Abstract
Despite the well-known benefits of the minimally invasive approach for the right colon cancer treatment, less is known about its feasibility and advantages in morbid obese patients. The aim of this study is to compare the postoperative outcomes after totally minimally invasive right colectomy between the obese and non-obese population. Data derived from a prospectively maintained multicenter colorectal database were analysed, dividing the enrolled patients into two groups: obese (BMI > 29.99) patient group and non-obese patient group. Data about gender, age, American Society of Anesthesiologists (ASA) Score, tumor characteristics, operative time, anastomosis time, extraction site, incision length, intraoperative complications, postoperative complications, postoperative recovery, specimen length and retrieved nodes were taken to assess the achievement of the oncologic standards. After a propensity score matching, a total of 184 patients was included, 92 in each group. No differences were found in terms of demographic data and tumor characteristics. Intraoperative data showed a significant difference in terms of anastomosis time in favour of non-obese group (p < 0.0001). No intraoperative complications were recorded and no conversion was needed in both groups. No differences were found in terms of postoperative complications. There were no differences in terms of first mobilization (p = 0.745), time to first flatus (p = 0.241) time to tolerance to liquid and solid diet (p = 0.241 and p = 0.06) and length of hospital stay (p = 0.817). The analysis of oncologic outcomes demonstrated adequate results in both groups. The results obtained by our study confirmed the feasibility and safety of the totally minimally invasive approach even in obese population.
Keywords: Right colon, Cancer, Intracorporeal, Obese, Minimally invasive colectomy, Surgery
Introduction
Obesity is a global pandemic, especially in industrialized countries. Bariatric surgery is nowadays considered as the most effective approach to obtain a weight loss and a reduction in the obesity-related conditions [1–4].
Nevertheless, the increased prevalence of obesity has caused an increase even in the prevalence of colorectal cancers [5].
In the setting of right colon cancer, totally minimally invasive approach should be considered the preferred way to perform right hemicolectomy [6–8]. In this setting, several reports have demonstrated the superiority of this approach in comparison with extracorporeal anastomosis, in terms of postoperative complications and recovery outcomes [7–13]. Despite the well-known benefits of the minimally invasive approach, less is known about its feasibility and advantages in certain conditions, such as in morbid obese patients [14–16].
The aim of this study is to compare the postoperative outcomes after totally minimally invasive right colectomy between the obese and non-obese population.
Materials and methods
After obtaining the Institutional Review Board approval of each Centre, all consecutive patients from January 2007 to December 2017 who underwent a totally minimally invasive right hemicolectomy were identified to be included in a multicenter experience. The study was conducted in compliance with the STROBE checklist [17].
Data from a prospectively maintained colorectal database, derived from high-volume colorectal centres [18], were analysed.
Each centre ensured the enrolment of at least 60 patients.
The enrolled patients were divided into two groups: obese (BMI ≥ 30 kg/m2) patient group and non-obese patient group.
All the included patients were operated on with a standardized totally intracorporeal right colectomy, as previously described [19, 20], and according to Enhanced Recovery After Surgery (ERAS) protocols [21, 22].
Briefly, after the division of the ileocolic pedicles and the right branches of the middle colic artery at their origin, a totally minimally invasive right hemicolectomy with an intracorporeal side-to-side ileocolic anastomosis was performed.
Demographic data (gender, age, Body Mass Index (BMI) American Society of Anesthesiologists (ASA) Score), tumor localization and TNM classification were recorded.
To assess any intraoperative challenge, data about operative time, anastomosis time, extraction site and incision length were recorded. Furthermore, intraoperative complications were recorded.
The analysed outcomes included postoperative complications, including postoperative nausea, pain, ileus, wound infection, intraluminal and extraluminal bleedings, anastomotic leakage, the need of Intensive Care Unit (ICU) and 30-day postoperative death. Furthermore, the complications were classified according their severity by the adoption of Clavien–Dindo (CD) Classification [23].
The term anastomotic leakage defined the conditions with clinical or radiologic anastomotic dehiscence, with or without the need of surgical revision. Specifically, the anastomotic leakage was classified as grade A, if resulting in no change in patients’ management; grade B, requiring active therapeutic intervention but without a surgical intervention; grade C, when a surgical re-operation was needed [24].
Any type of bleeding was considered relevant if required blood transfusions.
Other analysed outcomes were postoperative recovery, expressed as mobilization, time to first flatus and first stool, tolerance to a solid diet and length of hospital stay. Finally, data about specimen length and retrieved nodes to assess the achievement of the oncologic standards were registered.
