Abstract
Perfluoroalkyl and polyfluoroalkyl substances (PFAS) are emerging contaminants in water and soil. Electrospun membranes with open structure could treat PFAS in a gravity-driven mode with ultralow pressure needs. The electrospun ultrathin fibers (67 ± 27 nm) was prepared for the enhanced specific surface area; where polyvinylidene fluoride (PVDF) backbones and the grafted quaternary ammonium moieties (QA; PVDF-g-QA membranes) provided both hydrophobicity and anion-exchange ability (electrostatic interaction). High affinity towards the perfluorooctanoic acid (PFOA)/perfluorooctanesulfonic acid (PFOS) molecules (denoted as PFOX collectively) was observed, and >95% PFOX was removed from synthetic groundwater with a flux of 32.3 Lm−2h−1 at ΔPo = 313 Pa. With a higher octanol/water partitioning coefficient (Log Kow = 6.3) and close dispersion interaction parameter to the membrane backbones (16.6% difference in δd), the effective PFOS removal remained under alkaline and high conductivity conditions due to the intensive hydrophobic interaction compared to that of PFOA. Long-term studies exhibited >90% PFOX removal in an 8 h test with a capacity of 258 L/m2. Under mild regeneration conditions, PFOA and PFOS were concentrated by 35-fold and 39-fold, respectively. Overall, the gravity-driven electrospun PVDF-g-QA membranes, with adsorptive effectiveness and ease of regeneration, showed great potential in PFAS remediation.
Keywords: Electrospun membranes, PFAS Removal, Removal mechanism, Water treatment, Co-existing competitors
1. Introduction
Perfluoroalkyl and polyfluoroalkyl substances (PFAS) are man-made chemicals notorious for their toxicity, persistence and wide-spread presence [1]. In 2009, ubiquitous PFAS compounds, such as perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS), were listed in the Stockholm Convention on Persistent Organic Pollutants. Since then, stringent regulations were applied to control the production and usage of PFAS [2], where the drinking water advisory level of combined PFOA and PFOS (denoted as PFOX, collectively) was suggested as 70 ng/L from the US Environmental Protection Agency [3]. Hence, effective treatment methods are anticipated for the remediation of highly-contaminated PFAS sites, as well as for the controls on contaminant migration.
Due to the presence of trace concentrations in natural water sources (ranging from low to thousands μg/L) [4] and low molecular length of PFOX (ca. 1.0 nm and 1.2 nm for PFOA and PFOS, respectively) [5], ineffective PFAS removal was observed using conventional treatment methods in wastewater treatment plants (WWTPs), such as coagulation, biodegradation and advanced oxidation processes [1,6]. Membrane technologies provide promising measures for the removal of micropollutants [7-10], and could remove more than 95% PFOX using both commercial and state-of-art nanofiltration (NF)/reverse osmosis (RO) membranes (via size-exclusion and Donnan exclusion effects) [7].
The widespread use of NF/RO in PFOX remediation is, however, limited by the relatively high energy demand (requires large operating pressure ΔP to overcome natural osmotic pressure), low water recovery, and membrane fouling potential [7,11,12]. In contrast, the electrospun nanofibrous membranes provide an efficient alternative, due to their high specific surface area, versatile surface modification or lower clogging potential [13-20]. Certain studies have been reported to modify the nanofibrous membranes, in the selection of backbone materials or grafting functionalization, for the removal of PFAS from water [21,22]. Furthermore, the ability to tailor the loose structure of electrospun nanofibrous membranes could enable a lower energy-demand in a gravity-driven mode [14,23,24].
Specific to PFOX remediation, maintaining a high removal efficiency with a high flux of nanofibrous membranes is the concern. This difficulty is enhanced by the nano-scale molecule lengths of PFOX molecules. Hence, introduction of additional affinity was required to boost the removal efficiency under high throughput. Since the PFOX are negatively-charged over a broad pH range, anion-exchange resins and other amine-functionalized materials, with positively-charged amine or quaternary ammonium (QA) groups, showed prominent affinity towards PFOX molecules [25,26]. In addition to the anion-exchange ability, the hydrophobicity of the electrospun membranes should be tuned to achieve a high permeability and high affinity between electrospun membranes and the hydrophobic perfluorocarbon “tails” of PFOX molecules.
Other than the membrane synthesis and modification, the water composition should also be evaluated for the PFOX remediation. As pointed out by critical review articles of PFOX remediation technologies [4,27], higher concentrations of PFOX were often chosen (mg/L level rather than μg/L level) for the ease of observation as well as the simplification the complexity of water chemistries and co-existing natural organic matters (NOM). However, considering the low [PFOX]/[−NOM] ratio ([NOM] in the range of 0.5–10 mg/L in groundwater [28]), high selectivity and affinity towards PFOX should be achieved to suppress the competitive adsorption. The elucidation of removal mechanism is therefore significant to guide the development of the electrospun membranes to achieve high removal performance under complex water conditions.
Hence, gravity-driven electrospun membranes were developed to remove PFOX (with an environmentally relevant concentration of 0.12 μM) from synthetic groundwater. The objectives of the study include (1) fabricating ultrathin fibers with tunable hydrophobicity and high anion-exchange ability for electrospun membrane application; (2) the elucidation of the removal mechanism of PFOX molecules with the advanced characterization; (3) understanding the influences of water components and co-existing organics; and (4) evaluating the long-term performance and reusability of the fabricated electrospun membranes.
2. Material and methods
2.1. Materials
The polyethylene terephthalate (PET) nonwoven substrate was obtained from Iris International Trading Co., Ltd. The PVDF powder (Mw ~ 534,000), the QA agent, i.e., [2-(methacryloyloxy)ethyl]trimethylammonium chloride (75 wt% in H2O, CAS #5039-78-1), and organic solvents including dimethylformamide (DMF) and acetone, were purchased from Sigma-Aldrich. The polyethylene-polyamines (PEPA), and the organic and inorganic competitors, cetyltrimethylammonium bromide (CTAB), sodium dodecyl sulfate (SDS), octanoic acid (OA), 3-(perfluorobutyl)propanol (3-PP), Bovine serum albumin (BSA) and inorganic salts; as well as the feed stocks and analytical standards of PFOA and potassium perfluorooctanesulfonate, were ordered from Shanghai Macklin Biochemical Co., Ltd. The Suwannee river humic acid (3S101H) and fulvic acid substances (2S101F) were obtained from the International Humic Substances Society.
