Abstract
We examine whether the rate of delivery of photons from a UV radiation source has an effect on the inactivation of spores. We directly compare the output of a high-peak-power UV laser source at 248 nm to a low-power continuous lamp source (254 nm) in the inactivation of Bacillus subtilis spores. The two UV sources differ by a factor of 108 in peak power. Contrary to previous reports, no clear differences in spore survival were observed.
The identification and inactivation of biological warfare agents is a high priority for military and civilian defense authorities. The development of defenses against the release of these agents by rogue nations is under way. Decontamination of personnel, buildings, equipment, and the environment is also being addressed. UV irradiation has high potential to inactivate a wide variety of biological agents and is one of several technologies being studied (4). Another major application of UV irradiation is in the decontamination of foods. Understanding the fundamental interaction between photons and biological organisms could lead to significant advances in the effectiveness of UV irradiation for both of these applications. Characterization of energy density effects may reveal new, efficient pathways for UV inactivation. Here we focus on high-peak-power laser sources and whether they offer advantages in the inactivation of biological warfare agents. Energy density used in the context of this article refers to changes in the number of photons per unit area (energy/area) or the rate of delivery of photons per unit area (power/area) and is reported in joules per square meter or watts per square meter, respectively. Peak power from a pulse laser source is defined as the pulse energy divided by the pulse duration and is reported in watts.
This is a controversial issue because some of the information circulated on this topic implies a strong energy density effect but has not been published or peer reviewed, and two reports that have been published provide opposing conclusions. One report suggests that the efficiency of sterilization of food and packaging materials is fundamentally different when a pulsed light source with high peak power rather than a continuous lamp source with low power is used. The investigators reported the inactivation (disappearance) of the “tails” of the survival curves using pulsed light, a result which has never been reported using a continuous lamp source. These startling improvements were attributed to the pulsed properties of the light. However, one of the light sources was broadband and one was not, thus complicating the interpretation of the results (2). A more recent report using a flashlamp source with a higher repetition rate suggests only a small improvement in inactivation and little to no effect on the survival curve tails (6). This report compared the UV portion of two broadband sources. Therefore, a close examination of the inactivation efficiencies of a pulsed, high-peak-power source and a lamp source is needed.
The purpose of this article is to address whether the inactivation of spores exposed to UV irradiation exhibits a peak power dependence when narrow, nearly monochromatic light sources near 250 nm, a highly effective germicidal wavelength, are used. This study targets the parameter photons per unit time per unit area, which is the most likely source of any observed difference due to a pulsed light source. We were careful to control other variables, extend the range of inactivation to 6 orders of magnitude, and include error bars for a definitive result.
Varying energy density parameters serves to reveal additional inactivation mechanisms, which can lead to the development of much more efficient inactivation processes. If the rate at which photons are delivered is important, different light sources may be more effective than others. If an energy density effect is present, increasing the peak power of the light source may substantially improve the ability to inactivate biowarfare agents targeting military personnel or civilians during a terrorist attack. Bacillus subtilis is well suited for this study because it serves as a model for Bacillus anthracis and would provide proof of concept for one of the most difficult types of cells to inactivate under the most challenging applications of inactivation. B. subtilis spores require 7 to 50 times more UV fluence to be inactivated than vegetative cells at wavelengths near 250 nm (8–10). B. anthracis spores require about 75 times more UV fluence than vegetative cells (5). Fluence is a measure of energy per area and is reported in joules per square meter.
Inactivation of spores stems from the formation of thymine dimers, such as the spore photoproduct, a 5-thyminyl-5-6-dehydrothymine adduct, following the absorption of UV light by the DNA of the organism (12). It is the formation of these dimers that prevents replication. Systems with a high degree of heterogeneity and those with complicated photochemistry are good candidates for exhibiting energy density effects. The spores investigated here have several biological layers in the form of inner and outer spore coats (7). They have small acid-soluble proteins attached to the DNA (3, 3a, 6a, 9, 13), and they exhibit complicated photochemistry (1, 11, 14, 15), i.e., multiple reaction pathways. This is a likely system for finding energy density effects. In addition, systems with energy density effects are expected to produce nonlinear survival curves, and previous inactivation survival curves have exhibited an unexplained curvature (4, 6, 16).