To exclude any bias related to the allocation of each patient in the different study group, a propensity score was estimated using a multivariate logistic regression model based on age, gender, ASA Score, previous abdominal surgery, and tumour localization. One-to-one matching without replacement was performed with a 0.1 caliper width, and the resulting score-matched pairs were used in subsequent analyses.
Statistical analysis was performed using the IBM SPSS Statistics for Windows, Version 27.0 (IBM Corp, Armonk, NY). Continuous data were expressed as the means ± standard deviation (SD), and categorical variables were expressed as number and percentages. Continuous variables were compared by the Mann–Whitney U test and categorical variables with the Chi-square χ2 test. All results are presented as two-tailed values and a p < 0.05 defined a statistical significance.
Results
The whole analysed database included 1033 patients from ten departments of surgery.
Demographic data of patients before propensity score matching are summarized in Table 1.
Table 1.
Demographic data and tumor characteristics before propensity score matching
| Characteristics | Obese (n = 187) | Non-obese (n = 846) | p value |
|---|---|---|---|
| Gender | 0.019 | ||
| M | 113 (60.4) | 429 (50.7) | |
| F | 74 (39.6) | 417 (49.3) | |
| Age | 69.49 ± 11.55 | 69.21 ± 9.34 | 0.760 |
| ASA Score | 0.333 | ||
| I | 11 (5.9) | 62 (7.3) | |
| II | 102 (54.5) | 495 (58.6) | |
| III | 74 (39.6) | 283 (33.5) | |
| IV | 0 (0) | 4 (0.5) | |
| Previous abdominal surgery | 48 (27.9) | 350 (41.4) | < 0.0001 |
| Tumour localization | 0.181 | ||
| Ileo-cecal valve | 39 (20.9) | 176 (20.8) | |
| Ascending colon | 102 (54.5) | 515 (60.9) | |
| Hepatic flexure | 33 (17.6) | 120 (14.2) | |
| Proximal transverse colon | 13 (7.0) | 35 (4.1) | |
| T stage | 0.771 | ||
| Tx | 0 (0) | 3 (0.4) | |
| T0 | 2 (1.1) | 13 (1.5) | |
| Tis | 19 (10.2) | 85 (10) | |
| T1 | 17 (9.1) | 65 (7.7) | |
| T2 | 32 (17.1) | 185 (21.9) | |
| T3 | 98 (52.4) | 398 (47) | |
| T4a | 17 (9.1) | 87 (10.3) | |
| T4b | 2 (1.1) | 10 (1.2) | |
| N stage | 0.020 | ||
| Nx | 1 (0.5) | 1 (0.1) | |
| N0 | 125 (66.8) | 588 (69.5) | |
| N1 | 9 (4.8) | 52 (6.1) | |
| N1a | 12 (6.4) | 50 (5.9) | |
| N1b | 18 (9.6) | 64 (7.6) | |
| N1c | 0 (0) | 9 (1.1) | |
| N2 | 8 (4.3) | 32 (3.8) | |
| N2a | 4 (2.1) | 38 (4.5) | |
| N2b | 10 (5.3) | 12 (1.2) | |
| M stage | 0.543 | ||
| M0 | 178 (95.2) | 788 (93.1) | |
| M1 | 4 (2.1) | 32 (3.8) | |
| M1a | 5 (2.7) | 22 (2.6) | |
| M1b | 0 (0) | 4 (0.5) | |
Values are expressed as number and (percentage)
P-value considered significant if p < 0.05
M male, F Female, ASA American Society of Anesthesiologists, n number of patients in the group
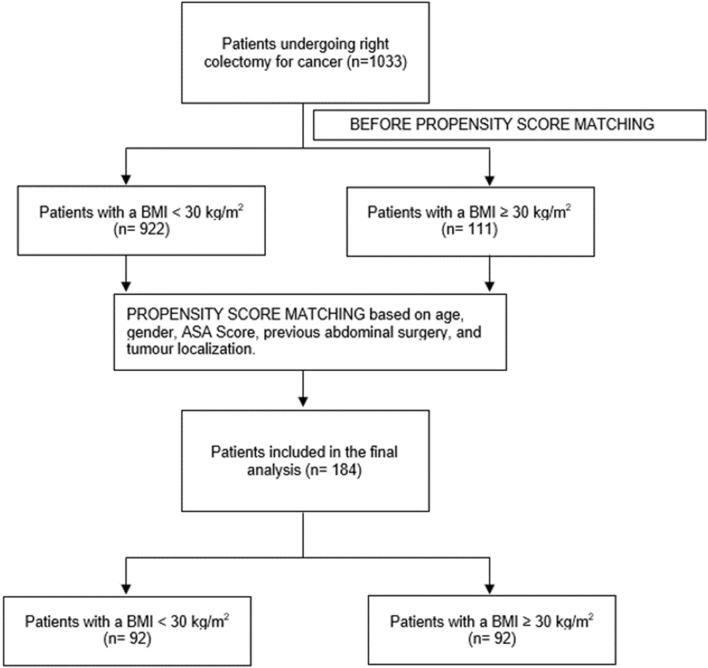
After the propensity score matching, a total of 184 patients was included, 92 in each group. The results of STROBE Flow diagram of the inlcuded patients is represented in Fig. 1.
