Abstract
Background and Objective
Venetoclax is an approved BCL-2 inhibitor, currently under evaluation in different hematological malignancies in adult and pediatric populations. Venetoclax is available as 10, 50, and 100 mg tablets. To provide an alternative to patients who find taking the commonly prescribed 100 mg tablet a challenge, the interchangeability of lower-strength tablets with the 100 mg tablet was investigated. Additionally, newly developed oral suspension powder formulations to facilitate dosing in pediatrics were evaluated.
Methods
Pharmacokinetic data from 80 healthy female participants from three phase I studies were utilized to evaluate the bioavailability of (1) 10 and 50 mg tablets relative to a 100 mg tablet; (2) 0.72 and 7.2% (drug to total weight) oral powder formulations relative to the 100 mg tablet; and (3) oral powder formulations administered using different vehicles (apple juice, apple sauce, and yogurt) relative to water under fed conditions.
Results
Bioavailability assessments at a 100 mg dose of venetoclax demonstrated bioequivalence across the 10, 50, and 100 mg tablet strengths. Oral powder formulations met the bioequivalence criteria (0.80–1.25) with respect to area under the concentration–time curve to time of the last measurable concentration (AUCt) and to infinite time (AUC∞) but exhibited a slightly lower maximum plasma concentration (Cmax). Exposure–response analyses were utilized to demonstrate that the lower Cmax observed with the powder formulations is not clinically meaningful. The delivery vehicles tested did not affect the bioavailability of venetoclax oral powder formulations.
Conclusions
The smaller-sized tablets (10 and 50 mg) and the newly developed oral powder formulations of venetoclax can be used interchangeably with the 100 mg tablets to improve the patients’ experience, while maintaining adequate exposure.
Clinical Trials Identifiers
NCT01682616, 11 September 2012; NCT02005471, 9 December 2013; NCT02242942, 17 September 2014; NCT02203773, 30 July 2014; NCT02287233, 10 November 2014; NCT02993523, 15 December 2016; NCT03069352, 3 March 2017.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40261-022-01172-4.
Key Points
| Venetoclax tablets (10, 50, and 100 mg) and newly developed oral powder formulations were evaluated to establish interchangeability of the different formulations with the 100 mg tablets and to enable administration with different dosing vehicles. |
| Bioavailability data and exposure–response analyses demonstrated that the smaller-sized venetoclax tablets and the oral powder formulations could be used interchangeably to administer appropriate doses of venetoclax based on patients’ preference. |
| Apple juice, apple sauce, and yogurt can be used to facilitate venetoclax powder formulation administration. |
Introduction
Venetoclax is a first-in-class, potent, orally bioavailable, small-molecule selective inhibitor of B-cell lymphoma 2 (BCL-2) in the biaryl acyl sulfonamide chemical class that restores programmed cell death in cancer cells. Venetoclax has received approvals in multiple countries for chronic lymphocytic leukemia (CLL) and for the treatment of newly diagnosed acute myeloid leukemia (AML) in certain adult populations [1]. Venetoclax is being studied in other hematologic malignancies such as non-Hodgkin’s lymphoma, multiple myeloma (MM), myelodysplastic syndromes in adult patients, and hematologic malignancies and solid tumors in pediatric patients.
Venetoclax is currently marketed as 10, 50, and 100 mg film-coated tablets. Approved doses are 400 and 600 mg/day and doses up to 800 mg are currently being investigated in different hematologic and solid malignancies, notably relapsed/refractory (R/R) MM [2]. Venetoclax is a biopharmaceutics classification system (BCS) class IV compound with poor aqueous solubility and high hydrophobicity (logP = 8.1) [3, 4]. An amorphous solid dispersion formulation is utilized within the currently marketed tablets to overcome the challenging physicochemical properties. Following oral administration, the maximum plasma concentration (Cmax) of venetoclax is attained 5–8 h after dosing, and the half-life (t½) ranged between 14 and 18 h [5]. Venetoclax is prescribed to be administered with food, as food increases the bioavailability of venetoclax by approximately 3- to 5-fold compared with fasting conditions [6].
The 100 mg tablet is the most commonly used formulation; however, due to the low drug loading, the 100 mg tablet is relatively large in size (17.2 mm long, 9.5 mm wide, oblong biconvex shape) [7]. The smaller, lower-strength 10 mg (6 mm diameter, round biconvex shape) [8] and 50 mg (14 mm long, 8 mm wide, oblong biconvex shape) [9] tablets may be preferred by patients who experience difficulty swallowing larger tablets. Additionally, to facilitate dosing in pediatric patients who may not be able to swallow tablets, two oral powder formulations were developed. The oral powder formulations would reduce the pill burden and enable mixing of venetoclax with liquids or soft foods such as apple juice and yogurt to deliver the dose. The new powder formulations utilize the same venetoclax drug substance and same amorphous solid dispersion extrudate intermediate as the currently marketed venetoclax tablets, with venetoclax active substance representing either 0.72 or 7.2% of the total powder weight to deliver a wide range of doses up to 600 mg.
To assess the interchangeability of the lower-strength tablets and support the development of the oral powder formulations, three phase I bioavailability studies in healthy female participants have been conducted in which the lower-strength tablets and oral powder formulations were compared with the 100 mg tablet. The impact of liquid and soft food vehicles (apple juice, apple sauce, and yogurt) on the bioavailability of the oral powder formulations was also evaluated. In addition, exposure–response analyses were conducted to address any differences in bioavailability observed between the tested formulations and to establish interchangeability with the 100 mg tablet formulation.
Methods
Participant Population and Study Designs
Three phase I, open-label, randomized, crossover studies (Studies 1, 2, and 3) were conducted to evaluate the bioavailability of various formulations of venetoclax. A summary of these studies is provided in Table 1. Briefly, Study 1 assessed the bioavailability across film-coated tablets with strengths of 10, 50, and 100 mg at a dose of 100 mg under low-fat conditions. Study 2 compared the bioavailability of the two oral powder formulations (0.72 and 7.2%) relative to the currently marketed 100 mg tablet under high-fat conditions and evaluated the effect of food (high-fat meal) on the bioavailability of the oral powder formulation at a dose of 100 mg. Study 3 characterized the effect of different dosing vehicles (apple juice, apple sauce, yogurt, and water as the reference vehicle) on the bioavailability of the 0.72 and 7.2% oral powder formulations under moderate-fat conditions.
Table 1.