A B. subtilis subsp. niger dry-spore sample (Pine Bluff Mix) was obtained from the Biological Sciences Division of Dugway Proving Grounds. It contained ∼2.7 × 1011 CFU per g or ∼2.8 × 1012 spores per g of dried material, as determined by direct counting. A solution of ∼108 spores per ml (determined by plate counting) in deionized, sterile H2O was made and vortexed. It was diluted before each irradiation experiment to a concentration of ∼107 CFU/ml (plate count) and loaded into sterile Suprasil cuvettes with a 2-mm path length. These were irradiated with a 248-nm UV pulsed excimer laser (Lambda Physik 240i) at two different peak powers or a mercury lamp (Spectroline ENF-240C) at 254 nm. Samples were then transferred to a UV-sterilized hood for plating. Difco Tripticase soy agar plates were incubated overnight at 30°C or at room temperature for 2 to 3 days before visual counting. Multiple serial dilutions and multiple plates of specific serial dilutions were used to obtain the counts and uncertainties. A control experiment to determine if protoreactivation was present was carried out independently of the peak power experiments. Photoreactivation was not observed using 254-nm lamp radiation for inactivation and 365-nm radiation for reactivation; hence, all samples were exposed to room light thereafter. Knudson also reported no observed photoreactivation from the spores of B. anthracis (5).
The minimum practical path length of the optical cells was 2 mm. The maximum spore concentration which resulted in “optically thin” samples was ∼107 CFU/ml. This value corresponds to an absorption at 250 nm of ∼0.10 or a transmission of ∼80% through the spore samples.
The high peak power source was an excimer laser operated with KrF at 248 nm. The energy per pulse was ∼120 mJ at 10 Hz in an area of ∼1 by 3 cm2. The pulse duration was nominally 14 ns. The laser energy was measured using a calibrated Scientech disk calorimeter detector (model 380403), which was calibrated to a National Institute of Standards and Technology traceable standard and had an accuracy of ∼3%.
The Spectroline lamp produced ∼90 W/m2 at the distance used. The uniformity of the lamp source had a maximum variation of ∼3% within the irradiation area. The output was measured with a calibrated UDT UV 100EC photodiode and read with a Keithley picoammeter (model 480). The photodiode was calibrated to a National Institute of Standards and Technology traceable standard between 200 and 400 nm and produced 0.114 A per W at 254 nm. The total uncertainty of the lamp energy used in the experiments was estimated to be ∼20%.
The survival of B. subtilis subsp. niger spores at two laser pulse energies was examined. The peak fluences of laser radiation were 35 and 250 J/m2 per pulse. These values were converted to peak powers of 2.5 × 109 and 1.8 × 1010 W/m2, respectively. The survival curves indicated no clear difference between the two laser power measurements, which differed by a factor of 7 in peak power. Survival was also examined using a mercury lamp with an output of ∼90 W/m2 at 254 nm. The 10% survival point for the lamp in this study was ∼100 J/m2. This finding is consistent with that observed for B. subtilis (PY79) by Riesenman and Nicholson (7), who reported 102 ± 14 J/m2, and lower than that found by Setlow (8), who reported 315 J/m2 for B. subtilis (wild type, average of 168 and GSY 1026).
The use of two monochromatic light sources with similar photon energies near the peak effective germicidal wavelength offers an advantage in this study. The spectral dependence of spore inactivation is removed, and direct comparisons of peak power and other energy density parameters can be made. We suspended spores in solution to reduce the degree of possible masking of spores and to increase the dynamic range of the inactivation to a 5- or 6-order-of-magnitude reduction. For these reasons, we believe that the experimental conditions used in this report provide the simplest, best intrinsic energy dependencies for the interaction of photons with bacterial spores in the literature to date.
