Abstract
Prostate carcinoma is a highly prevalent biologically and clinically diverse disease, generally associated with a consistent elevation of prostate-specific antigen levels. Castration-resistant prostate cancer represents a heterogeneous clinical setting that ranges from patients with an asymptomatic prostate-specific antigen elevation after hormone blockade failure and good performance status to patients with significant debilitating symptoms and rapidly progressive disease, leading to death. Nonmetastatic castration-resistant prostate cancer is a transient disease stage defined over specific criteria established within a sensitive time period. The majority of the patients with nonmetastatic castration-resistant prostate cancer will eventually develop metastatic lesions, associated with prostate cancer-specific morbidity and mortality. However, progression to metastatic disease is a heterogeneous process still not fully understood, with studies suggesting that younger age, high Gleason score (> 7), high prostate-specific antigen levels, reduced prostate-specific antigen doubling time (< 6 months), and a rapid alkaline phosphatase rise as potentially associated factors. Although the nonmetastatic castration-resistant prostate cancer treatment landscape has substantially evolved in recent years, the disease heterogeneity makes treatment decisions for this population challenging in the effort to achieve a balance between the risk of disease progression and the toxicity of new treatments in patients who often have associated comorbidities, yet are generally asymptomatic. The present article addresses the current main challenges in nonmetastatic castration-resistant prostate cancer management, including in diagnosis, owing to the development of new imaging modalities with a direct impact in disease detection, prognostic classification, as a result of the traditionally oversimplified definition of disease aggressiveness (mainly based on prostate-specific antigen doubling time), and patient selection for the most adequate treatment.
Key Points
| Nonmetastatic castration-resistant prostate cancer (M0 CRPC) is a transient disease stage defined over specific criteria established within a sensitive time period and the majority of the M0 CRPC patients will develop metastatic lesions, associated with prostate cancer-specific morbidity and mortality. |
| The emergence of highly sensitive imaging modalities will challenge the conceptual setting of M0 CRPC, as a growing number of patients will be diagnosed with early metastatic instead of M0 disease, with direct impact in their treatment plan. Nevertheless, considering the lack of clinical trials assessing the prognosis of patients with metastases detected only by PSMA warrants further investigation as to whether PSMA PET/CT should be extensively used in high-risk patients. |
| Recent studies have shown that PSA levels and PSADT are important tools in prognostic risk assessment in prostate cancer. However, patient risk stratification should be based not only on PSADT, but also in other factors, such as time since endocrine therapy, total PSA, Gleason score, N1 disease, and tumor histopathology. |
| The management of the long-time recognized pre-metastatic CRPC has been recently transformed with the approval of new-generation ARi darolutamide, apalutamide, and enzalutamide, which have shown a relevant capacity to delay metastatic disease, with a very favorable trade-off regarding adverse events. |
Introduction
Prostate carcinoma is a biologically and clinically heterogeneous disease with a high prevalence, generally associated with a consistent elevation of prostate-specific antigen (PSA) levels [1]. It accounts for 7.3% of all cancer globally and approximately 7% of patients have metastatic disease at the time of initial presentation [2].
According to the Global Cancer Observatory, prostate cancer was the second most commonly diagnosed cancer and the fifth leading cause of cancer death among men in 2020 [3]. Almost 1.4 million new cases and 375,000 deaths have been reported worldwide in that year alone, representing a substantial health burden. In industrialized countries, prostate cancer is the second most frequent non-dermatological cancer in male individuals, and the fourth most common cancer in the general population, after lung, breast, and colorectal cancer [3]. The disease represented the fifth leading cause of cancer death among men in 2020 [3]. In Portugal, the National Oncological Registry reported 5741 new cases and 1833 deaths due to prostate cancer in 2018, corresponding to an incidence rate of 78.3/105 [4], and GLOBOCAN 2020 reported 6759 new cases (20% of all cancers in men) and 1917 deaths from prostate cancer [3].
Androgen deprivation therapy (ADT), achieved through surgical or chemical castration, is the mainstay of treatment for advanced prostate cancer [5–7] and usually induces disease regression evidenced by radiographic response, PSA decline, and clinical improvement [8]. However, despite initial response, most patients eventually develop a progressive PSA rise or biochemical recurrence, and castration-sensitive prostate cancer gives rise to castration-resistant prostate cancer (CRPC) [9]. The development of resistance has been attributed to several mechanisms, spanning from those associated with androgen receptor signaling to novel pathways acting independently of the androgen axis, such as those involving RAS/MAP kinase, transforming growth factor-beta/SMAD pathway, fibroblast growth factor signaling, JAK/STAT pathway, Wnt-Beta catenin, and hedgehog signaling [10–12].
Although the understanding of CRPC has improved over the years, it remains a very heterogeneous clinical setting that ranges from patients with an asymptomatic PSA elevation after hormone blockade failure and good performance status to those with rapidly progressive disease, significant debilitating symptoms, and poor prognosis. Nonmetastatic castration-resistant prostate cancer (M0 CRPC) is a transient disease stage characterized by castration (testosterone levels < 50 ng/mL) resistance following ADT and the absence of detectable metastases in conventional imaging exams, together with a progressively rising PSA at an increase of 25% from nadir (starting PSA level ≥ 1.0 ng/mL), at a minimum rise of 2 ng/mL and confirmed by a second value [13–15]. The disease is hence defined by this very specific diagnosis established within a sensitive time period.
The real prevalence of M0 CRPC is currently unknown. A prevalence model using country-level data estimated that M0 CRPC accounted for a relatively small proportion (2−8%) of the total prostate cancer cases in 2013, anticipating an increase in the disease prevalence in upcoming years, owing to widespread screening and demographic changes [13].
A relevant proportion of those patients develop metastatic lesions, as documented in the study by Moreira et al. showing that around 60% of patients with M0 CRPC progress to metastatic disease within 5 years, most of whom within the first 3 years [16]. Additionally, Smith et al. reported that 46% of men with M0 CRPC develop metastases within 2 years [17, 18]. Progression to metastatic disease is a heterogeneous process still not fully understood. Evidence in the literature suggests that younger age, high Gleason score (> 7), high PSA levels, reduced PSA doubling time (PSADT; < 6 months), and a rapid alkaline phosphatase rise are associated with progression to metastatic disease [16–19].
The development of metastases is associated with prostate cancer-specific morbidity and mortality [18, 20–22]. Metastatic disease to bone, in particular, is linked to skeletal-related events, including severe pain, nerve and spinal cord compression, pathological fractures, skull base involvement, and the need for radiation and/or surgery, which significantly impair patients’ health-related quality of life (HRQoL) [23–25].
In Portugal, data regarding M0 CRPC are scarce and not systematized. The National Oncological Registry collects data on prostate cancer, with the last register being from 2018, but data specifically on M0 CRPC are not contemplated. This is an unmet need in the country that should be tackled in the near future, retrieving disease-specific data in a systematic and structured manner in health institutions, similarly to what is done in other European countries, such as the Netherlands [26].
Although the M0 CRPC treatment landscape has substantially evolved over recent years, the disease heterogeneity makes treatment decisions for these patients a challenge. The therapeutic decision for these patients is often difficult, as the balance between the risk of disease progression and toxicity of new treatments in patients who are generally asymptomatic and often have associated comorbidities is precarious. The current main challenges in the disease management include diagnosis, with the development of newer imaging modalities with a direct impact in disease detection—prognostic classification—as a result of the traditionally oversimplified definition of disease aggressiveness, mainly based on PSADT, and patient selection for the most adequate treatment, which will be addressed next.
Diagnostic Challenges: What is the Impact of New Imaging Modalities in M0 CRPC Detection?
