round and shading showing the 95% CI.
Participants played 5 practice and 60 formal rounds of the TG. In each round, investor i makes a single investment, ti (0 ≤ ti ≤ 100), which is applied to all neighboring trustees. Trustee j, connecting four investors, receives investment t [t = (3t1 + 3t2 + 3t3 + 3t4)/4] and decides on a single return rate, rj (0 ≤ rj ≤ 1), which is applied to all neighboring investors. Neighbors' decisions in the previous round are presented to participants before making their next decision. For each connected investor(1)–trustee(j) pair, the trustee keeps the remaining amount [i.e., 3ti × (1 – rj)] and the investor keeps what is left from the investment and the returns from the trustee [i.e., (100 – ti) + 3ti × rj]. Each participant's payoff in each round is averaged over all connected pairs.
Ultimatum bargaining game network.
The UBG network (Fig. 2a) had the same network structure as the TG network. There were three central responders, each connected to six neighboring proposers, and six peripheral responders, each connected to three neighbors. For proposers, each had a homogeneous neighborhood size of four responders (i.e., two central and two peripheral responders, ensuring an unbiased influence from both types of responders).
Figure 2.
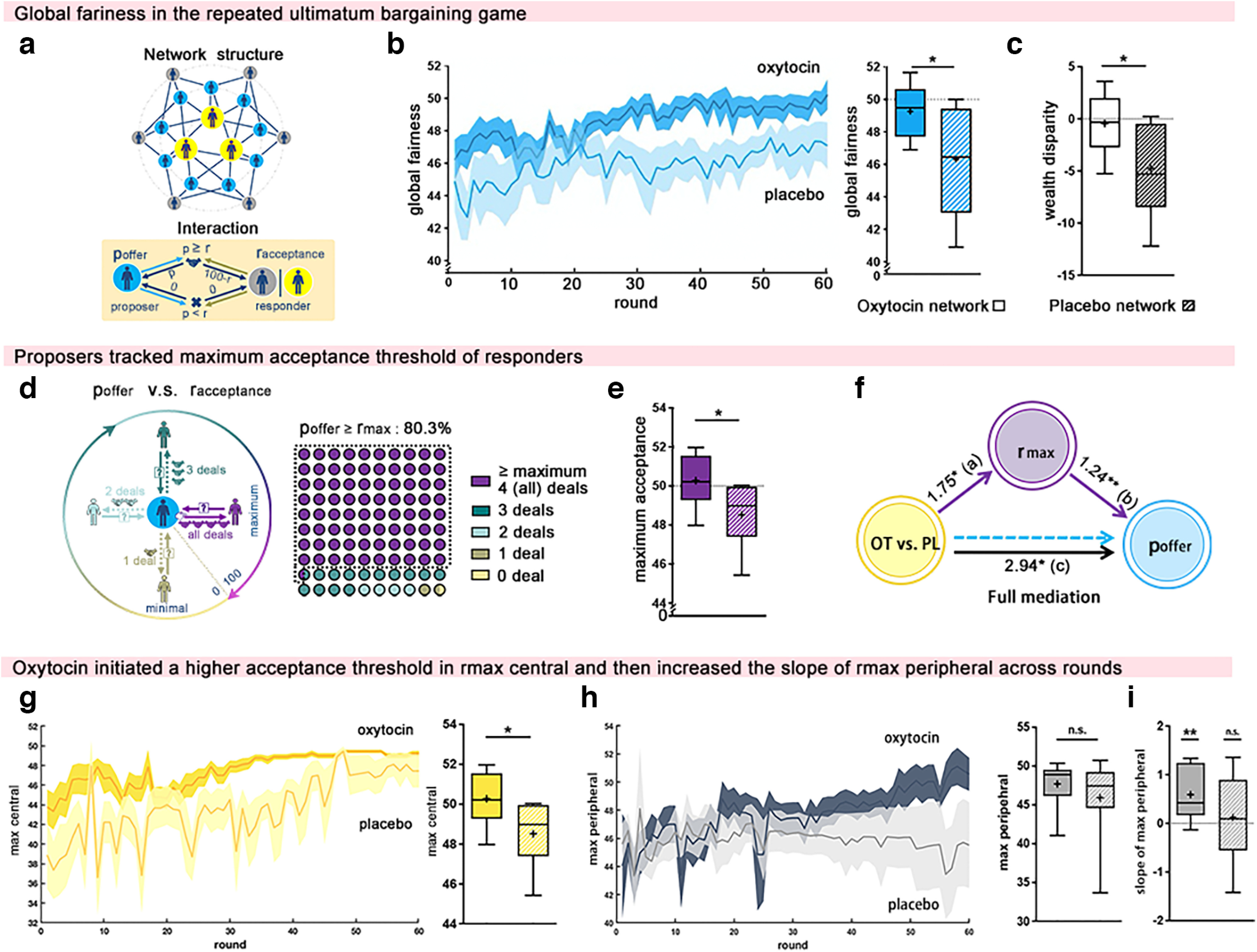
Effects of oxytocin on the ultimate bargaining network. a, Network structure and interaction rules. Each network consisted of 18 participants, with 9 proposers (blue-filled circle) and 9 responders (central responders, yellow-filled circles; peripheral responders, gray-filled circles). In each UBG round, a proposer made one single offer, poffer, to all of his/her neighboring responders, and a responder decided on one single minimum acceptance level, raccept, that would apply to all of their neighboring proposers. For each connection, the deal with one neighbor was made only if poffer ≥ raccept; otherwise, both received 0. b, Global fairness (poffer) dynamics across the 60 rounds of the UBG. In oxytocin (vs placebo) networks, global fairness was significantly higher. c, Oxytocin significantly increased payoff equality between proposers and responders. d, Conceptual illustration and real strategy choices of proposers. Each proposer received responses from four neighboring responders with different levels of acceptance. Proposers would make all deals succeed when their offer matched the highest acceptance level (rmax) among neighboring responders. Proposers indeed matched the highest acceptance level of responders in 80.3% of the rounds (poffer ≥ rmax, purple dots). e, f, Oxytocin enhanced rmax (e), and between-network mediation analyses (f) showed that the effect of oxytocin on changes in poffer was mediated by rmax. g, h, Oxytocin induced a higher rmax in central responders (rmax central; g) but not in peripheral responders (rmax peripheral; h). i, However, with the increased enforcement of fairness by central players given oxytocin, peripheral players also increased maximum demand for a fair distribution of resources over time (indicated by the increased slope of rmax peripheral). Data are plotted as box plots for oxytocin and placebo networks, with horizontal lines indicating median values, fixation indicating mean values, boxes indicating 25% and 75% quartiles, and whiskers indicating the 2.5–97.5% percentile range. The round-by-round dynamics of decisions over time are presented on the left side of the box plot, with each solid line representing the mean value of each round and shading showing the 95% CI. *p < 0.05, **p < 0.01, n.s., not significant.
Participants played 5 practice and 60 formal rounds of the UBG game. In each round, a proposer makes a single offer, poffer (0 ≤ poffer ≤ 100), which is simultaneously applied to all neighboring responders. Each responder decides on a single minimum amount that he or she accepts for all neighboring proposers, raccept (0 ≤ raccept ≤ 100). Proposers and responders make decisions simultaneously, and each connected proposer–responder pair shares a fixed number of resources (100 Money Unit). Each pair makes a deal if poffer ≥ raccept, then the proposer keeps 100 – poffer and the responder receives poffer. Otherwise, both get nothing. At the end of each trial, participants are presented with the decisions of neighbors and their own payoff. The payoff of each round was averaged over all connected pairs of each player.
Two-stage prisoner's dilemma game network.
Each tPDG network contained 12 players. In the tPDG, every player had the same role (i.e., deciding whether to cooperate or defect and to punish or not in each round; see below), but, because of the network structure, differed in the number of neighbors. There were three central players, each making decisions with eight unchanging neighbors (two central and six peripheral players), and nine peripheral players, each connected to four unchanging neighbors (two central and two peripheral players; Fig. 3a).
Figure 3.
