Abstract
Background
To explore the efficiency of ectopic thymectomy by the three surgical approaches of trans-sternum, right unilateral thoracoscopy and thoracoscopic subxiphoid in patients with non-thymomatous myasthenia gravis.
Methods
155 consecutive non-thymomatous myasthenia gravis patients who underwent extended thymectomy by 3 approaches including trans-sternum, right unilateral thoracoscopy and thoracoscopic subxiphoid in 1st affiliated hospital of Sun Yat-Sen University from January 2017 to October 2019 were reviewed. Differences of perioperative clinical characteristics in three surgical approaches were analyzed.
Results
Time to onset of myasthenia gravis (early or late) (p = 0.018), blood loss (p < 0.001), duration of operation (p = 0.031), duration and volume of thoracic drainage (p = 0.039 and p = 0.026), length of hospitalization (p = 0.039), the efficiency of ectopic thymectomy (p = 0.037), and the detection rate of ectopic thymus in the second quadrant (p = 0.018) were different among the three surgical approaches. In univariate logistic regression analysis, higher efficiency of ectopic thymectomy were associated with transsternal (OR 2.36, 95% CI 1.32–4.22, p = 0.011) and thoracoscopic subxiphoid approaches (OR 2.07, 95% CI 1.12–3.82, p = 0.033). In the multiple logistic regression analysis, the transsternal approach (OR 2.02, 95% CI 1.10–3.71, p = 0.024) was an independent protective factor for the efficiency of ectopic thymectomy.
Conclusions
Both the right unilateral thoracoscopic and thoracoscopic subxiphoid approaches have advantages over the transsternal approach in short-term postoperative recovery. Transsternal approach is still the best choice for ectopic thymectomy while thoracoscopic subxiphoid approach show the potential as an alternative way.
Keywords: Myasthenia gravis, Surgical approaches, Ectopic thymectomy
Introduction
Myasthenia gravis (MG) is an autoimmune disease mediated by antibodies and complement, involving the neuro-muscular junction acetylcholine receptors (AChRs) on the postsynaptic membrane [1]. Extended thymectomy which defined as thymectomy including adipose tissue clearance in anterior mediastinum has been considered as standard surgical treatment for MG [2]. The purpose of removing adipose tissue is to remove the ectopic thymus (ET), as residue of ET is thought to be contributed to poor response to surgical treatment [3].
Transsternal approach has been applied in extended thymectomy for decades [4]. With introduction of video-assisted thoracic surgery technique, thoracoscopic mini-invasive approaches have been chosen as alternative ways for extended thymectomy. However, whether the use of thoracoscopic approach, especially its ability to remove ET, can be as effective as the transsternal one is still controversial. Therefore, we retrospectively analyzed clinical characteristics and the efficiency of ectopic thymectomy in non-thymomatous MG patients who underwent extended thymectomy by three different surgical approaches (trans-sternum, right unilateral thoracoscopy and thoracoscopic subxiphoid).
Patients and methods
The study protocol was approved by the Ethics Review Board of the First Affiliated Hospital of Sun Yat-Sen University and conformed to the Declaration of Helsinki.
155 MG patients who underwent extended thymectomy at the First Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China, from January 2017 to October 2019 were reviewed. All patients underwent extended thymectomy in approaches of trans-sternum (TS), right unilateral thoracoscopy (RUT) or thoracoscopic subxiphoid (TSX). The surgeries were performed by three sophisticated surgeons. Doctor Zou performed TS, RUT and TSX in 18, 16, and 23 patients. Doctor Chen performed the three approaches in 22, 13 and 16 patients while Doctor Su performed those in 12, 21 and 14 cases, respectively. The differences of approaches chosen among three doctors was not significant (not shown in the article). The basic surgical procedure included complete resection of the thymus and clearance of adipose tissue in the anterior mediastinum. The range of adipose tissue clearance included bilateral to the phrenic nerve, up to the root of neck, and down to both sides of the cardio-diaphragmatic angle [5].
Clinical characteristics of age at surgery, sex, Myasthenia Gravis Foundation of America (MGFA) stage, pyridostigmine dose, time from disease onset to surgery, preoperative and postoperative myasthenic crisis, AchRs level, preoperative and postoperative immunoglobulin treatment, preoperative and postoperative plasmapheresis, perioperative applications of corticosteroid, surgical approaches, blood loss, operation time, length and volume of thoracic drainage, ratio of thymic parenchyma, incidence of ET removal, distribution of ET, time of hospitalization and postoperative complications were collected.
