Abstract
While life in urban environments may confer a number of benefits, it may also result in a variety of exposures, with toxic consequences for neurodevelopment and neuropsychological health. Neurotoxicants are any of a large number of chemicals or substances that interfere with normal function and/or compromise adaptation in the central and/or peripheral nervous system. Evidence suggests that neurotoxicant effects have a greater effect when occurring in utero and during early childhood. Recent findings exploring neural-level mechanisms provide a crucial opportunity to explore the ways in which environmental conditions may get “under the skin” to impact a number of psychological behaviors and cognitive processes, ultimately allowing for greater synergy between macro- and microlevel efforts to improve mental health in the presence of neurotoxicant exposures. In this review, we provide an overview of 3 types of neurotoxicants related to the built environment and relevant to brain development during childhood and adolescence: lead exposure, outdoor particulate matter pollution, and endocrine-disrupting chemicals. We also discuss mechanisms through which these neurotoxicants affect central nervous system function, including recent evidence from neuroimaging literature. Furthermore, we discuss neurotoxicants and mental health during development in the context of social determinants and how differences in the spatial distribution of neurotoxicant exposures result in health disparities that disproportionately affect low-income and minority populations. Multifaceted approaches incorporating social systems and their effect on neurotoxicant exposures and downstream mental health will be key to reduce societal costs and improve quality of life for children, adolescents, and adults.
Keywords: Endocrine-disrupting chemicals, Lead exposure, Mental health, Neurodevelopment, Neurotoxicants, Particulate matter
Within the past century, and increasingly in recent decades, more of the population has shifted to living in more urban city settings, up from 40% of the United States population in 1900 to 83% in 2018 (1). While cities and large urban areas can confer a large number of benefits, such as more diverse social networks, well-developed infrastructure, and access to resources and opportunities for social support (2), they also may subject inhabitants to a large number of exposures that may have repercussions for their physical and mental health. In addition, owing to the complex and dynamic spatial properties of cities, urban development is widely heterogeneous, leading to spatial and socioeconomic inequity in how these exposures are distributed (3), therefore making the issue of negative urban exposures and health of interest to epidemiologists, sociologists, and biologists alike.
Although environmental exposures in cities have been documented to have a number of effects on physical health, research on their impacts on mental health has, until recent years, been fairly scarce (4). In particular, recent findings have highlighted the importance of childhood environmental exposures on neurodevelopment (5), providing an additional piece of the complex puzzle through which these exposures may affect mental health. Recent neuroimaging methods have offered an opportunity to interrogate the ways in which various environmental exposures can get “under the skin” to affect neural structure and function. Thus, the focus of this review is to present current evidence supporting toxic childhood environmental exposures that affect neural structure and function, referred to here as neurotoxicants, and their impact on mental health.
Neurotoxicants are any chemicals or substances that interfere with normal function and/or compromise adaptation in the central and/or peripheral nervous system, either during development or at maturity (6,7). This definition may apply to more than 200 chemicals reported as neurotoxicants in humans (8), ranging from metals and inorganic compounds to air pollution to organic solvents (see http://braindrain.dk/known-chemical-brain-drainers/ for a list of known neurotoxicants). An additional key point from this definition is that it highlights time of exposure during development as critical to the severity of neurotoxicant effects, with increased vulnerability occurring in utero and during early childhood (9). While several effects have been reported for neurotoxicant exposures in adulthood, low levels of exposure during key developmental periods may be enough to cause irreparable brain injury. To reduce societal costs and improve quality of life for affected individuals, research on modifiable risk factors holds the promise to open new avenues for early prevention and intervention.
In the following sections, this article highlights 3 neurotoxicant exposures related to the built environment: lead exposure, outdoor particulate matter (PM) pollution, and endocrine-disrupting chemicals (EDCs). We will also discuss candidate mechanisms identified in the literature on how these neurotoxicants affect central nervous system (CNS) function and relate to neuropsychological mental health disruptions, with a particular focus on noninvasive neuroimaging results. While this article is not a systematic review of the literature on neurotoxicants, our goal is to bring awareness to the various ways in which certain environmental exposures that are often found in urban settings can impact individual-level neuropsychological development and function, so as not only to inspire change at legislative levels but also to leverage efforts of individuals within their communities by providing knowledge that may aid in advocating for their wellbeing. Furthermore, we would like to acknowledge that most (if not all) negative neurotoxicant exposure associations with mental health are exacerbated in racial and ethnic minority and low-income children (5), and although the coverage of the structural and social determinants of mental health involved are outside the scope of this article, further research is needed to interrogate the particular upstream mechanisms (e.g., institutionalized discriminatory practices, environmental racism) that give rise to these unequal exposures and disparities (10).
Lead Exposure
Relevance to Public Health
Metals are one of the most widely studied, and concerning, set of neurotoxicants, with annual costs for lead poisoning and methylmercury toxicity estimated at $60 billion in 2008 (11). Lead-based poisoning is particularly alarming given that evidence has shown that any amount of detectable lead can be harmful to neuropsychological outcomes at all ages (12). In 2021, the Center for Disease Control and Prevention updated the blood lead reference value to 3.5 μg/dL to identify children at ages 1 to 5 years in the top 2.5 percentile of blood lead level (BLL) distribution (13). Over the past decades, environmental legislation in the United States has dramatically reduced the number of cases of lead poisoning in children ages 1 to 5, from 88% in 1976–1980 to 0.8% during 2007–2010, using a BLL cutoff of ≥10 μg/dL (13). Yet, as of 2017, about 500,000 U.S. children are currently affected by elevated BLLs using a more stringent cutoff of ≥5 μg/dL (14). As is the case with many environmental neurotoxicants, the highest burden of lead poisoning is experienced by minority populations, with Black children being twice as likely to have elevated BLLs than White or Latinx children (15), highlighting the undeniable effect of upstream structural and sociopolitical factors in the distribution of neurotoxicant exposures.
Sources of Exposure
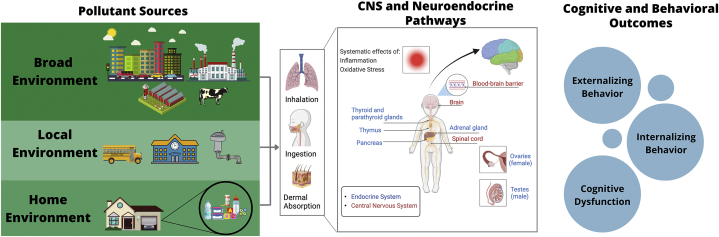
Sources of lead-based poisoning include lead-based paint in older homes, contaminated drinking water pipes, and residual lead in soil deposited from airborne emissions, which can recontaminate areas (16) (Figure 1; Table S1).
Figure 1.
Sources of major neurotoxicants and proposed physiological targets. Exposure to the 3 classes of neurotoxicants discussed in this article (lead, outdoor particulate matter air pollution, and endocrine-disrupting chemicals) can occur through multiple avenues, because the sources for these toxicants include broad environmental pollution from traffic, agriculture, industrial activities, etc.; local pollution in areas, such as parks and schools, where children spend time; and home pollution, where children may be exposed to household items containing neurotoxic chemicals. These pollution sources, encountered in children’s broad and local environments, may enter the body through inhalation, ingestion, and/or dermal absorption (see Table S1 for further details). Once in the bloodstream, these toxicants may cross the blood-brain barrier and directly act upon components of the central nervous system (CNS) or indirectly affect the brain by altering other systems, including air pollution–related increases in inflammation and oxidative stress or endocrine-disrupting chemicals acting to disrupt the endocrine system. A causal arrow is not drawn between the physiological diagram and the behavioral and cognitive outcomes, because no causal link has been confirmed. However, it is hypothesized that disruption of normal neurodevelopment and damage to neural and endocrine pathways may lead to changes in behavioral symptoms, cognitive development, and mental health in children.
