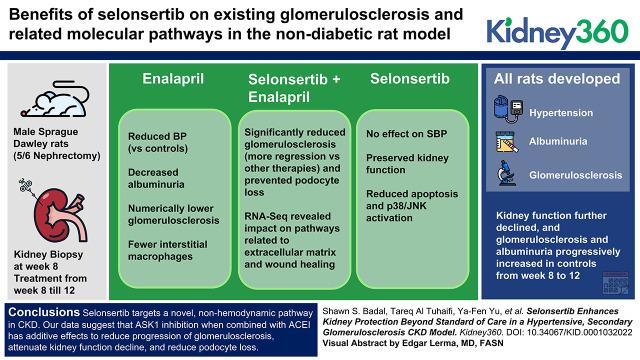
Key Points
Selonsertib (SEL), a selective apoptosis signal-regulating kinase 1 inhibitor, targets an oxidative stress pathway in CKD.
SEL plus enalapril reduces glomerulosclerosis, attenuates kidney function decline, and reduces podocyte loss more than either agent alone.
SEL has a nonhemodynamic mechanism and has additional effects on apoptosis and fibrosis in the kidney when combined with standard of care.
Keywords: chronic kidney disease, angiotensin-converting enzyme inhibitor, apoptosis signal-regulating kinase 1 inhibitor, basic science, diabetic kidney disease, selonsertib
Visual Abstract
Abstract
Background
Despite widespread use of renin-aldosterone-angiotensin system inhibitors and the benefits of lowering glomerular pressure in patients with CKD, there remains a major unmet need for therapies targeting underlying causes of CKD progression. Apoptosis signal-regulating kinase 1 (ASK1) promotes apoptosis and glomerulosclerosis, and is implicated in the progression of diabetic kidney disease (DKD), a major cause of CKD. Selonsertib is a selective ASK1 inhibitor currently in clinical development for the treatment of DKD. We examined the added benefits of selonsertib on existing glomerulosclerosis and related molecular pathways in the nondiabetic 5/6 nephrectomy (5/6 Nx) rat model in combination with the angiotensin-converting enzyme inhibitor (ACEI) enalapril.
Methods
Male Sprague Dawley rats underwent 5/6 Nx with kidney biopsy 8 weeks later for assessment of glomerulosclerosis, and were randomized to four treatment groups with equal glomerulosclerosis: selonsertib, enalapril, combination (selonsertib plus enalapril), and untreated controls. Serum creatinine, systolic BP (SBP), and urinary albumin were measured at intervals. Animals were euthanized at week 12 for histologic, biochemical, and molecular analyses.
Results
All rats developed hypertension, albuminuria, and glomerulosclerosis by week 8. Kidney function further declined, and glomerulosclerosis and albuminuria progressively increased in controls from week 8 to 12. Enalapril treatment alone from week 8 to 12 reduced SBP versus controls, decreased albuminuria, and resulted in numerically lower glomerulosclerosis. Selonsertib alone had no effect on SBP but preserved kidney function. Combined treatment significantly reduced glomerulosclerosis, with more regression than either monotherapy. Enalapril treatment resulted in fewer interstitial macrophages, whereas selonsertib treatment reduced apoptosis and podocyte loss. RNA-seq revealed that combined treatment influenced pathways related to extracellular matrix and wound healing.
Conclusions
Selonsertib targets a novel, nonhemodynamic pathway in CKD. Our data suggest that ASK1 inhibition, when combined with ACEI, has additive effects to reduce progression of glomerulosclerosis, attenuate kidney function decline, and reduce podocyte loss.
Introduction
CKD often leads to ESKD and remains a growing public health problem, despite treatment options designed to reduce both systemic and glomerular hypertension. A hallmark of late-stage CKD and diabetic kidney disease (DKD) is the significant and progressive loss of functional nephrons, a pathologic sequela that can be studied preclinically in rodents after 5/6 nephrectomy (5/6 Nx). The removal of renal mass in this model causes compensatory derangements within the remaining nephrons, including hypertrophy and proteinuria, triggering hypertension and the subsequent development of glomerulosclerosis lesions (1). Previous studies in this model have helped to elucidate the mechanisms underlying the benefit of standard CKD therapies, including angiotensin-converting enzyme inhibitors (ACEIs), angiotensin type 1 receptor blockers (ARBs), and aldosterone inhibitors (2). We previously demonstrated that high-dose ACEI or ARB therapy ameliorated the progression of glomerulosclerosis, and even induced regression of existing glomerulosclerosis, in about two thirds of rats treated from week 8 to 12 after 5/6 Nx (3). However, monotherapy with blockade of the renin-aldosterone-angiotensin system alone is insufficient to induce long-term regression of glomerulosclerosis (4). This suggests multipronged therapeutic approaches that go beyond hemodynamic effects will be necessary for the optimal treatment of CKD.
In progressive CKD, reactive oxygen species (ROS) in the kidney can drive cellular injury, apoptosis, inflammation, and fibrosis. ROS influence the activation of multiple cellular processes, including the activation of apoptosis signal-regulating kinase 1 (ASK1), a redox-sensitive mitogen-activated protein kinase kinase kinase (MAPKKK), which activates downstream terminal MAPK kinases p38 and c-Jun amino-terminal kinase (JNK) (5). The JNK/p38 pathway stimulates production of inflammatory cytokines/chemokines, promotes cell apoptosis, contributes to metabolic perturbations, and induces fibrosis (6,7). Increased kidney cortical and glomerular p38 and JNK activation has been observed in several kidney diseases, including DKD, mesangial proliferative glomerulonephritis (GN), and anti–glomerular basement membrane GN, in both humans and experimental models (8–10). Previous studies indicate that global knockout or pharmacologic inhibition of ASK1 can attenuate the activation of JNK and p38, reduce progressive podocyte apoptosis, improve podocyte morphology, attenuate renal fibrosis, and halt declining GFR in multiple rodent models of CKD (11–14).
The ASK1 inhibitor selonsertib is currently under clinical investigation as a potential treatment in DKD (ClinicalTrials.gov identifiers, NCT02177786 and NCT04026165) (15). Selonsertib initiation is associated with an acute decrease in eGFR that is hypothesized to be due to inhibition of tubular secretion of creatinine and not due to a hemodynamic mechanism (15). Therefore, we assessed the acute effect of selonsertib on renal hemodynamics in the setting of CKD. In a chronic 5/6 Nx model, we evaluated the renal benefits of selonsertib treatment when combined with an ACEI, the latter at a dose established to normalize glomerular hemodynamics.
Materials and Methods
Animals
Adult male Sprague Dawley rats (8–9 weeks old; 250–350 g; Charles River, Nashville, TN) were housed under normal conditions with a 12-hour light/dark cycle at 21°C with 40% humidity and 12 air exchanges per hour. All studies were approved by the Institutional Animal Care and Use Committee.
For chronic studies of CKD, glomerulosclerosis was induced by removing one kidney and ablating two thirds of the contralateral kidney by ligating branches of the renal arteries, as previously described (1). All rats underwent open-shave renal biopsy at 8 weeks to assess the severity of glomerulosclerosis, as previously described (3). On average, 15 glomeruli (range, 10–30 glomeruli) were available in these biopsy specimens. Rats were randomized at 8 weeks into four groups with similar levels of systemic BP, urinary protein levels, and glomerulosclerosis: the control group (n=11) received normal rat chow and water ad libitum (5001 diet, Purina Laboratory Rodent diet, 23.4% protein, 4.5% fat, 6.0% fiber, 0.40% sodium); the selonsertib group (n=12; ASK1 inhibitor, selonsertib; Gilead Sciences, Foster City, CA) received chow containing 0.009% selonsertib; the enalapril group (n=12) received normal rat chow and 50 mg/L enalapril in drinking water (antihypertensive dose); and the combination group (n=12) received both selonsertib and enalapril treatment. Doses were determined on the basis of efficacy in previous rodent studies (3). Selonsertib was provided at a dose designed to achieve >90% inhibition of ASK1 function (Supplemental Figure 1), similar to the 18-mg oral, once daily dose in humans (15). Animals were anesthetized for surgeries with sodium pentobarbital (Abbott Laboratories, North Chicago, IL; 25–30 mg/kg body wt, intraperitoneal). Animals were then euthanized at week 12 after 5/6 Nx.
