Abstract
The potential for aerobic methyl tert-butyl ether (MTBE) degradation was investigated with microcosms containing aquifer sediment and groundwater from four MTBE-contaminated sites characterized by oxygen-limited in situ conditions. MTBE depletion was observed for sediments from two sites (e.g., 4.5 mg/liter degraded in 15 days after a 4-day lag period), whereas no consumption of MTBE was observed for sediments from the other sites after 75 days. For sediments in which MTBE was consumed, 43 to 54% of added [U-14C]MTBE was mineralized to 14CO2. Molecular phylogenetic analyses of these sediments indicated the enrichment of species closely related to a known MTBE-degrading bacterium, strain PM1. At only one site, the presence of water-soluble gasoline components significantly inhibited MTBE degradation and led to a more pronounced accumulation of the metabolite tert-butyl alcohol. Overall, these results suggest that the effects of oxygen and water-soluble gasoline components on in situ MTBE degradation will vary from site to site and that phylogenetic analysis may be a promising predictor of MTBE biodegradation potential.
The magnitude and remediation cost of methyl tert-butyl ether (MTBE) contamination in drinking water have rapidly become a national concern. It has been estimated that 250,000 of the approximately 385,000 confirmed leaking underground storage tank (LUST) releases in the United States involve MTBE (15). In California, at least 10,000 LUST sites are estimated to be contaminated with MTBE (13). Several states, including California, have set primary maximum concentration levels for MTBE at or below 20 μg/liter, and at an even lower level of 12 μg/liter for tert-butyl alcohol (TBA), an MTBE metabolite. The U.S. Environmental Protection Agency has listed MTBE as a possible human carcinogen, whereas TBA is a known animal carcinogen (7). MTBE appears to be more mobile and less biodegradable than BTEX compounds (benzene, toluene, ethylbenzene, and xylenes), and consequently, MTBE plumes have extended over kilometer-scale distances, as is the case at Port Hueneme, Calif., and East Patchogue, N.Y.
Previous microcosm studies reported little or no biodegradation of MTBE under a variety of aerobic (11, 14) and anaerobic (18, 23, 26) conditions. More recent microcosm (3) and column (6) studies suggest that limited intrinsic biodegradation of MTBE may occur. One research group observed MTBE mineralization activity in stream-bed sediments from both contaminated and pristine sites under aerobic conditions (4, 5). Mixed cultures capable of MTBE degradation have been isolated from activated sludge (10, 20). Pure bacterial cultures capable of MTBE metabolism have been reported (12, 17, 22), including strain PM1, which uses MTBE as a sole carbon source and electron donor (12), and propane-oxidizing strains that cometabolize MTBE (22). In microcosm and field experiments, Salanitro et al. (21) showed that oxygenation in combination with bioaugmentation with an MTBE-degrading consortium resulted in more rapid MTBE degradation, although indigenous populations also degraded MTBE.
While these results are promising, there is still insufficient information concerning MTBE biodegradation, especially regarding the distribution of aerobic MTBE degradative activity across LUST sites. It is not clear whether simply adding oxygen to anoxic sediments at a given LUST site will result in MTBE degradation. Furthermore, the effect of water-soluble gasoline components on MTBE and TBA biodegradation in aquifer sediments has not been adequately addressed, although the effects of individual BTEX compounds on MTBE degradation by the pure culture PM1 have been investigated (9). In this article, the effects of the presence of oxygen and water-soluble gasoline components on metabolism of MTBE and TBA by aquifer bacteria were investigated along with the degree of MTBE mineralization and the effect of MTBE consumption on aquifer microbial communities.
Microcosm construction and analysis.
