Abstract
Breast cancer (BC) remains a leading cause of death among women today, and mortality among African American women in the US remains 40% higher than that of their White counterparts, despite reporting a similar incidence of disease over recent years. Previous meta-analyses and studies of BC mortality highlight that tumor characteristics, rather than socio-economic factors, drive excess mortality among African American women with BC. This is further complicated by the heterogeneity of BC, where BC can more appropriately be defined as a collection of diseases rather than a single disease. Molecular phenotyping and gene expression profiling distinguish subtypes of BC, and these subtypes have distinct prognostic outcomes. Racial disparities transcend these subtype-specific outcomes, where African American women suffer higher mortality rates among all BC subtypes. The most striking differences are observed among the most aggressive molecular subtype, triple-negative BC (TNBC), where incidence and mortality are significantly higher among African American women compared to all other race/ethnicity groups. We and others have shown that this predisposition for triple-negative disease may be linked to shared west African ancestry, where the highest rates of TNBC are observed among west African nations, and these high frequencies follow into the African diaspora. Genetic and molecular characterization of breast tumors among subtypes and racial/ethnic groups have begun to identify targets with future therapeutic potential, but more work needs to be done to identify targeted treatment options for all women who suffer from BC.
Keywords: Breast cancer heterogeneity, Breast cancer disparities
Social versus biological determinants in BC disparities
Over the past decade, significant progress has been made in identifying the multifactorial risk factors of Breast Cancer (BC) incidence and survival outcomes, paving the way to better screening and treatment options. Over the past 5 years, overall cancer mortality has steadily decreased, and this trend is expected to continue [1]. Despite these successes, BC is the most frequently diagnosed cancer globally and the leading cause of cancer-related death among women [2, 3], accounting for 25% of cancer cases and 15% of cancer-related deaths [3]. In the US, BC remains the second leading cause of death among women [4], and over 275,000 new BC cases are expected this year, with over 42,000 deaths from BC [1]. Among these deaths lurks persistent racial/ethnic disparities in outcomes over the past 50 years, and similar disparity trends are also observed globally [5, 6]. Historically, BC incidence rates have been lower for African American (AA) women compared to White/European American (EA) women, though incidence rates have recently increased in AA collapsing this incidence gap [7] (Fig. 1a). Disparities in BC mortality initially emerged during the mid-1980s and remains 40% higher among AA woman compared to EA women today [4] (Fig. 1b). The emergence of disparities in survival directly coincided with the advent of targeted endocrine therapies [8], where AA women present with tumors types that do not respond to these therapies (Fig. 1c). Factors associated with differences in mortality also include AA women more frequently presenting with higher grade and later stage tumors [2–4, 9].
Fig. 1.
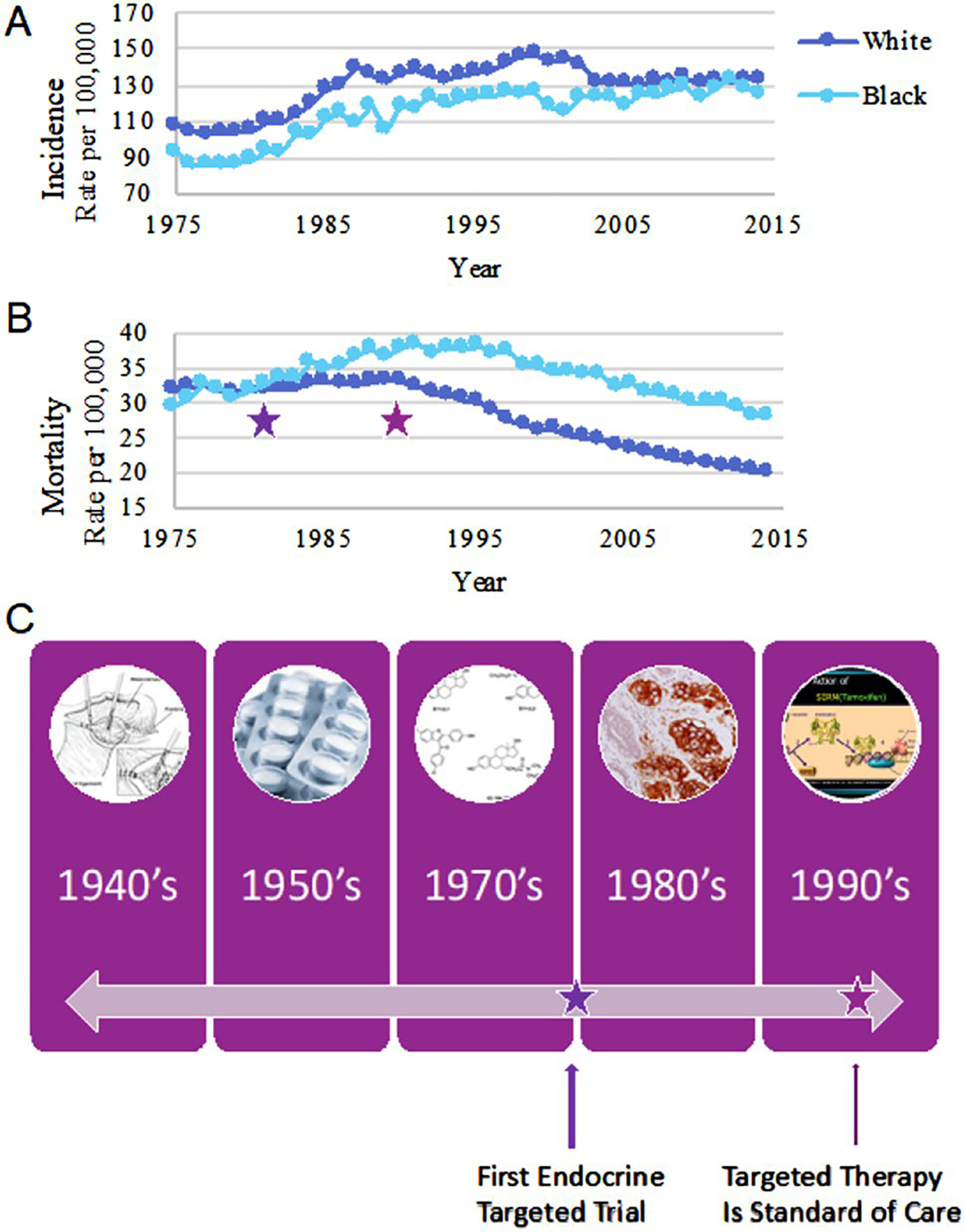
Emergence of BC diversity unmasked by advent of targeted endocrine therapy. BC incidence (a) and mortality (b) curves among females in the US from SEER data collected between 1975 and 2015. Incidence and mortality reported among White/European American women is shown in dark blue, and among Black/African American women is shown in light blue. Purple stars correspond with the timeline in panel C, highlighting the advent of targeted endocrine therapy in clinical trials (late 1970s/early 1980s, left), and its eventual use as standard of care beginning in the early to mid 1990s (right)
