Abstract
This scientific commentary refers to ‘Neurovascular injury with complement activation and inflammation in COVID-19’ by Lee et al. (https://doi.org/10.1093/brain/awac151).
This scientific commentary refers to ‘Neurovascular injury with complement activation and inflammation in COVID-19’ by Lee et al. (https://doi.org/10.1093/brain/awac151).
Shortly after the pandemic began in early 2020, it became clear that SARS-CoV-2 not only impairs lung function but also affects other organs including the brain. Early studies described signs of vascular abnormalities in the brains of severely ill COVID-19 patients that were verified by immunohistology in post-mortem tissue. With increasing evidence, it is now accepted that infection with SARS-CoV-2 affects the vasculature of various organs and that these effects may contribute to organ dysfunction. However, it is still unclear exactly what happens to the brain microvessels and which mechanisms are involved. In this issue of Brain, Lee and colleagues1 shed new light on the mystery of vascular effects of SARS-CoV-2, and thereby provide an explanation for why COVID-19 includes neurological symptoms.
The authors examined post-mortem tissue of patients who died during the first wave of the pandemic in 2020 in New York. While they have described some of their findings already,2 in the current study they used additional techniques to characterize in more detail vascular and immunological features of microvessels in the brain. Compared to controls, the brain tissue of patients who died from COVID-19 showed a dramatic increase in the extravasation of serum proteins, especially fibrinogen, indicating a disturbed blood–brain barrier. In addition, the authors observed platelet accumulation in combination with augmented expression of platelet endothelial cell adhesion molecule 1 (PECAM-1), as well as increased tissue factor and von Willebrand factor, in COVID-19 patients, suggesting that activation of the coagulation system in the brain vasculature most likely leads to occlusion and damage to small vessels. This fits with the authors’ earlier findings and also with evidence of injured cerebral blood vessels obtained using MRI.2,3
Interestingly, the authors detected immune complexes using multiplex fluorescence microscopy at the vascular wall. These complexes were positive for IgG/IgM antibodies and co-localized with complement factors. Complement activation has previously been demonstrated in COVID-19,4 but not directly in the brain. The resulting formation of the membrane attack complex (MAC), consisting of several activated complement factors (C5b-9) as shown by Lee et al.1 could contribute to the endothelial cell death that was detected in other studies.5
An activated endothelium and more permeable blood–brain barrier could result in infiltration of immune cells as seen in other neurological diseases. Indeed, the authors showed data supporting the infiltration of CD3- or CD8-positive T cells and CD68-positive macrophages.1 These infiltrates were present almost exclusively in the perivascular space and not in the brain parenchyma, indicating indirect effects, if any, on cells like neurons and glia.
As others have shown before, the neuropathological abnormalities in patients with severe COVID-19 were most prominent in the hindbrain, suggesting a more susceptible vasculature or increased entry of SARS-CoV-2 in this region.6 The authors even found morphological signs of neuronophagia in the hindbrain,1 suggesting neuronal cell death and phagocytosis by microglia. Finally, they used an elaborate technique to examine spatial transcriptomics in the hindbrain, focusing on regions enriched in the endothelial marker PECAM-1 or the immune cell marker CD45, thereby generating a dataset that could be useful for future studies. Notably, the expression of several genes encoding factors involved in perfusion regulation such as eNOS or Kir2.1 was differentially regulated in control versus COVID-19 patients.1
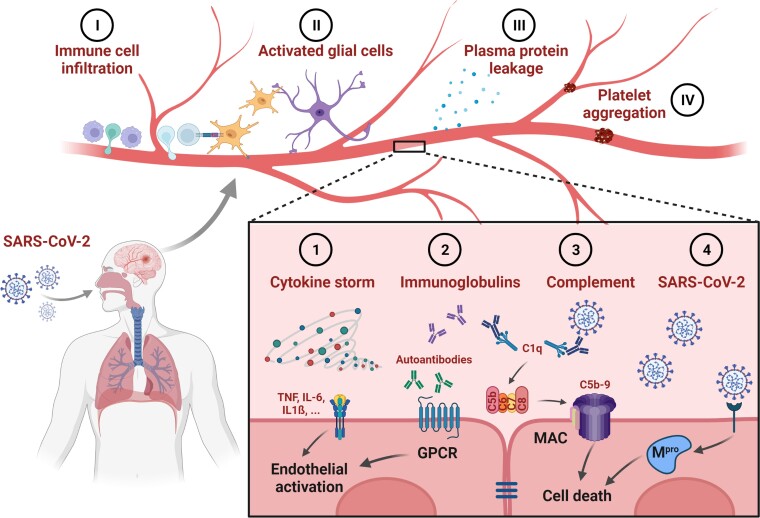