Prostate cancer staging has been traditionally performed through conventional imaging techniques, such as a technetium-99m bone scan and chest, abdomen, and pelvic computed tomography (CT). However, these methods have limited accuracy to detect prostate cancer metastases. The CT scan has a sensitivity of 42% in the detection of metastatic lymph nodes [27] and a bone scan has a sensitivity of 59–79% and a specificity of 75–82% in the detection of bone metastases, as shown in the meta-analysis by Shen et al. [28].
Novel and more sensitive next-generation imaging modalities have recently emerged as powerful adjuncts or even alternatives to conventional imaging methods, increasing the diagnostic accuracy in detecting metastatic disease in patients with prostate cancer. Among these are the 68Ga-labeled prostate-specific membrane antigen (PSMA) positron emission tomography (PET)/CT scan, sodium fluoride PET scan, 11C-choline PET/CT scan, and whole-body magnetic resonance imaging [29–32]. Eiber et al. showed a detection efficacy of 68Ga-PSMA ligand PET/CT from 96.8 to 57.9%, decreasing from high to low PSA levels [29]. In another study, 68Ga-PSMA detected 54% of the positive cases [30]. Mosavi and colleagues compared the whole-body magnetic resonance imaging with 18F-sodium fluoride PET/CT for the detection of bone metastases in patients with high-risk prostate carcinoma and found that 18F-sodium fluoride PET/CT showed a higher number of true-positive compared with diffusion weighted imaging (higher sensitivity) and a higher number of false-positive findings (lower specificity) [31]. In a meta-analysis of the available evidence of PET and PET/CT using 11C-choline and 18F-fluorocholine as tracers in imaging patients with prostate cancer in staging and restaging settings, in staging patients with proven but untreated prostate cancer, the results on a per-patient basis showed a pooled sensitivity of 84% and a specificity of 79%. On a per-lesion basis, these values were 66% and 92%. In restaging patients with biochemical failure after local treatment with curative intent, the meta-analysis results on a per-patient basis showed a pooled sensitivity of 85% and a specificity of 88% [32].
These tools have improved sensitivity in prostate cancer staging and are able to detect metastases earlier than conventional techniques [33, 34], leading to a restaging of the disease in patients thought to be in the M0 CRPC stage. Prostate-specific membrane antigen is a transmembrane glycoprotein that is overexpressed in prostate tumor cells. Prostate-specific membrane antigen PET is being progressively adopted as an alternative testing modality in specific settings, as it has the potential to more accurately identify nodal and distant/bone metastases and differentiate between patients with low-volume metastatic disease and M0 disease. The degree of PSMA expression has been correlated with disease aggressiveness [35, 36] and research results of restaging in patients with M0 CRPC using PSMA PET have been reported [37–39]. A retrospective analysis included 200 patients with M0 CRPC with no metastases as per conventional imaging and similar characteristics to those included in the pivotal trials of new-generation androgen receptor inhibitors (ARi), and assessed the presence of metastases with PSMA PET [40]. The analysis showed that 55% of patients had distant metastatic disease, being in fact incorrectly staged as M0. Moreover, pelvic disease was detected in 44% of patients. Another study included 30 patients with M0 CRPC and identified at least one malignant focus through PSMA PET in 90% of them [41]. Prostate-specific membrane antigen PET-positive findings were observed in 100% (n = 20) of patients with PSA levels > 2 ng/mL and in 70% (n = 10) of those with PSA levels < 2 ng/mL.
Given this body of evidence, the RADAR III group recommended that if traditional imaging fails to detect metastatic disease, next-generation imaging can be performed only if approved therapies in the low-volume metastatic space are being considered [42]. In Portugal, although there are no official figures, the use of this method is not uniform across the country, with a trend toward greater use in academic hospitals and oncology centers.
Prostate-specific membrane antigen PET is not routinely used in the context of CPRC disease [40]. Importantly, these new imaging modalities have not been included in the most recent clinical trials in M0 CRPC of new-generation ARi, the SPARTAN [43, 44], PROSPER [45, 46], and ARAMIS trials [47, 48]. Most patients in these trials would probably have had positive PSMA PET imaging results, as recently acknowledged by the European Association of Urology Consensus Panel in Advanced Prostate Cancer [49], the American Society of Clinical Oncology guidelines [50], and the Advanced Prostate Cancer Consensus Conference [51].
Overall, the emergence of these highly sensitive imaging modalities will challenge the conceptual setting of M0 CRPC, as a growing number of patients will be diagnosed with early metastatic disease instead of M0 disease, with a direct impact on their treatment plan. Nevertheless, considering the lack of clinical trials assessing the prognosis of patients with metastases detected only by PSMA, further investigation is needed as to whether PSMA PET/CT should be extensively used in high-risk patients [49].
Prognostic Assessment: Is PSADT Enough?
Prostate-specific antigen doubling time has been considered a prognostic marker in retrospective studies, but that has been difficult to validate in prospective assessments [52, 53]. The optimal time interval between PSA values for estimating PSADT and its optimal limits still need to be determined.
In the case of M0 CRPC, PSA indirectly monitors tumor activity from the androgen receptor signal, which can correlate with tumor growth. However, it should be noted that PSA decline has not been proven to be a marker of survival or outcome, and androgen receptor downregulation does not always represent tumor cell elimination. In fact, it is acknowledged that some aggressive prostate cancers are low PSA secretors [54].
Recent studies have shown that PSA levels and PSADT are important tools in prognostic risk assessment in prostate cancer. Baseline PSA level, PSA velocity, and PSADT have been associated with the time to bone metastases, metastasis-free survival (MFS), and overall survival (OS) in M0 CRPC [54–56]. However, while PSA levels are clearly defined through testing, a PSADT calculation can be less straightforward.
Prostate-specific antigen doubling time is a relevant issue in M0 CRPC treatment. In the pivotal studies of the new Ari apalutamide (SPARTAN trial [43, 44]), enzalutamide (PROSPER trial [45, 46]), and darolutamide (ARAMIS trial [47, 48]), the median PSADT was below 5 months, suggesting that the use of these drugs requires evidence of rapid disease progression, which ultimately translates into positive PET-PSMA imaging. Other imaging modalities may be used, such as CT of the thorax or chest X-ray, CT of the abdomen and pelvis or pelvic magnetic resonance imaging, and a bone scan, but have lower sensitivity. Additionally, PSADT measurement can vary significantly with the calculation method used, and standardization is crucial, as PSADT can be the only treatment-defining parameter in cases lacking radiographic evidence [57].
The US Food and Drug Administration currently does not specify PSADT as a clinical criterion, but the European Medicines Agency recommends a PSADT of less than 10 months, as used in trials, for selecting patients for these new hormonal therapies in the setting of M0 CPCR [58]. Patient risk stratification based only on PSADT, as several studies have done so far, may be an oversimplistic way of assessing disease aggressiveness in M0 CRPC. Other factors, such as time since endocrine therapy, total PSA, Gleason score, N1 disease, and tumor histopathology may also be relevant for risk stratification [16–19]. Molecular prognostic and predictive factors are also being investigated as adjuncts to improve risk stratification in patients with M0 CRPC, namely/including prostate cancer gene 3 and messenger RNA expression [59] and identification of genomic alterations in circulating tumor DNA, as PTEN loss, MYC and AR mutations, trans-membrane protease serine 2-ERG fusion (a fusion of the trans-membrane protease serine 2 and the ERG gene), and DNA repair gene deficiencies [60].
