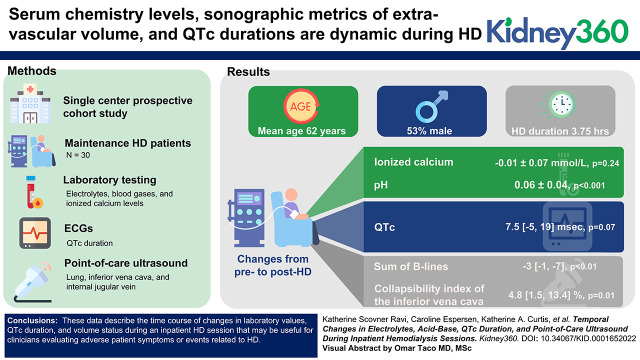
Key Points
Serum chemistry levels, sonographic metrics of extra-vascular volume, and QTc durations are dynamic during HD.
This research provides comprehensive data on the dynamic changes in physiology during the course of contemporary HD sessions.
This research illustrates methods for performing point-of-care ultrasound which may enhance volume management for HD patients in the future.
Keywords: dialysis, acid-base, ECG, electrolytes, hemodialysis, inpatients, point-of-care systems, QTc, ultrasound
Visual Abstract
Abstract
Background
Of the more than 550,000 patients receiving maintenance hemodialysis (HD) in the United States, each has an average of 1.6 admissions annually (>880,000 inpatient HD sessions). Little is known about the temporal changes in laboratory values, ECGs, and intravascular and extravascular volume during inpatient HD sessions.
Methods
In this prospective cohort study of hospitalized HD patients, we assessed intradialytic laboratory values (metabolic panels, blood gases, ionized calcium levels), ECGs, and sonographic measures of volume status.
Results
Among 30 participants undergoing HD (mean age 62 years; 53% men, 43% Black) laboratory values had the largest changes in the first hour of HD. There was no significant change in ionized calcium levels pre- to post-HD (change: –0.01±0.07, P=0.24); 12 of 30 and 17 of 30 patients had levels below the lower reference limit at the beginning and end of HD, respectively. The mean pH increased pre- to post-HD (change: 0.06±0.04, P<0.001); 21 of 30 had a pH above the upper reference limit post-HD. There was a trend toward longer median QTc duration from pre- to post-HD (change: 7.5 msec [–5 msec, 19 msec], P=0.07). The sum of B lines on lung ultrasound decreased from pre- to post-HD (median decrease: 3 [1, 7], P<0.01). The collapsibility index of the inferior vena cava increased pre- to post-HD (median increase: 4.8% [1.5%, 13.4%], P=0.01), whereas internal jugular vein diameter did not change (P=0.24).
Conclusions
Among hospitalized patients undergoing HD, we found dynamic changes in laboratory values, QTc duration, and volume status. Further research is required to assess whether HD prescriptions can be tailored to alter these variations to potentially improve patient outcomes.
Introduction
Of the more than 550,000 patients receiving maintenance hemodialysis (HD) in the United States (1), each has an average of 1.6 admissions per year (>880,000 inpatient HD sessions) (2). Despite in-hospital monitoring, little is known about the temporal changes in laboratory values, electrocardiograms (ECGs), and intravascular and extravascular volume status during inpatient HD sessions. The risk of complications during HD may be increased in the hospital setting, where patients often face acute illness that may compromise their hemodynamic stability. Understanding expected changes in electrolytes, pH, cardiac rhythms, and volume status may help the clinician respond more readily when complications such as intradialytic hypotension and arrhythmia occur during HD sessions. For example, both lower serum calcium and potassium levels are associated with longer QTc durations (3). Further, alkalemia promotes intracellular shift of serum potassium (4) and also leads to a change in the electrical charge of proteins, which promotes protein-to-calcium complex formation and results in lower ionized calcium concentrations (5); thus, alkalemia may also contribute to QTc prolongation. QTc prolongation represents an important biomarker because it is associated with ventricular arrhythmia (6) and bradyarrhythmia in patients with CKD (7) and is an independent predictor of sudden cardiac death (8) and mortality (9) in patients with ESKD.
Intradialytic hypotension affects 15%–50% of outpatient HD sessions and may be even more prevalent in the inpatient setting where acute illness may affect hemodynamic stability (10, 11). Point-of-care ultrasound provides a unique approach to assess volume shifts in both the extravascular and intravascular spaces. Knowledge of the timing of these shifts in the setting of dialysis may provide the clinician with improved understanding of the etiology of intradialytic hypotension when it occurs in their patients so that they may more readily respond. In the present study, lung ultrasound to assess for pulmonary congestion is used as a proxy for extravascular volume, and the internal jugular vein and inferior vena cava are used as markers of intravascular volume.
The aim of this work is to provide a comprehensive investigation of intradialytic changes with respect to laboratory markers, ECG findings, and point-of-care ultrasound measures of volume status in a cohort of maintenance HD inpatients.
Materials and Methods
Study Population
Patients admitted to Brigham and Women’s Hospital in Boston, Massachusetts, from September 2019 through February 2021 were screened for inclusion. Thirty ESKD maintenance HD patients were included. There was a 6-month pause in recruitment after 10 patients were enrolled due to the coronavirus disease 2019 pandemic. Patients were on floor level of care, had been on HD for >90 days, were ≥18 years of age, were on thrice-weekly HD with sessions at least 3 hours long, and had capacity to provide informed consent. Patients requiring vasopressor therapy other than midodrine were excluded. Patients with atrial fibrillation, supraventricular tachycardia, and left bundle branch block were excluded because these rhythm abnormalities may compromise the ability to quantify QTc durations. To prevent complications from potential anemia caused by blood draws, patients with acute coronary syndrome or stroke, or the possibility of these complications in the preceding 7 days were excluded, as were patients with hematocrit levels <24% and patients with active bleeding. Given the associated changes in hemodynamics, pregnant patients were also excluded. Patients with active coronavirus disease 2019 infection were excluded. In addition, patients with lung transplant (n=2) were excluded from the lung ultrasound image analysis because prior lung surgery could have affected lung ultrasound findings irrespective of volume status. As pneumonia and interstitial lung disease could affect B-line number (12), we performed a sensitivity analysis that additionally excluded patients (n=1) with these conditions to assess whether removing this subgroup affected B-line changes during HD.
Study Design
In this prospective cohort study, blood laboratory testing (electrolytes, blood gases, and ionized calcium levels) and ECGs were obtained pre, 1 hour into, and post-HD in a single HD session per participant. Patients underwent point-of-care ultrasound examinations for the assessment of volume status at several time points: lung and inferior vena cava ultrasound were obtained pre, post, and 1 hour post-HD, and internal jugular vein imaging was obtained pre and post-HD. HD prescriptions and ultrafiltration volumes were collected from the medical records. Dialysate prescriptions were not dictated by protocol but rather as deemed clinically indicated by the patient’s inpatient nephrology team.
Laboratory Analyses
Blood gas samples were transported on ice immediately to the hospital’s central laboratory for processing. As blood was collected via the HD machine, blood gas samples were considered central venous when HD was performed through internal jugular access (13) and arterial when arteriovenous access was used (14). At each time point, 6 ml of blood was collected in a lithium heparin tube with gel separator for complete metabolic panels, and 3 ml of blood was collected in a Luer lock syringe for blood gas samples. The lithium heparin tubes were placed on ice and processed at the completion of HD sessions. Blood was centrifuged at 1700g for 15 minutes at 4°C. Plasma supernatant was extracted and pipetted into 0.5 ml aliquots. Samples were stored immediately in a –80°C freezer. At the end of the study, all electrolytes and laboratory measurements, aside from blood gases and ionized calcium levels, which were immediately processed onsite, were batch-processed at Quest Diagnostics using standard techniques.