African American women suffer from disproportionately higher cancer mortality rates across all cancer types [10]. It is well-established that the consequences of lower socioeconomic status (SES), systemic racism and environmental exposures that affect wellness create barriers to equitable care [11–14]. Several of these factors, such as housing options [15, 16] are highly correlated with food insecurity [17, 18] and neighborhood deprivation [19], and are mediators of disparities in AA BC mortality compared to EA [20, 21]. The higher burden of interval disease (diagnosed between screening timepoints) can also be partially attributed to factors of health equity [22]. The resulting SES correlations with survival are ultimately an effect of bias in the health system [23], and include lack of effective options for clinical treatment and prohibited medical access and medical equity [24, 25], even in the context of progressive policies to address disparities [26]. Interestingly, despite suffering from similar socioeconomic barriers to access, Latina women with shared American Native (NA) ancestry have been found to have genetic factors that are protective in BC disease and outcomes [27–29]. Together, these data indicate that while SES-related barriers to access are associated with worse BC outcomes, they cannot completely explain the ongoing disparities in AA survival observed in clinic. In addition to factors linked to SES [11, 12], analyses that address other multifactorial variables that factor into disparities concluded that excess mortality is consistently found in AA patients, even after controlling for SES factors, and excess mortality can be connected to tumor characteristics instead [10, 30–33]. For example, among Hormone Receptor-positive (HR+) BC, which represent the highest proportion of cancers among all race/ethnic groups and have targeted therapies available, survival outcomes are significantly worse among AA women [4, 34–36] (Fig. 2). Hormone Receptor-negative (HR-) and TNBC disease is diagnosed higher rates among AA women [35], and increased prevalence of these biologically distinct and aggressive subtypes among premenopausal AA women, are a prime example of biased incidence of specific tumor characteristics [37, 38] (Fig. 2). Additionally, worse survival outcomes persist among AA women with HR-/TNBC compared to EA women, again after controlling for SES factors [39]. These observations indicate that SES factors cannot be the only drivers of the disparities we observe in the clinical setting [10, 30–33].
Fig. 2.
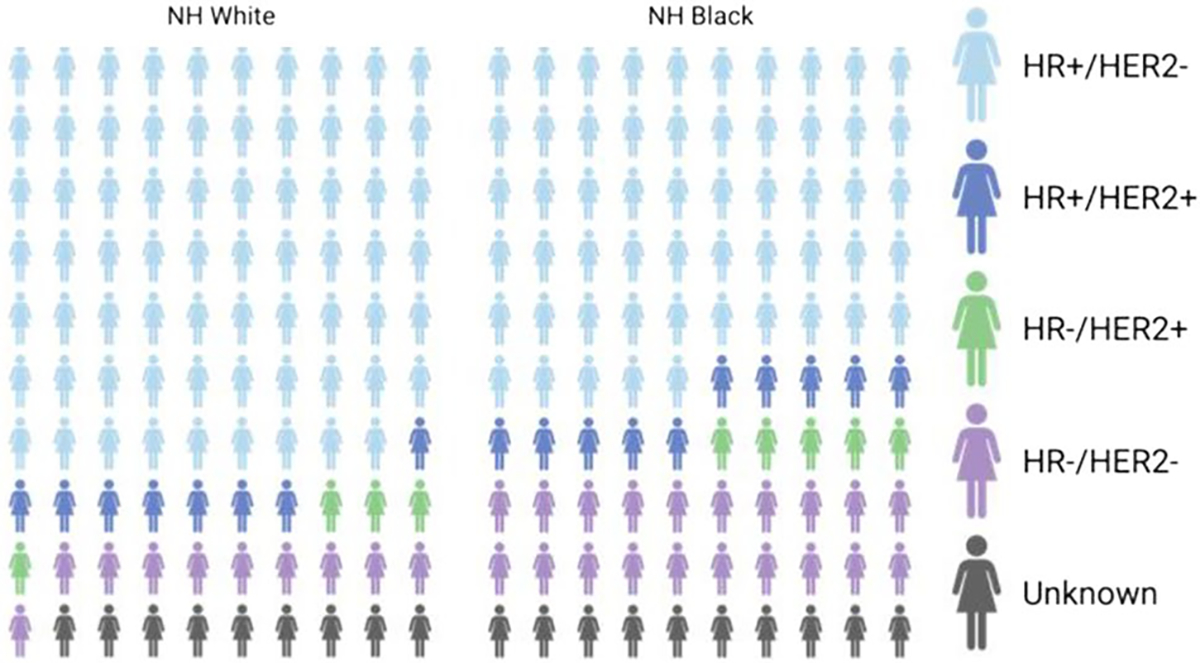
Distribution of BC subtypes have bias in prevalence between White and Black Americans. Distribution of BC subtypes among non-Hispanic White and non-Hispanic Black women in the US, 2012–2016. Data from SEER program and CDC National Program of Cancer Registries, frequencies reported from DeSantis et al. [4]
BC is a molecularly heterogeneous disease comprised of multiple subtypes with distinct prognostic outcomes
This disparity in BC outcomes is further complicated by the complexity of the disease, as BC represents a collection of heterogeneous cancer types characterized by molecular and intrinsic phenotypes. These tumor phenotypes are found at disparate frequencies across different racial/ethnic groups and have distinct prognostic outcomes which inform the clinical management of disease for the patient. Molecular subtypes of BC are defined by the presence or absence of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) on the tumor cell surface by immunohistochemistry (IHC) staining and/or in situ hybridization. ER and PR positivity in tumors represent HR + tumors, HER2 positive tumors represent their own molecular subtype, and triple-negative breast tumors (TNBC) are defined by the absence of these receptors on the breast tumor cell surface. HR + and HER2 + tumors have targeted molecular treatments available and have better survival outcomes compared to TNBC tumors, which lack targeted treatments, are more aggressive, and have worse survival outcomes [4, 7, 40].