Potential CNS Mechanisms
Lead does not seem to have any known physiological functions (17) but is able to cross the blood-brain barrier, thereby interfering with the functioning of many processes in the CNS (18). Based on limited studies in humans and many studies in rodents and nonhuman primate systems, lead seems to heavily affect dopaminergic neurotransmission. Primarily, studies in rats indicate that chronic exposure to lead may result in increased dopamine release in the nucleus accumbens while reducing synaptic clearance of dopamine (19,20). Studies on lead exposure have also indicated increased sensitivity in dopamine D1/D2 receptors, with possible additional disturbances in glutamate/dopamine interactions (21), although this may be dependent on the level of the dose, sex, and developmental stage of exposure, with earlier exposures resulting in stronger perturbations to dopaminergic systems (17,20,21). Furthermore, murine models of lead exposure and dopaminergic system perturbations have also indicated an enhanced sensitivity to rewarding stimuli and drugs, such as cocaine, for perinatal lead exposures (22), although further research is needed in this domain to determine the extent to which these results generalize to other rewarding stimuli following lead exposure.
Neuroimaging Findings
With the advent of neuroimaging techniques such as magnetic resonance imaging (MRI), researchers have also been able to better characterize the impact of lead exposure on human brain structure and function. Studies on adults with high childhood lead exposure found decreased gray matter volume and alteration to myelination and axonal integrity, suggesting altered white matter connectivity (23, 24, 25). Reductions in gray matter volume occurred in the anterior cingulate and ventrolateral prefrontal cortices in men and in the inferior parietal lobe in women (23). This effect of a global reduction in gray matter volume was replicated when associated with a proxy of lead risk exposure in a large, diverse sample of 9- to 10-year-old children from the Adolescent Brain Cognitive Development (ABCD) Study, although this study did not measure BLLs directly (26). More recently, evidence from a resting-state functional MRI study found that prenatal exposure to lead was related to different age-related fetal brain patterns, such that lead-exposed fetuses showed stronger age-related lateral to posterior cingulate functional connectivity and weaker functional connectivity between insular and temporal areas relative to lead-naive fetuses, suggesting early alteration in systems supporting higher-order cognitive and regulatory functions (27).
Impact on Mental Health
Exposure to heavy metals such as lead has been documented to have a substantive impact on the development of neurocognitive functions such as attention, memory, and general executive function (28, 29, 30, 31, 32, 33, 34, 35, 36) and has been reviewed elsewhere (29,37,38). Newer studies have expanded on these findings by exploring the impacts of lead exposure on mental health problems and developmental disabilities such as autism spectrum disorder (ASD) and learning disabilities (38). Both internalizing and externalizing behaviors have been reported to relate to lead exposure in children (39), as have schizophrenia (40) and childhood attention-deficit/hyperactivity disorder (ADHD) (21,37,41, 42, 43, 44, 45, 46, 47). More recent meta-analyses investigating the role of childhood lead exposure in increasing ADHD prevalence suggest an even more specific effect of lead on attention and response inhibition behaviors associated with ADHD (37). High levels of lead in red blood cells and lead urinary levels were also reported in at least 1 cohort study of children with ASD, although the design of the study did not allow for causal inferences in this relationship (48).
Overall, lead exposure has wide-ranging implications for a variety of neuropsychological processes that are often associated with mental health problems, particularly those associated with ADHD. Further research is needed to elucidate not only the particular pathways through which lead exposure results in mental health problems, but also the extent and magnitude of these effects, given recent calls to attention to the variability of these effects in verbal and performance tests [see (49,50) for a discussion within the context of lead-IQ relationships]. While legislative actions over the past few decades have greatly reduced the number of individuals affected by chronic lead exposure, it will take years of maintained efforts to eliminate the negative effects of lead, given that house dust and soil contaminated by remnants of lead paint continue to be the most common and persistent sources of lead exposure in children (51), thus highlighting a multidisciplinary concerted effort to combat the impact of heavy metals such as lead on mental health.
Outdoor Ambient PM
Relevance to Public Health
Outdoor air pollution is a mixture of gaseous and solid particles that includes components derived from both natural and anthropogenic sources. The largest proportion of the health impacts of outdoor air pollution are attributed to PM, which is defined by its aerodynamic diameter (PM10, <10 μm; PM2.5 <2.5 μm; ultrafine, PM0.1, <0.1 μm) (52). Children are likely to be the most vulnerable to potential neurotoxic effects of PM because brain maturation continues through the third decade of life (53,54). A recent literature review in adults concluded that there is evidence to support the hypothesis of links between PM exposure and depression, anxiety, and suicide risk, but further reviews with a focus on children are needed (55). In addition, social stratification of exposure to outdoor PM is common; low socioeconomic areas and neighborhoods with a high percentage of racial/ethnic minorities (i.e., Black, Latinx, Native American, and Asian populations in the United States) are more likely to be exposed to higher levels of PM than predominantly White neighborhoods (56).
Sources of Exposure
Primary sources of outdoor PM—especially PM2.5—vary by location and include traffic emissions, road dust, industrial processes, commercial cooking, agricultural processes, wildfires, and many other anthropogenic and natural sources (Figure 1; Table S1). Secondary PM is formed in the ambient atmosphere as gas-phase chemical compounds interact, form new particles, and condense onto existing particles (57).
Potential CNS Mechanisms
Mechanistically, outdoor ambient air pollution may affect the brain by acting either directly via transport of nanosized particles or secondarily through systemic changes leading to increased neuroinflammation and oxidative stress (58, 59, 60). At the neural level, exposure to small particles such as PM2.5 and nanosized PM during development alters dendritic spine density (61) and neurogenesis (62) and affects microglial cells (59,63). These are important neuronal processes underlying synaptic proliferation and “pruning” and are essential hallmarks of childhood and adolescent neurodevelopment (60). Moreover, distinct neuronal cell types have different biochemical sensitivity to oxidative stress, with key emotional regions, including the prefrontal cortex and amygdala, purported to be most susceptible (64). Finally, inhalation studies in animals have found that prenatal exposure to diesel exhaust alters neurotransmitter levels in various brain regions, including the amygdala (65,66).
Neuroimaging Findings
Similar to lead exposure, emerging cross-sectional human MRI findings suggest that prenatal and postnatal childhood PM2.5 exposure may affect brain structure and function (67). Using anatomical MRI, prenatal PM2.5 exposure has been linked to thinner gray matter cortices in the prefrontal and cuneus regions (68) and widespread smaller white matter surface area (69) and smaller corpus callosum volumes (70) in childhood. Beyond the prenatal period, childhood PM2.5 exposure has been linked to altered cortical thickness in gray matter of the frontal, parietal, and temporal lobes and subcortical volumes in 9- to 10-year-old children from the ABCD Study (71). More recent work has shown associations between prenatal air pollutant exposure and white matter microstructural differences using diffusion MRI at ages 9 to 12 years (72), as well as associations between air pollution mixtures during childhood and altered brain activity during a sensory task and differences in functional connectivity of large-scaled brain networks, including the default mode network (73).
Impact on Mental Health
Prenatal and childhood air pollution exposure has been associated with poor neurodevelopmental and emotional outcomes (53,54,74,75), including emotional behavioral problems (76), impaired emotional regulation (77), anxiety/depression (76, 77, 78, 79, 80, 81), ADHD symptoms (70,82,83), ASD (84,85), psychotic experience (86), and increased likelihood of being prescribed psychiatric medication (87). More research is needed to establish the strength of these associations and to determine which behavioral and mental health outcomes are most affected. However, considerable heterogeneity exists among associations observed in epidemiological studies, with some studies failing to observe these aforementioned associations between air pollution exposure and mental health problems (88,89). Inconclusive findings may stem from the inability to use identical methods of exposure assessment across development in heterogeneous cohorts of children (88,89). Furthermore, heterogeneity in the method of exposure assessment (spatial modeling, personal air monitors, etc.), limitations in study population size, variation in the lag time between exposure and outcome, and variation in exposure age contribute to ongoing uncertainty in establishing causal links between PM exposure and mental health outcomes in children.
Despite this, experimental animal studies have found that exposure to outdoor PM2.5 and nanosized PM in utero as well as during childhood and adulthood may result in long-term behavioral consequences, including greater anxious and depressive-like symptoms (61,62,65,90, 91, 92, 93, 94, 95). Additional studies have also found that prenatal exposure to diesel exhaust and early postnatal exposure to nanosized PM is associated with increased displays of aggression (65) and poor behavioral control (96,97) in mice.
In summary, emerging data suggest a link between outdoor PM and mental health. Further research is needed to clarify this link and to explore complex interactions with social stratification, outdoor air pollution, and mental health.
