Abstract
The recent development of solvent- and polymer-based brain clearing techniques has greatly advanced our ability to visualize the mammalian nervous system in 3D. However, it remains challenging to image the mammalian body en bloc as existing methods can be prohibitively expensive or difficult in practice. Here, we developed HYBRiD (HYdrogel-Based Reinforcement of DISCO), by strategically recombining components of organic- and polymer-based clearing pipelines and achieved superb simplicity, transparency, protein retention, and compatibility with direct fluorescent imaging as well as immunostaining in cleared mammalian bodies. Using parvalbumin- and somatostatin-Cre models, we demonstrated the utility of HYBRiD for whole-body imaging of genetically encoded fluorescent reporters without antibody enhancement of signals in newborn and juvenile mice. Using K18-hACE2 transgenic mice, HYBRiD enabled perfusion-free clearing and visualization of SARS-CoV-2 infection in a whole mouse chest, revealing macroscopic and microscopic features of viral pathology in the same sample. HYBRiD offers a simple, affordable, and universal solution to visualize large heterogeneous body parts or entire animals for basic and translational research.
Introduction
Understanding physiology and disease across multiple organ systems has long been a challenge in biology. Recent developments of tissue clearing techniques have enabled unprecedented 3D characterization of mammalian tissues. Primarily optimized for the brain, these methods are generally based on two distinct strategies. Organic solvent-based protocols (3DISCO1, iDISCO2–4, uDISCO5, FDISCO6, and vDISCO7) inspired by BABB and earlier methods, are rapid and robust in clearing tissues, but tend to quench fluorescence and often require additional signal amplification. On the other hand, aqueous-based methods developed through chemical screens (CUBIC8–11) or by polymer-reinforced scaffolds (CLARITY12–14, PACT/PARS15,16, SHIELD17,18, ACT-PRESTO19) allow better retention of endogenous signals but may require special devices or longer time to achieve complete clearing and are not suited to all tissue types (Extended Data Fig. 1A, also reviewed by Ueda et al.20).
While most of these methods clear rodent brains with ease, it remains much more difficult to visualize en bloc body parts (i.e., multiple organs with bone, muscle, blood vessels, and connective tissue intact in the same volume) with passive clearing as the balance of the transparency and fluorescence retention is harder to obtain. The recent approaches to achieve whole-body clearing in rodents require continuous, pressurized perfusion of hazardous solvents (uDISCO5, PEGASOS21) or nanobodies (vDISCO7) over a period of several days, which can be challenging and prohibitively expensive to adopt or scale up in routine experiments. Here, we developed HYBRiD, a simple and cost-effective clearing method suitable for visualization of essentially any organ or a whole juvenile rodent by rationally recombining organic and aqueous strategies. We achieved the long-sought goal of crossing hydrogel-based clearing techniques like CLARITY to solvent-based DISCO while completing whole-body clearing without the perfusion of organic solvent or antibodies. HYBRiD allows complete clearing of large heterogeneous tissues, superior retention of endogenous fluorescence and protein, and minimal use of expensive, delicate, or corrosive procedures in comparison to either parental method, and is fully compatible with direct fluorescence imaging as well as immunolabeling.
Results
Standard brain clearing is not optimal for body samples
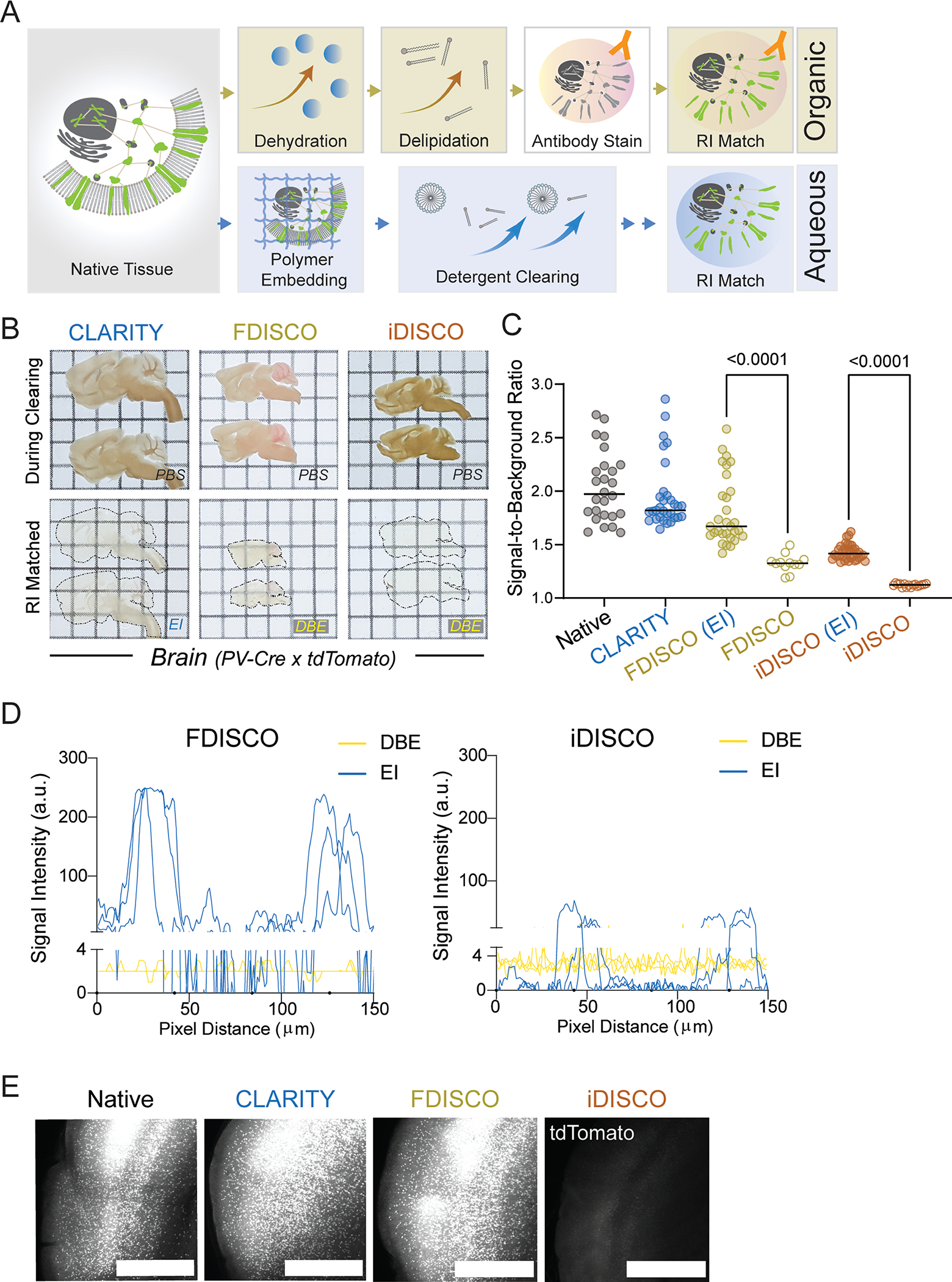
We performed a side-by-side comparison of existing brain clearing methods using transgenic mice that express the red fluorescent protein, tdTomato, under the control of parvalbumin-Cre recombinase (PV-Ai9). We chose PV-Cre as the mouse model because the expression is known to be active in both the central nervous system and peripheral neurons, thus allowing us to assess tdTomato signal across the whole animal. We benchmarked CLARITY and several DISCO variants. As expected, all tested methods readily cleared the brain (Extended Data Figs. 1B and 2B). The hind limbs of these mice were used as a model to evaluate clearing in large non-brain tissues. Before clearing, the legs were pretreated with previously established decalcification (CUBIC 3.010) and decoloring (PEGASOS21) reagents. Unmodified CLARITY alone did not fully delipidate the leg after 14 days, whereas iDISCO achieved the highest transparency (Fig. 1A). However, iDISCO, together with its milder iteration FDISCO, severely bleached the tdTomato signal as we evaluated the fluorescence of well-studied cortical PV+ neurons (Fig. 1B top row, 1C, Extended Data Fig. 1C) in brain tissue. This was mostly expected as iDISCO was not intended to preserve fluorophores but for use with immunolabeling. After stepwise time courses of both DISCO protocols, we found the final steps of methanol dehydration and dibenzyl ether (DBE) refractive index (RI) matching to be the two primary causes of fluorescence loss in DISCO (Fig. 1D–F), whereas replacing DBE with an aqueous RI matching solution (EasyIndex, EI, or PROTOS16) significantly rescued the tdTomato signal in FDISCO and iDISCO (Fig. 1B bottom row, 1C, Extended Data Fig. 1C–E).
Fig. 1: Development and characterization of HYBRiD method.
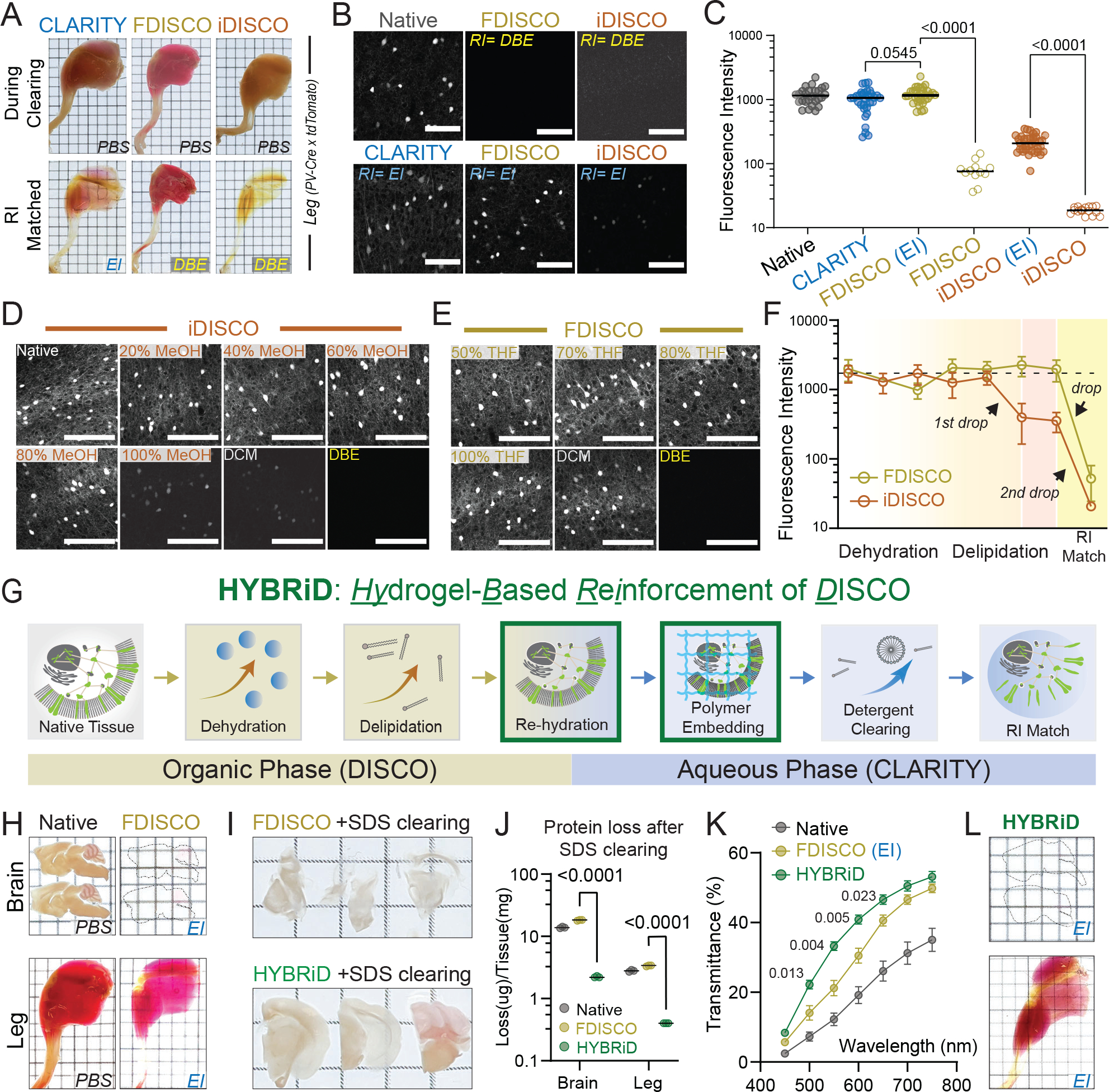
A. Representative images of whole-mount adult PV-Ai9 hindlimbs processed by indicated protocols. EI: EasyIndex; DBE: dibenzyl ether.