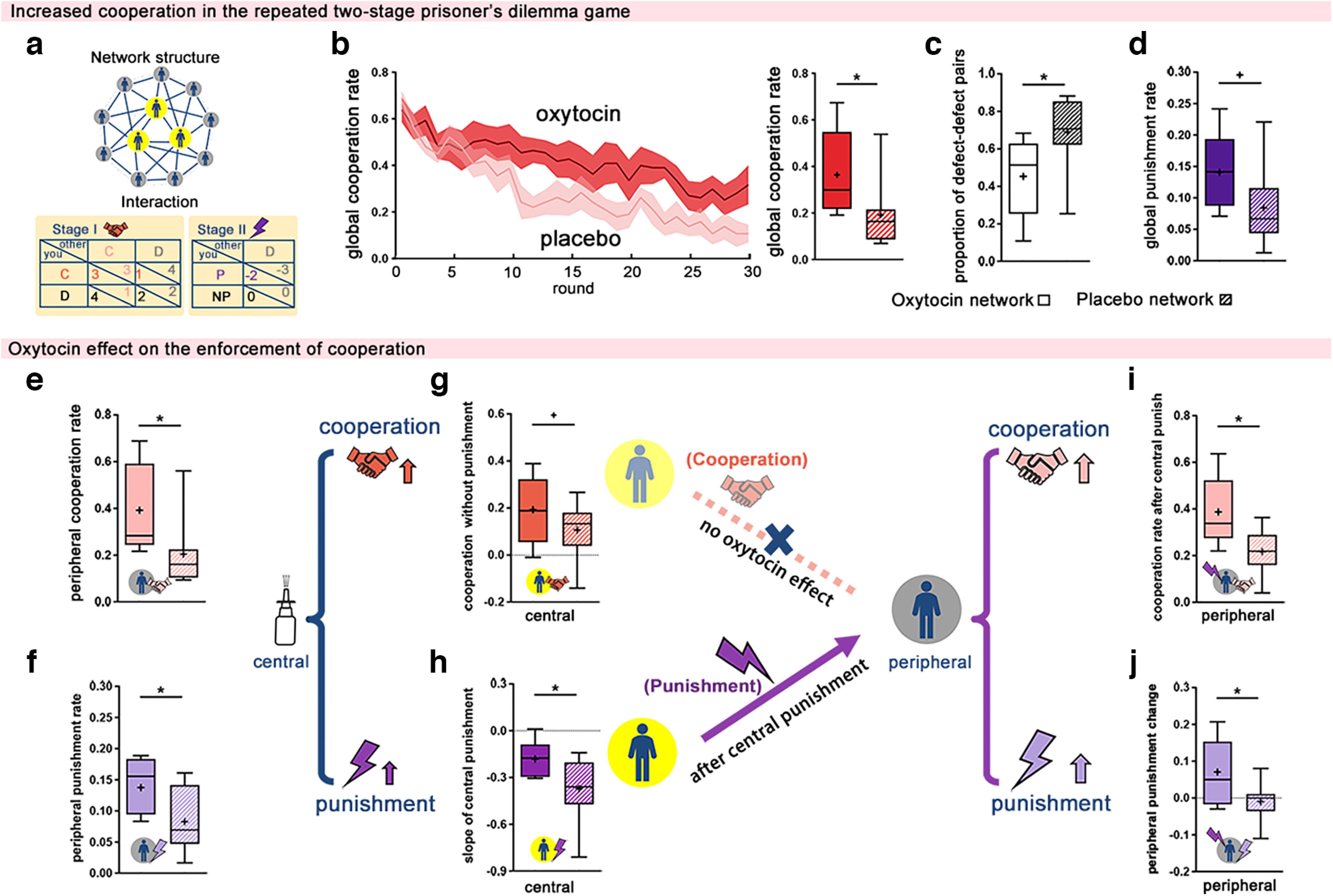
Effects of oxytocin on the two-stage prisoner's dilemma network. a, Network structure and interaction rules. Each tPDG network consisted of 12 participants: 3 central players (yellow-filled circle) played the tPDG game with 8 neighbors (2 central players and 6 peripheral players), and 9 peripheral players (gray-filled circles) played the tPDG game with 4 neighbors (2 central players and 2 peripheral players). In each tPDG round, participants made decisions to either cooperate (C) or defect (D) in stage I, received feedback on the number of neighbors choosing C/D and, then, in stage II, decided whether to punish (P) their defecting neighbors or not punish (NP). b, Cooperation rate of the network across the 30 tPDG rounds. b–d, The administration of oxytocin to the three central players increased the cooperation rate (b), decreased the number of defect–defect decision pairs (c), and elevated the punishment rate of the whole network (d). e, f, Peripheral players' cooperation rate (e) and punishment rate (f) were increased in the oxytocin (vs placebo) networks. g, h, Central players in the oxytocin (vs placebo) networks showed more nonpunishing cooperation (g), but also decreased their punishment behaviors less over time (h). i, j, Punishment from central players led to the increased cooperation (i) and punishment (j) of peripheral players in oxytocin (vs placebo) networks. Data are plotted as box plots for oxytocin and placebo networks, with horizontal lines indicating median values, fixation indicating mean values, boxes indicating 25% and 75% quartiles, and whiskers indicating the 2.5–97.5% percentile range. The round-by-round dynamics of decisions over time are presented on the left side of the box plot, with each solid line representing the mean value of each round and shading showing the 95% CI. *p < 0.05, † 0.05<p < 0.1.
Participants played 5 practice and 30 formal rounds of the tPDG. In each round, participants made the following two decisions: (1) in stage I, participants chose to either cooperate (C) or defect (D), with the chosen option being applied to all neighbors simultaneously; and (2) in stage II, participants were presented with the number of their neighbors choosing C or D and decided whether or not to costly punish the defectors in their neighborhood (cost-to-penalty ratio, 2:3). Reputation effects were ruled out by having participants decide whether or not to punish only all neighboring defectors rather than to punish a selection of players. Cost and punishment were normalized by neighborhood size (i.e., the cost was 2 MUs multiplied by the number of neighboring defectors, divided by neighborhood size, and the penalty was 3 MUs multiplied by the number of neighbors choosing punishment, divided by neighborhood size). At the end of each round, each participant was presented with their first-stage cooperation choice, second-stage punishment choice, own payoff, the number of neighbors' cooperation choices in stage I, and the number of their neighbors' punishment choices in stage II (which is not applicable if one chooses C in the first stage). Each participant's payoff in each round was averaged over all connected pairs (Fig. 3a, payoff matrix).
Experimental statistical analysis
All experiments were double blind (i.e., both participants and experimenters were blind to the treatment). We first compared the decisions in the individual version of the game (before entering the network) between the central players given oxytocin and placebo to examine the effects of oxytocin on trust behavior and fairness at an individual level.
To examine the effects of oxytocin on the network, we compared the indices in the oxytocin and placebo networks separately for different roles and different positions. We conducted the following two sets of analyses: (1) more conservative network-level independent-samples Student's t tests; and (2) generalized linear mixed model (GLMM) that the incorporated individual-nested effect. In the first set of analyses, data were aggregated at the network level, with treatment (oxytocin vs placebo) as a between-network factor, and the treatment effects were examined using two-tailed independent t tests (our data satisfied both the normal distributional assumption and equal variance assumption). For the null effects, we further conducted Bayesian analysis to assess whether and to what extent the null hypothesis of no differences between oxytocin and placebo conditions is indeed more likely than the alternative hypothesis. For the observed effects, alternatively, we analyzed the data using a GLMM with treatment as a fixed effect and individuals in each network as a random effect. These two sets of analyses obtained similar results, and we reported the results based on both analysis strategies.
To analyze the end-state of the network as a function of treatment, we performed analyses based on the final two-thirds of the rounds. This approach is similar to previous studies examining network dynamics (Gallo and Yan, 2015; Li et al., 2018), aiming to capture stable interaction strategies/states (the corresponding statistical tests across all rounds yielded the same pattern but with a smaller effect size). To examine the spreading dynamics in the network, we analyzed the time course (all rounds split into the first half vs second half) as a within-network factor. Specifically, we tested whether the oxytocin effects occurred in earlier rounds for central players who received oxytocin administration and whether these effects influenced peripheral players in later rounds.
Analysis of TG network.
We performed network-level comparisons between the oxytocin and placebo networks on (1) return (averaged among 9 trustees for their returns over the last 40 rounds) and (2) investment (averaged investment of investors over the last 40 rounds, both in all 9 investors and separately averaged in 3 central investors or 6 peripheral investors).
Analysis of UBG network.
Comparisons at the network level between the oxytocin and placebo networks were performed on (1) global fairness, indicated by poffer (averaged within 9 proposers and over the last 40 rounds; range, 0–100); (2) wealth disparity (i.e., payoff differences = averaged payoff of 9 proposers minus that of 9 responders across rounds); (3) maximum acceptance level of all neighboring responders for each proposer, rmax (averaged across 9 proposers and rounds for their neighboring maximum poffer); and (4) maximum acceptance level among central responders (i.e., rmax central) or peripheral responders (i.e., rmax peripheral) and the time slope (by regressing the averaged rmax peripheral in a network across all 30 rounds and comparing the network differences after Fisher z transformation of the regression coefficient). In addition, poffer and rmax were compared with the perfect fairness benchmark of 50 in the placebo and oxytocin networks, respectively, using one-sample t tests.
Finally, we performed a mediation analysis to test whether the oxytocin effect on the global fairness indicated by poffer was mediated by its effect on rmax. Specifically, we conducted Pearson correlation analysis to test the relationship between rmax and poffer and further tested whether the oxytocin-elevated rmax accounted for the effect of oxytocin on enhancing the offers of proposers. We used between-session mediation analysis (Preacher and Hayes, 2008) with 1000 bootstrapped samples to test whether the indirect path without rmax was less significant or even insignificant.
Analysis of tPDG network.
Network-level comparisons between the oxytocin and placebo networks were performed on the following: (1) the cooperation rate and punishment rate (range, 0–1; averaged across 12 players of each network and separately averaged over three central players or 9 peripheral players over the last 20 rounds); (2) the proportion of dilemma situations [i.e., DD pair (defect–defect decision pairs)]; and (3) the defect-switch-to-cooperate rate (proportion of switching to cooperation after choosing defection in the previous round). For central players, we calculated (4) the proportion of nonpunishing cooperation (round where participants chose to cooperate but not to punish), (5) the slope of the punishment rate (by regressing the averaged punishment rate in central players across all 30 rounds and comparing the network differences after Fisher z transformation of the regression coefficient), and (6) the punishment rate when more (or less) than half of the neighbors chose to defect. For peripheral players, we calculated (7) the cooperation rate changes after neighboring central players' nonpunishing cooperation, (8) the cooperation rate changes after being punished by central players in the previous round, and (9) the proportion of choosing to punish after being punished by central players in the previous round. In addition, we performed stepwise regression analysis to explain the influence of central cooperation with/without punishment on the cooperation of peripheral players across all networks.
Evolutionary modeling
Model structure.
We performed a two-stage prisoner's dilemma game in a finite population of a fixed structure, where agents interact only with their connected neighbors along the unweighted and bilateral edges. We used the same network structure, degree of heterogeneity, payoff matrix, and game interaction as those used in the behavioral experiment for our main simulations. We assumed that the population consists of the following two types of agents: classical agents and manipulated agents. While classical agents enter the network with a randomly picked strategy from the strategy pool, manipulated agents are provided with a smaller strategy pool to generate certain cooperation and punishment rates. We accordingly set different strategies to specify their behaviors.
Strategy setup.
For each classical agent, a strategy is randomly picked from the strategy pool, which contains 10 different strategies denoting the cooperation and punishment responses of agents in the first generation. Specifically, the strategy pools consist of four cooperation strategies (always-cooperate, always-defect, tit-for-tat, and punishment-driven cooperation) at stage I and three punishment strategies at stage II (always-punish, always-nonpunish, and conditionally punish). In stage I, agents using (1) the always-cooperate strategy will always choose to cooperate, (2) the always-defect strategy will always defect, (3) the tit-for-tat strategy will choose to cooperate in the first round and conditionally cooperate when the neighbors choose cooperation in the previous round and otherwise defect (i.e., the cooperation-driven strategy), and (4) the punishment-driven strategy will choose to defect in the first round and cooperate only after their neighbors choose to punish in the previous round. In stage II, agents using (1) the always-punish strategy will always-punish defection, (2) the always-nonpunish strategy will never choose to punish regardless of their own choice, and (3) conditionally punish strategy will choose to punish defection only when the agent itself chooses to cooperate in stage I (this strategy is not considered for always-defect agents and always-cooperate agents, as it is not a possible strategy for always-defect agents in stage II, and it is the same strategy for always-punish agents as it is for always-cooperate agents). Therefore, there are 10 different strategies in total. The strategy pool was balanced in the distribution of the initial cooperation/defection tendency and the punish/nonpunish tendency. Once a strategy was picked for one agent, he/she will maintain the strategy in a generation across rounds.
