CKD affects a large population of patients worldwide. It is characterized by gradual renal function loss resulting from persistent renal cell death, chronic inflammation, and fibrosis, and currently, effective treatment to reverse disease progression is lacking. In the recent study by Badal et al. (1), the authors examined the therapeutic effects of an apoptosis signal regulating kinase 1 (ASK1) inhibitor selonsertib in hypertension-induced glomerulosclerosis and renal function loss, which can be augmented in combination with an angiotensin-converting enzyme inhibitor (ACEi) enalapril. Their results shed new light on the potential clinical application of the ASK1 inhibitor in CKD treatment.
ASK1/MAP3K5, a member of the mitogen-activated protein kinase kinase kinase family, has been identified to play an indispensable role in stress- and cytokine-induced cell death, aberrant cell proliferation, and fibrosis in the last two decades. ASK1 activation mainly occurs during pathologic conditions when a redox responsive protein thioredoxin fails to suppress it after the cellular redox balance is broken. The downstream activated pathways include p38 mitogen-activated protein kinase (MAPK) and c-Jun amino terminal kinase, which are closely related to various diseases in diverse cell types (Figure 1). In tumors, ASK1 activation can lead to apoptosis of cancer cells, amplification of cytokine production in tumor-associated immune cells (primarily macrophages), and degradation of endothelial junction protein VE-cadherin to promote macrophage transmigration (2). In the liver, the inhibition of ASK1 by its inhibitors can attenuate hepatic inflammation and improve nonalcoholic fatty liver disease. It is also reported that ASK1 activation is essential for cardiac hypertrophy, which is verified in an Ang II–induced hypertension murine model by using ASK1 inhibitor selonsertib. In addition, both pharmacological and genetic inhibition of ASK1 in the mouse suppresses the development of bleomycin-induced pulmonary fibrosis (3).
Figure 1.
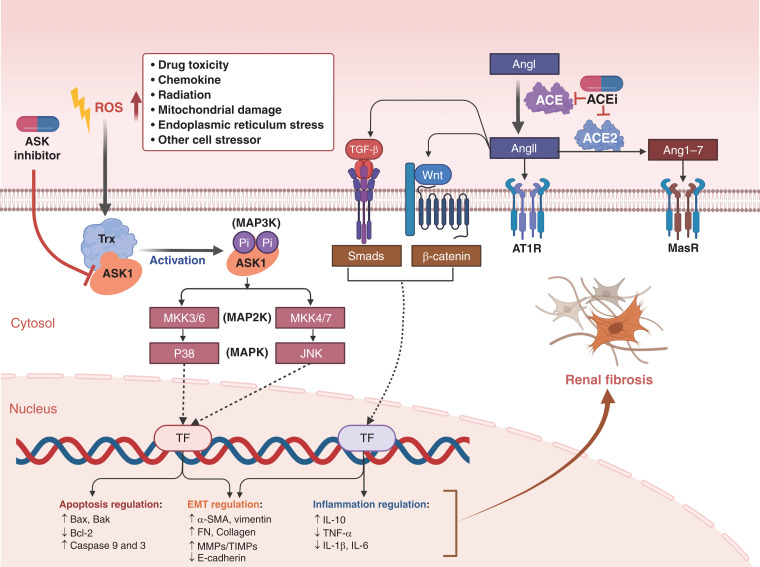
Apoptosis signal regulating kinase 1 (ASK1) inhibitor and angiotensin-converting enzyme inhibitor (ACEi) mediated protective effect on renal fibrosis. ASK1 inhibitor and ACEi can protect the kidney from fibrosis by blocking different pathways, and their combination has the potential to exert stronger function than monotherapy. When kidney cells are under stress, ASK1 activation will lead to P38 and c-Jun amino terminal kinase pathways activation, and further modulate the transcription of gene expression involved in apoptosis and epithelial/mesenchymal transition (EMT) regulation, which can be suppressed by ASK1 inhibitor. Meanwhile, ACEi may block the activation of TGF-β and WNT/β-catenin pathways through the modulation of the renin-angiotensin system, which will affect renal fibrosis by suppression of EMT and inflammation. Trx, thioredoxin; Ang, angiotensin; AT1R, angiotensin II type 1 receptor; MasR, Mas receptor; TF, transcription factor; FN, fibronectin.