2.2. Fabrication and characterization
The electrospun PVDF-g-QA membranes were prepared in a three-step process: partial defluorination, QA grafting, and electrospinning (Fig. 1). The partial defluorination method was described by Cheng et al. [29]., where the PVDF powder (8 wt%) and PEPA (25 wt% of PVDF) were dissolved in DMF and the polymer solution was agitated at the speed of 300 rpm (magnetic stirrer) overnight at 40 °C to form C=C double bond for the following grafting modification. The dehydration of QA agent was achieved via rotary evaporation at 35 °C and 4000 Pa, and the dehydrated QA was then mixed with the partial defluorinated solution for 0.5 h at room temperature. The APS (0.5 wt% of PVDF) was served as an initiator for grafting process to form PVDF-g-QA solution (75 °C for 2 h). After cooling, 25 vol% acetone was added to adjust the viscosity of the prepared PVDF-g-QA solution. The electrospinning was conducted at 23 kV with the 0.1 m work distance between the spinneret (needle) and the rotary collector, where the injection rate of prepared PVDF-g-QA solution was 0.15 mL/h (syringe pump). The rotational speed of the collector was 300 rpm and the needle ID was 23 with an inner diameter of 0.337 mm. Each nanofibrous membrane, with a size of 120 mm × 100 mm, was made after 4 h electrospinning with a 0.6 mL consumption of the dope solution. The relative humidity (RH) was controlled and maintained as 60%. The electrospun PVDF membranes were also made with the partial defluorination and electrospinning steps. The electrospun PVDF-g-QA membranes with different QA dosages (0%, 25%, 50%, 75%) were denoted as 0%QA, 25%QA, 50%QA and 75%QA, respectively.
Fig. 1.
Schematic of membrane fabrication methods from the preparation of dope solution in the electrospinning process.
Surface morphology was characterized using a scanning electron microscope (SEM, ZEISS GeminiSEM 500). The membrane composition was analyzed using an attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR, PerkinElmer), an energy dispersive spectroscopy (EDS, EDAX Falion 60S), and an X-ray photoelectron spectroscopy (XPS, Thermo ESCALAB 250Xi). The surface zeta potential (ζ-potential) was measured via a zeta potential analyzer (SurPASS 3, Anton Paar). The surface free energy was analyzed via a contact angle meter (JC2000D1, Shanghai Zhongchen Digital Technology Apparatus Co., Ltd). Details of characterization methods were included in SI section 1.
2.3. Analytical methods
A liquid chromatograph mass spectrometer (Shimadzu LCMS-8050) was used for PFOX measurement. A linear dynamic range of 0.5–50 μg/L was achieved (R2 = 0.997) based on eight calibration points. The LC-MS/MS method was modified based on our previous report [30] (SI section 2). No additional separation step was required before sample injecting, since the developed electrospun membranes could filter out the insoluble particles and less than 5% loss of PFOX was observed when the synthetic groundwater passing through the PET substrate.
2.4. Gravity-driven test and regeneration
The synthetic groundwater (pH 7.5 and 656 μS/cm) was prepared using 1 mM NaH2PO4 buffer and 5 mM NaCl ionic strength adjuster, where the PFOA and PFOS were then spiked sequentially to achieve a concentration of 0.12 μM, individually, to mimic the [PFOX] in contaminated sites. The 50 ml (Vremoval) PFOX spiked synthetic groundwater was treated in single gravity-driven run, where the electrospun membranes were placed in a glass sand core filtration apparatus (effective area: 11.3 cm2) for gravity-driven tests. The hydrostatic pressure (ΔP) is a function of the heigh of water surface (Δh0 = 0.032 m), thus it changes during filtration experimentation. The initial effective ΔP started at 313 Pa (ΔP0) and fell to 0 Pa at the end of filtration. The operation time was counted from the start point to the timepoint, where no additional permeate water drops were detected in 2 min of filtration. The removal efficiency was defined as following equation (1):
| (1) |
In addition, various pH levels (3.3–11.2), water conductivity conditions (from the levels of DI water to simulated industrial wastewater, 84 to 11150 μS/cm), and co-existing organic competitors (5000 μg/L for every competitor), were evaluated for PFOX removal under complex water matrices. A long-term study was also conducted by repeating the single gravity-driven filtration run for nine times continuously. After the long-term test, the spent membranes were regenerated by passing through 10 ml 5% methanol solution with pH 11 and 20 mM NaCl, where the volume of regenerated solution (Vregen) was 1/5 of the Vremoval.
3. Results and discussion
3.1. Morphology and composition
Under the same electrospinning conditions, the thickness of fibers was positively correlated with the QA dosage (Fig. 2), where the average diameter (dF) of fibers was increased from 31.7 ± 16.9 to 99.9 ± 22.9 nm when the QA dosage increased from 0 to 75 wt% of the PVDF (dF was calculated based on a broader imaging area of 56 μm × 40 μm, n = 100, Fig. S1). The increased QA dosage may enhance the Mw of PVDF-g-QA, resulting in an increased viscosity for the formation of thicker nanofibers (QA dosage from 0% to 75%). In addition, the developed ultrathin fibers can be attributed to the high voltage (23 kV) and the slow injection flowrate (0.15 mL/h) [31], and the ultrathin structure would also enhance specific surface area for abundant adsorption sites. The typical bead-on-string structures were observed at the electrospun PVDF membranes without QA grafting (Fig. 2a). The bead-on-string structures could be attributed to the insufficient viscosity compared to the strong surface tension at high applied voltage, where an unstable electrospun jet could further split into smaller drops and shrink into beads [32]. QA grafting may increase the entanglement of polymer chains, which could prevent the formation of bead-on-string structures, as well as the fracture of fibers (Fig. 2c and d), resulting in a uniform and stable ultrathin fiber structure for the following gravity-driven PFOX removal applications. Therefore, the increased QA not only increases fiber diameter but also hinders the formation of bead, this statement was also matched with published studies [33]. SEM images with higher resolution (50k) were also taken (Fig. S2), where no obvious pores and voids were observed on the developed nanofibers.
Fig. 2.
SEM imaging of electrospun PVDF-g-QA membranes with different QA dosages (0–75 wt% of the PVDF). The average diameter of nanofibers was calculated based on Fig. S1 (n = 100).
Increased N/F atomic ratio (from 0.33 to 0.92, via EDS mapping) was observed after QA grafting, where fluorine was used as a baseline for the comparison since the PVDF is the only fluorine source in membrane preparation processes (Fig. 3a, b, e). Due to the low pKa values of PFOA (−0.2–2.8) [34] and PFOS (−3.3) [35], the positively-charged QA can serve as a cationic center for the adsorption of ionized PFOX. In addition, the thickness of electrospun layer was observed as ca. 4.6 μm, according to the cross-section imaging (Fig. 3c). This was further verified with the distinctive distribution of O, F and N elements between the electrospun layer and the substrate layer, where the electrospun layer presented significant F (PVDF only) and N signal (PEPA and QA, collectively), but weaker O signal (mainly observed in the substrate layer). The measured average thickness was 5.5 ± 1.2 μm as quantified by a digital thickness gage among 10 replicates.
Fig. 3.
EDS mapping of membrane surfaces of (a) electrospun PVDF and (b) electrospun PVDF-g-QA membranes (50% QA); and of cross-section of electrospun PVDF-g-QA (50% QA) membranes (image c, d, e, and f). Oxygen in cyan, fluorine in pink, and nitrogen in green.