Genomic investigations of tumors have further defined the phenotypic differences of molecular subtypes, where distinct gene expression profiles were found across a broad range of breast tumors [41, 42]. These gene expression profiles were refined into a panel of 50 genes (PAM50), that could reproducibly classify tumors into intrinsic phenotypes [43]. Five intrinsic subtypes have been defined with the PAM50 classification, and they represent HRpositive cancers (Luminal A (LumA), Luminal B (LumB), HER2 enriched (HER2), Normal-like and Basal-like BCs (comprising TNBC tumors) (Fig. 3). The concordance of these molecular phenotypes with intrinsic subtypes has been investigated by multiple groups and cohorts, and meta-analyses of these studies show that there is agreement between these two methods [38, 44]. These intrinsic phenotypes have additional prognostic value to molecular subtypes, where risk of recurrence is lowest among LumA cancers, and relapse-free survival rates are lowest among HER2 + and basal-like tumors [42, 43, 45]. The prognostic value of the PAM50 intrinsic subtyping tool has been validated in longer follow up survival cohorts [46], indicating its value in the clinical setting.
Fig. 3.
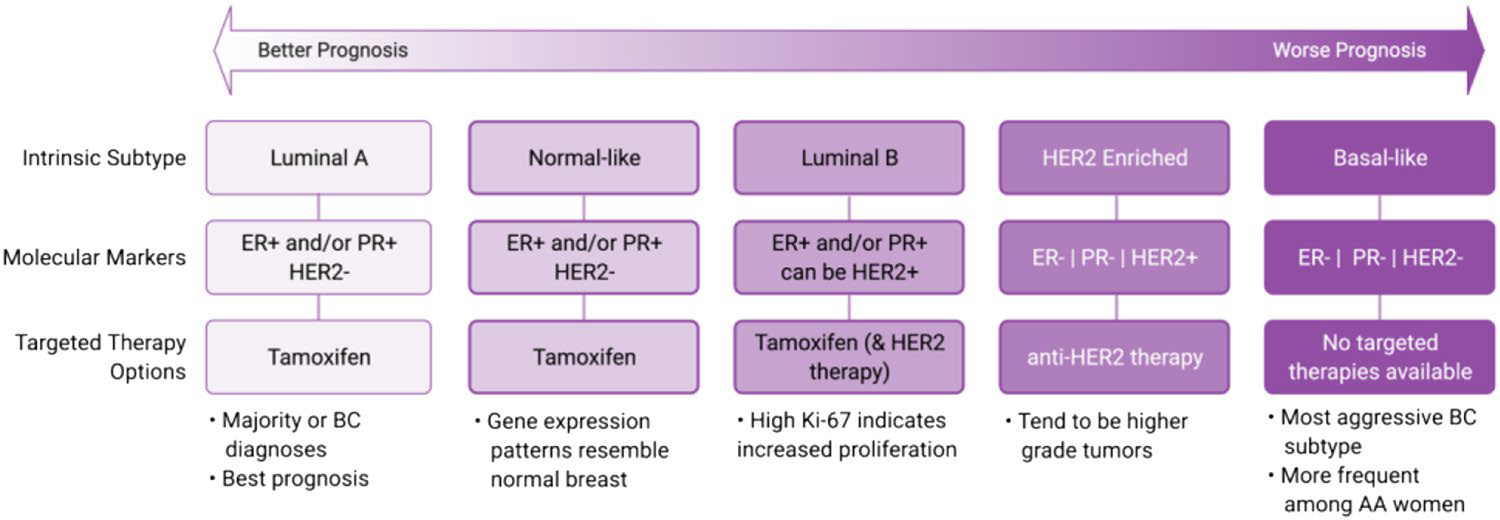
Relative prognosis and treatment options among BC subtypes. Adapted from Dai et al. [45]
Incidence, prevalence and survival outcomes of BC subtypes across racial/ethnic groups
HR + cancers typically represent the highest proportion of cancers among all race/ethnic groups, however 5-year survival outcomes are worse among AA women for these cancers when looking across multiple studies/cohorts [4, 34–36]. Genetic profiling of LumA (ER+ and/or PR+) tumors between AA and EA women has revealed a set of genes upregulated in AA tumors associated with poor prognostic outcomes [47], and a multi-gene score derived from expression values of these upregulated genes was associated with increased risk of recurrence [48]. HER2+ cancers are diagnosed at a similar frequency between race groups [4]. Higher odds ratio for HER2+ cancers are also observed among AA women [35], and relative to LumA/ER+ tumors, were more often late stage and had lymph node involvement [34, 37].
The widening gap in BC mortality among AA women began in the late 1970s, when targeted treatments for HR + BC became available, and subsequently standard of care [8]. With the advent of these targeted therapies, overall BC mortality began to decrease due to effective treatment response, but subsequently unmasked diversity in tumor phenotypes, where this widening of the mortality gap can be attributed to disproportionately higher prevalence of HR-/TNBC tumors among AA women compared to all other racial/ethnic groups in the US [4, 34, 37]. AA women have a higher likelihood of developing basal-like/TNBC disease compared to other subtypes [35], and also suffer from worse survival outcomes compared to other racial/ethnic groups [4, 37, 49]. Prevalence of TNBC is particularly high among premenopausal AA women [38], where a bimodal distribution of TNBC across age groups of AA women is observed [37]. Among young AA women (<35yo), over 50% of BC cases were determined to be TNBC, with the second peak of TNBC prevalence occurring among AA women aged 51–60 [37]. Similar to reports for overall BC survival, worse survival outcomes persist among AA women with TNBC disease after controlling for SES factors [39].