In this study, we simplified the ET distribution area into four quadrants using the anterior midline and the horizontal line at the start of the ascending aorta (Fig. 1). Adipose tissues removed in the quadrants were placed in buffered 10% formalin and labelled. They were then sliced and stained using haematoxilin and eosin. Final specimens were independently examined by two pathologists. ET was diagnosed as the un-encapsulated lobules of thymus, microscopic foci of thymic tissue or the Hassals corpuscles in adipose tissues [6]. Ratio of thymic parenchyma, which defined as volume of thymic parenchyma comparing to that of adipose tissue in the thymus, was also assessed by the pathologists. According to the ratio of thymic parenchyma, we categorized the patients into three groups (“< 40%”, “≥ 40% and “< 70%” and “≥ 70%”).
Fig. 1.
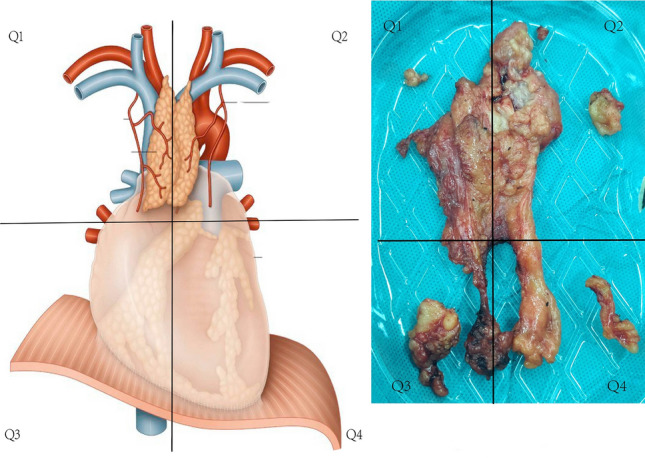
Four quadrants of anterior mediastinum and root of the neck which were separated by the anterior midline and the horizontal line at the start of the ascending aorta. Q1: Upper mediastinum and root of neck on the right; Q2: Upper mediastinum and root of neck on the left; Q3: Lower mediastinum and cardiophrenic angle on the right; Q4: Lower mediastinum and cardiophrenic angle on the left
Preoperative and postoperative myasthenic crisis which were defined as the complication characterized by worsening of muscle weakness resulting in respiratory failure presented within 1 month before surgery and 2 weeks after surgery. The application of Immunoglobin and plasmapheresis within 1 week before and 2 weeks after surgery was considered as perioperative treatment.
The median follow-up time was 27 months. According to MG symptoms, the patients were categorized into complete remission (CR), partial remission (PR), unchanging (UC) and progression disease (PD). The assessment should be performed and agreed by both the surgeon and neurologist.
Statistical analysis
Quantitative data were reported as mean ± standard deviation (SD) and compared by one-way ANOVA test. Qualitative data were reported as frequency (ratio) and compared by chi-square test or Fisher’s exact test. Univariate and multivariate logistic regression analysis (binomial) were applied to identify association between clinical factors and the efficiency of ectopic thymectomy. A p value ≤ 0.05 was considered significant. All statistical analyses were performed using IBM SPSS Statistics, version 22 (IBM Corp, Armonk, NY, USA).
Results
The study included a total of 155 patients, with 60.7% female and 39.3% male. Mean age at surgery was 27.3 ± 9.29 years old, and juvenile patients (< 18 years old) were accounted for 12.9%.
Depending on MGFA stage, 1 case (0.7%), 43 cases (27.7%), 40 cases (25.8%), 38 cases (24.5%), 27 cases (17.4%), 4 cases (2.6%) and 2 cases (1.2%) were at stage I, IIA, IIB, IIIA, IIIB, IVA and IVB, respectively. All patients were positive for AchRs antibodies. 11 patients (7.1%) experienced postoperative myasthenic crisis. The mean time from disease onset to surgery was 18.1 ± 6.20 months. All patients were treated with pyridostigmine (225.68 ± 53.22 mg/day); 113 (72.9%) cases were perioperatively treated with glucocorticoid. Before surgery, 31 (20.0%) and 13 (8.4%) were treated with immunoglobulin and plasmapheresis respectively.