ECG Analyses
QTc durations were adjudicated by two board-certified cardiologists. Reviewers were blinded to clinical information and imaging time point.
Ultrasound Image Acquisition and Analyses
Images were obtained using a Philips Lumify Ultrasound and were analyzed offline by a core imaging laboratory. Six-second clips were obtained for all images. All ultrasound videos were analyzed offline by a single investigator blinded to clinical data and measurement time point.
Lung ultrasound.
Images were acquired in eight chest zones with patients in the supine position, using a phased array transducer (4–1 MHz) in sagittal orientation as described previously (12). For B-line quantification, the highest number of B lines seen at any time during the clip in a single intercostal space was the number of B lines assigned to the image (12). As B-line count quantified in eight lung zones are highly related to 28 zones (Spearman’s ρ=0.93, P<0.001) and are equivalently predictive of cardiovascular events and mortality in HD patients (15), this more feasible method was used in our hospitalized population, consistent with other studies by our group and others (16–18). Zones 1, 2, 5, and 6 captured anterior lung fields, and zones 3, 4, 7, and 8 captured lateral lung fields. The sum of B lines in eight lung zones was used as the B-line count for each patient.
Pleural effusions.
The presence of pleural effusions was assessed with patients in the supine position, using the same transducer as for lung ultrasound images. Pleural effusions were assessed laterally on each hemithorax as described previously (19). For the semiquantitative assessment of pleural effusion size, a score was applied to each ultrasound clip (0, no pleural effusion visible; 1, pleural effusion visible in the costophrenic angle only; 2, pleural effusion extends over the costophrenic angle, no clear separation of the lung base from the diaphragm; 3, clear separation between the diaphragm and lung base at all points during the respiratory cycle; and 4, pleural effusion encompasses >50% of the basal pleural cavity) as described previously (19). Pleural effusion fluid burden was estimated for each person at each time point using the sum of pleural effusion scores from bilateral hemi-thoraces (score range: 0–8).
Inferior vena cava ultrasound.
The inferior vena cava was imaged with patients in the supine position, using the transthoracic echocardiographic subcostal view. It was visualized in the sagittal plane just proximal to the junction of the hepatic veins that lie 0.5–3 cm proximal to the ostium of the right atrium. Maximum and minimum diameters were measured from inner-to-inner edge at nonforced end-expiratory and end-inspiratory phases. The collapsibility index was calculated as (end-expiratory diameter–end-inspiratory diameter)/end-expiratory diameter×100 (20, 21).
Internal jugular vein ultrasound.
Images were acquired with a linear transducer (12–4 MHz) with patients sitting at 45° breathing normally. The right internal jugular vein diameter and area were assessed with the transducer held in transverse orientation and measured with the patient’s head turned to the left at two points: just below the jaw and at the level of the clavicle. The largest diameter in the anterior-to-posterior direction from inner-to-inner edge and largest cross-sectional area with the contour traced along the luminal side of the vessel during the clip were measured as the measurements corresponding to end expiration during normal respiration. Similar methods have been described previously (22–25).
Statistical Analyses
Summary statistics were performed using standard approaches according to data distribution. Changes in parameters were estimated by subtracting pre-HD values from post-HD values. Estimates of significant change between time points were performed using the paired t test for normally distributed outcomes and using the Wilcoxon signed-rank test for non-normally distributed outcomes. The intrareader agreement for the total number of B lines across all eight lung zones was tested in 15 patients using Bland–Altman analysis. Similarly, the intrareader agreement for the area and diameter of the internal jugular vein under the jaw and at the level of the clavicle during normal respiration was tested in 15 patients using Bland–Altman analysis. Two-tailed P values <0.05 were considered statistically significant without consideration of multiple testing. Missing data were not imputed. Analyses were completed using Stata v16.1 (StataCorp, College Station, TX).
Ethics
The study protocol was reviewed and approved by the Mass General Brigham Institutional Review Board (approval number: 2019P000727), which oversaw all study procedures. All participants provided written informed consent.
Results
Baseline Characteristics
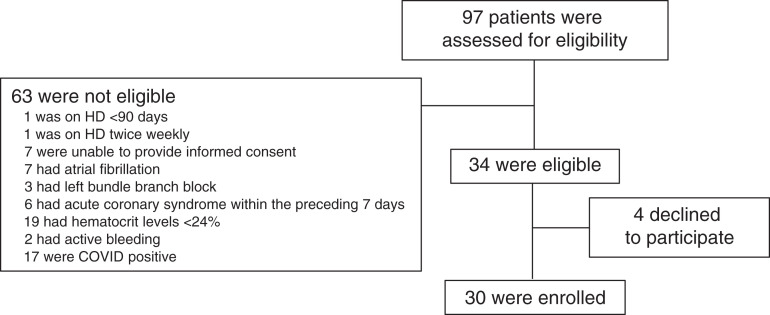
During the recruitment period, 63 patients on the floor level of care HD inpatient list did not meet the inclusion criteria. Thirty-four patients were approached, and 30 of these consented and ultimately participated in this study (see enrollment flow chart shown in Figure 1). The mean age of participants was 62±12 years; 53% were men; 43% were Black, and 13% were Hispanic. A total of 53% of participants had diabetes mellitus, 60% had history of heart failure, 53% had history of ischemic heart disease, and 53% had obstructive lung disease (chronic obstructive pulmonary disease, asthma, or obstructive sleep apnea; Table 1).
Figure 1.
Enrollment flow chart.
Table 1.
Characteristics of the participants
| Baseline Characteristics | All subjects (N=30) |
|---|---|
| Age, yr | 62±12 |
| Men, n (%) | 16 (53) |
| Race and ethnicity, n (%) | |
| White | 12 (40) |
| Black | 13 (43) |
| Other/unknown | 5 (17) |
| Hispanic | 4 (13) |
| Body mass index, kg/m2 | 28±7 |
| ESKD vintage, yr | 2.6 [1.1, 5.2] |
| Vascular access, n (%) | |
| AV fistula | 13 (43) |
| AV graft | 7 (23) |
| Catheter | 10 (33) |
| Comorbid conditions, n (%) | |
| Diabetes mellitus | 16 (53) |
| Ischemic heart disease | 16 (53) |
| Heart failure | 18 (60) |
| Stroke | 2 (7) |
| Peripheral vascular disease | 10 (33) |
| Obstructive lung disease | 16 (53) |
| Chronic obstructive lung disease | 8 (27) |
| Asthma | 6 (20) |
| Obstructive sleep apnea | 9 (30) |
| Pre-HD systolic blood pressure, mm Hg | 137±25 |
| Medication use, n (%) | |
| Statin | 18 (60) |
| ACEI or ARB | 3 (10) |
| β blockers | 17 (57) |
| Calcium channel blockers | 9 (30) |
| Loop diuretic | 0 (0) |
| Pre-HD QTc, msec | 468 [455, 486] |
| Admission for volume overload, n (%)a | 6 (2) |
| Number of Days Admitted on Study Date | 4 [3, 6] |
Continuous variables are presented as means±SD or median [25th, 75th percentiles]. Obstructive lung disease was classified as any of the following: chronic obstructive lung disease, asthma, or obstructive sleep apnea. AV, arteriovenous; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Volume overload was defined as “fluid overload, pleural effusion, heart failure, or pulmonary edema” as has been defined in a prior validation study (37).