Assessment of molecular determinants in M0 CRPC has also been proposed as a way to better identify clinical subgroups likely to benefit from specific therapies. In a post hoc analysis of the SPARTAN trial, the authors identified molecular subtypes associated with a decrease in PSA values and with survival outcomes [42]. Prostate-specific antigen responses were found to be deeper and faster in genomic classifier low-to-average risk gene expression and luminal subtypes, suggesting that the biomarker characteristics of these patients can disclose the presence of aggressive disease.
Treatment: For All or According to Patient Characteristics?
State-of-the-Art Treatment and Emerging Therapies
Treatment of M0 CRPC disease has historically been an unmet need in the management of prostate cancer. Until recently, the treatment strategy for M0 CRPC encompassed observation and maintenance with ADT monitored by PSADT with serial imaging until metastatic findings, or the use of first-generation Ari, such as bicalutamide or flutamide, followed by an anti-androgen withdrawal strategy, without a significant survival benefit [61–64]. However, the lack of robust prospective evidence guiding treatment decisions for these patients carried clinical uncertainty and suboptimal outcomes.
In recent years, the treatment landscape for patients with M0 CRPC changed with the addition of novel new-generation Ari to the therapeutic armamentarium, based on three pivotal clinical trials using MFS (defined as the time from randomization to the first detection of distant metastases on imaging or death from any cause, whichever occurred first) as the primary endpoint. Metastasis-free survival was accepted as a legitimate endpoint for clinical drug trials in M0 CRPC after the Food and Drug Administration acknowledged that a substantial delay in the onset of metastases is clinically relevant in this setting and a surrogate capable of predicting OS [65, 66].
The androgen receptor antagonists darolutamide, apalutamide, and enzalutamide all showed an improvement in MFS when added to ADT in the population of patients with M0 CRPC and are currently approved for disease treatment based on their pivotal phase III studies SPARTAN [43, 44], PROSPER [45, 46], and ARAMIS [47, 48] (Table 1). Although these studies targeted patients with slightly different characteristics, they have shown similar clinical outcomes and a pooled analysis of their data demonstrated significantly improved OS and MFS with these agents compared with placebo [67]. International guidelines (including the National Comprehensive Cancer Network guidelines Version 2.2021 [68] and the Advanced Prostate Cancer Consensus Conference consensus [51]) currently recommend darolutamide, apalutamide, or enzalutamide as the preferred choice in addition to ADT for the majority of patients with M0 CRPC with PSA levels ≥ 2 ng/mL and PSADT ≤ 10 months.
Table 1.
Comparison of phase III clinical trials’ population, efficacy, and safety of the androgen receptor antagonists apalutamide, enzalutamide, and darolutamide in nonmetastatic castration-resistant prostate cancer
| SPARTAN [43, 44] (NCT01946204) Apalutamide (n = 806) vs placebo (n = 401) |
PROSPER [45, 46] (NCT02003924) Enzalutamide (n = 933) vs placebo (n = 468) |
ARAMIS [47, 48] (NCT02200614) Darolutamide (n = 955) vs placebo (n = 554) |
|
|---|---|---|---|
| Inclusion criteria | M0N0-N1CRPC, PSADT < 10 months |
M0N0CRPC, PSADT < 10 months, PSA > 2 ng/mL |
M0N0-N1CRPC, PSADT < 10 months, PSA > 2 ng/mL |
| Baseline characteristics | |||
| Median age, years (range) | 74 (48−94) vs 74 (52−97) | Enzalutamide 74 (50−95) vs placebo 73 (53−92) | 74 (48−95) vs 74 (50−92) |
| Median PSA at baseline, ng/mL | 7.78 vs 7.96 | 11.1 vs 10.2 | 9.0 vs 9.7 |
| Median PSADT, months | 4.4 vs 4.5 | 3.8 vs 3.6 | 4.4 vs 4.7 |
| Primary analysis | |||
| Median follow-up, months | 20.3 | Enzalutamide: 18.5; placebo: 15.1 | 17.9 |
| Primary endpoint | Median MFS: 40.5 vs 16.2 months; HR 0.28 (95% CI 0.23–0.35); p < 0.001 | Median MFS: 36.6 vs 14.7 months; HR 0.29 (95% CI 0.24–0.35); p < 0.001 | Median MFS: 40.4 vs 18.4 months; HR 0.41 (95% CI 0.34–0.50); p < 0.001 |
| Secondary endpoints |
Median PFS: 40.5 vs 14.7 months; HR 0.29; 95% CI 0.24–0.36; p < 0.001 Median time to symptomatic progression: NR vs NR; HR 0.45; 95% CI 0.32–0.63; p < 0.001 Median OS: NR vs 39.0; HR 0.70; 95% CI 0.47–1.04; p = 0.07 Median time to first cytotoxic chemotherapy: NR vs NR; HR 0.44; 95% CI 0.29–0.66 |
Median time to PSA progression: 37.2 vs 3.9 months; HR 0.07; 95% CI 0.05–0.08; p < 0.001 Median time to first use of new antineoplastic therapy: 39.6 vs 17.7 months; HR 0.21; 95% CI 0.17–0.26; p < 0.001 Median OS: NR vs NR; HR 0.80; 95% CI 0.58–1.09; p = 0.15 |
Median OS: NR vs NR; HR 0.71; 95% CI 0.50–0.99; p = 0.045 Median time to pain progression: 40.3 vs 25.4 months; HR 0.65; 95% CI 0.53–0.79; p < 0.001 Median time to first use of cytotoxic chemotherapy: NR vs 38.2 months; HR 0.43; 95% CI 0.31–0.60; p < 0.001 Median time to first SSE: NR vs NR; HR 0.43; 95% CI 0.22–0.84; p < 0.01 |
| Final analysis | |||
| Median follow-up, months | 52.0 | 48.0 | 29.1 |
| Secondary endpoints |
Median OS: 73.9 vs 59.9 months; HR 0.78; 95% CI 0.64–0.96; p = 0.016 Median time to cytotoxic chemotherapy: NR vs NR; HR 0.63; 95% CI 0.49–0.81; p = 0.0002 Median time to symptomatic progression: NR vs NR; HR 0.57; 95% CI 0.44–0.73; p < 0.0001 |
Median OS: 67.0 vs 56.3 months; HR 0.73; 95% CI 0.61–0.89; p = 0.001 Median time to use of cytotoxic chemotherapy: NR vs NR; HR 0.54; 95% CI 0.44–0.67 Median time to first use of new subsequent antineoplastic therapy: 66.7 vs 19.1 months; HR 0.29; 95% CI 0.25–0.34 Chemotherapy-free survival: 58.3 vs 41.6 months; HR 0.62; 95% CI 0.52–0.72 |
Median OS: NR vs NR; HR 0.69; 95% CI 0.53–0.88; p = 0.003 Median time to first cytotoxic chemotherapy: NR vs NR; HR 0.58; 95% CI 0.44–0.76; p < 0.001 Median time to pain progression: 40.3 vs 25.4 months; HR 0.65; 95% CI 0.53–0.79; p < 0.001 Median time to first SSE: NR vs NR; HR 0.48; 95% CI 0.29–0.82; p = 0.005 |
| Safety endpoints | |||
| Treatment discontinuation because of AEs | 15 vs 8.4% | 17 vs 9% | 8.9 vs 8.7% |
| Grade 3/4 adverse events | 56 vs 36% | 48 vs 27% | 24.7 vs 19.5% |
| Most common AEs (> 10%) |
Fatigue: 33 vs 21% Hypertension: 28 vs 21% Diarrhea: 23 vs 15% Falls 22 vs 9.5% Nausea: 20 vs 16% Arthralgia: 20 vs 8.3% Weight loss: 20 vs 6.5% Back pain: 18 vs 15% Hot flashes: 15 vs 8.5% |
Fatigue: 46 vs 22% Musculoskeletal events: 34 vs 23% Fractures: 18 vs 6% Hypertension: 18 vs 6% Falls: 18 vs 5% |
Fatigue: 12.8 vs 8.7% |
AEs adverse events, CI confidence interval, HR hazard ratio, MFS metastasis-free survival, NR not reported, OS overall survival, PFS progression-free survival, PSA prostate-specific antigen, PSADT PSA doubling time, SSE symptomatic skeletal event
The ARAMIS trial investigated the addition of darolutamide to ongoing ADT and reported a significant increase of 22 months in MFS compared with placebo (median 40.4 vs 18.4 months; hazard ratio [HR] for metastases or death in the darolutamide group, 0.41; 95% confidence interval [CI] 0.34−0.50; p < 0.001) [47]. In the final survival analysis, the percentage of patients alive at 3 years was significantly higher with darolutamide versus placebo (83% [95% CI 80−86] vs 77% [95% CI 72−81]) [48]. In patients receiving darolutamide, the risk of death was significantly reduced (HR 0.69; 95% CI 0.53–0.88; p = 0.003) and the time to pain progression was extended by 14.9 months (HR 0.65; 95% CI 0.53–0.79; p < 0.001) [48]. Treatment discontinuation rates were similar between darolutamide (8.9%) and placebo (8.7%) [47]. The most common adverse event (AE) was fatigue (12.1% vs 8.7%), and no AE was reported in more than 15% of patients receiving darolutamide [47]. Grade 3/4 AEs were reported in 24.7% of patients in the darolutamide arm and 19.5% of patients in the placebo arm [47].