Surgical approaches of TS, RUT and TSX were performed in 57 (36.8%), 51 (32.9%) and 47 (30.3%) patients, respectively. Mean volume of blood loss was 103.3 ± 63.04 ml and mean time of operation was 146.3 ± 62.41 min. Mean length and volume of thoracic drainage were 2.63 ± 1.22 days and 269.93 ± 146.32 ml, respectively.
ET were found in 64 (41.3%) cases. ET appeared in Q1, Q2, Q3 and Q4 quadrants were 26 (40.0%), 36 (55.4%), 22 (33.4%) and 29 (44.6%), respectively. Ratio of thymic parenchyma of “40%”, “ > = 40% and < 70%” and “ > = 70%” were found in 72 (46.5%), 71 (45.8%) and 12 (7.7%) patients.
Postoperatively, 29 (18.7%) cases were treated with immunoglobin and 6 (3.9%) cases underwent plasmapheresis. 22 cases (8.2%) experienced postoperative myasthenic crisis. Mean time of hospitalization was 9.61 ± 3.91 days. 31 (20%) patients had postoperative complications, among them 13 (41.9%) were surgical incision infection, 14 (45.2%) were pneumonia and 9 (29.0%) were mediastinitis.
The follow-up data showed the patients with CR, PR, UC and PD were 9 (5.8%), 100 (64.5%), 36 (23.2%) and 10 (6.5%), respectively (Table 1).
Table 1.
Clinical and pathological characteristics of MG patients underwent extended thymectomy
| Characteristic | N | % |
|---|---|---|
| Number of patients | 155 | 100% |
| Age, years, mean ± SD | 27.33 ± 9.29 | |
| Juvenile (< 18 years) | 20 | 12.9% |
| Adults (>= 18 years) | 135 | 87.1% |
| MG onset | ||
| Early (< 40 years) | 138 | 89.0% |
| Late (>= 40 years) | 17 | 11.0% |
| Sex | ||
| Male | 61 | 39.4% |
| Female | 94 | 60.7% |
| MGFA stage | ||
| I | 1 | 0.7% |
| IIA | 43 | 27.7% |
| IIB | 40 | 25.8% |
| IIIA | 38 | 24.5% |
| IIIB | 27 | 17.4% |
| IVA | 4 | 2.6% |
| IVB | 2 | 1.3% |
| AchR level | ||
| Positive | 155 | 100.0% |
| Negative | 0 | 0.0% |
| Pulmonary function | ||
| FEV1 (mean ± SD) | 66.5% ± 8.8% | |
| FVC (mean ± SD) | 74.2% ± 10.3% | |
| Preoperative myasthenic crisis | ||
| Positive | 11 | 7.1% |
| Negative | 144 | 92.9% |
| Time between disease onset and surgery (months) | ||
| Mean ± SD | 18.81 ± 6.20 | |
| Pyridostigmine dose (mg) | ||
| Mean ± SD | 225.68 ± 53.22 | |
| Perioperative glucocorticoid usage | ||
| Positive | 113 | 72.9% |
| Negative | 42 | 27.1% |
| Preoperative immunoglobin usage | ||
| Positive | 31 | 20.0% |
| Negative | 124 | 80.0% |
| Preoperative plasmapheresis | ||
| Positive | 13 | 8.4% |
| Negative | 142 | 91.6% |
| Surgical approach | ||
| TS | 57 | 36.8% |
| RUT | 51 | 32.9% |
| TSX | 47 | 30.3% |
| Blood loss | ||
| Mean ± SD | 103.30 ± 63.04 | |
| < 50 ml | 33 | 21.3% |
| 50–100 ml | 41 | 26.5% |
| > 100 ml | 81 | 52.2% |
| Operation duration (min) | ||
| Mean ± SD | 146.30 ± 62.41 | |
| < 60 min | 5 | 3.2% |
| 60–120 min | 71 | 45.8% |
| > 120 min | 79 | 51.0% |
| Length of chest tube drainage (days) | ||
