Key Points
Low levels of drinking water lead contamination are associated with an increased risk of iron deficiency among those with kidney disease.
Black people seem particularly susceptible to the association of lead contamination and iron deficiency.
Keywords: chronic kidney disease, anemia, drinking water, ESKD, intoxication
Visual Abstract
Abstract
Background
Although those with kidney disease may have heightened susceptibility to heavy metal toxicity, whether low levels of drinking water lead contamination have clinical consequence is unknown.
Methods
Given that lead toxicity is known to associate with iron deficiency, we merged data from the Environmental Protection Agency (EPA) Safe Drinking Water Information and United States Renal Data Systems to examine whether municipal 90th percentile drinking water lead levels associate with iron deficiency among incident dialysis patients. Iron deficiency was defined across thresholds of transferrin saturation (<10% and 20%) and ferritin (<100 and <200 ng/ml), and simultaneous transferrin saturation <20% and ferritin <200 ng/ml, all obtained within 30 days of dialysis initiation. The average 90th percentile of drinking water lead samples per patient city of residence over a 5-year period before dialysis initiation was examined at the <1 μg/L level of detection, and at the 25th, 50th, and 100th percentile of the EPA’s actionable level (15 μg/L).
Results
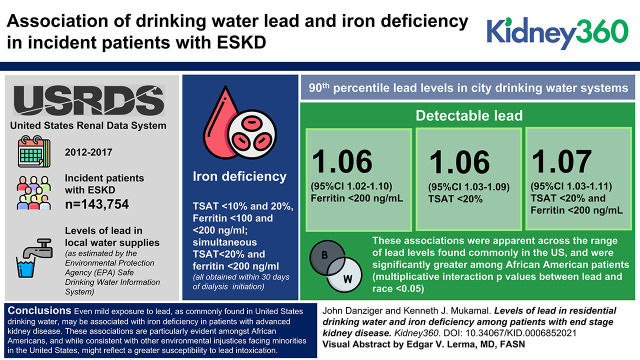
Among 143,754 incident ESKD patients, those in cities with drinking water lead contamination had 1.06 (95% CI, 1.03 to 1.09), 1.06 (95% CI, 1.02 to 1.10), and 1.07 (95% CI, 1.03 to 1.11) higher adjusted odds of a transferrin saturation <20%, ferritin <200 ng/ml, and simultaneous transferrin saturation <20% and ferritin <200 ng/ml, respectively. These associations were apparent across the range of lead levels found commonly in the United States and were significantly greater among Black patients (multiplicative interaction P values between lead and race <0.05).
Conclusions
Even exposure to low levels of lead contamination, as commonly found in US drinking water, may have adverse hematologic consequence in patients with advanced kidney disease. These associations are particularly evident among Black people and, although consistent with other environmental injustices facing minorities in the United States, might reflect a greater susceptibility to lead intoxication.
Introduction
Iron deficiency affects large proportions of the population worldwide, and has a range of pathophysiologic consequences, including anemia, fatigue, restless legs, and malaise. It occurs with particular frequency in CKD, where it is an important determinant of erythropoietin stimulating agent (ESA) use and overall health care utilization.
Lead toxicity is an important environmental determinant of iron deficiency. Although lead exposure most typically occurs from workplace contact, particulate inhalation, and paint ingestion, contamination of drinking water is an increasingly recognized source (1–9). The aging infrastructure of water delivery systems in the United States, including lead service lines and household plumbing, are susceptible to leaching, as most dramatically evidenced in Flint, Michigan (10). The Environmental Protection Agency (EPA) recognizes no safe limit of lead exposure and, although establishing a maximum contaminant goal level of zero, mandates regulatory action only when the 90th percentile of tested samples exceeds 15 μg/L. Accordingly, significant variability in lead contamination remains in US drinking water.
Recently, we showed that low levels of lead exposure, as found widely in drinking water systems across the United States, are associated with lower levels of hemoglobin and higher rates of ESA utilization among incident ESKD patients (11). To examine the potential hematologic consequences of lead exposure in this highly susceptible population further, herein we examine the association of drinking water lead levels and iron deficiency. In addition, given that Black people have higher rates of iron deficiency (12) and anemia (13,14) and, simultaneously, higher rates of lead exposure than other racial/ethnic groupings (15), we examine these associations across racial strata.
