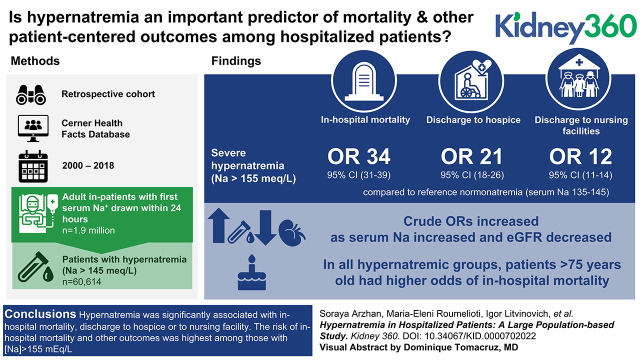
Key Points
Hypernatremia has been studied less than hyponatremia and may serve as an important predictor of outcomes among hospitalized patients.
This work addresses a key gap regarding outcomes of hypernatremia by assessing the relationship of hypernatremia to outcomes by eGFR or age groups.
Hypernatremia was significantly associated with in-hospital mortality and discharge to a hospice or nursing facility.
Keywords: chronic kidney disease, hypernatremia, mortality, outcomes
Visual Abstract
Abstract
Background
Hypernatremia is a frequently encountered electrolyte disorder in hospitalized patients. Controversies still exist over the relationship between hypernatremia and its outcomes in hospitalized patients. This study examines the relationship of hypernatremia to outcomes among hospitalized patients and the extent to which this relationship varies by kidney function and age.
Methods
We conducted an observational study to investigate the association between hypernatremia, eGFR, and age at hospital admission and in-hospital mortality, and discharge dispositions. We analyzed the data of 1.9 million patients extracted from the Cerner Health Facts databases (2000–2018). Adjusted multinomial regression models were used to estimate the relationship of hypernatremia to outcomes of hospitalized patients.
Results
Of all hospitalized patients, 3% had serum sodium (Na) >145 mEq/L at hospital admission. Incidence of in-hospital mortality was 12% and 2% in hyper- and normonatremic patients, respectively. The risk of all outcomes increased significantly for Na >155 mEq/L compared with the reference interval of Na=135–145 mEq/L. Odds ratios (95% confidence intervals) for in-hospital mortality and discharge to a hospice or nursing facility were 34.41 (30.59–38.71), 21.14 (17.53–25.5), and 12.21 (10.95–13.61), respectively (all P<0.001). In adjusted models, we found that the association between Na and disposition was modified by eGFR (P<0.001) and by age (P<0.001). Sensitivity analyses were performed using the eGFR equation without race as a covariate, and the inferences did not substantially change. In all hypernatremic groups, patients aged 76–89 and ≥90 had higher odds of in-hospital mortality compared with younger patients (all P<0.001).
Conclusions
Hypernatremia was significantly associated with in-hospital mortality and discharge to a hospice or nursing facility. The risk of in-hospital mortality and other outcomes was highest among those with Na >155 mEq/L. This work demonstrates that hypernatremia is an important factor related to discharge disposition and supports the need to study whether protocolized treatment of hypernatremia improves outcomes.
Introduction
Compared with hyponatremia, hypernatremia among hospitalized patients is a less prevalent electrolyte disorder (1–3) and its outcomes have been less studied (1). The prevalence of hypernatremia among hospitalized patients has been reported as being between 1% and 4% (1,2,4–7).
Although some studies have shown an increase in mortality in older patients with hypernatremia (8–10), others have not demonstrated this association (1,11). There have also been limitations in selecting the clinical setting, such as inpatient or outpatient (1,9,12), which has led to variations in the prevalence of hypernatremia (13–15). Additionally, little is known about the association between hypernatremia and discharge dispositions—specifically, discharge to a hospice or nursing facility in hospitalized patients (1). These discharge dispositions are key patient-centered outcomes. Moreover, most of the studies addressing the relationship between hypernatremia and selected outcomes of hospitalized patients were single-center studies and did not use a large diverse sample.
Impairment in kidney function and aging are predisposing factors for hypernatremia. Substantial knowledge gaps remain regarding prognostic implications of hypernatremia in hospitalized patients considering different levels of eGFR (16,17). Some studies have demonstrated that advanced CKD and lower eGFR have an apparent protective effect on hypernatremia-related mortality (18). However, controversies still exist over the outcomes of hypernatremia (17,18). The lack of population-based evidence supports the need to evaluate the relationship of hypernatremia to outcomes among hospitalized patients with various levels of kidney function.
To address the knowledge gaps regarding hypernatremia and its correlation with poor outcomes in hospitalized patients, we analyzed data on hospitalized patients from the Cerner Health Facts national database. Furthermore, we investigated the relationship between age and eGFR with outcomes among hospitalized patients.
Materials and Methods
We conducted an observational retrospective cohort study of patients hospitalized between 2000 and 2018 using the Cerner Health Facts database. The Institutional Review Board of the University of New Mexico approved this study protocol (no. 19-429). The requirement for informed consent was waived. This study was conducted in accordance with the principles of the Declaration of Helsinki.
We defined the index hospital admission as the first inpatient encounter during the study period for patients who met the following inclusion criteria: (1) age ≥18 years, and (2) first serum sodium concentration (Na) drawn within 24 hours of admission. The second inclusion criterion reduced the likelihood of the Na being affected by various treatments or iatrogenic causes after hospital admission. All laboratory results in Table 1 were taken within 24 hours of admission. The missing rate for laboratory results is reported in Supplemental Table 1.
Table 1.
Characteristics of hospitalized patients with hyper- or normonatremia
| Patient Characteristics | All Cohort, N=1,963,020 (100%) | Normonatremia, N=1,902,406 (97%) | Hypernatremia, N=60,614 (3%) | Standardized Mean Difference |
|---|---|---|---|---|
| Age, yr | 58.2 (±19.2) | 57.9 (±19.2) | 65.5 (±18.7) | 0.4 |
| BMI, kg/m2 | 29.6 (±8.1) | 29.6 (±8.1) | 27.8 (±8.1) | 0.23 |
| Systolic BP, mm Hg | 137.8 (±26) | 137.8 (±25.9) | 136.7 (±29.7) | 0.04 |
| Diastolic BP, mm Hg | 78 (±15.8) | 78 (±15.8) | 76.6 (±17.6) | 0.08 |
| eGFR, ml/min per 1.73 m2 | 81.2 (±30.2) | 81.7 (±30) | 65.9 (±32.2) | 0.51 |
| eGFR, ml/min per 1.73 m2 | 0.51 | |||
| <15 | 40,865 (2) | 37,957 (2) | 2908 (5) | |
| 15–29 | 76,993 (4) | 70,607 (4) | 6386 (11) | |
| 30–59 | 359,665 (18) | 341,726 (18) | 17,939 (30) | |
| 60–89 | 668,029 (34) | 649,686 (34) | 18,343 (30) | |
| ≥90 | 817,468 (42) | 802,430 (42) | 15,038 (25) | |
| Sex | ||||
| Women | 1,036,709 (53) | 1,005,506 (53) | 31,203 (52) | 0.03 |
| Race | 0.17 | |||
| White | 1,508,639 (77) | 1,465,674 (77) | 42,965 (71) | |
| Black | 283,213 (14) | 271,349 (14) | 11,864 (20) | |
| Native American | 18,502 (0.9) | 17,412 (0.9) | 1090 (2) | |