| Mean ± SD | 2.63 ± 1.22 | |
| Volume of chest tube drainage (ml) | ||
| Mean ± SD | 269.93 ± 146.32 | |
| ETT | ||
| Positive | 64 | 41.3% |
| Negative | 91 | 58.7% |
| Distribution of ETT | ||
| Q1 | 26 | 40.0% |
| Q2 | 36 | 55.4% |
| Q3 | 22 | 33.9% |
| Q4 | 29 | 44.6% |
| Ratio of thymus parenchyma | ||
| < 40% | 72 | 46.5% |
| >= 40% and < 70% | 71 | 45.8% |
| >= 70% | 12 | 7.7% |
| Postoperative immunoglobin usage | ||
| Positive | 29 | 18.7% |
| Negative | 126 | 81.3% |
| Postoperative plasmapheresis | ||
| Positive | 6 | 3.9% |
| Negative | 149 | 96.1% |
| Postoperative myasthenic crisis | ||
| Positive | 22 | 8.2% |
| Negative | 133 | 91.8% |
| Length of hospital stay (days) | ||
| Mean ± SD | 9.61 ± 3.91 | |
| Postoperative complications | ||
| Surgical incision infection | 13 | 41.9% |
| Pneumonia | 14 | 45.2% |
| Mediastinitis | 9 | 29.0% |
| None | 124 | 80.0% |
| Prognosis | ||
| CR | 9 | 5.8% |
| PR | 100 | 64.5% |
| UC | 36 | 23.2% |
| PD | 10 | 6.5% |
MG myasthenia gravis, MGFA Myasthenia Gravis Foundation of America, TS transsternal, RUT right unilateral thoracoscopy, TSX thoracoscopic subxiphoid, ETT ectopic thymic tissue, CR complete remission, PR partial remission, UC unchanging, PD progression disease
Comparison of clinical characteristics in the three surgical approaches groups (TS vs. RUT vs. TSX) showed volume of blood loss (145.3 ml vs. 87.1 ml vs. 70 ml, p < 0.001), operation time (159.5 min vs. 128.4 min vs. 149.8 min, p = 0.031), length of thoracic drainage (2.9 days vs. 2.5 days vs. 2.4 days, p = 0.047), volume of thoracic drainage (306.7 ml vs. 266.3 ml vs. 229.3 ml, p = 0.026), incidence of ET removal (10 (43.5%) vs. 13 (25.5%) vs. 20 (42.5%), p = 0.037), probability of ET found in Q2 quadrant (18 (64.3%) vs. 3 (23.1%) vs. 13 (65.0%), p = 0.032) and time of hospitalization (10.51 days vs. 9.57 days vs. 8.55 days, p = 0.039) were significantly different.
In TS group, the patients with CR, PR, UC and PD were 4 (7%), 38 (66.7%), 12 (21%) and 3 (5.3%). In RUT group, the patients with CR, PR, UC and PD were 2 (3.9%), 32 (62.6%), 14 (27.5%) and 3 (6%). In TSX group, the patients with CR, PR, UC and PD were 3 (6.4%), 30 (63.8%), 10 (21.3%) and 4 (8.5%), respectively. The differences of prognosis among three surgical approaches were not obvious statistically (Table 2).
Table 2.
Comparison of clinical characteristics in three surgical approaches
| Variables | TS | RUT | TSX | p values |
|---|---|---|---|---|
| Age | ||||
| Mean ± SD | 28.40 ± 10.24 | 28.35 ± 9.98 | 24.91 ± 6.67 | 0.102 |
| Juvenile (< 18) | 5 (8.8%) | 9 (17.7%) | 6 (12.8%) | 0.389 |
| Adults (>= 18) | 52 (91.2%) | 42 (82.3%) | 41 (87.2%) | |
| Sex | ||||
| Male | 25 (43.9%) | 22 (43.1%) | 14 (30.0%) | 0.273 |
| Female | 32 (56.1%) | 29 (56.9%) | 35 (70.0%) | |
| MG onset | ||||