Materials and Methods
Using data from the United States Renal Data System (USRDS), the national registry of patients with ESKD, we identified all patients who initiated dialysis between 2012 and 2017 (16). For each patient, we identified the city of residence, which was defined by a single ZIP code for ≥6 months preceding dialysis initiation. We used data from the ESKD Medical Evidence Report, a standardized form that the Centers for Medicare and Medicaid Services (CMS) required for the registration of all incident ESKD patients, to ascertain demographic information, comorbidities, pre-ESKD laboratory measurements, including serum creatinine and hemoglobin, and pre-ESKD ESA use. We used data in CROWNWeb, a CMS data management system that all Medicare-certified dialysis facilities use to supply laboratory information from the first month of dialysis therapy. CROWNWeb began to accept data in June 2012. As previously described (11), we used the Safe Drinking Water Information System (SDWIS) Federal Data Warehouse to describe concentrations of lead in city drinking water systems. We joined the USRDS and SDWIS datasets by city and state to create a complete dataset of 187,298 incident ESKD patients. Of these, 143,754 patients in 7349 cities had reported iron studies during the first month of dialysis, comprising the primary dataset.
Federal guidelines mandate representative lead sampling on the basis of the size of each water system and its previous lead concentrations, ranging from 5 to 100 samples per water system, over a 6-month to 3-year interval (17). If ≥10% of these samples exceed the EPA’s actionable level of 15 μg/L, the municipal utility system must take corrective action and increase the frequency of testing. The 90th percentile of all results are cataloged in the SDIWS according to the dates of water surveillance. We generated an annual lead level for each water system by matching the lead results to each year within the sampling period. Because of repeated measures per surveillance period, we averaged the maximum reported lead level per year of all water systems serving a city, weighted by the number of individuals served by each water system, to derive an annual city-wide lead level. For each patient, we calculated the average of city-wide levels during the 5 years preceding dialysis initiation. Given that the 90th percentile threshold may not accurately quantify the exposure at the end-user level, we compared patients in cities with any detectable drinking water lead (≥1 μg/L) to those with undetectable levels, and also categorized the exposure by percentiles (25th, 50th, and 100th) of the EPA’s current actionable level (15 μg/L).
Given the complexity of iron metabolism and multiple definitions of iron deficiency, we explored several primary outcomes, including transferrin saturation <10% and <20%, ferritin <100 and <200 ng/ml, and simultaneous transferrin saturation <20% and ferritin <200 ng/ml. Patient characteristics included age, sex, race (White, Black, Asian, other/unknown), diabetes mellitus, heart failure, hypertension, tobacco use, eGFR (as calculated by the Modification in Diet in Renal Disease four-factor equation), and year of dialysis initiation. Median household income and unemployment rate were ascertained at the ZIP code level using data from the United States Census American Community Survey 5-year estimates.
We summarized patient characteristics according to categories of drinking water lead concentrations. We used logistic regression, including all patient characteristics and socioeconomic factors, to examine the adjusted association of city-wide lead levels with thresholds of iron deficiency. Exposure was examined at the level of detection (90th percentile lead level ≥1 μg/L) and across EPA thresholds.
We explored the significance of multiplicative interactions between race (Black versus non-Black, excluding those with missing race categorization) and detectable drinking water lead and provide the stratified results. We also explored whether results differed according to pre-ESKD ESA use or nephrology care, hemoglobin concentrations (pre-ESKD), and household income. Institutional Review Board permission was obtained for research activities (BIDMC protocol 2020P000200).
Results
Among patients initiating dialysis in the United States from 2012 to 2017, 80% (n=114,696) lived in cities with measurable (≥1 μg/L) 90th percentile lead levels in municipal drinking water. Demographics, comorbidities, and socioeconomic factors were similar across categories of drinking water lead exposure (Table 1), except for higher proportions of Black patients and patients with heart failure in cities with higher drinking water lead.
Table 1.