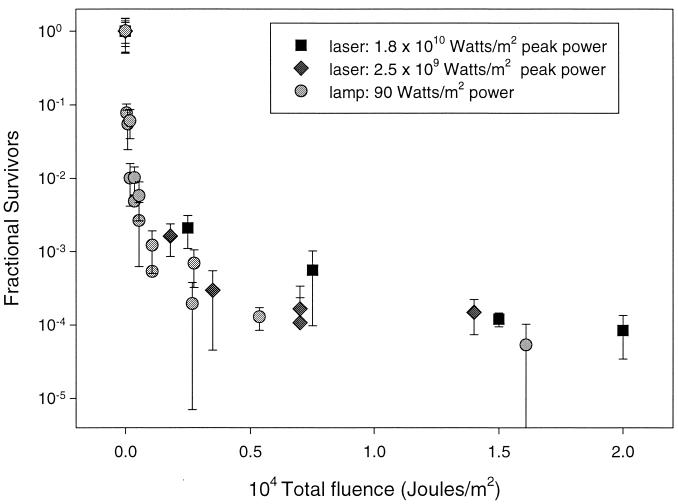
We observed no discernible peak power effect when we varied peak power by 8 orders of magnitude (Fig. 1). The maximum total fluence in all experiments exceeded 104 J/m2. Total fluences of 104 J/m2 resulted in a ∼5-order-of-magnitude reduction of viable spores in all three conditions. However, it took 3 h to deliver 104 J/m2 using the lamp and 40 s to deliver the same total fluence using the laser with a repetition rate of 10 Hz, indicating that the inactivation (delivery of photons) is much more rapid with the high peak power source. This more rapid delivery of photons from a pulsed source, in and of itself, is a significant advantage in the defense against biological weapons and in the sterilization of foods.
FIG. 1.
Semilog plot of fractional survival as a function of total fluence under three different peak power irradiation conditions. Laser fluences were 35 and 250 J/m2/pulse, corresponding to peak powers of 2.5 × 109 and 1.8 × 1010 W/m2, respectively. The lamp fluence had a power of ∼90 W/m2. The plot shows the lack of peak power dependence above the uncertainty of the measurements. Error bars show ±1 standard deviation.
Our high peak power measurements compare favorably to the spore survival curves reported by McDonald et al. (6), who used a flashlamp source and a lamp source. They reported a slight difference between the two, with the pulsed source being more effective than the lamp source. However, it is not clear if the difference was outside their experimental uncertainty. Although the results of Warriner et al., using only a high peak power source, are similar to ours in some general ways, their samples were spores dried on various surfaces and included masking effects that we should not have (16).
We conclude that peak power in the delivery of photon energy is not an important factor in the range studied and that high peak power sources offer no intrinsic advantage in the inactivation of bacterial spores over low peak power sources other than their much more rapid photon delivery time. Although there are a few energy density parameters that may show an effect, it appears from the data presented here that the total number of photons delivered is the important parameter and not the number of photons delivered per unit of time. Because an effect was previously reported using a broadband pulsed light source with a longer pulse width (2), these two parameters should also be examined more closely. A longer pulse width is related to increased integrated energy per pulse, and this factor could be an important parameter. It would greatly improve the ability to counter a biological attack if an additional inactivation mechanism becomes accessible as a result of one of these two parameters.
Acknowledgments
This work was supported by the DSO office at DARPA.