| Asian | 31,399 (2) | 30,342 (2) | 1057 (2) | |
| Hispanic | 35202 (2) | 34,173 (2) | 1029 (2) | |
| Pacific Islander | 3277 (0.2) | 3232 (0.2) | 45 (0.1) | |
| Other | 82,788 (4) | 80,224 (4) | 2564 (4) | |
| Quan-CCI categories | 0.29 | |||
| 0 | 716,464 (37) | 699,932 (37) | 16,532 (27) | |
| 1–2 (mild) | 477,687 (24) | 462,726 (24) | 14,961 (25) | |
| 3–4 (moderate) | 219,075 (11) | 209,590 (11) | 9485 (16) | |
| ≥5 (severe) | 196,545 (10) | 186,407 (10) | 10,138 (17) | |
| Unknown | 353,249 (18) | 343,751 (18) | 9498 (16) | |
| Quan-CCI | 1.8 (±2.3) | 1.7±2.3 | 2.4±2.7 | 0.28 |
| Comorbidities | ||||
| Hypertension | 648,371 (33) | 627,375 (33) | 20,996 (35) | 0.07 |
| Heart failure | 167,581 (9) | 158,554 (8) | 9027 (15) | 0.22 |
| Diabetes mellitus | 336,193 (17) | 321,721 (17) | 14,472 (24) | 0.18 |
| Peripheral vascular disease | 50,629 (3) | 48,794 (3) | 1835 (3) | 0.03 |
| Ischemic heart disease | 339,847 (17) | 326,996 (17) | 12,851 (21) | 0.1 |
| CKD | 113,837 (6) | 107,513 (6) | 6324 (10) | 0.19 |
| ESKD | 21,559 (1) | 20,763 (1) | 796 (1) | 0.02 |
| COPD | 275,385 (14) | 265,806 (14) | 9579 (16) | 0.04 |
| Adrenal hyperactivity | 750 (0) | 704 (0) | 46 (0.1) | 0.02 |
| Hyperthyroidism | 6475 (0.3) | 6219 (0.3) | 256 (0.4) | 0.02 |
| Hypothyroidism | 110,031 (6) | 106,415 (6) | 3616 (6) | 0.01 |
| Liver disease | 52,958 (3) | 50,561 (3) | 2397 (4) | 0.07 |
| Depression | 156,180 (8) | 150,750 (8) | 5430 (9) | 0.03 |
| Dementia | 29,178 (2) | 26,180 (1) | 2998 (5) | 0.22 |
| Hypercoagulopathy | 5226 (0.3) | 5108 (0.3) | 118 (0.2) | 0.02 |
| Leading diagnosis | ||||
| Head trauma | 30,341 (2) | 28,591 (2) | 1750 (3) | 0.1 |
| Ischemic stroke | 50,237 (3) | 48,243 (3) | 1994 (3) | 0.04 |
| Hemorrhagic stroke | 8872 (0.5) | 8,293 (0.4) | 579 (1) | 0.07 |
| Obstetrics/gynecologic conditions | 59,733 (3) | 59,528 (3) | 205 (0.3) | 0.24 |
| Pneumonia | 113,544 (6) | 107,142 (6) | 6402 (11) | 0.19 |
| Sepsis | 76,692 (4) | 70,196 (4) | 6496 (11) | 0.3 |
| Central diabetes insipidus | 716 (0) | 568 (0) | 148 (0.2) | 0.06 |
| Nephrogenic diabetes insipidus | 120 (0) | 81 (0) | 39 (0.1) | 0.04 |
| Urinary tract infection | 117,440 (6) | 109,994 (6) | 7446 (12) | 0.24 |
| Laboratory tests | ||||
| Serum albumin, g/dl | 3.8 (±0.6) | 3.7 (±0.6) | 3.6 (±0.7) | 0.24 |
| Serum creatinine, mg/dl | 1.1 (±1) | 1.1 (±1) | 1.4 (±1.2) | 0.28 |
| Serum potassium, mEq/L | 4 (±0.6) | 4 (±0.5) | 4.1 (±0.7) | 0.08 |
| Glucose, mg/dl | 134.8 (±73.3) | 133.5 (±70.5) | 172.7 (±124.4) | 0.39 |
| Hemoglobin A1C | 7 (±2.1) | 7 (±2.1) | 7.4 (±2.4) | 0.15 |
| CO2, meq/L | 25.3 (±3.9) | 25.3 (±3.8) | 24.8 (±5.7) | 0.12 |
| O2 saturation, % | 96.3 (±6.3) | 96.3 (±6.2) | 95.5 (±8) | 0.11 |
| Calcium, mg/dl | 9.1 (±0.6) | 9.1 (±0.6) | 9.1 (±0.9) | 0.06 |
| Phosphorus, mg/dl | 3.5 (±1.2) | 3.5 (±1.1) | 3.8 (±1.7) | 0.2 |
| Magnesium, mmol/L | 2 (±0.4) | 2 (±0.4) | 2.1 (±0.5) | 0.29 |
| Chloride, mmol/L | 103.6 (±4.3) | 103.5 (±4.1) | 108.2 (±5.9) | 0.93 |
| Serum osmolality, mOsm/L | 299 (±27.5) | 295.8 (±24.9) | 326.9 (±32.3) | 1.08 |
| Anion gap, mmol/L | 10.8 (±4.1) | 10.7 (±4) | 13.6 (±5.6) | 0.61 |
| Intact PTH, pg/ml | 195.8 (±299.7) | 195.8 (±299.4) | 196.6 (±305.5) | 0.003 |
| Ferritin, ng/ml | 228.5 (±359.6) | 226.1 (±357.5) | 293.4 (±404.8) | 0.18 |
| Iron saturation, % | 21.9 (±22.3) | 21.9 (±22.3) | 21.8 (±20.9) | 0.003 |
| Hemoglobin, g/dl | 13 (±2.2) | 13 (±2.2) | 12.8 (±2.5) | 0.09 |
| WBC, >103/ml | 10 (±4.7) | 10 (±4.6) | 11.2 (±6) | 0.22 |
| Platelet count | 233.4 (±83.8) | 233.7 (±83.5) | 225.9 (±92.4) | 0.09 |
| Serum uric acid, mmol/L | 96.6 (±37.1) | 95.9 (±36.3) | 115.3 (±49.5) | 0.45 |
| BUN, mmol/L | 18.8 (±13.7) | 18.5 (±13.2) | 28.7 (±22.7) | 0.55 |
| AST, IU/L | 43 (±94.3) | 42.4 (±92.2) | 62.7 (±142.7) | 0.17 |
| ALT, IU/L | 39.7 (±81.8) | 39.3 (±80.8) | 50 (±107.6) | 0.11 |
| Total bilirubin, μmol/L | 0.7 (±0.9) | 0.7 (±0.9) | 0.7 (±0.8) | 0.04 |
| Serum total protein, mg/dl | 6.9 (±0.8) | 6.9 (±0.8) | 6.8 (±1) | 0.12 |
Data shown as mean (±SD) or n (%). Normonatremia: serum sodium (Na) 135–145 mEq/L; hypernatremia: Na>145 mEq/L. Na levels corrected by adding 1.6 mEq/L for each 100 mg/dL above 100 mg/dL of the concomitantly measured serum glucose levels. BMI, body mass index; Quan-CCI, Quan-Charlson comorbidity index; COPD, chronic obstructive pulmonary disease; PTH, parathyroid hormone; WBC, white blood cell; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Patient demographics, comorbidities, causes of admission, laboratory studies, and disposition status at hospital discharge were collected. The comorbidities were identified using the International Classification of Diseases, 9th and 10th editions, Clinical Modification (ICD-9 and 10 CM) codes. Laboratory tests were identified using Logical Observation Identifiers Names and Codes (LOINC) (19). The Quan-Charlson Comorbidity Index (Quan-CCI)—a comorbidity index adapted from the Charlson comorbidity index for administrative databases—was also calculated (20).
We corrected the Na by adding 1.6 mEq/L for each 100 mg/dl above 100 mg/dl of the concomitantly measured serum glucose levels (21–23). To calculate eGFR, we applied the CKD-EPI Equation (24). The primary outcomes were determined as in-hospital mortality, discharge disposition of hospitalized patients (hospice, nursing facility, or home), and length of stay. We further excluded patients with Na <135 mEq/L, those with missing eGFR and Na values, and those with discharge dispositions other than home, a hospice, or a nursing facility.
Statistical Analyses
Categorical variables were expressed as percentages, and subgroups were compared using chi-squared tests. Continuous variables were summarized as mean±SD or as medians and interquartile ranges if distributions were skewed. Patient characteristics in normonatremic (Na 135–145 mEq/L) and different hypernatremic (Na >145 mEq/L) groups (mild, moderate, severe) were compared using the Kruskal–Wallis test for continuous variables and the chi-squared test for categorical variables.
Interrelated associations between Na levels and multiple disposition outcomes were evaluated using multinomial logistic regression model analysis while accounting for the competing risks of in-hospital mortality and other dispositions. Discharge to home was used as the reference outcome. For a descriptive, graphical analysis, we used restricted cubic splines in the model to estimate probabilities for in-hospital mortality and discharge to a hospice or nursing facility across the continuous range of Na. For our primary analyses, we discretized Na into four categories: normonatremia: Na 135–145 mEq/L (reference category); mild hypernatremia: Na >145–150 mEq/L; moderate hypernatremia: Na >150–155 mEq/L; and severe hypernatremia: Na >155 mEq/L. Analysis models without and with covariates were fitted for age, sex, race/ethnicity, CKD, and Quan-CCI.