The strategies for manipulated agents were determined from a different strategy pool to enable our manipulation of the cooperation and punishment rates of those individuals in central positions. We manipulated the strategies of central agents by varying the cooperation and punishment rates in the face of defection. A strategy consisted of a specific proportion of cooperation action (Ci) and a certain proportion of punishment action toward defection (Pi) in a generation. We set up the cooperation (punishment) strategy by varying the distance toward the always-cooperate (always-punish) strategy from 0 to 1 in increments of 0.1. The strategy of each manipulated agent was fixed in each generation.
Evolutionary dynamics.
In each life cycle (one generation), all pairs of directly connected agents engaged in 30 rounds of the two-stage prisoner's dilemma game consisting of cooperation and punishment stages. The payoff of each agent was based on the interaction outcomes with its neighbors and was accumulated across rounds within one generation as a representation of fitness. After each generation, the agent with the worst fitness was replaced by a new agent (Syswerda, 1991). Specifically, agent I, with the lowest payoff, was selected to substitute its strategy with a new strategy randomly picked from the corresponding strategy pool (i.e., the strategy of a classical agent was replaced by a new strategy randomly picked from the classical 10-strategy pool; the strategy of a manipulated agent was replaced by a new strategy randomly chosen from the restricted strategy pool). The intensity of the selection parameter ω denoted the dependency of choosing agent i according to its worst fitness. The intensity of the selection parameter ω denotes the strength of natural selection according to fitness. The higher the ω value is, the more likely it is that agents with worse payoffs in the population are to be substituted by new agents.
Cooperation index.
For a given network setting, we let the system evolve for 100,000 generations to reach a stable state in line with previous work (Szolnoki and Perc, 2015; Gross and De Dreu, 2019). Then, we computed the cooperative acts indicated by the strategies at the beginning of each generation to exclude the confounding factors in terms of neighbors' choice across rounds. We averaged the cooperative acts across the last 5 × 104 generations as the proportion of cooperative acts out of all actions in the population. We further calculated the cooperation index under four typical conditions (ChighPlow, ClowPlow, ChighPhigh, and ClowPhigh), with a cooperation rate ranging from 0 to 20% (80–100%) indicating low (high) cooperation and a punishment rate ranging from 0 to 20% (80–100%) indicating low (high) punishment. To calculate the impact of each manipulation, we conducted regression analyses, predicting the observed cooperation rate based on the manipulation of central agents. We also calculated the rate of each strategy for peripheral nodes under each condition. To further compare the impact of the cooperation and punishment manipulations, we plotted the isoclines of the cooperation index in a two-dimensional parameter space of manipulated cooperation and punishment rates and calculated the slope of the isoclines along the y-axis (i.e., the manipulated cooperation rate) as the gradient of the isoclines. Specifically, we focused on the slope equaling 0 as the 90° gradient of the isoclines, where central cooperation did not influence the proportion of cooperation in peripheral nodes, and the slope equaling 1 as the 45° gradient of the isoclines, where central cooperation and punishment had the same influence on peripheral cooperation.
Parameter space.
For the ease of calculation and comparability, our main analyses focus on moderate selection strength (i.e., ω = 8) as well as the same population size (i.e., N = 12), and same parameters (i.e., heterogeneous degree, 'rich-club' coefficient, and cost ratio) of the network as in the behavioral experiment. To examine the robustness of the evolutionary results, we also investigated the evolutionary dynamics across a wider parameter space. We ran simulations sampled with various parameter sets, including population size N ∈ {12, 40, 100}, heterogeneous degree: r ∈ {5:3, 2:1}, selection strength: ω ∈ {6, 8, 10}, cost ratio: ∈ {2:3, 2:4, 2:5}, and the 'rich-club' coefficient (i.e., the number of central-to-central connections): ∈ {3, 2, 1, 0}. The evolutionary outcome of changed heterogeneity, the cost ratio, and larger network size with the same agent-based simulation process yielded similar results, showing that the observations were generalizable across this wider parameter space.
Comparison models.
We employ additional evolutionary models to investigate the result of manipulating the cooperation and punishment rates on i) peripheral nodes (rather than central nodes) in the same degree-heterogeneous network; ii) different number of central nodes. The main difference between the peripheral manipulation model and our main model was the position of manipulated agents: we chose three unconnected (to keep the same number of manipulated nodes) or four peripheral nodes (to keep the same minimal coverage of manipulated nodes) as manipulated cooperators. The main difference between the altered number of manipulated central model and our main model was the number of manipulated central agents: we chose one or two central node as manipulated cooperators. All other modeling parameters were the same as in our main model, where we manipulated the central nodes. In addition, we ran additional models with only one or two central nodes surrounded by peripheral nodes. These alternative models allowed us to compare the importance of centrality in spreading cooperation.
Data availability
The custom routines for data analysis written in MATLAB are available from the corresponding author on reasonable request.
Results
Behavioral results
Oxytocin elevated local trust, but failed to increase global trust in social networks
Experiment 1 implements a network version of the TG in which an individual, as an investor, transfers resources to another individual (trustee). Transfers triple in value, and trustees decide how much to return to their investors. Thus, through trust and reciprocation, both investors and trustees can gain. Yet, trust can be exploited by a trustee who does not return any resources (Berg et al., 1995; Engelmann et al., 2019). Before entering the social network, central investors played a personal version of the TG (one-shot). We found that central investors who received oxytocin (relative to placebo) increased their investment (27 central investors in 9 oxytocin networks vs 30 central investors in 10 placebo networks: 44.33 ± 2.83 vs 32.44 ± 3.37; t(17) = 2.70; p = 0.015; Cohen's d = 1.27; 95% CI, 2.55, 21.23).
In the subsequent network trust game, each 18-person TG network had nine trustees connected to four investors each. Investors differed in their centrality. Three central investors were connected to six neighboring trustees, while six peripheral investors were connected to only three trustees (Fig. 1b). In each TG round, an investor made a single investment t (0 ≤ t ≤ 100), which was tripled and received by all neighboring trustees simultaneously. Each trustee then learned about the received investments from neighboring investors and decided on a single return rate r (0 ≤ r ≤ 1) for all of her neighbors. For each connected investor–trustee pair, the trustee earned the remaining amount [3t × [1 – r)], and the investor earned its remained resources as well as the returns from trustees [(100 – t) + 3t × r]. Participants learned about their neighbors' decisions at the end of each round. Payoffs were calculated as the average earnings across connected pairs.
Experimental instructions for experiments 1–3. Download Figure 1-1, DOCX file (27.9KB, docx) .
Central investors given oxytocin transferred more money to their connected trustees and, hence, trusted them more compared with those given the placebo (27 central investors in 9 oxytocin networks vs 30 central investors in 10 placebo networks: 48.79 ± 7.32 vs 30.22 ± 4.43; t(17) = 2.22; p = 0.040; Cohen's d = 1.01; 95% CI, 0.93, 36.20, Fig. 1c; results from GLMM analysis: t(55) = 2.02; p = 0.048; 95% CI, 0.13, 31.18). However, this increased investment did not influence their neighboring trustees' return rate (oxytocin vs placebo: 31.87 ± 2.07 vs 30.66 ± 1.27; t(17) = 0.51; p = 0.616; Cohen's d = 0.23; 95% CI, −3.80, 6.22; Bayes factor = 2.85 as the possibility ratio of null hypothesis to the alternative hypothesis; Fig. 1d). In addition, peripheral investors' decisions did not differ between oxytocin and placebo networks (oxytocin vs placebo: 40.92 ± 6.45 vs 36.01 ± 2.73; t(17) = 0.728; p = 0.477; Cohen's d = 0.33; 95% CI, −9.33, 19.15; Bayes factor = 2.56; Fig. 1e). These results indicate that oxytocin made the central nodes more trusting but did not increase global trust or reciprocity in the social network in which they were embedded.
Oxytocin promotes global fairness in the repeated ultimatum bargaining game
Experiment 2 examined an alternative mechanism through which the local administration of oxytocin to central nodes can increase global cooperation in degree-heterogeneous networks—the enforcement of a fairness norm. To this end, we implemented a network version of a UBG with the same heterogeneous structure as that of the TG network. In each of the 18-person UBG groups (324 participants), the network had three central responders, each connected to six proposer neighbors; six peripheral responders, each connected to three proposers; and nine proposers, each connected to four responders (Fig. 2a). Central responders received either oxytocin or placebo. In each round, each proposer made a single offer [poffer (0 ≤ poffer ≤ 100)] to all neighboring responders, and each responder indicated a single minimum amount [raccept (0 ≤ raccept ≤ 100)] that they would accept for all neighboring proposers. For each pair, if poffer ≥ raccept, the offer was accepted, with the proposer keeping 100 – poffer and the responder receiving poffer; otherwise, both received nothing. The payoff in each round was averaged over connected pairs. Accordingly, across trials and through deals made or not, investors and responders were able to converge at a mutually acceptable distribution of resources. It should be noted that, before entering the network, central responders given oxytocin (vs placebo) showed an increased acceptance level in the personal version of UBG (27 central responders in nine oxytocin networks vs 27 central responders in nine placebo networks: 37.44 ± 2.76 vs 27.89 ± 2.19; t(16) = 2.71; p = 0.015; Cohen's d = 1.28; 95% CI, 2.09, 17.02).