Summary of venetoclax bioavailability phase I studies
| Study number | N | Study design | Venetoclax treatment and regimensa |
|---|---|---|---|
| Study 1 | 40b | Single-dose, randomized, open-label, three-period, six-sequence, crossover |
Three single 100 mg doses of orally administered film-coated tablets under low-fat conditions consisted of the following regimens: A: 1 × 100 mg tabletc B: 2 × 50 mg tabletd C: 10 × 10 mg tabletd |
| Study 2 | 16 | Single-dose, open-label, randomized, five-period, four-sequence crossover |
Five single 100 mg doses under fasted (n = 8/regimen) or high-fat fed (n = 16/regimen) conditions consisted of the following regimens: A:100 mg film-coated tablet, fedc B:7.2% oral powder formulation, fedd C:0.72% oral powder formulation, fedd D:7.2% oral powder formulation, fastedc E:0.72% oral powder formulation, fastedc |
| Study 3 | 24 | Single-dose, open-label, randomized, two-cohort, four-period, eight-sequence crossover |
Four single 100 mg doses under moderate-fat fed conditions consisted of the following regimens: Cohort 1 (n = 12) A: 0.72% oral powder formulation in waterc B: 0.72% oral powder formulation in apple juiced C: 0.72% oral powder formulation in apple sauced D: 0.72% oral powder formulation in yogurtd Cohort 2 (n = 12) E: 7.2% oral powder formulation in waterc F: 7.2% oral powder formulation in apple juiced G: 7.2% oral powder formulation in apple sauced H: 7.2% oral powder formulation in yogurtd |
Note: Low-fat meals consisted of ≤ 700 Kcal with ≤ 20% of the total caloric intake from fat; moderate-fat (standardized diet) meals consisted of ~ 700 Kcal with ~ 30% of caloric intake from fat; and high-fat meals consisted of 800–1000 Kcal with ~ 50% of the caloric intake from fat
N number of participants
aEach regimen in a period consisted of a single oral dose with a washout interval of 5 days between regimens
bFour replacement participants were enrolled to ensure complete pharmacokinetic data from 36 participants across all regimens and were assigned to the same sequence group as the participant being replaced. Three participants discontinued from the study due to the occurrence of at least one AE (all mild [Grade 1] in severity) and 37 participants completed the study
cReference
dTest
For all studies, healthy females, between 18 and 60 years of age, who were postmenopausal, permanently surgically sterile, or perimenopausal or premenopausal and practicing at least two methods of birth control until at least 1 month after the last dose of study drug were selected for participation. Only female participants were enrolled in these clinical studies based on two reasons: (1) the potential for venetoclax-related testicular toxicity (germ cell loss) identified in non-clinical studies [10]; and (2) no effects of venetoclax on female reproductive tissues were observed in general repeat dose toxicology studies [11].
In all studies, enrolled participants were randomly assigned in equal numbers to crossover sequences in their respective studies, except in Study 2 where only half as many participants were assigned to the fasted regimens in the last period. Venetoclax was orally administered in the morning of Day 1 in each period to enable single-dose pharmacokinetic measurements up to 72 h with a 5-day washout interval between each dose/regimen. Participants received a standardized diet for all meals during the study except for their designated regimen breakfast on Day 1 of each period (see Table 1). The tablets were administered in Study 1 and Study 2 with 150 and 240 mL of water, respectively. Powder formulations were dispersed into the dosing vehicle prior to administration, followed by 240 mL of water. In Study 2, powder formulations were dispersed in 20 mL of vehicle prior to administration and then followed by 10 mL of vehicle for rinsing. In Study 3, 0.72 and 7.2% powder formulations were dispersed in 20 and 10 mL of vehicle, respectively, prior to administration and then followed by 10 mL of vehicle for rinsing. When study drug was administered in the fed state, administration occurred within approximately 30 min of starting the designated meal. When administered in the fasted state, administration occurred after a minimum of a 10-h fast and approximately 4 h before the following meal.
For all three studies, safety evaluations included adverse event (AE) monitoring, physical examinations, vital signs, 12-lead electrocardiogram (ECG), and clinical laboratory profiles throughout the study. AE intensity and laboratory evaluation changes were assessed using National Cancer Institute Common Terminology Criteria for Adverse Events version 5 [12]. These studies were conducted in accordance with Good Clinical Practice Guidelines and the ethical principles that have their origin in the Declaration of Helsinki [13]. The protocols were approved by the Institutional Review Board at the study site (AbbVie Clinical Pharmacology Research Unit, Grayslake, IL, USA) and each participant provided written informed consent before any study-related procedures were performed.
Sample size for each study was determined to provide at least 90% power to obtain a two-sided 90% confidence interval (CI) for the ratio of formulation means within the limits of 0.80–1.25. Sample size for Study 1 was based on the power to demonstrate bioequivalence. Sample size for Study 2 and Study 3 were screening studies and not powered to demonstrate bioequivalence. The calculations assumed a true within-participant standard deviation of 0.242 for a log-transformed pharmacokinetic parameter and is based on the observed variability in log-transformed area under the plasma concentration-time curve (AUC) in a previous venetoclax bioavailability study.
Pharmacokinetic Sampling and Bioanalysis
In all studies, blood samples for venetoclax assay were collected into dipotassium ethylenediaminetetraacetic acid (K2EDTA)-containing collection tubes prior to dosing (0 h) and at 1, 2, 4, 6, 8, 10, 12, 24, 48 and 72 h after dosing in each study period. Sufficient blood was collected to provide ~ 1.2 mL of plasma from each sample. Immediately after collection, the blood samples were inverted to ensure good mixing of blood and anticoagulant, placed in ice or a cryoblock, and centrifuged for 10–15 min (2–8 °C) to separate the plasma within 1 h of collection. Samples were placed in the freezer within 2 h after collection and maintained at − 20 °C or colder until analysis. Plasma concentrations of venetoclax were determined using a validated liquid chromatography-tandem mass spectrometric (LC-MS/MS) detection method [14]. The lower limit of quantitation (LLOQ) for venetoclax was established at 2.11 ng/mL. Precision of the assay has been previously reported [15]. Samples quantified below the lowest standard were reported as zero.
Pharmacokinetic and Statistical Analyses
Venetoclax pharmacokinetic parameters were calculated using non-compartmental analyses using Phoenix WinNonlin version 8.2.0.4383 (Certara L.P., St Louis, MO, USA). Pharmacokinetic parameters were determined by linear trapezoidal approach and included Cmax, time to Cmax (peak time, Tmax), terminal phase elimination half-life (t½), AUC from time zero to the time of the last measurable concentration (AUCt), and AUC extrapolated to infinite time (AUC∞). The relative bioavailability evaluated in the studies were estimated along with 90% CIs obtained from a linear mixed-effects analysis performed for log-transformed pharmacokinetic parameters Cmax, AUCt, and AUC∞. Bioequivalence was declared if the 90% CIs of the geometric mean ratios of the AUCt, AUC∞, and Cmax for the regimens compared were contained within the 0.80–1.25 range. All available data were included in these assessments.
Exposure–Response Analysis
Data from seven clinical studies (three in CLL and four in AML patients) evaluating venetoclax as a combination therapy were included in the exposure–response analysis. These studies included two studies in CLL patients evaluating venetoclax in combination with rituximab (RTX; studies NCT01682616 [16, 17] and NCT02005471 [18]), one in CLL patients evaluating venetoclax in combination with obinutuzumab (OBZ; study NCT02242942 [19]), two in AML patients evaluating venetoclax in combination with hypomethylating agents (HMAs; studies NCT02203773 [20] and NCT02993523 [21]), and two in AML patients evaluating venetoclax in combination with low-dose cytarabine (LDAC; studies NCT02287233 [22] and NCT03069352 [23]). Detailed descriptions as well as efficacy and safety results from these studies have been reported elsewhere [16, 18–24].