Potential effect modification of the relationship between eGFR and outcomes by Na was assessed by adding Na category×eGFR category interactions to models. We categorized eGFR levels into five different groups: eGFR ≥90 ml/min per 1.73 m2 (reference category), eGFR 60–89 ml/min per 1.73 m2, eGFR 30–59 ml/min per 1.73 m2, eGFR 15–29 ml/min per 1.73 m2, and eGFR <15 ml/min per 1.73 m2. Sensitivity of eGFR models to race was assessed by removing race from the model.
Potential effect modification of the relationship between age and outcomes by Na was assessed by adding Na category × age category interactions to models. To evaluate interrelated associations between age and outcome of Na, we categorized age into five different groups: 18–45, 46–65 (reference category), 66–75, 76–89, and ≥90 years old. Custom linear contrasts were used to obtain odds ratios (OR) by subgroup. Sensitivity analysis of results to patient comorbidities was conducted by replacing selected covariates with Quan-CCI. Predicted values from models were obtained to visualize relationships between Na and outcomes.
Finally, we assessed the relationships between Na levels and length of hospitalization (days) among those who were discharged to home. The distribution was right skewed, so log-transformed days were used in linear models of length of stay adjusted for CKD and the Quan-CCI, taking an approach that follows our multinomial logistic regression analyses. We report regression coefficients from the models to summarize the magnitude of the effect on log-transformed days. Moreover, we tested for the differential effect of age and eGFR on these associations. All statistical analyses were performed using R v3.4 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
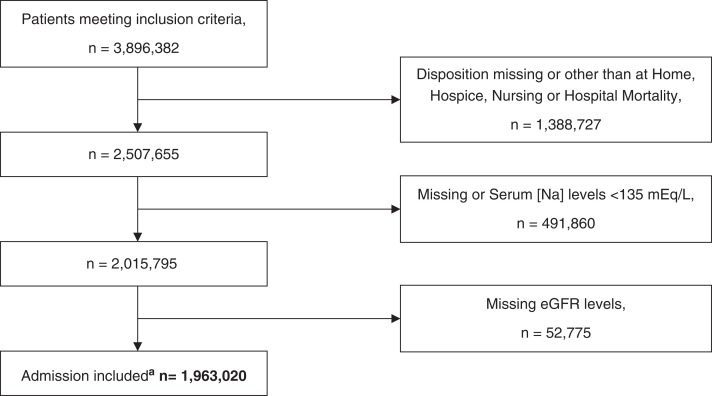
A total of 3,896,382 patients met our inclusion criteria. The final cohort included 1,963,020 patients (Figure 1), of whom 60,614 (3%) had hypernatremia (Na >145 mEq/L) at hospital admission.
Figure 1.
Flow chart of the sample selection process. aThe final cohort available for analysis. Serum sodium levels were corrected by adding 1.6 mEq/L for each 100 mg/dl above 100 mg/dl of the concomitantly measured serum glucose levels.
Hypernatremic patients had significantly lower eGFR and were older compared with normonatremic patients (both P<0.001). Patient characteristics are summarized in Table 1. We found statistically significant differences (P<0.001) for the comparison between hypernatremic and normonatremic patients for all patient characteristics. We also reported standardized mean differences. Some of the laboratory data in Table 1 had a high missing rate. Supplemental Table 1 details the missing rate. One reason for such a large percentage of missing laboratory values could be the time frame of blood test drawing (within 24 hours of admission). We did not include the laboratory tests taken after 24 hours of admission.
Frequencies of outcomes by hypernatremia status, eGFR category, and age group are summarized in Supplemental Table 2. The incidence of in-hospital mortality was 12% among hypernatremic patients compared with 2% in normonatremic patients. The relative frequencies of discharge to a hospice in hypernatremic and normonatremic patients were 2% and 0.7%, respectively. The relative frequencies of discharge to a nursing facility in hypernatremic and normonatremic patients were 26% and 11%, respectively.
Serum Sodium and the Outcomes
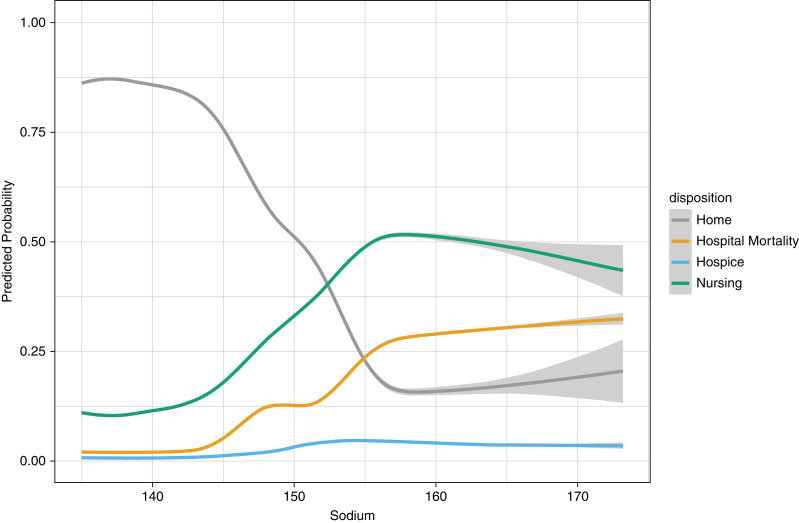
The crude probability of in-hospital mortality corresponding to Na was lowest between 135 and 145 mEq/L and continued to increase above this range (Figure 2). The probability of discharge to a hospice demonstrated a similar pattern, albeit less profound. The estimated probability of discharge to a nursing facility at lower Na levels appeared to show an up-trending pattern but started to decrease at Na above 155 mEq/L. The probability of discharge to home increased to an Na between 135 and 145 mEq/L, then decreased above this range, and again started to increase slightly above 155 mEq/L. Discharge to a hospice showed a slight increase at Na above 150 mEq/L and then remained steady (Figure 2).
Figure 2.
Restricted cubic splines of the crude probability of in-hospital mortality, discharge to a hospice, discharge to home, and discharge to a nursing facility as a function of serum sodium levels at hospital admission. These estimated probabilities were derived from a multinomial logistic regression model. Serum sodium levels were corrected by adding 1.6 mEq/L for each 100 mg/dl above 100 mg/dl of the concomitantly measured serum glucose levels.
For the Na categories, in the adjusted multinomial logistic regression analysis, we found that the OR for in-hospital mortality significantly increased as Na increased relative to normonatremia interval (P<0.001). We observed similar trends for discharge to a hospice or nursing facility (Table 2).
Table 2.
Relationships between serum sodium levels stratified by eGFR levels and outcomes
| Natremia Statusa at Hospital Admission | In-Hospital Mortality | Discharge to a Hospice | Discharge to a Nursing Facility |
|---|---|---|---|
| Adjustedb Odds Ratio (95% Confidence Interval) | Adjustedb Odds Ratio (95% Confidence Interval) | Adjustedb Odds Ratio (95% Confidence Interval) | |
| Normonatremia | 1 | 1 | 1 |
| Hypernatremia | |||
| Mild | 4.8 (4.6 to 4.9) | 2.1 (1.9 to 2.3) | 2.1 (2 to 2.1) |
| Moderate | 22.6 (20.9 to 24.4) | 10.41 (9 to 12) | 6.97 (6.5 to 7.5) |
| Severe | 34.4 (30.6 to 38.7) | 21.1 (17.5 to 25.5) | 12.2 (11 to 13.6) |
| eGFR level, ml/min per 1.73 m2 | |||
| Normonatremia | |||
| <15 | 5.4 (5.1 to 5.6) | 0.8 (0.7 to 0.9) | 1.4 (1.3 to 1.4) |
| 15–29 | 5.5 (5.3 to 5.8) | 1 (0.9 to 1.1) | 1.4 (1.3 to 1.4) |
| 30–59 | 2.3 (2.2 to 2.4) | 0.5 (0.5 to 0.5) | 1 (1 to 1) |
| 60–89 | 1.5 (1.4 to 1.5) | 0.6 (0.5 to 0.6) | 0.9 (0.9 to 1) |
| ≥90 | 1 | 1 | 1 |
| Mild hypernatremia | |||
| <15 | 14 (12.4 to 16) | 0.1 (0.1 to 0.3) | 2.7 (1.9 to 2.4) |
| 15–29 | 19.2 (17.6 to 21.1) | 0.4 (0.3 to 0.5) | 4 (3.7 to 4.3) |
| 30–59 | 11.9 (11.2 to 12.7) | 2 (1.7 to 2.1) | 2.5 (2.4 to 2.6) |
| 60–89 | 6 (5.5 to 6.4) | 1.7 (1.5 to 1.9) | 1.6 (1.6 to 1.7) |
| ≥90 | 8.1 (7.4 to 8.7) | 1.6 (1.3 to 2) | 2.2 (2 to 2.3) |
| Moderate hypernatremia | |||
| <15 | 54.6 (42.5 to 70.2) | 0.17 (0 to 1.9) | 6.6 (5.2 to 8.4) |
| 15–29 | 69.3 (55.8 to 86) | 3.91 (2.3 to 6.6) | 16.5 (13.6 to 20.1) |
| 30–59 | 22 (19 to 25.4) | 5 (3.9 to 6.4) | 5.4 (4.8 to 6.1) |
| 60–89 | 36.6 (30.6 to 43.9) | 36.4 (29.5 to 44.8) | 11.6 (10 to 13.5) |
| ≥90 | 15.5 (12.5 to 19.3) | 0 (NA) | 4 (3.3 to 4.8) |
| Severe hypernatremia | |||
| <15 | 656.5 (328.7 to 1311.1) | 4.2 (0.5 to 34.2) | 124.3 (62.8 to 246.6) |
| 15–29 | 849 (67.2 to 107.2) | 9.1 (6.1 to 13.7) | 10.7 (8.5 to 13.4) |
| 30–59 | 89.6 (73.4 to 109.3) | 12.2 (8.5 to 17.4) | 15.2 (12.6 to 18.4) |
| 60–89 | 300.5 (215.1 to 419.9) | 52.2 (32.2 to 84.7) | 33.6 (23.9 to 47.3) |
| ≥90 | 30 (19.9 to 45.4) | 34.6 (19.6 to 61) | 10 (7 to 14.2) |
Normonatremia: Na >135–145 mEq/L; hypernatremia: Na >145 mEq/L (mild hypernatremia: Na >145–150 mEq/L; moderate hypernatremia: Na >150–155 mEq/L; severe hypernatremia: Na >155 mEq/L). Na, serum sodium level; OR, odds ratio.