Fig. 1.
STROBE Flow Diagram of the included patients
Mean and median BMI of the non-obese and obese patients group were 24.67 ± 2.7 and 32.31 ± 2.5, respectively, and 24.35 (18.2–29.9) and 31.3 (30–42.57) respectively, with a p < 0.0001. No differences were found in terms of gender (p = 0.615), age (obese: 69.46 ± 8.45 vs non-obese: 70.08 ± 10.43, p = 0.659) ASA Score (p = 0.580), previous abdominal surgery (p = 0.181), tumour localization (p = 0.688), T, N and M classification (p = 0.209, p = 0.110 and p = 0.220, respectively). Patients’ and tumours’ characteristics were summarized in Table 2.
Table 2.
Demographic data and tumor characteristics after propensity score matching
| Characteristics | Obese (n = 92) | Non-obese (n = 92) | p value |
|---|---|---|---|
| Gender | 0.615 | ||
| M | 66 (71.7) | 70 (76.1) | |
| F | 26 (28.3) | 22 (23.9) | |
| Age | 69.46 ± 8.45 | 70.08 ± 10.43 | 0.659 |
| BMI | 32.31 ± 2.5 | 24.67 ± 2.7 | |
| BMI median (range) | 31.3 (30–42.57) | 24.35 (18.2–29.9) | |
| ASA score | 0.580 | ||
| I | 3 (3.3) | 4 (4.3) | |
| II | 52 (56.5) | 45 (48.9) | |
| III | 37 (40.2) | 42 (45.7) | |
| IV | 0 (0) | 1 (1.1) | |
| Previous abdominal surgery | 45 (48.9) | 35 (38) | 0.181 |
| Tumour localization | 0.688 | ||
| Ileo-cecal valve | 10 (10.9) | 14 (15.2) | |
| Ascending colon | 63 (68.5) | 60 (65.2) | |
| Hepatic flexure | 15 (16.3) | 12 (13) | |
| Proximal transverse colon | 4 (4.3) | 6 (6.5) | |
| T stage | 0.209 | ||
| Tx | 0 (0) | 0 (0) | |
| T0 | 4 (4.3) | 5 (5.4) | |
| Tis | 13 (14.1) | 8 (8.7) | |
| T1 | 7 (7.6) | 5 (5.4) | |
| T2 | 9 (9.8) | 22 (23.9) | |
| T3 | 49 (53.3) | 44 (47.8) | |
| T4a | 9 (9.8) | 8 (8.7) | |
| T4b | 1 (1.1) | 0 (0) | |
| N stage | 0.110 | ||
| Nx | 1 (1.1) | 0 (0) | |
| N0 | 52 (56.5) | 69 (75) | |
| N1 | 6 (6.5) | 4 (4.3) | |
| N1a | 8 (8.7) | 3 (3.3) | |
| N1b | 11 (12) | 6 (6.5) | |
| N1c | 0 (0) | 2 (2.2) | |
| N2 | 6 (6.5) | 4 (4.3) | |
| N2a | 1 (1.1) | 2 (2.2) | |
| N2b | 7 (7.6) | 2 (2.2) | |
| M stage | 0.220 | ||
| M0 | 83 (90.2) | 87 (94.5) | |
| M1 | 7 (7.6) | 2 (2.2) | |
| M1a | 2 (2.2) | 3 (3.3) | |
| M1b | 0 (0) | 0 (0) | |
Values are expressed as number and (percentage). Continuous variables are expressed as mean and standard deviation. BMI has been expressed also as median and range
M male, F Female, ASA American Society of Anesthesiologists
Intraoperative data showed no significant differences in terms of operative time between the groups (obese 176 ± 51 min vs non-obese 180 ± 54 min, p = 0.622), while a significant difference was retrieved in terms of anastomosis time in favour of non-obese group (obese 19 ± 3 min vs non-obese 16 ± 4 min, p < 0.0001). In terms of extraction site, a significant difference was found between the two groups (p = 0.006), as well as the incision length (p < 0.0001). In details, in both group the preferred extraction site was the Pfannenstiel incision, but in the non-obese group a larger number of patients underwent a ventral midline incision (obese 13 vs non-obese 26 patients). No intraoperative complications were recorded and no conversion was needed in both groups.