Baseline characteristics according to 90th percentile levels of lead in residential drinking water
| Characteristic | Lead in Drinking Water, μg/L | ||||
|---|---|---|---|---|---|
| <1 | 1 to <3.75 | 3.75 to <7.5 | 7.5 to <15 | ≥15 | |
| Number of patients | 28,178 | 63,765 | 33,584 | 15,639 | 2588 |
| Number of cities | 1903 | 4183 | 2522 | 857 | 254 |
| Pre-ESKD patient characteristics | |||||
| Age, yr | 63 (14.9) | 63.4 (15.4) | 63.5 (15.6) | 63.7 (15.6) | 62.9 (15) |
| Women | 42 | 42 | 42 | 42 | 42 |
| Race | |||||
| White | 65 | 68 | 74 | 66 | 63 |
| Black | 25 | 27 | 22 | 29 | 35 |
| Asian | 8 | 4 | 3 | 4 | 2 |
| Other/unknown | 3 | 2 | 1 | 1 | 1 |
| Comorbidities | |||||
| Diabetes | 60 | 58 | 59 | 57 | 57 |
| Hypertension | 89 | 89 | 89 | 89 | 89 |
| Congestive heart failure | 27 | 28 | 30 | 31 | 31 |
| Tobacco use | 6 | 7 | 6 | 6 | 6 |
| Body mass index | 29.9 (8) | 29.8 (7.9) | 29.6 (7.9) | 29.4 (8) | 30.1 (8.1) |
| eGFR, ml/min per m2 | 9.8 (4.5) | 10.2 (4.5) | 10.1 (4.5) | 10 (4.6) | 9.8 (4.5) |
| Peritoneal dialysis | 6 | 5 | 4 | 4 | 5 |
| Nephrology carea | 78 | 77 | 74 | 76 | 77 |
| Pre-ESKD ESA useb | 23 | 22 | 23 | 24 | 24 |
| Laboratory values, % | |||||
| Transferrin saturation | 23.4 (10.6) | 23 (10.4) | 23.1 (10.5) | 23.2 (10.7) | 22.7 (10.1) |
| Transferrin saturation <10% | 4 | 4.2 | 4.2 | 4.2 | 4.4 |
| Transferrin saturation <20% | 40.4 | 42.3 | 41.6 | 41.9 | 42.7 |
| Ferritin | 447 (382) | 443 (385) | 447 (388) | 460 (396) | 436 (370) |
| Ferritin <100 ng/ml | 10.1 | 10.7 | 10.8 | 9.7 | 10.8 |
| Ferritin <200 ng/ml | 27.8 | 28.9 | 28.6 | 27.2 | 29.2 |
| Transferrin saturation <20% and ferritin <200 ng/ml | 12.9 | 14 | 13.4 | 13 | 14.3 |
| Zip code characteristics | |||||
| Median household income, $1000K | 55.81 (20.9) | 54.3 (21.2) | 50.9 (23.4) | 53.4 (25.2) | 52.3 (22.3) |
| Unemployment rate | 7.8 (3.5) | 7.9 (4) | 8.8 (5.4) | 8.9 (4.8) | 8.6 (4.7) |
ESA, erythropoietin stimulating agent.
Missing in 16,109.
Missing in 40,941.