REFERENCES
- 1.Donnellan J E, Setlow R B. Thymine photoproducts but not thymine dimers fround in ultraviolet-irradiated bacterial spores. Science. 1965;149:308–310. doi: 10.1126/science.149.3681.308. [DOI] [PubMed] [Google Scholar]
- 2.Dunn J, Ott T, Clark W. Pulsed-light treatment of food and packaging. Food Technol. 1995;49:95–98. [Google Scholar]
- 3.Fairhead H, Setlow P. Binding of DNA to alpha/beta-type small, acid-soluble proteins from spores of Bacillus or Clostridium species prevents formation of cytosine dimers, cytosine-thymine dimers, and bipyrimidine photoadducts after UV irradiation. J Bacteriol. 1992;174:2874–2880. doi: 10.1128/jb.174.9.2874-2880.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Fairhead H, Setlow B, Setlow P. Prevention of DNA damage in spores and in vitro by small, acid-soluble proteins from Bacillus species. J Bacteriol. 1993;175:1367–1374. doi: 10.1128/jb.175.5.1367-1374.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irving F, McMurry T, Herbold J. Non-medical dispersed biological weapons countermeasures. Texas: Armstrong Lab, Occupational Environmental Health Directorate, Brooks Air Force Base; 1997. . Technical Report AL/OE-TR1997-0081. [Google Scholar]
- 5.Knudson G. Photoreactivation of ultraviolet-irradiated, plasmid-bearing, and plasmid-free strains of Bacillus anthracis. Appl Environ Microbiol. 1986;52:444–449. doi: 10.1128/aem.52.3.444-449.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonald K, Curry R, Clevenger T, Brazos B, Unklesbay K, Eisenstark A, Baker S, Golden J, Morgan R. The development of photosensitized pulsed and continuous ultraviolet decontamination techniques for surfaces and solutions. IEEE Trans Plasma Science. 2000;28:89–96. [Google Scholar]
- 6a.Nicholson W L, Setlow B, Setlow P. Ultraviolet irradiation of DNA complexed with alpha/beta-type small, acid-soluble proteins from spores of Bacillus or Clostridium species makes spore photoproduct but not thymine dimers. Proc Natl Acad Sci USA. 1991;88:8288–8292. doi: 10.1073/pnas.88.19.8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riesenman P J, Nicholson W L. Role of the spore coat layers in Bacillus subtilis spore resistance to hydrogen peroxide, artificial UV-C, UV-B, and solar UV radiation. Appl Environ Microbiol. 2000;66:620–626. doi: 10.1128/aem.66.2.620-626.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Setlow P. Resistance of bacterial spores to ultraviolet light. Comments Mol Cell Biophys. 1988;5:253–264. [Google Scholar]
- 9.Setlow P. I will survive: protecting and repairing spore DNA. J Bacteriol. 1992;174:2737–2741. doi: 10.1128/jb.174.9.2737-2741.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Setlow P. Mechanisms which contribute to the long-term survival of spores of Bacillus species. J Appl Bacteriol Symp Suppl. 1994;74:49s–60s. doi: 10.1111/j.1365-2672.1994.tb04357.x. [DOI] [PubMed] [Google Scholar]
- 11.Slieman T A, Nicholson W L. Artificial and solar UV radiation induces strand breaks and cyclobutane pyrimidine dimers in Bacillus subtilis spore DNA. Appl Environ Microbiol. 2000;66:199–205. doi: 10.1128/aem.66.1.199-205.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun Y B, Palasingam K, Nicholson W L. High-pressure liquid-chromatography assay for quantitatively monitoring spore photoproduct repair mediated by spore photoproduct lyase during germination of UV-irradiated Bacillus subtilis spores. Anal Biochem. 1994;221:61–65. doi: 10.1006/abio.1994.1379. [DOI] [PubMed] [Google Scholar]
- 13.Tovar-Rojo F, Setlow P. Effects of mutant small, acid-soluble spore proteins from Bacillus subtilis on DNA in vivo and in vitro. J Bacteriol. 1991;173:4827. doi: 10.1128/jb.173.15.4827-4835.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varghese A J. 5-Thyminyl-5,6-Dihydrothymine from DNA irradiated with ultraviolet light. Biochem Biophys Res Commun. 1970;38:484–490. doi: 10.1016/0006-291x(70)90739-4. [DOI] [PubMed] [Google Scholar]
- 15.Wang T-Z V, Rupert C S. Evidence for the monomerization of spore photoproduct to two thymines by the light-independent “spore repair” process in Bacillus subtilis. Photochem Photobiol. 1977;25:123–127. doi: 10.1111/j.1751-1097.1977.tb07432.x. [DOI] [PubMed] [Google Scholar]
- 16.Warriner K, Rysstad G, Murden A, Rumsby P, Thomas D, Waites W. Inactivation of Bacillus subtilis spores on packaging surfaces by U.V. excimer laser irradiation. J Appl Microbiol. 2000;88:678–685. doi: 10.1046/j.1365-2672.2000.01015.x. [DOI] [PubMed] [Google Scholar]