Endocrine-Disrupting Chemicals
Relevance to Public Health
EDCs are defined by the World Health Organization as exogenous chemicals that interfere with the endocrine system and thus cause adverse effects in an organism, including developmental malformation, increased risk of cancer, reproductive problems, and interference with normal function of the immune and nervous systems (98, 99, 100).
Two scientific statements published by the Endocrine Society have sought to provide a comprehensive review of EDC-related literature, including an in-depth look at the effects of EDCs on neuroendocrine systems and neurophysiology (101,102).
Exposure to these compounds—especially during critical windows such as fetal development, childhood, and puberty—may lead to permanent defects that do not appear until later in life (100). At a population level, exposure to EDCs has been observed as consistently higher among low-income individuals and ethnic/racial minorities compared with higher-income White participants (103), contributing to a greater economic burden of EDC-related disease for Black and Latinx populations in the United States (104).
Associations have been explored between EDCs and a number of psychiatric neurodevelopmental disorders, including ASD, ADHD, anxiety and stress disorders, depression, and others (105). Given that more than 800 chemicals are known or suspected to have endocrine-disrupting properties, a comprehensive discussion of associations between EDCs and mental health outcomes is outside the scope of this article (99,100,106). This article will focus on 3 types of EDCs—bisphenol A (BPA), phthalates, and polychlorinated biphenyl compounds (PCBs)—that are repeatedly identified in literature as disrupters of neurodevelopment.
Sources of Exposure
BPA is ubiquitous in the environment, and more than 90% of children in multiple regions around the world have confirmed exposure (107, 108, 109, 110, 111). BPA is commonly found in reusable bottles, microwave ovenware, food storage containers, the internal coatings of food and beverage cans, and children’s toys; exposure occurs through ingestion and dermal absorption (Figure 1) (112,113). Phthalates are a family of EDCs that are divided into two subgroups with distinct commercial uses and toxicological properties (114,115). Low molecular weight phthalates are used in pharmaceuticals, cosmetics, personal care products, and packaging (Figure 1) (114,116). Owing to their molecular structure and lack of covalent bonds within the polymer matrix, these chemicals easily leach from products into the environment, and human exposure occurs through food ingestion, dermal absorption, and inhalation (114,116). Finally, PCBs are synthetic organochlorine compounds that were banned in many countries, including the United States, in the 1970s, but they are still persistent and ubiquitous environmental contaminants (117, 118, 119). They were popular for multiple industrial uses in the early 1930s, including as capacitor and transformer oils, hydraulic fluids, lubricating oils, and plasticizers (Figure 1; Table S1) (119).
Potential CNS Mechanisms
EDCs do not necessarily lead to direct neurotoxic damage (i.e., poisoning neural tissue and causing cell death). Instead, they are capable of disrupting the structure and function of neural systems indirectly via neuroendocrine-related mechanisms (120). There are 2 major pathways by which EDCs may affect the brain: by disrupting neuroendocrine processes originating in the hypothalamus and/or by acting on steroid hormone receptors and signaling pathways throughout the brain (101). The developing human brain is especially vulnerable to EDCs during fetal development, during which these chemicals can impair critical development steps, including neurogenesis, neuronal migration, neuronal and glial cell differentiation, myelination, and synaptogenesis (120, 121, 122). Many EDCs can cross the placenta and enter the fetal bloodstream, where they may interact with steroid and xenobiotic receptors, impacting estrogen signaling, interfering with hormone transport, binding hormone receptors, and disrupting hormone synthesis (105,121,123,124).
The specific mechanisms by which EDCs may affect neurobehavioral development have been explored in depth elsewhere (99,101,120). Phthalate, PCB, and BPA exposure have all been associated with thyroid homeostasis interference and decreased thyroid maternal hormone levels. Maternal hypothyroxinemia—a state of low free maternal thyroid hormones—during the prenatal period has been associated with delayed cognitive and neuromotor development and increased ADHD symptoms in children (102,105,125, 126, 127, 128, 129). Research has suggested that the estrogen-mimicking capabilities of BPA make it capable of dysregulating endocrine signaling pathways during the perinatal period (105). Similarly, it has been proposed that some of the possible behavioral effects of BPA—especially those related to anxiety and depression—occur through its estrogenic activity, including its ability to bind to estrogen receptor subtypes, which are located in brain regions critical for stress response (105). In animal studies, associations between EDCs and mental health outcomes vary in strength, direction, and magnitude and depend on multiple factors, including species, dose, exposure timing, and outcome measurement (101). Yet, there is consensus that the expression of genes and proteins related to steroid hormone receptors in the brain are highly sensitive to EDC exposure, especially during critical windows of brain development (101).
Neuroimaging Findings
Emerging evidence suggests that prenatal exposure to phthalates, in particular, may be related to MRI brain morphometry and connectivity in adolescence. A study measuring maternal concentrations of phthalate esters during the third trimester was associated with reduced anterior cingulate and cerebellum volumes in adolescents and with reduced diffusion in white matter tracts in the corpus callosum, corona radiata, superior fronto-occipital fasciculus, and superior longitudinal fasciculus (130). Another study recruiting mother–child pairs found significant relationships between prenatal phthalate concentrations and regional brain activity at rest, with higher exposure related to differences in connectivity patterns seen for superior and middle frontal, middle and inferior temporal, anterior cingulum, and insular brain regions in female adolescents (131).
Impact on Mental Health
BPA has been linked to anxiety and hyperactivity in children, with some studies detecting disproportionate effects in boys (132, 133, 134) or girls (135,136). These studies collected maternal urinary samples during the prenatal period and evaluated anxiety symptoms and scores at 2 years of age (136) and at 10 and 12 years of age (137). Results varied by exposure window, suggesting different critical windows of exposure during fetal development for boys and girls (105). Similar links have been established between prenatal and early-life exposure to BPA and symptoms of depression, anxiety, and increased aggressive behavior during childhood and adolescence, with particular susceptibility noted in males (133,134,137,138). Animal studies have supported these findings, showing associations between prenatal BPA exposure and increased aggression and changes in the dopaminergic system in the limbic forebrain (136). Although these studies support potential associations between pre- and perinatal exposure to BPA and behavioral measures that suggest behavioral problems, BPA has not been definitively linked to diagnosis of any disorders.
Multiple epidemiological studies have detected associations—sometimes with sex-specific effects—between prenatal exposure to phthalates and increased behavioral problems in childhood (139, 140, 141, 142, 143). In a 2010 study, prenatal low molecular weight phthalate metabolites were associated with elevated aggression, attention problems, conduct problems, depression, and higher scores on an Externalizing Problems Composite score and the Behavioral Symptom Index (139). Similarly, a 2017 study using subscores from the Strength and Difficulties Questionnaire detected associations between the presence of two phthalate metabolites in maternal cord blood and internalizing behavior, relationship problems, and emotional symptoms in 3-year-old boys (100).
Multiple studies exist linking chronic exposure to low doses of PCBs to lower child cognition and other neuropsychological outcomes (119,144). Two 2010 studies detected significant associations between low-level prenatal exposure and behavioral outcomes in young children, including increased inattention in 11-month-old infants (118) and an increased state of unhappiness and anxiety during testing sessions in 5-year-olds (125). Finally, a 2003 Michigan study found associations between prenatal PCB exposure and greater impulsivity, poorer concentration, and poorer working memory in 11-year-old children (117); such symptoms are sometimes, but not always, impaired in individuals with ADHD (37).
Together, the literature suggests that there is concern that early-life exposure to EDCs may influence mental health difficulties in children. Additional neighborhood-level stressors may interact with EDC exposure and impact associations between EDCs and mental health outcomes (145,146). Further research is needed to confirm associations between EDCs and mental health outcomes and to investigate how disparities in exposure to EDCs may further impact risk.