B. Representative confocal images of 1mm PV-Ai9 brain slices processed by indicated protocols. Native samples were fixed in PFA without clearing. Showing tdTomato+ neurons in neocortex.
C. Quantification of fluorescence intensity of each condition in (B): Native (26), CLARITY (31), FDISCO (EI) (30), FDISCO (12), iDISCO (EI) (35), iDISCO (15). Data are presented as raw values and mean values. Statistical significance was determined by two-tailed t-tests.
D-E. Representative images and (F) quantification of time-course analysis of fluorescence quenching. 1mm brain slices were processed and stopped at individual timepoints. N = 10–16 for each step in each method. Data are presented as mean values ± SD.
G. Schematic of the HYBRiD protocol.
h. Representative images of PV-Ai9 brain slice and hindlimb before and after FDISCO.
I. Representative images of FDISCO (top) or HYBRiD (bottom) brain slices after 7 days of SDS treatment.
J. Quantification of protein loss during passive SDS clearing. Data are presented as raw values and mean values. Statistical significance was determined by two-tailed t-tests. N = 3 for each group.
K. Quantification of transparency of hindlimbs. All values are mean ± SEM, N =6 for each group. Statistical significance (false discovery rate) between HYBRiD and FDISCO was determined by multiple unpaired t-tests with two-stage step-up.
L. Representative images of PV-Ai9 brain slice and hindlimb processed by HYBRiD.
Grid size: 3mm; scale bar: 100 μm.
A hybrid strategy combining organic and hydrogel clearing
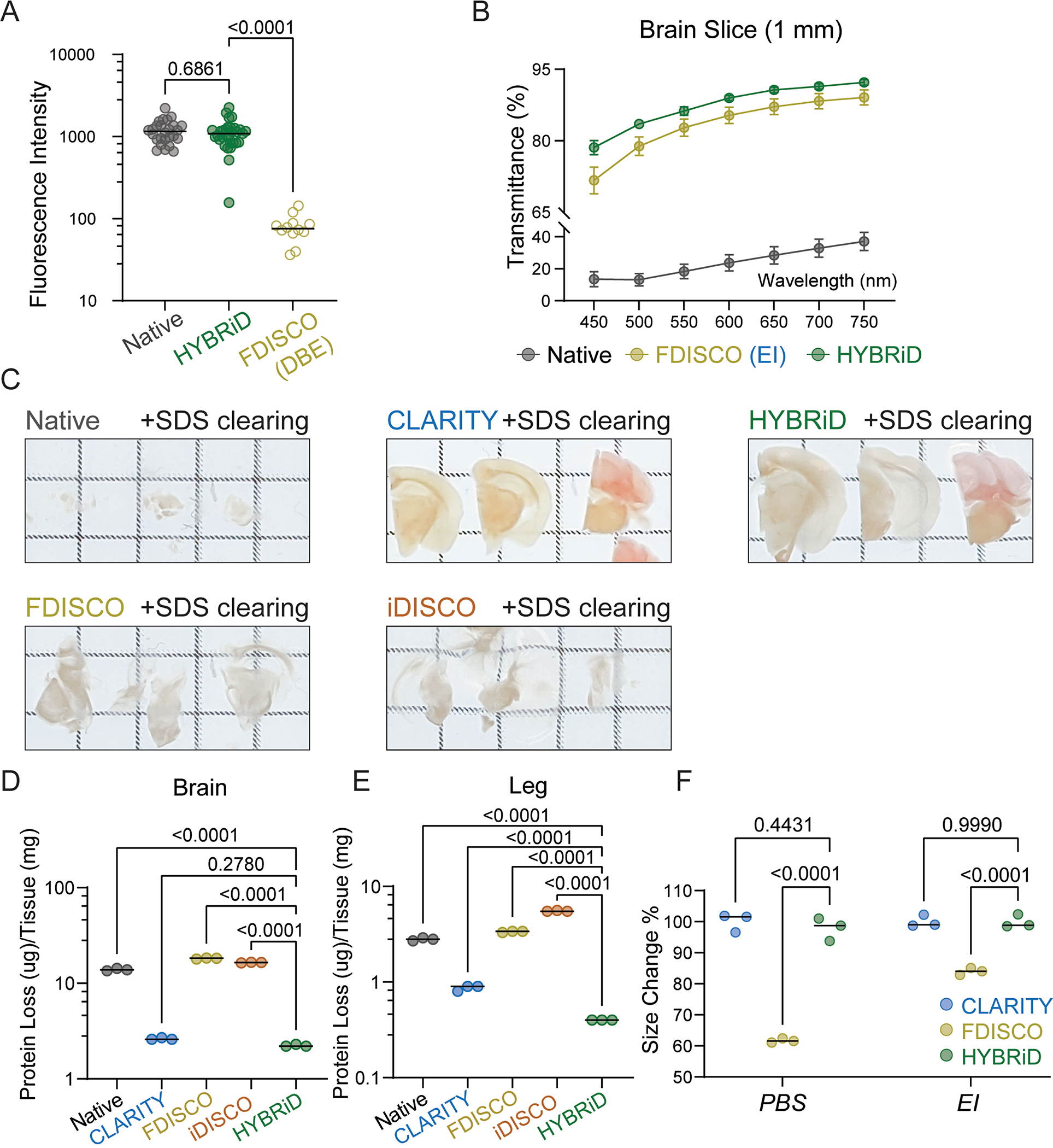
Since rehydration and replacing DBE in FDISCO greatly improved fluorescence but failed to render full transparency to a whole leg, we reasoned that stronger but non-quenching clearing steps are further needed for large heterogeneous FDISCO-processed tissues to be cleared (Fig. 1G–H). Aqueous methods preserve fluorophores better but have been developed in parallel to the DISCO methods and are not thought to be compatible with the latter (Extended Data Fig. 1A). After realizing that the fluorescence signal was still near 100% after DCM treatment in FDISCO (Fig. 1F), we reasoned that milder aqueous detergent could be used to further delipidate large samples. However, directly exposing rehydrated FDISCO samples to sodium dodecyl sulfate (SDS) resulted in disintegration of tissues and severe protein loss (Fig. 1I–J), suggesting additional reinforcement is necessary. Hence, we embedded rehydrated, FDISCO-cleared tissue in CLARITY hydrogel (1% acrylamide, 4% paraformaldehyde) before SDS delipidation (Fig. 1G). Indeed, sequential DISCO delipidation and CLARITY reinforcement greatly improved transparency (Fig. 1K–L) and fluorescence intensity (~10 fold higher than FDISCO, Extended Data Fig. 2A) while preserving structural integrity and tissue size (Fig. 1I, Extended Data Fig. 2C, F) and preventing SDS-induced protein loss (Fig. 1J, Extended Data Fig. 2D–E), in comparison to the parental methods. By combining the solvent strength of DISCO methods and the reinforcement of a polymer-based scaffolding, large heterogeneous tissue samples can be cleared with brief organic treatment followed by detergent clearing, which we have named the HYBRiD (HYdrogel-Based Reinforcement of DISCO) protocol.
HYBRiD clearing capability and fluorescence compatibility
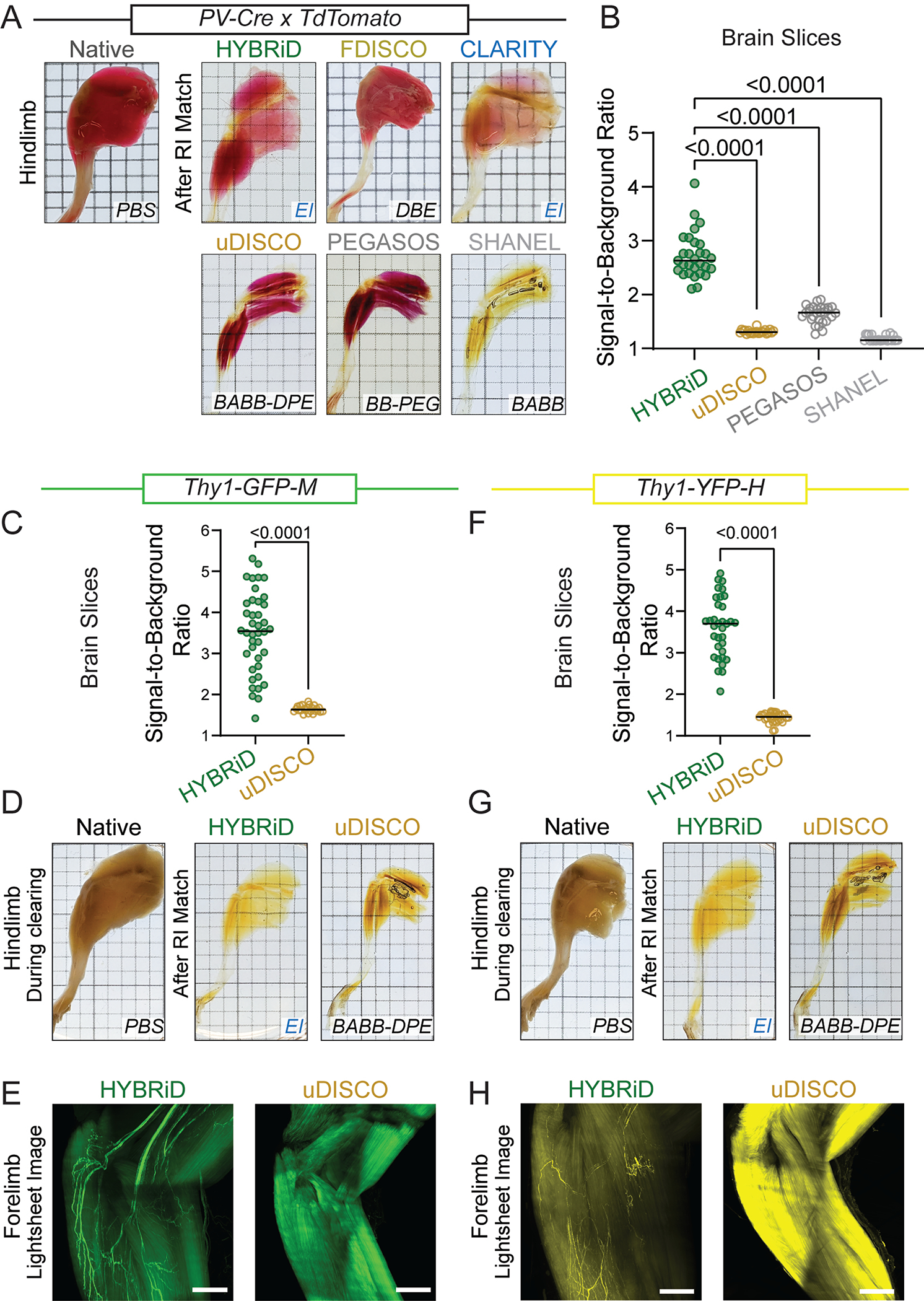
In addition to its parental methods, we also compared HYBRiD to methods recently developed for large tissue clearing, including uDISCO5, PEGASOS21, and SHANEL22. Again, all tested methods readily cleared the brain (Fig. 2A). Passive clearing alone (without continuous perfusion) was sufficient for SHANEL, uDISCO, and HYBRiD to clear a whole leg (Extended Data Fig. 3A). Next, we evaluated tdTomato fluorescence retention (Fig. 2A–B, Extended Data Fig. 3A–B). In our tests, SHANEL exhibited the highest overall clearing power, however, it was designed to be used with immunostaining22; thus, as expected, severely quenched tdTomato signals. uDISCO, as previously reported6, was incompatible with tdTomato and resulted in weaker fluorescence intensity compared to HYBRiD. Lastly, PEGASOS yielded an intermediate fluorescence intensity but failed to clear a whole leg without perfusion. Similar results were obtained using newborn PV-Ai9 mice (Extended Data Fig. 4A–E).