Na levels corrected by adding 1.6 mEq/L for each 100 mg/dl above 100 mg/dl of the concomitantly measured serum glucose levels.
The adjusted ORs were derived from a multinomial logistic regression model adjusted for age, sex, race, CKD, and the Quan-CCI (all P<0.001).
There was a significant association between Na categories and length of hospitalization (P<0.001). These overall relationships are shown in Table 4. For those discharged to their homes, median length of stay was 2.94 days. Length of stay was shortest for normonatremia.
Table 4.
Relationships between serum sodium levels at hospital admission and length of hospitalization among those discharged to home (stratified by eGFR/age)
| Natremia Statusa at Hospital Admission | Length of Stay among those Discharged to Home (N=1,664,500) | |
|---|---|---|
| Median, Interquartile Range | Coefficient and 95% Confidence Interval | |
| Normonatremia | 2.9 (1.9–4.6) | Reference |
| Hypernatremia | ||
| Mild | 3.3 (2.1–5.4) | 0.1 (0.1 to 0.1) |
| Moderate | 4.1 (2.7–7.0) | 0.3 (0.2 to 0.3) |
| Severe | 4.7 (3–7.7) | 0.4 (0.3 to 0.4) |
| eGFR level, ml/min per 1.73 m2 | ||
| Normonatremia | ||
| <15 | 4 (2.6–6.5) | 0.2 (0.2 to 0.3) |
| 15–29 | 3.5 (2.2–5.6) | 0.1 (0.1 to 0.1) |
| 30–59 | 3 (2–4.9) | 0.05 (0.04 to 0.05) |
| 60–89 | 2.9 (1.9–4.4) | −0.01 (-0.02 to 0.01) |
| ≥90 | 2.9 (1.9–4.4) | Reference |
| Mild hypernatremia | ||
| <15 | 4.3 (2.8–6.9) | 0.3 (0.2 to 0.3) |
| 15–29 | 4 (2.6–6.1) | 0.2 (0.2 to 0.3) |
| 30–59 | 3.4 (2.2–5.6) | 0.1 (0.1 to 0.2) |
| 60–89 | 3.2 (2.2–5.2) | 0.1 (0.1 to 0.1) |
| ≥90 | 3.2 (2–5.3) | 0.1 (0.1 to 0.1) |
| Moderate hypernatremia | ||
| <15 | 5.4 (3.7–9.1) | 0.4 (0.3 to 0.6) |
| 15–29 | 5 (3.3–7.7) | 0.4 (0.3 to 0.5) |
| 30–59 | 4.2 (2.8–7.0) | 0.3 (0.2 to 0.4) |
| 60–89 | 4 (2.5–6.9) | 0.3 (0.2 to 0.3) |
| ≥90 | 3.8 (2.2–6.3) | 0.2 (0.2 to 0.3) |
| Severe hypernatremia | ||
| <15 | 7 (4.9–12.4) | 0.6 (0.4 to 0.8) |
| 15–29 | 5 (3.7–7.5) | 0.4 (0.3 to 0.6) |
| 30–59 | 4.3 (2.9–6.9) | 0.3 (0.2 to 0.4) |
| 60–89 | 4.5 (3–8.5) | 0.4 (0.3 to 0.5) |
| ≥90 | 4 (2.7–6.8) | 0.3 (0.1 to 0.5) |
| Age group, yr | ||
| Normonatremia | ||
| 18–45 | 2.9 (1.9–4.4) | 0 (N/A) |
| 46–65 | 3 (1.9–4.7) | Reference |
| 66–75 | 3 (1.9–4.7) | −0 (N/A) |
| 76–89 | 3 (2–4.7) | 0 (N/A)b |
| ≥90 | 3 (2–4.6) | 0 (N/A)c |
| Mild hypernatremia | ||
| 18–45 | 3.1 (2–5.3) | 0.1 (0.9 to 0.1) |
| 46–65 | 3.4 (2.1–5.7) | 0.1 (0.1 to 0.1) |
| 66–75 | 3.3 (2.1–5.4) | 0.1 (0.1 to 0.1) |
| 76–89 | 3.3 (2.1–5.3) | 0.1 (0.1 to 0.1) |
| ≥90 | 3.3 (2.1–5.2) | 0.1 (0 to 0.2) |
| Moderate hypernatremia | ||
| 18–45 | 3.8 (2.4–6.1) | 0.2 (0.2 to 0.3) |
| 46–65 | 4.2 (2.7–7.2) | 0.3 (0.2 to 0.3) |
| 66–75 | 4.4 (2.5–7.9) | 0.3 (0.2 to 0.4) |
| 76–89 | 4.7 (3–6.9) | 0.3 (0.2 to 0.4) |
| ≥90 | 3.9 (2.8–6.1) | 0.2 (0.1 to 0.4)d |
| Severe hypernatremia | ||
| 18–45 | 4.3 (3.2–7.8) | 0.4 (0.2 to 0.5) |
| 46–65 | 4.7 (2.8–7.9) | 0.4 (0.2 to 0.5) |
| 66–75 | 4.4 (2.9–7.8) | 0.3 (0.8 to 0.5) |
| 76–89 | 4.8 (3.4–7.1) | 0.4 (0.2 to 0.5) |
| ≥90 | 5.8 (3–6.7) | 0.3 (0.1 to 0.5) |
Normonatremia: Na >135–145 mEq/L; hypernatremia: Na >145 mEq/L (mild hypernatremia: Na >145–150 mEq/L; moderate hypernatremia: Na >150–155 mEq/L; severe hypernatremia: Na >155 mEq/L). IQR, interquartile range.
Na levels corrected by adding 1.6 mEq/L for each 100 mg/dl above 100 mg/dl of the concomitantly measured serum glucose levels.