Exposure–response relationships of venetoclax efficacy in patients with CLL and AML were evaluated using quartile plots and logistic regression analyses using R version 3.6.1 (The R Foundation for Statistical Computing, Vienna, Austria) with steady-state Cmax (Cmax,ss) as an exposure metric. Individual patient Cmax,ss values were determined via NONMEM version 7.4.3 (Dublin, Ireland) based on a population pharmacokinetic model [25] that leveraged pharmacokinetic data from multiple clinical trials evaluating venetoclax in patients across different indications and in healthy participants. Best responses achieved while receiving treatment were used as the efficacy variable for the exposure–response analyses. For CLL, the response rates (overall response rate [ORR] and complete response [CR]) were selected for the purpose of the exposure–response analyses. For AML, best responses of CR or CR with incomplete marrow recovery (CR/CRi) were selected as the clinical endpoints of interest.
Results
Participants
A total of 80 adult female participants between 18 and 60 years of age and in general good health at enrollment participated in the phase I bioavailability studies. Demographic data for the healthy participants enrolled in these studies were similar across all studies and are shown in electronic supplementary Table S1.
Safety Assessments
Across all studies, treatment-emergent AEs (TEAE) possibly attributed to study drug experienced by the participants were Grade 1 (mild in severity), and the regimens tested were generally well tolerated by the participants. In Study 1, three participants prematurely discontinued the study due to TEAEs. These TEAEs included rash maculopapular (Grade 1, n = 1) and dermatitis allergic (Grade 1, n = 1) with reasonable possibility of being related to study drug, and blood glucose increase (Grade 1, n = 1) with no reasonable possibility of being related to study drug. One additional TEAE (face oedema) possibly attributed to study drug occurred in Study 1 in a participant who completed the study. In Study 2, five participants experienced TEAEs that were possibly attributed to study drug, with the most common event being gastrointestinal disorders (n = 3). In Study 3, two participants experienced TEAEs possibly attributed to study drug. In both cases, the TEAE was nausea. No AEs resulted in participant discontinuation in Studies 2 and 3. There was no pattern to the AEs reported, and no new safety issues were identified in Studies 1, 2, or 3.
Bioavailability of Lower-Strength Tablets (Study 1)
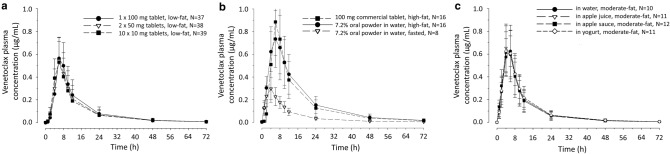
The mean plasma concentration–time profiles for each of the regimens of Study 1 are shown in Fig. 1a, and a summary of the pharmacokinetic parameters is provided in Table 2. The Tmax (6 h) and t½ (13.6 h) were the same for all three regimens. Two 50 mg venetoclax tablets were bioequivalent to one 100 mg venetoclax tablet, ten 10 mg venetoclax tablets were bioequivalent to two 50 mg venetoclax tablets, and ten 10 mg venetoclax tablets were bioequivalent to one 100 mg venetoclax tablet, as demonstrated by 90% CIs of the geometric mean ratios of Cmax and AUC within the 0.80–1.25 range (Table 3).
Fig. 1.
Venetoclax mean (± SD) plasma concentration–time profiles for a venetoclax tablet strength bioavailability assessments in Study 1; b venetoclax 7.2% oral powder formulation bioavailability assessments in Study 2; and c vehicle bioavailability assessments of venetoclax 7.2% oral powder formulation in Study 3. All groups in all three studies were administered a dose of 100 mg of venetoclax. N number of participants in each group, SD standard deviation
Table 2.
Plasma pharmacokinetic parameters of venetoclax administered to healthy non-childbearing adult females in bioavailability phase I studies
| Study regimen description | N | Fed state | Pharmacokinetic parameters | ||||
|---|---|---|---|---|---|---|---|
| Cmax, µg/mL | Tmaxa, h | t½b, h | AUCt, µg⋅h/mL | AUC∞, µg⋅h/mL | |||
| Study 1: 100 mg single dose administered as tablets of varying strengths under low-fat conditions | |||||||
| 1 × 100 mg tablet | 37 | Fed | 0.552 (0.590, 34) | 6 (4.0–8.0) | 13.6 (2.04) | 6.44 (7.07, 46) | 6.59 (7.26, 48) |
| 2 × 50 mg tablets | 38 | Fed | 0.522 (0.559, 35) | 6 (4.0–10.0) | 13.6 (3.06) | 5.82 (6.51, 48) | 5.96 (6.71, 51) |
| 10 × 10 mg tablets | 39 | Fed | 0.515 (0.550, 33) | 6 (4.0–8.0) | 13.6 (3.58) | 5.75 (6.37, 46) | 5.9 (6.57, 49) |
| Study 2: 100 mg single-dose tablet or oral powder under fasted or high-fat conditions | |||||||
| 1 × 100 mg tablet | 16 | Fed | 0.863 (0.913, 32) | 6 (4.0–10) | 14.7 (2.76) | 10.5 (11.4, 42) | 10.8 (11.8, 44) |
| 7.2% oral powder | 16 | Fed | 0.74 (0.780, 34) | 6 (4.0–8.0) | 13.4 (2.83) | 11.7 (12.6, 40) | 12 (13.0, 42) |
| 7.2% oral powder | 8 | Fasted | 0.283 (0.297, 33) | 4 (4.0–4.0) | 14.3 (4.87) | 3.5 (3.69, 34) | 3.63 (3.83, 34) |
| 0.72% oral powder | 16 | Fed | 0.69 (0.711, 26) | 6 (4.0–10) | 13 (1.86) | 11.3 (12.2, 41) | 11.6 (12.6, 43) |
| 0.72% oral powder | 8 | Fasted | 0.291 (0.310, 38) | 4 (4.0–6.0) | 16.2 (4.93) | 4.2 (4.71, 51) | 4.4 (4.97, 54) |
| Study 3: 100 mg single-dose oral powder in four vehicles under moderate-fat conditions | |||||||
| 7.2% oral powder in water | 10 | Fed | 0.637 (0.649, 20) | 6 (4.0–6.0) | 14.3 (2.28) | 7.11 (7.33, 26) | 7.24 (7.46, 26) |
| 7.2% oral powder in apple juice | 11 | Fed | 0.642 (0.671, 31) | 4 (4.0–6.0) | 13.6 (3.08) | 6.69 (7.16, 42) | 6.80 (7.29, 42) |
| 7.2% oral powder in apple sauce | 12 | Fed | 0.648 (0.669, 31) | 4 (4.0–6.0) | 14.7 (1.42) | 6.74 (7.14, 44) | 6.85 (7.26, 44) |
| 7.2% oral powder in yogurt | 11 | Fed | 0.623 (0.665, 35) | 4 (4.0–6.0) | 14.1 (2.72) | 6.93 (7.59, 43) | 7.05 (7.73, 44) |
| 0.72% oral powder in water | 12 | Fed | 0.692 (0.717, 29) | 6 (4.0–6.0) | 14.3 (1.92) | 8.60 (9.23, 40) | 8.77 (9.42, 40) |
| 0.72% oral powder in apple juice | 12 | Fed | 0.717 (0.738, 26) | 6 (4.0–6.0) | 14.2 (1.58) | 8.56 (8.97, 33) | 8.73 (9.16, 33) |
| 0.72% oral powder in apple sauce | 12 | Fed | 0.784 (0.793, 16) | 6 4.0–6.0) | 14.3 (1.59) | 8.96 (9.30, 29) | 9.15 (9.50, 29) |
| 0.72% oral powder in yogurt | 12 | Fed | 0.735 (0.766, 28) | 6 (4.0–6.0) | 15.3 (1.97) | 8.86 (9.50, 38) | 9.07 (9.74, 39) |
Note: Parameters are presented as geometric mean [mean (CV%)] unless otherwise noted. Low-fat meals consisted of ≤ 700 Kcal with ≤ 20% of the total caloric intake from fat; moderate-fat (standardized diet) meals consisted of ~ 700 Kcal with ~ 30% of caloric intake from fat; and high-fat meals consisted of 800–1000 Kcal with ~ 50% of the caloric intake from fat
AUC∞ area under the plasma concentration–time curve from time zero to infinite time, AUCt area under the plasma concentration–time curve from time zero to time of the last measurable concentration, Cmax maximum observed plasma concentration, CV coefficient of variation, t½ terminal phase elimination half-life, Tmax time to reach maximum observed plasma concentration
aMedian (minimum–maximum)
bHarmonic mean (pseudo-standard deviation)
Table 3.