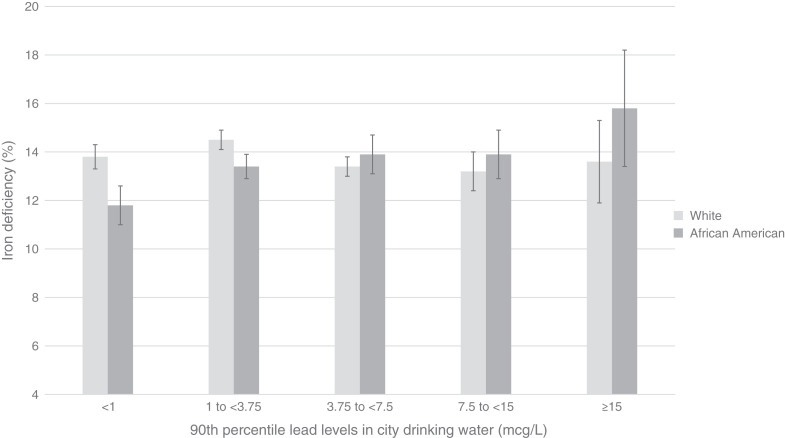
Iron deficiency was more common among ESKD patients residing in cities with lead-containing drinking water, particularly among Black people (Figure 1). In models that adjusted for a range of individual and environmental factors (Table 2), detectable levels of drinking water lead were associated with 1.06 (95% CI, 1.03 to 1.09) higher odds of transferrin saturation <20%, 1.06 (95% CI, 1.02 to 1.10) odds of ferritin <200 ng/ml, and 1.07 (95% CI, 1.03 to 1.11) odds of simultaneous transferrin saturation <20% and ferritin <200 ng/ml. These associations were significantly more robust among Black patients (Table 3).
Figure 1.
Proportions of Black and White ESKD patients in the United States with iron deficiency, as defined by a transferrin saturation <20% and ferritin <200, according to 90th percentiles of lead in municipal drinking water. Compared with undetectable levels of lead, residence in cities with higher lead levels was associated with significantly higher unadjusted risk of iron deficiency among Black patients (P<0.001) but not among White patients.
Table 2.
Adjusted association of residential water systems lead concentration and iron deficiency among patients starting dialysis between 2012 and 2017 in the United States
| Iron Deficiency | 90th Percentile Lead Levels in City Drinking Water Systems | |||||
|---|---|---|---|---|---|---|
| Thresholds of EPA’s Maximum Allowable Drinking Water Lead Content | Detectable Lead | |||||
| <1 μg/L | 1 to <3.75 μg/L | 3.75 to <7.5 μg/L | 7.5 to <15 μg/L | ≥15 μg/L | ≥1 μg/L | |
| Transferrin saturation <10% | Ref. | 1.02 (0.95 to 1.09) | 1.01 (0.93 to 1.1) | 1.02 (0.92 to 1.12) | 1.08 (0.89 to 1.31) | 1.03 (0.96 to 1.1) |
| Transferrin saturation <20% | Ref. | 1.07 (1.04 to 1.1) | 1.04 (1 to 1.07) | 1.05 (1.01 to 1.09) | 1.09 (1 to 1.17) | 1.06 (1.03 to 1.09) |
| Ferritin <100 ng/ml | Ref. | 1.07 (1.01 to 1.13) | 1.09 (1.02 to 1.16) | 0.97 (0.89 to 1.04) | 1.08 (0.93 to 1.26) | 1.06 (1.01 to 1.11) |
| Ferritin <200 ng/ml | Ref. | 1.07 (1.03 to 1.11) | 1.07 (1.02 to 1.12) | 0.99 (0.94 to 1.05) | 1.09 (0.99 to 1.21) | 1.06 (1.02 to 1.1) |
| Transferrin saturation <20% and ferritin <200 ng/ml | Ref. | 1.1 (1.05 to 1.14) | 1.04 (0.99 to 1.09) | 1.01 (0.95 to 1.08) | 1.12 (0.99 to 1.26) | 1.07 (1.03 to 1.11) |
Data shown as odds ratio (95% confidence intervals). Adjusted for age, sex, race, comorbidities, eGFR, median household income and unemployment rate of patient’s zip code of residence, and year of dialysis initiation. EPA, Environmental Protection Agency.
Table 3.