In kidney, ASK1 also shows its tremendous effect on injury and fibrosis regulation. Several in vitro studies have indicated the role of ASK1 in oxidative stress–induced apoptosis of different murine kidney cells, including mesangial cells, podocytes, and tubular epithelial cells (4). Moreover, in vivo studies have verified the importance of ASK1 in AKI and repair (5). In both ischemia/reperfusion and unilateral ureter obstruction models, Ask1−/− mice showed protective effect on renal injury with reduced activation of p38 MAPK and c-Jun amino terminal kinase compared with wild-type mice. GS-444217, a selective small-molecule inhibitor of ASK1, significantly reduces ischemia/reperfusion and unilateral ureter obstruction–induced AKI in rats. ASK1 is especially noticed to mediate renal injury and fibrosis in CKDs, including diabetic kidney disease (DKD). ASK1 is activated by high glucose and its advanced glycation end products both in vivo and in vitro, resulting in p38 MAPK signaling pathway activation and renal fibrosis (4). Notably, in renal biopsies from patients with DKD, ASK1 pathway is activated in both glomerular and tubular compartments. Furthermore, GS-444217 can attenuate albuminuria and ameliorate other pathologic features of DKD such as glomerulosclerosis, loss of podocytes, and tubulointerstitial fibrosis in db/db eNOS–/– mice (6).
The universal pathologic role of ASK1 activation inspires enthusiasm to develop ASK1 inhibitors for therapy, and selonsertib has been tested in a few clinical trials (Table 1). It is notable that as a first-in-class, selective, and potent small-molecule inhibitor of ASK1, selonsertib is confirmed to be safe and well tolerated in healthy humans (7). It has been mainly tested for the treatment of fibrotic diseases such as DKD (8), nonalcoholic steatohepatitis (NASH) (9–11), and pulmonary arterial hypertension (12). In the phase 2 clinical trial to evaluate selonsertib’s safety and efficacy in adults with treatment refractory moderate-to-advanced DKD, despite the results not meeting the primary efficacy end point (no significant difference of mean eGFR between selonsertib and placebo groups at 48 weeks), post hoc analyses showed that selonsertib appears to slow the decline of kidney function after adjusting the confounded eGFR differences at 48 weeks related to the inhibition of creatinine secretion by selonsertib (8). Meanwhile, the phase 2 clinical trial of selonsertib conducted in patients with NASH and stage 2–3 fibrosis shows its promising effect on reducing liver fibrosis (9). Unfortunately, two randomized, double-blind, placebo-controlled, phase 3 trials of selonsertib monotherapy demonstrated no antifibrotic effect in patients with bridging fibrosis or compensated cirrhosis due to NASH, leading to the termination of both trials (10).
Table 1.