HD Treatment Characteristics
The median duration of HD was 3.75 hours [3.5 hours, 4 hours] with a mean single-pool Kt/V of 1.7±0.3 and net ultrafiltration volume of 2.1±1.1 L. Thirteen patients were dialyzed against a 2 mEq/L potassium bath, 13 were dialyzed against a 3 mEq/L potassium bath, and four were dialyzed against a 4 mEq/L potassium bath. Twenty-eight were dialyzed on a 2.5 mEq/L calcium bath; one was on a 2 mEq/L calcium bath, and one was on a 3 mEq/L calcium bath. All patients were on a bicarbonate bath of 35 mEq/L (Table 2).
Table 2.
Characteristics of the dialysis prescription
| Baseline Characteristics | All Subjects (N=66) |
|---|---|
| Duration of hemodialysis, h | 3.75 [3.5, 4] |
| Session of week, n (%) | |
| 1st | 11 (37) |
| 2nd | 8 (27) |
| 3rd | 11 (37) |
| spKt/V | 1.7±0.3 |
| Predialysis weight, kg | 81±21 |
| kg over dry weight target before dialysis | 1.6 [0, 3.9] |
| Net ultrafiltration, L | 2.1±1.1 |
| Dialysate flow, ml/min | 750 [700, 800] |
| Blood flow, ml/min | 350 [350, 400] |
| Dialysate potassium, mEq/L, n (%) | |
| 2 | 13 (43) |
| 3 | 13 (43) |
| 4 | 4 (13) |
| Dialysate calcium, mEq/L, n (%) | |
| 2 | 1 (3) |
| 2.5 | 28 (93) |
| 3 | 1 (3) |
| Dialysate sodium, mEq/L, n (%) | |
| 140 | 30 (100) |
| Dialysate bicarbonate, mEq/L, n (%) | |
| 35 | 30 (100) |
Continuous variables are presented as means±SD or median [25th, 75th percentiles]. One patient was initially on a 3 K bath but was changed to a 4 K bath 60 minutes into her 4-hour-long HD session; this patient was included as a 4 K bath for our analyses. spKt/V, single-pool Kt/V where K=dialyzer clearance of urea, t=dialysis time, and V=volume of distribution of urea.
Laboratory Values
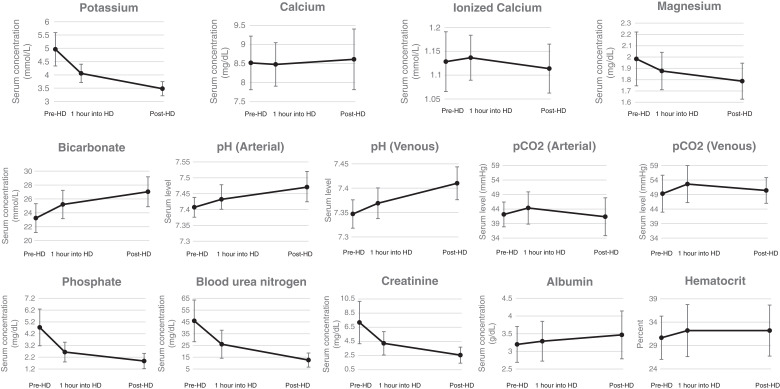
Plasma chemistry levels were dynamic, with the most profound changes occurring within the first hour for all biomarkers (shown in Figure 2). Pre-HD, 1 of 30 patients had a pH lower than the reference range (this patient had comorbid chronic obstructive pulmonary disease and obstructive sleep apnea), and 1 of 30 patients had a pH above the reference range (this patient had asthma). One hour into HD, 5 of 29 had a pH above the reference range, and one patient had a pH below the reference range. By the end of HD, 21 of 30 patients had a pH above the reference range, and none had a pH below the reference range. During HD, serum bicarbonate increased (mean increase 4±3 mEq/L, P<0.001), whereas pCO2 levels did not change (P=0.82). Nine of 30 patients were hyperkalemic (>5.3 mmol/L) pre-HD, whereas 15 of 30 patients were hypokalemic (<3.5 mmol/L) post-HD. Calcium and ionized calcium levels did not change from pre- to post-HD (P=0.56 and 0.24, respectively). Calcium levels also did not change when the two patients on dialysate calcium baths other than 2.5 mEq/L were excluded (P=0.42), but there was a drop in ionized calcium levels when patients only on 2.5 mEq/L dialysate calcium baths were assessed (decrease of 0.02±0.05 mmol/L, P=0.04). Full results of laboratory parameters at the pre, 1 hour into, and post-HD time points are shown in Tables 3–5, respectively.
Figure 2.
Intradialytic serum laboratory levels.
Table 3.
Electrolyte and laboratory values pre-HD
| Laboratory Parameter | Pre-HD Value, Mean±SD, n Subjects | Lower Limit of Normal | Frequency of Pre-HD Concentrations below the Lower Limit of Normal, n (%) | Upper Limit of Normal | Frequency of Pre-HD Concentrations above the Upper Limit of Normal, n (%) |
|---|---|---|---|---|---|
| Potassium, mEq/L | 5±0.6, 30 | <3.5 | 0 (0) | >5.3 | 9 (30) |
| Bicarbonate, mEq/L | 23±2, 30 | <20 | 2 (7) | >32 | 0 (0) |
| pH (arterial) | 7.41±0.03, 20 | <7.35 | 0 (0) | >7.45 | 1 (5) |
| Carbon dioxide (arterial), mm Hg | 42±4, 20 | <36 | 1 (5) | >47 | 4 (20) |
| pH (central venous) | 7.35±0.03, 10 | <7.3 | 1 (10) | >7.4 | 0 (0) |
| Carbon dioxide (central venous), mm Hg | 49±6, 10 | <38 | 0 (0) | >50 | 3 (30) |
| Calcium, mg/dl | 8.5±0.7, 30 | <8.6 | 16 (53) | >10.3 | 0 (0) |
| Ionized calcium, mmol/L | 1.13±0.06, 30 | <1.13 | 12 (40) | >1.32 | 0 (0) |
| Magnesium, mg/dl | 2±0.2, 30 | <1.5 | 0 (0) | >2.5 | 1 (3) |
| Phosphate, mg/dl | 4.7±1.6, 30 | <2.5 | 2 (7) | >4.5 | 16 (53) |
| BUN, mg/dl | 46±18, 30 | <7 | 0 (0) | >25 | 26 (87) |
| Creatinine, mg/dl | 7.1±3, 30 | <0.6 | 0 (0) | >1.35 | 30 (100) |
| Albumin, g/dl | 3.2±0.5, 30 | <3.6 | 23 (77) | >5.1 | 0 (0) |
| HCT, % | 31±5, 30 | <36 | 25 (83) | >48 | 0 (0) |
Percent of sessions with a value above or below the limits of normal are calculated as Nmeasurement outside of limits of normal/Ntotal measurements. pH and pCO2 were arterial if drawn from arteriovenous access and central venous if drawn from a central line (13, 14). HCT, hematocrit; HD, hemodialysis.
Table 5.