West African Ancestry and TNBC disease—race/ancestry-informed disparities research
Epidemiology of TNBC disease
Epidemiological data show higher rates of TNBC in subpopulations of shared west African ancestry, in most nations that track African ancestry through self-reported race (SRR) [30, 50–52]. In the ICSBCS cohort, prevalence of TNBC cancers is highest among west African and AA women compared to East African and EA women [53–55]. After quantifying west African ancestry in the ICSBCS cohort, we found that the percentage of west African ancestry is higher among TNBC cases compared to non-TNBC cases [54], which is concordant with other independent cohorts [56]. Global investigations of BC status indicate similar trends, where Great Britain [57, 58] and Switzerland [59] also show association between TNBC prevalence and west African ancestry.
Through comprehensive literature search, we have compiled TNBC prevalence rates reported from countries globally (Fig. 4). Higher frequency of TNBC disease is found among Sub-Saharan African nations, and we additionally observe higher rates of TNBC disease across the African diaspora in ad-mixed populations (Fig. 4). Compared to global BC mortality reports, countries with higher TNBC prevalence also report higher overall BC mortality. Despite reporting lower incidence of BC disease, low- and middleincome countries suffer from higher mortality rates, and these disparate outcomes are largely attributed to observed later stage at presentation and limited access to treatment options [3]. The highest frequencies of TNBC correlate with the highest mortality from BC, and likely indicates the poor prognoses from this aggressive molecular subtype, lacking effective and/or targeted therapies.
Fig. 4.
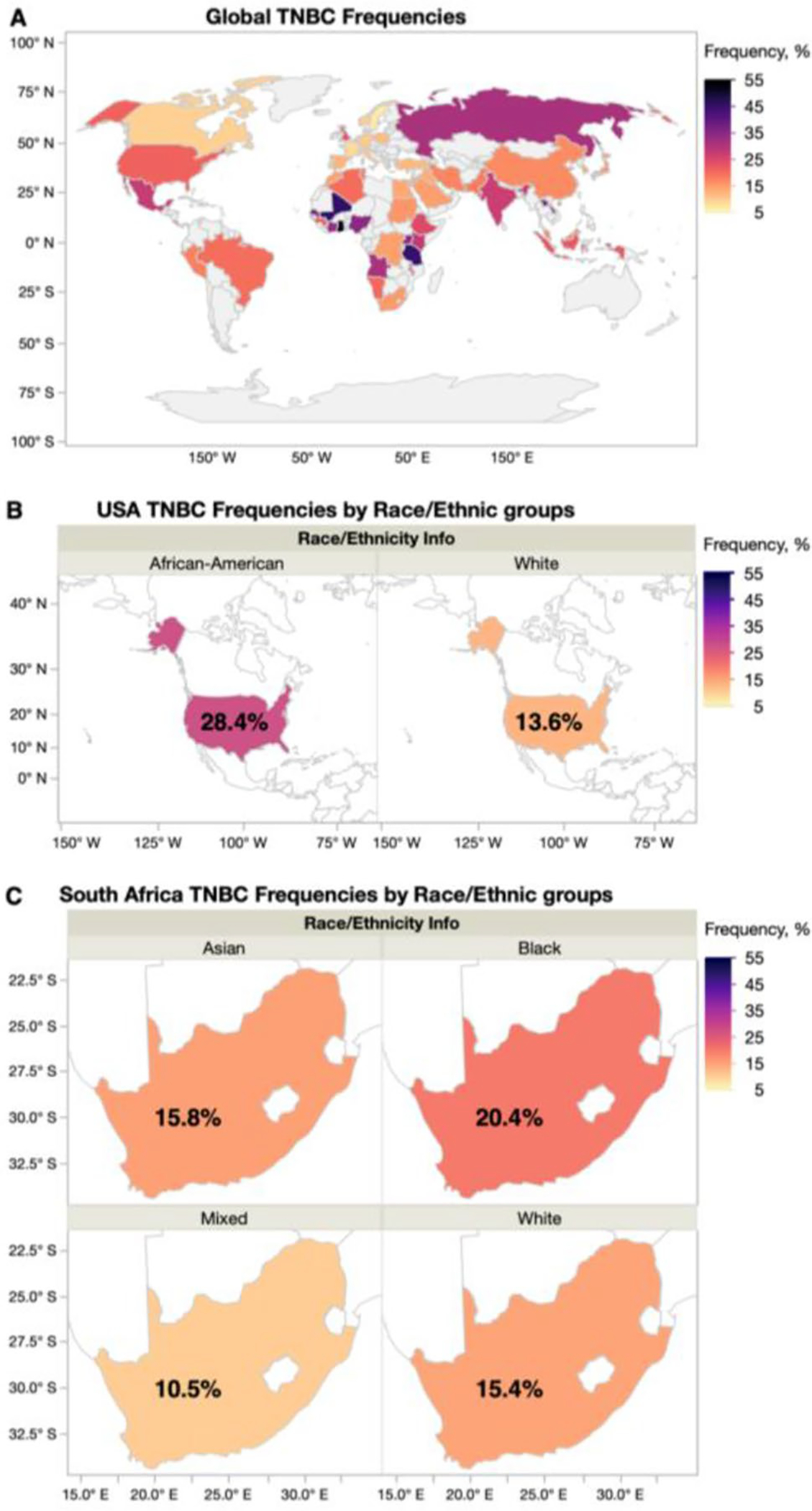
TNBC Frequencies globally and in ad-mixed populations. a Global TNBC frequencies reported from over 100 published studies. b Average TNBC frequency reported among African American and White BC patients in the USA. c Average TNBC frequency reported among Asian, Black, Mixed and White BC patients in South Africa
Genomic investigations of TNBC among individuals of African ancestry
In a case-series analysis comparing TNBC cases versus non-TNBC cases in an African ancestry enriched cohort, we found the Duffy-null allele to be significantly associated with TNBC risk among AA, after controlling for age and west African ancestry [54]. This suggests that TNBC in women of African descent, aside from just having African ancestry, may be influenced by the biological mechanisms that are related to DARC function [60]. We hypothesize that DARC is the driving factor for differences in TNBC mortality [39]. Intriguingly, we have observed that the underlying tumor biology of TNBC does differ among race groups, with higher prevalence of certain TNBC subtypes in women of African descent.