Aquifer sediment and groundwater were obtained from four different MTBE-contaminated LUST sites in California (from Palo Alto, Sacramento, Travis Air Force Base [AFB], and Sunnyvale). MTBE concentrations in the groundwater associated with the sediments were the following (in μg/liter): 1,200 for Palo Alto, 2,000 for Sacramento, 200 for Travis AFB, and 2,300 for Sunnyvale. The total BTEX concentrations in the groundwater were (in μg/liter): <5 for Palo Alto and Sunnyvale, 2,500 for Sacramento, and 8,300 for Travis AFB. For microcosms, groundwater samples were obtained from an upgradient well at each site that had no detectable MTBE, TBA, or BTEX. Sediment and groundwater samples were transported on ice, stored at 4°C, and used within 3 months of collection. Concentrations of dissolved oxygen (DO) in groundwater were measured colorimetrically (Chemetrics, Calverton, Va.) and indicated anoxic conditions for three sites tested (Palo Alto, Sacramento, and Travis AFB). Microcosms included 15 or 30 g of wet sediment, and 72 or 144 ml of groundwater, in either 125- or 250-ml amber glass, screw-cap bottles with Teflon-lined septa. Sterile microcosms were prepared by autoclaving sediment before adding groundwater and sodium azide (2 g/liter). MTBE was added as an aqueous stock solution to a final concentration of 4.2 − 4.8 mg/liter, and bottles were incubated at ambient temperature with end-over-end mixing. DO in the microcosms was measured using a Lazar DO-166 Dissolved Oxygen Probe (Lazar Research Laboratories, Inc., Los Angeles, Calif.) with reference to oxygen-saturated and N2-purged (anoxic) groundwater. For microcosms amended with water-soluble gasoline components, gasoline (without oxygenate additives) was equilibrated with groundwater (1:5, vol/vol) overnight, and 720 μl of the gasoline-saturated groundwater was added to yield a total BTEX concentration of approximately 1 mg/liter. For some experiments, groundwater was replaced with growth medium. The medium contained vitamins and trace elements (25) as well as the following salts (mM): NaCl (17.1), KCl (6.7), NH4Cl (4.6), MgCl2 (2.0), KH2PO4 (1.5), Na2SO4 (1.4), and CaCl2 (1.0).
Aliquots (100 to 200 μl) of water were sampled from microcosms with a glass syringe and transferred to VOA vials (I-Chem, New Castle, Del.) containing 40 ml of reagent water. MTBE and TBA were analyzed by purge-and-trap gas chromatography-mass spectrometry with selected ion monitoring; analytical instrumentation included an Archon AutoSampler System (Model 5100A; Varian, Walnut Creek, Calif.) and an OI Sample Concentrator (Model 4560; OI Analytical, College Station, Tex.), interfaced to a Hewlett-Packard Model 5973 Mass Selective Detector (Palo Alto, Calif.) fitted with an Rtx-502.2 column (0.32-mm inner diameter by 60-m length, 1.8-μm film thickness; Restek, Bellefonte, Pa.). Internal standard quantification with MTBE-d12 (Cambridge Isotope Laboratories, Cambridge, Mass.) was used for MTBE, and external standard quantification was used for TBA. The operational detection limits after 200-fold dilution were approximately 10 μg/liter for MTBE and 100 μg/liter for TBA.
Mineralization experiments.
In mineralization experiments, 1-g aliquots of wet sediment from MTBE-exposed microcosms were added to 40-ml VOA vials. Five milliliters of medium was added along with 0.3 μCi of [U-14C]MTBE (∼98% radiochemical purity; NEN Life Science Products, Boston, Mass.) and unlabeled MTBE to give an initial concentration of ca. 4 mg of total MTBE/liter. Sterile controls contained 2 g of sodium azide/liter. Each vial contained two 10-by-75-mm glass vials containing 0.5 ml of either 0.5 N NaOH (to trap 14CO2) or 0.5 M KH2PO4 (pH 4.3; to correct for MTBE in the CO2 trap). Samples were incubated aerobically with shaking (100 rpm) at ambient temperature. A parallel set of microcosms with unlabeled MTBE was sampled and analyzed by gas chromatography-mass spectrometry to determine when MTBE and TBA were depleted. The trap contents were removed after 5 to 7 days and added to 10 ml of liquid scintillation cocktail (Universol; ICN, Costa Mesa, Calif.) and analyzed with a Wallac liquid scintillation counter (Model 1409; PerkinElmer-Wallac, Inc., Gaithersburg, Md.) with quench correction. In addition to 14CO2 determination in the traps, 14CO2 in the medium was measured at the beginning and end of the experiment by sacrificial sampling of replicates, as described previously (2). Radioactivity in the solids was measured after removal of the liquid, and correction was made for quenching and for the sediment water content. Headspace samples (1 ml) were analyzed at the beginning and end of the experiment. Activity in the headspace (corrected for total volume) and solids (corrected for total solid dry mass) were included to determine the total activity.
DGGE methods.