The global fairness indicated by the whole network poffer increased significantly in those networks where central responders were given oxytocin (vs placebo: 49.26 ± 0.55 vs 46.32 ± 1.14; t(16) = 2.32; p = 0.034; Cohen's d = 1.09; 95% CI, 0.26, 5.62; Fig. 2b; GLMM results: t(160) = 2.46; p = 0.015; 95% CI, 0.58, 5.29). The mean offer of 49.26 in oxytocin networks closely matched the maximally fair offer of a 50–50 split (poffer in oxytocin network vs 50: t(8) = −1.35; p = 0.213; Cohen's d = −0.45; 95% CI, 47.99, 50.52; Bayes factor = 1.89), while in placebo networks the average poffer was significantly <50 (t(8) = −3.23; p = 0.012; Cohen's d = −1.08; 95% CI, 43.69, 48.95; Fig. 2b; GLMM results: t(80) = −3.42, p = 9.841 × 10−4, 95% CI, 44.18, 48.46). Accordingly, oxytocin networks achieved more fair deals (oxytocin vs placebo: 49.85 ± 0.46 vs 47.88 ± 0.62; t(16) = 2.55; p = 0.022; Cohen's d = 1.20; 95% CI, 0.33, 3.61; GLMM results: t(160) = 2.67; p = 0.008; 95% CI, 0.51, 3.39) and a more equal distribution of wealth between responders and proposers (oxytocin vs placebo: −0.45 ± 0.95 vs −4.76 ± 1.50; t(16) = 2.42; p = 0.028; Cohen's d = 1.14; 95% CI, 0.54, 8.09; Fig. 2c; GLMM results: t(160) = 2.57; p = 0.011; 95% CI, 0.99, 7.63; wealth disparity in oxytocin network vs 0: t(8) = −1.35; p = 0.652; Cohen's d = 0.16; 95% CI, −1.75, 2.65; Bayes factor = 3.71). In placebo networks, the responders' payoffs were significantly lower than those of proposers (t(8) = −3.16; p = 0.013; Cohen's d = 1.05; 95% CI, −8.22, −1.29; Fig. 2c; GLMM results: t(80) = −3.36; p = 0.001; 95% CI, −7.58, −1.94).
The establishment of network-wide fairness in oxytocin networks was driven by an increase in the acceptance threshold of neighboring responders. Each proposer received responses from four (two central and two peripheral) neighboring responders with different levels of acceptance, and the proposer would make all deals succeed when their offer met or exceeded the highest acceptance level set by any of their neighboring responders (referred to as rmax). We found that proposers tracked and matched the responders' highest acceptance level (poffer ≥ rmax) in 80.3% of the rounds (Fig. 2d). Intriguingly, responders in oxytocin network maintained a maximally fair 50–50 split, rmax (rmax = 50.27; vs 50: t(8) = 0.62; p = 0.553; Cohen's d = 0.21; 95% CI, 49.26, 51.29; Bayes factor = 3.44), which was not observed in placebo networks (rmax = 48.52 vs 50: t(8) = −2.81; p = 0.023; Cohen's d = −0.94; 95% CI, −2.69, −0.27; GLMM results: t(80) = −2.98; p = 0.004; 95% CI, −2.46, −0.49; treatment comparison: oxytocin vs placebo: 50.27 ± 0.44 vs 48.52 ± 0.52; t(16) = 2.55; p = 0.021; Cohen's d = 1.20; 95% CI, 0.30, 3.20; Fig. 2e; GLMM results: t(160) = 2.71; p = 0.008; 95% CI, 0.47, 3.03). Proposers, accordingly, adjusted their poffer upward in oxytocin networks. These adjustments, predicted by oxytocin administration, were fully mediated by changes in rmax (b18 = 0.371, SE = 0.185; 95% CI, 0.116, 0.821; Fig. 2f).
Further analyses tested whether the center (i.e., rmax central) or peripheral (i.e., rmax peripheral) responders required the highest acceptance threshold to keep proposers increasing their offer. This showed that oxytocin indeed induced a higher acceptance threshold in central responders (higher rmax central in oxytocin vs placebo networks: 47.50 ± 0.61 vs 43.58 ± 1.72; t(16) = 2.15; p = 0.048; Cohen's d = 1.01; 95% CI, 0.04, 7.79; Fig. 2g; GLMM results: t(52) = 2.27; p = 0.027; 95% CI, 0.46, 7.38), but not in peripheral responders (rmax peripheral in oxytocin vs placebo networks: 47.70 ± 0.96 vs 45.92 ± 1.70; t(16) = 0.914; p = 0.374; Cohen's d = 0.43; 95% CI, −2.36, 5.93; Bayes factor = 2.03; Fig. 2h). Importantly, with the increased enforcement of fairness by central players given oxytocin, peripheral players also increased their maximum demand for a fair distribution of resources over time (the slope of rmax peripheral in oxytocin networks: r(9) = 0.591; p = 0.012; Cohen's d = 1.09; Fig. 2i; GLMM results: t(53) = 2.29; p = 0.026; 95% CI, 0.03, 0.38), but not in placebo networks (r(9) = 0.122; p = 0.41; Fig. 2i; GLMM results: t(53) = 1.15; p = 0.26; 95% CI, −0.09, 0.34). Eventually, this led to the establishment of a network-wide fairness norm.
Oxytocin increased network cooperation in the two-stage prisoner's dilemma game
Together, experiments 1 and 2 suggest that oxytocin facilitates the spreading of cooperation in social networks not so much through a “pay it forward” effect but rather through stricter enforcement of a fairness norm. To test this possibility directly, experiment 3 implemented a tPDG with costly punishment (Fig. 3a), in which prosociality and norm enforcement could be observed in combination. In stage I, participants chose to either cooperate or defect, with the chosen option applied to all neighbors simultaneously. In stage II, each participant was shown the number of cooperation or defection choices of neighbors and was able to punish, at a personal cost, neighbors who chose defection in stage I (analogous to deciding to punish unfair offers by rejecting them in experiment 2; detailed in the Experimental design section).
We implemented 17 tPDG networks (204 participants, with 12 per network). In each degree-heterogeneous bipartite tPDG network, there were three central players and nine peripheral players, with unchanging neighbors (Fig. 3a). Each central player was connected to eight neighbors (two central players and six peripheral players), and each peripheral player was connected to only four neighbors (two central players and two peripheral players). Central players were given either oxytocin or placebo. Networks with central players administered oxytocin showed an increased rate of cooperation at the whole network level (27 central responders in nine oxytocin networks vs 24 central responders in eight placebo networks: 0.36 ± 0.06 vs 0.19 ± 0.05; t(15) = 2.14; p = 0.049; Cohen's d = 1.05; 95% CI, 0.001, 0.35; Fig. 3b; GLMM results: t(202) = 2.28; p = 0.024; 95% CI, 0.02, 0.32), with fewer defect–defect interactions (oxytocin vs placebo: 0.36 ± 0.06 vs 0.19 ± 0.05; t(15) = −2.42; p = 0.029; Cohen's d = −1.17; 95% CI, −0.45, −0.03; Fig. 3c; GLMM results: t(202) = −2.57; p = 0.011; 95% CI, −0.42, −0.06). Similar to UBG networks, we also found a trend of higher punishment rates in oxytocin (vs placebo) networks (oxytocin vs placebo: 0.14 ± 0.02 vs 0.08 ± 0.02; t(15) = 1.89; p = 0.079; Cohen's d = 0.91; 95% CI, −0.007, 0.12; Fig. 3d; GLMM results: t(202) = 2.01; p = 0.046; 95% CI, 0.001, 0.11). Moreover, untreated peripheral players in oxytocin (vs placebo) networks chose to cooperate to a greater degree (oxytocin vs placebo: 0.39 ± 0.06 vs 0.20 ± 0.05; t(15) = 2.27; p = 0. 039; Cohen's d = 1.11; 95% CI, 0.01, 0.36; Fig. 3e; GLMM results: t(151) = 2.41; p = 0.017; 95% CI, 0.03, 0.34), switched from defection to cooperation more often (oxytocin vs placebo: 0.24 ± 0.04 vs 0.11 ± 0.02; t(15) = 2.82; p = 0.013; Cohen's d = 1.40; 95% CI, 0.03, 0.22; GLMM results: t(147) = 2.35; p = 0.020; 95% CI, 0.03, 0.29), and punished free-riding more often (oxytocin vs placebo: 0.14 ± 0.01 vs 0.08 ± 0.02; t(15) = 2.35; p = 0.033; Cohen's d = 1.13; 95% CI, 0.01, 0.11; Fig. 3f; GLMM results: t(151) = 2.21; p = 0.028; 95% CI, 0.01, 0.10).
Central punishment rather than cooperation drives the spread of cooperation
Follow-up analyses revealed that oxytocin facilitated the spread of cooperation and punishment among peripheral players because of the increased enforcement of cooperation rather than reciprocal cooperation. First, although central players tended to exhibit more nonpunishing cooperation (i.e., choosing to cooperate without punishing others' defection) in oxytocin (vs placebo) networks (oxytocin vs placebo: 0.24 ± 0.04 vs 0.15 ± 0.02; t(15) =1.92; p = 0.074; Cohen's d = 0.94; 95% CI, −0.01, 0.19; Fig. 3g; GLMM results: t(49) = 2.04; p = 0.047; 95% CI, 0.001, 0.176), this increase in nonpunishing cooperation did not influence the peripheral cooperation rate (oxytocin vs placebo: −0.04 ± 0.02 vs −0.02 ± 0.02; t(15) = −0.68; p = 0.507; Cohen's d = −0.33; 95% CI, −0.08, 0.04; GLMM results: t(145) = −0.06; p = 0.950; 95% CI, −0.058, 0.054). Second, central players in oxytocin networks decreased their punishment rate across time more slowly than did those in placebo networks (oxytocin vs placebo: −0.18 ± 0.04 vs −0.37 ± 0.07; t(15) = 2.36; p = 0.032; Cohen's d = 1.12; 95% CI, 0.02, 0.36; Fig. 3h; GLMM results: t(48) = 4.58; p = 3.291 × 10−5; 95% CI, 0.102, 0.260) and punished defection to a greater degree, even when more than half of their neighbors had defected (oxytocin vs placebo: 0.15 ± 0.14 vs −0.36 ± 0.12; t(15) = 2.42; p = 0.032; Cohen's d = 1.29; 95% CI, 0.05, 0.96; GLMM analysis showed a similar trend: t(23) = 1.61; p = 0.121; 95% CI, −0.08, 0.64). More importantly, receiving punishment from neighboring central players not only increased the cooperation rate of peripheral players in oxytocin (vs placebo) networks (oxytocin vs placebo: 0.39 ± 0.05 vs 0.22 ± 0.03; t(15) = 2.74; p = 0.015; Cohen's d =1.35; 95% CI, 0.04, 030; Fig. 3i; GLMM analysis showed a similar trend: t(126) = 1.27; p = 0.207; 95% CI, −0.055, 0.252) but also increased the likelihood of peripheral players punishing defection (oxytocin vs placebo: 0.07 ± 0.03 vs −0.01 ± 0.02; t(15) = 2.16; p = 0. 047; Cohen's d = 1.07; 95% CI, 0.001, 0.16; Fig. 3j; GLMM results: t(126) = 2.53; p = 0.013; 95% CI, 0.024, 0.195). Similar to experiment 2, untreated peripheral players adopted the increased norm enforcement of their fellow central players who received oxytocin.