Bioavailability of the three tablet strengths: Study 1 point estimates and 90% CIs
| Study regimen description | Pharmacokinetic parametersa | Central valueb | Relative bioavailability | ||
|---|---|---|---|---|---|
| Test | Reference | Point estimatec | 90% CI | ||
| 2 × 50 mg tablets versus 1 × 100 mg tablet | Cmax | 0.522 | 0.557 | 0.937 | 0.852–1.031 |
| AUCt | 5.83 | 6.56 | 0.889 | 0.808–0.979 | |
| AUC∞ | 5.98 | 6.72 | 0.890 | 0.809–0.979 | |
| 10 × 10 mg tablets versus 2 × 50 mg tablets | Cmax | 0.515 | 0.522 | 0.986 | 0.896–1.085 |
| AUCt | 5.78 | 5.83 | 0.991 | 0.900–1.090 | |
| AUC∞ | 5.93 | 5.98 | 0.992 | 0.902–1.091 | |
| 10 × 10 mg tablets versus 1 × 100 mg tablet | Cmax | 0.515 | 0.557 | 0.924 | 0.840–1.017 |
| AUCt | 5.78 | 6.56 | 0.881 | 0.800–0.969 | |
| AUC∞ | 5.93 | 6.72 | 0.883 | 0.803–0.971 | |
Note: All groups were conducted under moderate-fat conditions consisting of ≤ 700 Kcal with ≤ 20% of the total caloric intake from fat
AUC∞ area under the plasma concentration–time curve from time zero to infinite time, AUCt area under the plasma concentration–time curve from time zero to time of the last measurable concentration, CIs confidence intervals, Cmax maximum observed plasma concentration
aUnits for Cmax and AUC are expressed as μg/mL and μg⋅h/mL, respectively
bAnti-logarithm of the least squares means for logarithms
cAnti-logarithm of the difference (test minus reference) of the least squares means of the logarithms
Bioavailability of Oral Powder Formulations (Study 2)
The mean plasma concentration–time profiles following oral administration of 100 mg of venetoclax via 1 × 100 mg currently marketed tablet or the 7.2% oral powder formulation at a 100 mg dose under high-fat conditions are presented in Fig. 1b (see electronic supplementary Fig. S1a for the 0.72% oral powder profiles). The pharmacokinetic parameters for the 0.72 and 7.2% oral powder formulations are summarized in Table 2, and point estimates of the relative bioavailability of the oral powder formulations under high-fat conditions are presented in Table 4. Compared with the currently marketed tablet, the areas under the curves (AUCt and AUC∞) met the bioequivalence criteria (0.80–1.25). The lower bound of the 90% CI for the Cmax of the 7.2 and 0.72% oral powder formulations extended slightly below 0.80 to 0.78 and 0.72, respectively, under fed conditions (Table 4).
Table 4.
7.2 and 0.72% oral powder formulation bioavailability: Study 2 point estimates and 90% CIs
| Study regimen description | Pharmacokinetic parametersa | Central valueb | Relative bioavailability | ||
|---|---|---|---|---|---|
| Test | Reference | Point estimatec | 90% CI | ||
| 7.2% oral powder (high fat) versus 100 mg tablet (high fat) | Cmax | 0.740 | 0.863 | 0.857 | 0.777–0.946 |
| AUCt | 11.7 | 10.5 | 1.109 | 1.056–1.164 | |
| AUC∞ | 12.0 | 10.8 | 1.107 | 1.053–1.162 | |
| 7.2% oral powder (high fat) versus 7.2% oral powder (fasted) | Cmax | 0.740 | 0.313 | 2.361 | 2.045–2.727 |
| AUCt | 11.7 | 4.01 | 2.915 | 2.595–3.274 | |
| AUC∞ | 12.0 | 4.18 | 2.871 | 2.559–3.221 | |
| 0.72% oral powder (high fat) versus 100 mg tablet (high fat) | Cmax | 0.690 | 0.863 | 0.799 | 0.724–0.881 |
| AUCt | 11.3 | 10.5 | 1.074 | 1.022–1.128 | |
| AUC∞ | 11.6 | 10.8 | 1.071 | 1.019–1.125 | |
| 0.72% oral powder (high fat) versus 0.72% oral powder (fasted) | Cmax | 0.690 | 0.263 | 2.617 | 2.267–3.022 |
| AUCt | 11.3 | 3.67 | 3.085 | 2.747–3.465 | |
| AUC∞ | 11.6 | 3.82 | 3.035 | 2.705–3.405 | |
Note: High-fat meals consisted of 800–1000 Kcal with ~ 50% of the caloric intake from fat
AUC∞ area under the plasma concentration–time curve from time zero to infinite time, AUCt area under the plasma concentration–time curve from time zero to time of the last measurable concentration, CIs confidence intervals, Cmax maximum observed plasma concentration
aUnits for Cmax and AUC are expressed as μg/mL and μg⋅h/mL, respectively
bAnti-logarithm of the least squares means for logarithms
cAnti-logarithm of the difference (test minus reference) of the least squares means of the logarithms
Food Effect Evaluation (Study 2)
The effect of food on the pharmacokinetics of the 0.72% and 7.2% oral powder formulation was also determined in Study 2. Figure 1b shows the mean pharmacokinetic profiles of the 7.2% oral powder formulation administered under fasted and high-fat conditions (see electronic supplementary Fig. S1a for the 0.72% oral powder formulation profiles). The pharmacokinetic parameters are summarized in Table 2, with point estimates and geometric mean ratios for these assessments summarized in Table 4. Food (high-fat meal) increased the Cmax and AUC of the oral powder formulations by 2.4- to 3-fold (see Table 4).