Conclusions
While urban life may confer several benefits, it may be accompanied by numerous exposures that may be harmful or unsafe for the CNS. Exposure to heavy metals such as lead, outdoor air pollution, and EDCs may affect neuropsychological factors, particularly when these negative exposures occur at an early age, either in utero or in early childhood. While a number of effects and candidate mechanisms have been discussed here, for many neurotoxicants it is difficult to establish causality between a single bad actor and diagnosable disorders in human epidemiological studies, owing to limits in sample size, interindividual variations in risk factors, gene-by-environment interactions, and the complex mixtures of exposures that different people experience (147). Furthermore, despite emerging evidence from epidemiological and animal model studies that suggest associations between neurotoxicants, such as metals, air pollution, and EDCs, and neural and behavioral outcomes, it can take years for causality to be widely accepted (120). Despite this (perhaps dismal) landscape, it is important to state the importance of awareness and advocacy surrounding the issue of neurotoxicant exposures in order to influence policy makers that can enact legislation to largely mitigate and eliminate these toxic environmental exposures. It is also important to leverage and highlight the efforts of environmental justice activists and community organizers, usually from historically marginalized communities, bringing attention to how these neurotoxicants disproportionately impact their communities. Mitigating and eliminating the negative effect of neurotoxicant exposures requires a multifaceted approach. Further research is needed that can explore mechanisms taking into account the complex dynamics in which environmental, social, and political factors occur (5), as well as elucidate how both the timing and accumulation of neurotoxicants impact the CNS and subsequent risk for the development of mental health problems.
Acknowledgments and Disclosures
This work was funded in part by the National Institute of Environmental Health Sciences (Grant No. R01 ES032295; principal investigator, MMH).
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2022.05.002.
Contributor Information
Carlos Cardenas-Iniguez, Email: carlosc2@usc.edu.
Megan M. Herting, Email: herting@usc.edu.
Supplementary Material
References
- 1.Ritchie H., Roser M. Urbanization. Our World in Data. 2018 https://ourworldindata.org/urbanization Available at: [Google Scholar]
- 2.Tost H., Champagne F.A., Meyer-Lindenberg A. Environmental influence in the brain, human welfare and mental health. Nat Neurosci. 2015;18:1421–1431. doi: 10.1038/nn.4108. [DOI] [PubMed] [Google Scholar]
- 3.Brelsford C., Lobo J., Hand J., Bettencourt L.M.A. Heterogeneity and scale of sustainable development in cities. Proc Natl Acad Sci U S A. 2017;114:8963–8968. doi: 10.1073/pnas.1606033114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reuben A., Manczak E.M., Cabrera L.Y., Alegria M., Bucher M.L., Freeman E.C., et al. The interplay of environmental exposures and mental health: Setting an agenda. Environ Health Perspect. 2022;130 doi: 10.1289/EHP9889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Payne-Sturges D.C., Cory-Slechta D.A., Puett R.C., Thomas S.B., Hammond R., Hovmand P.S. Defining and intervening on cumulative environmental neurodevelopmental risks: Introducing a complex systems approach. Environ Health Perspect. 2021;129 doi: 10.1289/EHP7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costa L.G. Neurotoxicity testing: A discussion of in vitro alternatives. Environ Health Perspect. 1998;106(suppl 2):505–510. doi: 10.1289/ehp.98106505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giordano G., Costa L.G. Developmental neurotoxicity: Some old and new issues. ISRN Toxicol. 2012;2012 doi: 10.5402/2012/814795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grandjean P., Landrigan P.J. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13:330–338. doi: 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rice D., Barone S., Jr. Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ Health Perspect. 2000;108(suppl 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Washington H.A. Little, Brown Spark; New York: 2019. A Terrible Thing to Waste: Environmental Racism and Its Assault on the American Mind. [Google Scholar]
- 11.Trasande L., Liu Y. Reducing the staggering costs of environmental disease in children, estimated at $76.6 billion in 2008. Health Aff (Millwood) 2011;30:863–870. doi: 10.1377/hlthaff.2010.1239. [DOI] [PubMed] [Google Scholar]
- 12.Landrigan P.J., Boffetta P., Apostoli P. The reproductive toxicity and carcinogenicity of lead: A critical review. Am J Ind Med. 2000;38:231–243. doi: 10.1002/1097-0274(200009)38:3<231::aid-ajim2>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 13.Blood lead reference value. Center for Disease Control and Prevention. https://www.cdc.gov/nceh/lead/data/blood-lead-reference-value.htm Available at:
- 14.Hauptman M., Bruccoleri R., Woolf A.D. An update on childhood lead poisoning. Clin Pediatr Emerg Med. 2017;18:181–192. doi: 10.1016/j.cpem.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeter D., Banks E.C., Aschner M. Disparity in risk factor severity for early childhood blood lead among predominantly African-American Black children: The 1999 to 2010 US NHANES. Int J Environ Res Public Health. 2020;17:1552. doi: 10.3390/ijerph17051552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahaffey K.R. Sources of lead in the urban environment. Am J Public Health. 1983;73:1357–1358. doi: 10.2105/ajph.73.12.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones D.C., Miller G.W. The effects of environmental neurotoxicants on the dopaminergic system: A possible role in drug addiction. Biochem Pharmacol. 2008;76:569–581. doi: 10.1016/j.bcp.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Bradbury M.W., Deane R. Permeability of the blood-brain barrier to lead. Neurotoxicology. 1993;14:131–136. [PubMed] [Google Scholar]
- 19.Zuch C.L., O’Mara D.J., Cory-Slechta D.A. Low-level lead exposure selectively enhances dopamine overflow in nucleus accumbens: An in vivo electrochemistry time course assessment. Toxicol Appl Pharmacol. 1998;150:174–185. doi: 10.1006/taap.1998.8396. [DOI] [PubMed] [Google Scholar]
- 20.Szczerbak G., Nowak P., Kostrzewa R.M., Brus R. Maternal lead exposure produces long-term enhancement of dopaminergic reactivity in rat offspring. Neurochem Res. 2007;32:1791–1798. doi: 10.1007/s11064-007-9306-0. [DOI] [PubMed] [Google Scholar]
- 21.Cory-Slechta D.A. Postnatal lead exposure and MK-801 sensitivity. Neurotoxicology. 1997;18:209–220. [PubMed] [Google Scholar]
- 22.Rocha A., Trujillo K.A. Neurotoxicity of low-level lead exposure: History, mechanisms of action, and behavioral effects in humans and preclinical models. Neurotoxicology. 2019;73:58–80. doi: 10.1016/j.neuro.2019.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brubaker C.J., Dietrich K.N., Lanphear B.P., Cecil K.M. The influence of age of lead exposure on adult gray matter volume. Neurotoxicology. 2010;31:259–266. doi: 10.1016/j.neuro.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cecil K.M., Brubaker C.J., Adler C.M., Dietrich K.N., Altaye M., Egelhoff J.C., et al. Decreased brain volume in adults with childhood lead exposure. PLoS Med. 2008;5:e112. doi: 10.1371/journal.pmed.0050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cecil K.M. Effects of early low-level lead exposure on human brain structure, organization and functions. J Dev Orig Health Dis. 2011;2:17–24. [Google Scholar]
- 26.Marshall A.T., Betts S., Kan E.C., McConnell R., Lanphear B.P., Sowell E.R. Association of lead-exposure risk and family income with childhood brain outcomes. Nat Med. 2020;26:91–97. doi: 10.1038/s41591-019-0713-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomason M.E., Hect J.L., Rauh V.A., Trentacosta C., Wheelock M.D., Eggebrecht A.T., et al. Prenatal lead exposure impacts cross-hemispheric and long-range connectivity in the human fetal brain. Neuroimage. 2019;191:186–192. doi: 10.1016/j.neuroimage.2019.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dietrich K.N., Succop P.A., Berger O.G., Hammond P.B., Bornschein R.L. Lead exposure and the cognitive development of urban preschool children: The Cincinnati Lead Study cohort at age 4 years. Neurotoxicol Teratol. 1991;13:203–211. doi: 10.1016/0892-0362(91)90012-l. [DOI] [PubMed] [Google Scholar]