Adjusted association of residential water systems lead concentrations and iron deficiency among patients starting dialysis between 2012 and 2017 in the United States
| Iron Deficiency | Race | 90th Percentile Lead Levels in City Drinking Water Systems | ||||||
|---|---|---|---|---|---|---|---|---|
| Thresholds of EPA’s Maximum Allowable Drinking Water Lead Content | Detectable Lead | P Valuea | ||||||
| <1 μg/L | 1 to <3.75 μg/L | 3.75 to <7.5 μg/L | 7.5 to <15 μg/L | ≥15 μg/L | ≥1 μg/L | |||
| Transferrin saturation <10% | White | Ref. | 1.02 (0.93 to 1.11) | 1 (0.91 to 1.1) | 1 (0.89 to 1.13) | 0.92 (0.71 to 1.19) | 1.01 (0.93 to 1.09) | 0.63 |
| Black | Ref. | 1 (0.86 to 1.16) | 1.07 (0.91 to 1.27) | 1.08 (0.89 to 1.31) | 1.41 (1.02 to 1.94) | 1.04 (0.91 to 1.19) | ||
| Transferrin saturation <20% | White | Ref. | 1.06 (1.03 to 1.10) | 1.02 (0.98 to 1.06) | 1.01 (0.96 to 1.06) | 0.99 (0.88 to 1.09) | 1.04 (1.01 to 1.08) | 0.03 |
| Black | Ref. | 1.1 (1.04 to 1.16) | 1.08 (1.01 to 1.16) | 1.16 (1.07 to 1.25) | 1.28 (1.1 to 1.46) | 1.11 (1.05 to 1.17) | ||
| Ferritin <100 ng/ml | White | Ref. | 1.03 (0.95 to 1.10) | 1.02 (0.95 to 1.1) | 0.89 (0.81 to 0.98) | 0.99 (0.82 to 1.2) | 1.01 (0.95 to 1.07) | 0.02 |
| Black | Ref. | 1.16 (1.03 to 1.29) | 1.21 (1.06 to 1.38) | 1.17 (1 to 1.36) | 1.34 (1.04 to 1.72) | 1.18 (1.06 to 1.31) | ||
| Ferritin <200 ng/ml | White | Ref. | 1.04 (0.99 to 1.09) | 0.98 (0.94 to 1.03) | 0.92 (0.86 to 0.97) | 1.04 (0.91 to 1.18) | 1.01 (0.96 to 1.05) | 0.001 |
| Black | Ref. | 1.11 (1.03 to 1.2) | 1.19 (1.08 to 1.3) | 1.16 (1.04 to 1.28) | 1.19 (1 to 1.42) | 1.14 (1.06 to 1.22) | ||
| Transferrin saturation <20% and ferritin <200 ng/ml | White | Ref. | 1.06 (1 to 1.12) | 0.98 (0.92 to 1.03) | 0.95 (0.88 to 1.03) | 0.99 (0.84 to 1.16) | 1.02 (0.97 to 1.07) | 0.01 |
| Black | Ref. | 1.14 (1.04 to 1.24) | 1.19 (1.03 to 1.31) | 1.16 (1.03 to 1.31) | 1.36 (1.11 to 1.66) | 1.16 (1.07 to 1.27) | ||
Data shown as odds ratios (95% confidence intervals). Adjusted for age, sex, comorbidities, eGFR, median household income and unemployment rate of patient’s zip code of residence, and year of dialysis initiation. EPA, Environmental Protection Agency.
Multiplicative interaction term P value testing Black versus other racial groupings and lead ≥1 μg/L.
There was no effect modification by ESA utilization or nephrology care before ESKD onset, pre-ESKD hemoglobin concentration, or household income (multiplicative interaction P values all >0.05).
Discussion
Among patients starting dialysis in the United States from 2012 to 2017, those living in cities with measurable amounts of lead in their residential drinking water had a higher prevalence of iron deficiency as estimated from iron, transferrin, and ferritin levels. These associations were observed at levels of drinking water lead well below the EPA’s current actionable level that mandates water remediation and were particularly apparent among Black people.
Although there is extensive epidemiologic evidence linking iron deficiency and lead toxicity (18–21), the complexity of iron metabolism renders pathophysiologic explanations speculative. Heavy metals absorption occurs competitively across the gastrointestinal tract, and iron supplementation may minimize the toxicity of environmental lead exposure (20,22). The binding of hepcidin to ferroportin on the membranes of iron-exporting cells, including enterocytes, induces endocytosis and proteolysis of ferroportin, thereby reducing iron delivery to plasma. Although there are known determinants of hepcidin activity, including inflammation, iron, and erythropoietin, whether it is modulated by lead has not been examined (23). Polymorphisms in a range of iron metabolism mediators have been linked to differences in lead susceptibility (24–27).