We have also identified differences in gene expression patterns among TNBC tumors that are associated with west African ancestry [61], where we have identified ~ 150 genes that are associated with west African ancestry across TNBC tumors. This differentially expressed gene signature indicated alteration in TP53, NFKB1 and AKT pathways, and we additionally identified west African ancestry associated upregulated genes that have targeted therapeutics currently in clinical trials. Approximately half of our gene list overlapped with SRR differentially expressing genes, indicating that by using quantified genetic ancestry, we are able to better account for differences in tumor gene expression due to genetic admixture of the samples, that was otherwise lost in our SRR analysis [61].
Gene expression profiling defines subtypes of TNBC
Similar to the PAM50 intrinsic subtyping approach, subtypes of TNBC have been defined using gene expression profiling, and the distribution of TNBC subtypes are different among AA and EA women [52, 61–63]. Lehmann et al. have identified four TNBC subtypes based on gene expression profiling: basal-like1 (BL1), basal-like 2 (BL2), mesenchymal (M), and luminal androgen receptor (LAR) [64, 65]. AA TNBC tumors are reported to be more frequently BL1/2 TNBC subtypes, [52, 61–63], where EA are more M and LAR [62]. In a TNBC cohort where patients received neoadjuvant chemotherapy, BL1 tumors have reported higher rates of pathological complete response (pCR), while BL2 and LAR have lowest rates of pCR [66].
Androgen receptor (AR) expression has also been investigated in the context of TNBC disease, where TNBC tumors that lack AR expression are classified as quadruple-negative BC (QNBC) [52, 67]. Comparing race/ethnicity groups, AA TNBC tumors were found to be more frequently ARor QNBC than EA (87% versus 56%, respectively). QNBC is associated with younger age at diagnosis compared to TNBC [52, 67], and worse survival outcomes for AA QNBC patients [52].
Conclusions—race and ancestry in cancer disparities; a way forward
Genetic ancestry of the modern African diaspora is largely representative of west African descendants of enslaved Africans, through the forced migration over hundreds of years and dozens of generations due to the Trans-Atlantic Slave Trade. In the US, while west African ancestry is predominant among individuals who self-identify as AA [68, 69], varying levels of geographic ancestry from African, European and Native American groups are unpredictable in each individual. This draws concern over the reproducibility and validity of ‘race-specific’ risk and warrants a more quantifiable approach that assesses genetic ancestry on an individual basis. In our own analyses, we have shifted our focus to study the specific impact of west African ancestry in BC tumor biology, within admixed populations. Specifically, an individual’s quantified African ancestry is utilized as a continuous variable in our gene network analyses, allowing for the selection of differential expression that is directly associated with shared west African ancestry. We do not disregard an individual self-reported race/ethnicity (SRR), but rather leverage their specific genetic ancestry composition to determine differences in BC risk, or tumor gene expression profiles. Using this approach, we and others have shown that west African ancestry among AA women is significantly with TNBC disease [50, 51, 54, 70]. This trend is also observed as we look globally, as comparison studies in countries such as Great Britain [57, 58] and Switzerland [59] also show increase risk of TNBC disease among individuals with African ancestry.
However, it is not necessary to totally disregard an individual’s self-reported race/ethnicity, but rather utilize aspects of identity and ancestry as is appropriate for the investigation. Specifically, with integrated clinical and demographic data annotations, we can leverage specific genetic ancestry composition with social determinants to interpret the effects leading to gene network changes. This perspective can allow us to discern how differences in BC risk, or tumor gene expression profiles are either related to heritable factors or social/cultural factors. Ultimately, we may be able to even pinpoint the impact of systemic racism on tumor biology as a function of social determinant impacts on tumor etiology and progression. Such progress could have profound impacts on health policy and disease prevention to improve disparities.
Recent agency-level public endeavors have drawn more focus on the importance of inclusion of diverse ancestry and ethnic populations in clinical trials and translational research. While we understand that BC is a heterogeneous disease, where both tumor characteristics and patient race/ethnicity play an important role in prognostic outcomes of a BC diagnosis, there is still a dearth of diversity in the data. We also understand that SES and the outcomes of racism have attributed to disparities in BC mortality, where mortality is persistently higher among AA women. Our extensive efforts to identify the underpinnings of higher TNBC incidence across the African diaspora has revealed a significant link to shared west African Ancestry. Specifically, while TNBC frequencies are highest among Sub Saharan African countries and remain higher across the African diaspora, additional efforts are needed to unravel the confounding genetic admixture in the contemporary descendants of the Trans-Atlantic Slave Trade, which is associated with increased risk of TNBC. However, naming unique etiologies of BC disparities, be it social or biological determinants, will not ameliorate these disparities. The key to changing the paradigm is discerning the modifiable social determinant factors, and the targetable biological determinant factors, and affecting them with concerted medical research efforts. And without a doubt, this requires the consistent participation of the marginalized communities.
Genetic investigations of TNBC that have focused on the impact of west African ancestry on tumor biology have identified gene signatures that may serve as therapeutic targets and have made clear a path for development of targeted therapies. While a significant amount of research has been conducted to address these BC disparities, it is imperative that as we conceive and conduct future BC studies, we plan for inclusion of diverse patient populations. This includes designing studies to fully characterize tumor heterogeneity across subtypes and racial/ethnic groups, and to additionally identify targets for treatment that are effective across all BC patients.