In the SPARTAN trial, apalutamide improved MFS by 24.3 months compared with placebo (median 40.5 vs 16.2 months; HR for metastases or death 0.28; 95% CI 0.23–0.35; p < 0.0001), as well as secondary endpoints of time to metastases, progression-free survival, and time to symptomatic progression [43]. In the prespecified final OS analysis, a 22% reduction in the hazard of death was reported in the apalutamide group compared with the placebo group (median 73.9 vs 59.9 months; HR 0.78; 95% CI 0.64−0.96; p = 0.016) [44]. In addition, apalutamide was associated with a significantly longer time to the first cytotoxic chemotherapy (median not reached in either group; HR 0.63; 95% CI 0.49–0.81; p = 0.0002). The most common cause of drug discontinuation was progressive disease (43% with apalutamide and 74% with placebo), and 15% of patients receiving apalutamide and 8.4% of those receiving placebo discontinued the study regimen because of AEs [44]. Adverse events occurring in 15% of patients receiving apalutamide (vs placebo) included fatigue (33% vs 21%), hypertension (28% vs 21%), diarrhea (23% vs 15%), falls (22% vs 9.5%), nausea (20% vs 16%), arthralgia (20% vs 8.3%), weight loss (20% vs 6.5%), back pain (18% vs 15%), and hot flashes (15% vs 8.5%) [44]. Grade 3/4 AEs were reported in 56% and 36% of patients in each group, respectively [44].
In the PROSPER trial, treatment with enzalutamide led to a clinically meaningful and statistically significant 71% lower risk of metastases or death compared with placebo (median MFS 36.6 vs 14.7 months; HR for metastases or death 0.29; 95% CI 0.24−0.35; p < 0.001) and also to a PSA progression-free survival benefit (median 37.2 vs 3.9 months; HR 0.07; 95% CI 0.05–0.08; p < 0.001) [45]. In the final OS analysis, enzalutamide was associated with a significant 27% decrease in the risk of death compared with placebo (median 67.0 vs 56.3 months; HR 0.73; 95% CI 0.61–0.89; p = 0.001) [46]. Regarding toxicities, 17% of patients in the enzalutamide group and 9% of patients in the placebo group discontinued the experimental drug because of AEs [46]. Grade ≥ 3 AEs were reported in 48% and 27% of patients, respectively. Adverse events occurring in more than 15% of patients receiving enzalutamide (vs placebo) included fatigue (46% vs 22%), musculoskeletal events (including back pain, arthralgia, myalgia, musculoskeletal pain, pain in the extremities, musculoskeletal stiffness, muscular weakness, and muscle spasms; 34% vs 23%), hypertension (18% vs 6%), falls (18% vs 5%), and fractures (18% vs 6%) [46].
Although the activity of the three agents in M0 CRPC has not been directly compared in head-to-head trials, it appears to be similar, given the similar trial designs and respective inclusion and exclusion criteria. The three pivotal trials targeted patients with high-risk M0 CRPC, defined by a baseline PSA level of 2 ng/mL and PSADT ≤ 10 months, and shared the same primary endpoint of MFS assessed by CT and a pelvic, chest, and abdomen bone scan every 16 weeks. All trials included patients with nodal disease; both ARAMIS and SPARTAN enrolled patients with malignant nodes of < 2 cm in diameter located below the aortic bifurcation. Additionally, all patients underwent ADT throughout the intervention phase. Similar clinical outcomes were achieved, with median MFS between 36 and 40 months in the three studies and a consistently positive OS signal with matured data.
Because of a decreased crossing of the blood–brain barrier by darolutamide, fatigue and asthenia were less frequently reported with this agent. Other AEs are difficult to compare between studies owing to the lack of a systematic reporting system and the low frequency of most events.
Although the three trials reported similar results, slight differences existed in their respective patient populations. Because of the increased risk of seizures with enzalutamide and apalutamide [69–72], patients with a history of this condition were excluded from PROSPER and SPARTAN but not from ARAMIS.
How to Select the Best Treatment for Each Patient
Pharmacological Interactions of Androgen Receptor Inhibitors
Adverse events of anticancer therapies are an important issue when making clinical decisions, as they may have a detrimental impact on patients’ quality of life (QoL). This QoL burden is particularly relevant in patients with M0 CRPC, given the mostly asymptomatic nature of their disease. Because M0 CRPC is usually diagnosed in older men, often with comorbidities and receiving multiple concomitant medications, the risk of AEs or loss of efficacy due to drug interactions is also a crucial aspect in clinical decision making [73–75]. Therefore, such interactions should be assessed before making clinical decisions for patients with M0 CRPC with polypharmacy and a reasonable life expectancy.
All three second-generation ARi carry a risk of drug interactions, which should be closely examined before choosing one agent over another. Apalutamide and enzalutamide have a similar molecular structure and mechanism of action, as well as similar cytochrome P450 (CYP) inhibition capability. Both agents are associated with a higher incidence of certain AEs, such as falls, fatigue, hypertension, rashes, and seizures compared with placebo [43–46], and have the potential for pharmacological interactions with other medications that are substrates for several metabolizing enzymes and drug transporters [43, 45, 75–79]. Because of their CYP inhibition capability, both agents have the potential for CYP-mediated drug interactions, mainly for those that are substrates to CYP3A4, CYP2C9, and CYP2C19 enzymes [80, 81], with resulting altered activity of comedications such as anticoagulants, antihypertensives, opioid analgesics, and proton pump inhibitors [74, 75, 80–83]. Apalutamide and enzalutamide are also able to penetrate the blood–brain barrier and thus achieve active central nervous system concentrations, where they inhibit gamma-aminobutyric acid receptors [84]. This may result in an increased risk of seizures [69, 71, 72]. In a retrospective study of pharmacy records in patients with metastatic CRPC, enzalutamide was also associated with potential drug interactions with central nervous system drugs (e.g., opioid analgesics) and hence with a potentially increased risk of cognitive adverse effects, falls, and fractures [75]. In another retrospective analysis of a large claims database, a high prevalence of potential drug interactions was identified among patients with M0 CRPC receiving apalutamide and enzalutamide, highlighting the need for close monitoring when initiating these therapies [85].