P<0.01 for all categories, except bP=0.87, cP=0.43, and dP=0.01.
eGFR and the Outcomes
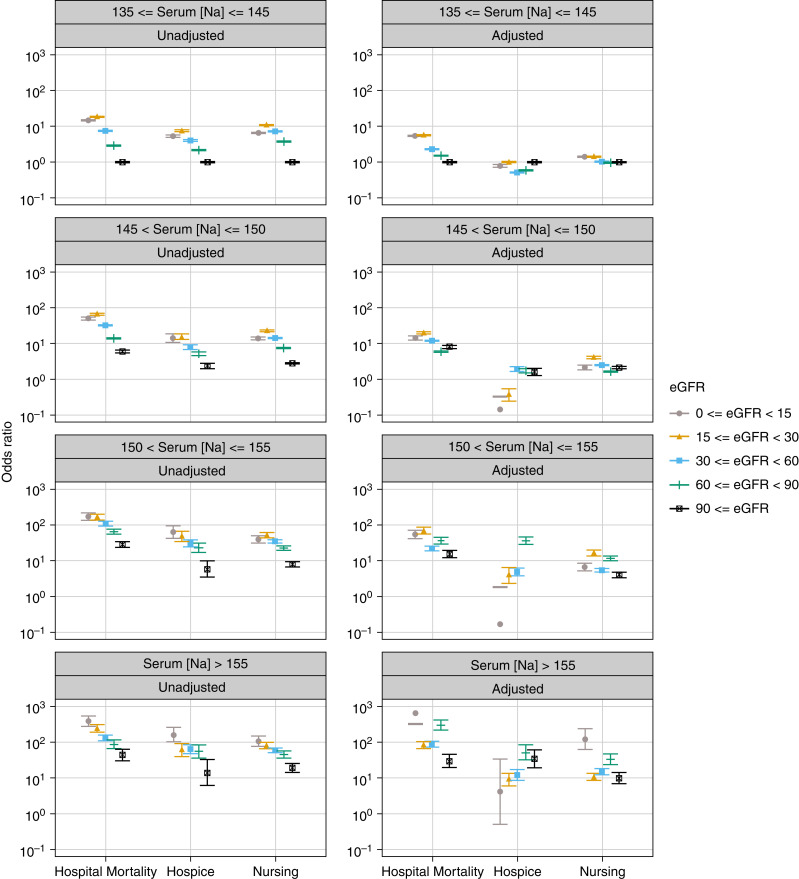
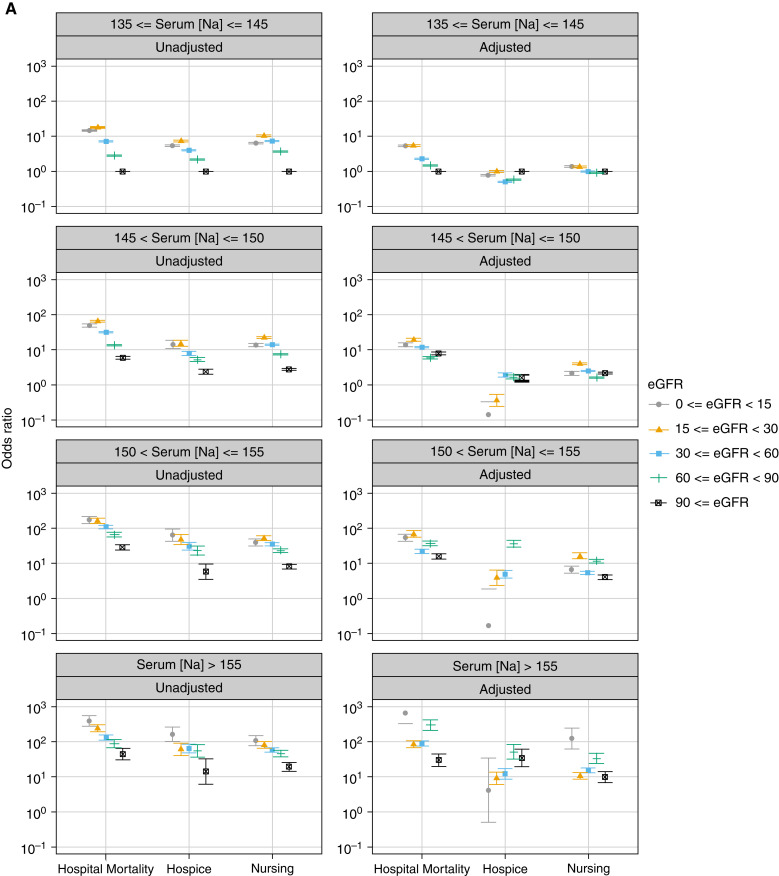
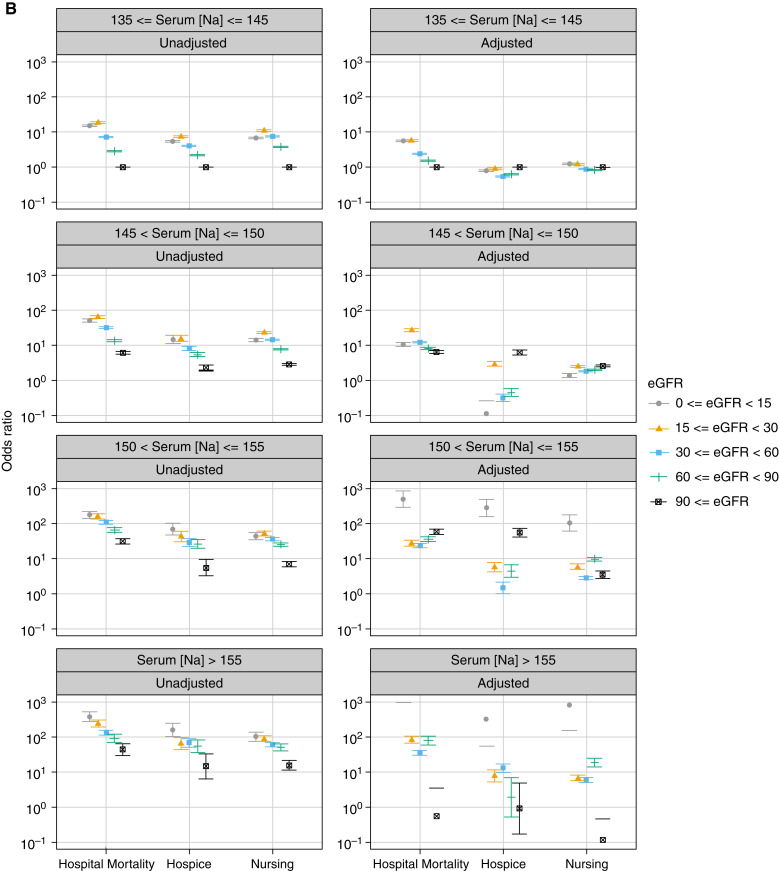
The main test for interaction between eGFR levels and Na categories demonstrated a significant association between these two factors and in-hospital mortality (P<0.001). Generally, crude ORs of in-hospital mortality increased as Na increased relative to the 135–145 mEq/L interval and as eGFR decreased below 90 ml/min per 1.73 m2. This was also true for discharge to a hospice or nursing facility (Figure 3, Table 2).
Figure 3.
Plot of odds ratios (95% CI) for in-hospital mortality and discharge to a hospice or nursing facility associated with different intervals of serum sodium levels (mEq/L) at hospital admission stratified by eGFR levels. The odds rations were derived from multinomial logistic regression models adjusted for age, sex, race, and the selected comorbidities and reasons for hospitalization. Discharge to home and serum sodium levels of 135–145 mEq/L served as the reference. Serum sodium levels were corrected by adding 1.6 mEq/L for each 100 mg/dl increase above 100 mg/dl of the concomitantly measured serum glucose levels. Error bars indicated 95% CI. 95% CI, 95% confidence interval.
The adjusted model showed that the ORs of in-hospital mortality significantly increased as Na rose above 145 mEq/L and eGFR decreased below 90 ml/min per 1.73 m2 compared with the reference Na interval and eGFR >90 ml/min per 1.73 m2 (P<0.001). Different trends were observed for each Na category (Table 2, Figure 3).
In the unadjusted model, discharge to a hospice showed the same trend as for in-hospital mortality. The highest ORs in normonatremia and mild hypernatremia were observed with an eGFR of 15–29 and in moderate and severe hypernatremia with an eGFR <15 ml/min per 1.73 m2. In all Na categories, the lowest ORs of discharge to a hospice were observed with an eGFR ≥90 ml/min per 1.73 m2 (Figure 3). Adjusted model showed different patterns in each Na category (Figure 3, Table 2).
With regard to discharge to a nursing facility, the unadjusted model showed that in normonatremia and mild and moderate hypernatremia, the highest ORs were observed with an eGFR of 15–29 ml/min per 1.73 m2, whereas the lowest ORs were observed with an eGFR ≥90 ml/min per 1.73 m2 (Figure 3).