Funding
This study was funded by National Cancer Institute (Grant No. 5R21CA210237-03).
Abbreviations
- BC
Breast Cancer
- EA
European American
- AA
African American
- SES
Socio-Economic Status
- HR
Hormone Receptor
- TNBC
Triple-Negative Breast Cancer
- pCR
Pathologic Complete Response
- QNBC
Quadruple-Negative Breast Cancer
Footnotes
Presented at the 8th International Cancer Metastasis Congress in San Francisco, CA, USA from October 25-27, 2019 (http://www.cancermetastasis.org). To be published in an upcoming Special Issue of Clinical and Experimental Metastasis: Novel Frontiers in Cancer Metastasis.
References
- 1.Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA Cancer J Clin 70(1):7–30 [DOI] [PubMed] [Google Scholar]
- 2.Global Burden of Disease Cancer C, Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T, Alizadeh-Navaei R, Allen C, Alsharif U, Alvis-Guzman N, Amini E et al. (2018) Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: a systematic analysis for the global burden of disease study. JAMA Oncol 4(11):1553–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torre LA, Islami F, Siegel RL, Ward EM, Jemal A (2017) Global cancer in women: burden and trends. Cancer Epidemiol Biomarkers Prev 26(4):444–457 [DOI] [PubMed] [Google Scholar]
- 4.DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, Jemal A, Siegel RL (2019) Breast cancer statistics, 2019. CA Cancer J Clin 69(6):438–451 [DOI] [PubMed] [Google Scholar]
- 5.Oluwasanu M, Olopade OI (2020) Global disparities in breast cancer outcomes: new perspectives, widening inequities, unanswered questions. Lancet Glob Health 8(8):e978–e979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu K, Ding P, Wu Y, Tian W, Pan T, Zhang S (2019) Global patterns and trends in the breast cancer incidence and mortality according to sociodemographic indices: an observational study based on the global burden of diseases. BMJ Open 9(10):e028461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A (2016) Breast cancer statistics, 2015: convergence of incidence rates between black and white women. CA Cancer J Clin 66(1):31–42 [DOI] [PubMed] [Google Scholar]
- 8.Newman LA (2016) Parsing the etiology of breast cancer disparities. J Clin Oncol 34(9):1013–1014 [DOI] [PubMed] [Google Scholar]
- 9.Shoemaker ML, White MC, Wu M, Weir HK, Romieu I (2018) Differences in breast cancer incidence among young women aged 20–49 years by stage and tumor characteristics, age, race, and ethnicity, 2004–2013. Breast Cancer Res Treat 169(3):595–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69(1):7–34 [DOI] [PubMed] [Google Scholar]
- 11.Alcaraz KI, Wiedt TL, Daniels EC, Yabroff KR, Guerra CE, Wender RC (2020) Understanding and addressing social determinants to advance cancer health equity in the United States: a blueprint for practice, research, and policy. CA Cancer J Clin 70(1):31–46 [DOI] [PubMed] [Google Scholar]
- 12.Warnecke RB, Campbell RT, Vijayasiri G, Barrett RE, Rauscher GH (2019) Multilevel examination of health disparity: the role of policy implementation in neighborhood context, in patient resources, and in healthcare facilities on later stage of breast cancer diagnosis. Cancer Epidemiol Biomarkers Prev 28(1):59–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jee-Lyn Garcia J, Sharif MZ (2015) Black lives matter: a commentary on racism and public health. Am J Public Health 105(8):e27–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams DR, Lawrence JA, Davis BA (2019) Racism and health: evidence and needed research. Annu Rev Public Health 40:105–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beyer KM, Zhou Y, Matthews K, Bemanian A, Laud PW, Nattinger AB (2016) New spatially continuous indices of redlining and racial bias in mortgage lending: links to survival after breast cancer diagnosis and implications for health disparities research. Health Place 40:34–43 [DOI] [PubMed] [Google Scholar]
- 16.Bailey ZD, Krieger N, Agenor M, Graves J, Linos N, Bassett MT (2017) Structural racism and health inequities in the USA: evidence and interventions. Lancet 389(10077):1453–1463 [DOI] [PubMed] [Google Scholar]
- 17.Pisegna J, Xu M, Spees C, Krok-Schoen JL (2020) Mental health-related quality of life is associated with diet quality among survivors of breast cancer. Support Care Cancer 29:2021–2028 [DOI] [PubMed] [Google Scholar]
- 18.Farquharson WH, Thornton CJ (2020) Debate: exposing the most serious infirmity - racism’s impact on health in the era of COVID-19. Child Adolesc Ment Health 25(3):182–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coughlin SS (2019) Social determinants of breast cancer risk, stage, and survival. Breast Cancer Res Treat 177(3):537–548 [DOI] [PubMed] [Google Scholar]
- 20.Warner ET, Tamimi RM, Hughes ME, Ottesen RA, Wong YN, Edge SB, Theriault RL, Blayney DW, Niland JC, Winer EP et al. (2015) Racial and ethnic differences in breast cancer survival: mediating effect of tumor characteristics and sociodemographic and treatment factors. J Clin Oncol 33(20):2254–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cerdena JP, Plaisime MV, Tsai J (2020) From race-based to race-conscious medicine: how anti-racist uprisings call us to act. Lancet 396(10257):1125–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson B, Hohl SD, Molina Y, Paskett ED, Fisher JL, Baltic RD, Washington CM (2018) Breast cancer disparities among women in underserved communities in the USA. Curr Breast Cancer Rep 10(3):131–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yonas MA, Jones N, Eng E, Vines AI, Aronson R, Griffith DM, White B, DuBose M (2006) The art and science of integrating Undoing Racism with CBPR: challenges of pursuing NIH funding to investigate cancer care and racial equity. J Urban Health 83(6):1004–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson B (2020) How structural racism can kill cancer patients: black patients with breast cancer and other malignancies face historical inequities that are ingrained but not inevitable. In this article, the second of a 2-part series, we explore the consequences of and potential solutions to racism and inequality in cancer care. Cancer Cytopathol 128(2):83–84 [DOI] [PubMed] [Google Scholar]