Fig. 2: Comparing HYBRiD with other clearing methods.
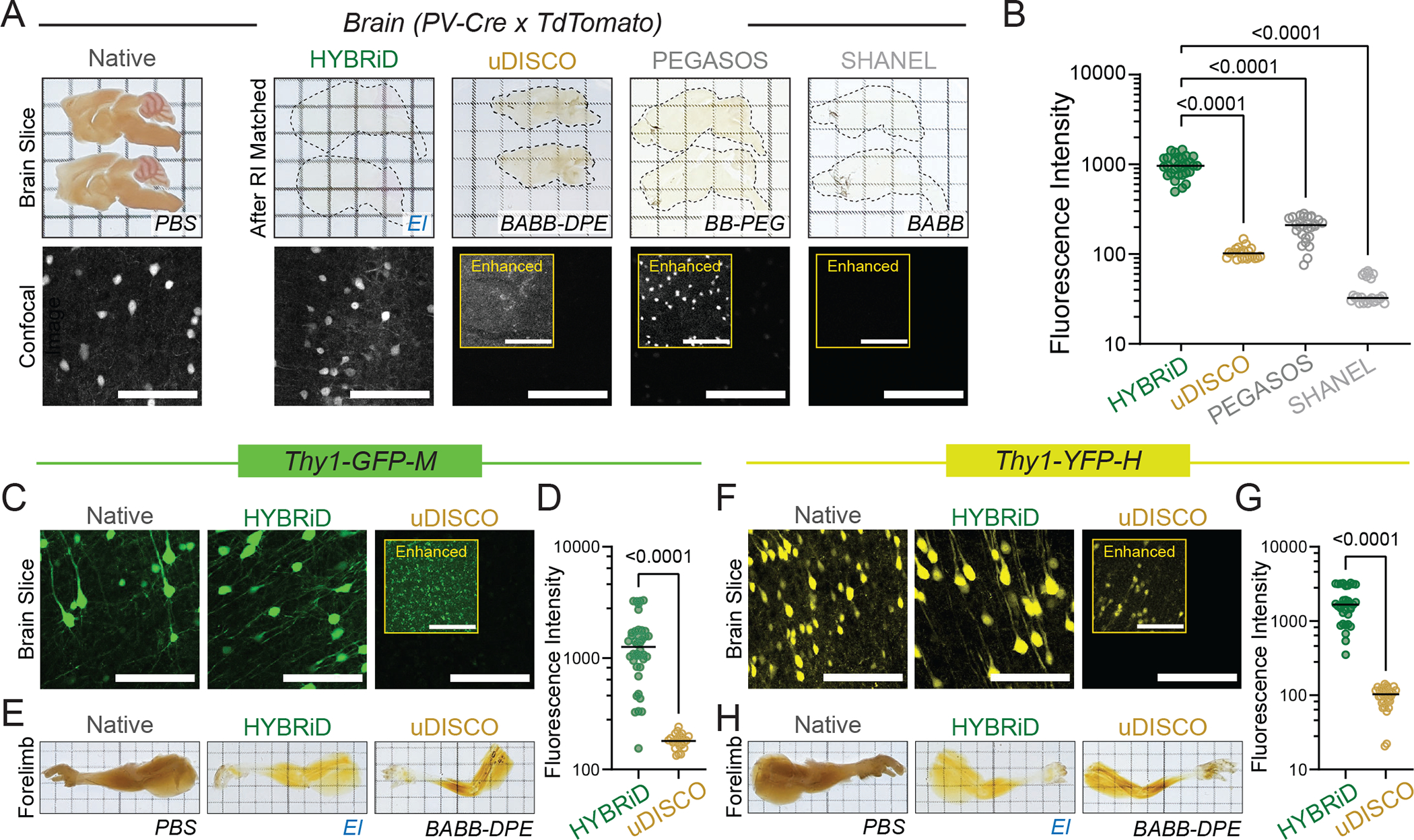
A. Representative backlit (top) and confocal (bottom) images of 1mm PV-Ai9 brain slices processed by indicated protocols. Showing tdTomato+ neurons in neocortex. Contrast enhanced (8-fold) images are shown in insets. HYBRiD samples were imaged with 1/4 laser power relative to other methods to avoid oversaturation.
B. Quantification of tdTomato fluorescence intensity for HYBRiD (29), uDISCO (22), PEGASOS (28), SHANEL (24) (N=4 samples per condition). Statistical significance was determined by one-way analysis of variance (ANOVA).
C – D. Comparison of HYBRiD, uDISCO on GFP fluorescence preservation. Representative images (C) and quantification (D) of GFP fluorescence intensity in Thy1-GFP-M brain slices cleared by HYBRiD (40) or uDISCO (24) (N=5 samples per condition). Statistical significance was determined by two-tailed t-test.
E. Representative images of forelimb from Thy1-GFP-M mice cleared by HYBRiD or uDISCO.
F – G. Comparison of HYBRiD, uDISCO on YFP fluorescence preservation. Representative images (F) and quantification (G) of YFP fluorescence intensity in brain slices cleared by HYBRiD (34) or uDISCO (32) (N=5 samples per condition). Statistical significance was determined by two-tailed t-test.
H. Representative images of forelimb from Thy1-YFP-H mice cleared by HYBRiD or uDISCO.
Grid size: 3mm; scale bar: 100 μm.
We also tested HYBRiD in maintaining GFP and YFP signals using brains and limbs from the Thy1-GFP (M-line) and Thy1-YFP (H-line) mice, two models widely used to evaluate clearing compatibility with sparely expressed fluorophores. Similar to what has been reported on one of its parental methods (FDISCO) 6 and in Extended Data Fig. 2A, HYBRiD better preserved both GFP (Fig. 2C–E, Extended Data Fig. 3C–E) and YFP (Fig. 2F–H, Extended Data Fig. 3F–H) compared to uDISCO under comparable passive clearing conditions.
HYBRiD reveals transgenic fluorophores in whole animals
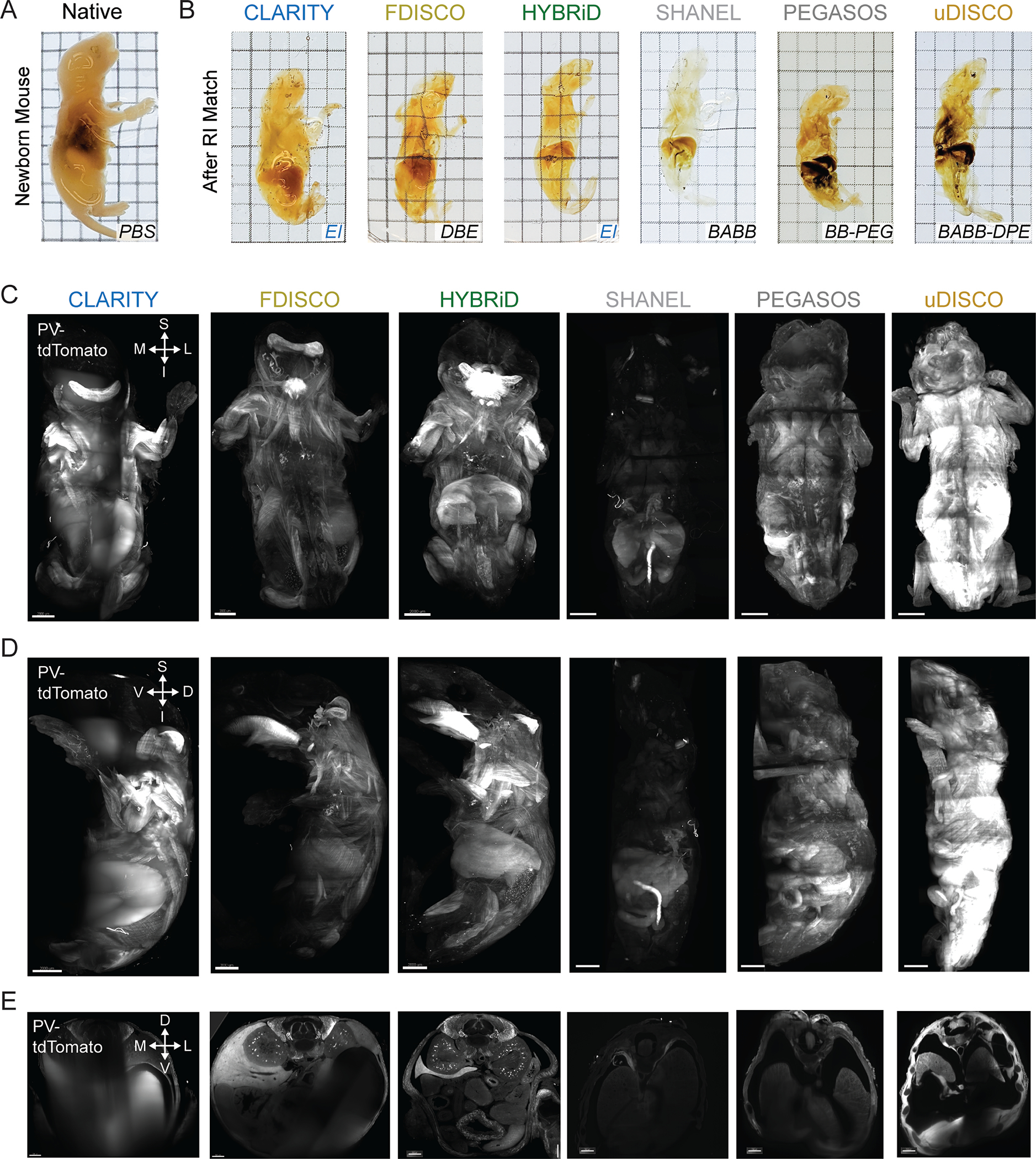
Transgenic animals are widely used in biology to target and manipulate specific cell types. However, potential “off-target” leakage of transgene expression (for example, Cre recombinase or fluorophores) has been a long-standing challenge for the development and application of transgenic models. Comprehensive profiling and identification of transgene expression across all organ systems are crucial to interpret phenotypes of genetic mouse models accurately. For example, the aP2-Cre mouse was initially designed as an adipocyte-specific driver, but years later it was revealed this mouse line also has leaky Cre expression in macrophages and the lymphatic system, confounding a decade of diabetes research using this mouse model23. Traditionally, people relied on histology or tissue PCR to detect transgene expression in mouse lines but this can be prohibitively tedious and unreliable, especially for cell types that penetrate many different tissues and organs (such as blood cells and nerves). After establishing the HYBRiD protocol, we next tested if this method could be used to efficiently profile whole-body Cre expression. PV-Cre and Somatostatin (SST)-Cre mice have long been used to target interneuron populations in the brain, but their expression in the rest of the body is less characterized. We applied HYBRiD to whole newborn PV-Ai9 and SST-Ai9 mice and examined the bodies by fluorescent light-sheet microscopy (Fig. 3A). HYBRiD outperformed the parental methods for achieving transparency in a newborn animal (Fig. 3B–C, Extended Data Fig. 4). This allowed 3D profiling of Cre-expressing cells across the body at high resolution (Fig. 3D–G, Supplementary Video 1); we identified tdTomato+ cells in the cochlea and vestibular system of the inner ear (Fig. 3E), neurons in the dorsal root ganglia (DRG) (Fig. 3F), epithelial cells of the kidneys, wrapping the pulmonary vasculature, as well as in the colon and bladder (Fig. 3G). In comparison, distinct patterns of SST-Cre expression in the inner ear, DRGs, kidneys, colon, and bladder were observed (Fig. 3H–K).