- 29.Banks E.C., Ferretti L.E., Shucard D.W. Effects of low level lead exposure on cognitive function in children: A review of behavioral, neuropsychological and biological evidence. Neurotoxicology. 1997;18:237–281. [PubMed] [Google Scholar]
- 30.Lanphear B.P., Dietrich K., Auinger P., Cox C. Cognitive deficits associated with blood lead concentrations <10 microg/dL in US children and adolescents. Public Health Rep. 2000;115:521–529. doi: 10.1093/phr/115.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiodo L.M., Covington C., Sokol R.J., Hannigan J.H., Jannise J., Ager J., et al. Blood lead levels and specific attention effects in young children. Neurotoxicol Teratol. 2007;29:538–546. doi: 10.1016/j.ntt.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Bellinger D.C. Very low lead exposures and children’s neurodevelopment. Curr Opin Pediatr. 2008;20:172–177. doi: 10.1097/MOP.0b013e3282f4f97b. [DOI] [PubMed] [Google Scholar]
- 33.Bao Q.S., Lu C.Y., Song H., Wang M., Ling W., Chen W.Q., et al. Behavioural development of school-aged children who live around a multi-metal sulphide mine in Guangdong Province, China: A cross-sectional study. BMC Public Health. 2009;9:217. doi: 10.1186/1471-2458-9-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Searle A.K., Baghurst P.A., van Hooff M., Sawyer M.G., Sim M.R., Galletly C., et al. Tracing the long-term legacy of childhood lead exposure: A review of three decades of the Port Pirie Cohort study. Neurotoxicology. 2014;43:46–56. doi: 10.1016/j.neuro.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Shah-Kulkarni S., Ha M., Kim B.M., Kim E., Hong Y.C., Park H., et al. Neurodevelopment in early childhood affected by prenatal lead exposure and iron intake [published correction appears in Medicine (Baltimore) 2016; 95:e077f] Medicine (Baltimore) 2016;95 doi: 10.1097/MD.0000000000002508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lanphear B.P., Hornung R., Khoury J., Yolton K., Baghurst P., Bellinger D.C., et al. Low-level environmental lead exposure and children’s intellectual function: An international pooled analysis [published correction appears in Environ Health Perspect 2019; 127:99001] Environ Health Perspect. 2005;113:894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eubig P.A., Aguiar A., Schantz S.L. Lead and PCBs as risk factors for attention deficit/hyperactivity disorder. Environ Health Perspect. 2010;118:1654–1667. doi: 10.1289/ehp.0901852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rauh V.A., Margolis A.E. Research Review: Environmental exposures, neurodevelopment, and child mental health – New paradigms for the study of brain and behavioral effects. J Child Psychol Psychiatry. 2016;57:775–793. doi: 10.1111/jcpp.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiodo L.M., Jacobson S.W., Jacobson J.L. Neurodevelopmental effects of postnatal lead exposure at very low levels. Neurotoxicol Teratol. 2004;26:359–371. doi: 10.1016/j.ntt.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 40.Guilarte T.R., Opler M., Pletnikov M. Is lead exposure in early life an environmental risk factor for schizophrenia? Neurobiological connections and testable hypotheses. Neurotoxicology. 2012;33:560–574. doi: 10.1016/j.neuro.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braun J.M., Kahn R.S., Froehlich T., Auinger P., Lanphear B.P. Exposures to environmental toxicants and attention deficit hyperactivity disorder in U.S. children. Environ Health Perspect. 2006;114:1904–1909. doi: 10.1289/ehp.9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Froehlich T.E., Lanphear B.P., Auinger P., Hornung R., Epstein J.N., Braun J., Kahn R.S. Association of tobacco and lead exposures with attention-deficit/hyperactivity disorder. Pediatrics. 2009;124:e1054–e1063. doi: 10.1542/peds.2009-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Daneshparvar M., Mostafavi S.A., Zare Jeddi M., Yunesian M., Mesdaghinia A., Mahvi A.H., Akhondzadeh S. The role of lead exposure on attention-deficit/hyperactivity disorder in children: A systematic review. Iran J Psychiatry. 2016;11:1–14. [PMC free article] [PubMed] [Google Scholar]
- 44.Ha S., Yeung E., Bell E., Insaf T., Ghassabian A., Bell G., et al. Prenatal and early life exposures to ambient air pollution and development. Environ Res. 2019;174:170–175. doi: 10.1016/j.envres.2019.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nigg J.T., Nikolas M., Knottnerus G.M., Cavanagh K., Friderici K. Confirmation and extension of association of blood lead with attention-deficit/hyperactivity disorder (ADHD) and ADHD symptom domains at population-typical exposure levels. J Child Psychol Psychiatry. 2010;51:58–65. doi: 10.1111/j.1469-7610.2009.02135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang H.L., Chen X.T., Yang B., Ma F.L., Wang S., Tang M.L., et al. Case–control study of blood lead levels and attention deficit hyperactivity disorder in Chinese children. Environ Health Perspect. 2008;116:1401–1406. doi: 10.1289/ehp.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roy A., Bellinger D., Hu H., Schwartz J., Ettinger A.S., Wright R.O., et al. Lead exposure and behavior among young children in Chennai, India. Environ Health Perspect. 2009;117:1607–1611. doi: 10.1289/ehp.0900625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adams J.B., Audhya T., McDonough-Means S., Rubin R.A., Quig D., Geis E., et al. Toxicological status of children with autism vs. neurotypical children and the association with autism severity. Biol Trace Elem Res. 2013;151:171–180. doi: 10.1007/s12011-012-9551-1. [DOI] [PubMed] [Google Scholar]
- 49.Shaffer R.M., Sellers S.P., Baker M.G., de Buen Kalman R., Frostad J., Suter M.K., et al. Improving and expanding estimates of the global burden of disease due to environmental health risk factors. Environ Health Perspect. 2019;127 doi: 10.1289/EHP5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lanphear B.P., Hornung R., Khoury J., Yolton K., Baghurst P., Bellinger D.C., et al. Erratum: “low-level environmental lead exposure and children’s intellectual function: an international pooled analysis.”. Environ Health Perspect. 2019;127 doi: 10.1289/EHP5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prevent children’s exposure to lead. Centers for Disease Control and Prevention. https://www.cdc.gov/nceh/features/leadpoisoning/index.html Available at:
- 52.World Health Organization Ambient air pollution: A global assessment of exposure and burden of disease. 2016. https://apps.who.int/iris/handle/10665/250141 Available at:
- 53.Brockmeyer S., D’Angiulli A. How air pollution alters brain development: The role of neuroinflammation. Transl Neurosci. 2016;7:24–30. doi: 10.1515/tnsci-2016-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Prado Bert P., Mercader E.M.H., Pujol J., Sunyer J., Mortamais M. The effects of air pollution on the brain: A review of studies interfacing environmental epidemiology and neuroimaging. Curr Environ Health Rep. 2018;5:351–364. doi: 10.1007/s40572-018-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Braithwaite I., Zhang S., Kirkbride J.B., Osborn D.P.J., Hayes J.F. Air pollution (particulate matter) exposure and associations with depression, anxiety, bipolar, psychosis and suicide risk: A systematic review and meta-analysis. Environ Health Perspect. 2019;127 doi: 10.1289/EHP4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kravitz-Wirtz N., Crowder K., Hajat A., Sass V. The long-term dynamics of racial/ethnic inequality in neighborhood air pollution exposure, 1990-2009. Du Bois Rev. 2016;13:237–259. doi: 10.1017/S1742058X16000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Integrated Science Assessment (ISA) for Particulate Matter (Final report, Dec 2019) US Environmental Protection Agency. https://cfpub.epa.gov/ncea/isa/recordisplay.cfm?deid=347534#tab-3 Available at: [PubMed]