In addition, a separate group of male Sprague Dawley rats (6–7 weeks old, 176–200 g; Charles River Laboratories, Wilmington, MA) were used for acute studies of selonsertib in an ablation, nonligation, 5/6 Nx model. These rats were acclimatized under standard conditions at Plato BioPharma, Inc. (PBI; Westminster, CO) with a 12-hour light/dark cycle and fed a standard chow diet (22% protein, 0.4% sodium; 8640 diet; Envigo, Indianapolis, IN) for at least 7 days before the study. Rats (n=56) underwent right unilateral nephrectomy via a retroperitoneal approach, and a group of weight-matched controls (n=16) underwent sham surgery. One week later (day −7), the uninephrectomized rats underwent subtotal left nephrectomy by targeted renal ablation via pole resection, using a retroperitoneal approach, and the control rats underwent a second sham surgery before recovery for 1 week in standard housing conditions. After 7 weeks (day 49), kidney function was assessed and rats with disease induction, defined as plasma BUN >30 mg/dl and plasma creatinine >0.65 mg/dl, were entered into further intervention studies. Starting on day 53, the 5/6 Nx rats were treated orally with 10-mg/kg selonsertib or 5 ml/kg-vehicle (0.5% carboxymethyl cellulose sodium, medium viscosity, 0.25% polysorbate-80 in pH 2.0 deionized water) twice daily for seven consecutive doses over 4 days. Clearance measurements were taken on day 56, 2 hours after the final morning dose of selonsertib. The same dose of selonsertib (10 mg/kg, twice daily) was used on the basis of pharmacokinetic/pharmacodynamic data in rats (Supplemental Figure 1) demonstrating comparable profile to an 18-mg selonsertib dose in humans (15). Control rats (sham surgery) received vehicle only.
Hemodynamics in Resection 5/6 Nx Rats
Kidney hemodynamic measurements were done by steady-state, bladder-catheterized urinary clearances of tritium-inulin and carbon-14–p-aminohippurate (PAH; PerkinElmer, Hopkinton, MA). Renal blood flow was calculated as effective renal plasma flow/(1−hematocrit), and effective renal plasma flow was calculated as the urinary clearance of PAH. BP and pulse were measured using an arterial catheter and transducer.
Renal Function in Chronic Ligation 5/6 Nx Rats
Systolic BP (SBP), albuminuria, serum creatinine, and creatinine clearance (Ccr) were assessed at weeks 0, 8, and 12. SBP was measured using tail-cuff plethysmography in conscious, trained rats (IITC Life Science Inc., Woodland Hills, CA). Animals were placed in metabolic cages for 24 hours for urine collection. Urine albumin was measured by ELISA, and serum and urine creatinine were measured by VitrosCREA slides (Johnson & Johnson Clinical Diagnostics Inc., Rochester, NY). Ccr was calculated as urinary creatinine×urine volume/serum creatinine, and was expressed as microliters per minute per body weight (1). Blood counts (reticulocyte, hemoglobin, hematocrit, and red blood cells) were assessed at euthanasia (Supplemental Table 1).
Morphologic Assessment
Kidney tissue from biopsy and autopsy was immersion fixed in 4% paraformaldehyde/PBS solution and routinely processed, and 3-μm sections were cut and stained with Periodic acid–Schiff. A semiquantitative score was used to evaluate the degree of glomerulosclerosis. Sclerosis was defined as obliteration of glomerular capillary loops by extracellular matrix. The severity of sclerosis in each glomerulus was graded from 0 to 4+ as follows: 0, no lesion; 1+, sclerosis of <25%; 2+, sclerosis of 25%–50%; 3+, sclerosis of >50%–75%; and 4+, sclerosis of >75% of the glomerulus, respectively. A whole kidney average sclerosis index for each rat was obtained by averaging scores from all glomeruli (range, 10–30) of biopsy specimen slides and all glomeruli on the section (n≥60) of autopsy specimen slides. All sections were examined without knowledge of the treatment. Change of sclerosis from biopsy specimen to autopsy specimen was calculated for each animal, as previously described (3).
Immunohistochemistry
For assessment of Wilms tumor-1 (WT1) and ED1 positivity, sections were microwaved in citrate buffer (pH 6.0) for 5 minutes. Endogenous peroxidase was quenched with 3% hydrogen peroxidase for 10 minutes, and slides were then exposed to Power Block (BioGenex Laboratories, San Ramon, CA) for 45 minutes. The primary antibodies used were rabbit anti-rat WT1 (1:800; Santa Cruz Biotechnology Inc., Dallas, TX) and mouse anti-rat ED1 (1:50; Dako North America, Carpinteria, CA), incubated overnight at 4°C. Immunoperoxidase staining was performed with the Vectastain ABC kit (Vector Laboratories, Burlingame, CA), with diaminobenzidine as a chromogen. Hematoxylin was used as a counterstain. WT1+ cell number per glomerulus area and percentage of ED1+ area in glomeruli or cortex were assessed. Positive and negative controls showed appropriate staining.
Terminal Deoxynucleotidyl Transferase–Mediated Digoxigenin-Deoxyuridine Nick-End Labeling Staining
The terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) staining technique was used for detecting DNA breaks using ApopTag Peroxidase In Situ Apoptosis Detection kit (Intergen Co., Purchase, NY) (16). In brief, 3-μm sections from paraffin-embedded tissue were pretreated by incubation with proteinase K (2 μg/ml) for 15 minutes at room temperature. Endogenous peroxidase was inhibited by 3% hydrogen peroxide in PBS for 5 minutes, and sections were rinsed with PBS, immersed in terminal deoxynucleotidyl transferase equilibration buffer, and then incubated with terminal deoxynucleotidyl transferase and digoxigenin–deoxyuridine triphosphate at 37°C for 60 minutes. The reaction was blocked with buffer, and anti–digoxigenin peroxidase conjugate was applied and incubated for 30 minutes. The slides were developed by using 3,3′-diaminobenzidine substrate. Apoptosis was assessed by scoring TUNEL+ cell number per glomerulus or tubulointerstitial area. All sections were examined without knowledge of the treatment. Positive and negative controls showed appropriate staining.
Western Blot
Frozen kidney tissue samples were transferred in radioimmunoprecipitation buffer containing 1:100 phosphatase inhibitor cocktail I, 1:100 phosphatase inhibitor cocktail II (Sigma-Aldrich, St. Louis, MO), and 1:100 proteinase inhibitor cocktail tablet (Roche Diagnostics, Mannheim, Germany). Samples were homogenized and centrifuged, and the protein concentration was measured using the DC Protein Assay kit (Bio-Rad Laboratories, Hercules, CA). Connective tissue growth factor (CTGF; 1:400; Abcam PLC, Cambridge, MA), plasminogen activator inhibitor 1 (PAI-1; 1:400; R&D Systems, Minneapolis, MN), phosphorylated JNK (p-JNK; 1:1000; Cell Signaling Technology, Inc., Danvers, MA), p-p38 (1:1000; Cell Signaling Technology Inc.), and glyceraldehyde 3-phosphate dehydrogenase (1:500; Cell Signaling Technology, Inc.) were detected by incubating with the corresponding antibody overnight at 4°C. Horseradish peroxidase–labeled anti-goat or anti-rabbit IgG secondary antibody (1:2500 in 5% milk Tris-buffered saline/Tween 20) was added and incubated at room temperature for 45 minutes. Protein bands on Western blots were visualized by ECL Plus (Amersham, Arlington Heights, IL) according to the manufacturer’s instructions, and were developed on film. Analysis was shown as p-JNK and p-p38 compared with total JNK (1:1000; Cell Signaling Technology, Inc.) and p38 (1:1000; Cell Signaling Technology, Inc.), respectively, in all groups, units expressed as arbritrary densitometric units (AU).
Statistical Analysis
Data are expressed as means±SEM, and as change in each rat from start of intervention until euthanasia. Differences among groups were examined by one-way ANOVA followed by Bonferroni correction for multiple comparisons. Nonparametric data were compared by Mann–Whitney U test. Differences among groups were examined by Kruskal–Wallis followed by the Dunn correction for multiple comparisons. All P values <0.05 were considered significant. Continuous variables were then tested with paired t test to compare week 8 with week 12. For data that were not normally distributed, the Wilcoxon matched-pairs signed rank test was used for comparisons of week 8 and week 12 outcomes.
RNA Sample Preparation and Sequencing
Up to six sections of 10-µm-thick formalin-fixed and paraffin-embedded scrolls were processed for RNA according to the manufacturer’s instructions (RNeasy FFPE Kit, Qiagen, MD). RNA quality was determined on an Agilent Bioanalyzer for RNA integrity and quantitated by Nanodrop. RNA samples were converted into cDNA libraries using the Illumina TruSeq Stranded Total RNA sample preparation kit (RS-122-2303; Illumina, San Diego, CA). Briefly, total RNA samples were concentration normalized, and ribosomal RNA (rRNA) was removed using biotinylated probes that selectively bind rRNA species. The resulting rRNA-depleted RNA was fragmented using heat in the presence of divalent cations. Fragmented RNA was converted into double-stranded cDNA, with deoxyuridine triphosphate used in place of deoxythymidine triphosphate in the second strand master mix. Final libraries were quantified, normalized, and pooled. Sequencing was performed using an Illumina sequencing platform, resulting in a median number of 92.6 million reads across samples after removal of sequencing artifacts.