Fig. 3: HYBRiD Profiling of PV- and SST-tdTomato expression in newborn mice.
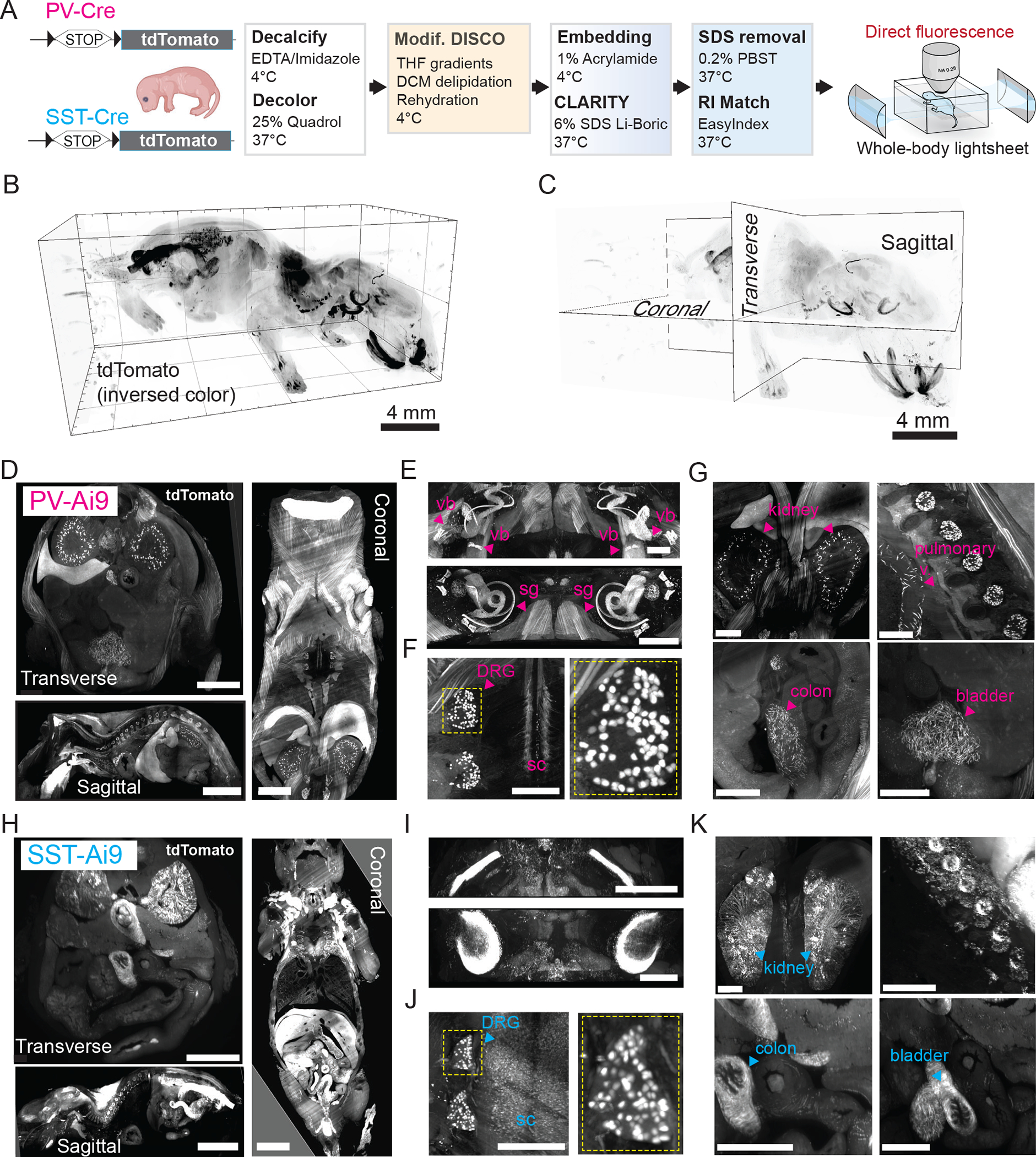
A. Workflow of HYBRiD clearing of whole-body newborn mice.
B. 3D lightsheet image volume of newborn mouse after HYBRiD (12 × 12 × 10mm), in-plane resolution=1.75μm, z step=4μm.
C. Schematic showing digital slicing planes on 3D volume image to collect images in (D) and (H).
D. 500μm digital slice views of PV-Ai9 cleared newborn mouse in planes shown in (C). Clear structures throughout dorsal to ventral regions. Scale: Transverse – 1mm, Sagittal – 4mm, Coronal – 2mm.
E. 3D block of inner ear of PV-Ai9 mouse showing detailed structures of the cochlea (vb-vestibular bodies; sg-spiral ganglia).
F. Slice view of PV-Ai9 mouse showing expression in the dorsal root ganglia (DRG) neurons and intermediate spinal cord pathways (sc-spinal cord).
G. PV expression in multiple abdominal organs and pulmonary vasculature.
H-K. The equivalent structures in SST-Ai9 newborn mice as in D-G.
Scale bar: 1 mm unless specified.
We further tested HYBRiD in clearing whole mice at different developmental stages. By tuning the duration of clearing steps (see Methods), HYBRiD cleared 2-week and 3-week old PV-Ai9 juveniles while preserving the tdTomato fluorescence (Fig. 4A–F). The cleared bodies can be imaged at single-cell resolution (lateral resolution= 1.75 μm/pixel, z step=4 μm) to visualize the whole chest (23×20×14mm) using a commercial lightsheet microscope (Fig. 4C, F, Extended Data Fig. 5A–N); or as an entire torso (50×30×14mm) with the help of a customized sample chamber (Fig. 4G–Q, Extended Data Fig. 5O–P, see Methods). Fluorescence signals were well preserved throughout the body over 14,000 μm imaging depth (>3,500 z-steps), revealing detailed anatomical structures both in coronal views (in-plane) and transverse views (reconstructed x-z planes, Fig. 4H–J, Supplementary Video 2). Notably, we resolved individual DRG neurons (Fig. 4M–N) and renal cortex structures (Fig. 4P–Q) in deep tissue >10,000 μm from the imaging surface (Supplementary Video 3).
Fig. 4: HYBRiD Profiling of PV-tdTomato expression in juvenile mice.
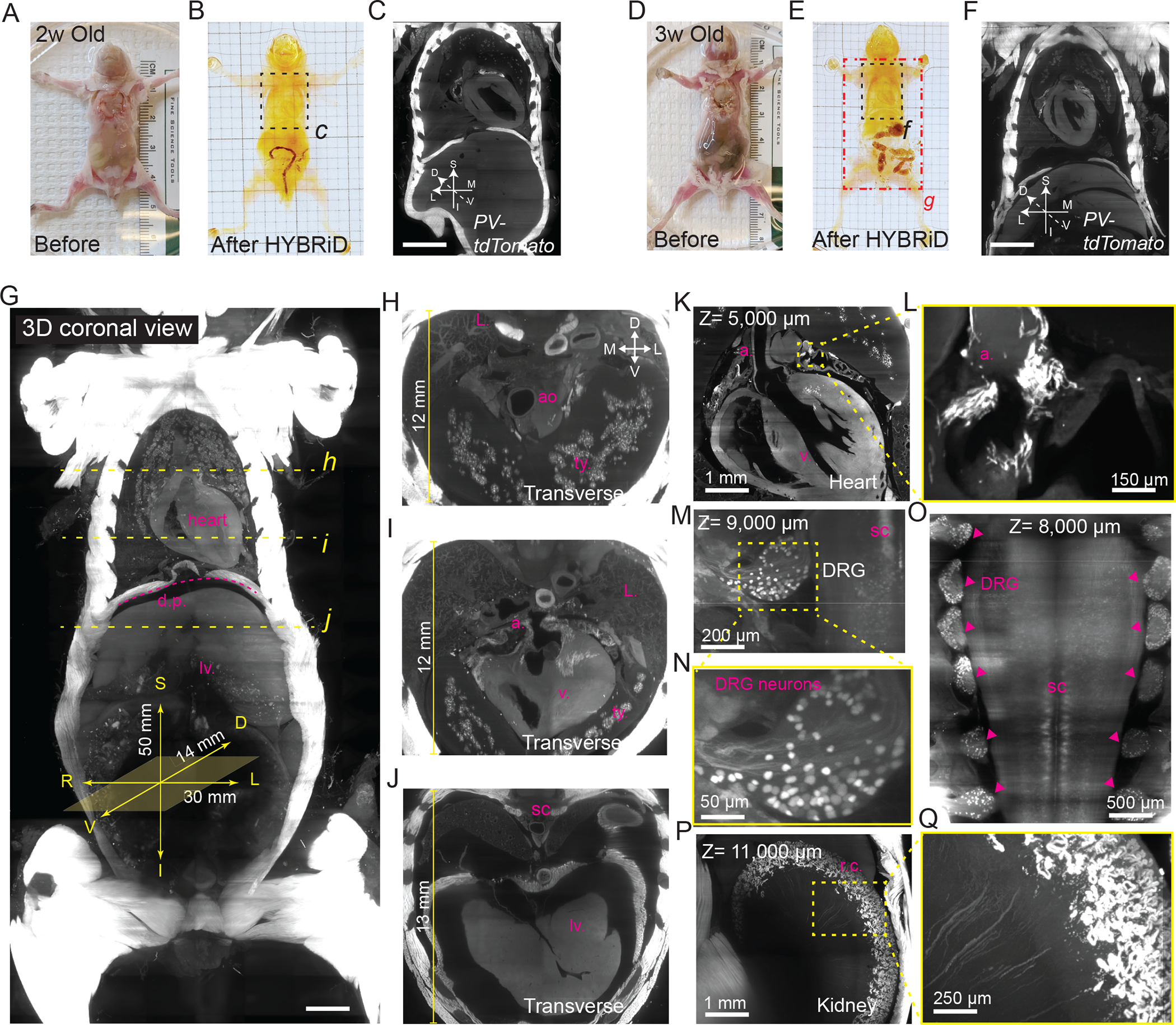
A-F. Representative images of 2-week-old (A-C) and 3-week-old (D-F) PV-Ai9 mice before (A and D) and after HYBRiD clearing (B and E). 3D lightsheet image volumes (as maximum intensity projection, MIP) of the outlined chest areas were shown in C and D.
G. MIP of 3D lightsheet image volume of the 3-week PV-Ai9 mouse as indicated in the red rectangle area in (E). Yellow dashed line indicated where digital transverse sections (H, I, and J) were taken, showing detailed structures of the thymus (t.y.), aorta (ao), atrium (a.), ventricle (v.), lung (L.), diaphragm (d.p.), spinal cord (s.c), and liver (lv.). D, dorsal; V, ventral; S, superior; I, inferior; L: left; R: right.