Vehicle Effect Evaluation (Study 3)
The mean plasma concentration–time profiles following oral administration of 100 mg of venetoclax 7.2% oral powder formulation in four different vehicles are shown in Fig. 1c (see electronic supplementary Fig. S1b for the 0.72% oral powder formulation profiles). A summary of the pharmacokinetic parameters for Study 3 are shown in Table 2. Pharmacokinetic parameters were comparable across all regimens. Pharmacokinetic analysis shows that compared with the oral powder formulation in water, the Cmax, AUCt, and AUC∞ of the oral powder formulation in apple juice, apple sauce, and yogurt met the bioequivalence criteria, as summarized in Table 5.
Table 5.
Vehicle effect on 7.2 and 0.72% oral powder formulation bioavailability: Study 3 point estimates and 90% CIs
| Study regimen description | Pharmacokinetic parametersa | Central valueb | Relative bioavailability | ||
|---|---|---|---|---|---|
| Test | Reference | Point estimatec | 90% CI | ||
| 7.2% oral powder formulation | |||||
| Apple juice versus water | Cmax | 0.631 | 0.630 | 1.001 | 0.894–1.121 |
| AUCt | 6.67 | 6.93 | 0.963 | 0.869–1.065 | |
| AUC∞ | 6.79 | 7.06 | 0.962 | 0.869–1.064 | |
| Apple sauce versus water | Cmax | 0.648 | 0.630 | 1.028 | 0.919–1.150 |
| AUCt | 6.74 | 6.93 | 0.973 | 0.879–1.076 | |
| AUC∞ | 6.85 | 7.06 | 0.971 | 0.878–1.074 | |
| Yogurt versus water | Cmax | 0.629 | 0.630 | 0.998 | 0.891–1.117 |
| AUCt | 6.78 | 6.93 | 0.978 | 0.884–1.083 | |
| AUC∞ | 6.90 | 7.06 | 0.978 | 0.884–1.082 | |
| 0.72% oral powder formulation | |||||
| Apple juice versus water | Cmax | 0.717 | 0.692 | 1.037 | 0.960–1.120 |
| AUCt | 8.56 | 8.60 | 0.995 | 0.937–1.057 | |
| AUC∞ | 8.73 | 8.77 | 0.994 | 0.936–1.056 | |
| Apple sauce versus water | Cmax | 0.784 | 0.692 | 1.134 | 1.050–1.224 |
| AUCt | 8.96 | 8.60 | 1.042 | 0.981–1.107 | |
| AUC∞ | 9.15 | 8.77 | 1.042 | 0.981–1.107 | |
| Yogurt versus water | Cmax | 0.735 | 0.692 | 1.063 | 0.984–1.148 |
| AUCt | 8.87 | 8.60 | 1.031 | 0.970–1.095 | |
| AUC∞ | 9.07 | 8.77 | 1.034 | 0.973–1.098 | |
Note: All groups were conducted under moderate-fat conditions (standardized diet) consisting of ~ 700 Kcal with ~ 30% of caloric intake from fat
AUC∞ area under the plasma concentration–time curve from time zero to infinite time, AUCt area under the plasma concentration–time curve from time zero to time of the last measurable concentration, CIs confidence intervals, Cmax maximum observed plasma concentration
aUnits for Cmax and AUC are expressed as μg/mL and μg⋅h/mL, respectively
bAnti-logarithm of the least squares means for logarithms
cAnti-logarithm of the difference (test minus reference) of the least squares means of the logarithms
Exposure–Response Analyses for Oral Powder Formulations
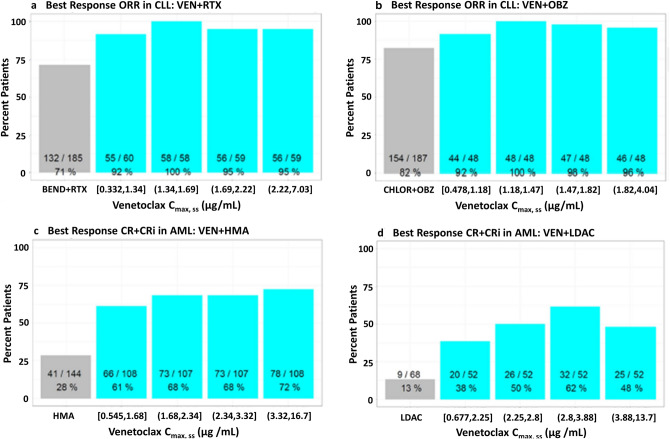
Exposure–response analyses with Cmax,ss as an exposure metric and clinical response in CLL and AML were performed using data from clinical studies investigating venetoclax in patients with CLL and AML. In CLL, the analysis demonstrated a flat exposure–response relationship between Cmax,ss and clinical response endpoints (ORR or CR) across the Cmax,ss range observed in the clinical studies. Specifically, quartile analysis and logistic regression analysis (data not shown) demonstrated that within the range of exposures observed, a slightly lower Cmax,ss does not impact ORR in patients with CLL receiving venetoclax in combination with RTX (NCT01682616 and NCT02005471) (Fig. 2a) or with OBZ (NCT02242942) (Fig. 2b). Similarly, a flat exposure–response relationship was also observed when CR was used as an efficacy endpoint within the same CLL populations (data on file at AbbVie).
Fig. 2.
Exposure–response quartile plots of venetoclax Cmax,ss vs. efficacy endpoints in CLL or AML patients receiving a venetoclax with rituximab (Studies NCT01682616 and NCT02005471); b venetoclax with obinutuzumab (Study NCT02242942); c venetoclax with hypomethylating agent (Studies NCT02203773 and NCT02993523); and d venetoclax with low-dose cytarabine (Studies NCT02287233 and NCT03069352). Note: Mean venetoclax Cmax,ss and 28% lower Cmax,ss for patients receiving 400 mg of venetoclax with RTX were 1.85 and 1.33 μg/mL, respectively. Mean venetoclax Cmax,ss and 28% lower Cmax,ss for patients receiving 400 mg of venetoclax with OBZ were 1.58 and 1.14 μg/mL, respectively. Mean venetoclax Cmax,ss and 28% lower Cmax,ss for patients receiving 400 mg of venetoclax in combination with HMA were 2.83 and 2.04 μg/mL, respectively. Mean venetoclax Cmax,ss and 28% lower Cmax,ss for patients receiving 600 mg of venetoclax in combination with LDAC were 3.42 and 2.46 μg/mL, respectively. AML acute myeloid leukemia, BEND bendamustine, CHLOR chlorambucil, CLL chronic lymphocytic leukemia, Cmax,ss steady-state maximum plasma concentration, CR complete response, CRi complete response with incomplete marrow recovery, HMA hypomethylating agent, LDAC low-dose cytarabine, OBZ obinutuzumab, ORR overall response rate, RTX rituximab, VEN venetoclax
Exposure–response analyses conducted using data from AML studies also demonstrated a flat exposure–response relationship between venetoclax Cmax,ss and a best response of CR or CRi (CR/CRi) in patients with AML. This was observed in patients receiving venetoclax with HMAs (NCT02203773 and NCT02993523) (Fig. 2c) or with LDAC (NCT02287233 and NCT03069352) (Fig. 2d).