Microcosm sediments from Palo Alto and Travis AFB were exposed to a total of 25 and 15 mg of MTBE/liter, respectively, as 5- or 10-mg/liter additions every 4 to 8 weeks prior to DNA extraction, whereas control microcosms were treated as described above. Total community DNA was extracted and purified from microcosm sediment using a Soil DNA Mega Prep Kit (Bio 101, Vista, Calif.). Highly enriched MTBE-degrading cultures were obtained from Palo Alto microcosms by performing multiple transfers of 5% inoculum (vol/vol) into medium containing 50 mg of MTBE/liter as the sole carbon source and electron donor. Genomic DNA was isolated from enrichment cultures by the method of Ausubel et al. (1). 16S ribosomal DNA (rDNA) was amplified by the PCR using the universal primers f968 and r1401 according to the method of Weisburg et al. (24), including a 40-bp 5′-GC clamp. Controls either lacking template or primers were analyzed on a 1% agarose gel to confirm the absence of detectable PCR artifacts. PCRs were run on a denaturing gradient gel (40 to 80% gradient) according to the method of Muyzer et al. (19) using a D-Code apparatus (Bio-Rad, Hercules, Calif.). The gel was stained with ethidium bromide, and DNA was extracted from bands, reamplified, and sequenced; 16S rDNA sequences were compared to the most similar sequences in the Ribosomal Database Project (RDP-II) database using Similarity Matrix version 1.1 (16).
Aerobic MTBE degradation results.
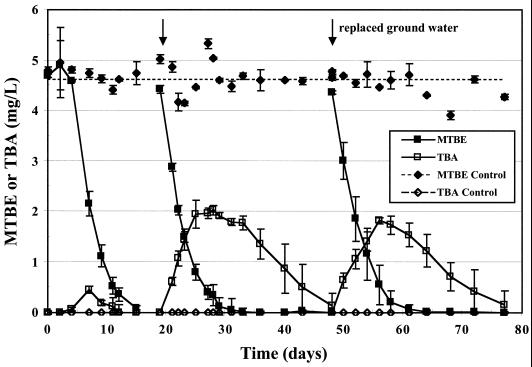
Rapid MTBE degradation was observed in microcosms containing sediments from a LUST site in Palo Alto, Calif.; 4.5 mg of MTBE/liter was degraded to <0.1 mg/liter in 15 days after an apparent lag period of 4 days (Fig. 1). TBA was transiently produced and reached a maximum concentration of ca. 0.5 mg/liter. When the microcosms were respiked with MTBE, rapid degradation was again observed; however, TBA accumulated to a concentration of approximately 2 mg/liter (one-half the initial molar MTBE concentration) and persisted for 27 days. MTBE degradation and TBA production did not change discernibly when the liquid was replaced with fresh groundwater (Fig. 1). In contrast, MTBE concentrations in sterile controls remained constant, and there was no TBA production. These data indicate that TBA, a known carcinogen, can accumulate in sediment-water systems with environmentally relevant MTBE concentrations. Similar MTBE degradation kinetics were observed for microcosms containing Palo Alto sediment and groundwater that had been stored at 4°C for 3 months.
FIG. 1.
MTBE degradation and TBA formation in microcosms containing aquifer sediment and groundwater from a LUST site in Palo Alto, Calif. Datum points represent averages, and error bars represent 1 SD of triplicate microcosm results. Data from day 0 to 15 are from a separate but identical experiment. Arrows denote when MTBE was added.
Rapid MTBE degradation also occurred in microcosms containing sediments from a LUST site at Travis AFB: 4.2 mg of MTBE/liter was degraded to <0.2 mg/liter in 20 days after a 10-day lag period. In contrast with the Palo Alto sediments, no accumulation of TBA was observed. Under similar incubation conditions, quite different results were obtained for sediment-groundwater microcosms from two other LUST sites. Microcosms from a Sacramento site did not show significant MTBE degradation relative to controls over a period of 75 days; throughout the experiment, MTBE concentrations averaged 4.6 ± 0.2 mg/liter (mean ± standard deviation [SD]) for controls and 4.2 ± 0.2 mg/liter for live samples. Similarly, no significant degradation was observed in sediment from a Sunnyvale LUST site relative to results for controls (P>0.05) over a 23-day period. In all microcosm experiments, measurements of DO in the groundwater at the beginning and end of the experiment indicated that aerobic conditions were maintained throughout. These data demonstrate that supplying ample oxygen was not sufficient to promote aerobic MTBE degradation in all sediments.