RNA-Sequencing Data Analysis
Low quality reads, bases, and adapter sequences were removed using fastq-mcf (http://expressionanalysis.github.io/ea-utils/). Trimmed and filtered sequencing data were aligned to the rat reference genome RGSC Rnor_6.0 using STAR (version 2.4) and quantified using RSEM (version 1.2.14), accounting for reads which align to multiple genes and/or isoforms. Gene expression profiles were normalized using edgeR (version 3.24.3) using trimmed mean of M-values normalization. Differential gene expression analysis was performed using the limma-voom package (version 3.38.3), and differential expression was defined as fold change >1.5× and <0.67× for upregulation and downregulation, respectively, at 5% false discovery rate in comparison with the control group. Longitudinal comparisons were defined as the changes in a respective treatment group over time (i.e., autopsy versus biopsy) relative to the expression differences in the vehicle-treated group over time. Gene ontology and pathway enrichment analysis was performed using Metascape (https://metascape.org) with differentially expressed genes to identify selonsertib, enalapril, and combination specific transcriptional effects.
Results
Selonsertib Does Not Affect Renal Hemodynamics in the 5/6 Nx Model
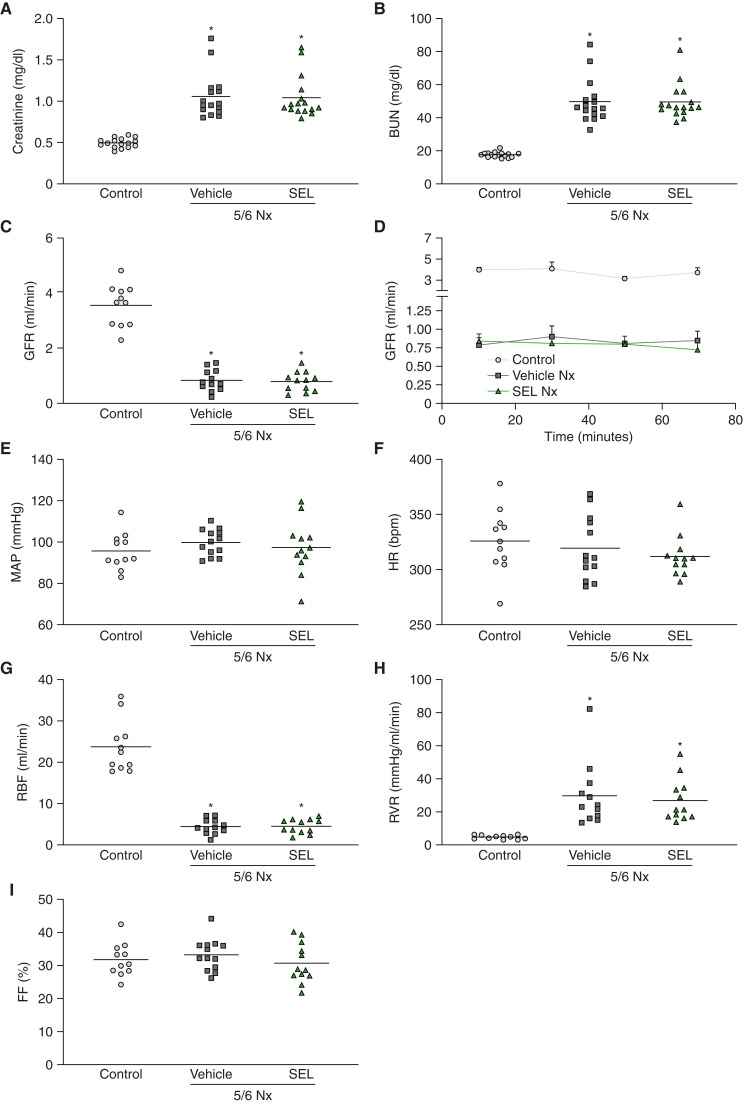
We first assessed if selonsertib had acute effects on systemic or kidney hemodynamic parameters. We used classic radiolabeled clearance techniques for precise measurements of GFR, renal blood flow, filtration fraction, and systemic BP in 5/6 Nx rats at week 8 after kidney mass resection (17). For these acute studies, we used a resection ablation method of 5/6 Nx, where the additional renal mass was surgically removed via polar excision 1 week after uninephrectomy; this approach causes similar impairment in renal function but induces only modest hypertension compared with the ligation method used for the chronic studies, and was, therefore, less likely to mask any potential hemodynamic effects of selonsertib (18). At 7 weeks after 5/6 Nx by resection, these rats had elevated plasma creatinine (0.80 mg/dl versus 0.39 mg/dl in controls) and plasma BUN concentrations (32.56 mg/dl versus 15.08 mg/dl in controls). After 4 days of treatment with selonsertib or vehicle, plasma levels of creatinine or BUN did not change in either group (Figure 1, A and B). Selonsertib did not cause any changes in GFR, systemic BP, or heart rate (Figure 1, C–F). Additionally, renal blood flow, renal vascular resistance, and filtration fraction, as measured by clearance of radiolabeled inulin and PAH, were also unchanged after selonsertib treatment (Figure 1, G–I), confirming that selonsertib does not change systemic or renal hemodynamics.
Figure 1.
Selonsertib does not have a hemodynamic effect in renal disease. Renal function parameters in 5/6 Nx rats treated with selonsertib or vehicle for 4 days at week 8 after surgery (n=11–16 animals per group). (A) Plasma creatinine concentration. (B) Plasma BUN concentration. (C) GFR represented as an average of four clearance periods. (D) GFR over time during four clearance periods. (E and F) Mean arterial pressure (MAP) and heart rate (HR), respectively, shown as an average of four clearance periods. (G) Renal blood flow (RBF). (H) Renal vascular resistance (RVR). (I) filtration fraction (FF). Data are presented as individual data points per animal (horizontal bar shows mean), except for (D), which are mean [SEM]. *P<0.05 versus control. 5/6 Nx, 5/6 nephrectomy; SEL, selonsertib.
Selonsertib and Enalapril Combined Further Attenuate Kidney Function Decline
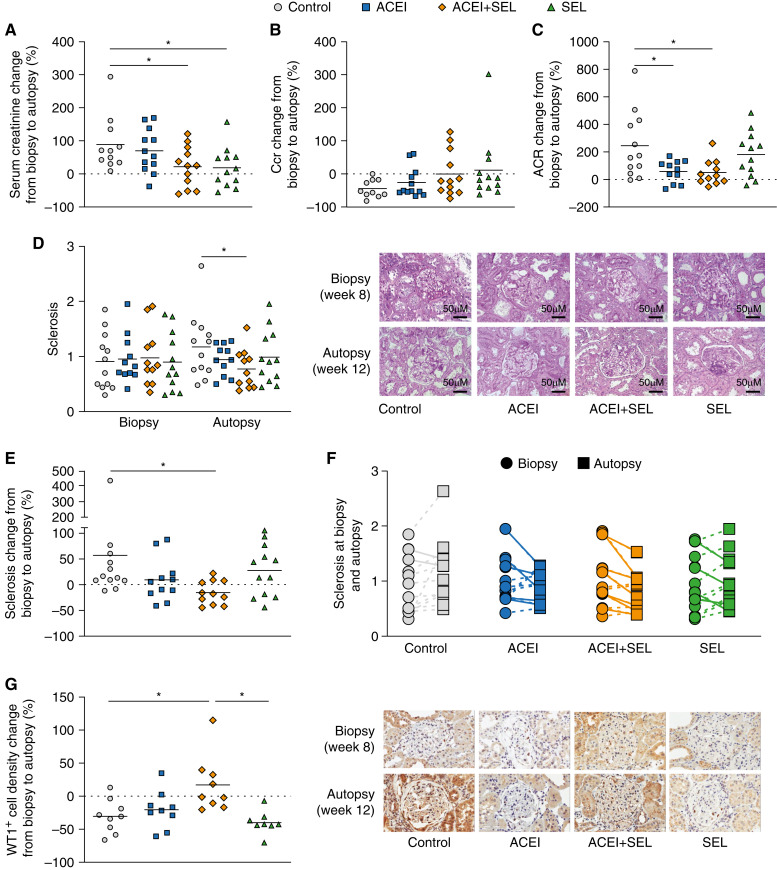
We next investigated the efficacy of selonsertib and the ACEI enalapril alone and in combination in the chronic ligation 5/6 Nx model. At 8 weeks after 5/6 Nx, BP was significantly increased, as expected (SBP 202.6 mm Hg, compared with baseline of 123.8 mm Hg). Selonsertib was well tolerated, evidenced by the fact that animals in all selonsertib treatment groups gained weight during the duration of treatment (Table 1). Serum creatinine increase from biopsy at week 8 to autopsy at week 12 was attenuated in animals treated with selonsertib (+19%±18%), or the combination (+22%±18%), but not with enalapril alone, compared with controls (+98%±26%; P<0.05; Figure 2A, Table 1). The selonsertib and combination groups maintained Ccr (selonsertib, +12%±29% [P<0.05] versus control; combination, +0.2%±20% [P=0.07] versus control), whereas Ccr decreased from week 8 to week 12 in the control (−49%±8%) and enalapril (–35%±11%) groups (Figure 2B, Table 1). Albuminuria, measured by urine albumin-creatinine ratio, was similar by study design among groups at week 8 before treatment initiation. In nontreated, diseased control rats, albumin-creatinine ratio increased by 253%±76% from week 8 to week 12. Selonsertib alone showed a similar increase in proteinuria (+181%±47%), whereas both enalapril alone and the combination of selonsertib plus enalapril resulted in significantly attenuated albuminuria increases (enalapril, +58%±24%; combination, +50%±28%; both P<0.05 versus control; Figure 2C, Table 2). Enalapril significantly reduced SBP at 12 weeks compared with control. Selonsertib had no significant effect on SBP, and combined treatment did not further reduce SBP versus enalapril alone (Table 1).