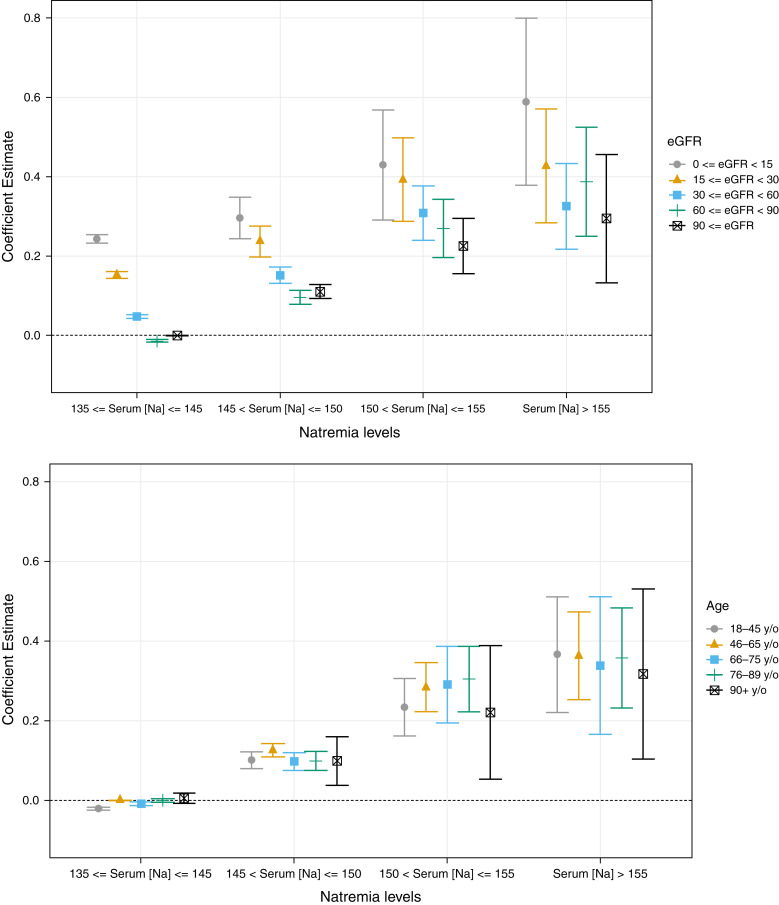
Length of stay showed an up-trending pattern as function of eGFR in all Na categories, with the two exceptions of patients with an eGFR of 60–89 ml/min per 1.73 m2in normonatremia and mild hypernatremia, who had the shortest length of stay. For moderate and severe hypernatremia, the shortest length of stay was observed for eGFRs ≥90 ml/min per 1.73 m2. The longest length of stay was observed for those with an eGFR <15 ml/min per 1.73 m2 in all Na categories (Figure 4, Table 4).
Figure 4.
Plot of relationships between serum sodium levels at hospital admission and length of hospitalization among those discharged to home stratified by eGFR/age.
The sensitivity analysis was performed using the eGFR equation without race as a covariate. The inferences did not substantially change after removing race from our eGFR model (Figure 5).
Figure 5.
Plot of the relative risk ratios (95% CI) for in-hospital mortality and discharge to a hospice or nursing facility associated with different intervals of serum sodium levels (mEq/L) at hospital admission stratified by eGFR levels with developing race/ethnicity in the model. (A) Before removing race from model. (B) After removing race from model. The sensitivity analyses were performed using the eGFR equation without a race covariate.
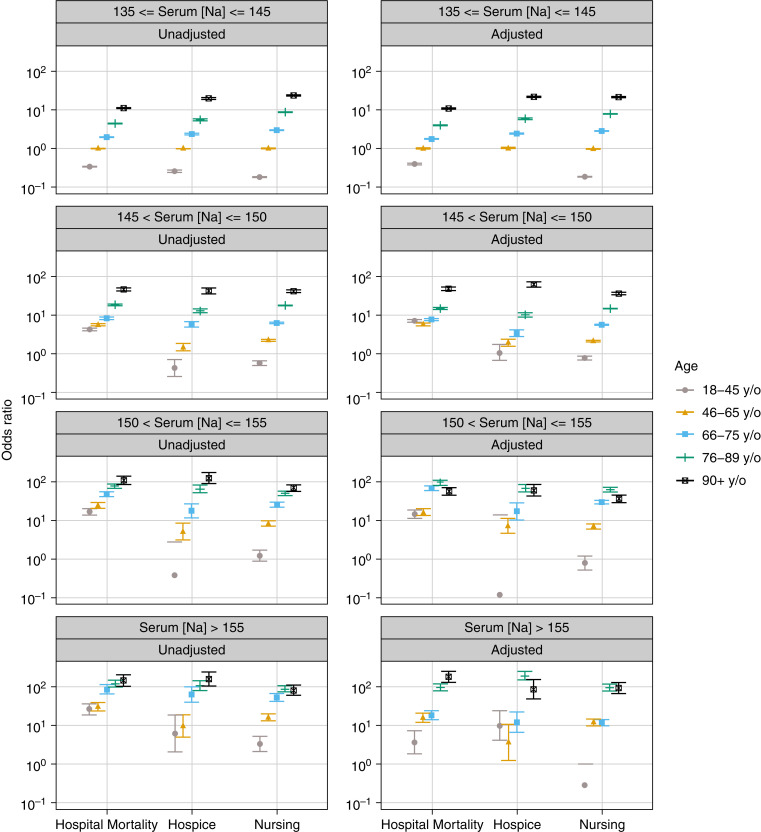
Age and the Outcomes
In all Na categories, we found a significant association between age and outcomes (all P<0.001). The crude results showed that older age groups had higher ORs of in-hospital mortality and discharge to a hospice or nursing facility in all Na categories (Figure 6). In the adjusted model, in each Na category, the relationship between age group and outcomes was largely similar to those in the crude models, with an increased risk of mortality and discharge to a hospice or nursing facility with older age (Figure 6, Table 3).
Figure 6.
Plot of the relative risk ratios (95% CI) for in-hospital mortality and discharge to a hospice or nursing facility associated with different intervals of serum sodium levels (mEq/L) at hospital admission stratified by age groups.
Table 3.
Relationships between serum sodium levels, stratified by age, and outcomes
| Natremia Statusa at Hospital Admission | Age Group, yr | In-Hospital Mortality | Discharge to a Hospice | Discharge to a Nursing Facility |
|---|---|---|---|---|
| Adjustedb Odds Ratio (95% Confidence Interval) | Adjustedb Odds Ratio (95% Confidence Interval) | Adjustedb Odds Ratio (95% Confidence Interval) | ||
| Normonatremia | 18–45 | 0.4 (0.4 to 0.4) | 0 (N/A) | 0.2 (0.2 to 0.2) |
| 46–65 | 1 | 1 | 1 | |
| 66–75 | 1.8 (1.76 to 1.9) | 2.4 (2.3 to 2.6) | 2.9 (2.8 to 2.9) | |
| 76–89 | 4 (3.9 to 4.1) | 5.9 (5.6 to 6.3) | 8.1 (8 to 8.2) | |
| ≥90 | 11 (10.6 to 11.5) | 21.8 (20.5 to 23.3) | 21.7 (21.2 to 22.1) | |
| Mild hypernatremia | 18–45 | 7.1 (6.5 to 7.8) | 1.1 (0.7 to 1.7) | 0.8 (0.7 to 0.9) |
| 46–65 | 5.7 (5.4 to 6.1) | 2 (1.6 to 2.4) | 2.1 (2 to 2.2) | |
| 66–75 | 7.7 (7.2 to 8.3) | 3.4 (2.8 to 4.2) | 5.7 (5.4 to 6) | |
| 76–89 | 14.9 (14.1 to 15.8) | 10.3 (9 to 11.7) | 15 (14.4 to 15.5) | |
| ≥90 | 48.3 (43.5 to 53.6) | 62.8 (53.7 to 73.4) | 36.2 (33.3 to 39.3) | |
| Moderate hypernatremia | 18–45 | 14.8 (12 to 18.2) | 0.1 (0 to 14.1) | 0.8 (0.5 to 1.2) |
| 46–65 | 16.1 (13.8 to 18.8) | 7.2 (4.7 to 11.2) | 7 (6.1 to 8) | |
| 66–75 | 66.7 (55.8 to 79.8) | 16.9 (10.1 to 28.4) | 29.5 (25.1 to 34.7) | |
| 76–89 | 96.2 (83.3 to 111.1) | 68.3 (53.4 to 87.4) | 61.3 (54.1 to 69.5) | |
| ≥90 | 57.3 (45.7 to 71.7) | 59.3 (41.7 to 84.5) | 36.5 (30.3 to 43.9) | |
| Severe hypernatremia | 18–45 | 3.7 (1.9 to 7.3) | 10.2 (4.2 to 24.6) | 0.3 (0.1 to 1) |
| 46–65 | 16.2 (12.3 to 21.4) | 3.7 (1.3 to 10.8) | 12.7 (9.9 to 14.9) | |
| 66–75 | 18.5 (14.2 to 24.2) | 12.3 (6.8 to 22.3) | 11.7 (9.7 to 14.7) | |
| 76–89 | 98.4 (79.4 to 122) | 194.8 (150 to 252.9) | 99.6 (83.1 to 119.3) | |
| ≥90 | 181.7 (130.6 to 252.7) | 87.5 (49.1 to 156.1) | 94 (69.4 to 127.3) |
Normonatremia: Na >135–145 mEq/L; hypernatremia: Na >145 mEq/L (mild hypernatremia: Na >145–150 mEq/L; moderate hypernatremia: Na >150–155 mEq/L; severe hypernatremia: Na >155 mEq/L). CI, confidence interval; Na, serum sodium level; OR, odds ratio.