Membrane composition was further analyzed via ATR-FTIR (Fig. 4a). The characteristic peak of terephthalate group (1240 cm−1) [36] on the PET substrate was diminished after electrospinning. In contrast, the strong peak of C-F stretching (1175 cm−1, validated using PVDF powder) [37] was observed on the electrospun PVDF and PVDF-g-QA membranes, which was consistent with the occurrence of F 1s peak in XPS spectra after electrospinning process (Fig. 4b). The characteristic peaks of C─O stretching (1714 cm−1, strong peak for ester) and N–H stretching (3039-3722 cm−1, strong and broad peak for amine salt) were enhanced after the QA grafting [38]. However, the evidence for the successful QA introduction was not sufficient due to the existence of ester and amine groups from the PET substrate and the PEPA molecules, respectively. Thus, XPS analysis was used to validate the introduction of quaternary ammonium groups. As shown in Fig. 4c, the N 1s peak of the PVDF-g-QA membranes could be deconvoluted into two components: amine N–H (399.8 eV) and quaternary ammonium N+ (403.1 eV) [39], and the N+/N–H ratio is 0.59. In contrast, negligible N+ peak was observed in the electrospun PVDF membranes and the PET substrate. Overall, the occurrence of N+ peak (403.1 eV), after QA grafting, proved the existence of quaternary ammonium moiety on the electrospun PVDF-g-QA membranes.
Fig. 4.
The composition analysis of electrospun PVDF and PVDF-g-QA (QA 50%) membranes using (a) ATR-FTIR spectra, (b) XPS spectra, and (c) specific N 1s peak.
The mechanical strength of fabricated PVDF-g-QA membranes were also tested. Negligible difference was observed in the Young’s modulus between the PET nonwoven substrate (1369 ± 31 MPa, n = 5) and electrospun PVDF-g-QA (50% QA) membranes (1362 ± 41 MPa, n = 10). Due to the lack of strong interactions (such as covalent bond), the electrospun layer can be partly peeled by the use of Scotch tape, where the membrane thickness was decreased from 5.5 ± 1.2 to 4.1 ± 1.6 μm and the permeate flux was increased by 22%. Methods such as heat-press should be considered in future studies to improve the interactions between nanofibrous layer and membrane substrate.
3.2. PFOX removal
Insignificant PFOX removal was observed using the PET substrate only, and the removal was slightly increased (from less than 5.0% to more than 16.2%, Table 1) after the electrospinning of PVDF polymers (without QA grafting). This increase can be attributed to (1) the increased operation time (12-fold) caused by the smaller pore size and the enhanced specific surface area; and (2) the hydrophobic interaction between the electrospun PVDF membranes (contact angle about 123.4°) and hydrophobic perfluoro tails of PFOX molecules (both PFOA and PFOS exhibit high octanol-water partition coefficient, Kow [40]). The PFOX removal was then greatly improved (>95%) using the electrospun PVDF-g-QA membranes (QA dosage was 50 wt% of PVDF), where the QA grafting granted the anion-exchange ability towards ionized PFOX. The QA grafting is expected to enhance the hydrophilicity of PVDF backbone, where the measured membrane contact angle was negatively correlated with the increased QA. The enhanced hydrophilicity could also increase the water flux, resulting in the increased permeate flux from 21.5 to 32.3 Lm−2h−1 at the QA dosage from 0% to 50%. Although the QA grafting could increase PFOX removal via electrostatic interaction, the excess use of hydrophilic QA could hinder the hydrophobic interaction between PFOX and membranes (details of electrostatic/hydrophobic interactions were discussed in section 3.4), which led to the decreases in PFOX removal (from 50% QA to 75% QA). In addition, the thicker nanofibers and compact structure, from 67.2 ± 27.3 to 99.9 ± 22.9 nm (50% QA to 75% QA, Fig. 2c-d) would hinder the permeance through electrospun membranes. Thus, QA dosage was selected as 50 wt% of PVDF for the enhanced removal efficiency and permeate flux.
Table 1.
Parameters of gravity-driven PFOX removal tests with various membranes (effective membrane area: 11.3 cm2; throughput per run: 50 ml PFOX spiked synthetic groundwater; Δh0 = 0.032 m; ΔP0 = 313 Pa and T = 22 °C).
| Type | Contact angle (degree) |
Permeate flux (Lm−2h−1) |
Operation time (h) |
PFOA removal (%) |
PFOS removal (%) |
|---|---|---|---|---|---|
| Substrate | 47.1 ± 3.5 | 258.6 | 0.17 | 3.1 ± 2.1 | 4.5 ± 2.4 |
| PVDF | 123.4 ± 3.3 | 21.5 | 2.05 | 16.2 ± 3.5 | 37.1 ± 3.1 |
| 25%QA | 115.4 ± 2.1 | 27.8 | 1.55 | 79.5 ± 2.8 | 86.1 ± 3.5 |
| 50%QA | 113.6 ± 1.5 | 32.3 | 1.33 | 97.9 ± 1.4 | 99.1 ± 0.4 |
| 75%QA | 99.8 ± 3.6 | 30.4 | 1.41 | 94.1 ± 3.4 | 97.3 ± 2.4 |
This high PFOX removal was also reported using activated carbon (in Table 2), where the smaller mesh size of activated carbon was favorable to achieve high PFOX removal, due to the less diffusion resistance to the micropores of activated carbon [34,41-43]. Compared to the activated carbon, the observed sub-micron macropores of the electrospun membranes should enhance the mass transfer of PFOX molecules to the positively-charged surface and thereby facilitate the PFOX removal. Furthermore, the gravity-driven electrospun PVDF-g-QA membranes showed much better removal of PFOX compared to that of the pressure-driven UF membranes with MWCO of 10 kDa (40–60% PFOX removal) [44], and the removal efficiency was comparable with the commercial NF 270 membranes (>95% PFOX removal) [41]. However, one should note that PFOX removal in pressure-driven UF/NF membranes is via contaminant rejection, instead of via contaminant adsorption, where the former is based on size-exclusion and Donnan effects [7].
Table 2.
The comparison of PFOA removal among different membrane technologies and adsorbents (C0 suggests initial concentration of PFOA).
| Method | C0 (μmol/ L) |
Water matrix | Operation | Removal (%) |
Reference |
|---|---|---|---|---|---|
| Nanofibrous PVDF-g-QA | 0.12 | Synthetic groundwater | Gravity-driven | 97.9 ± 1.4 | This study |
| Nanofibrous PVA/PDDA | 0.024 | DI water | Gravity-driven | >99.0 | [21] |
| Nanofiltration | 0.0024 | Synthetic groundwater | Pressure-driven | >95.0 | [41] |
| Reverse osmosis | 0.24 | River water | Pressure-driven | >99.0 | [45] |
| Ultrafiltration | 0.024 | DI water | Pressure-driven | <60.0 | [44] |
| Activated carbon | 0.024 | Synthetic groundwater | Batch mode | >99.0a | [43] |
| Amine-modified | 1.21 | Synthetic lake water | Batch mode | >95.0b | [46] |
The weight ratio of adsorbent/PFOA was 10000.