K – Q. Representative 3D views of 3-week PV-Ai9 mice showing PV-tdTomato expression in the heart (K), DRG (M), intermediate spinal cord spinal cord (O), and kidney (P-Q).
L. Zoom-in view of the yellow square in K, showing PV-tdTomato expression restricted in the atrium.
M. Zoom-in view of the yellow square in M, showing single cell resolution DRG neurons.
P. Zoom-in view of the yellow square in P, showing PV-tdTomato expression restricted in renal cortex (r.c.).
Scale bar: G: 2,500 μm; C,F: 2,000 μm.
Visualizing SARS-CoV-2 infection in mouse chests.
HYBRiD not only maintains the integrity of individual organs, but also keeps the entirety of organ systems en bloc, making it a particularly suitable tool for studying systemic diseases such as viral infections. With its unique combinatory clearing power, HYBRiD clarified the whole chest cavity of SARS-CoV-2 infected K18-hACE2 transgenic mice, remarkably, without perfusion to remove blood or perfusion or organic solvent for clearing (Fig. 5A–D). We were able to immuno-stain and image through the entire adult mouse chest (Fig. 5E–F, H–J, Supplementary Video 4), despite a large amount of blood accumulated in the diseased tissues before HYBRiD (Fig. 5B–C). We observed multifocal pulmonary infection in K18-hACE2 mice (Fig. 5F–J), while their WT counterparts did not show significant viral deposition as detected by SARS-CoV-2 nucleoprotein immunostaining (Fig. 5E). The SARS-CoV-2 antigen was predominantly seen in alveolar and airway epithelial cells24,25 but not in the pulmonary vasculature26 (Fig. 5G). To examine if these foci contain multi-nucleated regions previously reported in vitro27 and in humans28, the lungs were subsequently dissected out of the cleared chest for DAPI staining and re-imaged at higher magnifications (Fig. 5K–N). Areas with high levels of SARS-CoV-2 antigen were characterized by frequent syncytium, which are distinct to the K18-hACE2 mice and absent in the WT lungs (Fig. 5M–N, Extended Data Fig. 6). As these experiments were conducted in a Biosafety Level 3 (BLS3) facility where equipment and delicate procedures were minimally accessible, the simplicity of the HYBRiD protocol is precious.
Fig. 5: Visualization of a SARS-CoV-2 infected whole mouse chest by HYBRiD.

A. Workflow of HYBRiD clearing and immunostaining of WT or K18-hACE2 transgenic mice 5 days post infection (DPI) with SARS-CoV-2
B – D. Representative image of the chest at different stages of HYBRiD clearing.
E – F. MIP of 3D lightsheet image volume of immunostained WT (E) or K18-hACE2 (F) chest (19mm × 19mm × 13mm).
G. 100μm slice view from (F). White arrows indicate absence of nucleoprotein, yellow arrows indicate clusters of viral signal.
H - J. Transverse (H), coronal (I) and sagittal (J) view of the chest of SARS-CoV-2 infected mouse. b, bronchi; cw, chest wall; pc, pleural cavity; pa, pulmonary artery; pv, pulmonary vein; sc, spinal cord; tao, thoracic aorta. ao, aorta; be, bronchiole; es, esophagus; il, inferior lobe; sl, superior lobe; th, thymus; icm, intercostal muscles; ll, left lung; ri, ribs.
K. MIP of 3D lightsheet image volume of a half chest sample from another K18-hACE2 mouse.
L. 10X confocal imaging of the white square region in K, indicating high levels of nucleoprotein indicating rampant infection.
M. 40X confocal imaging of the white square region in L further stained with DAPI. Yellow arrows show multinucleated cells in K18-hACE2 but not WT lungs (N).
Grid size: 3mm in all images
Discussion
Together, by redesigning and recombining the seemingly contrasting principles underlying two mainstream brain clearing techniques, we developed a versatile clearing method for large, complex body parts and whole juvenile rodents. The ability of passive clearing a large body part or a whole juvenile mouse while preserving endogenous fluorescence makes HYBRiD uniquely suitable for early screening of new mouse models and studying multi-organ interactions across broad fields of biomedical research. It also enables a new opportunity for researchers who like to take advantage of the efficiency of organic clearing but hesitate to use organic solvent on their microscopes. Limited by the size of imaging hardware and practical considerations of clearing time, HYBRiD in its current format is prioritized for whole juvenile mice or an adult chest (or similarly sized body parts). However, it is conceivable that, similar to what has been demonstrated feasible here, one could integrate shrinkage-based or more aggressive organic clearing methods (such uDISCO or SHANEL) with polymer reinforcements to further balance fluorescence retention and transparency for even larger samples. Nonetheless, HYBRiD overcomes many practical limitations of the parental organic and hydrogel-based methods and offers a uniquely simple (without continuous perfusion or device) and scalable (passive and cost-effective) option for routine organ and whole-body imaging/screening. These features could greatly accelerate the dissemination of organ and body clearing technologies to the broader biomedical research community beyond neuroscience.
METHODS
Animals
Mice were group-housed on a 12-hr light/dark cycle and fed a standard rodent chow diet. Room temperature was kept at 22 °C with humidity between 30–80% (not controlled). The following strains were purchased from The Jackson Laboratory (Bar Harbor, ME) for this study: wild-type (WT) C57BL/6J (Stock No: 000664), B6.129P2-Pvalbtm1(cre)Arbr/J (Stock No: 017320, PV-Cre), B6.Cg-Ssttm2.1(cre)Zjh/J (Stock No: 013044, SST-Cre), and B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J (Stock No: 007909, Ai9). PV-Cre and SST-Cre lines were crossed with Ai9 animals to generate the PV-Ai9 and SST-Ai9 animals used in this study. B6.Cg-Tg(K18-ACE2)2Prlmn/J (Stock No: 034860, K18 hACE2) hemizygotes were utilized for SARS-CoV-2 infection experiments. B6.Cg-Tg(Thy1-EGFP)MJrs/J (Stock No: 007788, Thy1-GFP-M) and B6.Cg-Tg(Thy1-YFP)HJrs/J (Stock No: 003782, Thy1-YFP-H) animals were gift from the Deisseroth lab at Stanford University. Both male and female mice were used for anatomical assays. All experimental protocols were approved by The Scripps Research Institute Institutional Animal Care and Use Committee (Animal protocol 18-0001) and were in accordance with the guidelines from the NIH.
Sample Collection
For brain, leg, and torso samples, adult PV-Ai9/Thy1-GFP/Thy1-YFP mice (n=4–6 each genotype) were heavily anesthetized with isofluorane then transcardially perfused with ice-cold PBS followed by ice-cold 4% PFA in PBS with 4% sucrose (Electron Microscopy Perfusion Fixative, 1224SK). For juvenile whole body clearing, littermates at 2 weeks (P12, n=4) and 3 weeks (P21, n=3) of age were prepared using the above procedures. For newborn whole body clearing, PV-Ai9/SST-Ai9 animals age P0-P3 (n=4–6 each genotype) were anesthetized by hypothermia, then the front of the ribcage was removed, and animals were sacrificed by puncture of the right atrium. All samples were washed with PBS and then placed in 4% PFA (in PBS) for post-fixation overnight at 4°C. For SARS-CoV-2 infected mice, animals were euthanized at 5 days post infection and bodies then submerged in zinc-formalin for fixation without perfusion at 4°C for 3 days before transfer from the BSL3 facility. After post-fixation, all samples were washed in 1X PBS at RT for 1 hr three times to remove residual PFA. All skin and hair was removed. Intact chest samples were prepared by removal of the chest from the body (diaphragm to cervical spine inside ribcage) using scissors, then the scapula and the brown adipose tissue were trimmed off. One sample from each group (WT and K18-hACE2) was cut in half after clearing (along the central plane of the spine) to facilitate DAPI staining, while the rest were kept intact for fluorescent light-sheet microscopy.
Decalcification and Decoloring
Samples (all except 1mm brain slices, PEGASOS and SHANEL samples) were decalcified in 10% EDTA/15% Imidazole solution (in water) with gentle shaking at 4°C for 4 days1. Subsequently, the samples were washed in PBS with gentle shaking at RT overnight followed by 1 hr washes three times at RT. All samples were decolored using 25% N,N,N′,N′-Tetrakis(2-Hydroxypropyl)ethylenediamine (Quadrol) in 1X PBS with gentle shaking at 37°C for 2 days (4 days for 3w-juveniles), with solution refreshed after 8 hours, then again at 24hrs. Intact SARS-CoV-2 infected chest samples were decolored for 6 days to aid in the removal of substantial amounts of pigment (heme) left in the tissues since they were not perfused. Subsequently, the samples were washed in PBS with gentle shaking at RT overnight followed by 1 hr washes three times at RT. Pretreated tissues were stored in 1X PBS with 0.02% sodium azide at 4°C until clearing was completed.
CLARITY Processing
CLARITY samples were first processed fixed with 4% PFA (in PBS) then incubated in A1P4 hydrogel (1% acrylamide, 0.125% Bis, 4% PFA, 0.025% VA-044 initiator (w/v), in 1X PBS) at 4°C for CLARITY embedding and subsequent passive clearing as previously published2. Samples were transferred to hydrogel for 48–72 hours to allow monomer diffusion. The samples were degassed with nitrogen and polymerized (4 hours at 37°C) with gentle agitation. Samples were removed from hydrogel and washed with 20 mM LiOH-Boric buffer pH 8 containing 6% SDS at 37°C overnight to remove residual PFA and monomers. Buffer was refreshed the next day, and every 3–4 days following during passive clearing. Passive clearing took place at 37°C with continuous shaking until samples appeared translucent. After clearing, samples were washed in PBST (0.2% Triton-X100) for 2 hr, then the buffer was refreshed and samples were washed overnight at 37°C to remove residual SDS. Cleared samples were refractive index matched (EasyIndex, RI=1.52, LifeCanvas, or PROTOS3) and incubated at 37°C for 8 hours (up to 3 days) and then 6–8 hours at room temperature. A vacuum chamber was used to remove any air bubbles within the cleared samples before mounting and imaging.
iDISCO Processing
We used a recent iteration of the iDISCO method, Adipoclear4 as previously published. All procedures were carried out at RT with shaking. Fixed samples were washed in 20%, 40%, 60%, 80% methanol in H2O/0.1% Triton X-100/0.3 M glycine (B1N buffer, pH 7), and 100% methanol for wash times outlined below. Whole-mount samples were incubated in 100% methanol overnight at 4°C before delipidation, with omission of the original peroxide bleaching step to preserve maximum fluorescence signal. Samples were then delipidated with 100% dichloromethane (DCM; Sigma-Aldrich), washed in 100% methanol twice, then in 80%, 60%, 40%, 20% methanol in B1N buffer. Samples were then washed in PBS/0.1% Triton X-100/0.05% Tween 20/2 mg/ml heparin (PTxwH buffer) for 1 hr three times, then overnight.
FDISCO Processing
All procedures were carried out at 4°C with shaking as previously published5. Fixed samples were washed in 50%, 70%, 80% tetrahydrofuran (THF) in 25% Quadrol (in 1X PBS to adjust to pH 9), then 95% THF twice. Timing of organic delipidation washes followed the outline given below, with three week old juvenile whole body samples treated with dichloromethane three times. Samples were delipidated with 100% dichloromethane (DCM; Sigma-Aldrich) then washed in 95% THF twice followed by 80%, 70%, 50% THF. Samples were then washed in 0.2% PBST for 1 hr then 2 hr at RT, followed by 1X PBS for 1 hr three times then overnight at RT to thoroughly wash out any remaining organic solvent.
uDISCO/ PEGASOS/ SHANEL Processing
In comparison experiments, all procedures were completed as previously published using their passive protocols6–8. For uDISCO, brain slices matched with BABB-D4 and larger samples (legs and bodies) matched with BABB-D15 as suggested by the original paper. For PEGASOS, hard tissue protocol was used to clear limbs and newborn samples. For SHANEL, the protocol for passive clearing was followed, with modifications to timing due to the difference in sample size from the original publication which was geared toward extremely large tissues. Timing of organic washes followed the outline given below.