Summary of the clinical trials for selonsertib
| Year | Status | Phase | Disease Tested | Trial Objective | ClinicalTrials.gov Identifier | Reference |
|---|---|---|---|---|---|---|
| 2015 | Completed | 1 | DKD | To evaluate the pharmacokinetics of selonsertib in participants with normal and impaired hepatic function | NCT02509624 | |
| 2014–2016 | Completed | 2 | DKD | To determine the efficacy, safety, and tolerability of selonsertib in participants with DKD | NCT02177786 | (8) |
| 2019–2021 | Completed | 2 | DKD | To evaluate the efficacy and safety of selonsertib in participants with moderate to advanced DKD (MOSAIC) | NCT04026165 | (8) |
| 2014–2016 | Completed | 2 | PAH | To evaluate the effect of selonsertib in adults with PAH (ARROW) | NCT02234141 | (12) |
| 2016–2020 | Completed | 2 | AH | Selonsertib in combination with prednisolone versus prednisolone alone in participants with severe AH | NCT02854631 | |
| 2015–2016 | Completed | 2 | NASH | Safety, tolerability, and efficacy of selonsertib alone or in combination with simtuzumab (SIM) in adults with NASH and fibrosis stages F2–F3 | NCT02466516 | (9) |
| 2016–2018 | Completed | 2 | NASH | Safety, tolerability, and efficacy of selonsertib, firsocostat, and cilofexor in adults with NASH | NCT02781584 | (11) |
| 2018–2019 | Completed | 2 | NASH | To evaluate the safety and efficacy of selonsertib, firsocostat, cilofexor, and combinations in participants with bridging fibrosis or compensated cirrhosis due to NASH (ATLAS) | NCT03449446 | (11) |
| 2017–2019 | Terminated | 3 | NASH | Safety and efficacy of selonsertib in adults with NASH and bridging (F3) fibrosis (STELLAR-3) | NCT03053050 (STELLAR-3) | (10) |
| 2017–2019 | Terminated | 3 | NASH | Safety and efficacy of selonsertib in adults with compensated cirrhosis due to NASH (STELLAR-4) | NCT03053063 (STELLAR-4) | (10) |
DKD, diabetic kidney disease; PAH, pulmonary arterial hypertension; AH, alcoholic hepatitis; NASH, nonalcoholic steatohepatitis.
The reason for the failure of these clinical trials remains obscure. One possibility is that injuries in humans are more complicated compared with rodents and may involve redundant pathways other than ASK1 activation. For example, in a study by Sledz et al., selonsertib inhibited murine but not human platelet aggregation (13). Similarly, selonsertib monotherapy suppressed fibrosis in preclinical murine model studies but failed in human clinical trials. In this case, more preclinical research is necessary to identify the potential redundant mechanisms in humans compared with mice, and the combination therapy of ASK1 inhibitor with other drugs may be a new pathway for selonsertib to work for fibrosis treatment.
In this issue of Kidney360, Badal et al. provide new evidence for the potential combination treatment of ASK1 inhibitor with ACEi enalapril (1). In a hypertensive glomerulosclerosis CKD rat model (5/6 nephrectomy or 5/6 Nx), they evaluated the severity of glomerulosclerosis development in rats with selonsertib, enalapril, combination (selonsertib+enalapril), and untreated controls. Although selonsertib or enalapril alone can attenuated serum creatinine induction or lower systolic BP and albuminuria, respectively, the combined treatment significantly reduced glomerulosclerosis, renal function decline, podocyte loss, and overall renal apoptosis compared with monotherapy. Their results indicate that selonsertib and ACEi may offer protection from chronic renal injury through different pathways (Figure 1) or work on different renal cells. Selonsertib does not affect the systemic or renal hemodynamics but can inhibit renal cell apoptosis. Meanwhile, enalapril does not suppress cell apoptosis but reduces macrophage infiltration and lowers systolic BP. Overall, it provides prospective evidence for future clinical trials to combine ACEi with selonsertib in CKD patients with hypertensive glomerulosclerosis.
However, there are still some critical concerns to be addressed before moving forward. First, it should be noted that all of these results are still based on rodent models. ACEi may inhibit renal fibrosis by suppression of different pathways (Figure 1) (14), such as TGF-β pathways or WNT/β-catenin pathways, which may further regulate various gene expression, including inflammation and epithelial/mesenchymal transition (EMT) related genes. Although the role of complete EMT in vivo in renal fibrosis has been questioned, some studies implicate that partial EMT (epithelial cells expressing some mesenchymal markers and with partial phenotype change) may potentially regulate fibroblast activation and fibrosis induction. However, it is unknown whether these pathways are involved in the redundant regulation of renal fibrosis development in humans in addition to ASK1 activation. Second, will further data analysis of previous clinical trial results provide some new information? In the phase 2 DKD clinical trial, the patients also received traditional treatment (ACEis or angiotensin receptor blockers) together with placebo or selonsertib (8). Although ACEis and angiotensin receptor blockers all target the renin-angiotensin-aldosterone system, is there any difference between them when used in combination with selonsertib? In addition, in the phase 3 clinical trial for NASH (10), did any of the patients take ACEi during the treatment, and how was the effect of selonsertib on them compare with others? Third, it is unknown whether the combined effect of ACEi and selonsertib is limited to hypertension-induced CKD. It is reported that single-nucleotide polymorphisms in the Ask1 gene are closely related to insulin sensitivity (15). Would this be a reason for the failure of selonsertib monotherapy in DKD patients? Because ACEi has been shown to reduce liver fibrosis (9), can we expect stronger effect from ACEi/selonsertib combination intervention for NASH too?