In Palo Alto microcosms constructed with growth medium in lieu of site groundwater, MTBE degradation was more rapid; MTBE degraded to <0.1 mg/liter in 7 days, compared to 15 days (data not shown). This suggests either that the groundwater lacked an essential nutrient or that it contained an inhibitory compound.
Effects of water-soluble gasoline components.
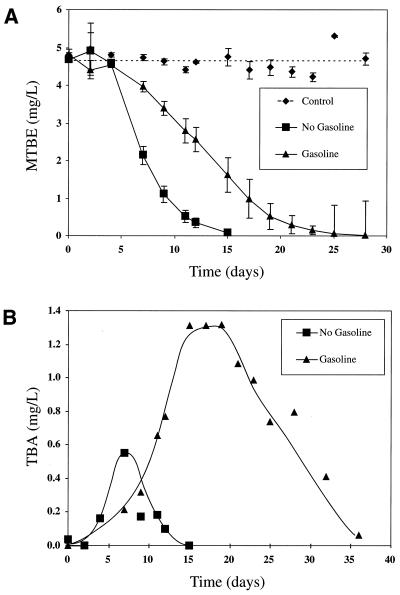
The effect of water-soluble gasoline components on MTBE degradation was determined for Palo Alto and Travis AFB sediments. In Palo Alto microcosms, dissolved gasoline components retarded MTBE degradation (Fig. 2A). The effect of gasoline components was even more pronounced for TBA (Fig. 2B); TBA accumulated to higher concentrations and persisted >21 days longer in the presence of gasoline components. In contrast, there was no effect of dissolved gasoline components on MTBE degradation or TBA accumulation in Travis AFB microcosms (data not shown). The bacteria indigenous to Travis AFB aquifer sediment may have been more adapted to gasoline components, since the Travis AFB sampling location was closer to the gasoline source and had higher concentrations of BTEX in groundwater than the Palo Alto sampling location. Although BTEX degradation was rapid in microcosms from both sites, BTEX compounds were degraded more rapidly in Travis AFB microcosms than in Palo Alto microcosms (within 1 day compared to 2 to 3 days); therefore, Travis microcosms had a shorter period of BTEX exposure.
FIG. 2.
Effect of water-soluble gasoline components on MTBE (A) and TBA (B) metabolism in Palo Alto microcosms. Datum points represent averages, and error bars (A) represent 1 SD of triplicate microcosm results. Note that different time intervals are shown in panels A and B.
The use of water-soluble gasoline components in this study, while representative of actual LUST site conditions, makes it difficult to determine which components were responsible for the observed inhibition. Deeb et al. (9) reported that BTEX compounds (added without other water-soluble gasoline components) inhibited degradation of MTBE by strain PM1; ethylbenzene and xylenes were more inhibitory than benzene and toluene. Our Palo Alto data support their findings; however, the lack of inhibition observed for the Travis AFB microcosms shows that this effect cannot be generalized to all LUST sites. As shown by other groups (8, 14), no inhibition of BTEX degradation was observed in the presence of MTBE (4.2 to 4.8 mg/liter). In Palo Alto microcosms, 425 to 475 μg of benzene/liter was degraded in 2 days, regardless of the presence of MTBE. Likewise, in Travis AFB microcosms, benzene (450 μg/liter) was degraded in 1 day with or without MTBE present.
MTBE mineralization results.
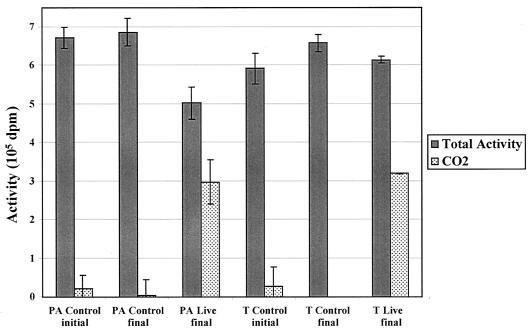
Mineralization experiments using Palo Alto and Travis AFB sediments showed that 43 ± 9% and 54 ± 4% of the total activity (mean ± SD; relative to controls at time zero) was converted to 14CO2, respectively (Fig. 3). Experiments with unlabeled MTBE showed that no MTBE or TBA remained when the final 14CO2 sample was collected. These data are within the range reported for mineralization of MTBE by the pure culture PM1 (46%) (12). The extent of conversion of [U-14C]MTBE to 14CO2 in this study was greater than that reported for three MTBE-degrading isolates belonging to the genera Methylobacterium, Rhodococcus, and Arthrobacter (8%) (17). Recent studies of surface water sediments reported that 20 to 79% of the total MTBE was consumed after 50 days, and the CO2 yield was 65 to 100% of the MTBE consumed relative to controls (5); however, the extent of mineralization for the one groundwater system studied was significantly less than that of the surface-water sediments (5% of the total [U-14C]MTBE added).