Na levels corrected by adding 1.6 mEq/L for each 100 mg/dl above 100 mg/dl of the concomitantly measured serum glucose levels.
The adjusted ORs were derived from a multinomial logistic regression model adjusted for age, sex, race, CKD, and the Quan-CCI (all P<0.001).
Across all Na categories, older age was associated with greater length of stay (Figure 4, Table 4).
Discussion
In this cohort study composed from a data warehouse for a large number of hospitals, we found that 3% of hospitalized patients had hypernatremia (Na >145 mEq/L). We demonstrated that in-hospital mortality was associated with higher levels of hypernatremia. The observation that hypernatremia was associated with a higher mortality rate (12%) was found to be independent of observed chronic disease. We also noticed a significant association between hypernatremia and increased odds of discharge to a hospice or nursing facility.
This report confirms previous observations relating hypernatremia to mortality in a large and diverse sample and hospitalized patients and extends previous research by examining discharge disposition. Tsipotis et al. (1) conducted a cohort study of 19,072 unselected hospitalized adults to investigate the crude relationship between clinical outcomes and community-acquired hypernatremia, defined as Na >142 mEq/L, demonstrating a relationship between hypernatremia and mortality among these patients. In addition, Tsipotis et al.’s study revealed a decrement in ORs of in-hospital mortality for Na >157 mEq/L in both the unadjusted and adjusted models (1). Our findings in terms of increment of in-hospital mortality along with the increase in the severity of hypernatremia are similar to the findings by Jin Jung et al. (3), who studied 79,998 patients, including 180 patients with hypernatremia, admitted to an urban tertiary care hospital in Korea. However, our conclusion is in contrast with the findings of Bataille et al. (25), who did not find a statistical relationship between the level of hypernatremia and mortality. In line with our results, previous studies by Hu et al. (13,26) in China and Funk et al. (14) in Austria demonstrated that there are independent associations between hypernatremia and the risk of in-hospital mortality, even after adjusting for potential confounding factors.
The results of this report revealed that eGFR was significantly lower in hypernatremic patients compared with normonatremic patients. With regard to the different levels of hypernatremia stratified by eGFR levels, the general pattern showed that by increasing Na and decreasing eGFR level, the risk of outcomes increased. Tsipotis et al. (1) showed that the highest ORs of in-hospital mortality in patients with hypernatremia on admission belonged to eGFR 30–59 ml/min per 1.73 m2. Han et al. (27) investigated associations between Na and CKD and observed that hypernatremia in patients with CKD in the outpatient setting were short- and long-term risk factors for mortality. Chiu et al. (28) reported no significant association with hypernatremia and mortality in an outpatient population with CKD. Sun et al. also found that the stage of CKD did not appear to affect the mortality associated with hypernatremia (29). In contrast, Kovesdy et al. (18) demonstrated that more advanced CKD displayed a relatively lower mortality associated with hypernatremia compared with patients with less severe stages of CKD. These differences can result from the nature of our inpatient population, who suffer from more acute conditions.
Patients with hypernatremia were older than normonatremic patients (65.51±18.70 years and 57.93±19.18 years, respectively; P<0.001). The results of our analyses generally revealed an up-trending pattern of ORs for all outcomes in higher Na and older age groups. In accordance with other studies (30–32), our study demonstrated that the odds of in-hospital mortality in all Na groups increased with aging. This can be attributed to the fact that elderly patients with hypernatremia present with fewer symptoms of hypernatremia compared with younger patients. A delay in diagnosis and treatment of hypernatremia can carry higher rates of mortality and poorer outcomes. Moreover, age-related impairment in organ function, decreased thirst drive, impaired urinary concentrating ability, reduced total body water, chronic illnesses, and disabilities predispose older adults to dehydration and hypernatremia (30). Our results also demonstrated that older patients with higher Na had greater ORs of discharge to a nursing facility.
Our study has several strengths. First, to the best of our knowledge, the association between hypernatremia and its outcomes (i.e., in-hospital mortality and discharge dispositions) has not been studied systematically at a large population level. Additionally, our study addressed a key gap in knowledge because we investigated the relationship between different levels of hypernatremia and selected outcomes through stratifying by eGFR level and age group in a large diverse population. Our study also has some limitations. First, we were unable to include a disease severity scoring modality such as the Acute Physiology and Chronic Health Evaluation or Sequential Organ Failure Assessment. However, this study included the identification of the comorbidities and reason for hospitalization using ICD-9 and -10 codes to account for the presence of medical conditions, and we were able to adjust for the Quan-CCI, which has been widely used to predict survival of hospitalized patients and predicts in-hospital mortality in critically ill patients well (20). Second, we did not have information about outpatient medication that might predispose them to hypernatremia. Because of the observational nature of our study, we cannot preclude the possibility of residual confounding and cannot draw any causal interpretations from our results. However, conducting large randomized controlled trails to overcome this limitation may not be easily practicable. Furthermore, we did not account for AKI in this report, and this is a limitation. However, the aim of our study was to investigate the relationships between hypernatremia and outcomes considering the level of kidney function (based on eGFR). Nevertheless, these limitations should not undermine the importance of the finding that hypernatremia, regardless of the cause, is significantly associated with increased in-hospital mortality.
Hypernatremia is relatively common among hospitalized patients, and regardless of the cause, it is significantly related to mortality and discharge to a nursing facility or hospice. Our study underscores the need for more awareness about hypernatremia and emphasizes the need for addressing water depletion among patients presenting to hospital with hypernatremia. We found that all levels of hypernatremia independently increased the odds of in-hospital mortality and discharge to a hospice or nursing facility. The prognosis for hypernatremic patients may improve if hypernatremia is corrected properly and in a timely manner. Further studies are needed to evaluate and develop the best treatment and management of hypernatremia in order to decrease the rate of adverse outcomes considering the level of kidney function and age. Duration of hypernatremia and its effect on poor outcomes is another issue that merits further study.
Disclosures
M.L. Unruh reports consultancy for Cara Therapeutics to chair of Data Monitoring Committee; a consulting agreement between Cara and the University of New Mexico; research funding from Dialysis Clinic, Inc.; and honoraria from the American Society of Nephrology, NKF, and the Renal Research Institute related to lectures. M.-E. Roumelioti reports participating in Dialysis Clinic, Inc., quality meetings and receiving financial support. All remaining authors have nothing to disclose.
Funding
This study has been supported and funded by Dialysis Clinic, Inc. (Grant ID: 3RGX8-FP00007518, Study ID: #C-4165).
Footnotes
See related editorial, “Outcomes Associated with Hypernatremia at Admission in Hospitalized Persons,” on pages 1122–1123.
Author Contributions
S. Arzhan was responsible for the investigation and project administration and wrote the original draft of the manuscript; S. Arzhan, C.G. Bologa, I. Litvinovich, and O.B. Myers were responsible for data curation; S. Arzhan, O.B. Myers, M.-E. Roumelioti, and M.L. Unruh were responsible for conceptualization; S. Arzhan and M.L. Unruh were responsible for funding acquisition, resources, and visualization; C.G. Bologa, I. Litvinovich, and O.B. Myers were responsible for software; C.G. Bologa, I. Litvinovich, O.B. Myers, M.-E. Roumelioti, and M.L. Unruh were responsible for validation; C.G. Bologa and O.B. Myers were responsible for the formal analysis; O.B. Myers and M.L. Unruh were responsible for supervision; and all authors were responsible for the methodology and reviewed and edited the manuscript.