Conversely, darolutamide has a distinct molecular structure, being metabolized mainly by CYP3A4 with no clinically relevant CYP induction or inhibition effect, and thus without major interactions with comedications metabolized by CYP enzymes [86]. In contrast to apalutamide and enzalutamide, darolutamide has limited blood–brain barrier penetration and a low affinity for the gamma-aminobutyric acid type A receptor, with a potentially lower risk of AEs in the brain compared with the other two compounds [47, 87]. In the study by Moilanen et al., the blood–brain barrier penetration rate of apalutamide and enzalutamide was ten times higher that of darolutamide [88]. Because of this, darolutamide has a limited effect on mental status, as demonstrated in preclinical studies [89, 90], and is considered safe in patients with a history of seizures [91]. The interaction between darolutamide and the breast cancer resistance protein transporter may lead to an increase in the levels of rosuvastatin, potentially increasing its secondary effects on the muscle and the liver [89]. However, a post hoc analysis of patients who participated in the ARAMIS trial showed limited potential for clinically relevant drug interactions between darolutamide and comedications frequently used to treat age-related comorbidities in patients with M0 CRPC, such as lipid-modifying agents, β-blockers, antithrombotics, and systemic antibiotics [92]. A similar proportion of patients in each treatment arm discontinued the study drug because of AEs in this study (8.9% in the darolutamide arm vs 8.7% in the placebo arm) [47]. All these aspects are relevant when determining the risk-benefit balance of ARi in patients with M0 CRPC receiving multiple comedications and should be considered in clinical decision making for these patients.
Clinical Approach to Each Individual Patient
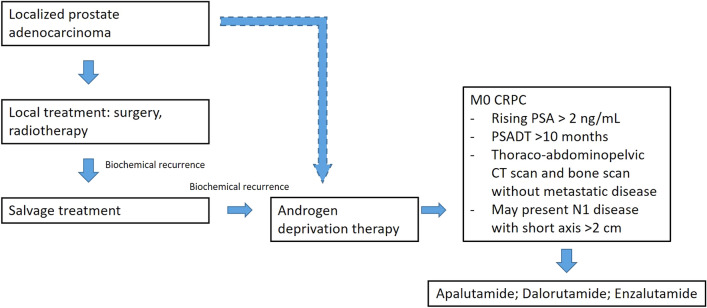
Nonmetastatic castration-resistant prostate cancer is a disease state with a variable course and patients have heterogeneous clinical characteristics. The clinical approach for each individual should be based on drug efficacy, but also treatment toxicity and pharmacological interactions, as well as patient comorbidities and QoL, and adapted to each individual patient (Fig. 1).
Fig. 1.
Individual patient clinical approach algorithm. CT computed tomography, M0 CRPC nonmetastatic castration-resistant prostate cancer, PSA prostate-specific antigen, PSADT PSA doubling time
The therapeutic goals for patients with M0 CRPC are to delay the development of metastases and increase OS, while preserving QoL. Until now, no standard of care has been formally endorsed for patients with M0 CRPC. The treatment landscape for M0 CRPC will predictably change with the introduction of the new anti-androgens darolutamide, apalutamide, and enzalutamide in clinical practice, which will widen treatment options for these patients. However, a desirable individualized treatment selection is hampered by the fact that there are no head-to-head studies comparing the safety and efficacy of these new agents.
Of note, a PSADT > 10 months was not part of the inclusion criteria in PROSPER, SPARTAN, and ARAMIS trials. Hence, observation without therapeutic intervention remains an option for patients with PSADT > 10 months [55]. Among AEs reported with the new agents with an impact on QoL are fatigue, falls, fractures, cardiovascular complications, hypertension, nausea, loss of appetite, hot flashes, seizures, rashes, and mental-impairment disorders, among others. Patients with a PSA level ≥ 8 ng/mL or PSADT ≤ 10 months are considered to be at high risk for rapid progression and should start systemic treatment [55].
The drug of choice for each patient with M0 CRPC will vary according to specific drug toxicities, patient comorbidities, and clinical settings. The fact that a significant proportion of patients with M0 CRPC, as defined by conventional imaging, show metastases upon access to PSMA PET suggests that PROSPER, SPARTAN, and ARAMIS populations actually had (minimal) metastatic disease, highlighting the severity of this disease stage and the indication for early treatment. In two independent systematic reviews and network meta-analyses, apalutamide and enzalutamide were more effective than darolutamide regarding MFS and PSA progression-free survival, while darolutamide showed benefits in OS and also as the best tolerated of the three agents [70, 93]. This agrees with preclinical data reporting a higher therapeutic index for apalutamide, with a greater opportunity for dose escalation [87].
Health-related quality of life is a relevant endpoint when defining an individualized clinical approach. While all three ARi trials evaluated HRQoL, it was a secondary endpoint in PROSPER and an exploratory endpoint in SPARTAN and ARAMIS. All trials used validated tools to evaluate patients’ QoL, including the Functional Assessment of Cancer Therapy-Prostate (FACT-P) and the European Quality of Life-5 Dimensions (EQ-5D-3L) questionnaires. Overall, findings from these trials indicate that treatment with ARi does not appear to negatively impact HRQoL in asymptomatic patients with high-risk M0 CRPC [42].
PROSPER showed that enzalutamide maintained HRQoL with a minimal decline and a similar median time to degradation to placebo [45], while SPARTAN and ARAMIS demonstrated that apalutamide and darolutamide had generally similar effects on QoL as those seen with placebo and over time [43, 47]. A potential disadvantage to consider in darolutamide is the fact that it has to be taken twice a day and with foods, which may increase undercompliance.
As a class effect, higher rates of falls, fractures, fatigue, cardiovascular events, and even death have been reported in clinical trials with the agents compared with placebo [94]. In the clinical practice, rash and hypothyroidism have been more associated with apalutamide, while hypertension and central nervous system-related adverse effects have been more linked to enzalutamide or apalutamide [95, 96]. Additionally, the risk of drug interactions associated with the three agents should be closely examined before choosing one agent over another.
Conclusions
Over recent decades, prostate cancer has been an ever-changing disease, with its course evolving with each new treatment and imaging method, leading to an increased complexity in its clinical approach. The management of the long-time recognized pre-metastatic CRPC has been recently transformed with the approval of new-generation ARi darolutamide, apalutamide, and enzalutamide. These agents have shown a relevant capacity to delay metastatic disease, with a very favorable trade-off regarding AEs. With advances in the diagnosis and treatment of the disease, the choice between these agents will not be straightforward, reinforcing the need for a thorough evaluation of patient characteristics, disease progression, and drug profiles in order to achieve the desired outcomes in M0 CRPC.
Declarations
Funding
Bayer financially supported the medical writing company Prime Focus by providing writing assistance.
Conflict of interest
AF received honoraria as a consultant/speaker for Astellas, AstraZeneca, Bayer, BMS, Janssen, MSD, Novartis, Pfizer, Recordati, and Roche. LC received honoraria as a consultant/speaker for Astellas, Bayer, BMS, Janssen, MSD, Roche, and Servier. MJM received honoraria as a consultant/speaker for Bayer, BMS, Janssen, MSD, Ipsen, and Pfizer. RR received honoraria as a consultant/speaker for Bayer, Janssen, and Pfizer. LF and CMS have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ contributions
All authors contributed to the review of the literature, article elaboration, and revision. All authors gave their final approval of the submitted version.