Explanations for the significantly larger associations between drinking water lead levels and iron deficiency in Black than White patients are similarly hypothesis generating. Ongoing socioeconomic inequities likely contribute to differences in exposure (28). Additional environmental toxins that associate with drinking water contamination, not accounted for in our analysis, may confound our findings. In addition, given the nonrandom sampling of water systems, 90th percentile values likely misclassify individual levels of exposure, particularly for those most vulnerable to lead contamination (29,30). Such systematic underestimation of environmental lead and its negative effects is particularly concerning for Black people, among whom socioeconomic disadvantage increases vulnerability, and higher rates of kidney disease increase susceptibility, to lead toxicity (5,31,32). Whether unrecognized lead toxicity may contribute to the higher rates of anemia and ESA utilization among Black people than other ethnic groupings requires further study (33,34). Finally, the role of genetic variations in iron and lead metabolism requires further study.
Our findings further highlight the heightened susceptibility to environmental toxins among patients with kidney disease (35). Although most individuals absorb only a small fraction of ingested lead, mitigating the toxicity of water contamination, unique pathophysiologic complications of CKD, including hypocalcemia, vitamin D deficiency (36,37), and low protein diets (38), increase the proportion of lead absorbed across the gastrointestinal tract. Furthermore, because excretion of lead primarily occurs through glomerular filtration, patients with CKD are particularly susceptible to lead accumulation and, accordingly, have circulating blood levels several fold higher than those with normal function (39,40).
Our study has several important limitations. Without individual patient dietary practices, including use of tap versus bottled water, direct assay of water lead content, and residential stability, lead exposure cannot be accurately adjudicated. Further studies with direct measurement of household water are needed. Although we could not accurately characterize determinants likely to affect iron metabolism, including iron supplementation, underlying inflammation, nutrition, and a range of other disease states, the overall levels of comorbidity burden, pre-ESKD care, and ESA use were generally similar across strata of lead exposure. In addition, given no standard definition of iron deficiency in patients with kidney disease and competing pathophysiologic forces that modify iron storage and handling, misclassification is possible.
In conclusion, for patients with advanced kidney disease, especially Black people, even low levels of lead contamination may have hematologic consequence. Further studies with more precise quantification of lead exposure and body measures of lead accumulation are needed to understand the importance of environmental lead.
Disclosures
J. Danziger reports consultancy for Healthmap Solutions; ownership interest in Healthmap Solutions; and is the regional medical director at Healthmap Solutions. K.J. Mukamal reports other interests or relationships with US Highbush Blueberry Council and Wolters Kluwer.
Funding
None.
Acknowledgments
The data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
Author Contributions
J. Danziger was responsible for conceptualization, data curation, formal analysis, investigation, and methodology, and wrote the original draft of the manuscript; and both authors reviewed and edited the manuscript.