| Early (< 40 years) | 51 (89.5%) | 41 (80.4%) | 46 (97.9%) | 0.018 |
| Late (>= 40 years) | 6 (10.5%) | 10 (19.6%) | 1 (2.13%) | |
| MGFA stage | ||||
| I | 0 (0%) | 0 (0%) | 1 (2.13%) | 0.408 |
| IIA | 17 (29.5%) | 13 (25.5%) | 13 (27.7%) | |
| IIB | 9 (15.8%) | 19 (37.3%) | 12 (25.5%) | |
| IIIA | 17 (29.5%) | 10 (19.6%) | 11 (23.4%) | |
| IIIB | 11 (19.3%) | 7 (13.7%) | 9 (19.2%) | |
| IVA | 1 (1.7%) | 2 (3.9%) | 1 (2.13%) | |
| IVB | 2 (3.5%) | 0 (0%) | 0 (0%) | |
| Pyridostigmine dose (mg) | ||||
| Mean ± SD | 217.89 ± 55.99 | 224.12 ± 52.08 | 236.81 ± 50.13 | 0.191 |
| Time between disease onset and surgery (months) | ||||
| Mean ± SD | 18.96 ± 5.68 | 19.22 ± 5.08 | 18.17 ± 7.80 | 0.814 |
| Pulmonary function (%) | ||||
| FEV1 (mean ± SD) | 67.66 ± 7.82 | 65.50 ± 11.06 | 66.32 ± 6.97 | 0.439 |
| FVC (mean ± SD) | 76.02 ± 9.13 | 72.43 ± 12.59 | 73.77 ± 8.58 | 0.185 |
| Preoperative myasthenic crisis | ||||
| Positive | 4 (7%) | 5 (9.8%) | 2 (4.3%) | 0.628 |
| Negative | 53 (93%) | 46 90.2%) | 45 (95.7%) | |
| Preoperative immunoglobin treatment | ||||
| Positive | 16 (28.1%) | 9 (17.7%) | 6 (12.8%) | 0.133 |
| Negative | 41 (71.9%) | 42 (82.3%) | 41 (87.2%) | |
| Preoperative plasmapheresis | ||||
| Positive | 6 (10.5%) | 5 (9.8%) | 2 (4.3%) | 0.512 |
| Negative | 51 (89.5%) | 46 (90.2%) | 45 (95.7%) | |
| Perioperative glucocorticoid usage | ||||
| Positive | 40 (70.2%) | 37 (72.5%) | 36 (76.6%) | 0.762 |
| Negative | 17 (29.8%) | 14 (27.5%) | 11 (23.4%) | |
| Blood loss (ml) | ||||
| Mean ± SD | 145.79 ± 57.57 | 86.47 ± 55.67 | 70 ± 47.09 | < 0.001 |
| < 50 ml | 1 (1.7%) | 13 (25.5%) | 19 (40.4%) | < 0.001 |
| 50–100 ml | 11 (19.3%) | 17 (33.3%) | 13 (27.7%) | |
| > 100 ml | 15 (79%) | 21 (41.2%) | 15 (31.9%) | |
| Operation duration (min) | ||||
| Mean ± SD | 159.5 ± 61.05 | 128.43 ± 56.79 | 149.78 ± 66.55 | 0.031 |
| < 60 min | 0 (0%) | 2 (3.9%) | 3 (6.4%) | 0.146 |
| 60–120 min | 24 (42.1%) | 28 (54.9%) | 19 (40.4%) | |
| > 120 min | 33 (57.9%) | 21 (41.2%) | 25 (53.2%) | |
| Length of chest tube drainage (days) | ||||
| Mean ± SD | 2.95 ± 1.38 | 2.49 ± 1.05 | 2.38 ± 1.13 | 0.039 |
| Volume of chest tube drainage (ml) | ||||
| Mean ± SD | 311.75 ± 140.71 | 260.59 ± 135.93 | 229.36 ± 153.43 | 0.026 |
| Ratio of thymus parenchyma | ||||
| < 40% | 33 (57.9%) | 18 (35.3%) | 21 (44.7%) | 0.149 |
| >= 40% and < 70% | 22 (38.6%) | 27 (52.9%) | 22 (46.8%) | |
| >= 70% | 2 (3.5%) | 6 (11.76%) | 4 (8.5%) | |
| ETT | ||||
| Positive | 28 (49.1%) | 11 (25.5%) | 22 (46.8%) | 0.005 |
| Negative | 29 (50.9%) | 38 (74.5%) | 26 (53.2%) | |
| Distribution of ETT | ||||
| Q1 | 12 (41.4%) | 8 (57.1%) | 6 (27.3%) | 0.200 |
| Q2 | 19 (65.5%) | 3 (21.4%) | 13 (63.6%) | 0.018 |
| Q3 | 9 (31.0%) | 7 (50.0%) | 5 (27.27%) | 0.340 |
| Q4 | 12 (41.4%) | 6 (42.9%) | 11 (50.0%) | 0.819 |
| Postoperative immunoglobin usage | ||||