References
- 1.Kessler B, Albertsen P. The natural history of prostate cancer. Urol Clin N Am. 2003;30:219–226. doi: 10.1016/S0094-0143(02)00182-9. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute. SEER stat fact sheets: prostate cancer. 2021. https://seer.cancer.gov/statfacts/html/prost.html. Accessed 23 Sept 2021.
- 3.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 4.Miranda AC, Mayer-da-Silva A, Glória L, Brito C. Registo Oncológico Nacional de Todos os Tumores na População Residente em Portugal, em 2018. 2021. https://ron.min-saude.pt/media/2196/2021-0518_publica%C3%A7%C3%A3o-ron_2018.pdf. Accessed 10 Jan 2022.
- 5.Perlmutter A, Mark A, Lepor H. Androgen deprivation therapy in the treatment of advanced prostate cancer. Rev Urol. 2007;9:S3–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Sun M, Choueiri TK, Hamnvik O-PR, et al. Comparison of gonadotropin-releasing hormone agonists and orchiectomy. JAMA Oncol. 2016;2:500. 10.1001/jamaoncol.2015.4917. [DOI] [PubMed]
- 7.Byar DP, Corle DK. Hormone therapy for prostate cancer: results of the Veterans Administration Cooperative Urological Research Group Studies. J Urol. 1989;141:1032–1033. doi: 10.1016/S0022-5347(17)41100-1. [DOI] [PubMed] [Google Scholar]
- 8.Huggins C, Hodges CV. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–297. [Google Scholar]
- 9.Shafi AA, Yen AE, Weigel NL. Androgen receptors in hormone-dependent and castration-resistant prostate cancer. Pharmacol Ther. 2013;140:223–238. doi: 10.1016/j.pharmthera.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Crowley F, Sterpi M, Buckley C, et al. A review of the pathophysiological mechanisms underlying castration-resistant prostate cancer. Res Rep Urol. 2021;13:457–472. doi: 10.2147/RRU.S264722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandrasekar T, Yang JC, Gao AC, et al. Targeting molecular resistance in castration-resistant prostate cancer. BMC Med. 2015;13:206. doi: 10.1186/s12916-015-0457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karantanos T, Evans CP, Tombal B, et al. Understanding the mechanisms of androgen deprivation resistance in prostate cancer at the molecular level. Eur Urol. 2015;67:470–479. doi: 10.1016/j.eururo.2014.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liede A, Arellano J, Hechmati G, et al. International prevalence of nonmetastatic (M0) castration-resistant prostate cancer (CRPC) J Clin Oncol. 2013;31:e16052. doi: 10.1200/jco.2013.31.15_suppl.e16052. [DOI] [Google Scholar]
- 14.Schaeffer E, Srinivas S, Antonarakis ES, et al. NCCN guidelines insights: prostate cancer, Version 1.2021. J Natl Compr Cancer Netw. 2021;19:134–143. doi: 10.6004/jnccn.2021.0008. [DOI] [PubMed] [Google Scholar]
- 15.Scher HI, Morris MJ, Stadler WM, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016;34:1402–1418. doi: 10.1200/JCO.2015.64.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moreira DM, Howard LE, Sourbeer KN, et al. Predictors of time to metastasis in castration-resistant prostate cancer. Urology. 2016;96:171–176. doi: 10.1016/j.urology.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith MR, Cook R, Lee K-A, et al. Disease and host characteristics as predictors of time to first bone metastasis and death in men with progressive castration-resistant nonmetastatic prostate cancer. Cancer. 2011;117:2077–2085. doi: 10.1002/cncr.25762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith MR, Kabbinavar F, Saad F, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23:2918–2925. doi: 10.1200/JCO.2005.01.529. [DOI] [PubMed] [Google Scholar]
- 19.Metwalli AR, Rosner IL, Cullen J, et al. Elevated alkaline phosphatase velocity strongly predicts overall survival and the risk of bone metastases in castrate-resistant prostate cancer. Urol Oncol. 2014;32:761–768. doi: 10.1016/j.urolonc.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 21.Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152–160. doi: 10.1016/S1470-2045(14)71205-7. [DOI] [PubMed] [Google Scholar]
- 22.Crawford ED, Stone NN, Yu EY, et al. Challenges and recommendations for early identification of metastatic disease in prostate cancer. Urology. 2014;83:664–669. doi: 10.1016/j.urology.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Roghmann F, Antczak C, McKay RR, et al. The burden of skeletal-related events in patients with prostate cancer and bone metastasis. Urol Oncol Semin Orig Investig. 2015;33:17.e9–17.e18. doi: 10.1016/j.urolonc.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Tsuzuki S, Park SH, Eber MR, et al. Skeletal complications in cancer patients with bone metastases. Int J Urol. 2016;23:825–832. doi: 10.1111/iju.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broder MS, Gutierrez B, Cherepanov D, et al. Burden of skeletal-related events in prostate cancer: unmet need in pain improvement. Support Care Cancer. 2015;23:237–247. doi: 10.1007/s00520-014-2437-3. [DOI] [PubMed] [Google Scholar]
- 26.Westgeest HM, Uyl-de Groot CA, van Moorselaar RJA, et al. Differences in trial and real-world populations in the Dutch Castration-Resistant Prostate Cancer Registry. Eur Urol Focus. 2018;4:694–701. doi: 10.1016/J.EUF.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Hövels AM, Heesakkers RAM, Adang EM, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol. 2008;63:387–395. doi: 10.1016/j.crad.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 28.Shen G, Deng H, Hu S, et al. Comparison of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: a meta-analysis. Skelet Radiol. 2014;43:1503–1513. doi: 10.1007/s00256-014-1903-9. [DOI] [PubMed] [Google Scholar]
- 29.Eiber M, Maurer T, Souvatzoglou M, et al. Evaluation of hybrid 68 Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56:668–674. doi: 10.2967/jnumed.115.154153. [DOI] [PubMed] [Google Scholar]
- 30.Morigi JJ, Stricker PD, van Leeuwen PJ, et al. Prospective comparison of 18 F-fluoromethylcholine versus 68 Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J Nucl Med. 2015;56:1185–1190. doi: 10.2967/jnumed.115.160382. [DOI] [PubMed] [Google Scholar]
- 31.Mosavi F, Johansson S, Sandberg DT, et al. Whole-body diffusion-weighted MRI compared with 18 F-NaF PET/CT for detection of bone metastases in patients with high-risk prostate carcinoma. Am J Roentgenol. 2012;199:1114–1120. doi: 10.2214/AJR.11.8351. [DOI] [PubMed] [Google Scholar]
- 32.Umbehr MH, Müntener M, Hany T, et al. The role of 11C-choline and 18F-fluorocholine positron emission tomography (PET) and PET/CT in prostate cancer: a systematic review and meta-analysis. Eur Urol. 2013;64:106–117. doi: 10.1016/j.eururo.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 33.Rauscher I, Düwel C, Haller B, et al. Efficacy, predictive factors, and prediction Nnmograms for 68 Ga-labeled prostate-specific membrane antigen-ligand positron-emission tomography/computed tomography in early biochemical recurrent prostate cancer after radical prostatectomy. Eur Urol. 2018;73:656–661. doi: 10.1016/j.eururo.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Fendler W, Calais J, Gartmann J, et al. Accuracy of 68 Ga-PSMA11 PET/CT on recurrent prostate cancer: preliminary results from a phase 2/3 prospective trial. J Clin Oncol. 2018;36:5001. doi: 10.1200/JCO.2018.36.15_suppl.5001. [DOI] [Google Scholar]
- 35.Eder M, Schäfer M, Bauder-Wüst U, et al. 68 Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug Chem. 2012;23:688–697. doi: 10.1021/bc200279b. [DOI] [PubMed] [Google Scholar]