The experimental results, combined, resonate with earlier work showing that oxytocin increases both cooperation (De Dreu et al., 2010; Israel et al., 2012) and the punishment of noncooperators (Aydogan et al., 2017; Daughters et al., 2017; Stallen et al., 2018). We further reveal how oxytocin-enhanced willingness to cooperate and to costly punish free riding facilitates the spread of cooperation throughout social networks. In fact, across experiments, the results suggest that both enhanced cooperation and enhanced norm enforcement are needed for central players to influence and facilitate the emergence of network-wide cooperation.
Evolutionary modeling
Evolutionary results of manipulating central enforcement in agent-based simulation
To further illustrate and validate the importance of the behavior of central nodes for the emergence of network cooperation, we implemented agent-based simulations grounded in evolutionary game theory (Santos et al., 2006; Iyer and Killingback, 2016; Allen et al., 2017). In particular, we tested to what degree network-wide cooperation hinges on the prosocial preferences of central nodes and their willingness to enforce a norm of cooperation through punishment in isolation and in combination. This approach allowed us to see whether and how our empirical findings (1) contribute to understand the social evolution of cooperation and (2) generalize across a wider parameter space than we could implement experimentally. To this end, we simulated computer agents that interacted in a heterogeneous network following the structure in experiment 3, playing repeated two-stage prisoner's dilemma games, with central nodes that differed in terms of their cooperation and punishment level and peripheral nodes using strategies randomly selected from a pool of possible strategies.
In line with our experimental results, these simulations revealed that cooperative central nodes with a high punishment rate (ChighPhigh) induced the highest cooperation rate in the rest of the population (i.e., the periphery of the network; Fig. 4a,b). In contrast, and resonating with the results from experiment 1, cooperation failed to invade the rest of the population when central cooperators seldomly punished (ClowPlow and ChighPlow; Fig. 4b). Instead, and consistent with the results of experiments 2 and 3, punishment by central nodes (ClowPhigh and ChighPhigh) promoted the emergence of peripheral cooperators (Fig. 4b). Indeed, when we consider the isoclines of cooperation rate in two-dimensional parameter space (C and P; Fig. 4a), we can see that punishment is more influential than cooperation in facilitating the spread of cooperation to the rest of the population. When central nodes punish only slightly (p < 0.2), the increased cooperation of central nodes does not result in increased cooperation in the periphery (i.e., the slope of isoclines is ∼0; Fig. 4a). With mild punishment (0.2 < p < 0.4), punishment accounts for the increasing cooperation in peripheral agents to a greater degree than does cooperation (Fig. 4a). Only with moderately harsh punishment (p > 0.4), does the cooperation of central nodes appear as efficient as punishment in inducing network cooperation (i.e., the slope of isoclines = 1; Fig. 4a).
Figure 4.
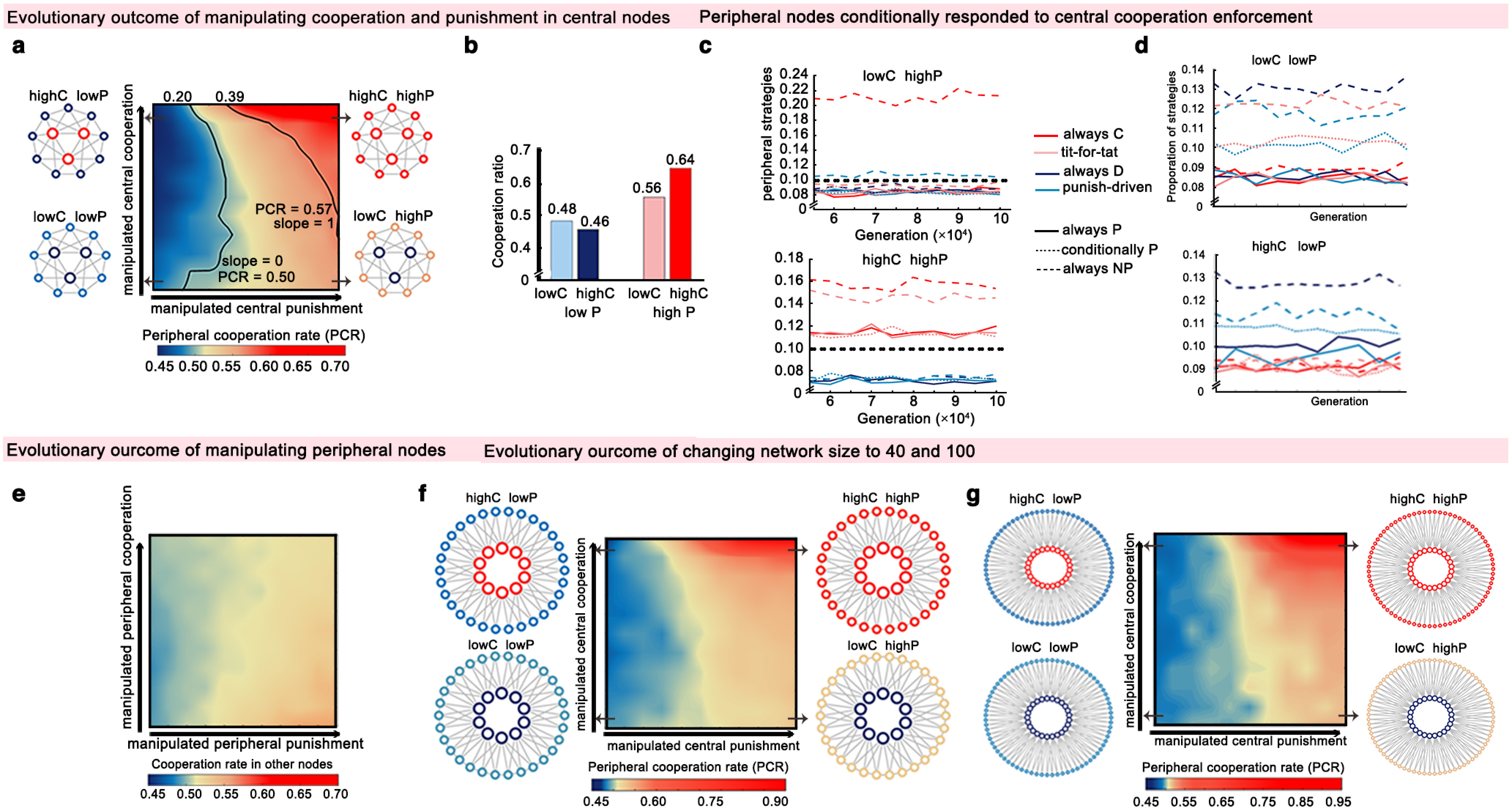
Evolution of peripheral cooperation when central nodes vary in cooperation and punishment rate. a, b, Cooperation rate of the periphery of the network depending on the punishment rate and cooperation rate of central nodes in the simulated agent-based networks. The networks in each corner illustrate the cooperation rate of all peripheral agents in the network based on high/low central cooperation/punishment. The slope analysis of isoclines (of cooperation rate in a two-dimensional parameter space of cooperation and punishment) showed that central cooperators failed to affect the rest of the population when they seldomly punished (slope = 0 when p = 0.20). In contrast, central cooperation is as effective as punishment only if cooperation is enforced by moderate to strong punishment (slope = 1 when p = 0.39). c, d, The evolutionary dynamics of the strategies used by the peripheral nodes with high (c) or low (d) central punishment. Whereas the always-cooperate strategy was favored in the ClowPhigh condition, punitive cooperators (ChighPhigh) in the central position facilitated the use of conditional cooperation (i.e., tit-for-tat strategy) and punishment strategies (i.e., always-punish and conditionally punish strategies) in the peripheral nodes. With low central punishment, only always-defect combined with always-nonpunish strategy slightly outperformed other strategies in peripheral players, regardless of the cooperation level of central nodes (ClowPlow and ChighPlow). e, An alternative evolutionary model that manipulates peripheral nodes shows a much weaker effect on global cooperation. f, g, Cooperation rate of the periphery of the network depending on central punishment and cooperation rates in networks with population sizes of 40 (f) and 100 (g). Cooperation outcomes of alternative population sizes reveal the same pattern. highC, high cooperation rate; lowC, low cooperation rate; highP, high punishment rate; lowP, low punishment rate; always C, always-cooperate; always D, always-defect; always P, always-punish; conditionally P, conditionally-punish; always NP, always-non-punish.
In line with experiments 2 and 3, our simulations reveal that peripheral nodes conditionally adopt the cooperative norm and increase their own punishment rate in response to the cooperation enforcement of central nodes. The punishment itself (ClowPhigh) appeared sufficient for promoting network cooperation (Fig. 4b) and increased the likelihood that peripheral nodes switch to a strategy of always-cooperate (Fig. 4c,d; for strategies used when punishment of central nodes was high or low). However, only punitive cooperators in central positions (ChighPhigh) lead peripheral nodes to switch to conditional cooperation (i.e., tit-for-tat) strategies and punish noncooperation (Fig. 4c).
Control models confirm the role of centrally enforced cooperation
We ran additional models in which we exogenously manipulated the cooperation and punishment rate of peripheral nodes rather than central nodes in the network. The results revealed a much weaker effect on altering global cooperation when manipulating peripheral nodes (Fig. 4e). Neither altering peripheral cooperation nor altering punishment dramatically changed network-wide cooperation. This supports the critical role of norm enforcement by central, as opposed to peripheral, players in the cooperation dynamic in the network. The observed dynamics generalize to large-scale networks, as the effect of manipulating the central nodes remains when the population size is increased to 40 agents (Fig. 4f) and 100 agents (Fig. 4g). Moreover, our findings were qualitatively insensitive to heterogeneity degree (Fig. 5a), selection strength (Fig. 5b), cost ratio (Fig. 5c), and the rich-club coefficient (i.e., the number of central-to-central connections; Fig. 5d–f).
Figure 5.