Although for ASK1 inhibitor the road from bench to bedside seems narrowed after the failed clinical trials, the study by Badal et al. (1) suggests that it may find a new way to success in therapy while working together with other drugs. Nevertheless, further clinical trials should depend on more preclinical study to identify the potential redundant pathways in humans and reanalysis of the existing clinical data to identify more evidence for combination use with ACEi.
Disclosures
All authors have nothing to disclose.
Funding
Q. Wei was supported by grant from NIDDK (1 RO1 DK18061-01A1).
Acknowledgments
The content of this article reflects the personal experience and views of the authors and should not be considered medical advice or recommendation. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or Kidney360. Responsibility for the information and views expressed herein lies entirely with the authors.
Footnotes
See related article, “Selonsertib Enhances Kidney Protection Beyond Standard of Care in a Hypertensive, Secondary Glomerulosclerosis CKD Model,” on pages 1169–1182.
Author Contributions
Q. Wei reviewed and edited the manuscript, and L. Wen wrote the original draft of the manuscript.
References
- 1.Badal SS, Al Tuhaifi T, Yu Y-F, Lopez D, Plato CT, Joly K, Breckenridge DG, Yang H-C, Liles JT, Fogo AB: Selonsertib enhances kidney protection beyond standard of care in a hypertensive, secondary glomerulosclerosis CKD model [published online ahead of print April 11, 2022]. Kidney360 10.34067/KID.0001032022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yin M, Zhou HJ, Zhang J, Lin C, Li H, Li X, Li Y, Zhang H, Breckenridge DG, Ji W, Min W: ASK1-dependent endothelial cell activation is critical in ovarian cancer growth and metastasis. JCI Insight 2: e91828, 2017. 10.1172/jci.insight.91828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogier JM, Nayagam BA, Lockhart PJ: ASK1 inhibition: A therapeutic strategy with multi-system benefits. J Mol Med (Berl) 98: 335–348, 2020. 10.1007/s00109-020-01878-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tesch GH, Ma FY, Nikolic-Paterson DJ: ASK1: A new therapeutic target for kidney disease. Am J Physiol Renal Physiol 311: F373–F381, 2016. 10.1152/ajprenal.00208.2016 [DOI] [PubMed] [Google Scholar]
- 5.Tesch GH, Ma FY, Nikolic-Paterson DJ: Targeting apoptosis signal-regulating kinase 1 in acute and chronic kidney disease. Anat Rec (Hoboken) 303: 2553–2560, 2020. 10.1002/ar.24373 [DOI] [PubMed] [Google Scholar]
- 6.Liles JT, Corkey BK, Notte GT, Budas GR, Lansdon EB, Hinojosa-Kirschenbaum F, Badal SS, Lee M, Schultz BE, Wise S, Pendem S, Graupe M, Castonguay L, Koch KA, Wong MH, Papalia GA, French DM, Sullivan T, Huntzicker EG, Ma FY, Nikolic-Paterson DJ, Altuhaifi T, Yang H, Fogo AB, Breckenridge DG: ASK1 contributes to fibrosis and dysfunction in models of kidney disease. J Clin Invest 128: 4485–4500, 2018. 10.1172/JCI99768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson CH, Etchevers K, Yi S, Breckenridge D, Hepner M, Patel U, Ling J, Mathias A: Pharmacokinetics, safety, and tolerability of selonsertib, an apoptosis signal-regulating kinase 1 (ASK1) inhibitor, following first-in-human single and multiple ascending doses in healthy subjects. Clin Pharmacokinet 59: 1109–1117, 2020. 10.1007/s40262-020-00878-y [DOI] [PubMed] [Google Scholar]