References
- 1.Shannon M, Graef JW: Lead intoxication from lead-contaminated water used to reconstitute infant formula. Clin Pediatr (Phila) 28: 380–382, 1989 [DOI] [PubMed] [Google Scholar]
- 2.Levallois P, St-Laurent J, Gauvin D, Courteau M, Prévost M, Campagna C, Lemieux F, Nour S, D'Amour M, Rasmussen PE: The impact of drinking water, indoor dust and paint on blood lead levels of children aged 1-5 years in montréal (Québec, Canada). J Expo Sci Environ Epidemiol 24: 185–191, 2014. 23361441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ngueta G, Prévost M, Deshommes E, Abdous B, Gauvin D, Levallois P: Exposure of young children to household water lead in the Montreal area (Canada): The potential influence of winter-to-summer changes in water lead levels on children’s blood lead concentration. Environ Int 73: 57–65, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Levallois P, St-Laurent J, Gauvin D, Courteau M, Prévost M, Campagna C, Lemieux F, Nour S, D’Amour M, Rasmussen PE: The impact of drinking water, indoor dust and paint on blood lead levels of children aged 1–5 years in Montréal (Québec, Canada). J Expo Sci Environ Epidemiol 24: 185–191, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gleason JA, Nanavaty JV, Fagliano JA: Drinking water lead and socioeconomic factors as predictors of blood lead levels in New Jersey’s children between two time periods. Environ Res 169: 409–416, 2019 [DOI] [PubMed] [Google Scholar]
- 6.Tests LS; Centers for Disease Control and Prevention (CDC) : Blood lead levels in residents of homes with elevated lead in tap water—District of Columbia, 2004. MMWR Morb Mortal Wkly Rep 53: 268–270, 2004 [PubMed] [Google Scholar]
- 7.Jennings B, Duncan LL: Water safety and lead regulation: Physicians’ community health responsibilities. AMA J Ethics 19: 1027–1035, 2017 [DOI] [PubMed] [Google Scholar]
- 8.Brown MJ, Margolis S: Lead in drinking water and human blood lead levels in the United States. MMWR Suppl 61: 1–9, 2012 [PubMed] [Google Scholar]
- 9.Watt GCM, Britton A, Gilmour WH, Moore MR, Murray GD, Robertson SJ, Womersley J: Is lead in tap water still a public health problem? An observational study in Glasgow. BMJ 313: 979–981, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roy S, Edwards MA: Preventing another lead (Pb) in drinking water crisis: Lessons from the Washington D.C. and Flint MI contamination events. Curr Opin Environ Sci Health 7: 34–44, 2019 [Google Scholar]
- 11.Danziger J, Mukamal KJ, Weinhandl E: Associations of community water lead concentrations with hemoglobin concentrations and erythropoietin-stimulating agent use among patients with advanced CKD. J Am Soc Nephrol 32: 2425–2434, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barton JC, Wiener HH, Acton RT, Adams PC, Eckfeldt JH, Gordeuk VR, Harris EL, McLaren CE, Harrison H, McLaren GD, Reboussin DM: Prevalence of iron deficiency in 62,685 women of seven race/ethnicity groups: The HEIRS Study. PLoS One 15: e0232125, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zakai NA, McClure LA, Prineas R, Howard G, McClellan W, Holmes CE, Newsome BB, Warnock DG, Audhya P, Cushman M: Correlates of anemia in American Blacks and Whites: The REGARDS Renal Ancillary Study. Am J Epidemiol 169: 355–364, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le CHH: The prevalence of anemia and moderate-severe anemia in the US population (NHANES 2003–2012). PLoS One 11: e0166635, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention : National Report on Human Exposure to Environmental Chemicals. Available at: https://www.cdc.gov/exposurereport. https://www.cdc.gov/exposurereport. Accessed July 2, 2021
- 16.United States Renal Data System : 2019 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Available at https://www.usrds.org/annual-data-report. Accessed August 10, 2021 [Google Scholar]
- 17.United States Environmental Protection Agency : Lead and Copper Rule. Available at: https://www.epa.gov/dwreginfo/lead-and-copper-rule. Accessed September 12, 2021
- 18.Clark M, Royal J, Seeler R: Interaction of iron deficiency and lead and the hematologic findings in children with severe lead poisoning. Pediatrics 81: 247–254, 1988 [PubMed] [Google Scholar]