Electrolyte and laboratory values pre and immediately post-HD
| Laboratory Parameter | Pre-HD Value, Mean±SD, n Subjects | Post-HD Value, Mean±SD, N Subjects | Δ (Post-HD minus Pre-HD), Mean±SD, n Subjects | P Value (Post- versus Pre-HD) | Lower Limit of Normal | Frequency of Post-HD Concentrations below the Lower Limit of Normal, n Subjects | Upper Limit of Normal | Frequency of Post-HD Concentrations above the Upper Limit of Normal, n Subjects |
|---|---|---|---|---|---|---|---|---|
| Potassium, mEq/L | 5±0.6, 30 | 3.5±0.3, 30 | −1.5±0.7, 30 | <0.001 | <3.5 | 15 (50) | >5.3 | 0 (0) |
| Bicarbonate, mEq/L | 23±2, 30 | 27±2, 30 | 4±3, 30 | <0.001 | <20 | 0 (0) | >32 | 0 (0) |
| pH (arterial) | 7.41±0.03, 20 | 7.47±0.05, 20 | 0.06±0.04, 20 | <0.001 | <7.35 | 0 (0) | >7.45 | 14 (70) |
| Carbon dioxide (arterial), mm Hg | 42±4, 20 | 41±7, 20 | −1±4, 20 | 0.38 | <36 | 3 (15) | >47 | 4 (20) |
| pH (central venous) | 7.35±0.03, 10 | 7.41±0.03, 10 | 0.06±0.03, 10 | <0.001 | <7.3 | 0 (0) | >7.4 | 7 (70) |
| Carbon dioxide (central venous), mm Hg | 49±6, 10 | 50±4, 10 | 1±3, 10 | 0.34 | <38 | 0 (0) | >50 | 4 (40) |
| Calcium, mg/dl | 8.5±0.7, 30 | 8.6±0.8, 30 | 0.1±0.9, 30 | 0.56 | <8.6 | 12 (40) | >10.3 | 0 (0) |
| Ionized calcium, mmol/L | 1.13±0.06, 30 | 1.11±0.05, 29 | −0.01±0.07, 29 | 0.24 | <1.13 | 17 (59) | >1.32 | 0 (0) |
| Magnesium, mg/dl | 2±0.2, 30 | 1.8±0.2, 30 | −0.2±0.2, 30 | <0.001 | <1.5 | 1 (3) | >2.5 | 0 (0) |
| Phosphate, mg/dl | 4.7±1.6, 30 | 1.9±0.6, 30 | −2.9±1.2, 30 | <0.001 | <2.5 | 26 (87) | >4.5 | 0 (0) |
| BUN, mg/dl | 46±18, 30 | 13±6, 30 | −33±14, 30 | <0.001 | <7 | 6 (20) | >25 | 1 (3) |
| Creatinine, mg/dl | 7.1±3, 30 | 2.5±1.1, 30 | −4.6±2.2, 30 | <0.001 | <0.6 | 0 (0) | >1.35 | 24 (80) |
| Albumin, g/dl | 3.2±0.5, 30 | 3.5±0.7, 30 | 0.3±0.3, 30 | <0.001 | <3.6 | 18 (60) | >5.1 | 0 (0) |
| HCT, % | 31±5, 30 | 32±5, 30 | 2±5, 30 | 0.1 | <36 | 24 (80) | >48 | 1 (3) |
Mean differences (Δ) were calculated using data from subjects who had both pre and post measurement for each given parameter. Percent of sessions with a value above or below the limits of normal are calculated as Nmeasurement outside of limits of normal/Ntotal measurements. Two participants had phosphate levels <1 post-HD; for calculation of the intra-HD change and post-HD mean, post-HD phosphate was replaced with 1 mg/dL in these individuals. pH and pCO2 were arterial if drawn from arteriovenous access and central venous if drawn from a central line (13, 14). HCT, hematocrit; HD, hemodialysis.
Table 4.
Electrolyte and laboratory values pre- and 1 hour into HD
| Laboratory Parameter | Pre-HD Value, Mean±SD, n Subjects | 1 Hour into HD Value, mean±SD, n Subjects | Δ (1 Hour into HD minus Pre-HD), Mean±SD, n Subjects | P Value (1 Hour into versus Pre-HD) | Lower Limit of Normal | Frequency of 1 Hour into HD Concentrations Below the Lower Limit of Normal, n (%) | Upper Limit of Normal | Frequency of 1 Hour into HD Concentrations above the Upper Limit of Normal, n (%) |
|---|---|---|---|---|---|---|---|---|
| Potassium, mEq/L | 5±0.6, 30 | 4.1±0.3, 30 | −0.9±0.4, 30 | <0.001 | <3.5 | 0 (0) | >5.3 | 0 (0) |
| Bicarbonate, mEq/L | 23±2, 30 | 25±2, 30 | 2±3, 30 | <0.001 | <20 | 0 (0) | >32 | 0 (0) |
| pH (arterial) | 7.41±0.03, 20 | 7.43±0.05, 19 | 0.02±0.03, 19 | <0.01 | <7.35 | 1 (5) | >7.45 | 5 (26) |
| Carbon dioxide (arterial), mm Hg | 42±4, 20 | 44±6, 19 | 2±4, 19 | 0.03 | <36 | 1 (5) | >47 | 5 (26) |
| pH (central venous) | 7.35±0.03, 10 | 7.37±0.03, 10 | 0.02±0.02, 10 | 0.01 | <7.3 | 0 (0) | >7.4 | 0 (0) |
| Carbon dioxide (central venous), mm Hg | 49±6, 10 | 53±6, 10 | 3±3, 10 | <0.01 | <38 | 0 (0) | >50 | 6 (60) |
| Calcium, mg/dl | 8.5±0.7, 30 | 8.5±0.6, 30 | 0±0.5, 30 | 0.67 | <8.6 | 15 (50) | >10.3 | 0 (0) |
| Ionized calcium, mmol/L | 1.13±0.06, 30 | 1.14±0.05, 30 | 0.01±0.06, 30 | 0.43 | <1.13 | 12 (40) | >1.32 | 0 (0) |
| Magnesium, mg/dl | 2±0.2, 30 | 1.9±0.2, 30 | −0.1±0.1, 30 | <0.001 | <1.5 | 0 (0) | >2.5 | 0 (0) |
| Phosphate, mg/dl | 4.7±1.6, 30 | 2.6±0.8, 30 | −2.1±1, 30 | <0.001 | <2.5 | 13 (43) | >4.5 | 1 (3) |
| BUN, mg/dl | 46±18, 30 | 26±12, 30 | −20±8, 30 | <0.001 | <7 | 1 (3) | >25 | 16 (53) |
| Creatinine, mg/dl | 7.1±3, 30 | 4.2±1.7, 30 | −2.9±1.6, 30 | <0.001 | <0.6 | 0 (0) | >1.35 | 30 (100) |
| Albumin, g/dl | 3.2±0.5, 30 | 3.3±0.6, 30 | 0.1±0.2, 30 | <0.01 | <3.6 | 21 (70) | >5.1 | 0 (0) |
| HCT, % | 31±5, 30 | 32±6, 29 | 2±6, 29 | 0.13 | <36 | 22 (76) | >48 | 0 (0) |
Mean differences (Δ) were calculated using data from subjects who had both pre and post measurement for each given value. Percent of sessions with a value above or below the limits of normal are calculated as Nmeasurement outside of limits of normal/Ntotal measurements. pH and pCO2 were arterial if drawn from arteriovenous access and central venous if drawn from a central line (13, 14). HCT, hematocrit; HD, hemodialysis.