The weight ratio of adsorbent/PFOA was 50.
The physical and chemical properties of the spent membranes were also investigated to verify the PFOX adsorption. The peak of sulfonate (169.4 eV [47]) was observed in XPS analysis after the gravity-driven tests (Fig. S3), which demonstrated the existence of adsorbed PFOS. The decreased contact angle (from 113.6° to 84.3°) after the PFOX removal test (after nine continuous runs) also proved the adsorption of PFOX on the membranes (Fig. 5a), since the adsorbed surfactant-like PFOX molecules also enhance the wettability of the hydrophobic membrane surface. In addition, the small Δh0 (0.032 m) required a low energy demand to go against the gravitational potential energy, which was calculated as 213 J per m3 of PFOX contaminated water.
Fig. 5.
(a) Contact angle measurements of the electrospun PVDF-g-QA membranes (50%QA) at initial stage, after continuous nine runs, and after regeneration; and (b) long-term performance (runs 1 to 9) and after regeneration (runs r1 to r3). The throughput per run was 50 ml PFOX spiked synthetic groundwater. ([PFOX]ini = 0.12 μM, Δh0 = 0.032 m; ΔP0 = 313 Pa and T = 22 °C).
3.3. Long-term study and regeneration
The long-term performance of PVDF-g-QA membranes was also tested (Fig. 5b), where more than 90.1% PFOA and 96.4% PFOS were removed, simultaneously, within the continuous six runs (8 h continuously, total treatment capacity 258 L per m2 of membrane surface). The removal efficiency was significantly decreased (41.8% removal for PFOA and 73.7% removal for PFOS) when the adsorption sites with higher affinity were occupied after the ninth runs (after 12 h tests). Considering the thickness of the electrospun membrane (ca. 5.5 μm) and the ultra-low ΔP0 (313 Pa), the life-time of effective filtration use with more than 90% removal efficiency could be further extended by either stacking the electrospun membranes (increasing effective thickness) or arranging the membranes in series (mimicking the commercial packed bed adsorption columns [48]) to increase the contact time between PFOX molecules and membranes.
The decreased removal efficiency was recovered via membrane regeneration. Mild regeneration conditions, with the combination of pH adjuster (pH 11) and organic solvent (5 vol% methanol), were applied after the ninth run, and the effective desorption of PFOX was achieved. The removal efficiency was recovered back to 97.4% and 98.4% for PFOA and PFOS, respectively (Fig. 5b). This high removal efficiency maintained consistent for another three runs (r1 to r3) after regeneration. Furthermore, no harsh conditions were required in regeneration compared with commercial activated carbons or strong alkaline anion-exchange resins [27].
In addition to the robust reusability, the less solution applied in regeneration (Vregen was 1/5 of Vremoval applied in the single tests) could largely concentrate the PFOX for further remediation treatments. In this study, 92.5% and 94.5% of adsorbed PFOA and PFOS were desorbed, respectively, in the regeneration process after the ninth gravity-driven run, which resulted in a 35.4-fold and 39.8-fold increase of [PFOA] and [PFOS] accordingly. This high concentration factor could reduce the volume of regeneration solution on the one hand, but also largely enhance the [PFOX]ini for the subsequent destruction techniques, such as advanced oxidation and thermal methods [49,50], with enhanced energy efficiency and dynamics. The spent methanol could be reused by collecting via a small distillation unit. Hence, the electrospun membranes presented great potential in the integration with advanced destruction methods to effectively degrade PFAS molecules.
The stability of the electrospun PVDF-g-QA membranes was also investigated using ATR-FTIR and contact angle methods (Fig. S4). The characteristic peaks of N–H stretching (3039-3722 cm−1), C─O stretching (1714 cm−1), and C–F stretching (1175 cm−1) remained not only after the long-term tests but also after regeneration steps. In addition, the contact angle of membrane was increased to the original state when the adsorbed PFOX molecules were desorbed after regeneration (Fig. 5a). These two characterizations overall demonstrated the stability of the electrospun PVDF-g-QA membranes after the long-term tests and regeneration.
3.4. Removal mechanism
According to the PFOX removal studies using benchmark activated carbons/anion-exchange resins, the adsorption mechanism were generally attributed to the electrostatic, hydrophobic, and Van Der Waals interactions [27]. The high positive surface zeta potential (ζ-potential) of electrospun PVDF membrane below pH 7.0 can be attributed to the addition of PEPA (polyamide) in the polymer solution. The QA grafting further increased the isoelectric point of the membrane surface (pHiep from 7.6 to more than 11.9, Fig. 6), where the ζ-potential was observed as +1.5 and + 60.5 mV at pH 7.5 (synthetic groundwater), respectively, for electrospun PVDF (without QA grafting) and PVDF-g-QA membranes. The positively-charged membrane surface, after QA grafting, could serve as an anion-exchanger with ionized PFOX (electrostatic attraction), and thus results in the significant improvement in PFOX removal (Table 1). In this regard, the decreased ζ-potential at higher pH (less than +20.0 mV at pH 11) would impair the electrostatic attraction, which, in turn, would be favorable for the desorption process in the regeneration of the spent membranes. This statement was also verified by the increased ζ-potential at pH 11–12 of the spent membranes (from −6.61 mV to + 12.4 mV), since the adsorbed negatively-charged PFOX molecules would desorb at this pH level.
Fig. 6.
Surface ζ-potential of the electrospun PVDF membrane (square), the PVDF-g-QA membrane (50% QA) (circle), and the post-test PVDF-g-QA membrane for PFOX removal (triangle).
Besides the electrostatic attraction, other driving forces should exist due to the better removal of PFOS compared to that of PFOA (both monoacid with the similar perfluoro carbon chains) (Fig. 5b). As discussed in section 3.2, the hydrophobic membrane surface (via electrospinning of PVDF layer) showed improved PFOX removal, especially for the more hydrophobic PFOS molecules (Log Kow(PFOS) = 6.3, and Log Kow(PFOA) = 5.3) [40]. The hydrophobic interaction was further analyzed by comparing the dispersion interaction (δd) between the hydrophobic PVDF backbone and PFOX molecules. As a conventional membrane matrix, the δd of PVDF was reported as 17.2 MPa1/2 [51,52], whereas the δd of PFOX molecules can be calculated using a group contribution method (equation (2)) developed by Hansen and Beerbower [53]. The reported group contribution values of molar volumes and molar attraction constants were summarized in Table 3 [37,53-55], and the δd of PFOA and PFOS were calculated as 13.8 MPa1/2 and 14.3 MPa1/2, respectively. The small difference in δd between membrane backbone and PFOX molecules, as well as the high Kow of PFOX molecules, jointly implies the strong hydrophobic interactions.
| (2) |
where V (cm3/mol) is the molar volume and F ((J cm−3)1/2 mol−1) is the molar attraction constant.