Organic Protocol Timing
1mm Brain sections: 30 min. Whole Mount Limbs: 45 min. Newborn Mouse (P0-P3): 90 min. Adult Mouse Chest: 90 min. Juvenile Whole Body: 180 min. Each organic clearing method (iDISCO/FDISCO/uDISCO/PEGASOS/ SHANEL/HYBRiD) has provided a wide range of options for the organic washing length according to sample types and fluorescence labels. To enable a direct and efficient comparison among them, we used a fixed wash duration (applied to both organic dehydration and delipidation) across all tested methods when processing the same type of tissues, as summarized in the above table.
HYBRiD CLARITY embedding and additional clearing
HYBRiD method samples were first processed with the rehydrated FDISCO procedure outlined above before gel embedding. See CLARITY procedure outlined above. The intact chest samples contained high levels of excess blood which required an increased duration of passive clearing to reduce. Chest samples were passively cleared for at least 28 days (up to 40 days). Active clearing using a SmartClear machine (LifeCanvas Technologies) could accelerate the clearing, but it is not required. To remove all SDS from the tissue, samples were washed with 0.2% PBST at 37°C with shaking twice for 2 hrs then overnight, twice.
Immunostaining
Monoclonal rabbit anti-SARS-CoV nucleoprotein (Sinobiological, 40143-R019)9–10 was directly conjugated to AlexaFluor 647 using the APEX Antibody Labeling Kit (ThermoFisher, A10475) to allow single step detection of viral infection in the cleared chest samples. Intact chest samples were incubated with the conjugated antibody at 37°C for 4 days in 10% DMSO/0.2% PBST/0.02% sodium azide (1:700 dilution from the direct yield of APEX kit), then for an additional 4 days with an increased concentration (1:350 dilution) to maximize penetration of the antibody. Excess antibody was washed off with 0.2% PBST at 37°C with shaking twice for 2 hr then overnight twice.
Viral Infection
B6.Cg-Tg(K18-ACE2)2Prlmn/J (Stock No: 034860, K18 hACE2) hemizygotes, or wild-type (WT) C57BL/6J control mice, were purchased from The Jackson Laboratory (Bar Harbor, ME). WT mice (n=3) were used as mock-infected controls and K18-hACE2 transgenic mice (n=3, six total) were infected for tissue clearing experiments. All mice were maintained in parallel in a BSL-3 facility for SARS-CoV-2 infection until sacrifice. Mice were infected intranasally with 1 × 105 PFU of SARS-CoV-2 in a final volume of 50 μl following isoflurane sedation. After viral infection, mice were monitored daily and euthanized at 5 DPI by CO2.
Overall Timeline
| Sample Type | Fixation | Pre-Treatment | Organic Clearing | Embedding | Aqueous Clearing* | Immunostaining | Refractive Index Match |
|---|---|---|---|---|---|---|---|
| 1mm Brain Sections | 1 d | - | 1 d | 2 d | 7 d | - | 1 d |
| Whole Mount Limbs | 1 d | 6 d | 1 d | 2 d | 14 d | - | 2 d |
| Newborn Whole Body | 2 d | 6 d | 2 d | 3 d | 21 d | - | 2 d |
| Adult Chest (without perfusion) | 4 d | 10 d (4+6) | 2 d | 3 d | 28 d | 8 d | 3 d |
| Juvenile Whole Body (perfused) | 2 d | 8 d (4+4) | 3 d | 3 d | 28 d | - | 3 d |
Optional use of active clearing will shorten this step by 25–50%
Imaging
Light-sheet imaging:
Whole body and intact chest images were acquired with a light-sheet microscope (SmartSPIM, LifeCanvas). Samples were mounted using 1% agarose/EasyIndex. Whole body newborns were mounted with the dorsal side (spine) up, while intact chest samples were mounted dorsal side down (spine at the bottom of the Z-range). Samples were securely mounted to the holder after mounting gel solidified (~20 minutes at 4°C). Mounted samples were imaged inside the standard SmartSPIM chamber (90 × 65 × 45mm) filled with 250ml of EasyIndex after overnight equilibration. All the samples used in this study can be mounted using the standard chamber, however, for >2-week old mice only the chests can be visualized due to the size limit of the standard chamber. To image the whole-body of juvenile mice (2 and 3-week old), a custom oversized chamber (170 × 90 × 60 mm) was used and filled with 750ml of EasyIndex. All images were acquired using a 3.6X, 0.2 NA objective (LifeCanvas), with Z-step set to 4 μm, exposure time of 2 ms, and sampling at full resolution (2048 × 2048, 1.75/1.75/4 μm XYZ voxel size). Image acquisition was completed with bilateral illumination along the central plane of symmetry within the sample.
Confocal microscopy:
Cleared samples were incubated in RI matching media then mounted to a glass microscope slide using spacers (1mm, Sunjin Lab). Spacers were coated with silicone (KWIK-SIL, WPI) for use with samples in organic solvent to prevent degradation of the plastic. Tissues were then imaged with the Olympus FV3000 confocal microscope with one of the following objectives: 4X, 0.28 NA, air (XLFluor, Olympus); 10X, 0.6 NA, water immersion (XLUMPlanFI, Olympus); or 40X, 1.25 NA, silicone oil immersion (UPlanSApo, Olympus).
Image Post-Processing
Raw data from the SmartSPIM was destriped and stitched using commercial software from LifeCanvas Technologies. Destriped and stitched images were converted to .ims format for 3D visualization in IMARIS (Bitplane, v9.2.1). Maximum intensity projections were captured as snapshots, and digital slice views were generated using the ortho slicer tool and movies using the recording feature in IMARIS. Confocal images were viewed and adjusted using Fiji (ImageJ v2.1.0).
Quantifications and Image Analysis
Transmittance
A 6 mm diameter biopsy punch was used to cut 1mm thick brain slices to fit into the wells of a 96 well plate. For leg transparency measurements, 3mm thick cross section blocks were cut from the center of the upper hindleg to minimize tissue heterogeneity between samples, then the punch was used to trim excess tissue. Samples were placed into wells with the minimum volume of RI matching media needed to submerge fully. Absorbance was measured by multi-area (5×5 grid across each well) at each wavelength 450nm-750nm with Cytation 3 plate reader (BioTek). Central 9 areas (3×3) of reading were used for quantification, as the edge of the wells gave varying results due to high viscosity of RI matching media. Blank was subtracted from raw absorbance values, then converted to transmittance (each condition n=5).
Signal profile
Signal profile data was generated using Fiji. After opening a Z-projection (MIP) of an image, a straight line was drawn across an area which includes signal and background. The plot profile tool was used, and the signal plot data recorded. Likewise, the same procedure was applied to areas of only background with no signal present. All signal profile plot data was normalized by dividing signal plot by the background plot values.
Tissue Size Measurement
Size measurement of linear change were completed using bright-field images of 1mm brain slices over a grid from each method in RI matching media, subtracted from PFA only slices in PBS. Calculations were completed using the square root of the area size change as previously published5.
Fluorescence intensity and signal-to-background ratio
Fluorescence intensity and signal-to-background ratio were quantified using FIJI based on previous uDISCO and FDISCO publications5,8. For each image, a max intensity projection over 50 μm of a region of interest (ROI) of 150 – 200 μm × 150 – 200 μm was generated on the surface of the tissue (within 150 μm from the top). Region of signal was identified by auto-thresholding, and the rest of image pixels were designated as region of background. For region of signal and region of background, total area, mean intensity, and sum of intensity were measured by FIJI. Fluorescence intensity was then calculated by , and signal-to-background ratio was calculated by . For each condition, 8–12 random ROI in the cortex per tissue sample were quantified and 4–6 tissue samples per condition were used in each analysis.
Protein Loss
Samples treated with FDISCO and iDISCO protocols were evaluated for protein loss dependent on sequential hydrogel embedding in comparison to native and CLARITY embedded tissue with no pretreatment. Half coronal 1mm PV-Ai9 brain slices were briefly dabbed on a kimwipe to remove excess moisture before mass of tissue in each condition was recorded. All samples placed into LiOH-Boric SDS buffer following their respective treatments and were placed at 37°C with shaking to begin passively clearing. On the fourth day of clearing, buffer was sampled from each tube, then fresh clearing buffer was added to each tube before placing samples back on the 37°C shaker. Samples continued passively clearing until day 7, when clearing buffer was once again collected. Pierce BCA Protein Assay Kit (Cat no. 23227, ThermoFisher Scientific) was used for colorimetric quantification of protein content. Total protein loss values are the sum of day 4 and day 7 quantifications.
Statistics and Reproducibility
Statistical significance was evaluated with Prism 8 (Graphpad) using t-tests and one-way or two-way ANOVA followed by Tukey’s multiple comparison post hoc test. All data presented as individual datapoint or mean ± SEM with n given in figure legend as biological replicates.
All fluorescence intensity, transparency, size, protein loss experiments were repeated 3 times with N=6. Newborn clearing experiments were repeated 3 times with N=3 of each method, and genotype for Ai9 samples. For juvenile clearing experiments, 3–4 biological replicates were compared at ages P12 (2 wk) and at P21 (3 wk); for SARS-CoV-2 samples, infection was performed once with 3 biological replicates in each genotype.
Data Availability
All data supporting the findings of this study are available from the corresponding author upon request.
Extended Data
Extended Data Fig. 1. Development of HYBRiD.
A. Schematic of aqueous versus organic clearing protocols. Yellow: organic phase. Blue: aqueous phase. B. Representative images of 1 mm sagittal PV-Ai9 brain slices processed by indicated clearing protocols from 6 samples per group. EI: EasyIndex; DBE: dibenzyl ether. Grid size: 3 mm. C. Quantification of signal-to-background ratio of TdTomato fluorescence signal in PV-Ai9 brain slices processed by indicated clearing protocols. Native (n = 26), CLARITY (n = 31), FDISCO (EI) (n = 30), FDISCO (n = 12), iDISCO (EI) (n = 35), iDISCO (n = 15). (n indicates numbers of measurements from 6 samples per condition). Statistical significance was determined by two-tailed t-tests. D. Representative signal intensity profiles of FDISCO (left) and iDISCO (right) processed PV-Ai9 brain slices in either organic (DBE) or aqueous (EI) RI matching solution. N = 3 for each condition. E. Representative MIP confocal images of 1 mm PV-Ai9 brain slices processed by the indicated clearing methods from 6 samples per condition. Scale bar: 3 mm.
Extended Data Fig. 2. Comparison of HYBRiD and parental methods.
A. Quantification of fluorescence intensity of 1 mm PV-Ai9 brain slices of Native (n = 26), cleared by HYBRiD (n = 30) or FDISCO (n = 12). (n indicates numbers of measurements from 6 samples per condition). Statistical significance of indicated comparison was determined by one-way ANOVA. B. Quantification of transparency of brain slices. All values are mean ± SEM, N = 4 for each group. No significance between HYBRiD and FDISCO, determined by multiple unpaired t-tests. C. Representative images of brain slices processed by the indicated clearing method from 3 samples per condition after 7 days of SDS treatment. D - E. Quantification of protein loss in brain slices (D) and hindlimb (E) during passive SDS clearing. N = 3 per group. Statistical significance was determined by one-way ANOVA. F. Size changes of brain slices after CLARITY, FDISCO and HYBRiD. No significant size changes were detected between CLARITY and HYBRiD. N = 3 per group. Statistical significance was determined by one-way ANOVA.