Overall, this study is an important step towards understanding what is going on in the brains of severely ill COVID-19 patients. However, the initial trigger of vascular damage remains hypothetical. According to the authors, immunoglobulin complexes stimulate the classical complement cascade, activate endothelial cells, and subsequently induce blood–brain barrier leakage and immune cell infiltration. In addition, autoantibodies were detected in the blood and CSF of severely ill COVID-19 patients,7 but also in patients suffering from post-acute sequelae of COVID-19.8 The autoantibodies were able to bind directly to vascular antigens, and may even show agonist activity.7,8 However, it is unclear whether endothelial cells in the brain are directly affected. The immunoglobulin complexes bound to the vascular wall that Lee et al.1 describe in their study may be a hint in this direction (Fig. 1).
Figure 1.
Effects of SARS-CoV-2 on brain endothelial cells. Several direct and indirect effects of SARS-CoV-2 infection on brain endothelial cells have been described and may contribute to neurological symptoms in patients suffering from COVID-19 and its sequelae. (1) Elevated levels of cytokines [e.g. tumour necrosis factor (TNF), or interleukins like IL-6 or IL-1β] activate receptors on brain endothelial cells, leading to a pro-inflammatory phenotype. In severely ill patients, a cytokine storm—dramatically increased cytokine levels—is associated with disease burden. (2) Immunoglobulins raised after SARS-CoV-2 infection can have direct effects on cells, as described for autoantibodies acting on G protein-coupled receptors (GPCR), but also indirect effects (3) as shown for the activation of the complement system. The formation of the membrane attack complex (MAC) as the ultimate effect of the classical complement cascade results in the death of the affected cell. (4) Cell death can also be induced by direct infection of endothelial cells, leading to the expression of the main protease of SARS-CoV-2 (Mpro). Consequences of cell activation or death at the endothelium include (I) infiltration of immune cells including macrophages and T cells; (II) activation of astrocytes and microglia with the latter shown to interact directly with T cells in COVID-19 patients; (III) leakage of plasma proteins such as fibrinogen as a consequence of a disturbed blood–brain barrier; and (IV) platelet aggregation causing occlusion of microvessels in the brain. Altogether, endothelial cells act as gatekeepers for the interaction of blood components with the brain parenchyma and are central to COVID-19 disease and its post-acute sequelae. Created with BioRender.com
Besides autoantibodies, the endothelium in the brain can be activated by cytokines like interleukins or TNF that are known to be released in large quantities during acute COVID-19 (Fig. 1). These cytokines act directly on endothelial cells, leading to adhesion of blood cells and opening of the blood–brain barrier with subsequent immune cell infiltration.
A third possibility for how SARS-CoV-2 could affect microvessels in the brain is via a direct effect of the virus on the endothelium (Fig. 1). The authors did not find signs of direct viral infection of brain tissue. However, other studies have detected viral particles in the brains of patients and in vascular cells. An explanation for the rare detection of SARS-CoV-2 could be the rapid induction of cell death in infected endothelial cells. Recently, it was shown that macrophages in the lung undergo cell death after SARS-CoV-2 infection, explaining the depletion of these cells in the respiratory tract.9 The death of pulmonary macrophages was secondary to an inflammasome activation leading to the release of cytokines and the induction of a hyperinflammatory state. Another interesting finding in that study was that induced cell death prevented SARS-CoV-2 replication in infected cells and therefore viral spreading. In principle, one could imagine a similar mechanism applying to endothelial cells in the brain. Viral infection and expression of SARS-CoV-2 proteins has been shown to induce cell death in the brain endothelium,5 and the same mechanisms trigger a neuroinflammatory state. As a side effect, the death of endothelial cells will activate the vasculature in the brain, leading to a leaky blood–brain barrier, adhesion of plasma proteins, and infiltration of immune cells (Fig. 1).