Intraoperative data were summarized in Table 3.
Table 3.
Intraoperative data
| Intraoperative data | Obese (n = 92) | Non-obese (n = 92) | p value |
|---|---|---|---|
| Operative time | 176 ± 51 | 180 ± 54 | 0.622 |
| Anastomosis time | 19 ± 3 | 16 ± 4 | < 0.0001 |
| Extraction site | 0.006 | ||
| Not specified | 0 (0) | 2 (1.1) | |
| Pfannenstiel incision | 77 (83.7) | 57 (61.9) | |
| Ventral midline incision | 13 (14.1) | 26 (28.2) | |
| Ventral out-midline incision | 2 (2.2) | 7 (7.6) | |
| Incision length | < 0.0001 | ||
| Not specified | 1 (1.1) | 13 (14.1) | |
| < 5 cm | 23 (25) | 42 (45.6) | |
| > 5 cm but < 10 cm | 68 (73.9) | 35 (38) | |
| > 10 cm | 0 (0) | 2 (2.2) | |
| Intraoperative complications | 0 (0) | 0 (0) | 1.000 |
| Conversion | 0 (0) | 0 (0) | 1.000 |
Continuous variables are expressed as mean ± standard deviation, values are expressed as number and (percentage)
P-value considered significant if p < 0.05
Cm centimetres, n number of patients in the group
n= number of patients in the group
The analysis of postoperative complications showed no differences between the two groups in terms of postoperative nausea (p = 0.305), pain (p = 0.246), ileus (p = 0.354), wound infection (p = 0.444), intra- and extra-luminal bleeding (p = 0.212 and p = 1.000, respectively), anastomotic leakage (p = 1.000), the need of ICU (p = 0.368) and postoperative mortality (p = 1.000).
In terms of treatment, one leakage required no further management, two a radiologic drainage and two a surgical re-intervention in the non-obese patients group, while two no changes in postoperative management, one a radiologic intervention and two a surgical revision in the obese patients group, respectively.
Considering the Clavien–Dindo Classification for postoperative complications, no significant differences were found in terms of CD-1 complications (obese 22 vs non-obese 20, p = 0.123), CD-2 complications (obese 1 vs non-obese 4, p = 0.368), CD-3 complications (obese 2 vs non-obese 4, p = 0.689), CD-4 complications (obese: 3 vs non-obese: 0, p = 0.311), CD-5 (obese 1 vs non-obese 1, p = 1.000) (Fig. 1).
The analysis of postoperative recovery outcomes showed no differences in terms of first mobilization (obese 25 ± 13 h vs non-obese 27 ± 26, p = 0.745), time to first flatus (obese 27 ± 26 h vs non-obese: 25 ± 12 h, p = 0.241) time to tolerance to liquid and solid diet (obese 39 ± 23 h vs non-obese 41 ± 25 h, p = 0.241 and obese 72 ± 60 h vs non-obese 89 ± 46 h, p = 0.06) and length of hospital stay (obese 8 ± 6 days vs non-obese 7 ± 4 days, p = 0.817), while a significant difference was retrieved in terms of time to first stool in favour of obese group (obese 70 ± 35 h vs non-obese 93 ± 35 h, p < 0.0001).
The analysis of oncologic outcomes demonstrated adequate results in both groups. In details, the length of the extracted specimen was significantly longer in the obese group (obese 29.3 ± 11.2 cm vs non-obese 24.2 ± 9 cm, p = 0.002), as well as the number of harvested nodes was significantly higher in the obese group (obese 24.8 ± 11.3 cm vs non-obese 19 ± 8 cm, p < 0.0001).
Data on postoperative complications, recovery outcomes and oncologic data are shown in Table 4.
Table 4.