Extended Data Fig. 3. Comparison of HYBRiD and organic clearing methods.
A. Representative images of hindlimb from PV-Ai9 adult mice processed by the indicated clearing protocols from 6 samples per condition. RI matching media is labeled. B. Quantification of signal-to-background ratio of TdTomato fluorescence signal in PV-Ai9 brain slices processed by HYBRiD (n = 29), uDISCO (n = 22), PEGASOS (n = 28), SHANEL (n = 24). (n indicates numbers of measurements from 4 samples per condition). Statistical significance was determined by one-way ANOVA. C. Quantification of signal-to-background ratio of GFP fluorescence signal in Thy1-GFP-M brain slices processed by HYBRiD (n = 40) or uDISCO (n = 24) (n indicates numbers of measurements from 5 samples per condition). Statistical significance was determined by two-tailed t-test. D. Representative images of hindlimb from Thy1-GFP-M adult mice cleared by HYBRiD or uDISCO (4 samples per group). E. Representative lightsheet images (3D MIP) of forelimb from Thy1-GFP-M adult mice cleared by HYBRiD or uDISCO. F. Quantification of signal to background ratio of YFP fluorescence signal in Thy1-YFP-H brain slices processed by HYBRiD (n = 34) or uDISCO (n = 32) (n indicates numbers of measurements from 5 samples per condition). Statistical significance was determined by two-tailed t-test. G. Representative images of hindlimb from Thy1-YFP-H adult mice cleared by HYBRiD or uDISCO (4 samples per group). H. Representative lightsheet images (3D MIP) of forelimb from Thy1-YFP-H adult mice cleared by HYBRiD or uDISCO from 4 samples per group. Grid size: 3 mm. Scale bar: 1 mm.
Extended Data Fig. 4. Comparison of HYBRiD and other methods on clearing of newborn mice.
A,B. Representative brightfield images of PV-Ai9 newborn mice processed by indicated clearing methods and RI matched in the indicated media (3 samples per condition). Grid size: 3 mm. C - E. Overview of lightsheet imaging of PV-Ai9 newborn mice cleared by the indicated clearing protocols. 3D volume of cleared PV-Ai9 mice from each clearing protocol is shown in coronal view (C), sagittal view (D), and transverse view (E). Note HYBRiD, SHANEL, and uDISCO sample were imaged fully through the D-V axis. D, dorsal; V, ventral; S, superior; I, inferior; L, lateral; M, medial. Scale bar: 2 mm.
Extended Data Fig. 5. HYBRiD clearing of juvenile mice.
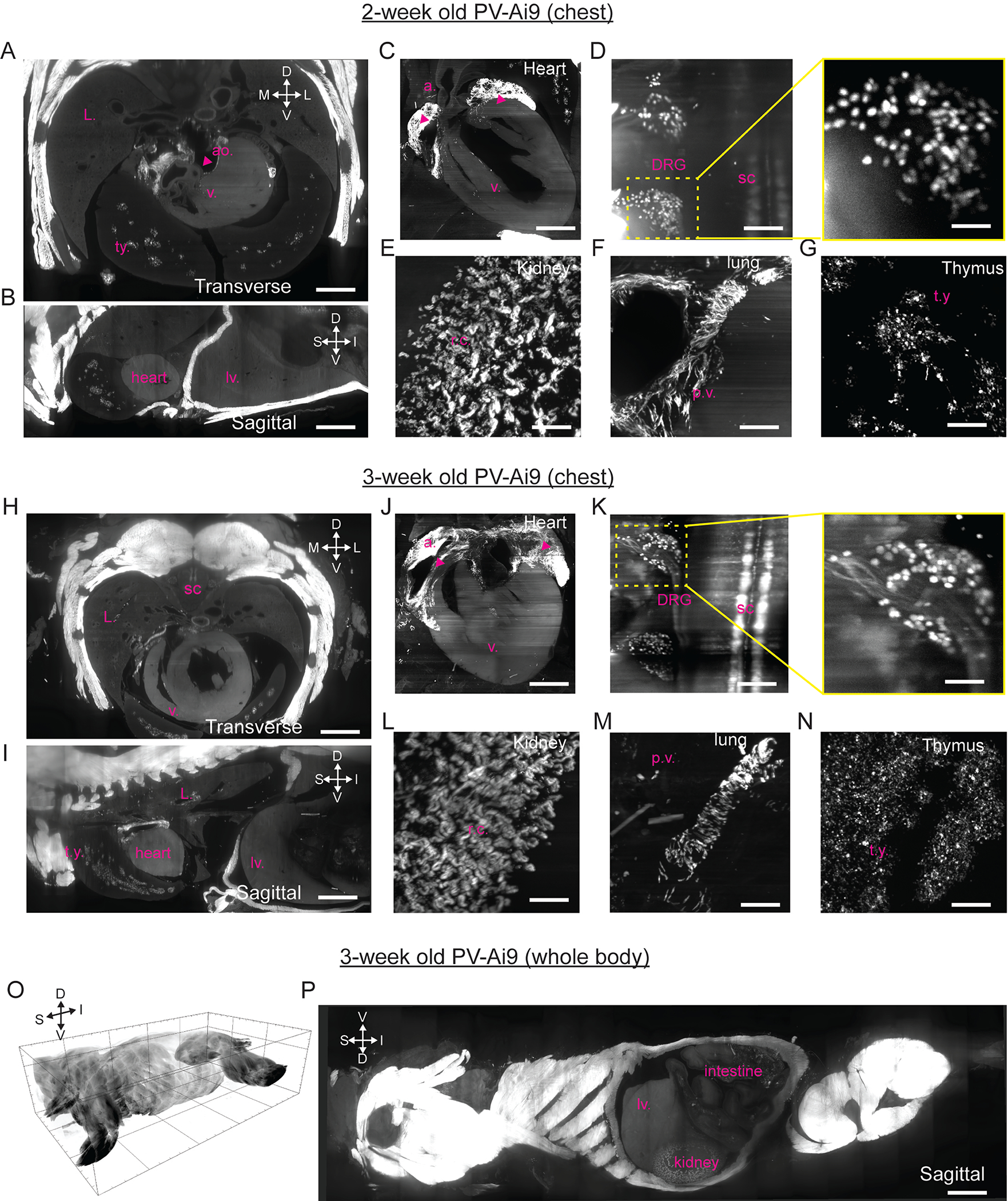
A – G. Overview of lightsheet imaging of 2-week-old PV-Ai9 mice cleared by HYBRiD from 4 samples. Transverse (A) and sagittal (B) views showing the chest and heart. Zoom-in views of multiple tissues including heart (C), DRG (D), kidney (E), lung (F) and thymus (G). Labels: atrium(a.), thymus (t.y.), aorta (ao), ventricle (v.), lung (L.), spinal cord (sc), pulmonary vasculature (p.v.), renal cortex (r.c.), and liver (lv.) H – N. Overview of lightsheet imaging of 3-week-old PV-Ai9 mice cleared by HYBRiD from 3 samples. Transverse (H) and sagittal (I) views showing the chest and heart. Zoom-in views of multiple tissues including heart (J), DRG (K), kidney (L), lung (M) and thymus (N). O – P. 3D volume of lightsheet imaging of 3-week-old PV-Ai9 mice cleared by HYBRiD. D, dorsal; V, ventral; S, superior; I, inferior; L, lateral; M, medial. Scale bar: O: 5,000 μm; P: 3,000 μm; A, B, H, I: 1,500 μm; C, J: 1,000 μm; D, F, G, K, M, N: 200 μm; E, L: 150 μm. Inserts in D and K: 50 μm.
Extended Data Fig. 6. SARS-CoV nucleoprotein in WT and K18-hACE2 lung.
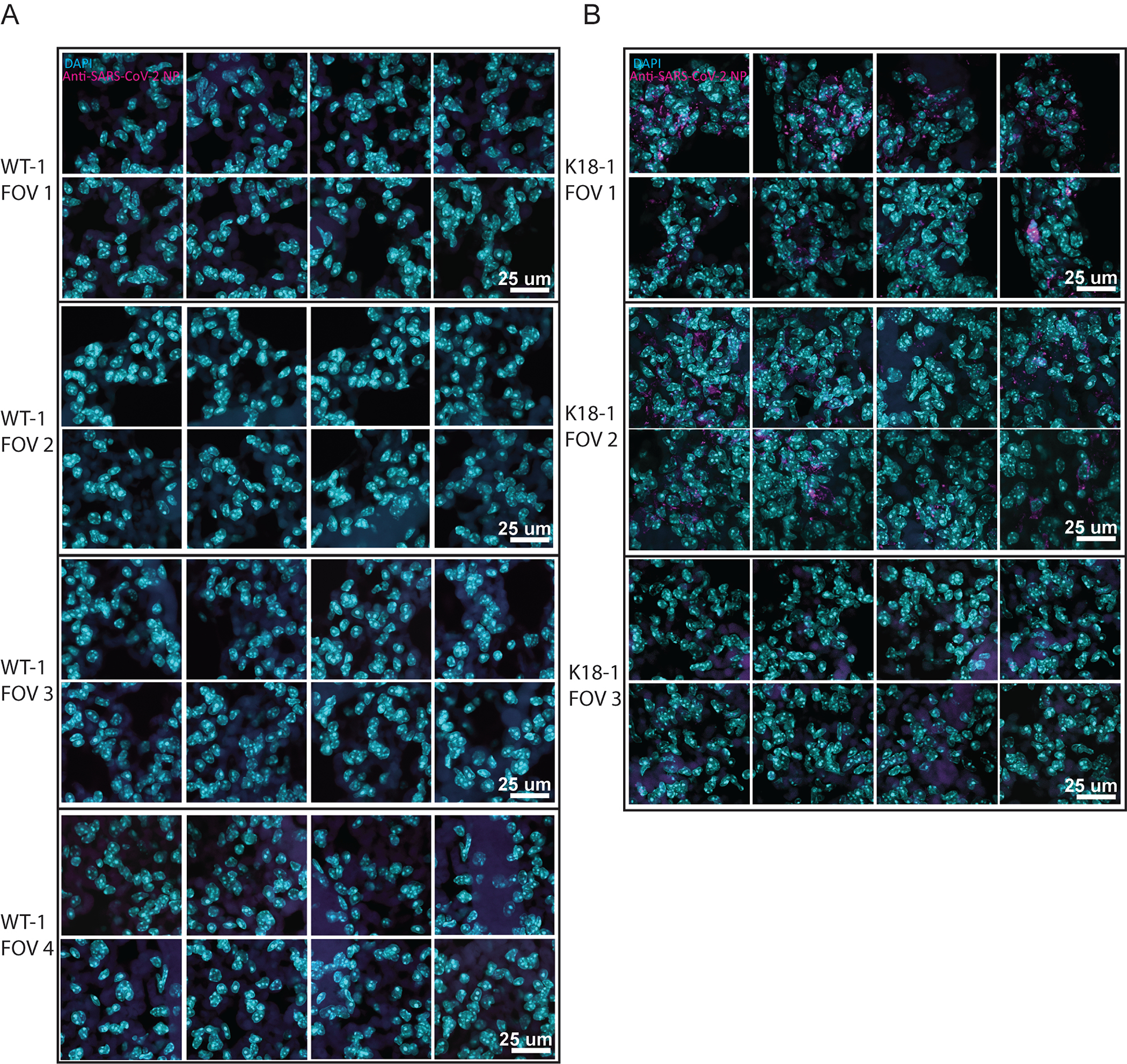
MIP of 40x confocal imaging of anti-SARS-CoV nucleoprotein stained lung from WT (A) or K18-hACE2 (B) (3 samples per group). FOV 3 in (B) shows a region with low viral infection as an internal negative control.