- 25.Pallok K, De Maio F, Ansell DA (2019) Structural racism—a 60-year-old black woman with breast cancer. N Engl J Med 380(16):1489–1493 [DOI] [PubMed] [Google Scholar]
- 26.Thornton RL, Glover CM, Cene CW, Glik DC, Henderson JA, Williams DR (2016) Evaluating strategies for reducing health disparities by addressing the social determinants of health. Health A?(Millwood) 35(8):1416–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fejerman L, Ahmadiyeh N, Hu D, Huntsman S, Beckman KB, Caswell JL, Tsung K, John EM, Torres-Mejia G, Carvajal-Carmona L et al. (2014) Genome-wide association study of breast cancer in Latinas identifies novel protective variants on 6q25. Nat Commun 5:5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffman J, Fejerman L, Hu D, Huntsman S, Li M, John EM, Torres-Mejia G, Kushi L, Ding YC, Weitzel J et al. (2019) Identification of novel common breast cancer risk variants at the 6q25 locus among Latinas. Breast Cancer Res 21(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hines LM, Sedjo RL, Byers T, John EM, Fejerman L, Stern MC, Baumgartner KB, Giuliano AR, Torres-Mejia G, Wolff RK et al. (2017) The interaction between genetic ancestry and breast cancer risk factors among hispanic women: the breast cancer health disparities study. Cancer Epidemiol Biomarkers Prev 26(5):692–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newman LA (2017) Breast cancer disparities: socioeconomic factors versus biology. Ann Surg Oncol 24(10):2869–2875 [DOI] [PubMed] [Google Scholar]
- 31.Albain KS, Unger JM, Crowley JJ, Coltman CA Jr, Hershman DL (2009) Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst 101(14):984–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menashe I, Anderson WF, Jatoi I, Rosenberg PS (2009) Underlying causes of the black-white racial disparity in breast cancer mortality: a population-based analysis. J Natl Cancer Inst 101(14):993–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newman LA, Griffith KA, Jatoi I, Simon MS, Crowe JP, Colditz GA (2006) Meta-analysis of survival in African American and white American patients with breast cancer: ethnicity compared with socioeconomic status. J Clin Oncol 24(9):1342–1349 [DOI] [PubMed] [Google Scholar]
- 34.Allott EH, Geradts J, Cohen SM, Khoury T, Zirpoli GR, Bshara W, Davis W, Omilian A, Nair P, Ondracek RP et al. (2018) Frequency of breast cancer subtypes among African American women in the AMBER consortium. Breast Cancer Res 20(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huo D, Hu H, Rhie SK, Gamazon ER, Cherniack AD, Liu J, Yoshimatsu TF, Pitt JJ, Hoadley KA, Troester M et al. (2017) Comparison of breast cancer molecular features and survival by African and European ancestry in the cancer genome atlas. JAMA Oncol 3(12):1654–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Troester MA, Sun X, Allott EH, Geradts J, Cohen SM, Tse CK, Kirk EL, Thorne LB, Mathews M, Li Y et al. (2018) Racial differences in PAM50 subtypes in the carolina breast cancer study. J Natl Cancer Inst 110(2):176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ihemelandu CU, Leffall LD Jr., Dewitty RL, Naab TJ, Mezghebe HM, Makambi KH, Adams-Campbell L, Frederick WA (2007) Molecular breast cancer subtypes in premenopausal and postmenopausal African-American women: age-specific prevalence and survival. J Surg Res 143(1):109–118 [DOI] [PubMed] [Google Scholar]
- 38.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S et al. (2006) Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 295(21):2492–2502 [DOI] [PubMed] [Google Scholar]
- 39.Lund MJ, Trivers KF, Porter PL, Coates RJ, Leyland-Jones B, Brawley OW, Flagg EW, O’Regan RM, Gabram SG, Eley JW (2009) Race and triple negative threats to breast cancer survival: a population-based study in Atlanta, GA. Breast Cancer Res Treat 113(2):357–370 [DOI] [PubMed] [Google Scholar]
- 40.Costa RLB, Gradishar WJ (2017) Triple-negative breast cancer: current practice and future directions. J Oncol Pract 13(5):301–303 [DOI] [PubMed] [Google Scholar]
- 41.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA et al. (2000) Molecular portraits of human breast tumours. Nature 406(6797):747–752 [DOI] [PubMed] [Google Scholar]
- 42.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS et al. (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98(19):10869–10874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, Davies S, Fauron C, He X, Hu Z et al. (2009) Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol 27(8):1160–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prat A, Pineda E, Adamo B, Galvan P, Fernandez A, Gaba L, Diez M, Viladot M, Arance A, Munoz M (2015) Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast 24(Suppl 2):S26–35 [DOI] [PubMed] [Google Scholar]
- 45.Dai X, Li T, Bai Z, Yang Y, Liu X, Zhan J, Shi B (2015) Breast cancer intrinsic subtype classification, clinical use and future trends. Am J Cancer Res 5(10):2929–2943 [PMC free article] [PubMed] [Google Scholar]
- 46.Caan BJ, Sweeney C, Habel LA, Kwan ML, Kroenke CH, Weltzien EK, Quesenberry CP Jr., Castillo A, Factor RE, Kushi LH et al. (2014) Intrinsic subtypes from the PAM50 gene expression assay in a population-based breast cancer survivor cohort: prognostication of short- and long-term outcomes. Cancer Epidemiol Biomarkers Prev 23(5):725–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.D’Arcy M, Fleming J, Robinson WR, Kirk EL, Perou CM, Troester MA (2015) Race-associated biological differences among Luminal A breast tumors. Breast Cancer Res Treat 152(2):437–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parada H Jr, Sun X, Fleming JM, Williams-DeVane CR, Kirk EL, Olsson LT, Perou CM, Olshan AF, Troester MA (2017) Race-associated biological differences among luminal A and basal-like breast cancers in the Carolina Breast Cancer Study. Breast Cancer Res 19(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ding YC, Steele L, Warden C, Wilczynski S, Mortimer J, Yuan Y, Neuhausen SL (2019) Molecular subtypes of triple-negative breast cancer in women of different race and ethnicity. Oncotarget 10(2):198–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palmer JR, Ruiz-Narvaez EA, Rotimi CN, Cupples LA, Cozier YC, Adams-Campbell LL, Rosenberg L (2013) Genetic susceptibility loci for subtypes of breast cancer in an African American population. Cancer Epidemiol Biomarkers Prev 22(1):127–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Newman LA, Kaljee LM (2017) Health disparities and triple-negative breast cancer in African American Women: a review. JAMA Surg 152(5):485–493 [DOI] [PubMed] [Google Scholar]