Supplemental Material
This article contains the following supplemental material online at https://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0000702022/-/DCSupplemental
Missing laboratory data profile. Download Supplemental Table 1, PDF file, 229 KB (162.7KB, pdf)
Profile of hospitalized patients with and without hypernatremia. Download Supplemental Table 2, PDF file, 229 KB (162.7KB, pdf)
References
- 1.Tsipotis E, Price LL, Jaber BL, Madias NE: Hospital-associated hypernatremia spectrum and clinical outcomes in an unselected cohort. Am J Med 131: 72–82.e1, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Lombardi G, Ferraro PM, Calvaruso L, Naticchia A, D’Alonzo S, Gambaro G: Sodium fluctuations and mortality in a general hospitalized population. Kidney Blood Press Res 44: 604–614, 2019 [DOI] [PubMed] [Google Scholar]
- 3.Jung WJ, Lee HJ, Park S, Lee SN, Kang HR, Jeon JS, Noh H, Han DC, Kwon SH: Severity of community acquired hypernatremia is an independent predictor of mortality. Intern Emerg Med 12: 935–940, 2017. 28474207 [DOI] [PubMed] [Google Scholar]
- 4.Thongprayoon C, Cheungpasitporn W, Yap JQ, Qian Q: Increased mortality risk associated with serum sodium variations and borderline hypo- AND hypernatremia in hospitalized adults. Nephrol Dial Transplant 35: 1746–1752, 2020. 31219584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akirov A, Diker-Cohen T, Steinmetz T, Amitai O, Shimon I: Sodium levels on admission are associated with mortality risk in hospitalized patients. Eur J Int Med 46: 25–29, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Girardeau Y, Jannot A-S, Chatellier G, Saint-Jean O: Association between borderline dysnatremia and mortality insight into a new data mining approach. BMC Med Inform Decis Mak 17: 152, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liamis G, Filippatos TD, Elisaf MS: Evaluation and treatment of hypernatremia: A practical guide for physicians. Postgrad Med 128: 299–306, 2016. 26813151 [DOI] [PubMed] [Google Scholar]
- 8.Imaizumi T, Nakatochi M, Fujita Y, Nomura R, Watanabe K, Maekawa M, Yamakawa T, Katsuno T, Maruyama S: The association between intensive care unit-acquired hypernatraemia and mortality in critically ill patients with cerebrovascular diseases: A single-centre cohort study in Japan. BMJ Open 7: e016248, 2017. 28821524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salahudeen AK, Doshi SM, Shah P: The frequency, cost, and clinical outcomes of hypernatremia in patients hospitalized to a comprehensive cancer center. Support Care Cancer 21: 1871–1878, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Darmon M, Pichon M, Schwebel C, Ruckly S, Adrie C, Haouache H, Azoulay E, Bouadma L, Clec'h C, Garrouste-Orgeas M, Souweine B, Goldgran-Toledano D, Khallel H, Argaud L, Dumenil A-S, Jamali S, Allaouchiche B, Zeni F, Timsit J-F: Influence of early dysnatremia correction on survival of critically ill patients. Shock 41: 394–399, 2014. 24667611 [DOI] [PubMed] [Google Scholar]
- 11.Lopes IF, Dezelée S, Brault D, Steichen O: Prevalence, risk factors and prognosis of hypernatraemia during hospitalisation in internal medicine. Neth J Med 73: 448–454, 2015 [PubMed] [Google Scholar]
- 12.Cecconi M, Hochrieser H, Chew M, Grocott M, Hoeft A, Hoste A, Jammer I, Posch M, Metnitz P, Pelosi P, Moreno R, Pearse RM, Vincent JL, Rhodes A: Preoperative abnormalities in serum sodium concentrations are associated with higher in-hospital mortality in patients undergoing major surgery. Br J Anaes 116: 63–69, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Hu J, Wang Y, Geng X, Chen R, Zhang P, Lin J, Teng J, Zhang X, Ding X: Dysnatremia is an independent indicator of mortality in hospitalized patients. Med Sci Monit 23: 2408–2425, 2017. 28528344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Funk G-C, Lindner G, Druml W, Metnitz B, Schwarz C, Bauer P, Metnitz PGH: Incidence and prognosis of dysnatremias present on ICU admission. Intensive Care Med 36: 304–311, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Liamis G, Rodenburg EM, Hofman A, Zietse R, Stricker BH, Hoorn EJ: Electrolyte disorders in community subjects: Prevalence and risk factors. Am J Med 126: 256–263, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Khan S, Floris M, Pani A, Rosner MH: Sodium and volume disorders in advanced chronic kidney disease. Adv Chronic Kidney Dis 23: 240–246, 2016. 27324677 [DOI] [PubMed] [Google Scholar]
- 17.Kovesdy CP: Significance of hypo- AND hypernatremia in chronic kidney disease. Nephrol Dial Transplant 27: 891–898, 2012. 22379183 [DOI] [PubMed] [Google Scholar]
- 18.Kovesdy CP, Lott EH, Lu JL, Malakauskas SM, Ma JZ, Molnar MZ, Kalantar-Zadeh K: Hyponatremia, hypernatremia, and mortality in patients with chronic kidney disease with and without congestive heart failure. Circulation 125: 677–684, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huff SM, Rocha RA, McDonald CJ, De Moor GJ, Fiers T, Bidgood WD, Forrey AW, Francis WG, Tracy WR, Leavelle D, Stalling F, Griffin B, Maloney P, Leland D, Charles L, Hutchins K, Baenziger J: Development of the logical observation identifier names and codes (LOINC) vocabulary. J Am Med Inform Assoc 5: 276–292, 1998. 9609498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, Januel J-M, Sundararajan V: Updating and validating the charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 173: 676–682, 2011. 21330339 [DOI] [PubMed] [Google Scholar]
- 21.Katz MA: Hyperglycemia-induced hyponatremia-calculation of expected serum sodium depression. N Engl J Med 289: 843–844, 1973. 4763428 [DOI] [PubMed] [Google Scholar]
- 22.Hillier TA, Abbott RD, Barrett EJ: Hyponatremia: evaluating the correction factor for hyperglycemia. Am J Med 106: 399–403, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Al Mawed S, Pankratz VS, Chong K, Sandoval M, Roumelioti M-E, Unruh M: Low serum sodium levels at hospital admission: Outcomes among 2.3 million hospitalized patients. PLoS ONE 13: e0194379, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J: A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bataille S, Baralla C, Torro D, Buffat C, Berland Y, Alazia M, Loundou A, Michelet P, Vacher-Coponat H: Undercorrection of hypernatremia is frequent and associated with mortality. BMC Nephrol 15: 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu B, Han Q, Mengke N, He K, Zhang Y, Nie Z, Zeng H: Prognostic value of ICU-acquired hypernatremia in patients with neurological dysfunction. Medicine (Baltimore) 95: e3840, 2016. 27583842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han S-W, Tilea A, Gillespie BW, Finkelstein FO, Kiser MA, Eisele G, Kotanko P, Levin N, Saran R: Serum sodium levels and patient outcomes in an ambulatory clinic-based chronic kidney disease cohort. Am J Nephrol 41: 200–209, 2015 [DOI] [PubMed] [Google Scholar]
- 28.Chiu DYY, Kalra PA, Sinha S, Green D: Association of serum sodium levels with all-cause and cardiovascular mortality in chronic kidney disease: Results from a prospective observational study. Nephrology 21: 476–482, 2016 [DOI] [PubMed] [Google Scholar]
- 29.Sun L, Hou Y, Xiao Q, Du Y: Association of serum sodium and risk of all-cause mortality in patients with chronic kidney disease: A meta-analysis and sysematic review. Sci Rep 7: 15949, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turgutalp K, Özhan O, Gök Oğuz E, Yılmaz A, Horoz M, Helvacı İ, Kiykim A: Community-acquired hypernatremia in elderly and very elderly patients admitted to the hospital: Clinical characteristics and outcomes. Med Sci Monit 18: CR729–CR734, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snyder NA, Feigal DW, Arieff AI: Hypernatremia in elderly patients. A heterogeneous, morbid, and iatrogenic entity. Ann Intern Med 107: 309–319, 1987. 3619220 [DOI] [PubMed] [Google Scholar]
- 32.Grangeon-Chapon C, Dodoi M, Esnault VL, Favre G: Osmotic stress and mortality in elderly patients with kidney failure: A retrospective study. Clin Interv Aging 14: 225–229, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Missing laboratory data profile. Download Supplemental Table 1, PDF file, 229 KB (162.7KB, pdf)
Profile of hospitalized patients with and without hypernatremia. Download Supplemental Table 2, PDF file, 229 KB (162.7KB, pdf)