Table 3.
Dispersion interaction parameters of functional groups of PFOX molecules (data were collected from reported literatures [37,53-55]).
| Functional group | V (cm3/mol) | Fd [(J cm−3)1/2mol−1] |
|---|---|---|
| —CF3 | 46.1 | 561 |
| —CF2— | 23.0 | 307 |
| —SO3H | 23.6 | 597 |
| —COOH | 28.5 | 530 |
The key role of hydrophobic interaction in PFOX removal was also reported: a positively correlation was established for the removal efficiency and the log Kow of different PFAS molecules [56]. Hence, PFOX removal is driven by both electrostatic attraction and hydrophobic interactions (Fig. 7). The effective PFOX removal can be attributed to (1) the high specific surface area provided by the ultrathin electrospun fibers (67 ± 27 nm); (2) the increased hydrophobic interaction between the hydrophobic PVDF backbone and PFOX molecules (similar δd and high partitioning coefficient); and (3) the introduced quaternary ammonium anion-exchange ability (electrostatic attraction) granted by the QA grafting.
Fig. 7.
Schematic of PFOX removal mechanisms using the electrospun PVDF-g-QA membranes.
3.5. Influences of water compositions and organic competitors
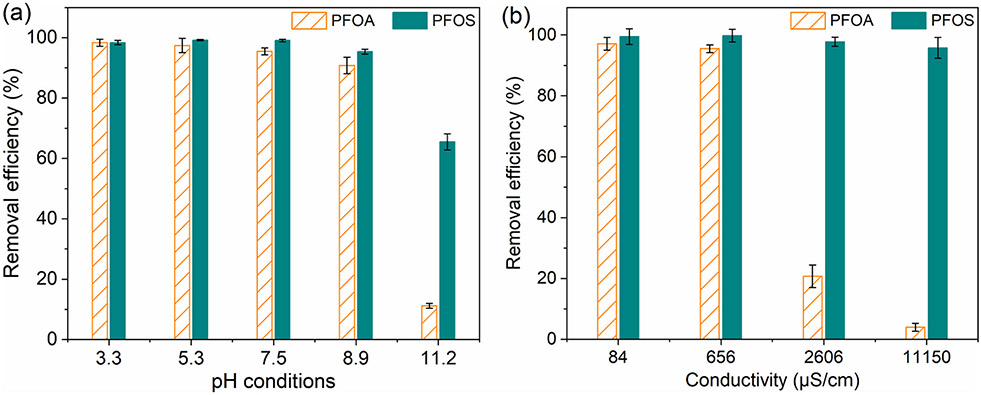
Considering the trace concentration of PFAS in natural water sources (low μg/L level), the water composition and the co-existing organic competitors should be evaluated. As shown in Fig. 8a, the electrospun membranes showed high PFOX removal (>90.0%) at a broad pH range (from 3.3 to 8.9). This was consistence with the ζ-potential measurement, where the high surface ζ-potential (more than +40 mV from pH 3.3 to 8.9) would trigger the strong electrostatic interaction. Furthermore, the dramatically decreased removal efficiency at pH 11.2 (merely 11.2% removal for PFOA and 65.5% removal for PFOS) was therefore caused by the reduced electrostatic attraction, where the ζ-potential was decreased from +60.5 mV to less than + 20.0 mV. The less drastic decrease on the removal of PFOS, compared to PFOA, also indicated the role of hydrophobic interaction, since the PFOS exhibited higher Kow and relatively close δd to the membrane backbone.
Fig. 8.
Influences of (a) pH and (b) water conductivity on PFOX removal using the electrospun PVDF-g-QA membranes (50%QA). [PFOX]ini = 0.12 μM and T = 22 °C.
With the increased water conductivity (NaCl adjuster), distinctive influences were observed on the removal of PFOA and PFOS: the PFOA removal was largely hampered (from 97.1% to 3.9%) when the conductivity level increased from the DI water level (84 μS/cm from the pH buffer) to the industrial wastewater level (11150 μS/cm), whereas negligible influence was observed on PFOS removal with the same increased conductivity (Fig. 8b). The hindrance on PFOA removal could be attributed to the competitive adsorption of anionic ions, where the anion-exchange sites were occupied and the electrostatic interaction between electrospun membranes and PFOA molecules was reduced. The negligible influence on PFOS removal could be explained by the highly intensive hydrophobic interaction, which compensated for the impaired electrostatic interaction. Hence, the high removal efficiency of more hydrophobic PFOS molecules was achieved under the high conductivity conditions, since the hydrophobic interaction was more intense with PFOS compared to PFOA molecules.
The influences of co-existing organic competitors were evaluated with representative compounds, including natural organic matters (HA and FA substances), cationic and anionic surfactants (CTAB and SDS), and compounds with partly similar structure of PFOX (OA and 3-PP) (Fig. 9a). The concentration level of individual organic competitors was prepared as 5000 μg/L to simulate the groundwater conditions, which made the [competitor]/[PFOX] ratio was nearly 100-fold. For anionic organic competitors, macromolecule HA and FA substances showed less negative influences on PFOX removal compared to that of anionic surfactant SDS (smaller molecule, 288 Da), which could be explained by the lower adsorption affinity caused by the hydrophilic moieties of HA and FA substances [57]. The cationic surfactant CTAB, however, enhanced the PFOX removal by either the enhanced electrostatic attraction or the precipitation potential with PFOX molecules [27]. For the competitors with the partial similar structure of PFOX, OA (carboxylic group, anionic) and 3-PP (perfluoro group, hydrophobic) both showed hindrance on PFOA removal (from 95.5% to about 79.5%). Weak influence on PFOS removal was observed in the presence of OA (Log Kow = 3.03), whereas more hydrophobic 3-PP (Log Kow = 4.15) presented significant negative influence on PFOS removal (from 99.1% to 68.1%). The distinctive influences of anionic competitor and hydrophobic compounds on PFOX removal demonstrated that the PFOS adsorption relies more on hydrophobic interaction compared to that of PFOA. The negative correlation between PFOS removal and increased Kow of organic competitors (neutral or negatively charged) that was also observed (Fig. 9b). Overall, 76.3% PFOA and 87.5% PFOS were removed when all the studied organic competitors were included, and this information was essential for the environmental application of the electrospun PVDF-g-QA membranes.
Fig. 9.
Influences of organic competitors on PFOX removal using the electrospun PVDF-g-QA membranes (50%QA): (a) removal performance and (b) correlation with the Kow of individual organic competitors. [Individual competitors] = 5000 μg/L, [PFOX]ini = 0.12 μM and T = 22 °C.
In addition to PFOX removal performance, the effects of water chemistry on membrane fouling were also evaluated on permeate flux, using a mixture of NOM and protein as organics (50,000 μg/L humic acid and bovine serum albumin) [58], inorganic salts (100 mM NaCl), and scaling ions (5 mM CaCl2). As shown in Fig. S5, no significant decrease on permeate flux was observed at the first eight consecutive runs in a 10.8 h period (from 32.3 to 31.4 Lm−2h−1) and less than 15% decreases in permeate flux was observed after a total sixteen consecutive runs in a total throughput of 707.9 L/m2 during 22.8 h running time.