QTc Durations
The median pre-HD QTc was 468 msec [455 msec, 486 msec], the median QTc duration 1 hour into HD was 475 msec [460 msec, 501 msec], and the median post-HD QTc was 480 msec [467 msec, 502 msec]. QTc durations were >500 msec in 6 of 30 patients pre-HD, 8 of 30 patients 1 hour into HD, and 8 of 30 patients post-HD. The median increase in QTc duration on ECG (post-HD QTc minus pre-HD QTc) was 7.5 msec [–5 msec, 19 msec].
Volume Status
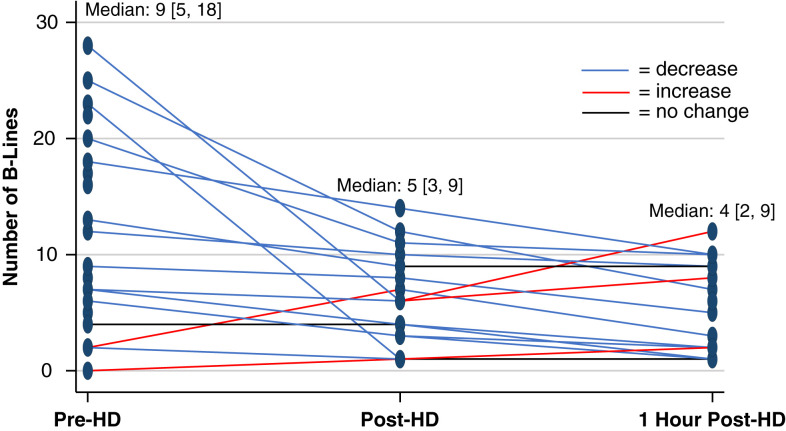
The median sum of B lines on lung ultrasound in eight zones was nine [5, 18] pre-HD, five [3, 9] post-HD, and four [2, 9] 1 hour post-HD (Figure 3, Table 6). The sum of B lines in eight zones decreased from pre- to post-HD (median decrease: 3 [1, 7], P<0.01) and from pre to 1 hour post-HD (median decrease: 4 [0, 8], P=0.02). The sum of B lines in eight zones also showed a trend toward continued decrease from post-HD to 1 hour post-HD (median decrease: 2 [0, 3], P=0.05). Pre-HD, 17 of 28 individuals had at least one lung zone with three or more B lines. Post-HD, 11 of 24 individuals had at least one lung zone with three or more B lines. One hour post-HD, 7 of 22 individuals had at least one lung zone with three or more B lines (Table 6). In sensitivity analyses in which one patient was excluded for having pneumonia, B lines still decreased from pre- to post-HD (median decrease: 2 [1, 4], P=0.01) and from pre to 1 hour post-HD (median decrease: 4 [0, 8], P=0.02).
Figure 3.
Spaghetti plot demonstrating individual changes in B lines over the course of hemodialysis.
Table 6.
Trajectory of ultrasound measures during the course of HD
| Ultrasound Category | Pre-HD Median [25th, 75th Percentiles], n Subjects | Post-HD Median [25th, 75th Percentiles], n Subjects | 1 Hour Post-HD Median [25th, 75th percentiles], n Subjects | P Value Difference Pre- to Post-HD |
|---|---|---|---|---|
| Lung | ||||
| B-line count in eight lung zones | 9 [5, 18], 23 | 5 [3, 9], 18 | 4 [2, 9], 18 | <0.01 |
| Pleural effusion score | 0 [0, 0], 18 | 0 [0, 0.5], 16 | 0 [0, 0], 17 | 1 |
| Inferior vena cava | ||||
| Diameter at end-expiration, cm | 2 [1.5, 2.3], 21 | 1.8 [1.4, 2], 15 | 2.1 [1.8, 2.2], 14 | 0.27 |
| Diameter at end inspiration, cm | 1.6 [1, 1.8] 20 | 1.4 [0.8, 1.6], 15 | 1.5 [1.1, 1.8], 12 | <0.01 |
| Inferior vena cava collapsibility index, % | 24.7 [15.6, 32.5], 20 | 26.2 [16.6, 38.5], 15 | 23.1 [16.3, 33.3], 12 | 0.01 |
| Internal jugular vein | ||||
| Diameter level of clavicle, cm | 1 [0.7, 1.3], 27 | 0.9 [0.8, 1.3], 23 | 0.24 | |
| Diameter below jaw, cm | 0.7 [0.4, 1], 26 | 0.7 [0.4, 0.9], 21 | 0.67 | |
| Area level of clavicle, cm2 | 1 [0.6, 1.4], 26 | 0.9 [0.6, 1.5], 23 | 0.37 | |
| Area below jaw, cm2 | 0.6 [0.2, 1], 25 | 0.4 [0.3, 0.8], 21 | 0.26 | |
Pleural effusions were rare at any point during HD (median scores: pre-HD 0 [0, 0], post-HD 0 [0, 0.5], and 1 hour post-HD 0 [0, 0]), and the median pleural effusion score did not change from pre- to post-HD (P=1; Table 6).
Although the median end-expiratory inferior vena cava diameter did not change from pre- to post-HD (n=13, P=0.27), the median end-inspiratory diameter decreased (n=13, median decrease: 0.2 cm [0.0 cm, 0.3 cm], P<0.01), and the inferior vena cava collapsibility index increased (n=13, median increase: 4.8% [1.5%, 13.4%], P=0.01). The collapsibility index trended toward being higher post-HD compared with 1 hour post-HD (n=10, median decrease: 1 hour post compared with post-HD 10.5% [–2.2%, 20.2%], P=0.08). The collapsibility index pre compared with 1 hour post-HD was unchanged (N=9, P=0.5; Table 6).
The internal jugular vein diameter did not change from pre- to post-HD just below the jaw (P=0.67) or at the level of the clavicle (P=0.24). Similarly, the area did not change from pre- to post-HD just below the jaw (P=0.26) or at the level of the clavicle (P=0.37; Table 6; example images shown in Figure 4). Results were unchanged when patients with right internal jugular dialysis catheters were excluded (data not shown).
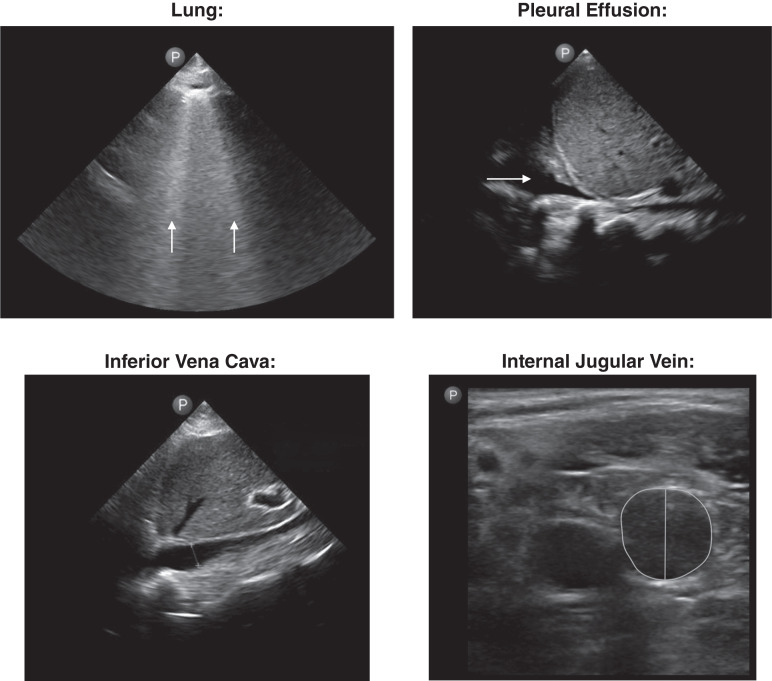
Figure 4.