- 58.Costa L.G., Cole T.B., Coburn J., Chang Y.C., Dao K., Roque P. Neurotoxicants are in the air: Convergence of human, animal, and in vitro studies on the effects of air pollution on the brain. Biomed Res Int. 2014;2014 doi: 10.1155/2014/736385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Genc S., Zadeoglulari Z., Fuss S.H., Genc K. The adverse effects of air pollution on the nervous system. J Toxicol. 2012;2012 doi: 10.1155/2012/782462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Allen J.L., Oberdorster G., Morris-Schaffer K., Wong C., Klocke C., Sobolewski M., et al. Developmental neurotoxicity of inhaled ambient ultrafine particle air pollution: Parallels with neuropathological and behavioral features of autism and other neurodevelopmental disorders. Neurotoxicology. 2017;59:140–154. doi: 10.1016/j.neuro.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fonken L.K., Xu X., Weil Z.M., Chen G., Sun Q., Rajagopalan S., Nelson R.J. Air pollution impairs cognition, provokes depressive-like behaviors and alters hippocampal cytokine expression and morphology. Mol Psychiatry. 2011;16:987–995. doi: 10.1038/mp.2011.76. 973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Woodward N.C., Haghani A., Johnson R.G., Hsu T.M., Saffari A., Sioutas C., et al. Prenatal and early life exposure to air pollution induced hippocampal vascular leakage and impaired neurogenesis in association with behavioral deficits. Transl Psychiatry. 2018;8:261. doi: 10.1038/s41398-018-0317-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Costa L.G., Cole T.B., Coburn J., Chang Y.C., Dao K., Roqué P.J. Neurotoxicity of traffic-related air pollution. Neurotoxicology. 2017;59:133–139. doi: 10.1016/j.neuro.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bolton J.L., Marinero S., Hassanzadeh T., Natesan D., Le D., Belliveau C., et al. Gestational exposure to air pollution alters cortical volume, microglial morphology, and microglia-neuron interactions in a sex-specific manner. Front Synaptic Neurosci. 2017;9:10. doi: 10.3389/fnsyn.2017.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yokota S., Oshio S., Moriya N., Takeda K. Social isolation-induced territorial aggression in male offspring is enhanced by exposure to diesel exhaust during pregnancy. PLoS One. 2016;11 doi: 10.1371/journal.pone.0149737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mallya A.P., Wang H.D., Lee H.N.R., Deutch A.Y. Microglial pruning of synapses in the prefrontal cortex during adolescence. Cereb Cortex. 2019;29:1634–1643. doi: 10.1093/cercor/bhy061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herting M.M., Younan D., Campbell C.E., Chen J.C. Outdoor air pollution and brain structure and function from across childhood to young adulthood: A methodological review of brain MRI studies. Front Public Health. 2019;7:332. doi: 10.3389/fpubh.2019.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guxens M., Lubczyńska M.J., Muetzel R.L., Dalmau-Bueno A., Jaddoe V.W.V., Hoek G., et al. Air pollution exposure during fetal life, brain morphology, and cognitive function in school-age children. Biol Psychiatry. 2018;84:295–303. doi: 10.1016/j.biopsych.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 69.Peterson B.S., Rauh V.A., Bansal R., Hao X., Toth Z., Nati G., et al. Effects of prenatal exposure to air pollutants (polycyclic aromatic hydrocarbons) on the development of brain white matter, cognition, and behavior in later childhood [published correction appears in JAMA Psychiatry 2015; 72:625] JAMA Psychiatry. 2015;72:531–540. doi: 10.1001/jamapsychiatry.2015.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mortamais M., Pujol J., Martínez-Vilavella G., Fenoll R., Reynes C., Sabatier R., et al. Effects of prenatal exposure to particulate matter air pollution on corpus callosum and behavioral problems in children. Environ Res. 2019;178 doi: 10.1016/j.envres.2019.108734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cserbik D., Chen J.C., McConnell R., Berhane K., Sowell E.R., Schwartz J., et al. Fine particulate matter exposure during childhood relates to hemispheric-specific differences in brain structure. Environ Int. 2020;143 doi: 10.1016/j.envint.2020.105933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lubczyńska M.J., Muetzel R.L., El Marroun H., Basagaña X., Strak M., Denault W., et al. Exposure to air pollution during pregnancy and childhood, and white matter microstructure in preadolescents. Environ Health Perspect. 2020;128 doi: 10.1289/EHP4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pujol J., Martínez-Vilavella G., Macià D., Fenoll R., Alvarez-Pedrerol M., Rivas I., et al. Traffic pollution exposure is associated with altered brain connectivity in school children. Neuroimage. 2016;129:175–184. doi: 10.1016/j.neuroimage.2016.01.036. [DOI] [PubMed] [Google Scholar]
- 74.Clifford A., Lang L., Chen R., Anstey K.J., Seaton A. Exposure to air pollution and cognitive functioning across the life course—A systematic literature review. Environ Res. 2016;147:383–398. doi: 10.1016/j.envres.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 75.Suades-González E., Gascon M., Guxens M., Sunyer J. Air pollution and neuropsychological development: A review of the latest evidence. Endocrinology. 2015;156:3473–3482. doi: 10.1210/en.2015-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Forns J., Dadvand P., Foraster M., Alvarez-Pedrerol M., Rivas I., López-Vicente M., et al. Traffic-related air pollution, noise at school, and behavioral problems in Barcelona schoolchildren: A cross-sectional study. Environ Health Perspect. 2016;124:529–535. doi: 10.1289/ehp.1409449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Margolis A.E., Herbstman J.B., Davis K.S., Thomas V.K., Tang D., Wang Y., et al. Longitudinal effects of prenatal exposure to air pollutants on self-regulatory capacities and social competence. J Child Psychol Psychiatry. 2016;57:851–860. doi: 10.1111/jcpp.12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fan S.J., Heinrich J., Bloom M.S., Zhao T.Y., Shi T.X., Feng W.R., et al. Ambient air pollution and depression: A systematic review with meta-analysis up to 2019. Sci Total Environ. 2020;701 doi: 10.1016/j.scitotenv.2019.134721. [DOI] [PubMed] [Google Scholar]
- 79.Roberts S., Arseneault L., Barratt B., Beevers S., Danese A., Odgers C.L., et al. Exploration of NO2 and PM2.5 air pollution and mental health problems using high-resolution data in London-based children from a UK longitudinal cohort study. Psychiatry Res. 2019;272:8–17. doi: 10.1016/j.psychres.2018.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yolton K., Khoury J.C., Burkle J., LeMasters G., Cecil K., Ryan P. Lifetime exposure to traffic-related air pollution and symptoms of depression and anxiety at age 12 years [published correction appears in Environ Res 2019; 176:108519] Environ Res. 2019;173:199–206. doi: 10.1016/j.envres.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brunst K.J., Ryan P.H., Altaye M., Yolton K., Maloney T., Beckwith T., et al. Myo-inositol mediates the effects of traffic-related air pollution on generalized anxiety symptoms at age 12 years. Environ Res. 2019;175:71–78. doi: 10.1016/j.envres.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Newman N.C., Ryan P., Lemasters G., Levin L., Bernstein D., Hershey G.K.K., et al. Traffic-related air pollution exposure in the first year of life and behavioral scores at 7 years of age. Environ Health Perspect. 2013;121:731–736. doi: 10.1289/ehp.1205555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Perera F.P., Chang H.W., Tang D., Roen E.L., Herbstman J., Margolis A., et al. Early-life exposure to polycyclic aromatic hydrocarbons and ADHD behavior problems. PLoS One. 2014;9 doi: 10.1371/journal.pone.0111670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Talbott E.O., Arena V.C., Rager J.R., Clougherty J.E., Michanowicz D.R., Sharma R.K., Stacy S.L. Fine particulate matter and the risk of autism spectrum disorder. Environ Res. 2015;140:414–420. doi: 10.1016/j.envres.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 85.Volk H.E., Lurmann F., Penfold B., Hertz-Picciotto I., McConnell R. Traffic-related air pollution, particulate matter, and autism. JAMA Psychiatry. 2013;70:71–77. doi: 10.1001/jamapsychiatry.2013.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Newbury J.B., Arseneault L., Beevers S., Kitwiroon N., Roberts S., Pariante C.M., et al. Association of air pollution exposure with psychotic experiences during adolescence. JAMA Psychiatry. 2019;76:614–623. doi: 10.1001/jamapsychiatry.2019.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oudin A., Bråbäck L., Åström D.O., Strömgren M., Forsberg B. Association between neighbourhood air pollution concentrations and dispensed medication for psychiatric disorders in a large longitudinal cohort of Swedish children and adolescents. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2015-010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Forns J., Sunyer J., Garcia-Esteban R., Porta D., Ghassabian A., Giorgis-Allemand L., et al. Air pollution exposure during pregnancy and symptoms of attention deficit and hyperactivity disorder in children in Europe. Epidemiology. 2018;29:618–626. doi: 10.1097/EDE.0000000000000874. [DOI] [PubMed] [Google Scholar]