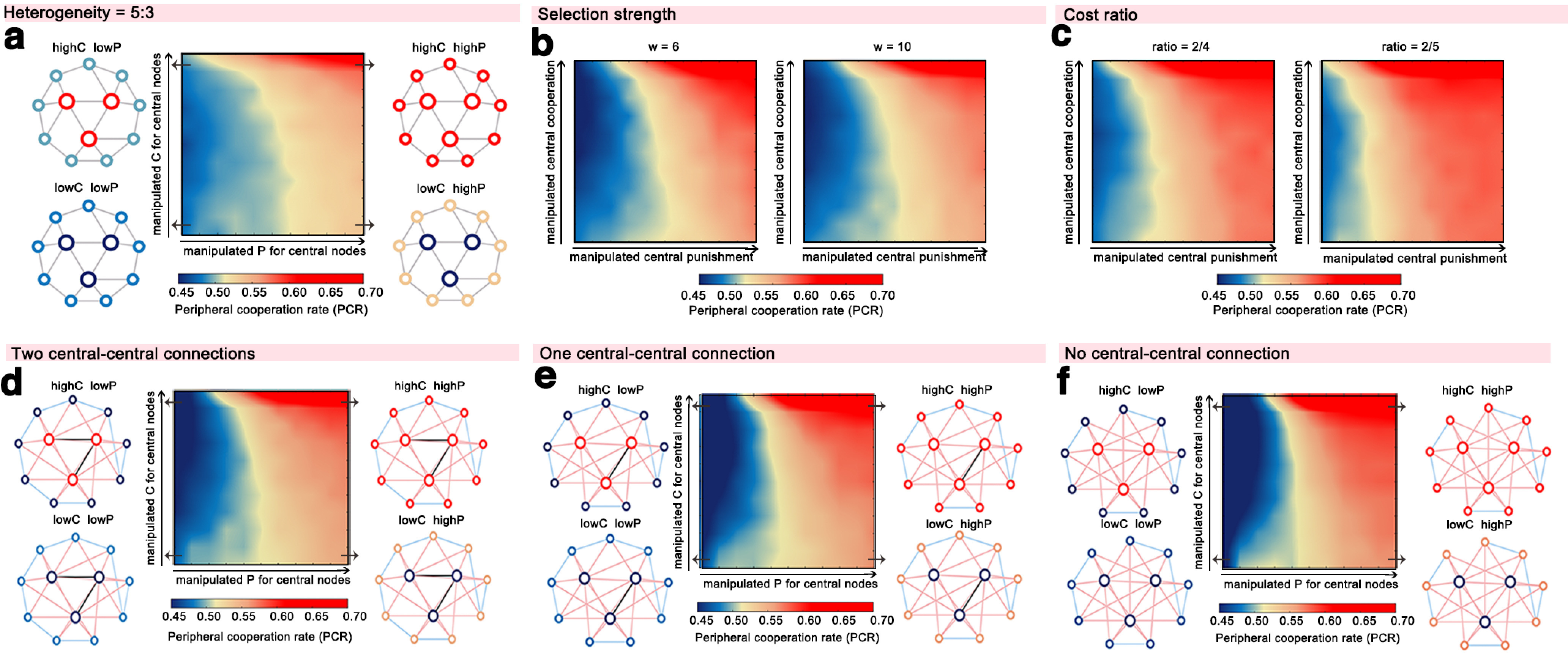
Consistent results of the alternative agent-based models with varied heterogeneity degree, selection strength, cost ratio, and the number of central-to-central connections. a–c, Cooperation rate of the periphery of the network depending on the punishment rate (x-axis) and cooperation rate (y-axis) of central nodes in the simulated agent-based networks where the heterogeneity degree equals 5:3 (i.e., central nodes are connected with five nodes and peripheral nodes are connected with three nodes; a), selection strength equals 6 or 10 (b), or cost ratio equals 2:4 or 2:5 (c), while other parameters remain unchanged. Changes in these parameters revealed the same pattern that altering the punishment propensity of central nodes is more powerful than altering cooperation rates in increasing peripheral cooperation. d–f, Results of the alternative agent-based models with 2 (d), 1 (e), and 0 (f) central-to-central connections, with descending synchronization effect of the central community (central community even disappeared in models in e and f). The patterns of cooperation spread was qualitatively insensitive to the rich-club coefficient and remained similar as the original model in these alternative models (black lines, central–central connections; pink lines, central–peripheral connections; blue lines, peripheral–peripheral connections). highC, high cooperation rate; lowC, low cooperation rate; highP, high punishment rate; lowP, low punishment rate.
Finally, we examined whether and how the number of manipulated central nodes and its coverage in the network influenced cooperation spreading by manipulating different numbers of central nodes with alternative models. We observed a similar pattern as in the original model when manipulating the cooperation and punishment rate of two-thirds of the central nodes (i.e., two central nodes in the current network structure), as follows: (1) two cooperative central nodes with a high punishment rate (ChighPhigh) induced the highest cooperation rate in the rest of the population; and (2) punishment by two central nodes (ClowPhigh and ChighPhigh) promoted the emergence of peripheral cooperators (Fig. 6a,b). However, manipulating the cooperation and punishment rate of one central node was not sufficient to alter global cooperation (Fig. 6c,d). In addition, the number of manipulated central nodes covaried with the coverage of manipulated nodes (i.e., directly influenced connections: the connections between each manipulated node and other nodes). Thus, it is possible that the coverage (i.e., the number of directly influenced connections) alone could be essential for cooperation spreading. We tested this possibility in a model that kept the same minimal required number of directly influenced connections (i.e., 15 of 30 connections) but manipulated the cooperation and punishment rate of four peripheral nodes rather than two central nodes in the network (Fig. 6e). The results revealed a much weaker effect on altering global cooperation when manipulating peripheral nodes with the same coverage (Fig. 6f). The results suggest that broad coverage, especially central coverage, is necessary and more efficient in facilitating network-wide cooperation. Together, influential nodes cannot only facilitate network-wide cooperation but can also foster network-wide norm enforcement through their punishment behavior in heterogeneous structures.
Figure 6.
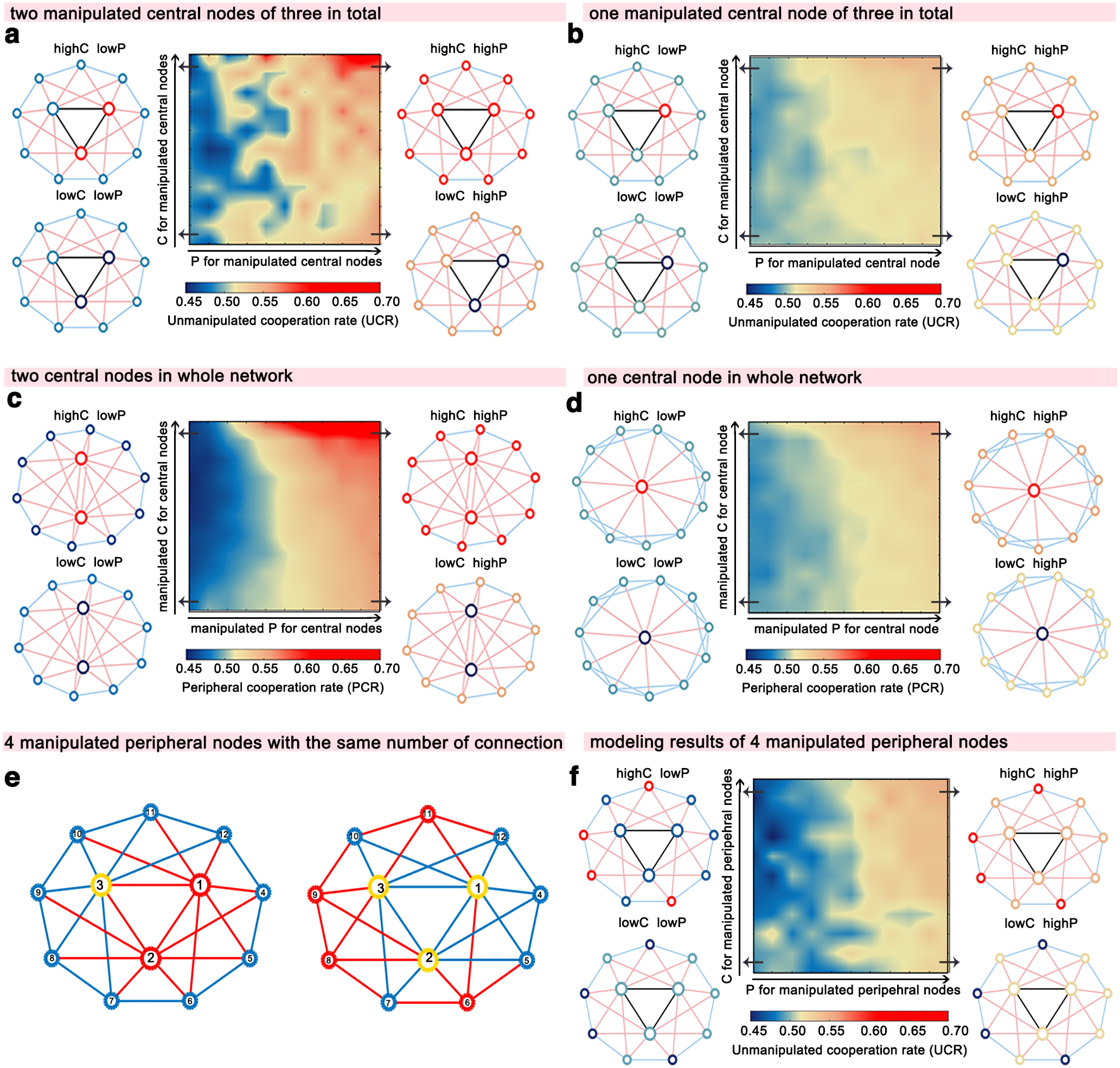
Results of the alternative agent-based models with varied numbers of manipulated central nodes and central coverage. a, b, With the same network structure as the original model, we manipulated cooperation/punishment rates only for two central nodes (a) or one central node (b). c, d, With the same number of 12 nodes and 30 edges as the original model, we changed the number of central nodes: 2 central nodes (c) or 1 central node (d) in alternative models. We found central nodes to be less influential on altering global cooperation when decreasing the number of central nodes. Specifically, the pattern remained similar when manipulating cooperation/punishment rated for two central nodes (a, c). However, when the cooperation/punishment rates were manipulated on only one central node, the cooperation spreading effect disappeared (b, d). e, f, Results of alternative agent-based models when manipulating peripheral nodes with the same coverage. e, Network structures with the same 15 (of 30) directly influenced connections (red lines). To achieve 50% coverage of manipulated nodes, we manipulated the cooperation/punishment rate of two central nodes (i.e., nodes 1 and 2) or four peripheral nodes (i.e., nodes 6, 8, 9, 11; red circles). The number of central, peripheral nodes, and the number of directly influenced connections, and the structures were the same as in these two models. Yellow circles, Unmanipulated central nodes; blue circles, unmanipulated peripheral nodes; blue lines, connections between unmanipulated nodes. f, Although we manipulated more peripheral nodes, manipulations of peripheral nodes resulted in a similar but much weaker tendency of the cooperation/punishment influence. Black lines, Central–central connections; pink lines, central–peripheral connections; blue lines, peripheral–peripheral connections. highC, high cooperation rate; lowC, low cooperation rate; highP, high punishment rate; lowP, low punishment rate.