Example lung, diaphragm, inferior vena cava, and internal jugular vein images obtained by the nephrologist investigator for this study. Arrows indicate B lines on the lung image and a pleural effusion on the pleural effusion image. The diameter is labeled on the image of the inferior vena cava, and the diameter and area are labeled on the image of the internal jugular vein.
With regard to the intrareader agreement analysis, the mean B-line difference for the total number of B lines in 15 patients was 0.67 (95% CI, –3.63 to 4.96; Spearman’s ρ=0.98; P<0.001). Moreover, the mean difference in area and diameter of the internal jugular vein under the jaw during normal respiration in 15 patients was –0.012 cm2 (95% CI, –0.18 to 0.20 cm2) and –0.036 cm (95% CI, –0.23 to 0.15 cm), respectively (Spearman’s ρ=0.93 [P<0.001] and 0.92 [P<0.001], respectively). The mean difference in area and diameter of the internal jugular vein at the level of the clavicle during normal respiration in 15 patients was –0.008 cm2 (95% CI, –0.11 to 0.13 cm2) and –0.006 cm (95% CI, –0.08 to 0.07 cm), respectively (Spearman’s ρ=0.98 [P<0.001] and 1 [P<0.001], respectively).
In this comprehensive physiology study of individuals receiving inpatient HD, marked changes in electrolytes, acid-base, electrocardiography, and sonographic measures of volume status are described. Significant proportions of patients finish HD with electrolyte abnormalities and with evidence of pulmonary congestion, despite having just undergone ultrafiltration.
One of the novel aspects of our study is that it includes data on serum laboratory parameters 1 hour into HD and post-HD. These time points have rarely been studied in research settings, are infrequently obtained in clinical practice, and add to our prior data describing rebound changes in electrolytes in the hours just post-HD (26). Further research is required to determine how changes in laboratory parameters during and just at the end of HD may relate to patient symptoms and outcomes.
The frequency of abnormal acid-base status during HD is noteworthy. One patient presented to HD with acidosis and one with alkalosis, respectively, whereas more than two thirds of participants had alkalosis post-HD. Concomitantly, serum bicarbonate increased during HD, whereas pCO2 did not change. Predialysis acidosis in HD patients is associated with catabolism of protein consumed as part of a normal diet, whereas relative alkalosis in this population may be a marker of inadequate nutrition (27). Alkalosis (pre-HD pH ≥7.40 compared with 7.30–7.34) was associated with higher risk of all-cause and cardiovascular mortality in 15,132 outpatients observed in Japan (28). Especially in the setting of acute illness, poor nutrition and associated alkalosis may be a concern. For hospitalized patients, acid-base physiology is a highly relevant issue affected not only by nutrition, but also by pathologic acid production (e.g., lactic acidosis), HD prescriptions, and other factors in the setting of acute illness. Patients in the inpatient setting may have a greater tendency to be undernourished, with less acid generation between HD sessions and subsequently higher pre-HD serum bicarbonate, compared with stable outpatients. The post-HD alkalosis appears to be mostly driven by the bicarbonate load delivered during HD. Further research is required to determine optimal pH balance, whether dialysate bicarbonate prescriptions can be personalized to reach this optimal balance, and whether these manipulations can improve outcomes in the inpatient setting.
Ionized calcium concentrations, which provide information about free calcium levels available to cardiac myocytes that facilitate normal cardiac conduction, are rarely measured in clinical practice. Rather, serum calcium, which includes calcium bound to protein, is typically measured. In the present study, it appears that calcium, measured as total or ionized serum calcium levels, remains relatively stable during HD, and is low in about half of these patients throughout the HD session. Due to concerns for vascular calcification, dialysate calcium prescriptions have decreased in recent years (29). Prior research suggests that a dialysate calcium of 2.75 mEq/L may lead to a net zero intradialytic calcium balance (29), and the lower dialysate calcium prescriptions seen in this study could be contributing to the low ionized calcium levels observed in these patients. The long-term risks for vascular calcification may be decreased by lower calcium baths; however, given that the mortality rate of ESKD patients is very high at about 15%–20% per year (30), further studies should assess whether this long-term benefit outweighs the short-term risk for increased arrhythmia and hypotension associated with lower dialysate calcium concentrations in the maintenance HD patient population (31, 32). Although changing dialysate concentrations appropriately may be limited in the inpatient setting by short times between morning blood draws and HD sessions, research on this is especially indicated in the hospital setting where patients may face compromise to their hemodynamics and cardiac electrophysiology in the setting of acute illness that may place them at even higher risk for arrhythmia.
In our cohort, QTc durations were >500 msec in a substantial proportion of patients. QTc prolongation is associated with arrhythmia in patients with CKD (6, 7) and is an independent predictor of sudden cardiac death (8) and mortality (9) in patients with ESKD. Lower serum calcium and potassium levels are associated with longer QTc durations (3). Further, alkalosis, which we noted occurred frequently, drives potassium intracellularly and increases calcium-to-protein binding, thus also decreasing these electrolyte levels (4, 5). This may partially explain prior observations of QTc prolongation during HD with higher dialysate bicarbonate levels (33). Although dialysate potassium prescriptions were commonly adjusted in response to pre-HD serum concentrations in this study, dialysate bicarbonate and calcium baths had little variation. Given that changing dialysate concentrations is simple and cost neutral and that higher dialysate bicarbonate and lower dialysate calcium are associated with greater intra-HD QTc prolongation (as are lower potassium baths) (33), robust clinical trials are urgently needed to delineate how HD prescriptions may contribute to QTc prolongation and dysrhythmia in the peri-HD setting and whether dialysate prescriptions with personalized bicarbonate and calcium baths may prevent dysrhythmia from occurring.
As expected, volume status was also dynamic during HD sessions. Many patients had sonographic evidence of pulmonary congestion and continued to have evidence of this post-HD. There was a decrease in the sum of pulmonary B lines in eight lung zones in post compared with pre-HD, suggesting that extravascular volume in the lungs is removed during HD, as described previously (34). However, many patients continued to demonstrate at least three B lines in one or more lung zone—a finding that is associated with higher risk of rehospitalization for hypervolemia in patients with chronic heart failure (35). Our data show that the internal jugular vein diameter did not change during HD. This is consistent with prior findings that showed the internal jugular vein aspect ratio (anteroposterior diameter/lateral diameter) did not change significantly from pre- to post-HD (36). Given the observed decrease in B lines, without change in internal jugular vein diameter, it is possible that plasma refilling occurred quickly during HD in our cohort. However, our findings also demonstrate increased inferior vena cava collapsibility post compared with pre-HD. Interestingly, this collapsibility decreased back to pre-HD levels 1-hour post-HD. The trend toward continued decrease in B lines 1 hour post compared with post-HD with the simultaneous rebound decrease in collapsibility of the inferior vena cava may suggest continued mobilization of volume from the extra- to intravascular space in the hour after HD. Further studies to measure intracardiac filling pressures invasively would be helpful in assessing intravascular volume during HD and whether internal jugular vein and inferior vena cava ultrasound can be used as accurate surrogates of intravascular volume status.