- 36.Leek J, Lench N, Maraj B, et al. Prostate-specific membrane antigen: evidence for the existence of a second related human gene. Br J Cancer. 1995;72:583–588. doi: 10.1038/bjc.1995.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perera M, Papa N, Roberts M, et al. Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions: a systematic review and meta-analysis. Eur Urol. 2020;77:403–417. doi: 10.1016/j.eururo.2019.01.049. [DOI] [PubMed] [Google Scholar]
- 38.Ost P. PSMA PET-CT redefines nonmetastatic castration-resistant prostate cancer. Nat Rev Urol. 2020;17:133–134. doi: 10.1038/s41585-019-0268-1. [DOI] [PubMed] [Google Scholar]
- 39.Onal C, Guler OC, Torun N, et al. The effect of androgen deprivation therapy on 68Ga-PSMA tracer uptake in non-metastatic prostate cancer patients. Eur J Nucl Med Mol Imaging. 2020;47:632–641. doi: 10.1007/s00259-019-04581-4. [DOI] [PubMed] [Google Scholar]
- 40.Fendler WP, Weber M, Iravani A, et al. Prostate-specific membrane antigen ligand positron emission tomography in men with nonmetastatic castration-resistant prostate cancer. Clin Cancer Res. 2019;25:7448–7454. doi: 10.1158/1078-0432.CCR-19-1050. [DOI] [PubMed] [Google Scholar]
- 41.Fourquet A, Aveline C, Cussenot O, et al. 68Ga-PSMA-11 PET/CT in restaging castration-resistant nonmetastatic prostate cancer: detection rate, impact on patients’ disease management and adequacy of impact. Sci Rep. 2020;10:2104. doi: 10.1038/s41598-020-58975-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao SG, Chang SL, Erho N, et al. Associations of luminal and basal subtyping of prostate cancer with prognosis and response to androgen deprivation therapy. JAMA Oncol. 2017;3:1663–1672. doi: 10.1001/jamaoncol.2017.0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378:1408–1418. doi: 10.1056/NEJMoa1715546. [DOI] [PubMed] [Google Scholar]
- 44.Smith MR, Saad F, Chowdhury S, et al. Apalutamide and overall survival in prostate cancer. Eur Urol. 2021;79:150–158. doi: 10.1016/j.eururo.2020.08.011. [DOI] [PubMed] [Google Scholar]
- 45.Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378:2465–2474. doi: 10.1056/NEJMoa1800536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sternberg CN, Fizazi K, Saad F, et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020;382:2197–2206. doi: 10.1056/NEJMoa2003892. [DOI] [PubMed] [Google Scholar]
- 47.Fizazi K, Shore N, Tammela TL, et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2019;380:1235–1246. doi: 10.1056/NEJMoa1815671. [DOI] [PubMed] [Google Scholar]
- 48.Fizazi K, Shore N, Tammela TL, et al. Nonmetastatic, castration-resistant prostate cancer and survival with darolutamide. N Engl J Med. 2020;383:1040–1049. doi: 10.1056/NEJMoa2001342. [DOI] [PubMed] [Google Scholar]
- 49.Fanti S, Goffin K, Hadaschik BA, et al. Consensus statements on PSMA PET/CT response assessment criteria in prostate cancer. Eur J Nucl Med Mol Imaging. 2021;48:469–476. doi: 10.1007/s00259-020-04934-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trabulsi EJ, Rumble RB, Jadvar H, et al. Optimum imaging strategies for advanced prostate cancer: ASCO guideline. J Clin Oncol. 2020;38:1963–1996. doi: 10.1200/JCO.19.02757. [DOI] [PubMed] [Google Scholar]
- 51.Gillessen S, Attard G, Beer TM, et al. Management of patients with advanced prostate cancer: report of the Advanced Prostate Cancer Consensus Conference 2019. Eur Urol. 2020;77:508–547. doi: 10.1016/j.eururo.2020.01.012. [DOI] [PubMed] [Google Scholar]
- 52.Maffezzini M, Bossi A, Collette L. Implications of prostate-specific antigen doubling time as indicator of failure after surgery or radiation therapy for prostate cancer. Eur Urol. 2007;51:605–613. doi: 10.1016/j.eururo.2006.10.062. [DOI] [PubMed] [Google Scholar]
- 53.Ruffion A, Rebillard X, Grima F. PSA doubling time and method of calculation. Prog Urol. 2005;15:1035–1041. [PubMed] [Google Scholar]
- 54.Mateo J, Fizazi K, Gillessen S, et al. Managing nonmetastatic castration-resistant prostate cancer. Eur Urol. 2019;75:285–293. doi: 10.1016/j.eururo.2018.07.035. [DOI] [PubMed] [Google Scholar]
- 55.Smith MR, Saad F, Oudard S, et al. Denosumab and bone metastasis-free survival in men with nonmetastatic castration-resistant prostate cancer: exploratory analyses by baseline prostate-specific antigen doubling time. J Clin Oncol. 2013;31:3800–3806. doi: 10.1200/JCO.2012.44.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Howard LE, Moreira DM, De Hoedt A, et al. Thresholds for PSA doubling time in men with non-metastatic castration-resistant prostate cancer. BJU Int. 2017;120:E80–E86. doi: 10.1111/bju.13856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vickers AJ, Brewster SF. PSA velocity and doubling time in diagnosis and prognosis of prostate cancer. Br J Med Surg Urol. 2012;5:162–168. doi: 10.1016/j.bjmsu.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mottet N, van den Bergh RCN, Briers E, et al. EAU guidelines: prostate cancer 2019. https://uroweb.org/guideline/prostate-cancer/. Accessed 7 Jul 2022.
- 59.Kohaar I, Petrovics G, Srivastava S. A rich array of prostate cancer molecular biomarkers: opportunities and challenges. Int J Mol Sci. 2019;20:1813. doi: 10.3390/ijms20081813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Di Nunno V, Gatto L, Santoni M, et al. Recent advances in liquid biopsy in patients with castration resistant prostate cancer. Front Oncol. 2018;8:397. doi: 10.3389/fonc.2018.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yokomizo Y, Kawahara T, Miyoshi Y, et al. Efficacy of immediate switching from bicalutamide to flutamide as second-line combined androgen blockade. Biomed Res Int. 2016;2016:1–7. doi: 10.1155/2016/4083183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scher HI, Liebertz C, Kelly WK, et al. Bicalutamide for advanced prostate cancer: the natural versus treated history of disease. J Clin Oncol. 1997;15:2928–2938. doi: 10.1200/JCO.1997.15.8.2928. [DOI] [PubMed] [Google Scholar]
- 63.Scher HI, Kelly WK. Flutamide withdrawal syndrome: its impact on clinical trials in hormone-refractory prostate cancer. J Clin Oncol. 1993;11:1566–1572. doi: 10.1200/JCO.1993.11.8.1566. [DOI] [PubMed] [Google Scholar]
- 64.Kevin Kelly W, Scher HI. Prostate specific antigen decline after antiandrogen withdrawal: the flutamide withdrawal syndrome. J Urol. 1993;149:607–609. doi: 10.1016/S0022-5347(17)36163-3. [DOI] [PubMed] [Google Scholar]
- 65.Beaver JA, Kluetz PG, Pazdur R. Metastasis-free survival: a new end point in prostate cancer trials. N Engl J Med. 2018;378:2458–2460. doi: 10.1056/NEJMp1805966. [DOI] [PubMed] [Google Scholar]
- 66.Xie W, Regan MM, Buyse M, et al. Metastasis-free survival is a strong surrogate of overall survival in localized prostate cancer. J Clin Oncol. 2017;35:3097–3104. doi: 10.1200/JCO.2017.73.9987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fallah J, Zhang L, Weinstock C, et al. An FDA pooled analysis: characteristics and outcomes of patients with nonmetastatic castration-resistant prostate cancer, based on prior history of prostatectomy and/or radiation therapy. J Clin Oncol. 2021;39:197. doi: 10.1200/JCO.2021.39.6_suppl.197. [DOI] [Google Scholar]
- 68.National Comprehensive Cancer Network. Prostate Cancer (Version 4.2022). 2022. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed 10 Jan 2022.