FIG. 3.
Mineralization data for Palo Alto (PA) and Travis AFB (T) microcosms amended with ca. 6.5 × 105 dpm of [U-14C]MTBE. Data represent the averages and standard deviations from triplicate microcosms (except for T Live final, which indicates the results of duplicate microcosms). Mass balances were 75 ± 7% for Palo Alto and 104 ± 8% for Travis AFB microcosms (relative to initial controls).
DGGE results.
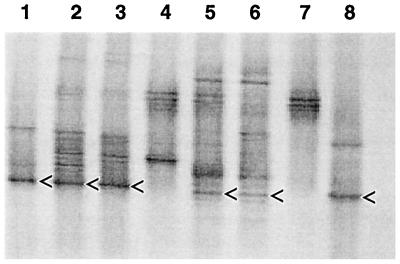
A denaturing gradient gel of 16S rDNA amplified from microcosms and the enrichment culture is shown in Fig. 4. Incubation of microcosms with MTBE resulted in a significant shift in the DGGE profiles of cultures from the Palo Alto and Travis AFB sites. A dominant band (denoted by arrows) was evident in MTBE-consuming microcosms from both sites and in the highly enriched mixed culture derived from Palo Alto sediments. The DNA sequence of this band in all profiles of active cultures most closely matched that of the Rubrivivax gelatinosus subgroup, of which the MTBE-degrading bacterium PM1 is a member (GenBank accession no. AF176594); similarity values relative to PM1 ranged from 93.3 to 96.2% (Table 1). The corresponding DNA sequences in Palo Alto and Travis AFB microcosms were highly similar to one another (Table 1). The corresponding band was not evident in control microcosms, suggesting that the Rubrivivax spp. constituted less than 1% of the total indigenous populations (the 1% lower limit of detection for DGGE was discussed by Muyzer et al. [19]). Furthermore, a corresponding band was not apparent in a live Sacramento microcosm (data not shown). It is noteworthy that such closely related bacteria became enriched during MTBE degradation even though they originated from such diverse environments: two geographically distinct LUST sites (this study) and a municipal compost biofilter treating exhaust air (strain PM1) (12). Although we have yet to confirm that the enriched Rubrivivax spp. degrade MTBE, two lines of evidence suggest their involvement: (i) bands corresponding to Rubrivivax spp. became more dominant in microcosms during MTBE consumption, and (ii) the band representing Rubrivivax spp. was predominant in the highly enriched culture that rapidly degraded MTBE (25 mg of MTBE/liter degraded in 2 days) as a sole carbon source and electron donor.
FIG. 4.
Denaturing gradient gel showing profiles of 16S rDNA fragments amplified from total microcosm DNA. Lanes 1 and 8, MTBE-degrading enrichment culture derived from Palo Alto microcosms; lanes 2 and 3, replicate Palo Alto microcosms incubated with MTBE; lane 4, Palo Alto sterile control microcosm; lanes 5 and 6, replicate Travis AFB microcosms incubated with MTBE; and lane 7, Travis AFB sterile control microcosm. The arrows highlight bands whose 16S rDNA sequences were similar to sequences of members of the Rubrivivax gelatinosus subgroup, of which the MTBE-degrading bacterium PM1 is a member. The sequence of the upper band in lanes 1 and 8 most closely matched the sequence of an uncultured β-proteobacterium (GenBank accession no. AF351224).
TABLE 1.