- 89.Jorcano A., Lubczyńska M.J., Pierotti L., Altug H., Ballester F., Cesaroni G., et al. Prenatal and postnatal exposure to air pollution and emotional and aggressive symptoms in children from 8 European birth cohorts. Environ Int. 2019;131 doi: 10.1016/j.envint.2019.104927. [DOI] [PubMed] [Google Scholar]
- 90.Davis D.A., Bortolato M., Godar S.C., Sander T.K., Iwata N., Pakbin P., et al. Prenatal exposure to urban air nanoparticles in mice causes altered neuronal differentiation and depression-like responses. PLoS One. 2013;8 doi: 10.1371/journal.pone.0064128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jia Z., Wei Y., Li X., Yang L., Liu H., Guo C., et al. Exposure to ambient air particles increases the risk of mental disorder: Findings from a natural experiment in Beijing. Int J Environ Res Public Health. 2018;15:160. doi: 10.3390/ijerph15010160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu X., Qian X., Xing J., Wang J., Sun Y., Wang Q., Li H. Particulate matter triggers depressive-like response associated with modulation of inflammatory cytokine homeostasis and brain-derived neurotrophic factor signaling pathway in mice. Toxicol Sci. 2018;164:278–288. doi: 10.1093/toxsci/kfy086. [DOI] [PubMed] [Google Scholar]
- 93.Zhang T., Zheng X., Wang X., Zhao H., Wang T., Zhang H., et al. Maternal exposure to PM2.5 during pregnancy induces impaired development of cerebral cortex in mice offspring. Int J Mol Sci. 2018;19:257. doi: 10.3390/ijms19010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ehsanifar M., Tameh A.A., Farzadkia M., Kalantari R.R., Zavareh M.S., Nikzaad H., Jafari A.J. Exposure to nanoscale diesel exhaust particles: Oxidative stress, neuroinflammation, anxiety and depression on adult male mice. Ecotoxicol Environ Saf. 2019;168:338–347. doi: 10.1016/j.ecoenv.2018.10.090. [DOI] [PubMed] [Google Scholar]
- 95.Chu C., Zhang H., Cui S., Han B., Zhou L., Zhang N., et al. Ambient PM2.5 caused depressive-like responses through Nrf2/NLRP3 signaling pathway modulating inflammation. J Hazard Mater. 2019;369:180–190. doi: 10.1016/j.jhazmat.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 96.Allen J.L., Conrad K., Oberdörster G., Johnston C.J., Sleezer B., Cory-Slechta D.A. Developmental exposure to concentrated ambient particles and preference for immediate reward in mice. Environ Health Perspect. 2013;121:32–38. doi: 10.1289/ehp.1205505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Allen J.L., Liu X., Weston D., Prince L., Oberdörster G., Finkelstein J.N., et al. Developmental exposure to concentrated ambient ultrafine particulate matter air pollution in mice results in persistent and sex-dependent behavioral neurotoxicity and glial activation. Toxicol Sci. 2014;140:160–178. doi: 10.1093/toxsci/kfu059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.What is endocrine disruption? U.S. Environmental Protection Agency. https://www.epa.gov/endocrine-disruption/what-endocrine-disruption Available at:
- 99.Bergman Å., Heindel J.J., Jobling S., Kidd K.A., Zoeller R.T. What is endocrine disruption all about? In: State of the Science of Endocrine Disrupting Chemicals−2012. 2013 https://www.who.int/publications/i/item/state-of-the-science-of-endocrine-disrupting-chemicals-summary Geneva, Switzerland: WHO Press,11–13. Available at: [Google Scholar]
- 100.Rivollier F., Krebs M.O., Kebir O. Perinatal exposure to environmental endocrine disruptors in the emergence of neurodevelopmental psychiatric diseases: A systematic review. Int J Environ Res Public Health. 2019;16:1318. doi: 10.3390/ijerph16081318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gore A.C., Chappell V.A., Fenton S.E., Flaws J.A., Nadal A., Prins G.S., et al. EDC-2: The Endocrine Society’s second scientific statement on endocrine-disrupting chemicals. Endocr Rev. 2015;36:E1–E150. doi: 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Diamanti-Kandarakis E., Bourguignon J.P., Giudice L.C., Hauser R., Prins G.S., Soto A.M., et al. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ruiz D., Becerra M., Jagai J.S., Ard K., Sargis R.M. Disparities in environmental exposures to endocrine-disrupting chemicals and diabetes risk in vulnerable populations. Diabetes Care. 2018;41:193–205. doi: 10.2337/dc16-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Attina T.M., Malits J., Naidu M., Trasande L. Racial/ethnic disparities in disease burden and costs related to exposure to endocrine-disrupting chemicals in the United States: An exploratory analysis. J Clin Epidemiol. 2019;108:34–43. doi: 10.1016/j.jclinepi.2018.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wiersielis K.R., Samuels B.A., Roepke T.A. Perinatal exposure to bisphenol A at the intersection of stress, anxiety, and depression. Neurotoxicol Teratol. 2020;79 doi: 10.1016/j.ntt.2020.106884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ghassabian A., Vandenberg L., Kannan K., Trasande L. Endocrine-disrupting chemicals and child health. Annu Rev Pharmacol Toxicol. 2022;62:573–594. doi: 10.1146/annurev-pharmtox-021921-093352. [DOI] [PubMed] [Google Scholar]
- 107.Calafat A.M., Ye X., Wong L.Y., Reidy J.A., Needham L.L. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Heffernan A.L., Aylward L.L., Toms L.M.L., Eaglesham G., Hobson P., Sly P.D., Mueller J.F. Age-related trends in urinary excretion of bisphenol A in Australian children and adults: Evidence from a pooled sample study using samples of convenience. J Toxicol Environ Health A. 2013;76:1039–1055. doi: 10.1080/15287394.2013.834856. [DOI] [PubMed] [Google Scholar]
- 109.Ha M., Kwon H.J., Leem J.H., Kim H.C., Lee K.J., Park I., et al. Korean Environmental Health Survey in Children and Adolescents (KorEHS-C): Survey design and pilot study results on selected exposure biomarkers. Int J Hyg Environ Health. 2014;217:260–270. doi: 10.1016/j.ijheh.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 110.Li X., Ying G.G., Zhao J.L., Chen Z.F., Lai H.J., Su H.C. 4-Nonylphenol, bisphenol-A and triclosan levels in human urine of children and students in China, and the effects of drinking these bottled materials on the levels. Environ Int. 2013;52:81–86. doi: 10.1016/j.envint.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 111.Frederiksen H., Nielsen J.K.S., Mørck T.A., Hansen P.W., Jensen J.F., Nielsen O., et al. Urinary excretion of phthalate metabolites, phenols and parabens in rural and urban Danish mother–child pairs. Int J Hyg Environ Health. 2013;216:772–783. doi: 10.1016/j.ijheh.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 112.Healy B.F., English K.R., Jagals P., Sly P.D. Bisphenol A exposure pathways in early childhood: Reviewing the need for improved risk assessment models. J Expo Sci Environ Epidemiol. 2015;25:544–556. doi: 10.1038/jes.2015.49. [DOI] [PubMed] [Google Scholar]
- 113.Geens T., Aerts D., Berthot C., Bourguignon J.P., Goeyens L., Lecomte P., et al. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem Toxicol. 2012;50:3725–3740. doi: 10.1016/j.fct.2012.07.059. [DOI] [PubMed] [Google Scholar]
- 114.Qian X., Li J., Xu S., Wan Y., Li Y., Jiang Y., et al. Prenatal exposure to phthalates and neurocognitive development in children at two years of age. Environ Int. 2019;131 doi: 10.1016/j.envint.2019.105023. [DOI] [PubMed] [Google Scholar]
- 115.Polanska K., Ligocka D., Sobala W., Hanke W. Phthalate exposure and child development: The Polish Mother and Child Cohort Study. Early Hum Dev. 2014;90:477–485. doi: 10.1016/j.earlhumdev.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 116.Miodovnik A., Edwards A., Bellinger D.C., Hauser R. Developmental neurotoxicity of ortho-phthalate diesters: Review of human and experimental evidence. Neurotoxicology. 2014;41:112–122. doi: 10.1016/j.neuro.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 117.Jacobson J.L., Jacobson S.W. Prenatal exposure to polychlorinated biphenyls and attention at school age. J Pediatr. 2003;143:780–788. doi: 10.1067/S0022-3476(03)00577-8. [DOI] [PubMed] [Google Scholar]