Table 1.
Functional parameters at week 0 and week 12
| Parameter | Week 0 | Week 8 | Week 12 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | ACEI | ACEI+SEL | SEL | Control | ACEI | ACEI+SEL | SEL | Control | ACEI | ACEI+SEL | SEL | |
| SBP, mm Hg | 123.8±3.8 | 118.9±3.5 | 122.1±2.3 | 122.2±4.0 | 202.6±10.9 | 200.0±9.0 | 194.0±7.8 | 201.0±6.7 | 210.4±8.5 | 175.0±9.2a,b | 170.5±9.3a | 184.7±9.2 |
| Body weight, g | 329.1±2.5 | 340.4±4.9 | 328.0±5.3 | 347.1±8.8 | 367.5±9.1 | 373.5±9.9 | 364.4±12.3 | 390.5±10.4 | 394.6±10.9c | 418.3±7.9b | 396.4±9.0d | 416.3±10.9e |
| UACR, µg/mg | 4.3±0.7 | 3.3±0.6 | 4.5±0.9 | 3.1±0.3 | 55.5±11.9 | 75.7±15.7 | 80.1±35.2 | 62.4±15.7 | 144.6±27.1c | 110.2±28.3 | 71.6±15.8a | 125.4±19.7e |
| Scr, mg/dl | 0.24±0.02 | 0.23±0.03 | 0.25±0.03 | 0.28±0.03 | 0.44±0.06 | 0.45±0.06 | 0.79±0.16 | 0.78±0.09 | 0.72±0.05c | 0.69±0.09b | 0.74±0.13 | 0.81±0.09 |
| Ccr, ml/min per 100 g body wt | 0.70±0.07 | 0.86±0.12 | 0.81±0.13 | 0.61±0.06 | 0.60±0.07 | 0.52±0.07 | 0.45±0.09 | 0.31±0.03 | 0.28±0.02c | 0.29±0.03b | 0.30±0.03 | 0.29±0.05 |
Data are shown as mean±SEM. n=9–12 animals per group. ACEI, angiotensin-converting enzyme inhibitor; SEL, selonsertib; SBP, systolic BP; UACR, urine albumin-creatinine ratio; Scr, serum creatinine; Ccr, creatinine clearance.
P<0.05 versus control at week 12.
P<0.05 versus ACEI at week 8.
P<0.05 versus at baseline (week 0).
P<0.05 versus ACEI+SEL at week 8.
P<0.05 versus SEL at week 8.
Figure 2.
Combination of selonsertib and enalapril prevents decline of kidney function and progression of glomerulosclerosis. 5/6 Nx rats were biopsied and randomized to treatment groups (n=9–12 animals per group) at week 8 and euthanized at week 12. (A) Change in serum creatinine concentration. (B) Change in creatinine clearance (Ccr). (C) Change in urine albumin-creatinine ratio (ACR). (D) Glomerulosclerosis at biopsy and autopsy, assessed using a semiquantitative 0 to 4+ score. (E and F) Change in glomerulosclerosis. (G) Change in density of WT1+ glomerular cells. Data are presented as individual data points per animal (horizontal bar shows mean). *P<0.05 versus control. ACEI, angiotensin-converting enzyme inhibitor; SEL, selonsertib; WT1, Wilms tumor-1.
Table 2.
Structural parameters at week 8 and 12
| Parameter | Week 8 | Week 12 | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | ACEI | ACEI+SEL | SEL | Control | ACEI | ACEI+SEL | SEL | |
| Sclerosis index (0–4 scale) | 0.95±0.15 | 0.95±0.13 | 0.98±0.16 | 0.90±0.15 | 1.24±0.17a | 0.94±0.09 | 0.77±0.11b,c | 0.99±0.14 |
| Glomerular WT1+ cell density, ×10−3/µm2 | 6.33±0.64 | 5.79±0.62 | 6.05±0.55 | 5.59±0.45 | 4.12±0.61a | 4.38±0.57 | 6.59±0.33b,d,e | 3.51±0.45f |
| ED1+ area/glomerular area, % | 0.68±0.13 | 0.56±0.13 | 0.58±0.21 | 0.49±0.18 | 1.07±0.16a | 0.41±0.08b,e | 0.64±0.11 | 1.22±0.44f |
| ED1+ area/tubulointerstitial area, % | 0.27±0.05 | 0.17±0.03 | 0.27±0.07 | 0.47±0.13 | 1.23±0.21a | 0.67±0.11b,e,g | 0.94±0.25 | 1.24±0.16f |
| TUNEL+ cell number/tubulointerstitial area, 10−5/μm2 | 0.92±0.11 | 1.03±0.13 | 0.98±0.09 | 0.77±0.07 | 2.07±0.21a | 2.74±0.37g | 1.28±0.20b,d | 1.25±0.22b,d,f |
Data are shown as mean±SEM. n=9–12 animals per group. ACEI, angiotensin-converting enzyme inhibitor; ED1, Ectodysplasin A; SEL, selonsertib; WT1, Wilms tumor-1; TUNEL, terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling.
P<0.05 versus baseline (week 0) at week 8.
P<0.05 versus control at week 12.
P<0.05 versus ACEI+SEL at week.
P<0.05 versus ACEI at week 12.
P<0.05 versus SEL at week 12.
P<0.05 versus SEL at week 8.
P<0.05 versus ACEI at week 8.
Selonsertib and Enalapril Combination Reduced Glomerulosclerosis Progression and Attenuated Podocyte Cell Loss
In untreated, diseased controls, glomerulosclerosis increased by 57%±35% (Figure 2, D and E, Table 2) from biopsy at 8 weeks to autopsy at 12 weeks. Enalapril or selonsertib only numerically attenuated progression of glomerulosclerosis (enalapril, +9%±13%; selonsertib, +27%±14%) versus week 8. However, combined treatment not only halted the progressive increase in glomerulosclerosis, but even showed less sclerosis, on average, at the end of study than at biopsy (combination, −22%±31%). Specifically, seven of 12 rats in the combination group demonstrated less sclerosis at autopsy versus biopsy, compared with only two of 12 in the controls, five of 12 in the enalapril group, and four of 12 in the selonsertib group (Figure 2F, Table 2).
The density of WT1+ glomerular cells, a marker of mature podocytes, decreased from biopsy to autopsy in control 5/6 Nx rats, with minimal numeric improvement with enalapril or selonsertib monotherapy. By contrast, the combination group had higher WT1+ cell density at 12 weeks (6.59±0.33 × 10−3/µm2) than controls (4.21±0.55 × 10−3/µm2). Higher WT1+ cell density from biopsy was significantly enhanced (P<0.05) at 12 weeks with combination treatment versus control or selonsertib alone (Figure 2G, Table 2).