4. Conclusion
In this study, the electrospun PVDF-g-QA membranes were designed, with ultrathin fibers (67 ± 27 nm), surface hydrophobicity (contact angle 113.6°) and highly positive surface ζ-potential (+60.5 mV at pH 7.5), for effective removal of PFOX in a gravity-driven process (95.5% removal of PFOA and 99.1% removal of PFOS, simultaneously, from synthetic groundwater). The key findings were listed as below:
The grafting of quaternary ammonium greatly enhanced the surface ζ-potential from +1.5 mV to +60.5 mV at pH 7.5, which enabled the anion-exchange ability (electrostatic attraction). The electrospun ultrathin fibers also provides high specific surface area for adsorption sites;
The hydrophobic interaction was demonstrated as a key parameter for PFOX removal since the high Kow (5.3 for PFOA and 6.3 for PFOS) and the close δd between PFOX molecules and membrane backbone (ca. 16.6% difference);
PFOA removal was largely impeded by either the alkaline condition or high conductivity, whereas effective PFOS removal was maintained due to the more intensive hydrophobic interaction between contaminant and membrane backbone.
Long-term study showed more than 90.1% PFOA and 96.4% PFOS were removed in a totally 8 h operation time with the treatment capacity of 258 L/m2. The regeneration and reuse were also achieved with mild conditions, where PFOA and PFOS were concentrated 35.4-fold and 39.8-fold, respectively.
Considering the merits of low energy demand (213 J per m3 of PFOX contaminated water against the gravitational potential energy), stable performances under various water compositions, ease of regeneration, and high concentration factor, the gravity-driven electrospun PVDF-g-QA membranes showed great potential to be applied in the environmental treatment of PFAS-like micropollutants.
Supplementary Material
Acknowledgments
This work was supported by China Postdoctoral Science Foundation [2020M681204]; the National Natural Science Foundation of China [21908054 & 22075076]; and NIEHS-SRP grant [P42ES007380]. We highly appreciate the collaborations with the University of Kentucky NIEHS Superfund center.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.memsci.2021.120180.
The authors declare no competing financial interest.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Lenka SP, Kah M, Padhye LP, A review of the occurrence, transformation, and removal of poly- and perfluoroalkyl substances (PFAS) in wastewater treatment plants, Water Res. 199 (2021) 117187, 10.1016/j.watres.2021.117187. [DOI] [PubMed] [Google Scholar]
- [2].Yan B, Munoz G, Sauvé S, Liu J, Molecular mechanisms of per- and polyfluoroalkyl substances on a modified clay: a combined experimental and molecular simulation study, Water Res. 184 (2020) 116166, 10.1016/j.watres.2020.116166. [DOI] [PubMed] [Google Scholar]
- [3].Hu XC, Andrews DQ, Lindstrom AB, Bruton TA, Schaider LA, Grandjean P, Lohmann R, Carignan CC, Blum A, Balan SA, Higgins CP, Sunderland EM, Detection of poly- and perfluoroalkyl substances (PFASs) in U.S. Drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants, Environ. Sci. Technol. Lett 3 (10) (2016) 344–350, 10.1021/acs.estlett.6b00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ateia M, Alsbaiee A, Karanfil T, Dichtel W, Efficient PFAS removal by amine-functionalized sorbents: critical review of the current literature, Environ. Sci. Technol. Lett 6 (12) (2019) 688–695, 10.1021/acs.estlett.9b00659. [DOI] [Google Scholar]
- [5].Xiao F, Davidsavor KJ, Park S, Nakayama M, Phillips BR, Batch and column study: sorption of perfluorinated surfactants from water and cosolvent systems by Amberlite XAD resins, J. Colloid Interface Sci 368 (1) (2012) 505–511, 10.1016/j.jcis.2011.11.011. [DOI] [PubMed] [Google Scholar]
- [6].Wang X, Yu N, Qian Y, Shi W, Zhang X, Geng J, Yu H, Wei S, Non-target and suspect screening of per- and polyfluoroalkyl substances in Chinese municipal wastewater treatment plants, Water Res. 183 (2020) 115989, 10.1016/j.watres.2020.115989. [DOI] [PubMed] [Google Scholar]
- [7].Boo C, Wang Y, Zucker I, Choo Y, Osuji CO, Elimelech M, High performance nanofiltration membrane for effective removal of perfluoroalkyl substances at high water recovery, Environ. Sci. Technol 52 (13) (2018) 7279–7288, 10.1021/acs.est.8b01040. [DOI] [PubMed] [Google Scholar]
- [8].Li X, Xu Y, Goh K, Chong TH, Wang R, Layer-by-layer assembly based low pressure biocatalytic nanofiltration membranes for micropollutants removal, J. Membr. Sci 615 (2020) 118514, 10.1016/j.memsci.2020.118514. [DOI] [Google Scholar]
- [9].Wang S, Wang F, Jin Y, Meng X, Meng B, Yang N, Sunarso J, Liu S, Removal of heavy metal cations and co-existing anions in simulated wastewater by two separated hydroxylated MXene membranes under an external voltage, J. Membr. Sci 638 (2021) 119697, 10.1016/j.memsci.2021.119697. [DOI] [Google Scholar]
- [10].Wan H, Islam MS, Briot NJ, Schnobrich M, Pacholik L, Ormsbee L, Bhattacharyya D, Pd/Fe nanoparticle integrated PMAA-PVDF membranes for chloro-organic remediation from synthetic and site groundwater, J. Membr. Sci 594 (2020) 117454, 10.1016/j.memsci.2019.117454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Park HB, Kamcev J, Robeson LM, Elimelech M, Freeman B, Maximizing the right stuff: the trade-off between membrane permeability and selectivity, Science 356 (6343) (2017). [DOI] [PubMed] [Google Scholar]
- [12].Van der Bruggen B, Mänttäri M, Nyström M, Drawbacks of applying nanofiltration and how to avoid them: a review, Separ. Purif. Technol 63 (2) (2008) 251–263, 10.1016/j.seppur.2008.05.010. [DOI] [Google Scholar]
- [13].Jiang H, Zhao Q, Wang P, Chen M, Wang Z, Ma J, Inhibition of algae-induced membrane fouling by in-situ formed hydrophilic micropillars on ultrafiltration membrane surface, J. Membr. Sci 638 (2021), 10.1016/j.memsci.2021.119648, 119648. [DOI] [Google Scholar]