- 52.Davis M, Tripathi S, Hughley R, He Q, Bae S, Karanam B, Martini R, Newman L, Colomb W, Grizzle W et al. (2018) AR negative triple negative or “quadruple negative” breast cancers in African American women have an enriched basal and immune signature. PLoS ONE 13(6):e0196909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jemal A, Fedewa SA (2012) Is the prevalence of ER-negative breast cancer in the US higher among Africa-born than US-born black women? Breast Cancer Res Treat 135(3):867–873 [DOI] [PubMed] [Google Scholar]
- 54.Newman LA, Jenkins B, Chen Y, Oppong JK, Adjei E, Jibril AS, Hoda S, Cheng E, Chitale D, Bensenhaver JM et al. (2019) Hereditary susceptibility for triple negative breast cancer associated with western Sub-Saharan African Ancestry: results from an International surgical breast cancer collaborative. Ann Surg 270(3):484–492 [DOI] [PubMed] [Google Scholar]
- 55.Jiagge E, Jibril AS, Chitale D, Bensenhaver JM, Awuah B, Hoenerhoff M, Adjei E, Bekele M, Abebe E, Nathanson SD et al. (2016) Comparative analysis of breast cancer phenotypes in African American, White American, and west versus east African patients: correlation between African ancestry and triple-negative breast cancer. Ann Surg Oncol 23(12):3843–3849 [DOI] [PubMed] [Google Scholar]
- 56.Wang S, Pitt JJ, Zheng Y, Yoshimatsu TF, Gao G, Sanni A, Oluwasola O, Ajani M, Fitzgerald D, Odetunde A et al. (2019) Germline variants and somatic mutation signatures of breast cancer across populations of African and European ancestry in the US and Nigeria. Int J Cancer 145(12):3321–3333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bowen RL, Duffy SW, Ryan DA, Hart IR, Jones JL (2008) Early onset of breast cancer in a group of British black women. Br J Cancer 98(2):277–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Copson E, Maishman T, Gerty S, Eccles B, Stanton L, Cutress RI, Altman DG, Durcan L, Simmonds P, Jones L et al. (2014) Ethnicity and outcome of young breast cancer patients in the United Kingdom: the POSH study. Br J Cancer 110(1):230–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rapiti E, Pinaud K, Chappuis PO, Viassolo V, Ayme A, Neyroud-Caspar I, Usel M, Bouchardy C (2017) Opportunities for improving triple-negative breast cancer outcomes: results of a population-based study. Cancer Med 6(3):526–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jenkins BD, Martini RN, Hire R, Brown A, Bennett B, Brown I, Howerth EW, Egan M, Hodgson J, Yates C et al. (2019) Atypical Chemokine Receptor 1 (DARC/ACKR1) in breast tumors is associated with survival, circulating chemokines, tumor-infiltrating immune cells, and African ancestry. Cancer Epidemiol Biomarkers Prev 28(4):690–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davis M, Martini R, Newman L, Elemento O, White J, Verma A, Datta I, Adrianto I, Chen Y, Gardner K et al. (2020) Identification of distinct heterogenic subtypes and molecular signatures associated with African ancestry in triple negative breast cancer using quantified genetic ancestry models in admixed race populations. Cancers (Basel) 12(5):1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lindner R, Sullivan C, Offor O, Lezon-Geyda K, Halligan K, Fischbach N, Shah M, Bossuyt V, Schulz V, Tuck DP et al. (2013) Molecular phenotypes in triple negative breast cancer from African American patients suggest targets for therapy. PLoS ONE 8(11):e71915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Keenan T, Moy B, Mroz EA, Ross K, Niemierko A, Rocco JW, Isakoff S, Ellisen LW, Bardia A (2015) Comparison of the genomic landscape between primary breast cancer in African American versus white women and the association of racial differences with tumor recurrence. J Clin Oncol 33(31):3621–3627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 121(7):2750–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lehmann BD, Jovanovic B, Chen X, Estrada MV, Johnson KN, Shyr Y, Moses HL, Sanders ME, Pietenpol JA (2016) Refinement of triple-negative breast cancer molecular subtypes: implications for neoadjuvant chemotherapy selection. PLoS ONE 11(6):e0157368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Masuda H, Baggerly KA, Wang Y, Zhang Y, Gonzalez-Angulo AM, Meric-Bernstam F, Valero V, Lehmann BD, Pietenpol JA, Hortobagyi GN et al. (2013) Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin Cancer Res 19(19):5533–5540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Angajala A, Mothershed E, Davis MB, Tripathi S, He Q, Bedi D, Dean-Colomb W, Yates C (2019) Quadruple Negative Breast Cancers (QNBC) demonstrate subtype consistency among primary and recurrent or metastatic breast cancer. Transl Oncol 12(3):493–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mersha TB, Abebe T (2015) Self-reported race/ethnicity in the age of genomic research: its potential impact on understanding health disparities. Hum Genom 9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Newman LA, Carpten J (2018) Integrating the genetics of race and ethnicity into cancer research: trailing Jane and John Q. Pulic. JAMA Surg 153(4):299–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Davis MB, Newman LA (2018) Breast cancer disparities: how can we leverage genomics to improve outcomes? Surg Oncol Clin N Am 27(1):217–234 [DOI] [PubMed] [Google Scholar]