Discussion
Large-scale cooperation among genetically unrelated individuals is not only a core building block of both ancestral and modern societies but also one of the most puzzling challenges from the ecological, evolutionary, and societal perspectives. Here, we identify costly punishment and network heterogeneity as important mechanisms that facilitate the propagation of cooperation in social networks. Through a series of laboratory experiments (combining economic decision-making paradigms with pharmacological challenge and spatially heterogeneous network structure) and evolutionary agent-based simulations, the current study examined how cooperative decisions spread from locally manipulated central individuals to untreated peripheral individuals and permeate social networks. The behavioral observations in the laboratory and evolutionary modeling results, combined, demonstrate that a joint effect of heterogeneous social ties and (oxytocin-initiated) costly punishment of central cooperators counters free riding allows the permeation of the network and enables the evolution of network cooperation.
The spread of cooperation in human social networks appears to rely not only on displays of cooperative behaviors but also needs a credible threat of punishment that discourages defection and unfair behavior. Oxytocin has been implicated in prosocial and cooperative behaviors (Israel et al., 2012; Spengler et al., 2017; Liu et al., 2019) and the enforcement of cooperative norms by punishing free riders of fairness norm violators (Aydogan et al., 2017; Stallen et al., 2018). Neuroimaging studies have shown that oxytocin administration increased prosocial behavior, which was associated with increased amygdala representation of social values (Liu et al., 2019) and enhanced anterior insula activity when signaling the willingness to punish norm violations (Stallen et al., 2018). We thus speculate that oxytocin could facilitate cooperation spreading, possibly through modulating the activity in the amygdala and anterior insula. This hypothesis would be interesting for future research to address.
The cascading effect of oxytocin-initiated prosociality was not observed in the repeated trust game: oxytocin increased trust, but no downstream effects were observed, possibly because investors in the trust game lacked an opportunity to punish exploitation, and this may discourage imitation (Engelmann et al., 2019). It thus seems that leading-by-example and imitation alone are not sufficient for promoting and sustaining cooperation in human social networks. Instead, we find in both our behavioral experiments and evolutionary modeling that cooperation only spreads as a joint function of influential cooperators who are willing to punish and enforce norms of fairness (Baldassarri and Grossman, 2011; Gavrilets and Richerson, 2017). Possibly, costly punishment (1) enables direct and indirect reciprocity through the sending of costly altruistic signals to galvanize the trace of network cooperation and (2) acts as a negative reinforcer to enforce conditional cooperation. Through costly punishment signals, network-central individuals act as paragons for peripheral individuals and induce a threat of loss that drives those who have been punished to switch from self-regarding behaviors to conditional cooperation. Our work reconciles the seemingly contradictory observations of the prevailing existence of costly punishment despite its implied costs for the individual and for the group as a whole (Dreber et al., 2008; Ohtsuki et al., 2009), providing a justification for such enforcement mechanisms in different cooperative systems. Accordingly, our results and simulations identify costly sanctioning as a crucial element in the evolution and spreading of cooperation in human networks, something seen also in other group-living species (Ågren et al., 2019). Crucially, our work suggests that oxytocin is a key element in the biological mechanism that helps (human) network-wide cooperation to emerge. Indeed, oxytocin increased not only individuals' prosociality, but also, and critically, their willingness to punish free riders and norm violators.
Prior studies have shown that cooperators are often promoted to influential positions in part because they are more successful in making connections with other cooperators (Rand et al., 2011), receive more support from fellow group members when taking on leadership positions (Hardy and van Vugt, 2006), and are entrusted with more punishment power (Gross et al., 2016). In our experiments, we did not give oxytocin (or placebo) to peripheral nodes of the network, which would further show whether the oxytocin effect on cooperation spreading is constrained to central nodes. However, we provided evidence for the critical role of central (rather than peripheral) nodes in cooperation spreading in social networks, both empirically and theoretically. First, we found that norm enforcement of central (but not peripheral) players positively predicts fairness at the level of the entire network, providing evidence of the quantitative influence of central enforcement on network-wide cooperation. Second, alternative simulation models manipulated the cooperation and punishment rates of (1) peripheral nodes and (2) a different number of central nodes. These simulations revealed that altering neither peripheral cooperation nor punishment dramatically changed network-wide cooperation, suggesting a much weaker effect on altering global cooperation when manipulating peripheral nodes (even with the same coverage). In addition, when two-thirds of the central agents were cooperators and willing to punish noncooperation, this allowed global cooperation to emerge in the network. Thus, our evolutionary agent-based simulations show that cooperative agents placed at the most connected central place are more efficient at enforcing and spreading cooperation (than peripheral cooperators), possibly because of their efficiency in enforcing cooperative norms via punishment. Accordingly, our findings offer a mechanistic explanation for the formation of cooperation through the effective alignment of degree centrality with prosociality and norm enforcement (Gürerk et al., 2006; Baldassarri and Grossman, 2011; Kleineberg, 2017). It explains the observation that prosocial individuals disproportionally occupy influential positions in a range of group-living species (Pike et al., 2008), humans included (Rand and Nowak, 2013; Lyle and Smith, 2014). It suggests that natural selection and cultural institutions favor individuals with specific (oxytocin-mediated) traits to not only act prosocially, but also to punish norm violations at a personal cost.
Together, present experiments and agent-based simulations reveal that increasing oxytocin in individuals having central network positions can propagate the spreading of cooperation in heterogeneous social networks. Crucially, we find that the impact of (pharmacologically enhanced) social preferences is constrained by the spatial structure of the network and the enforcement of cooperation through peer punishment. This biobehavioral mechanism aligns individual neurocognitive states and preferences with social institutions and cooperation norms in the dynamic shaping of large-scale cooperation in human societies and, possibly, the evolution of social organization in group-living species more generally.
Footnotes
This work was supported by the National Natural Science Foundation of China (Grant 32125019, 31771204 to Y.M.; Grant 72131003 to B.Z.); the National Key Research and Development Program of China (Grant 2022ZD0211000 to Y.M.); the Major Project of National Social Science Foundation (Grant 19ZDA363 to Y.M.); the Fundamental Research Funds for the Central Universities (Grant 2018EYT04 to Y.M.); the start-up funding from the State Key Laboratory of Cognitive Neuroscience and Learning, IDG/McGovern Institute for Brain Research, Beijing Normal University (to Y.M.); and the European Research Council under the European Union's Horizon 2020 Research and Innovation Programme (ADG agreement 785635 to C.K.W.D.D.).
The authors declare no competing financial interests.