- 19.Townsend RR, Cohen DL: Use of diuretics with ACE inhibitors or angiotensin receptor blockers and NSAIDs increases the risk of acute kidney injury. Evid Based Med 18: 232–233, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Park S, Sim CS, Lee H, Kim Y: Effects of iron therapy on blood lead concentrations in infants. J Trace Elem Med Biol 28: 56–59, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Muwakkit S, Nuwayhid I, Nabulsi M, al Hajj R, Khoury R, Mikati M, Abboud MR: Iron deficiency in young Lebanese children: Association with elevated blood lead levels. J Pediatr Hematol Oncol 30: 382–386, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Zimmermann MB, Muthayya S, Moretti D, Kurpad A, Hurrell R: Iron fortification reduces blood lead levels in children: A randomized, double‐blind, controlled trial in Bangalore, India. Pediatrics 117: 2014–2021, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Arnold J, Busbridge M, Sangwaiya A, Bhatkal B, Paskaran P, Pal A, Geoghegan F, Kealey T: Prohepcidin levels in refractory anaemia caused by lead poisoning. Case Rep Gastroenterol 2: 49–54, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cantonwine D, Hu H, Téllez-Rojo M, Sánchez BN, Lamadrid-Figueroa H, Ettinger AS, Mercado-García A, Hernández-Avila M, Wright RO: HFE gene variants modify the association between maternal lead burden and infant birthweight: A prospective birth cohort study in Mexico City, Mexico. Environ Health 9: 43, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaik AP, Alsaeed AH, Haque MFU, Alsaeed MA, Shaik AS: Human serum paraoxonase (PON-1) and hemochromatosis gene (HFE) gene polymorphisms in occupationally exposed lead workers from Saudi Arabia. Genet Mol Res 18: GMR18317, 2019 [Google Scholar]
- 26.Karwowski MP, Just AC, Bellinger DC, Jim R, Hatley EL, Ettinger AS, Hu H, Wright RO: Maternal iron metabolism gene variants modify umbilical cord blood lead levels by gene-environment interaction: A birth cohort study. Environ Health 13: 77, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kayaaltı Z, Akyüzlü DK, Söylemezoğlu T: Evaluation of the effect of divalent metal transporter 1 gene polymorphism on blood iron, lead and cadmium levels. Environ Res 137: 8–13, 2015 [DOI] [PubMed] [Google Scholar]
- 28.Yeter D, Banks EC, Aschner M: Disparity in risk factor severity for early childhood blood lead among predominantly African-American black children: The 1999 to 2010 US NHANES. Int J Environ Res Public Health 17: E1552, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santucci RJ, Scully JR: The pervasive threat of lead (Pb) in drinking water: Unmasking and pursuing scientific factors that govern lead release. Proc Natl Acad Sci U S A 117: 23211–23218, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Triantafyllidou S, Edwards M: Lead (Pb) in tap water and in blood: Implications for lead exposure in the United States. Crit Rev Environ Sci Technol 42: 1297–1352, 2012 [Google Scholar]
- 31.Lanphear BP, Weitzman M, Eberly S: Racial differences in Urban children’s environmental exposures to lead. Am J Public Health 86: 1460–1463, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mielke HW, Gonzales CR, Smith MK, Mielke PW: The urban environment and children’s health: Soils as an integrator of lead, zinc, and cadmium in New Orleans, Louisiana, U.S.A. Environ Res 81: 117–129, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Barton JC, Conrad ME, Nuby S, Harrison L: Effects of iron on the absorption and retention of lead. J Lab Clin Med 92: 536–547, 1978 [PubMed] [Google Scholar]
- 34.Lacson E Jr, Rogus J, Teng M, Lazarus JM, Hakim RM: The association of race with erythropoietin dose in patients on long-term hemodialysis. Am J Kidney Dis 52: 1104–1114, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Moody EC, Coca SG, Sanders AP: Toxic metals and chronic kidney disease: A systematic review of recent literature. Curr Environ Health Rep 5: 453–463, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barton JC, Conrad ME, Harrison L, Nuby S: Effects of calcium on the absorption and retention of lead. J Lab Clin Med 91: 366–376, 1978 [PubMed] [Google Scholar]
- 37.Blake KCH, Mann M: Effect of calcium and phosphorus on the gastrointestinal absorption of 203Pb in man. Environ Res 30: 188–194, 1983 [DOI] [PubMed] [Google Scholar]
- 38.Abdulla M: Nutritional factors in lead poisoning. Nutr Rev 40: 255–256, 1982 [DOI] [PubMed] [Google Scholar]
- 39.Palaneeswari MS, Rajan PMAS, Silambanan S, Ramalingam J: Blood lead in end-stage renal disease (ESRD) patients who were on maintainence haemodialysis. J Clin Diagnostic Res 6: 1633–1635, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muntner P, He J, Vupputuri S, Coresh J, Batuman V: Blood lead and chronic kidney disease in the general United States population: Results from NHANES III. Kidney Int 63: 1044–1050, 2003 [DOI] [PubMed] [Google Scholar]