- 118.Verner M.A., Plusquellec P., Muckle G., Ayotte P., Dewailly E., Jacobson S.W., et al. Alteration of infant attention and activity by polychlorinated biphenyls: Unravelling critical windows of susceptibility using physiologically based pharmacokinetic modeling. Neurotoxicology. 2010;31:424–431. doi: 10.1016/j.neuro.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 119.Boucher O., Muckle G., Bastien C.H. Prenatal exposure to polychlorinated biphenyls: A neuropsychologic analysis. Environ Health Perspect. 2009;117:7–16. doi: 10.1289/ehp.11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Patisaul H.B., Belcher S.M. Endocrine Disruptors, Brain, and Behavior. Oxford University Press; New York: 2017. Endocrine disruptors and neurobehavioral disorders; pp. 149–190. [Google Scholar]
- 121.Grandjean P., Landrigan P.J. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167–2178. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- 122.Demeneix B. Losing Our Minds: How Environmental Pollution Impairs Human Intelligence and Mental Health. Oxford University Press; New York: 2014. Autism spectrum disorders, attention-deficit/hyperactivity disorder, and congenital hypothyroidism: The case for gene × environment interactions; pp. 197–241. [Google Scholar]
- 123.Shanle E.K., Xu W. Endocrine disrupting chemicals targeting estrogen receptor signaling: Identification and mechanisms of action. Chem Res Toxicol. 2011;24:6–19. doi: 10.1021/tx100231n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zoeller R.T., Brown T.R., Doan L.L., Gore A.C., Skakkebaek N.E., Soto A.M., et al. Endocrine-disrupting chemicals and public health protection: A statement of principles from the Endocrine Society. Endocrinology. 2012;153:4097–4110. doi: 10.1210/en.2012-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Plusquellec P., Muckle G., Dewailly E., Ayotte P., Bégin G., Desrosiers C., et al. The relation of environmental contaminants exposure to behavioral indicators in Inuit preschoolers in Arctic Quebec. Neurotoxicology. 2010;31:17–25. doi: 10.1016/j.neuro.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 126.Vermiglio F., Lo Presti V.P., Moleti M., Sidoti M., Tortorella G., Scaffidi G., et al. Attention deficit and hyperactivity disorders in the offspring of mothers exposed to mild-moderate iodine deficiency: A possible novel iodine deficiency disorder in developed countries. J Clin Endocrinol Metab. 2004;89:6054–6060. doi: 10.1210/jc.2004-0571. [DOI] [PubMed] [Google Scholar]
- 127.Berbel P., Mestre J.L., Santamaría A., Palazón I., Franco A., Graells M., et al. Delayed neurobehavioral development in children born to pregnant women with mild hypothyroxinemia during the first month of gestation: The importance of early iodine supplementation. Thyroid. 2009;19:511–519. doi: 10.1089/thy.2008.0341. [DOI] [PubMed] [Google Scholar]
- 128.Li Y., Shan Z., Teng W., Yu X., Li Y., Fan C., et al. Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25–30 months. Clin Endocrinol (Oxf) 2010;72:825–829. doi: 10.1111/j.1365-2265.2009.03743.x. [DOI] [PubMed] [Google Scholar]
- 129.Pop V.J., Kuijpens J.L., van Baar A.L., Verkerk G., van Son M.M., de Vijlder J.J., et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf) 1999;50:149–155. doi: 10.1046/j.1365-2265.1999.00639.x. [DOI] [PubMed] [Google Scholar]
- 130.Shen C.Y., Weng J.C., Tsai J.D., Su P.H., Chou M.C., Wang S.L. Prenatal exposure to endocrine-disrupting chemicals and subsequent brain structure changes revealed by voxel-based morphometry and generalized Q-sampling MRI. Int J Environ Res Public Health. 2021;18:4798. doi: 10.3390/ijerph18094798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Weng J.C., Hong C.I., Tasi J.D., Shen C.Y., Su P.H., Wang S.L. The association between prenatal endocrine-disrupting chemical exposure and altered resting-state brain fMRI in teenagers. Brain Struct Funct. 2020;225:1669–1684. doi: 10.1007/s00429-020-02089-4. [DOI] [PubMed] [Google Scholar]
- 132.Perera F.P., Tang D., Wang S., Vishnevetsky J., Zhang B., Diaz D., et al. Prenatal polycyclic aromatic hydrocarbon (PAH) exposure and child behavior at age 6–7 years. Environ Health Perspect. 2012;120:921–926. doi: 10.1289/ehp.1104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Perera F., Vishnevetsky J., Herbstman J.B., Calafat A.M., Xiong W., Rauh V., Wang S. Prenatal bisphenol A exposure and child behavior in an inner-city cohort. Environ Health Perspect. 2012;120:1190–1194. doi: 10.1289/ehp.1104492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Roen E.L., Wang Y., Calafat A.M., Wang S., Margolis A., Herbstman J., et al. Bisphenol A exposure and behavioral problems among inner city children at 7–9 years of age. Environ Res. 2015;142:739–745. doi: 10.1016/j.envres.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Braun J.M., Kalkbrenner A.E., Calafat A.M., Yolton K., Ye X., Dietrich K.N., Lanphear B.P. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics. 2011;128:873–882. doi: 10.1542/peds.2011-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Braun J.M., Yolton K., Dietrich K.N., Hornung R., Ye X., Calafat A.M., Lanphear B.P. Prenatal bisphenol A exposure and early childhood behavior. Environ Health Perspect. 2009;117:1945–1952. doi: 10.1289/ehp.0900979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Perera F., Roen Nolte E.L., Wang Y., Margolis A.E., Calafat A.M., Wang S., et al. Bisphenol A exposure and symptoms of anxiety and depression among inner city children at 10–12 years of age. Environ Res. 2016;151:195–202. doi: 10.1016/j.envres.2016.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Harley K.G., Gunier R.B., Kogut K., Johnson C., Bradman A., Calafat A.M., Eskenazi B. Prenatal and early childhood bisphenol A concentrations and behavior in school-aged children. Environ Res. 2013;126:43–50. doi: 10.1016/j.envres.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Engel S.M., Miodovnik A., Canfield R.L., Zhu C., Silva M.J., Calafat A.M., Wolff M.S. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environ Health Perspect. 2010;118:565–571. doi: 10.1289/ehp.0901470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Whyatt R.M., Liu X., Rauh V.A., Calafat A.M., Just A.C., Hoepner L., et al. Maternal prenatal urinary phthalate metabolite concentrations and child mental, psychomotor, and behavioral development at 3 years of age. Environ Health Perspect. 2012;120:290–295. doi: 10.1289/ehp.1103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Philippat C., Nakiwala D., Calafat A.M., Botton J., De Agostini M., Heude B., et al. Prenatal exposure to nonpersistent endocrine disruptors and behavior in boys at 3 and 5 years [published correction appears in Environ Health Perspect 2018; 126:129001] Environ Health Perspect. 2017;125 doi: 10.1289/EHP1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kobrosly R.W., Evans S., Miodovnik A., Barrett E.S., Thurston S.W., Calafat A.M., Swan S.H. Prenatal phthalate exposures and neurobehavioral development scores in boys and girls at 6–10 years of age. Environ Health Perspect. 2014;122:521–528. doi: 10.1289/ehp.1307063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lien Y.J., Ku H.Y., Su P.H., Chen S.J., Chen H.Y., Liao P.C., et al. Prenatal exposure to phthalate esters and behavioral syndromes in children at 8 years of age: Taiwan Maternal and Infant Cohort Study. Environ Health Perspect. 2015;123:95–100. doi: 10.1289/ehp.1307154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schantz S.L., Widholm J.J., Rice D.C. Effects of PCB exposure on neuropsychological function in children. Environ Health Perspect. 2003;111:357–576. doi: 10.1289/ehp.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Morello-Frosch R., Shenassa E.D. The environmental “riskscape” and social inequality: Implications for explaining maternal and child health disparities. Environ Health Perspect. 2006;114:1150–1153. doi: 10.1289/ehp.8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Barrett E.S., Padula A.M. Joint impact of synthetic chemical and non-chemical stressors on children’s health. Curr Environ Health Rep. 2019;6:225–235. doi: 10.1007/s40572-019-00252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sprinkle R.H., Payne-Sturges D.C. Mixture toxicity, cumulative risk, and environmental justice in United States federal policy, 1980–2016: Why, with much known, was little done? Environ Health. 2021;20:104. doi: 10.1186/s12940-021-00764-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.