Similarity matrix for the 16S rDNA sequences of DGGE fragments
| Sequence no. | Strain or DGGE fragment in specified Fig. 4 lanea | % 16S rDNA sequence similarity with:
|
|||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| 1 | PA,b lane 1 | ||||||
| 2 | PA, lane 2 | 94.9 | |||||
| 3 | PA, lane 3 | 94.9 | 99.2 | ||||
| 4 | T,c lane 5 | 94.9 | 100 | 99.2 | |||
| 5 | T, lane 6 | 94.4 | 98.7 | 99.6 | 98.7 | ||
| 6 | PM1d | 96.2 | 93.3 | 94.1 | 93.3 | 93.7 | |
| 7 | Rub.gelat2e | 95.8 | 93.8 | 93.3 | 93.8 | 92.8 | 93.9 |
Sequence comparisons were made among selected 16S rDNA fragments (∼380 bp) from DGGE analysis (denoted by arrows in Fig. 4) and the two most closely related sequences in the RDP-II database (16).
PA, Palo Alto.
T, Travis AFB.
GenBank accession no. AF176594.
R. gelatinosus strain ATH 2.2.1 (GenBank accession no. D16213).
Concluding remarks.
Overall, these results suggest that caution is warranted for generalizations about in situ MTBE degradation; simply adding oxygen to anoxic sediments does not always result in aerobic MTBE degradation, and water-soluble gasoline components inhibit MTBE and TBA degradation in some sediments and not in others. Furthermore, our data show that TBA, a known carcinogen, may accumulate and persist in some sediments even with relatively low concentrations of MTBE and that TBA accumulation may be exacerbated in the presence of water-soluble gasoline components. Finally, although microcosm studies are currently the most reliable means of predicting the potential for in situ MTBE biodegradation at LUST sites, molecular phylogenetic analyses may serve as more rapid and potentially powerful diagnostic tools. Real-time, quantitative PCR methods may be more sensitive for these phylogenetic analyses than DGGE, such that enrichment on MTBE would not be required for detection of strains capable of MTBE degradation. However, further research is needed to confirm the apparent relationship between phylogeny (in particular, Rubrivivax spp. related to strain PM1) and the ability to degrade MTBE suggested by this study.
Acknowledgments
We sincerely thank M. Peterson and D. Oram (ETIC Engineering, Walnut Creek, Calif.) and W. Day (Travis AFB) for supplying LUST site data, aquifer sediment, and groundwater.
This work was supported in part by the Department of Energy Fossil Energy Program under contract FEW0048. This work was performed under the auspices of the U.S. Department of Energy by University of California Lawrence Livermore National Laboratory under contract no. W-7405-Eng-48.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1989. [Google Scholar]
- 2.Beller H R, Grbic-Galic D, Reinhard M. Microbial degradation of toluene under sulfate-reducing conditions and the influence of iron on the process. Appl Environ Microbiol. 1992;58:786–793. doi: 10.1128/aem.58.3.786-793.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borden R C, Daniel R A, Lebrun L E, Davis C W. Intrinsic biodegradation of MTBE and BTEX in a gasoline-contaminated aquifer. Water Resour Res. 1997;33:1105–1115. [Google Scholar]
- 4.Bradley P M, Landmeyer J E, Chapelle F H. Aerobic mineralization of MTBE and tert-butyl alcohol by stream-bed sediment microorganisms. Environ Sci Technol. 1999;33:1877–1879. [Google Scholar]
- 5.Bradley P M, Landmeyer J E, Chapelle F H. Widespread potential for microbial MTBE degradation in surface-water sediments. Environ Sci Technol. 2001;35:658–662. doi: 10.1021/es0015489. [DOI] [PubMed] [Google Scholar]
- 6.Church C D, Isabelle L M, Pankow J F, Rose D L, Tratnyek P G. Method for determination of methyl tert-butyl ether and its degradation products in water. Environ Sci Technol. 1997;31:3723–3726. [Google Scholar]
- 7.Cirvello J D, Radovsky A, Heath J E, Farnell D R, Landamond C., III Toxicity and carcinogenicity of t-butyl alcohol in rats and mice following chronic exposure in drinking water. Toxicol Ind Health. 1995;11:151–166. doi: 10.1177/074823379501100203. [DOI] [PubMed] [Google Scholar]
- 8.Deeb R A, Alvarez-Cohen L. Aerobic biotransformation of gasoline aromatics in multicomponent mixtures. Bioremed J. 2000;4:171–179. [Google Scholar]