There are several strengths to the present study. It is novel for its comprehensive and serial collection of laboratory tests during HD, including blood gases and ionized calcium levels. Further, it provides not only pre- and post-HD electrolyte data, but also an assessment during HD at the 1-hour mark. Additionally, ultrasound techniques to assess both intra- and extravascular volume during HD sessions in the inpatient setting were applied with the reader blinded to intradialytic time point. Further, we demonstrate the feasibility of the simplified eight lung zone method employed by a nephrologist to identify volume changes that occur during HD sessions. Despite these strengths, some limitations deserve consideration. First, guidelines in relation to HD prescriptions were not specified because the present study aimed to observe real-world changes. In addition, obtaining inferior vena cava data was only feasible in 12 patients at all time points. These data should therefore be interpreted with caution. Although this marker provided useful information regarding intravascular volume for our study, difficulty positioning the inpatient dialysis patient during HD compromised optimal imaging. To save the patient from additional study time, images were obtained during HD. In an ideal clinical setting, these images should be obtained with the patient off the HD machine for optimal positioning. It should also be noted that we do not have data regarding the degree of tricuspid regurgitation in this cohort, which could affect internal jugular vein measurements. Further, with regard to lung ultrasound, our analysis of B lines in anterior and lateral lung fields may have missed B lines in posterior lung fields, which may be particularly relevant in inpatients who spent most hours before their lung ultrasound exams in bed. Additionally, given concerns that contributing to anemia with additional blood draws might contribute to ischemia, patients with recent acute coronary syndrome or stroke were excluded from this study, which may compromise generalizability to the larger maintenance HD inpatient population. A final concern is that as this was a single-center study on 30 patients, the results may not be generalizable to the wider hospitalized maintenance HD population.
In conclusion, among hospitalized patients undergoing HD, dynamic changes in blood chemistry parameters, QTc durations, and volume status occur during their HD sessions. These data describe the time course of changes in important clinical parameters during an inpatient HD session that may be useful for clinicians evaluating adverse patient symptoms or events related to HD. Further research is required to assess how variations in these changes during HD are associated with clinical outcomes and whether HD prescriptions can be tailored to optimize patient care.
Disclosures
F.R. Mc Causland reports consultancy for GlaxoSmithKline, and research funding paid to institution from Advanced Medical and Fifth Eye. E. Platz reports consultancy for Novartis and ScPharmaceuticals; research funding from the National Institutes of Health; and other interests or relationships with Women as One. All remaining authors have nothing to disclose.
Funding
K.S. Ravi was supported by the American Society of Nephrology Ben J. Lipps Fellowship grant, by the National Institutes of Health T32 grant (DK 007527), and by the National Institutes of Health K23 grant (DK 127248). F.R. Mc Causland was supported by the National Institute of Diabetes and Digestive and Kidney Diseases grant (R03DK122240) and receives research funding from Advanced Instrument and Fifth Eye paid directly to his institution. K.S. Jering received support from the National Institutes of Health (Training Grant 5-T32 HL007604).
Acknowledgments
The authors would like to thank the staff of Quest Diagnostics for their support in ensuring laboratory data quality in the present study. The authors would also like to thank Philips for loaning the Lumify ultrasound that made volume assessment possible for this study.
Author Contributions
K.A. Curtis and K.S. Ravi were responsible for project administration; F.R. McCausland and E. Platz were responsible for supervision; K.S. Ravi was responsible for funding acquisition; all authors were responsible for conceptualization, data curation, formal analysis, investigation, and methodology, wrote the original draft of the manuscript and reviewed and edited the manuscript; and all authors agree to be accountable for ensuring that all questions related to the accuracy and integrity of any part to the work are appropriately investigated and resolved.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
- 1.United States Renal Data System : 2020 USRD System Annual Data Report: Epidemiology of Kidney Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Health, 2020. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8148988/pdf/nihms-1699563.pdf. Accessed April 1, 2022 [Google Scholar]
- 2.United States Renal Data System : Hospitalization. 2015, Ch. 5. Available at: https://www.usrds.org/media/1566/vol2_05_hospitalization_15.pdf. Accessed April 1, 2022
- 3.Al-Akchar M, Siddique MS: Long QT Syndrome. In: StatPearls [Internet]. Treasure Island, FL, StatPearls Publishing, 2020 [Google Scholar]
- 4.Aronson PS, Giebisch G: Effects of pH on potassium: New explanations for old observations. J Am Soc Nephrol 22: 1981–1989, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basile C, Rossi L, Lomonte C: The choice of dialysate bicarbonate: Do different concentrations make a difference? Kidney Int 89: 1008–1015, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Di Iorio B, Torraca S, Piscopo C, Sirico ML, Di Micco L, Pota A, Tartaglia D, Berardino L, Morrone LF, Russo D: Dialysate bath and QTc interval in patients on chronic maintenance hemodialysis: Pilot study of single dialysis effects. J Nephrol 25: 653–660, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Silva RT, Martinelli Filho M, Peixoto GL, Lima JJ, Siqueira SF, Costa R, Gowdak LH, Paula FJ, Kalil Filho R, Ramires JA: Predictors of arrhythmic events detected by implantable loop recorders in renal transplant candidates. Arq Bras Cardiol 105: 493–502, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalra PA, Green D, Poulikakos D: Arrhythmia in hemodialysis patients and its relation to sudden death. Kidney Int 93: 781–783, 2018 [DOI] [PubMed] [Google Scholar]
- 9.Hage FG, de Mattos AM, Khamash H, Mehta S, Warnock D, Iskandrian AE: QT prolongation is an independent predictor of mortality in end-stage renal disease. Clin Cardiol 33: 361–366, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orofino L, Marcén R, Quereda C, Villafruela JJ, Sabater J, Matesanz R, Pascual J, Ortuño J: Epidemiology of symptomatic hypotension in hemodialysis: Is cool dialysate beneficial for all patients? Am J Nephrol 10: 177–180, 1990 [DOI] [PubMed] [Google Scholar]