Discussion
Patient experience with a therapeutic dosing regimen has a considerable impact on medication adherence, potentially impacting therapeutic benefit. Measures to improve patients’ compliance include counseling, reducing dosing frequency, reducing pill burden, and understanding the overall patient experience [26]. Venetoclax is a novel and highly effective drug in the treatment of hematological malignancies. With venetoclax, the relatively long t½ supports a simplified once-daily dosing regimen; however, the poor solubility results in low bioavailability. The tablet utilizes an amorphous solid dispersion formulation that improves the bioavailability of venetoclax but yields a relatively large tablet (physical size of the 100 mg tablet > 1 g) due to low tablet drug loading. Low drug loading by weight is essential to ensure robust stability and clinical performance of the venetoclax tablets [27]. This may present a challenge related to swallowability in some populations such as elderly or pediatric patients. As part of the continuous efforts to improve the patient experience [27], additional dosing regimens in the three bioavailability studies presented within were evaluated. This included options to interchange two 50 mg or ten 10 mg tablets for one 100 mg tablet as well as new oral powder formulations that can be administered in liquid or soft food vehicles such as water, apple juice, apple sauce, or yogurt. The capability to provide patients options in their dosage forms and vehicles to facilitate swallowing is anticipated to enable patients to be chronically administered a formulation without significant difficulty.
In Study 1, we sought to assess the interchangeability of the film-coated tablets (10, 50 or 100 mg). Study 1 demonstrated bioequivalence across tablet strengths, thus allowing patients to take either 50 or 10 mg tablets to achieve their therapeutic dose. The ability to interchange smaller-sized tablets with the 100 mg tablet provides an alternative for patients with a preference towards oral solid dosage forms but find swallowing the 100 mg tablet challenging.
While interchangeability of smaller lower-strength tablets addresses the challenge of the larger 100 mg tablet, younger pediatric patients are usually considered incapable of swallowing tablets and another form of delivery is preferred in this setting, such as a liquid or an orodispersible formulation [28]. As an alternative to the venetoclax tablets, two oral powder formulations at strengths of 0.72 and 7.2% have also been developed to facilitate administration of venetoclax in patients (especially pediatric patients) who are unable to swallow the tablets. The two strengths (0.72 and 7.2%) were essential to enable delivery of a wide range of doses (3–600 mg) across the different pediatric subgroups. Bioavailability data from Study 2 demonstrated that the extent of absorption (based on AUC) of both oral powder formulations are bioequivalent to the tablets (Table 4). However, the rate of venetoclax absorption (based on Cmax) was slightly lower than the tablets with the lower bound of the 90% CI for the Cmax of the oral powder formulation extending outside the bioequivalence criteria of 0.80 (0.78 and 0.72 for the 7.2 and 0.72% oral powder formulations, respectively).
The lower bound of the 90% CI for Cmax of the powder extended only slightly below the bioequivalence limits. This difference in Cmax between the formulations is potentially attributed to the high variability in venetoclax pharmacokinetics and the relatively small number of participants (n = 16) enrolled in the study. A larger study with more participants could demonstrate bioequivalence of the powder formulation to the tablets with respect to Cmax. If the powder formulations have indeed 14–20% lower Cmax, a possible explanation could be a slightly shorter absorption window with the powder formulations compared with the tablet formulation. The powder formulations may be quickly exiting the stomach through the stomach road or ‘Magenstrasse’ [29] and traveling quicker through the duodenum where most of venetoclax absorption is thought to take place. While this would result in a quicker onset of absorption with the powder formulations, as evident by higher venetoclax concentrations at earlier sampling times (1 and 2 h), it might ultimately result in a lower Cmax value due to the shorter residence time within the duodenum.
To thoroughly address this and determine the impact of this slightly lower Cmax observed with the oral powder formulations on the clinical efficacy of venetoclax, the exposure–response relationship of venetoclax Cmax,ss and clinical endpoints were explored using data from clinical studies testing venetoclax in patients with CLL and AML. With respect to CLL, ORR and CR were selected as the clinical endpoints of interest for the exposure–response analyses. These were the most sensitive efficacy endpoints in the exposure–response analyses that were previously conducted for the selection of venetoclax dose in CLL [30, 31]. The analyses demonstrated that within the range of Cmax,ss observed in patients with CLL, a flat relationship between venetoclax Cmax,ss and clinical efficacy (demonstrated by ORR and CR rates) is evident.
For exposure–response analysis in the AML setting, best responses of CR or CRi were selected as the efficacy endpoints of interest as these endpoints are correlated with long-term survival in AML [32]. Similar to CLL, exposure–response analyses demonstrated a flat relationship between venetoclax Cmax,ss and achieving a best response of CR or CRi in patients with AML receiving venetoclax with either HMAs or LDAC. Based on these flat exposure–response relationships in both CLL and AML, the slightly lower Cmax observed with the oral powder formulation is unlikely to have an impact on clinical response.
Moreover, it should be noted that for chronic therapy, steady-state total exposures are considered more important for efficacy. Since venetoclax is used for chronic therapy, total exposure metrics such as AUC or average concentrations of the dosing period (Cavg) are expected to be better correlators to clinical efficacy compared with Cmax. These metrics also better reflect venetoclax dose interruptions or dose reductions over the course of treatment. Clinical responses to venetoclax have been previously correlated to venetoclax steady-state AUC (AUCss) or average steady-state concentrations (Cavg,ss) [30, 33, 34] for which the oral powder formulations meet the bioequivalence criteria with the tablets.
Taken collectively, based on the exposure–response analyses and the setting in which venetoclax is used, the slight differences in Cmax observed with the powder formulations would not translate into meaningful differences in clinical outcome and the powder formulations could be used interchangeably with the tablets to achieve the therapeutic dose.
Venetoclax is labeled to be administered with food but without specific recommendations for caloric or fat content. Under fasted conditions, venetoclax demonstrates low oral bioavailability, and administration of venetoclax with low- and high-fat meals results in approximately 3- and 5-fold increases in bioavailability, respectively [6]. These increases in bioavailability are believed to be a product of enhanced venetoclax intestinal lymphatic transport that is facilitated by the fat in food [4]. The enhanced lymphatic uptake increases systemic exposure by increasing the fraction of venetoclax absorbed as well as bypassing the hepatic first-pass effect [6].