Postoperative complications, recovery outcomes and oncologic outcomes
| Postoperative outcomes | Obese (n = 92) | Non-obese (n = 92) | p value |
|---|---|---|---|
| Postoperative complications | |||
| Nausea | 11 (12) | 17 (18.5) | 0.305 |
| Pain | 3 (3.3) | 0 (0) | 0.246 |
| Ileus | 8 (8.7) | 13 (14.1) | 0.354 |
| Wound infection | 5 (5.4) | 2 (2.2) | 0.444 |
| Intraluminal bleeding | 3 (3.3) | 8 (8.7) | 0.212 |
| Extra-luminal bleeding | 1 (1.1) | 0 (0) | 1.000 |
| Anastomotic leakage | 5 (5.4) | 5 (5.4) | 1.000 |
| ICU | 4 (4.3) | 1 (1.1) | 0.368 |
| Death | 1 (1.1) | 1 (1.1) | 1.000 |
| Clavien–Dindo | |||
| I | 22 (23.9) | 20 (21.7) | 0.123 |
| II | 1 (1.1) | 4 (4.3) | 0.368 |
| III | 2 (2.2) | 4 (4.3) | 0.689 |
| IV | 3 (3.3) | 0 (0) | 0.311 |
| V | 1 (1.1) | 1 (1.1) | 1.000 |
| Recovery outcomes | |||
| Time to first mobilization (hrs) | 25 ± 13 | 27 ± 26 | 0.745 |
| Time to first flatus (hrs) | 27 ± 26 | 25 ± 12 | 0.241 |
| Time to first stool (hrs) | 70 ± 35 | 93 ± 35 | < 0.0001 |
| Time to tolerance to liquid diet (hrs) | 39 ± 23 | 41 ± 25 | 0.241 |
| Time to tolerance to solid diet (hrs) | 72 ± 60 | 89 ± 46 | 0.06 |
| Length of hospital stay (days) | 8 ± 6 | 7 ± 4 | 0.817 |
| Oncologic outcomes | |||
| Length of the extracted specimen (cm) | 29.3 ± 11.2 | 24.2 ± 9 | 0.002 |
| Number of harvested nodes (cm) | 24.8 ± 11.3 | 19 ± 8 | < 0.0001 |
Continuous variables are expressed as mean ± standard deviation, values are expressed as number and (percentage)
P-value considered significant if p < 0.05
ICU Intensive Care Unit, hrs hours, cm centimetres, n number of patients in the group
Discussion
Minimally invasive right hemicolectomy can be considered nowadays as the gold standard procedure for the treatment of right colon cancer [9, 12, 13]. As known, after right colectomy the anastomosis could be performed in two different ways: in a totally intracorporeal way (totally laparoscopic intracorporeal right colectomy or laparoscopic right colectomy with intracorporeal anastomosis) or in a laparoscopic-assisted way (laparoscopic-assisted right colectomy or laparoscopic right colectomy with extracorporeal anastomosis).
Furthermore, several studies have demonstrated the superiority of the intracorporeal anastomosis over the extracorporeal reconstruction, in terms of safety and efficacy [7–13]. Similarly, several meta-analyses have shown that intracorporeal anastomosis is associated with similar postoperative outcomes over extracorporeal approach, but with significantly faster recovery, in terms of length of stay and bowel movements [6, 25–31].
However, despite these evidences, extracorporeal anastomosis continued to be performed by a large number of surgeons, probably because of the technical challenges of the intracorporeal anastomosis [32].
Furthermore, despite current data on intracorporeal anastomosis on the general population are clearly in favour of intracorporeal approach, data on the totally intracorporeal anastomosis in certain unfavourable condition, i.e. morbid obesity, are scarce and anecdotal [15, 16, 33, 34].
In 2006, Raftopoulos et al. [15] demonstrated on 45 patients, which of 13 obese, that totally laparoscopic right colectomy was safe and effective and that obesity had no effect on operative time, incision length, estimated blood loss, complications and length of hospital stay. The authors concluded that this technique had equally successful outcomes in both thin and obese patients.
In 2016, Keller et al. [16] performed a case-matched study on the adoption on single-incision laparoscopic colectomy, comparing 80 obese and 80 non-obese patients. Results showed no differences in terms of conversion rates, length of stay, complications and readmission, demonstrating that single-incision laparoscopic colectomy is safe and feasible even in the obese population.
The advantages of an intracorporeal anastomosis after right colectomy in the obese population were demonstrated by Vignali et al. [33] in their case-matched analysis on 128 patients, 64 who underwent intracorporeal anastomosis and 64 who underwent extracorporeal anastomosis. Intra-corporeal and extracorporeal anastomosis were associated with similar conversion rate, overall 30 day mortality and anastomotic leakage, while intra-corporeal anastomosis was associated with shorter recovery of bowel function, although no differences were observed in terms of length of hospital stay.
More recently, Lendzion et al. [34] proposed in 11 obese the adoption of the intra-corporeal anastomosis and the specimen extraction through natural orifice (vagina or anus). Registering only one seroma and one wound infection as postoperative complications, the authors concluded that intra-corporeal anastomosis with natural orifice specimen extraction is a good alternative in the obese patients.
According to the current literature, the intra-corporeal anastomosis seems to be feasible and safe even in the obese population. However, because of the paucity of the current data, we decided to perform this case-matched comparison to confirm the feasibility and safety of the intra-corporeal anastomosis.
In our study the obesity had no impact on the postoperative, recovery and oncologic outcomes.