- [14].Pronk W, Ding A, Morgenroth E, Derlon N, Desmond P, Burkhardt M, Wu B, Fane AG, Gravity-driven membrane filtration for water and wastewater treatment: a review, Water Res. 149 (2019) 553–565, 10.1016/j.watres.2018.11.062. [DOI] [PubMed] [Google Scholar]
- [15].Liu C, Song D, Zhang W, He Q, Huangfu X, Sun S, Sun Z, Cheng W, Ma J, Constructing zwitterionic polymer brush layer to enhance gravity-driven membrane performance by governing biofilm formation, Water Res. 168 (2020) 115181, 10.1016/j.watres.2019.115181. [DOI] [PubMed] [Google Scholar]
- [16].Ozcan S, Kaner P, Thomas D, Cebe P, Asatekin A, Hydrophobic antifouling electrospun mats from Zwitterionic Amphiphilic copolymers, ACS Appl. Mater. Interfaces 10 (21) (2018) 18300–18309, 10.1021/acsami.8b03268. [DOI] [PubMed] [Google Scholar]
- [17].Thavasi V, Singh G, Ramakrishna S, Electrospun nanofibers in energy and environmental applications, Energy Environ. Sci 1 (2) (2008) 205–221. [Google Scholar]
- [18].Peng S, Jin G, Li L, Li K, Srinivasan M, Ramakrishna S, Chen J, Multi-functional electrospun nanofibres for advances in tissue regeneration, energy conversion & storage, and water treatment, Chem. Soc. Rev 45 (5) (2016) 1225–1241, 10.1039/C5CS00777A. [DOI] [PubMed] [Google Scholar]
- [19].Chiao Y-H, Yap Ang MB, Huang Y-X, DePaz SS, Chang Y, Almodovar J, Wickramasinghe SR, A “graft to” electrospun Zwitterionic Bilayer membrane for the separation of hydraulic fracturing-produced water via membrane distillation, Membranes 10 (12) (2020), 10.3390/membranes10120402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Liu C, Liu J, Wang J, Li J, Luo R, Shen J, Sun X, Han W, Wang L, Electrospun mulberry-like hierarchical carbon fiber web for high-performance supercapacitors, J. Colloid Interface Sci 512 (2018) 713–721, 10.1016/j.jcis.2017.10.093. [DOI] [PubMed] [Google Scholar]
- [21].Guo H, Zhang J, Peng LE, Li X, Chen Y, Yao Z, Fan Y, Shih K, Tang CY, High-efficiency capture and recovery of anionic perfluoroalkyl substances from water using PVA/PDDA nanofibrous membranes with near-zero energy consumption, Environ. Sci. Technol. Lett 8 (4) (2021) 350–355, 10.1021/acs.estlett.1c00128. [DOI] [Google Scholar]
- [22].Mantripragada S, Deng D, Zhang L, Remediation of GenX from water by amidoxime surface-functionalized electrospun polyacrylonitrile nanofibrous adsorbent, Chemosphere 283 (2021) 131235, 10.1016/j.chemosphere.2021.131235. [DOI] [PubMed] [Google Scholar]
- [23].Zhao J, Wang W, Ye C, Li Y, You J, Gravity-driven ultrafast separation of water-in-oil emulsion by hierarchically porous electrospun Poly(L-lactide) fabrics, J. Membr. Sci 563 (2018) 762–767, 10.1016/j.memsci.2018.06.053. [DOI] [Google Scholar]
- [24].Wang R, Zhang L, Chen B, Zhu X, Low-pressure driven electrospun membrane with tuned surface charge for efficient removal of polystyrene nanoplastics from water, J. Membr. Sci 614 (2020) 118470, 10.1016/j.memsci.2020.118470. [DOI] [Google Scholar]
- [25].Maimaiti A, Deng S, Meng P, Wang W, Wang B, Huang J, Wang Y, Yu G, Competitive adsorption of perfluoroalkyl substances on anion exchange resins in simulated AFFF-impacted groundwater, Chem. Eng. J 348 (2018) 494–502. [Google Scholar]
- [26].Klemes MJ, Ling Y, Ching C, Wu C, Xiao L, Helbling DE, Dichtel WR, Reduction of a tetrafluoroterephthalonitrile-β-cyclodextrin polymer to remove anionic micropollutants and perfluorinated alkyl substances from water, Angew. Chem 131 (35) (2019) 12177–12181. [DOI] [PubMed] [Google Scholar]
- [27].Gagliano E, Sgroi M, Falciglia PP, Vagliasindi FG, Roccaro P, Removal of poly- and perfluoroalkyl substances (PFAS) from water by adsorption: role of PFAS chain length, effect of organic matter and challenges in adsorbent regeneration, Water Res. 171 (2020) 115381. [DOI] [PubMed] [Google Scholar]
- [28].Bolto B, Dixon D, Eldridge R, King S, Linge K, Removal of natural organic matter by ion exchange, Water Res. 36 (20) (2002) 5057–5065. [DOI] [PubMed] [Google Scholar]
- [29].Cheng B, Li Z, Li Q, Ju J, Kang W, Naebe M, Development of smart poly (vinylidene fluoride)-graft-poly(acrylic acid) tree-like nanofiber membrane for pH-responsive oil/water separation, J. Membr. Sci 534 (2017) 1–8, 10.1016/j.memsci.2017.03.053. [DOI] [Google Scholar]
- [30].Saad A, Mills R, Wan H, Mottaleb MA, Ormsbee L, Bhattacharyya D, Thermo-responsive adsorption-desorption of perfluoroorganics from water using PNIPAm hydrogels and pore functionalized membranes, J. Membr. Sci 599 (2020) 117821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhang J, Liu L, Si Y, Yu J, Ding B, Electrospun nanofibrous membranes: an effective arsenal for the purification of emulsified oily wastewater, Adv. Funct. Mater 30 (25) (2020) 2002192, 10.1002/adfm.202002192. [DOI] [Google Scholar]
- [32].Jarusuwannapoom T, Hongrojjanawiwat W, Jitjaicham S, Wannatong L, Nithitanakul M, Pattamaprom C, Koombhongse P, Rangkupan R, Supaphol P, Effect of solvents on electro-spinnability of polystyrene solutions and morphological appearance of resulting electrospun polystyrene fibers, Eur. Polym. J 41 (3) (2005) 409–421, 10.1016/j.eurpolymj.2004.10.010. [DOI] [Google Scholar]
- [33].Nezarati RM, Eifert MB, Cosgriff-Hernandez E, Effects of humidity and solution viscosity on electrospun fiber morphology, Tissue Eng. C Methods 19 (10) (2013) 810–819, 10.1089/ten.TEC.2012.0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yu Q, Zhang R, Deng S, Huang J, Yu G, Sorption of perfluorooctane sulfonate and perfluorooctanoate on activated carbons and resin: kinetic and isotherm study, Water Res. 43 (4) (2009) 1150–1158. [DOI] [PubMed] [Google Scholar]
- [35].Deng S, Shuai D, Yu Q, Huang J, Yu G, Selective sorption of perfluorooctane sulfonate on molecularly imprinted polymer adsorbents, Front. Environ. Sci. Eng. China 3 (2) (2009) 171–177, 10.1007/s11783-009-0017-4. [DOI] [Google Scholar]