Combination of Selonsertib and Enalapril Modifies Gene Expression
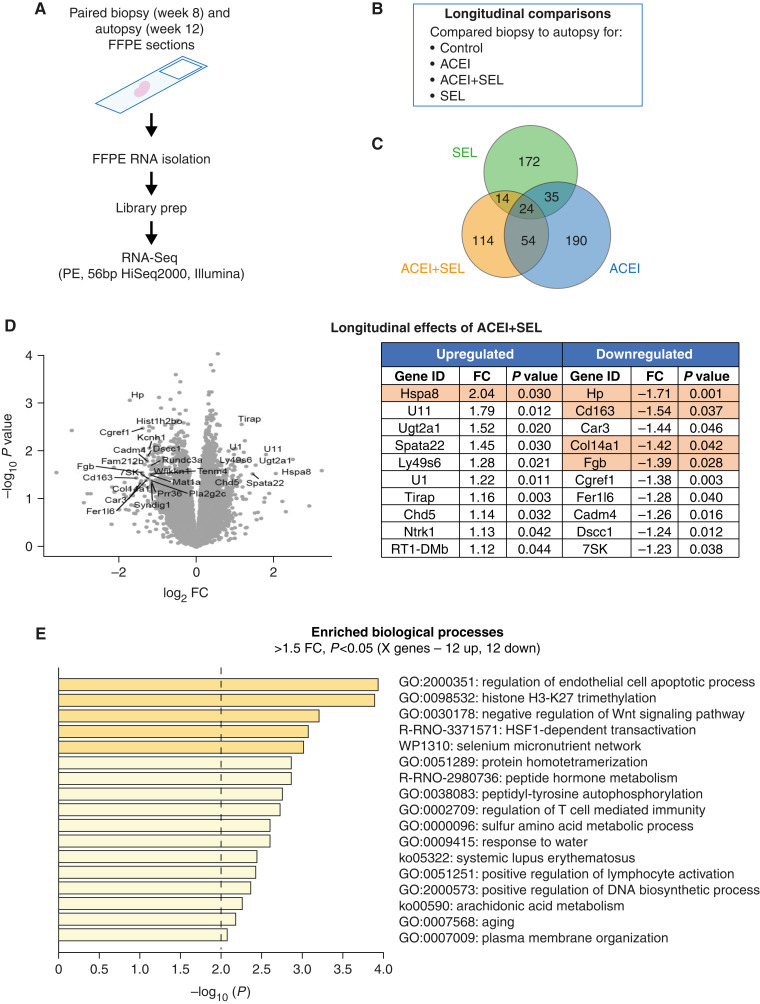
We next assessed effects of the combination of selonsertib and enalapril on molecular signatures versus enalapril or selonsertib alone by RNA-sequencing (RNA-seq) analysis. We compared biopsy (week 8) to autopsy (week 12) samples in the same rats, and also compared changes in gene expression over time across groups (longitudinal comparisons within each paired animal across groups; Figure 3, A and B). In longitudinal comparisons, 172 genes were specifically modified by selonsertib alone, 190 genes by enalapril alone, and 114 genes by the combination of both agents alone, suggesting different protection mechanisms by these interventions (Figure 3C). We observed a significant number of downregulated genes in the combination group over time (Figure 3D, left). The top ten significantly (P<0.05) differentially regulated genes from this analysis are shown in Figure 3D (right). The most downregulated gene after combination treatment was for haptoglobin (Hp), which has been previously proposed to be a biomarker of DKD progression (19, 20). In the combination group, significantly affected pathways included genes related to regulation of apoptotic processes, Wnt signaling, and general inflammation (Figure 3E). However, the comparison between treated and control groups at 12 weeks (end of study) revealed fewer statistically significant changes in the treatment groups (Supplemental Figures 2 and 3). The longitudinal comparisons suggest that interventions predominantly modified the trajectory of gene expression over time, as opposed to resulting in significant changes across groups at the end of the study. To understand expression patterns of genes significantly modulated by the selonsertib and enalapril combination, we examined publicly available single-cell sequencing data from the Kidney Precision Medicine Atlas (Supplementary Figures 4 and 5), which revealed that the affected genes were broadly expressed in different cell types and not enriched for one particular cell type.
Figure 3.
Treatment with selonsertib, enalapril, or combination leads to differential gene ontology response in CKD progression. 5/6 Nx rats were biopsied and randomized to treatment groups (n=9–12 animals per group) at week 8 and euthanized at week 12. (A) Preparation of samples for RNA-sequencing (RNA-seq) analysis. (B) Longitudinal comparisons. (C) Number of genes modified by each treatment. (D) Genes specifically modified by the combination treatment. (E) Significantly affected molecular pathways. FC, fold change; FFPE, formalin-fixed paraffin-embedded; SEL, selonsertib.
Different Effects of Selonsertib, Enalapril, and Combination on Apoptosis, MAPK, Inflammation, and Fibrosis
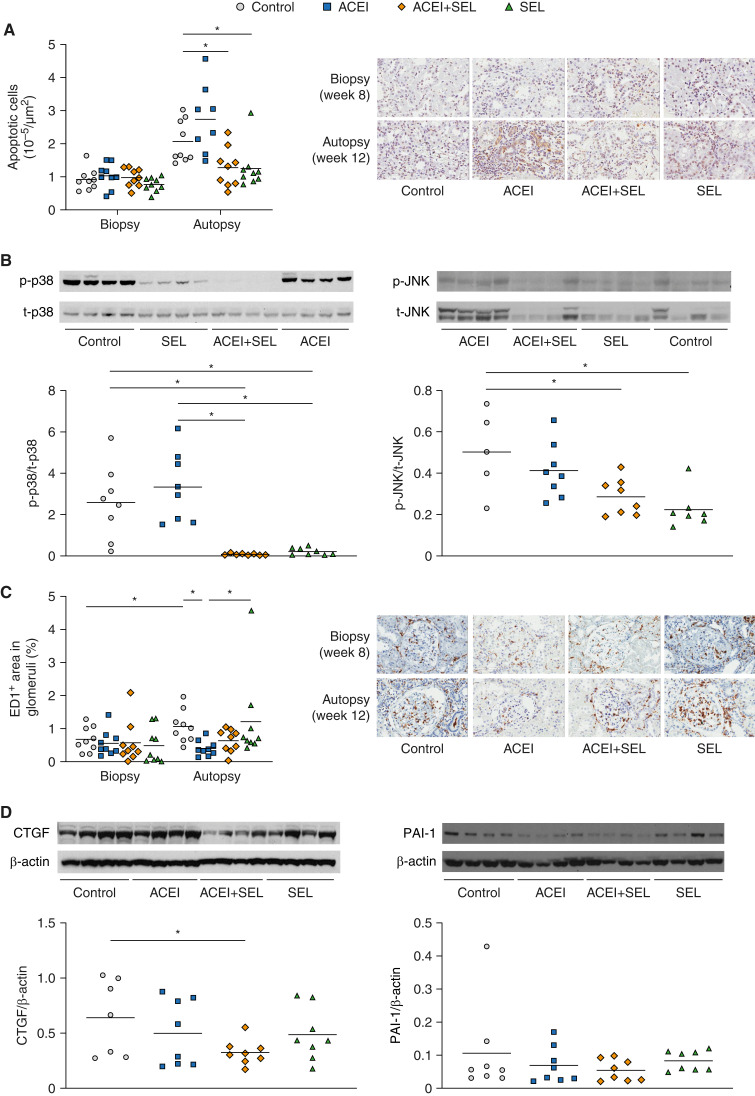
To further investigate the effects of selonsertib versus enalapril on apoptosis, MAPK, inflammation, and fibrosis, we measured related protein expression by Western blot and immunostaining. Both selonsertib alone and combination therapy, but not enalapril alone, decreased the density of apoptotic cells in the kidney, mainly in the tubulointerstitial area (selonsertib, 1.25±0.22 × 10−5/µm2; combination, 1.28±0.2 × 10−5/µm2; both P<0.05 versus controls) compared with controls (2.07±0.21 × 10−5/µm2) and enalapril alone (2.74±0.37 × 10−5/µm2) (Figure 4A). Significant inhibition (>90%) of p-JNK and p-p38 (both downstream targets of ASK1) was observed by Western blot of the kidney cortex after selonsertib treatment, confirming activity of the ASK1 inhibitor (Figure 4B).
Figure 4.
Treatment with selonsertib, enalapril, or the combination have differential effects on apoptosis, MAPK, inflammation, and fibrosis. 5/6 Nx rats were biopsied and randomized to treatment groups (n=8–12 animals per group) at week 8 and euthanized at week 12. (A) Density of apoptotic cells. (B) Relative expression of activated (p-) and total (t-) p38 and JNK at week 12. Western blot densitometry data expressed as arbitrary units (AU) (C) Glomerular ED1 positivity. (D) Expression of connective tissue growth factor (CTGF) and plasminogen activator inhibitor-1 (PAI-1) at week 12. Data are presented as individual data points per animal (horizontal bar shows mean). *P<0.05 versus control. JNK, c-Jun amino-terminal kinase; MAPK, mitogen-activated protein kinase; SEL, selonsertib.