First, our results confirmed the safety of the intracorporeal anastomosis in the obese population.
In fact, despite the morbid obesity could be considered a risk factor for intraoperative technical challenges, no intraoperative complications occurred in both groups, as well as the need for conversions. As expected the extraction incision was longer in the obese patients group, probably due to the rate of fatty tissue surrounding the colonic stump. Of interest, a significant difference was found between the two groups (p = 0.006), being a midline incision was preferred in the non-obese group. These data are in contradiction with the current literature, which demonstrated a higher rate of incisional rate in case of midline laparotomy [35]. However, this result is not dependent by any specific reason, but only by surgeons’ habits.
In terms of postoperative complications there were no differences in the two groups, i.e. postoperative nausea (p = 0.305), pain (p = 0.246), ileus (p = 0.354), wound infection (p = 0.444), intra- and extra-luminal bleeding (p = 0.212 and p = 1.000, respectively), anastomotic leakage (p = 1.000), the need of ICU (p = 0.368) and postoperative mortality (p = 1.000).
Even the oncologic radicality is ensured in the obese population. In fact, in both groups, the number of harvested nodes was higher than the threshold of a correct oncologic resection. Of interest, the length of colonic specimen was significantly longer in the obese group (obese 29.3 ± 11.2 cm vs non-obese 24.2 ± 9 cm, p = 0.002) and there is a paradoxical higher number of harvested nodes in the obese group (obese 24.8 ± 11.3 cm vs non-obese 19 ± 8 cm, p < 0.0001), strengthening the oncologic efficacy of a totally minimally invasive approach in these patients.
About this aspect, there are no evidences in the current literature. The longer extracted specimen, as well as the number of harvested nodes could be casual, but it could be interesting to perform further studies to confirm a correlation between obesity and colonic length or an augmented number of lymph nodes surrounding the visceral vessels.
Additionally, our results confirmed that this technique should be considered effective even in the obese patients. In fact, the comparison between the two groups demonstrated that no significance differences were found in terms of postoperative recovery outcomes, expressed as time to first mobilization (obese 25 ± 13 h vs non-obese 27 ± 26, p = 0.745), time to first flatus (obese 27 ± 26 h vs non-obese 25 ± 12 h, p = 0.241) time to tolerance to liquid and solid diet (obese 39 ± 23 h vs non-obese 41 ± 25 h, p = 0.241 and obese: 72 ± 60 h vs non-obese 89 ± 46 h, p = 0.06) and length of hospital stay (obese 8 ± 6 days vs non-obese 7 ± 4 days, p = 0.817).
Although the encouraging results, some limitation of this study should be addressed.
First, the retrospective design has some intrinsic inherent bias. Then, the small sample size could not ensure definitive conclusions. Finally, although several measurements have been proposed to mark technical challenges during colorectal procedures in obese patients (abdominal fat ratio, waist circumference and waist-to-hip ratio), the retrievable data obtained from our database were only on BMI. Thus, this represents an important selection bias.
Nevertheless, the results obtained by our study should be considered as a stimulus to apply a totally minimally invasive approach to right hemicolectomy even in the obese population.
However, data in the current literature remain scarce. For this reason, further high quality studies should be proposed to confirm these favourable outcomes.
Author contributions
Conceptualization: MM; methodology: MM, MM, GDDP, MM; formal analysis and investigation: all Authors; writing—original draft preparation: MM, MM; writing—review and editing: MM, MM, GDDP, MM; supervision: GDDP, MM; final approval: all authors.
Funding
Open access funding provided by Università degli Studi di Napoli Federico II within the CRUI-CARE Agreement. None.
Availability of data and material
data are available to the corresponding Author.
Declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Consent to participate
Informed consent was obtained from all participants included in the study.