- 11.Macedo E, Karl B, Lee E, Mehta RL: A randomized trial of albumin infusion to prevent intradialytic hypotension in hospitalized hypoalbuminemic patients. Crit Care 25: 18, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Platz E, Jhund PS, Girerd N, Pivetta E, McMurray JJV, Peacock WF, Masip J, Martin-Sanchez FJ, Miró Ò, Price S, Cullen L, Maisel AS, Vrints C, Cowie MR, DiSomma S, Bueno H, Mebazaa A, Gualandro DM, Tavares M, Metra M, Coats AJS, Ruschitzka F, Seferovic PM, Mueller C; Study Group on Acute Heart Failure of the Acute Cardiovascular Care Association and the Heart Failure Association of the European Society of Cardiology : Expert consensus document: Reporting checklist for quantification of pulmonary congestion by lung ultrasound in heart failure. Eur J Heart Fail 21: 844–851, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.London Health Sciences Centre : Procedure for Venous Blood Gas Sampling. Available at: https://www.lhsc.on.ca/critical-care-trauma-centre/procedure-for-venous-blood-gas-sampling. Accessed March 22, 2022
- 14.Santiago-Delpin EA, Buselmeier TJ, Simmons RL, Najarian JS, Kjellstrand CM: Blood gases and pH in patients with artificial arteriovenous fistulas. Kidney Int 1: 131–133, 1972 [DOI] [PubMed] [Google Scholar]
- 15.Torino C, Tripepi R, Loutradis C, Sarafidis P, Tripepi G, Mallamaci F, Zoccali C: Can the assessment of ultrasound lung water in haemodialysis patients be simplified? Nephrol Dial Transplant 36: 2321–2326, 2020 [DOI] [PubMed] [Google Scholar]
- 16.Platz E, Lewis EF, Uno H, Peck J, Pivetta E, Merz AA, Hempel D, Wilson C, Frasure SE, Jhund PS, Cheng S, Solomon SD: Detection and prognostic value of pulmonary congestion by lung ultrasound in ambulatory heart failure patients. Eur Heart J 37: 1244–1251, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivas-Lasarte M, Álvarez-García J, Fernández-Martínez J, Maestro A, López-López L, Solé-González E, Pirla MJ, Mesado N, Mirabet S, Fluvià P, Brossa V, Sionis A, Roig E, Cinca J: Lung ultrasound-guided treatment in ambulatory patients with heart failure: A randomized controlled clinical trial (LUS-HF study). Eur J Heart Fail 21: 1605–1613, 2019 [DOI] [PubMed] [Google Scholar]
- 18.Palazzuoli A, Ruocco G, Beltrami M, Nuti R, Cleland JG: Combined use of lung ultrasound, B-type natriuretic peptide, and echocardiography for outcome prediction in patients with acute HFrEF and HFpEF. Clin Res Cardiol 107: 586–596, 2018 [DOI] [PubMed] [Google Scholar]
- 19.Lindner M, Thomas R, Claggett B, Lewis EF, Groarke J, Merz AA, Silverman MB, Swamy V, Rivero J, Hohenstein C, Solomon SD, McMurray JJ, Steigner ML, Platz E: Quantification of pleural effusions on thoracic ultrasound in acute heart failure. Eur Heart J Acute Cardiovasc Care 9: 513–521, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vitturi N, Dugo M, Soattin M, Simoni F, Maresca L, Zagatti R, Maresca MC: Lung ultrasound during hemodialysis: The role in the assessment of volume status. Int Urol Nephrol 46: 169–174, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Porter TR, Shillcutt SK, Adams MS, Desjardins G, Glas KE, Olson JJ, Troughton RW: Guidelines for the use of echocardiography as a monitor for therapeutic intervention in adults: A report from the American Society of Echocardiography. J Am Soc Echocardiogr 28: 40–56, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Murthi SB, Fatima S, Menne AR, Glaser JJ, Galvagno SM, Biederman S, Fang R, Chen H, Scalea TM: Ultrasound assessment of volume responsiveness in critically ill surgical patients: Two measurements are better than one. J Trauma Acute Care Surg 82: 505–511, 2017 [DOI] [PubMed] [Google Scholar]
- 23.Lucas BP, D’Addio A, Clark J, Block C, Manning H, Remillard B, Leiter JC: Reproducibility of point-of-care ultrasonography for central vein diameter measurement: Separating image acquisition from interpretation. J Clin Ultrasound 45: 488–496, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon MA, Kliner DE, Girod JP, Moguillansky D, Villanueva FS, Pacella JJ: Detection of elevated right atrial pressure using a simple bedside ultrasound measure. Am Heart J 159: 421–427, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Simon MA, Schnatz RG, Romeo JD, Pacella JJ: Bedside ultrasound assessment of jugular venous compliance as a potential point-of-care method to predict acute decompensated heart failure 30-day readmission. J Am Heart Assoc 7: e008184, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Correa S, Scovner KM, Tumlin JA, Roy-Chaudhury P, Koplan BA, Costea AI, Kher V, Williamson D, Pokhariyal S, McClure CK, Mc Causland FR, Charytan DM: Electrolyte changes in contemporary hemodialysis: A secondary analysis of the Monitoring in Dialysis (MiD) Study. Kidney360 2: 695–707, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viegas M, Cândido C, Felgueiras J, Clemente J, Barros S, Farbota R, Vera F, Matos A, Sousa F: Dialysate bicarbonate variation in maintenance hemodiafiltration patients: Impact on serum bicarbonate, intradialytic hypotension and interdialytic weight gain. Hemodial Int 21: 385–392, 2017 [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto T, Shoji S, Yamakawa T, Wada A, Suzuki K, Iseki K, Tsubakihara Y: Predialysis and postdialysis pH and bicarbonate and risk of all-cause and cardiovascular mortality in long-term hemodialysis patients. Am J Kidney Dis 66: 469–478, 2015 [DOI] [PubMed] [Google Scholar]
- 29.van der Sande FM, Ter Meulen KJA, Kotanko P, Kooman JP: Dialysate calcium levels: Do they matter? Blood Purif 47: 230–235, 2019 [DOI] [PubMed] [Google Scholar]
- 30.UCSF Schools of Pharmacy and Medicine : The Kidney Project: Creating a Bioartificial Kidney as a Permanent Solution to End Stage Renal Disease. Available at: https://pharm.ucsf.edu/kidney/need/statistics. Accessed February 15, 2022
- 31.Roy-Chaudhury P, Tumlin JA, Koplan BA, Costea AI, Kher V, Williamson D, Pokhariyal S, Charytan DM; MiD investigators and committees : Primary outcomes of the Monitoring in Dialysis Study indicate that clinically significant arrhythmias are common in hemodialysis patients and related to dialytic cycle. Kidney Int 93: 941–951, 2018 [DOI] [PubMed] [Google Scholar]
- 32.Toussaint N, Cooney P, Kerr PG: Review of dialysate calcium concentration in hemodialysis. Hemodial Int 10: 326–337, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Beaubien ER, Pylypchuk GB, Akhtar J, Biem HJ: Value of corrected QT interval dispersion in identifying patients initiating dialysis at increased risk of total and cardiovascular mortality. Am J Kidney Dis 39: 834–842, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Mallamaci F, Benedetto FA, Tripepi R, Rastelli S, Castellino P, Tripepi G, Picano E, Zoccali C: Detection of pulmonary congestion by chest ultrasound in dialysis patients. JACC Cardiovasc Imaging 3: 586–594, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Platz E, Campbell RT, Claggett B, Lewis EF, Groarke JD, Docherty KF, Lee MMY, Merz AA, Silverman M, Swamy V, Lindner M, Rivero J, Solomon SD, McMurray JJV: Lung ultrasound in acute heart failure: Prevalence of pulmonary congestion and short- and long-term outcomes. JACC Heart Fail 7: 849–858, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sekiguchi H, Seaburg LA, Suzuki J, Astorne WJ, Patel AS, Keller AS, Gajic O, Kashani KB: Central venous pressure and ultrasonographic measurement correlation and their associations with intradialytic adverse events in hospitalized patients: A prospective observational study. J Crit Care 44: 168–174, 2018 [DOI] [PubMed] [Google Scholar]
- 37.Assimon MM, Nguyen T, Katsanos SL, Brunelli SM, Flythe JE: Identification of volume overload hospitalizations among hemodialysis patients using administrative claims: A validation study. BMC Nephrol 17: 173, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.