Glomerular macrophage infiltration, assessed as the percentage of glomerular area with ED1 positivity, increased from biopsy to autopsy in control animals (0.68%±0.13% versus 1%±0.16%, respectively) and in animals treated with selonsertib alone (0.49%±0.18% versus 1%±0.44%, respectively), whereas only minor changes were observed in animals treated with enalapril alone (0.56%±0.13% versus 0.41%±0.07%, respectively) or the combination (0.58%±0.21% versus 0.64%±0.11%, respectively; Figure 4C, Table 2).
TGF-β is a major profibrotic molecule linked with progression of glomerulosclerosis and tubulointerstitial fibrosis. Persistent activation of TGF-β is associated with kidney scarring. CTGF is induced by TGF-β and mediates many of its fibrotic effects (21). CTGF protein levels relative to β-actin were only numerically reduced by monotherapy with enalapril (0.50±0.11) or selonsertib (0.49±0.08) versus control (0.64±0.13). Combined treatment with selonsertib and enalapril significantly decreased CTGF expression (0.32±0.04; P<0.05) versus control (Figure 4D). Increased PAI-1 expression is also associated with inhibition of matrix degradation. Combination treatment only numerically decreased PAI-1 expression relative to β-actin (0.05±0.01) versus control (0.11±0.05; Figure 4D).
Discussion
In this study, selonsertib in combination with the ACEI enalapril attenuated glomerulosclerosis progression and renal function decline when treatment was started when renal injury and decreased GFR were already established, mimicking the clinical setting. These beneficial effects were greater than those observed with each single agent alone, and were not attributable to additional hemodynamic effects by selonsertib. Importantly, selonsertib did not influence systemic BP or albuminuria in this chronic 5/6 Nx model, demonstrating an independent, but complementary, mechanism to renin-aldosterone-angiotensin system inhibition. Selonsertib was also well tolerated in the chronic study. Our acute studies in a 5/6 Nx model further showed that selonsertib does not acutely change GFR, renal blood flow, BP, or heart rate. Of note, both ACEI and sodium-glucose cotransporter-2 inhibitors are known to cause acute decreases in eGFR (“eGFR dip”) due to acute lowering of systemic and/or glomerular hemodynamics, whereas selonsertib has also been shown to cause acute decreases in eGFR that are attributed to the inhibition of the tubular creatinine transporters MATE1/2K (22,23). Although these transporters do not have a prominent role in regulating plasma creatinine in rodents (24), this study and previously published studies demonstrating no effect on hemodynamics with selonsertib or ASK1 inhibition are consistent with the blockade of creatinine secretion as a cause for eGFR changes; however, more clinical studies are needed to fully address this. Our data are thus consistent with previously published findings in ASK1-knockout mice and from studies with ASK1 inhibitors in rodent models of renal dysfunction in which no changes in hemodynamics were detected (25). This nonhemodynamic mechanism differentiates selonsertib from current CKD therapies such as ACEIs, ARBs, and the novel sodium-glucose cotransporter-2 inhibitors, which reduce intraglomerular pressure, among other actions (26). ACEIs have well-defined beneficial hemodynamic, anti-inflammatory, and antifibrotic effects in CKD/DKD, and our results highlight the potential benefits of combining a glomerular pressure–lowering therapy with an agent that targets glomerular inflammation and apoptosis (13,27).
In normal physiologic conditions, ASK1 is maintained in an inactive state by binding to the redox-sensitive protein thioredoxin and is only activated via autophosphorylation of the activation loop upon stimulation by factors such as ROS, hypoxia, proinflammatory cytokines, and angiotensin II (27,28). ASK1 affects downstream MAPK signaling through p38 and JNK, both of which play roles in renal inflammation, apoptosis, and fibrosis (11,29). In both human and experimental CKD/DKD, ASK1 pathway activation is increased, as evidenced by higher levels of p-ASK1 and p-p38 (11). ASK1−/− mice are well characterized and do not have altered cardiac or systemic hemodynamics compared with wild-type mice, but are protected from heart failure and cardiac injury (30,31). In previous models of CKD, treatment with small-molecule inhibitors of ASK1 improved GFR without an associated effect on BP, similar to the current rat studies (11). Importantly, this study further demonstrates that ASK1 inhibition has additive effects on reducing glomerulosclerosis, which translate to less functional decline over ACEIs alone.
Combination treatment with enalapril and selonsertib also resulted in a greater preservation of podocytes, which have limited, if any, regenerative potential after injury. ACEIs can reduce intraglomerular pressure and thereby reduce mechanical stress on the podocyte; we observed significant podocyte protection, as measured by WT1+ staining density, in animals treated with the combination of enalapril and selonsertib. The loss of podocytes is strongly associated with progressive kidney disease. Additional studies from our group and others have reported that ASK1 inhibition can play a role to preserve podocyte numbers in the setting of kidney injury (11,12). Interestingly, single agents previously showed efficacy on podocyte loss in milder models of CKD, including the mild diabetic nephropathy model of db/db eNOS−/− mice. In contrast, a single agent alone initiated after disease is established is not sufficient to reduce podocyte loss in a more severe, hypertensive model of glomerulosclerosis, like the 5/6 Nx model, highlighting the need for a combination of therapeutic agents to attenuate podocyte loss in most human CKD settings. Additionally, our results show significant reductions in overall kidney apoptotic cells, including tubular epithelial cells, as measured by TUNEL staining, after selonsertib or combination treatments, but not with enalapril alone. This suggests the effect of selonsertib on apoptosis in the 5/6 Nx model is not limited to glomerular cells and that the effect on apoptosis with combination therapy is primarily driven by selonsertib. Although selonsertib treatment induced an accumulation in ED1+ cells, there may be shifts in macrophage subtypes between pro- and anti-inflammatory subsets (i.e., M1 versus M2). Future studies are required to understand whether such population shifts occur in this setting.
In addition to demonstrating a combination effect on glomerulosclerosis and kidney function, unbiased transcriptomic analysis revealed several pathways modulated by enalapril and selonsertib alone or in combination. Both enalapril and selonsertib affected a similar number of genes when analyzed over time (longitudinal comparison between autopsy and biopsy); however, the combination treatment affected additional genes and pathways that may underlie the current efficacy. Pathways affected by the combination include those involved in regulating apoptosis, Wnt signaling, and immunity/inflammation. Several of these genes, including Hp, CD163, Col14a1, and Fgb, have been linked to kidney injury, inflammation, and fibrosis, and to kidney disease progression in various CKDs (19,20,32–38); this subset of genes and pathways may explain, in part, the beneficial effects of combination treatment, although further evidence would be needed to confirm.
A limitation of this study is that comparison between biopsy and autopsy RNA-seq data was challenging because histopathology scores at biopsy, not gene expression profiles, were used to normalize study groups before treatment, introducing an additional level of variability into the end-of-study comparison, which may affect statistical power. However, these data still serve as an initial starting point to provide insight and hypothesis generation for further investigations. Additionally, the RNA-seq was performed on bulk tissue, which may confound the heterogeneity of cell types detected. However, single-cell RNA-seq data from public-domain data banks confirmed there was not one specific cell type enriched for the genes that were significantly modulated by the selonsertib and enalapril combination.
In summary, our results demonstrating an additive effect of selonsertib with enalapril to reduce glomerulosclerosis progression and functional decline were observed without any additional hemodynamic effects beyond those of ACEI alone, coupled with an unbiased examination of molecular pathways affected by combination therapy. These data advance understanding of how ASK1 inhibition contributes to kidney protection and support previous observations in humans, demonstrating potential for selonsertib in reducing eGFR slope decline in patients with DKD who are receiving ACEI therapies (15). These findings provide further rationale for the continued investigation of selonsertib for the treatment of kidney disease when added to current standard of care.
Disclosures
T. Al Tuhaifi reports having ownership interest in Butterfly Network and Merck. S.S. Badal reports being employed by, and having ownership interest in, Gilead Sciences. D.G. Breckenridge reports being employed by, and serving in an advisory or leadership role for Actio Biosciences; and having ownership interest in Actio Biosciences and Gilead Sciences. A.B. Fogo reports serving as a speaker at various national nephrology meetings for Associate Editor Kidney International, and as president elect of the International Society of Nephrology (president as of April 19, 2021); serving on the editorial boards of the American Journal of Pathology, American Journal of Physiology–Renal Physiology, Human Pathology, and JASN; receiving honoraria from Amgen, GlaxoSmithKline, and Novartis; serving on the advisory committee of Bayer; receiving research funding from Bayer, Gilead, and Novartis; serving as guest editor for the yearly pathology focus of Current Opinion in Nephrology and Hypertension, associate editor of Kidney International and Laboratory Investigation, and subject editor of Nephrology Dialysis Transplantation; and having consultancy agreements with Novartis. K. Joly reports being employed by, having ownership interest in, and receiving research funding from, PBI. J.T. Liles reports being employed by, having ownership interest in, and receiving research funding from Gilead Sciences. D. Lopez reports being employed by, and having ownership interest in, Gilead Sciences. C.T. Plato reports acquiring restricted stock (not yet unrestricted) in Inotiv, Inc. as part of the acquisition of PBI; being granted stock options in Inotiv, Inc. as part of an employment offer with Inotiv, Inc. (these stock options have not yet vested in any way); and being employed by, formerly having ownership interest in, receiving research funding from, serving in an advisory or leadership role (on the board of directors), and being the founder and president of, PBI (Inotiv, Inc. acquired PBI on October 4, 2021, so ownership interest no longer exists; C.T. Plato is no longer an officer or board member of any company and is now the senior vice president of physiologic sciences at Inotiv, Inc.). All remaining authors have nothing to disclose.