- 9.Deeb R A, Hu H Y, Hanson J R, Scow K M, Alvarez-Cohen L. Substrate interactions in BTEX and MTBE mixtures by an MTBE-degrading isolate. Environ Sci Technol. 2001;35:312–317. doi: 10.1021/es001249j. [DOI] [PubMed] [Google Scholar]
- 10.Fortin N Y, Deshusses M A. Treatment of methyl tert-butyl ether vapors in biotrickling filters. 1. Reactor startup, steady-state performance, and culture characteristics. Environ Sci Technol. 1999;33:2980–2986. [Google Scholar]
- 11.Fujiwara T, Kinoshita T, Sato H, Kojima I. Biodegradation and bioconcentration of alkylethers. Yukagaku. 1984;33:111–115. [Google Scholar]
- 12.Hanson J R, Ackerman C E, Scow K M. Biodegradation of methyl tert-butyl ether by a bacterial pure culture. Appl Environ Microbiol. 1999;65:4788–4792. doi: 10.1128/aem.65.11.4788-4792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Happel A M, Dooher B, Beckenbach E H. Methyl tertiary butyl ether (MTBE) impacts to California groundwater. UCRL-AR-130897. Livermore, Calif: Lawrence Livermore National Laboratory, University of California; 1998. [Google Scholar]
- 14.Jensen H M, Arvin E. Solubility and degradability of the gasoline additive MTBE, methyl tert-butyl-ether, and gasoline compounds in water. In: Arndt F, Hinsenveld M, van den Brink W J, editors. Contaminated soils '90. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1990. pp. 445–448. [Google Scholar]
- 15.Johnson R, Pankow J, Bender D, Price C, Zogorski J. MTBE: to what extent will past releases contaminate community water supply wells? Environ Sci Technol. 2000;34:210A–217A. doi: 10.1021/es003268z. [DOI] [PubMed] [Google Scholar]
- 16.Maidak B L, Cole J R, Lilburn T G, Parker C T, Saxman P R, Farris R J, Garrity G M, Olsen G J, Schmidt T M, Tiedje J M. The RDP-II (Ribosomal Database Project) Nucleic Acids Res. 2001;29:173–174. doi: 10.1093/nar/29.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mo K, Lora C O, Wanken A E, Javanmardian M, Yang W, Kulpa C F. Biodegradation of methyl-tert-butyl ether by pure bacterial cultures. Appl Microbiol Biotechnol. 1997;47:69–72. doi: 10.1007/s002530050890. [DOI] [PubMed] [Google Scholar]
- 18.Mormile M R, Liu S, Suflita J M. Anaerobic biodegradation of gasoline oxygenates: extrapolation of information to multiple sites and redox conditions. Environ Sci Technol. 1994;28:1727–1732. doi: 10.1021/es00058a026. [DOI] [PubMed] [Google Scholar]
- 19.Muyzer G, de Waal E C, Uitterlinden A G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol. 1993;59:695–700. doi: 10.1128/aem.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salanitro J P, Diaz L A, Williams M P, Wisniewski H L. Isolation of a bacterial culture that degrades methyl t-butyl ether. Appl Environ Microbiol. 1994;60:2593–2596. doi: 10.1128/aem.60.7.2593-2596.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salanitro J P, Johnson P C, Spinnler G E, Maner P M, Wisniewski H L, Bruce C. Field-scale demonstration of enhanced MTBE bioremediation through aquifer bioaugmentation and oxygenation. Environ Sci Technol. 2000;34:4152–4162. [Google Scholar]
- 22.Steffan R J, McClay K, Vainberg S, Condee C W, Zhang D. Biodegradation of the gasoline oxygenates methyl tert-butyl ether, ethyl tert-butyl ether, and tert-amyl methyl ether by propane-oxidizing bacteria. Appl Environ Microbiol. 1997;63:4216–4222. doi: 10.1128/aem.63.11.4216-4222.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suflita J M, Mormile M R. Anaerobic biodegradation of known and potential gasoline oxygenates in the terrestrial subsurface. Environ Sci Technol. 1993;27:976–978. [Google Scholar]
- 24.Weisburg W G, Barns S M, Pelletier D A, Lane D J. 16S Ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Widdel F, Bak F. Gram-negative mesophilic sulfate-reducing bacteria. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. New York, N.Y: Springer-Verlag; 1992. pp. 3352–3378. [Google Scholar]
- 26.Yeh C K, Novak J T. Anaerobic biodegradation of gasoline oxygenates in soils. Water Environ Res. 1994;66:744–752. [Google Scholar]