Examining the vasculature and its interactions with the immune system is thus proving key to understanding organ dysfunction in COVID-19. These interactions may be responsible for severe acute illness but also for the post-acute sequelae of COVID-19.10 Hypoperfusion and immune cell infiltration as well as glial activation in the brain parenchyma may trigger long-lasting neurological symptoms in COVID-19 patients. The study by Lee and colleagues1 is another hint that SARS-CoV-2 infection impairs vascular function, and provides valuable insights into the molecular players involved in the interaction between the vasculature and the immune system. Future efforts to find an effective therapy for the acute and post-acute phases of COVID-19 should consider targeting the cerebrovascular effects of the disease.
Contributor Information
Jan Wenzel, Institute for Experimental and Clinical Pharmacology and Toxicology, Center for Brain, Behavior and Metabolism, University of Lübeck, Lübeck, Germany; DZHK, German Center for Cardiovascular Research, Partner site Hamburg/Kiel/Lübeck, Lübeck, Germany.
Markus Schwaninger, Institute for Experimental and Clinical Pharmacology and Toxicology, Center for Brain, Behavior and Metabolism, University of Lübeck, Lübeck, Germany; DZHK, German Center for Cardiovascular Research, Partner site Hamburg/Kiel/Lübeck, Lübeck, Germany.
Funding
This work has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 813294 (ENTRAIN) and was supported by grants from the European Research Council (Synergy grant no. 2019-WATCH-810331 to M.S.) and the Deutsche Forschungsgemeinschaft (WE 6456/1-1 to J.W.).
Competing interests
The authors report no competing interests.
References
- 1. Lee MH, Perl DP, Steiner J, et al. . Neurovascular injury with complement activation and inflammation in COVID-19. Brain. 2022;145(7):2555–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee MH, Perl DP, Nair G, et al. . Microvascular injury in the brains of patients with COVID-19. N Engl J Med. 2021;384:481–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Conklin J, Frosch MP, Mukerji SS, et al. . Susceptibility-weighted imaging reveals cerebral microvascular injury in severe COVID-19. J Neurol Sci. 2021;421:117308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carvelli J, Demaria O, Vely F, et al. . Association of COVID-19 inflammation with activation of the c5a-c5ar1 axis. Nature. 2020;588:146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wenzel J, Lampe J, Muller-Fielitz H, et al. . The sars-cov-2 main protease m(pro) causes microvascular brain pathology by cleaving nemo in brain endothelial cells. Nat Neurosci. 2021;24:1522–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Matschke J, Lutgehetmann M, Hagel C, et al. . Neuropathology of patients with COVID-19 in germany: A post-mortem case series. Lancet Neurol. 2020;19:919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang EY, Mao T, Klein J, et al. . Diverse functional autoantibodies in patients with COVID-19. Nature. 2021;595:283–288. [DOI] [PubMed] [Google Scholar]
- 8. Wallukat G, Hohberger B, Wenzel K, et al. . Functional autoantibodies against g-protein coupled receptors in patients with persistent long-COVID-19 symptoms. J Transl Autoimmun. 2021;4:100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sefik E, Qu R, Junqueira C, et al. . Inflammasome activation in infected macrophages drives COVID-19 pathology. Nature. Published online 28 April 2022. doi: 10.1038/s41586-022-04802-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Merad M, Blish CA, Sallusto F, Iwasaki A. The immunology and immunopathology of COVID-19. Science. 2022;375:1122–1127. [DOI] [PubMed] [Google Scholar]