Funding
This work was supported, in part, by a Gilead Sciences (Gilead) grant, and by National Institute of Diabetes and Digestive and Kidney Diseases grant DK 56942 (to A.B. Fogo).
Acknowledgments
The authors would like to thank Swati Kaushik for bioinformatics support. Editorial support was provided by Sarah Graham of PharmaGenesis London (London, United Kingdom), and funded by Gilead Sciences, Inc. Hadramout Establishment for Human Development is thanked for sponsoring T. Al Tuhaifi’s research fellowship program at Vanderbilt University.
PBI is a leading preclinical drug discovery contract research organization (CRO). As such, PBI was compensated for providing research services resulting in the generation of data supporting submitted abstracts and potential full publications. Inotiv, Inc. acquired PBI on October 4, 2021, and is a CRO, and was thus also compensated for providing research services resulting in generation of data supporting submitted abstracts and potential publications.
Footnotes
See related editorial, “ASK1 Inhibitor in Chronic Kidney Disease Therapy: From Bench to Bedside,” on pages 1128–1131.
Author Contributions
T. Al Tuhaifi, S.S. Badal, K. Joly, C.T. Plato, H.-C. Yang, and Y.-F. Yu were responsible for investigation; S.S. Badal, D.G. Breckenridge, A.B. Fogo, J. T. Liles, D. Lopez, and H.-C. Yang were responsible for formal analysis; S.S. Badal, D.G. Breckenridge, A.B. Fogo, and H.-C. Yang conceptualized the study and wrote the original draft; D.G. Breckenridge, A.B. Fogo, and J. T. Liles were responsible for methodology; and all authors reviewed and edited the manuscript.
Data Sharing
All data is included in the manuscript and/or supporting information.
Supplemental Material
This article contains the following supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0001032022/-/DCSupplemental.
Hematocrit and hemoglobin at week 12. Download Supplemental Table 1, PDF file, 229 KB (1,011.2KB, pdf)
Selonsertib PK/PD relationship in a rat model of fibrosis. Download Supplemental Figure 1, PDF file, 229 KB (1,011.2KB, pdf)
Differentially expressed genes with selonsertib and ACEI treatment from longitudinal comparisons. Download Supplemental Figure 2, PDF file, 229 KB (1,011.2KB, pdf)
Differentially expressed genes with selonsertib, ACEI or combination treatment from end-of-study comparisons. Download Supplemental Figure 3, PDF file, 229 KB (1,011.2KB, pdf)
Single-Cell RNA-Seq violin plots of genes down-regulated in selonsertib and enalapril combination longitudinal comparisons. Download Supplemental Figure 4, PDF file, 229 KB (1,011.2KB, pdf)
Single-Cell RNA-Seq violin plots of genes up-regulated in selonsertib and enalapril combination longitudinal comparisons. Download Supplemental Figure 5, PDF file, 229 KB (1,011.2KB, pdf)
References
- 1.Yang HC, Zuo Y, Fogo AB: Models of chronic kidney disease. Drug Discov Today Dis Models 7: 13–19, 2010. 10.1016/j.ddmod.2010.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naito T, Ma L-J, Yang H, Zuo Y, Tang Y, Han JY, Kon V, Fogo AB: Angiotensin type 2 receptor actions contribute to angiotensin type 1 receptor blocker effects on kidney fibrosis. Am J Physiol Renal Physiol 298: F683–F691, 2010. 10.1152/ajprenal.00503.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma LJ, Nakamura S, Aldigier JC, Rossini M, Yang H, Liang X, Nakamura I, Marcantoni C, Fogo AB: Regression of glomerulosclerosis with high-dose angiotensin inhibition is linked to decreased plasminogen activator inhibitor-1. J Am Soc Nephrol 16: 966–976, 2005. 10.1681/ASN.2004060492 [DOI] [PubMed] [Google Scholar]
- 4.Yang HC, Fogo AB: Mechanisms of disease reversal in focal and segmental glomerulosclerosis. Adv Chronic Kidney Dis 21: 442–447, 2014. 10.1053/j.ackd.2014.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H: ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep 2: 222–228, 2001. 10.1093/embo-reports/kve046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grynberg K, Ma FY, Nikolic-Paterson DJ: The JNK signaling pathway in renal fibrosis. Front Physiol 8: 829, 2017. 10.3389/fphys.2017.00829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canovas B, Nebreda AR: Diversity and versatility of p38 kinase signalling in health and disease. Nat Rev Mol Cell Biol 22: 346–366, 2021. 10.1038/s41580-020-00322-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagai H, Noguchi T, Takeda K, Ichijo H: Pathophysiological roles of ASK1-MAP kinase signaling pathways. J Biochem Mol Biol 40: 1–6, 2007. 10.5483/bmbrep.2007.40.1.001 [DOI] [PubMed] [Google Scholar]
- 9.Adhikary L, Chow F, Nikolic-Paterson DJ, Stambe C, Dowling J, Atkins RC, Tesch GH: Abnormal p38 mitogen-activated protein kinase signalling in human and experimental diabetic nephropathy. Diabetologia 47: 1210–1222, 2004. 10.1007/s00125-004-1437-0 [DOI] [PubMed] [Google Scholar]
- 10.Sakai N, Wada T, Furuichi K, Iwata Y, Yoshimoto K, Kitagawa K, Kokubo S, Kobayashi M, Hara A, Yamahana J, Okumura T, Takasawa K, Takeda S, Yoshimura M, Kida H, Yokoyama H: Involvement of extracellular signal-regulated kinase and p38 in human diabetic nephropathy. Am J Kidney Dis 45: 54–65, 2005. 10.1053/j.ajkd.2004.08.039 [DOI] [PubMed] [Google Scholar]
- 11.Liles JT, Corkey BK, Notte GT, Budas GR, Lansdon EB, Hinojosa-Kirschenbaum F, Badal SS, Lee M, Schultz BE, Wise S, Pendem S, Graupe M, Castonguay L, Koch KA, Wong MH, Papalia GA, French DM, Sullivan T, Huntzicker EG, Ma FY, Nikolic-Paterson DJ, Altuhaifi T, Yang H, Fogo AB, Breckenridge DG: ASK1 contributes to fibrosis and dysfunction in models of kidney disease. J Clin Invest 128: 4485–4500, 2018. 10.1172/JCI99768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen A, Xu J, Lai H, D’Agati VD, Guan T-J, Badal S, Liles J, He JC, Lee K: Inhibition of apoptosis signal-regulating kinase 1 mitigates the pathogenesis of human immunodeficiency virus-associated nephropathy. Nephrol Dial Transplant 36: 430–441, 2021. 10.1093/ndt/gfaa198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amos LA, Ma FY, Tesch GH, Liles JT, Breckenridge DG, Nikolic-Paterson DJ, Han Y: ASK1 inhibitor treatment suppresses p38/JNK signalling with reduced kidney inflammation and fibrosis in rat crescentic glomerulonephritis. J Cell Mol Med 22: 4522–4533, 2018. 10.1111/jcmm.13705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma FY, Tesch GH, Nikolic-Paterson DJ: ASK1/p38 signaling in renal tubular epithelial cells promotes renal fibrosis in the mouse obstructed kidney. Am J Physiol Renal Physiol 307: F1263–F1273, 2014. 10.1152/ajprenal.00211.2014 [DOI] [PubMed] [Google Scholar]
- 15.Chertow GM, Pergola PE, Chen F, Kirby BJ, Sundy JS, Patel UD; GS-US-223-1015 Investigators : Effects of selonsertib in patients with diabetic kidney disease. J Am Soc Nephrol 30: 1980–1990, 2019. 10.1681/ASN.2018121231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawarazaki Y, Ichijo H, Naguro I: Apoptosis signal-regulating kinase 1 as a therapeutic target. Expert Opin Ther Targets 18: 651–664, 2014. 10.1517/14728222.2014.896903 [DOI] [PubMed] [Google Scholar]
- 17.Luippold G, Beilharz M, Mühlbauer B: Chronic renal denervation prevents glomerular hyperfiltration in diabetic rats. Nephrol Dial Transplant 19: 342–347, 2004. 10.1093/ndt/gfg584 [DOI] [PubMed] [Google Scholar]