- [36].Pereira A.P.d.S., Silva M.H.P.d., Lima ÉP, Paula A.d.S., Tommasini FJ, Processing and characterization of PET composites reinforced with geopolymer concrete waste, Mater. Res 20 (2017) 411–420. [Google Scholar]
- [37].Wan H, Briot NJ, Saad A, Ormsbee L, Bhattacharyya D, Pore functionalized PVDF membranes with in-situ synthesized metal nanoparticles: material characterization, and toxic organic degradation, J. Membr. Sci 530 (2017) 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kowalczyk I, Synthesis, molecular structure and spectral properties of quaternary ammonium derivatives of 1,1-Dimethyl-1,3-propylenediamine, Molecules 13 (2) (2008), 10.3390/molecules13020379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wang H, Chen M, Jin C, Niu B, Jiang S, Li X, Jiang S, Antibacterial [2-(Methacryloyloxy) ethyl] trimethylammonium chloride functionalized reduced graphene oxide/poly(ethylene-co-vinyl alcohol) multilayer barrier film for food packaging, J. Agric. Food Chem 66 (3) (2018) 732–739, 10.1021/acs.jafc.7b04784. [DOI] [PubMed] [Google Scholar]
- [40].Deng S, Zhang Q, Nie Y, Wei H, Wang B, Huang J, Yu G, Xing B, Sorption mechanisms of perfluorinated compounds on carbon nanotubes, Environ. Pollut 168 (2012) 138–144. [DOI] [PubMed] [Google Scholar]
- [41].Appleman TD, Dickenson ERV, Bellona C, Higgins CP, Nanofiltration and granular activated carbon treatment of perfluoroalkyl acids, J. Hazard Mater 260 (2013) 740–746, 10.1016/j.jhazmat.2013.06.033. [DOI] [PubMed] [Google Scholar]
- [42].Xiao X, Ulrich BA, Chen B, Higgins CP, Sorption of poly- and perfluoroalkyl substances (PFASs) relevant to aqueous film-forming foam (AFFF)-Impacted groundwater by biochars and activated carbon, Environ. Sci. Technol 51 (11) (2017) 6342–6351, 10.1021/acs.est.7b00970. [DOI] [PubMed] [Google Scholar]
- [43].Xiao X, Ulrich BA, Chen B, Higgins CP, Sorption of poly-and perfluoroalkyl substances (PFASs) relevant to aqueous film-forming foam (AFFF)-impacted groundwater by biochars and activated carbon, Environ. Sci. Technol 51 (11) (2017) 6342–6351. [DOI] [PubMed] [Google Scholar]
- [44].Rattanaoudom R, Visvanathan C, Removal of PFOA by hybrid membrane filtration using PAC and hydrotalcite, Desalinat. Water Treat 32 (1–3) (2011) 262–270, 10.5004/dwt.2011.2709. [DOI] [Google Scholar]
- [45].Loi-Brügger A, Panglisch S, Hoffmann G, Buchta P, Gimbel R, Nacke CJ, Removal of trace organic substances from river bank filtrate – performance study of RO and NF membranes, Water Supply 8 (1) (2008) 85–92, 10.2166/ws.2008.021. [DOI] [Google Scholar]
- [46].Wan H, Mills R, Qu K, Hower JC, Mottaleb MA, Bhattacharyya D, Xu Z, Rapid removal of PFOA and PFOS via modified industrial solid waste: mechanisms and influences of water matrices, Chem. Eng. J (2021) 133271, 10.1016/j.cej.2021.133271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wan H, Islam MS, Qian D, Ormsbee L, Bhattacharyya D, Reductive degradation of CC14 by sulfidized Fe and Pd-Fe nanoparticles: kinetics, longevity, and morphology aspects, Chem. Eng. J 394 (2020) 125013, 10.1016/j.cej.2020.125013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chularueangaksorn P, Tanaka S, Fujii S, Kunacheva C, Adsorption of perfluorooctanoic acid (PFOA) onto anion exchange resin, non-ion exchange resin, and granular-activated carbon by batch and column, Desalinat. Water Treat 52 (34–36) (2014) 6542–6548, 10.1080/19443994.2013.815589. [DOI] [Google Scholar]
- [49].Gagliano E, Falciglia PP, Zaker Y, Karanfil T, Roccaro P, Microwave regeneration of granular activated carbon saturated with PFAS, Water Res. 198 (2021) 117121, 10.1016/j.watres.2021.117121. [DOI] [PubMed] [Google Scholar]
- [50].Trojanowicz M, Bojanowska-Czajka A, Bartosiewicz I, Kulisa K, Advanced Oxidation/Reduction Processes treatment for aqueous perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS) – a review of recent advances, Chem. Eng. J 336 (2018) 170–199, 10.1016/j.cej.2017.10.153. [DOI] [Google Scholar]
- [51].Marino T, Russo F, Figoli A, The Formation of polyvinylidene fluoride membranes with tailored properties via vapour/non-solvent induced phase separation, Membranes 8 (3) (2018), 10.3390/membranes8030071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bottino A, Capannelli G, Munari S, Turturro A, Solubility parameters of poly (vinylidene fluoride). 10.1002/polb.1988.090260405, 1988, 26, 4, 785–794. [DOI] [Google Scholar]
- [53].Hansen CM, Hansen Solubility Parameters: a User’s Handbook, CRC press, 2007. [Google Scholar]
- [54].Brandrup J, Immergut EH, Grulke EA, Abe A, Bloch DR, Polymer Handbook, Wiley, New York, 1999. [Google Scholar]
- [55].Tamaki K, Watanabe S, Daikyoji Y, Partial molar volumes of sodium perfluoroalkanoates and lithium perfluoro-1-alkanesulfonates in aqueous solutions, Bull. Chem. Soc. Jpn 63 (12) (1990) 3681–3682, 10.1246/bcsj.63.3681. [DOI] [Google Scholar]
- [56].Park M, Wu S, Lopez IJ, Chang JY, Karanfil T, Snyder SA, Adsorption of perfluoroalkyl substances (PFAS) in groundwater by granular activated carbons: roles of hydrophobicity of PFAS and carbon characteristics, Water Res. 170 (2020) 115364. [DOI] [PubMed] [Google Scholar]
- [57].Du Z, Deng S, Zhang S, Wang B, Huang J, Wang Y, Yu G, Xing B, Selective and high sorption of perfluorooctanesulfonate and perfluorooctanoate by fluorinated alkyl chain modified montmorillonite, J. Phys. Chem. C 120 (30) (2016) 16782–16790, 10.1021/acs.jpcc.6b04757. [DOI] [Google Scholar]
- [58].Cai W, Gao Z, Yu S, Lv M, Shi Y, Wang J, New insights into membrane fouling formation during ultrafiltration of organic wastewater with high salinity, J. Membr. Sci 635 (2021) 119446, 10.1016/j.memsci.2021.119446. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.