The effect of a high-fat meal on the bioavailability of the powder formulations was evaluated in Study 2. The bioavailability of the new oral powder formulations was less impacted by food than the tablets. A high-fat meal resulted in a 2- to 3-fold increase in the Cmax and AUC of the oral powder formulations compared with fasting conditions. This food effect is relatively smaller compared with what is observed with the tablets (~ 5-fold increase with a high-fat meal) [6]. These data suggest that the pharmacokinetic variability due to differences in the meal type consumed would be relatively lower for the oral powder formulations than the tablets, and supports similar administration recommendations for the oral powder formulations [14].
With the development of the oral powder formulations, providing appropriate vehicles, other than water, to disperse and administer the formulation and adjust the taste becomes important. This is more especially important in younger children who are not able to weigh the long-term benefit of therapy over the short-term discomfort of any unpleasant taste experience [35]. We aimed to qualify different liquid and soft food vehicles to provide options for the administration of the oral powder formulations. Apple juice, apple sauce, and yogurt were selected as the preferred dosing vehicles for further development based on a panel taste assessment, global availability, and in-use stability studies, with assay and impurity results meeting predetermined specifications. The impact of the different vehicles (apple juice, apple sauce, or yogurt) on the bioavailability of the oral powder formulations was explored in Study 3. All tested vehicles did not affect the bioavailability of either powder formulations compared with water (Table 5). These data support the use of any of the tested vehicles for administration of the venetoclax oral powder formulation.
Conclusions
In this study, we present options to facilitate venetoclax administration using data from three bioavailability studies. The smaller-sized 10 and 50 mg venetoclax tablets are bioequivalent to the larger 100 mg tablet and can be used interchangeably. Alternatively, new oral powder formulations have been qualified and can also be used interchangeably with the 100 mg tablet. Moreover, the bioavailability of the new oral powder formulations was less impacted by food compared with the tablet and was not affected when different vehicles were used to administer the oral powder formulation. These results demonstrate that, based on patients’ preference, any of the formulations discussed within this manuscript can be used to deliver the therapeutic dose of venetoclax in adult and pediatric patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Kelly Calabrese, MS, an employee of AbbVie, for her contributions supporting the execution and analysis of the bioavailability studies. The authors would also like to thank Stormy Koeniger, PhD, an employee of AbbVie, for medical writing support.
Declarations
Funding
The studies were supported by AbbVie in collaboration with Genentech/Roche. Venetoclax (ABT-199/GDC-0199) is being developed in collaboration between AbbVie and Genentech. AbbVie and Genentech provided financial support for the studies and participated in the design of the studies, study conduct, analysis and interpretation of data, and the writing, review, and approval of the manuscript.
Conflict of interest
Mohamed Badawi, Xin Chen, Patrick Marroum, Ahmed A. Suleiman, Sven Mensing, Anette Koenigsdorfer, Julia Teresa Schiele, David Hoffman, Rajeev Menon, and Ahmed Hamed Salem are employees of AbbVie Inc. and may hold AbbVie stock and/or stock options. Divya Samineni is an employee of Genentech and may hold Genentech stock and/or stock options. Tammy Palenski is a former employee of AbbVie and may hold AbbVie stock and/or stock options.
Ethics approval
The studies reported herein were conducted in accordance with the International Council for Harmonisation (ICH) guidelines, applicable regulations, and guidelines governing clinical study conduct and the ethical principles that have their origin in the Declaration of Helsinki. Approval was granted by Institutional Review Boards and Independent Ethics Committees at participating institutions.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Code availability
Not applicable.
Availability of data and material
AbbVie is committed to responsible data sharing regarding the clinical trials they sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (e.g., protocols and clinical study reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
Footnotes
Ahmed A. Suleiman and Tammy Palenski: Affiliation of the author at the time these studies were conducted.
References
- 1.AbbVie Inc. Venclexta (venetoclax) [package insert]. US Food and Drug Administration. Revised November 2020. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208573s023lbl.pdf. Accessed Jan 2022.
- 2.Kaufman JL, Gasparetto C, Schjesvold FH, Moreau P, Touzeau C, Facon T, et al. Targeting BCL-2 with venetoclax and dexamethasone in patients with relapsed/refractory t(11;14) multiple myeloma. Am J Hematol. 2021;96(4):418–427. doi: 10.1002/ajh.26083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alaarg A, Menon R, Rizzo D, Liu Y, Bien J, Elkinton T, et al. A microdosing framework for absolute bioavailability assessment of poorly soluble drugs: a case study on cold-labeled venetoclax, from chemistry to the clinic. Clin Transl Sci. 2022;15(1):244–254. doi: 10.1111/cts.13144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choo EF, Boggs J, Zhu C, Lubach JW, Catron ND, Jenkins G, et al. The role of lymphatic transport on the systemic bioavailability of the Bcl-2 protein family inhibitors navitoclax (ABT-263) and ABT-199. Drug Metab Dispos. 2014;42(2):207–212. doi: 10.1124/dmd.113.055053. [DOI] [PubMed] [Google Scholar]
- 5.Salem AH, Agarwal SK, Dunbar M, Enschede SL, Humerickhouse RA, Wong SL. Pharmacokinetics of venetoclax, a novel BCL-2 inhibitor, in patients with relapsed or refractory chronic lymphocytic leukemia or non-Hodgkin lymphoma. J Clin Pharmacol. 2017;57(4):484–492. doi: 10.1002/jcph.821. [DOI] [PubMed] [Google Scholar]
- 6.Salem AH, Agarwal SK, Dunbar M, Nuthalapati S, Chien D, Freise KJ, et al. Effect of low- and high-fat meals on the pharmacokinetics of venetoclax, a selective first-in-class BCL-2 inhibitor. J Clin Pharmacol. 2016;56(11):1355–1361. doi: 10.1002/jcph.741. [DOI] [PubMed] [Google Scholar]
- 7.AbbVie Inc. Summary of product characteristics: venclyxto film-coated tablets 100mg (Great Britain). Electronic Medicines Compendium (EMC). Last updated 3 March 2022. Available at: https://www.medicines.org.uk/emc/product/10476/smpc. Accessed 25 May 2022.
- 8.AbbVie Inc. Summary of product characteristics: venclyxto film-coated tablets 10mg (Great Britain). Electronic Medicines Compendium (EMC). Last updated 3 March 2022. Available at: https://www.medicines.org.uk/emc/medicine/32650#gref. Accessed 25 May 2022.
- 9.AbbVie Inc. Summary of product characteristics: venclyxto 50 mg film-coated tablets (Great Britain). Electronic Medicines Compendium (EMC). Last updated 3 March 2022. Available at: https://www.medicines.org.uk/emc/product/10475/smpc. Accessed 25 May 2022.
- 10.AbbVie, Inc. VENCLEXTA (venetoclax) [package insert]. US Food and Drug Administration. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/208573s026lbl.pdf. Revised October 2021. Accessed 25 Mar 2022.