- 69.Pilon D, Behl AS, Ellis LA, et al. Assessment of real-world central nervous system events in patients with advanced prostate cancer using abiraterone acetate, bicalutamide, enzalutamide, or chemotherapy. Am Health Drug Benef. 2017;10:143–153. [PMC free article] [PubMed] [Google Scholar]
- 70.Kumar J, Jazayeri SB, Gautam S, et al. Comparative efficacy of apalutamide darolutamide and enzalutamide for treatment of non-metastatic castrate-resistant prostate cancer: a systematic review and network meta-analysis. Urol Oncol Semin Orig Investig. 2020;38:826–834. doi: 10.1016/j.urolonc.2020.03.022. [DOI] [PubMed] [Google Scholar]
- 71.Higano CS, Beer TM, Taplin M-E, et al. Long-term safety and antitumor activity in the phase 1–2 study of enzalutamide in pre- and post-docetaxel castration-resistant prostate cancer. Eur Urol. 2015;68:795–801. doi: 10.1016/j.eururo.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Slovin S, Clark W, Carles J, et al. Seizure rates in enzalutamide-treated men with metastatic castration-resistant prostate cancer and risk of seizure. JAMA Oncol. 2018;4:702. doi: 10.1001/jamaoncol.2017.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guthrie B, Makubate B, Hernandez-Santiago V, et al. The rising tide of polypharmacy and drug–drug interactions: population database analysis 1995–2010. BMC Med. 2015;13:74. doi: 10.1186/s12916-015-0322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Benoist GE, van Oort IM, Burger DM, et al. The combination of enzalutamide and opioids: a painful pitfall? Eur Urol. 2019;75:351–352. doi: 10.1016/j.eururo.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 75.Benoist GE, van Oort IM, Smeenk S, et al. Drug-drug interaction potential in men treated with enzalutamide: mind the gap. Br J Clin Pharmacol. 2018;84:122–129. doi: 10.1111/bcp.13425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Janssen Ortho LLC. Erleada (apalutamide) US prescribing information. 2018.
- 77.European Medicines Agency. Summary of product characteristics: Erleada. 2019.
- 78.European Medicines Agency. Summary of product characteristics: Xtandi. 2019.
- 79.Astellas Pharma US Inc. Xtandi (enzalutamide): US prescribing information. 2018.
- 80.European Medicines Agency. Erleada CHMP assessment report. 2018. https://www.ema.europa.eu/en/documents/assessment-report/erleada-epar-public-assessment-report_en.pdf. Accessed 10 Jan 2022.
- 81.European Medicines Agency. Xtandi CHMP assessment report. 2013. https://www.ema.europa.eu/en/documents/smop-initial/chmp-summary-positive-opinion-xtandi_en.pdf. Accessed 10 Jan 2022.
- 82.US Center for Drug Evaluation and Research. NDA/BLA multidisciplinary review and evaluation NDA 210951 Erleada (apalutamide). 2018. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210951Orig1s000MultidisciplineR.pdf. Accessed 10 Jan 2022.
- 83.US Center for Drug Evaluation and Research. NDA/BLA clinical pharmacology and biopharmaceutics review NDA 203415 Xtandi (enzalutamide). 2012. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/203415Orig1s000ClinPharmR.pdf. Accessed 10 Jan 2022.
- 84.Labrize F, Cany L, Massard C, et al. Enzalutamide and sleep apnea: an emerging central nervous system side-effect? Ann Oncol 2016;27:206. 10.1093/annonc/mdv481. [DOI] [PubMed]
- 85.Appukkuttan S, Fu C, Du Y, et al. Prevalence of potential drug-drug interactions among nonmetastatic castration-resistant prostate cancer patients treated with apalutamide and enzalutamide. J Clin Oncol. 2021;39:e18690. doi: 10.1200/JCO.2021.39.15_suppl.e18690. [DOI] [Google Scholar]
- 86.Zurth C, Graudenz K, Denner K, et al. Drug-drug interaction (DDI) of darolutamide with cytochrome P450 (CYP) and P-glycoprotein (P-gp) substrates: results from clinical and in vitro studies. J Clin Oncol. 2019;37:297. doi: 10.1200/JCO.2019.37.7_suppl.297. [DOI] [Google Scholar]
- 87.Clegg NJ, Wongvipat J, Joseph JD, et al. ARN-509: a novel antiandrogen for prostate cancer treatment. Cancer Res. 2012;72:1494–1503. doi: 10.1158/0008-5472.CAN-11-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moilanen A-M, Riikonen R, Oksala R, et al. Discovery of ODM-201, a new-generation androgen receptor inhibitor targeting resistance mechanisms to androgen signaling-directed prostate cancer therapies. Sci Rep. 2015;5:12007. doi: 10.1038/srep12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zurth C, Koskinen M, Fricke R, et al. Drug–drug interaction potential of darolutamide: in vitro and clinical studies. Eur J Drug Metab Pharmacokinet. 2019;44:747–759. doi: 10.1007/s13318-019-00577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shore ND, Tammela TL, Massard C, et al. Safety and antitumour activity of ODM-201 (BAY-1841788) in chemotherapy-naïve and CYP17 inhibitor-naïve patients: follow-up from the ARADES and ARAFOR Trials. Eur Urol Focus. 2018;4:547–553. doi: 10.1016/j.euf.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 91.Rice MA, Malhotra SV, Stoyanova T. Second-generation antiandrogens: from discovery to standard of care in castration resistant prostate cancer. Front Oncol. 2019;9:801. doi: 10.3389/fonc.2019.00801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shore N, Zurth C, Fricke R, et al. Evaluation of clinically relevant drug–drug interactions and population pharmacokinetics of darolutamide in patients with nonmetastatic castration-resistant prostate cancer: results of pre-specified and post hoc analyses of the phase III ARAMIS trial. Target Oncol. 2019;14:527–539. doi: 10.1007/s11523-019-00674-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mori K, Mostafaei H, Pradere B, et al. Apalutamide, enzalutamide, and darolutamide for non-metastatic castration-resistant prostate cancer: a systematic review and network meta-analysis. Int J Clin Oncol. 2020;25:1892–1900. doi: 10.1007/s10147-020-01777-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reis LO. Metastasis-free survival: progress or lowering the bar on nonmetastatic prostate cancer? Eur Urol. 2018;74:682–683. doi: 10.1016/j.eururo.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 95.Drago JZ, Kantoff PW, Stopsack KH. Adverse event profiles of apalutamide, enzalutamide, and darolutamide in SPARTAN, PROSPER, and ARAMIS: how confident are we about which drug is safest? J Clin Oncol. 2020;38:318. doi: 10.1200/JCO.2020.38.6_suppl.318. [DOI] [Google Scholar]
- 96.Gupta R, Sheng IY, Barata PC, et al. Non-metastatic castration-resistant prostate cancer: current status and future directions. Expert Rev Anticancer Ther. 2020;20:513–522. doi: 10.1080/14737140.2020.1772759. [DOI] [PubMed] [Google Scholar]