- 8.Chertow GM, Pergola PE, Chen F, Kirby BJ, Sundy JS, Patel UD; GS-US-223-1015 Investigators : Effects of selonsertib in patients with diabetic kidney disease. J Am Soc Nephrol 30: 1980–1990, 2019. 10.1681/ASN.2018121231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loomba R, Lawitz E, Mantry PS, Jayakumar S, Caldwell SH, Arnold H, Diehl AM, Djedjos CS, Han L, Myers RP, Subramanian GM, McHutchison JG, Goodman ZD, Afdhal NH, Charlton MR; GS-US-384-1497 Investigators : The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: A randomized, phase 2 trial. Hepatology 67: 549–559, 2018. 10.1002/hep.29514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrison SA, Wong VW, Okanoue T, Bzowej N, Vuppalanchi R, Younes Z, Kohli A, Sarin S, Caldwell SH, Alkhouri N, Shiffman ML, Camargo M, Li G, Kersey K, Jia C, Zhu Y, Djedjos CS, Subramanian GM, Myers RP, Gunn N, Sheikh A, Anstee QM, Romero-Gomez M, Trauner M, Goodman Z, Lawitz EJ, Younossi Z; STELLAR-3 and STELLAR-4 Investigators : Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: Results from randomized phase III STELLAR trials. J Hepatol 73: 26–39, 2020. 10.1016/j.jhep.2020.02.027 [DOI] [PubMed] [Google Scholar]
- 11.Loomba R, Noureddin M, Kowdley KV, Kohli A, Sheikh A, Neff G, Bhandari BR, Gunn N, Caldwell SH, Goodman Z, Wapinski I, Resnick M, Beck AH, Ding D, Jia C, Chuang JC, Huss RS, Chung C, Subramanian GM, Myers RP, Patel K, Borg BB, Ghalib R, Kabler H, Poulos J, Younes Z, Elkhashab M, Hassanein T, Iyer R, Ruane P, Shiffman ML, Strasser S, Wong VW, Alkhouri N; ATLAS Investigators : Combination therapies including cilofexor and firsocostat for bridging fibrosis and cirrhosis attributable to NASH. Hepatology 73: 625–643, 2021. 10.1002/hep.31622 [DOI] [PubMed] [Google Scholar]
- 12.Rosenkranz S, Feldman J, McLaughlin VV, Rischard F, Lange TJ, White RJ, Peacock AJ, Gerhardt F, Ebrahimi R, Brooks G, Satler C, Frantz RP; ARROW Study Group : Selonsertib in adults with pulmonary arterial hypertension (ARROW): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir Med 10: 35–46, 2022. 10.1016/S2213-2600(21)00032-1 [DOI] [PubMed] [Google Scholar]
- 13.Sledz KM, Moore SF, Vijayaragavan V, Mallah S, Goudswaard LJ, Williams CM, Hunter RW, Hers I: Redundant role of ASK1-mediated p38MAPK activation in human platelet function. Cell Signal 68: 109528, 2020. 10.1016/j.cellsig.2020.109528 [DOI] [PubMed] [Google Scholar]
- 14.Yoshiji H, Kuriyama S, Fukui H: Blockade of renin-angiotensin system in antifibrotic therapy. J Gastroenterol Hepatol 22[Suppl 1]: S93–S95, 2007. 10.1111/j.1440-1746.2006.04663.x [DOI] [PubMed] [Google Scholar]
- 15.Bian L, Hanson RL, Ossowski V, Wiedrich K, Mason CC, Traurig M, Muller YL, Kobes S, Knowler WC, Baier LJ, Bogardus C: Variants in ASK1 are associated with skeletal muscle ASK1 expression, in vivo insulin resistance, and type 2 diabetes in Pima Indians. Diabetes 59: 1276–1282, 2010. 10.2337/db09-1700 [DOI] [PMC free article] [PubMed] [Google Scholar]