References
- Ågren JA, Davies NG, Foster KR (2019) Enforcement is central to the evolution of cooperation. Nat Ecol Evol 3:1018–1029. 10.1038/s41559-019-0907-1 [DOI] [PubMed] [Google Scholar]
- Allen B, Lippner G, Chen YT, Fotouhi B, Momeni N, Yau ST, Nowak MA (2017) Evolutionary dynamics on any population structure. Nature 544:227–230. 10.1038/nature21723 [DOI] [PubMed] [Google Scholar]
- Apicella CL, Silk JB (2019) The evolution of human cooperation. Curr Biol 29:R447–R450. 10.1016/j.cub.2019.03.036 [DOI] [PubMed] [Google Scholar]
- Apicella CL, Marlowe FW, Fowler JH, Christakis NA (2012) Social networks and cooperation in hunter-gatherers. Nature 481:497–501. 10.1038/nature10736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydogan G, Furtner NC, Kern B, Jobst A, Müller N, Kocher MG (2017) Oxytocin promotes altruistic punishment. Soc Cogn Affect Neurosci 12:1740–1747. 10.1093/scan/nsx101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassarri D, Grossman G (2011) Centralized sanctioning and legitimate authority promote cooperation in humans. Proc Natl Acad Sci U S A 108:11023–11027. 10.1073/pnas.1105456108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battu B, Pammi VC, Srinivasan N (2018) Evolution of cooperation with heterogeneous conditional cooperators. Sci Rep 8:4524. 10.1038/s41598-018-22593-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg J, Dickhaut J, McCabe K (1995) Trust, reciprocity, and social history. Games Econ Behav 10:122–142. 10.1006/game.1995.1027 [DOI] [Google Scholar]
- Bird RB, Power EA (2015) Prosocial signaling and cooperation among Martu hunters. Evol Hum Behav 36:389–397. 10.1016/j.evolhumbehav.2015.02.003 [DOI] [Google Scholar]
- Bowles S, Gintis H (2004) The evolution of strong reciprocity: cooperation in heterogeneous populations. Theor Popul Biol 65:17–28. 10.1016/j.tpb.2003.07.001 [DOI] [PubMed] [Google Scholar]
- Boyd R, Gintis H, Bowles S (2010) Coordinated punishment of defectors sustains cooperation and can proliferate when rare. Science 328:617–620. 10.1126/science.1183665 [DOI] [PubMed] [Google Scholar]
- Buchan NR, Grimalda G, Wilson R, Brewer M, Fatas E, Foddy M (2009) Globalization and human cooperation. Proc Natl Acad Sci U|S|A 106:4138–4142. 10.1073/pnas.0809522106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS (2014) Oxytocin pathways and the evolution of human behavior. Annu Rev Psychol 65:17–39. 10.1146/annurev-psych-010213-115110 [DOI] [PubMed] [Google Scholar]
- Centola D (2010) The spread of behavior in an online social network experiment. Science 329:1194–1197. 10.1126/science.1185231 [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T (2009) Cooperation between non-kin in animal societies. Nature 462:51–57. 10.1038/nature08366 [DOI] [PubMed] [Google Scholar]
- Daughters K, Manstead AS, Ten Velden FS, De Dreu CK (2017) Oxytocin modulates third-party sanctioning of selfish and generous behavior within and between groups. Psychoneuroendocrinology 77:18–24. 10.1016/j.psyneuen.2016.11.039 [DOI] [PubMed] [Google Scholar]
- De Dreu CK, Greer LL, Handgraaf MJ, Shalvi S, Van Kleef GA, Baas M, Ten Velden FS, Van Dijk E, Feith SW (2010) The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science 328:1408–1411. 10.1126/science.1189047 [DOI] [PubMed] [Google Scholar]
- Dreber A, Rand DG, Fudenberg D, Nowak MA (2008) Winners don't punish. Nature 452:348–351. 10.1038/nature06723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann JB, Schmid B, De Dreu CK, Chumbley J, Fehr E (2019) On the psychology and economics of antisocial personality. Proc Natl Acad Sci U|S|A 116:12781–12786. 10.1073/pnas.1820133116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A (2007) G* Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–191. 10.3758/bf03193146 [DOI] [PubMed] [Google Scholar]
- Fehl K, Post DJ, Semmann D (2011) Co-evolution of behaviour and social network structure promotes human cooperation. Ecol Lett 14:546–551. 10.1111/j.1461-0248.2011.01615.x [DOI] [PubMed] [Google Scholar]
- Fehr E, Schurtenberger I (2018) Normative foundations of human cooperation. Nat Hum Behav 2:458–468. 10.1038/s41562-018-0385-5 [DOI] [PubMed] [Google Scholar]
- Fischer-Shofty M, Levkovitz Y, Shamay-Tsoory SG (2013) Oxytocin facilitates accurate perception of competition in men and kinship in women. Soc Cogn Affect Neurosci 8:313–317. 10.1093/scan/nsr100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo E, Yan C (2015) The effects of reputational and social knowledge on cooperation. Proc Natl Acad Sci U|S|A 112:3647–3652. 10.1073/pnas.1415883112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilets S, Richerson PJ (2017) Collective action and the evolution of social norm internalization. Proc Natl Acad Sci U|S|A 114:6068–6073. 10.1073/pnas.1703857114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracia-Lázaro C, Ferrer A, Ruiz G, Tarancón A, Cuesta JA, Sánchez A, Moreno Y (2012) Heterogeneous networks do not promote cooperation when humans play a Prisoner's Dilemma. Proc Natl Acad Sci U|S|A 109:12922–12926. 10.1073/pnas.1206681109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, De Dreu CK (2019) The rise and fall of cooperation through reputation and group polarization. Nat Commun 10:776. 10.1038/s41467-019-08727-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Méder ZZ, Okamoto-Barth S, Riedl A (2016) Building the Leviathan–Voluntary centralisation of punishment power sustains cooperation in humans. Sci Rep 6:20767. 10.1038/srep20767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gürerk O, Irlenbusch B, Rockenbach B (2006) The competitive advantage of sanctioning institutions. Science 312:108–111. 10.1126/science.1123633 [DOI] [PubMed] [Google Scholar]
- Hardy CL, Van Vugt M (2006) Nice guys finish first: the competitive altruism hypothesis. Pers Soc Psychol Bull 32:1402–1413. 10.1177/0146167206291006 [DOI] [PubMed] [Google Scholar]
- Hauert C, Traulsen A, Brandt H, Nowak MA, Sigmund K (2007) Via freedom to coercion: the emergence of costly punishment. Science 316:1905–1907. 10.1126/science.1141588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbe C, Chatterjee K, Nowak MA (2018) Partners and rivals in direct reciprocity. Nat Hum Behav 2:469–477. 10.1038/s41562-018-0320-9 [DOI] [PubMed] [Google Scholar]
- Hill KR, Walker RS, Božičević M, Eder J, Headland T, Hewlett B, Hurtado AM, Marlowe F, Wiessner P, Wood B (2011) Co-residence patterns in hunter-gatherer societies show unique human social structure. Science 331:1286–1289. 10.1126/science.1199071 [DOI] [PubMed] [Google Scholar]
- Israel S, Weisel O, Ebstein RP, Bornstein G (2012) Oxytocin, but not vasopressin, increases both parochial and universal altruism. Psychoneuroendocrinology 37:1341–1344. 10.1016/j.psyneuen.2012.02.001 [DOI] [PubMed] [Google Scholar]
- Kleineberg KK (2017) Metric clusters in evolutionary games on scale-free networks. Nat Commun 8:1888. 10.1038/s41467-017-02078-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurvers RH, Krause J, Croft DP, Wilson AD, Wolf M (2014) The evolutionary and ecological consequences of animal social networks: emerging issues. Trends Ecol Evol 29:326–335. 10.1016/j.tree.2014.04.002 [DOI] [PubMed] [Google Scholar]
- Li X, Jusup M, Wang Z, Li H, Shi L, Podobnik B, Stanley HE, Havlin S, Boccaletti S (2018) Punishment diminishes the benefits of network reciprocity in social dilemma experiments. Proc Natl Acad Sci U|S|A 115:30–35. 10.1073/pnas.1707505115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Li S, Lin W, Li W, Yan X, Wang X, Pan X, Rutledge RB, Ma Y (2019) Oxytocin modulates social value representations in the amygdala. Nat Neurosci 22:633–641. 10.1038/s41593-019-0351-1 [DOI] [PubMed] [Google Scholar]
- Iyer S, Killingback T (2016) Evolution of cooperation in social dilemmas on complex networks. PLoS Comput Biol 12:e1004779. 10.1371/journal.pcbi.1004779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyle HF, Smith EA (2014) The reputational and social network benefits of prosociality in an Andean community. Proc Natl Acad Sci U|S|A 111:4820–4825. 10.1073/pnas.1318372111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Li S, Wang C, Liu Y, Li W, Yan X, Chen Q, Han S (2016) Distinct oxytocin effects on belief updating in response to desirable and undesirable feedback. Proc Natl Acad Sci U|S|A 113:9256–9261. 10.1073/pnas.1604285113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciejewski W, Fu F, Hauert C (2014) Evolutionary game dynamics in populations with heterogenous structures. PLoS Comput Biol 10:e1003567. 10.1371/journal.pcbi.1003567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAvoy A, Allen B, Nowak MA (2020) Social goods dilemmas in heterogeneous societies. Nat Hum Behav 4:819–831. 10.1038/s41562-020-0881-2 [DOI] [PubMed] [Google Scholar]
- Newman ME, Barabási AL, Watts DJ (2006) The structure and dynamics of networks. Princeton, NJ: Princeton UP. [Google Scholar]
- Ohtsuki H, Iwasa Y, Nowak MA (2009) Indirect reciprocity provides only a narrow margin of efficiency for costly punishment. Nature 457:79–82. 10.1038/nature07601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike TW, Samanta M, Lindström J, Royle NJ (2008) Behavioural phenotype affects social interactions in an animal network. Proc Biol Sci 275:2515–2520. 10.1098/rspb.2008.0744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF (2008) Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods 40:879–891. 10.3758/brm.40.3.879 [DOI] [PubMed] [Google Scholar]
- Rand DG, Nowak MA (2013) Human cooperation. Trends Cogn Sci 17:413–425. 10.1016/j.tics.2013.06.003 [DOI] [PubMed] [Google Scholar]
- Rand DG, Arbesman S, Christakis NA (2011) Dynamic social networks promote cooperation in experiments with humans. Proc Natl Acad Sci U|S|A 108:19193–19198. 10.1073/pnas.1108243108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand DG, Nowak MA, Fowler JH, Christakis NA (2014) Static network structure can stabilize human cooperation. Proc Natl Acad Sci U|S|A 111:17093–17098. 10.1073/pnas.1400406111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Young LJ (2014) The biology of mammalian parenting and its effect on offspring social development. Science 345:771–776. 10.1126/science.1252723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, DeMarco AC, Hackett PD, Chen X, Gautam P, Stair S, Haroon E, Thompson R, Ditzen B, Patel R, Pagnoni G (2014) Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology 39:237–248. 10.1016/j.psyneuen.2013.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos FC, Pacheco JM, Lenaerts T (2006) Evolutionary dynamics of social dilemmas in structured heterogeneous populations. Proc Natl Acad Sci U|S|A 103:3490–3494. 10.1073/pnas.0508201103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos FC, Santos MD, Pacheco JM (2008) Social diversity promotes the emergence of cooperation in public goods games. Nature 454:213–216. 10.1038/nature06940 [DOI] [PubMed] [Google Scholar]
- Shirado H, Fu F, Fowler JH, Christakis NA (2013) Quality versus quantity of social ties in experimental cooperative networks. Nat Commun 4:2814. 10.1038/ncomms3814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler FB, Scheele D, Marsh N, Kofferath C, Flach A, Schwarz S, Stoffel-Wagner B, Maier W, Hurlemann R (2017) Oxytocin facilitates reciprocity in social communication. Soc Cogn Affect Neurosci 12:1325–1333. 10.1093/scan/nsx061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallen M, Rossi F, Heijne A, Smidts A, De Dreu CK, Sanfey AG (2018) Neurobiological mechanisms of responding to injustice. J Neurosci 38:2944–2954. 10.1523/JNEUROSCI.1242-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syswerda G (1991) A study reproduction in generational and steady-state genetic algorithms. Found Genet Algorithms 1:94–101. [Google Scholar]
- Szolnoki A, Perc M (2015) Conformity enhances network reciprocity in evolutionary social dilemmas. J R Soc Interface 12:20141299. 10.1098/rsif.2014.1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van IJzendoorn MH, Bakermans-Kranenburg MJ (2012) A sniff of trust: meta-analysis of the effects of intranasal oxytocin administration on face recognition, trust to in-group, and trust to out-group. Psychoneuroendocrino 37:438–443. 10.1016/j.psyneuen.2011.07.008 [DOI] [PubMed] [Google Scholar]
- Zhang H, Gross J, De Dreu CK, Ma Y (2019) Oxytocin promotes coordinated out-group attack during intergroup conflict in humans. Elife 8:e40698. 10.7554/eLife.40698 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental instructions for experiments 1–3. Download Figure 1-1, DOCX file (27.9KB, docx) .
Data Availability Statement
The custom routines for data analysis written in MATLAB are available from the corresponding author on reasonable request.