- 18.Lim BJ, Yang H-C, Fogo AB: Animal models of regression/progression of kidney disease. Drug Discov Today Dis Models 11: 45–51, 2014. 10.1016/j.ddmod.2014.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhensdadia NM, Hunt KJ, Lopes-Virella MF, Michael Tucker J, Mataria MR, Alge JL, Neely BA, Janech MG, Arthur JM; Veterans Affairs Diabetes Trial (VADT) study group : Urine haptoglobin levels predict early renal functional decline in patients with type 2 diabetes. Kidney Int 83: 1136–1143, 2013. 10.1038/ki.2013.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orchard TJ, Sun W, Cleary PA, Genuth SM, Lachin JM, McGee P, Paterson AD, Raskin P, Anbinder Y, Levy AP; DCCT/EDIC Research Group : Haptoglobin genotype and the rate of renal function decline in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes 62: 3218–3223, 2013. 10.2337/db13-0256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta S, Clarkson MR, Duggan J, Brady HR: Connective tissue growth factor: potential role in glomerulosclerosis and tubulointerstitial fibrosis. Kidney Int 58: 1389–1399, 2000. 10.1046/j.1523-1755.2000.00301.x [DOI] [PubMed] [Google Scholar]
- 22.Meraz-Muñoz AY, Weinstein J, Wald R: eGFR decline after SGLT2 inhibitor initiation: the tortoise and the hare reimagined. Kidney360 2: 1042–1047, 2021. 10.34067/kid.0001172021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neuen B, Tighiouart H, Heerspink H, Vonesh E, Chaudhari J, Miao S, Chan TM, Fervenza F, Floege J, Goicoechea M, Herrington W, Imai E, Jafar T, Lewis J, Li P, Locatelli F, Maes B, Perrone R, Praga M, Perna A, Schena F, Wanner C, Wetzels J, Woodward M, Xie D, Greene T, Inker L: Acute treatment effects on GFR in randomized clinical trials of kidney disease progression. J Am Soc Nephrol 33: 291–303, 2022. 10.1681/asn.2021070948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radi ZA: Kidney transporters and drug-induced injury in drug development. Toxicol Pathol 48: 721–724, 2020. 10.1177/0192623320937012 [DOI] [PubMed] [Google Scholar]
- 25.Yamashita T, Yamamoto E, Kataoka K, Nakamura T, Matsuba S, Tokutomi Y, Dong Y-F, Ichijo H, Ogawa H, Kim-Mitsuyama S: Apoptosis signal-regulating kinase-1 is involved in vascular endothelial and cardiac remodeling caused by nitric oxide deficiency. Hypertension 50: 519–524, 2007. 10.1161/HYPERTENSIONAHA.107.092049 [DOI] [PubMed] [Google Scholar]
- 26.Holtkamp FA, de Zeeuw D, Thomas MC, Cooper ME, de Graeff PA, Hillege HJL, Parving H-H, Brenner BM, Shahinfar S, Lambers Heerspink HJ: An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int 80: 282–287, 2011. 10.1038/ki.2011.79 [DOI] [PubMed] [Google Scholar]
- 27.Shiizaki S, Naguro I, Ichijo H: Activation mechanisms of ASK1 in response to various stresses and its significance in intracellular signaling. Adv Biol Regul 53: 135–144, 2013. 10.1016/j.jbior.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 28.Arthur JSC, Ley SC: Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol 13: 679–692, 2013. 10.1038/nri3495 [DOI] [PubMed] [Google Scholar]
- 29.Tesch GH, Ma FY, Han Y, Liles JT, Breckenridge DG, Nikolic-Paterson DJ: ASK1 inhibitor halts progression of diabetic nephropathy in Nos3-deficient mice. Diabetes 64: 3903–3913, 2015. 10.2337/db15-0384 [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi O, Higuchi Y, Hirotani S, Kashiwase K, Nakayama H, Hikoso S, Takeda T, Watanabe T, Asahi M, Taniike M, Matsumura Y, Tsujimoto I, Hongo K, Kusakari Y, Kurihara S, Nishida K, Ichijo H, Hori M, Otsu K: Targeted deletion of apoptosis signal-regulating kinase 1 attenuates left ventricular remodeling. Proc Natl Acad Sci USA 100: 15883–15888, 2003. 10.1073/pnas.2136717100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeda K, Noguchi T, Naguro I, Ichijo H: Apoptosis signal-regulating kinase 1 in stress and immune response. Annu Rev Pharmacol Toxicol 48: 199–225, 2008. 10.1146/annurev.pharmtox.48.113006.094606 [DOI] [PubMed] [Google Scholar]
- 32.Gurung RL, Dorajoo R, Liu S, M Y, Liu JJ, Wang L, Guo L, Yu X, Liu JJ, Lim SC: Genetic markers for urine haptoglobin is associated with decline in renal function in type 2 diabetes in East Asians. Sci Rep 8: 5109, 2018. 10.1038/s41598-018-23407-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mejia-Vilet JM, Zhang XL, Cruz C, Cano-Verduzco ML, Shapiro JP, Nagaraja HN, Morales-Buenrostro LE, Rovin BH: Urinary soluble CD163: A novel noninvasive biomarker of activity for lupus nephritis. J Am Soc Nephrol 31: 1335–1347, 2020. 10.1681/asn.2019121285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Randles M, Lausecker F, Kong Q, Suleiman H, Reid G, Kolatsi-Joannou M, Tian P, Falcone S, Davenport B, Potter P, Van Agtmael T, Norman J, Long D, Humphries M, Miner J, Lennon R: Identification of an altered matrix signature in kidney aging and disease. J Am Soc Nephrol 32: 1713–1732, 2021. 10.1681/asn.2020101442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuppe C, Ibrahim MM, Kranz J, Zhang X, Ziegler S, Perales-Patón J, Jansen J, Reimer KC, Smith JR, Dobie R, Wilson-Kanamori JR, Halder M, Xu Y, Kabgani N, Kaesler N, Klaus M, Gernhold L, Puelles VG, Huber TB, Boor P, Menzel S, Hoogenboezem RM, Bindels EMJ, Steffens J, Floege J, Schneider RK, Saez-Rodriguez J, Henderson NC, Kramann R: Decoding myofibroblast origins in human kidney fibrosis. Nature 589: 281–286, 2021. 10.1038/s41586-020-2941-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu C, Sun L, Xiao L, Han Y, Fu X, Xiong X, Xu X, Liu Y, Yang S, Liu F, Kanwar YS: Insights into the mechanisms involved in the expression and regulation of extracellular matrix proteins in diabetic nephropathy. Curr Med Chem 22: 2858–2870, 2015. 10.2174/0929867322666150625095407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang H, Zheng C, Lu Y, Jiang Q, Yin R, Zhu P, Zhou M, Liu Z: Urinary fibrinogen as a predictor of progression of CKD. Clin J Am Soc Nephrol 12: 1922–1929, 2017. 10.2215/cjn.01360217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goicoechea M, de Vinuesa SG, Gómez-Campderá F, Aragoncillo I, Verdalles U, Mosse A, Luño J: Serum fibrinogen levels are an independent predictor of mortality in patients with chronic kidney disease (CKD) stages 3 and 4. Kidney Int Suppl 74: S67–S70, 2008. 10.1038/ki.2008.519 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hematocrit and hemoglobin at week 12. Download Supplemental Table 1, PDF file, 229 KB (1,011.2KB, pdf)
Selonsertib PK/PD relationship in a rat model of fibrosis. Download Supplemental Figure 1, PDF file, 229 KB (1,011.2KB, pdf)
Differentially expressed genes with selonsertib and ACEI treatment from longitudinal comparisons. Download Supplemental Figure 2, PDF file, 229 KB (1,011.2KB, pdf)
Differentially expressed genes with selonsertib, ACEI or combination treatment from end-of-study comparisons. Download Supplemental Figure 3, PDF file, 229 KB (1,011.2KB, pdf)
Single-Cell RNA-Seq violin plots of genes down-regulated in selonsertib and enalapril combination longitudinal comparisons. Download Supplemental Figure 4, PDF file, 229 KB (1,011.2KB, pdf)
Single-Cell RNA-Seq violin plots of genes up-regulated in selonsertib and enalapril combination longitudinal comparisons. Download Supplemental Figure 5, PDF file, 229 KB (1,011.2KB, pdf)