- 11.AbbVie Inc. Prescribing information for venclexta. North Chicago: AbbVie Inc; 2018.
- 12.US Department of Health and Human Services, National Institutes of Health. National Cancer Institute DCTD Division of Cancer Treatment and Diagnosis. Common terminology criteria for adverse events (CTCAE) version 5.0. Published 27 November 2017. Available at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_50. Accessed 20 May 2022.
- 13.World Medical Association Declaration of Helsinki Ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 14.Liu H, Michmerhuizen MJ, Lao Y, Wan K, Salem AH, Sawicki J, et al. Metabolism and disposition of a novel B-cell lymphoma-2 inhibitor venetoclax in humans and characterization of its unusual metabolites. Drug Metab Dispos. 2017;45(3):294–305. doi: 10.1124/dmd.116.071613. [DOI] [PubMed] [Google Scholar]
- 15.Salem AH, Hu B, Freise KJ, Agarwal SK, Sidhu DS, Wong SL. Evaluation of the pharmacokinetic interaction between venetoclax, a selective BCL-2 inhibitor, and warfarin in healthy volunteers. Clin Drug Investig. 2017;37(3):303–309. doi: 10.1007/s40261-016-0485-9. [DOI] [PubMed] [Google Scholar]
- 16.Seymour JF, Ma S, Brander DM, Choi MY, Barrientos J, Davids MS, et al. Venetoclax plus rituximab in relapsed or refractory chronic lymphocytic leukaemia: a phase 1b study. Lancet Oncol. 2017;18(2):230–240. doi: 10.1016/S1470-2045(17)30012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts AW, Ma S, Kipps TJ, Coutre SE, Davids MS, Eichhorst B, et al. Efficacy of venetoclax in relapsed chronic lymphocytic leukemia is influenced by disease and response variables. Blood. 2019;134(2):111–122. doi: 10.1182/blood.2018882555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seymour JF, Kipps TJ, Eichhorst B, Hillmen P, D’Rozario J, Assouline S, et al. Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378(12):1107–1120. doi: 10.1056/NEJMoa1713976. [DOI] [PubMed] [Google Scholar]
- 19.Al-Sawaf O, Zhang C, Tandon M, Sinha A, Fink AM, Robrecht S, et al. Venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab for previously untreated chronic lymphocytic leukaemia (CLL14): follow-up results from a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020;21(9):1188–1200. doi: 10.1016/S1470-2045(20)30443-5. [DOI] [PubMed] [Google Scholar]
- 20.DiNardo CD, Pratz KW, Letai A, Jonas BA, Wei AH, Thirman M, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19(2):216–228. doi: 10.1016/S1470-2045(18)30010-X. [DOI] [PubMed] [Google Scholar]
- 21.DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617–629. doi: 10.1056/NEJMoa2012971. [DOI] [PubMed] [Google Scholar]
- 22.Wei AH, Strickland SA, Jr, Hou JZ, Fiedler W, Lin TL, Walter RB, et al. Venetoclax combined with low-dose cytarabine for previously untreated patients with acute myeloid leukemia: results from a phase Ib/II study. J Clin Oncol. 2019;37(15):1277–1284. doi: 10.1200/JCO.18.01600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei AH, Montesinos P, Ivanov V, DiNardo CD, Novak J, Laribi K, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood. 2020;135(24):2137–2145. doi: 10.1182/blood.2020004856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer K, Al-Sawaf O, Bahlo J, Fink AM, Tandon M, Dixon M, et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med. 2019;380(23):2225–2236. doi: 10.1056/NEJMoa1815281. [DOI] [PubMed] [Google Scholar]
- 25.Gong JQX, Suleiman AA, Mensing S, Salem AH. Pooled population pharmacokinetic analyses of venetoclax in multiple patient populations and healthy subjects: analyses of phase 1, 2 and 3 clinical trials. In preparation. [DOI] [PubMed]
- 26.Atreja A, Bellam N, Levy SR. Strategies to enhance patient adherence: making it simple. MedGenMed. 2005;7(1):4. [PMC free article] [PubMed] [Google Scholar]
- 27.Salem AH, Tao ZF, Bueno OF, Chen J, Chen S, Edalji R, et al. Expanding the repertoire for “Large Small Molecules”: prodrug ABBV-167 efficiently converts to venetoclax with reduced food effect in healthy volunteers. Mol Cancer Ther. 2021;20(6):999–1008. doi: 10.1158/1535-7163.MCT-21-0077. [DOI] [PubMed] [Google Scholar]
- 28.Nunn T, Williams J. Formulation of medicines for children. Br J Clin Pharmacol. 2005;59(6):674–676. doi: 10.1111/j.1365-2125.2005.02410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pal A, Brasseur JG, Abrahamsson B. A stomach road or "Magenstrasse" for gastric emptying. J Biomech. 2007;40(6):1202–1210. doi: 10.1016/j.jbiomech.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Deng R, Gibiansky L, Lu T, Li X, Lu D, Li C, et al. Exposure-response analysis of venetoclax in combination with rituximab in patients with relapsed or refractory chronic lymphocytic leukemia: pooled results from a phase 1b study and the phase 3 MURANO study. Leuk Lymphoma. 2020;61(1):56–65. doi: 10.1080/10428194.2019.1657575. [DOI] [PubMed] [Google Scholar]
- 31.Freise KJ, Jones AK, Menon R, Verdugo M, Humerickhouse R, Awni W, et al. Relationship between venetoclax exposure, rituximab coadministration, and progression-free survival in patients with relapsed or refractory chronic lymphocytic leukemia: demonstration of synergy. Hematol Oncol. 2017;35(4):679–684. doi: 10.1002/hon.2373. [DOI] [PubMed] [Google Scholar]
- 32.Agarwal SK, Mangal N, Menon RM, Freise KJ, Salem AH. Response rates as predictors of overall survival: a meta-analysis of acute myeloid leukemia trials. J Cancer. 2017;8(9):1562–1567. doi: 10.7150/jca.18686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freise KJ, Jones AK, Eckert D, Mensing S, Wong SL, Humerickhouse RA, et al. Impact of venetoclax exposure on clinical efficacy and safety in patients with relapsed or refractory chronic lymphocytic leukemia. Clin Pharmacokinet. 2017;56(5):515–523. doi: 10.1007/s40262-016-0453-9. [DOI] [PubMed] [Google Scholar]
- 34.Agarwal S, Gopalakrishnan S, Mensing S, Potluri J, Hayslip J, Kirschbrown W, et al. Optimizing venetoclax dose in combination with low intensive therapies in elderly patients with newly diagnosed acute myeloid leukemia: an exposure-response analysis. Hematol Oncol. 2019;37(4):464–473. doi: 10.1002/hon.2646. [DOI] [PubMed] [Google Scholar]
- 35.Coupland JN, Hayes JE. Physical approaches to masking bitter taste: lessons from food and pharmaceuticals. Pharm Res. 2014;31(11):2921–2939. doi: 10.1007/s11095-014-1480-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.