Supplementary Material
Supplementary Video 1
3D rendering of a HYBRiD-cleared PV-Ai9 newborn mouse (P2) imaged by a lightsheet microscope (SmartSPIM). White signal was from endogenous tdTomato fluorescence. In-plane resolution, 1.75 μm/pixel; z-step size, 4 μm. Image volume: 13 mm × 13 mm × 10 mm.
Supplementary Video 2
Slice view of HYBRiD-cleared 3-week-old PV-Ai9 mouse (P21) imaged as a whole torso by a lightsheet microscope (SmartSPIM). White signal was from endogenous tdTomato fluorescence. The sample was imaged as coronal view (X–Y, 3,540 in-plane frames). Transverse views were digitally resliced from the 3D volumes.
Supplementary Video 3
3D rendering of a HYBRiD cleared 3-week-old PV-Ai9 mouse (P21) imaged as a whole torso by a lightsheet microscope (SmartSPIM). White signal was from endogenous tdTomato fluorescence. In-plane resolution 3.5 μm/pixel (2× downsampled to enable 3D movie rendering), z-step size, 4 μm, 3,540 steps. Image volume: 50 mm × 30mm × 14mm.
Supplementary Video 4
3D rendering of a HYBRiD-cleared chest from a SARS-CoV-2-infected K18-hACE2 transgenic mouse. Blue is the autofluorescence signal used to outline the organs. Red is the anti-SARS-CoV NP AlexaFluor 647 signal. In-plane resolution, 1.75 μm/pixel; z-step size, 4 μm. Image volume: 19 mm × 19 mm × 13 mm.
ACKNOWLEDGEMENTS:
This work was supported by the National Institutes of Health Director’s New Innovator Award DP2DK128800 (L.Y.), National Institute of Diabetes and Digestive and Kidney K01DK114165 (L.Y.), Dana Foundation (L.Y.), and Baxter Foundation (L.Y). Y.W. was supported by the Dorris Scholars fellowship. We thank Drs. Paul Cohen, Ardem Patapoutian, Vinny Augustine, and Brian Hsueh for discussion. We are grateful to Dr. Zhuhao Wu for advice on iDISCO protocols. We also thank Drs. Deli Huang, Fangzhu Zhao, Xin Sun, and Dennis Burton for their input on immunostaining of the SARS-CoV-2 samples. We thank Dr. Karl Deisseroth and Sally Pak of Stanford University for gifting the Thy1-YFP and Thy1-GFP tissue samples. We are grateful to Dr. Jeffrey Stirman for the imaging support. We thank all members of the Ye lab and the Dorris Neuroscience Center (specially Drs. Kathryn Spencer and Filip de Souza Polli) for their support and feedback.
Footnotes
COMPETING INTERESTS: The authors declare that they have no competing interests.
REFERENCES
- 1.Erturk A et al. Three-dimensional imaging of solvent-cleared organs using 3DISCO. Nat Protoc 7, 1983–1995, doi: 10.1038/nprot.2012.119 (2012). [DOI] [PubMed] [Google Scholar]
- 2.Renier N et al. iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell 159, 896–910, doi: 10.1016/j.cell.2014.10.010 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Chi J, Crane A, Wu Z & Cohen P Adipo-Clear: A Tissue Clearing Method for Three-Dimensional Imaging of Adipose Tissue. J Vis Exp, doi: 10.3791/58271 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perin P, Voigt FF, Bethge P, Helmchen F & Pizzala R iDISCO+ for the Study of Neuroimmune Architecture of the Rat Auditory Brainstem. Front Neuroanat 13, 15, doi: 10.3389/fnana.2019.00015 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan C et al. Shrinkage-mediated imaging of entire organs and organisms using uDISCO. Nat Methods 13, 859–867, doi: 10.1038/nmeth.3964 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Qi Y FDISCO: Advanced solvent-based clearing method for imaging whole organs. Science Advances (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai R et al. Panoptic imaging of transparent mice reveals whole-body neuronal projections and skull-meninges connections. Nat Neurosci 22, 317–327, doi: 10.1038/s41593-018-0301-3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Susaki EA et al. Whole-brain imaging with single-cell resolution using chemical cocktails and computational analysis. Cell 157, 726–739, doi: 10.1016/j.cell.2014.03.042 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Tainaka K et al. Whole-body imaging with single-cell resolution by tissue decolorization. Cell 159, 911–924, doi: 10.1016/j.cell.2014.10.034 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Tainaka K et al. Chemical Landscape for Tissue Clearing Based on Hydrophilic Reagents. Cell Rep 24, 2196–2210 e2199, doi: 10.1016/j.celrep.2018.07.056 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Susaki EA et al. Versatile whole-organ/body staining and imaging based on electrolyte-gel properties of biological tissues. Nat Commun 11, 1982, doi: 10.1038/s41467-020-15906-5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung K et al. Structural and molecular interrogation of intact biological systems. Nature 497, 332–337, doi: 10.1038/nature12107 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomer R, Ye L, Hsueh B & Deisseroth K Advanced CLARITY for rapid and high-resolution imaging of intact tissues. Nat Protoc 9, 1682–1697, doi: 10.1038/nprot.2014.123 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Treweek JB et al. Whole-body tissue stabilization and selective extractions via tissue-hydrogel hybrids for high-resolution intact circuit mapping and phenotyping. Nat Protoc 10, 1860–1896, doi: 10.1038/nprot.2015.122 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neckel PH, Mattheus U, Hirt B, Just L & Mack AF Large-scale tissue clearing (PACT): Technical evaluation and new perspectives in immunofluorescence, histology and ultrastructure. Scientific Reports 6, doi: 10.1038/srep34331 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang B et al. Single-cell phenotyping within transparent intact tissue through whole-body clearing. Cell 158, 945–958, doi: 10.1016/j.cell.2014.07.017 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park YG et al. Protection of tissue physicochemical properties using polyfunctional crosslinkers. Nat Biotechnol, doi: 10.1038/nbt.4281 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ku T et al. Multiplexed and scalable super-resolution imaging of three-dimensional protein localization in size-adjustable tissues. Nat Biotechnol 34, 973–981, doi: 10.1038/nbt.3641 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee E et al. ACT-PRESTO: Rapid and consistent tissue clearing and labeling method for 3-dimensional (3D) imaging. Sci Rep 6, 18631, doi: 10.1038/srep18631 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ueda HR et al. Tissue clearing and its applications in neuroscience. Nat Rev Neurosci 21, 61–79, doi: 10.1038/s41583-019-0250-1 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Jing D et al. Tissue clearing of both hard and soft tissue organs with the PEGASOS method. Cell Res 28, 803–818, doi: 10.1038/s41422-018-0049-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao S et al. Cellular and Molecular Probing of Intact Human Organs. Cell 180, 796–812.e719, doi: 10.1016/j.cell.2020.01.030 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee KY et al. Lessons on conditional gene targeting in mouse adipose tissue. Diabetes 62, 864–874, doi: 10.2337/db12-1089 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winkler ES et al. SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat Immunol 21, 1327–1335, doi: 10.1038/s41590-020-0778-2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yinda CK et al. K18-hACE2 mice develop respiratory disease resembling severe COVID-19. PLoS Pathog 17, e1009195, doi: 10.1371/journal.ppat.1009195 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carossino M et al. Fatal neuroinvasion of SARS-CoV-2 in K18-hACE2 mice is partially dependent on hACE2 expression. bioRxiv, doi: 10.1101/2021.01.13.425144 (2021). [DOI] [Google Scholar]
- 27.Buchrieser J et al. Syncytia formation by SARS-CoV-2-infected cells. EMBO J 39, e106267, doi: 10.15252/embj.2020106267 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bussani R et al. Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology. EBioMedicine 61, 103104, doi: 10.1016/j.ebiom.2020.103104 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye L et al. Wiring and Molecular Features of Prefrontal Ensembles Representing Distinct Experiences. Cell 165, 1776–1788, doi: 10.1016/j.cell.2016.05.010 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan C et al. Shrinkage-mediated imaging of entire organs and organisms using uDISCO. Nat Methods 13, 859–867, doi: 10.1038/nmeth.3964 (2016). [DOI] [PubMed] [Google Scholar]
METHOD REFERENCES
- 1.Tainaka K et al. Chemical Landscape for Tissue Clearing Based on Hydrophilic Reagents. Cell Rep 24, 2196–2210 e2199, doi: 10.1016/j.celrep.2018.07.056 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Ye L et al. Wiring and Molecular Features of Prefrontal Ensembles Representing Distinct Experiences. Cell 165, 1776–1788, doi: 10.1016/j.cell.2016.05.010 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park YG et al. Protection of tissue physicochemical properties using polyfunctional crosslinkers. Nat Biotechnol, doi: 10.1038/nbt.4281 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chi J, Crane A, Wu Z & Cohen P Adipo-Clear: A Tissue Clearing Method for Three-Dimensional Imaging of Adipose Tissue. J Vis Exp, doi: 10.3791/58271 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qi Y FDISCO: Advanced solvent-based clearing method for imaging whole organs. Science Advances (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jing D et al. Tissue clearing of both hard and soft tissue organs with the PEGASOS method. Cell Res 28, 803–818, doi: 10.1038/s41422-018-0049-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao S et al. Cellular and Molecular Probing of Intact Human Organs. Cell 180, 796–812.e719, doi: 10.1016/j.cell.2020.01.030 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan C et al. Shrinkage-mediated imaging of entire organs and organisms using uDISCO. Nat Methods 13, 859–867, doi: 10.1038/nmeth.3964 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Carossino M et al. Fatal neuroinvasion of SARS-CoV-2 in K18-hACE2 mice is partially dependent on hACE2 expression. bioRxiv, doi: 10.1101/2021.01.13.425144 (2021). [DOI] [Google Scholar]
- 10.Buchrieser J et al. Syncytia formation by SARS-CoV-2-infected cells. EMBO J 39, e106267, doi: 10.15252/embj.2020106267 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Video 1
3D rendering of a HYBRiD-cleared PV-Ai9 newborn mouse (P2) imaged by a lightsheet microscope (SmartSPIM). White signal was from endogenous tdTomato fluorescence. In-plane resolution, 1.75 μm/pixel; z-step size, 4 μm. Image volume: 13 mm × 13 mm × 10 mm.
Supplementary Video 2
Slice view of HYBRiD-cleared 3-week-old PV-Ai9 mouse (P21) imaged as a whole torso by a lightsheet microscope (SmartSPIM). White signal was from endogenous tdTomato fluorescence. The sample was imaged as coronal view (X–Y, 3,540 in-plane frames). Transverse views were digitally resliced from the 3D volumes.
Supplementary Video 3
3D rendering of a HYBRiD cleared 3-week-old PV-Ai9 mouse (P21) imaged as a whole torso by a lightsheet microscope (SmartSPIM). White signal was from endogenous tdTomato fluorescence. In-plane resolution 3.5 μm/pixel (2× downsampled to enable 3D movie rendering), z-step size, 4 μm, 3,540 steps. Image volume: 50 mm × 30mm × 14mm.
Supplementary Video 4
3D rendering of a HYBRiD-cleared chest from a SARS-CoV-2-infected K18-hACE2 transgenic mouse. Blue is the autofluorescence signal used to outline the organs. Red is the anti-SARS-CoV NP AlexaFluor 647 signal. In-plane resolution, 1.75 μm/pixel; z-step size, 4 μm. Image volume: 19 mm × 19 mm × 13 mm.
Data Availability Statement
All data supporting the findings of this study are available from the corresponding author upon request.