Consent for publication
Informed consent for publication was obtained from all participants included in the study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Milone M, Lupoli R, Maietta P, et al. Lipid profile changes in patients undergoing bariatric surgery: a comparative study between sleeve gastrectomy and mini-gastric bypass. Int J Surg. 2015;14:28–32. doi: 10.1016/J.IJSU.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 2.Musella M, Apers J, Rheinwalt K, et al. Efficacy of bariatric surgery in type 2 Diabetes mellitus remission: the role of mini gastric bypass/one anastomosis gastric bypass and sleeve gastrectomy at 1 year of follow-up. A European survey. Obes Surg. 2016;26:933–940. doi: 10.1007/S11695-015-1865-6. [DOI] [PubMed] [Google Scholar]
- 3.Velotti N, Elisa De Palma FD, Sosa Fernandez LM, et al. Effect of bariatric surgery on in vitro fertilization in infertile men with obesity. Surg Obes Relat Dis. 2021;17:1752–1759. doi: 10.1016/j.soard.2021.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Milone M, De Placido G, Musella M, et al. Incidence of successful pregnancy after weight loss interventions in infertile women: a systematic review and meta-analysis of the literature. Obes Surg. 2016;26:443–451. doi: 10.1007/s11695-015-1998-7. [DOI] [PubMed] [Google Scholar]
- 5.Ciccioriccio MC, Iossa A, Boru CE, et al. Colorectal cancer after bariatric surgery (Cric-Abs 2020): Sicob (Italian society of obesity surgery) endorsed national survey. Int J Obes (Lond) 2021;45:2527–2531. doi: 10.1038/S41366-021-00910-6. [DOI] [PubMed] [Google Scholar]
- 6.Carnuccio P, Jimeno J, Parés D. Laparoscopic right colectomy: a systematic review and meta-analysis of observational studies comparing two types of anastomosis. Tech Coloproctol. 2014;18:5–12. doi: 10.1007/S10151-013-1029-4. [DOI] [PubMed] [Google Scholar]
- 7.Milone M, Elmore U, Di Salvo E, et al. Intracorporeal versus extracorporeal anastomosis. Results from a multicentre comparative study on 512 right-sided colorectal cancers. Surg Endosc. 2015;29:2314–2320. doi: 10.1007/s00464-014-3950-7. [DOI] [PubMed] [Google Scholar]
- 8.Scatizzi M, Kröning KC, Borrelli A, et al. Extracorporeal versus intracorporeal anastomosis after laparoscopic right colectomy for cancer: a case-control study. World J Surg. 2010;34:2902–2908. doi: 10.1007/s00268-010-0743-6. [DOI] [PubMed] [Google Scholar]
- 9.Zhang M, Lu Z, Zheng Z, et al. Comparison of short-term outcomes between totally laparoscopic right colectomy and laparoscopic-assisted right colectomy: a retrospective study in a single institution on 300 consecutive patients. Surg Endosc. 2022;36:176–184. doi: 10.1007/s00464-020-08252-6. [DOI] [PubMed] [Google Scholar]
- 10.Kornmann VNN, Hagendoorn J, Van Koeverden S, et al. Totally laparoscopic right hemicolectomy with intracorporeal anastomosis is a technically and oncologically safe procedure. Acta Chir Belg. 2013;113:439–443. doi: 10.1080/00015458.2013.11680960. [DOI] [PubMed] [Google Scholar]
- 11.Magistro C, Di LS, Ferrari G, et al. Totally laparoscopic versus laparoscopic-assisted right colectomy for colon cancer: is there any advantage in short-term outcomes? A prospective comparative assessment in our center. Surg Endosc. 2013;27:2613–2618. doi: 10.1007/S00464-013-2799-5. [DOI] [PubMed] [Google Scholar]
- 12.Milone M, Desiderio A, Velotti N, et al. Surgical stress and metabolic response after totally laparoscopic right colectomy. Sci Rep. 2021 doi: 10.1038/s41598-021-89183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veneroni S, Palini GM, Pirrera B, et al. Intracorporeal anastomosis versus extracorporeal anastomosis after laparoscopic right hemicolectomy for colon cancer: morbidity comparison at long-term follow-up. Minerva Surg. 2022 doi: 10.23736/s2724-5691.22.09281-4. [DOI] [PubMed] [Google Scholar]
- 14.Iorio T, Blumberg D. Laparoscopic colectomy is feasible in the mega-obese patient using a standardized technique. Surg Obes Relat Dis. 2014;10:1005–1008. doi: 10.1016/J.SOARD.2014.02.041. [DOI] [PubMed] [Google Scholar]
- 15.Raftopoulos I, Courcoulas AP, Blumberg D. Should completely intracorporeal anastomosis be considered in obese patients who undergo laparoscopic colectomy for benign or malignant disease of the colon? Surgery. 2006;140:675–683. doi: 10.1016/j.surg.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Keller DS, Ibarra S, Flores-Gonzalez JR, et al. Outcomes for single-incision laparoscopic colectomy surgery in obese patients: a case-matched study. Surg Endosc. 2016;30:739–744. doi: 10.1007/s00464-015-4268-9. [DOI] [PubMed] [Google Scholar]
- 17.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008 doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Mroczkowski P, Kube R, Ptok H, et al. Low-volume centre vs high-volume: the role of a quality assurance programme in colon cancer surgery. Colorectal Dis. 2011 doi: 10.1111/J.1463-1318.2011.02680.X. [DOI] [PubMed] [Google Scholar]
- 19.Minno M, Milone M, Mastronardi P, et al. Perioperative handling of antiplatelet drugs. A critical appraisal. Curr Drug Targets. 2013;14:880–888. doi: 10.2174/1389450111314080008. [DOI] [PubMed] [Google Scholar]
- 20.Milone M, Elmore U, Allaix ME, et al. Fashioning enterotomy closure after totally laparoscopic ileocolic anastomosis for right colon cancer: a multicenter experience. Surg Endosc. 2020;34:557–563. doi: 10.1007/S00464-019-06796-W. [DOI] [PubMed] [Google Scholar]
- 21.Nygren J, Thacker J, Carli F, et al. Guidelines for perioperative care in elective rectal/pelvic surgery: enhanced recovery after surgery (ERAS®) society recommendations. World J Surg. 2012;372(37):285–305. doi: 10.1007/S00268-012-1787-6. [DOI] [PubMed] [Google Scholar]
- 22.Gustafsson UO, Scott MJ, Schwenk W, et al. Guidelines for perioperative care in elective colonic surgery: enhanced recovery after surgery (ERAS®) society recommendations. World J Surg. 2013;37:259–284. doi: 10.1007/S00268-012-1772-0/TABLES/1. [DOI] [PubMed] [Google Scholar]
- 23.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205. doi: 10.1097/01.SLA.0000133083.54934.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rahbari NN, Weitz J, Hohenberger W, et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery. 2010;147:339–351. doi: 10.1016/j.surg.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Aiolfi A, Bona D, Guerrazzi G, et al. Intracorporeal versus extracorporeal anastomosis in laparoscopic right colectomy: an updated systematic review and cumulative meta-analysis. J Laparoendosc Adv Surg Tech. 2020;30:402–412. doi: 10.1089/lap.2019.0693. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H, Sun N, Fu Y, Zhao C. Intracorporeal versus extracorporeal anastomosis in laparoscopic right colectomy: updated meta-analysis of randomized controlled trials. BJS Open. 2021 doi: 10.1093/bjsopen/zrab133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milone M, Elmore U, Vignali A, et al. Recovery after intracorporeal anastomosis in laparoscopic right hemicolectomy: a systematic review and meta-analysis. Langenbeck’s Arch Surg. 2018 doi: 10.1007/S00423-017-1645-Y. [DOI] [PubMed] [Google Scholar]
- 28.Cirocchi R, Trastulli S, Farinella E, et al. Intracorporeal versus extracorporeal anastomosis during laparoscopic right hemicolectomy-Systematic review and meta-analysis. Surg Oncol. 2013;22:1–13. doi: 10.1016/j.suronc.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Zheng JC, Zhao S, Chen W, et al. Comparison of intracorporeal and extracorporeal anastomosis and resection in right colectomy: a systematic review and meta-analysis. Langenbeck’s Arch Surg. 2021;406:1789–1801. doi: 10.1007/s00423-021-02235-4. [DOI] [PubMed] [Google Scholar]
- 30.Wu Q, Jin C, Hu T, et al. Intracorporeal versus extracorporeal anastomosis in laparoscopic right colectomy: a systematic review and meta-analysis. J Laparoendosc Adv Surg Tech. 2017;27:348–357. doi: 10.1089/lap.2016.0485. [DOI] [PubMed] [Google Scholar]
- 31.van Oostendorp S, Elfrink A, Borstlap W, et al. Intracorporeal versus extracorporeal anastomosis in right hemicolectomy: a systematic review and meta-analysis. Surg Endosc. 2017;31:64–77. doi: 10.1007/s00464-016-4982-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Taher M, Okamoto N, Mutter D, et al. International survey among surgeons on laparoscopic right hemicolectomy: the gap between guidelines and reality. Surg Endosc. 2022 doi: 10.1007/s00464-022-09044-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vignali A, De Nardi P, Ghirardelli L, et al. Short and long-term outcomes of laparoscopic colectomy in obese patients. World J Gastroenterol. 2013;19:7405–7411. doi: 10.3748/wjg.v19.i42.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lendzion RJ, Gilmore AJ. Laparoscopic right hemicolectomy with intracorporeal anastomosis and natural orifice surgery extraction/minimal extraction site surgery in the obese. ANZ J Surg. 2021;91:1180–1184. doi: 10.1111/ANS.16416. [DOI] [PubMed] [Google Scholar]
- 35.Pereira JA, Pera M, López-Cano M, et al. Hernias at the extraction incision after laparoscopic colon and rectal resection: Influence of incision location and use of prophylactic mesh. Cir Esp. 2019;97:20–26. doi: 10.1016/J.CIRESP.2018.08.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
data are available to the corresponding Author.