| Positive | 13 (22.8%) | 9 (17.7%) | 7 (14.9%) | 0.572 |
| Negative | 44 (77.2%) | 42 (82.3%) | 40 (85.1%) | |
| Postoperative plasmapheresis | ||||
| Positive | 2 (3.5%) | 2 (3.9%) | 2 (4.3%) | 1.00 |
| Negative | 55 (96.5%) | 49 (96.1%) | 45 (95.7%) | |
| Postoperative myasthenic crisis | ||||
| Positive | 6 (10.5%) | 9 (17.7%) | 7 (14.9%) | 0.563 |
| Negative | 51 (89.5%) | 42 (82.3%) | 40 (85.1%) | |
| Length of hospital stay (days) | ||||
| Mean ± SD | 10.61 ± 3.93 | 9.45 ± 4.12 | 8.55 ± 3.41 | 0.039 |
| Postoperative complications | ||||
| Surgical incision infection | 7 (12.3%) | 1 (2.0%) | 5 (10.6%) | 0.105 |
| Pneumonia | 5 (8.8%) | 6 (11.76%) | 3 (6.4%) | 0.687 |
| Mediastinitis | 6 (10.5%) | 1 (2.0%) | 2 (4.3%) | 0.154 |
| None | 42 (73.58%) | 44 (86.3%) | 38 (80.9%) | 0.26 |
| Prognosis | ||||
| CR | 4 (7%) | 2 (3.9%) | 3 (6.4%) | 0.667 |
| PR | 38 (66.7%) | 32 (62.6%) | 30 (63.8%) | |
| UC | 12 (21%) | 14 (27.5%) | 10 (21.3%) | |
| PD | 3 (5.3%) | 3 (6%) | 4 (8.5%) | |
MG myasthenia gravis, MGFA Myasthenia Gravis Foundation of America, TS transsternal, RUT right unilateral thoracoscopy, TSX thoracoscopic subxiphoid, ETT ectopic thymic tissue, CR complete remission, PR partial remission, UC unchanging, PD progression disease
Univariate logistics regression test (binomial) revealed surgical approaches of TS (OR 2.36, 95% CI 1.32–4.22, p = 0.011) and TSX (OR 2.07, 95% CI 1.12–3.82, p = 0.033) both had higher efficiency of ectopic thymectomy (Table 3). While multivariate logistics regression test (binomial) revealed only TS had higher efficiency of ectopic thymectomy (OR 2.02, 95% CI 1.10–3.71, p = 0.024) (Table 4).
Table 3.
Univariate Logistic Regression Analysis (Binomial) of Risk Factors associated with ETT Removal in Non-thymomatous MG Patients
| Variables | OR | 95% confidence interval | P-value |
|---|---|---|---|
| Sex | |||
| Male | 1.00 | ||
| Female | 1.33 | 0.88–2.00 | 0.175 |
| Age at surgery (years) | 0.99 | 0.97–1.02 | 0.603 |
| Juvenile or adult | |||
| Juvenile | 1.00 | ||
| Adult | 1.21 | 0.64–2.26 | 0.559 |
| MG onset | |||
| Early | 1.00 | ||
| Late | 0.69 | 0.32–1.47 | 0.336 |
| MGFA stage | |||
| Stage IA + IIA | 1.00 | ||
| Stage IIB | 0.72 | 0.41–1.24 | 0.233 |
| Stage IIIA | 1.04 | 0.65–1.66 | 0.862 |
| Stage IIIB + IVA + IVB | 0.87 | 0.51–1.48 | 0.599 |
| Preoperative Myasthenic Crisis | |||
| Yes | 1.00 | ||
| No | 1.55 | 0.58–4.15 | 0.380 |
| Time between disease onset and surgery (months) | 1.01 | 0.98–1.04 | 0.436 |
| FEV1 (%) | 1.00 | 0.98–1.01 | 0.751 |
| FVC (%) | 1.00 | 0.98–1.02 | 0.767 |
| Pyridostigmine dose (mg) | 1.00 | 0.99–1.00 | 0.967 |
| Perioperative glucocorticoid usage | |||
| No | 1.00 | ||
| Yes | 1.03 | 0.67–1.57 | 0.901 |
| Preoperative immunoglobin usage | |||
| Yes | 1.00 | ||
| No | 1.08 | 0.66–1.77 | 0.748 |
| Preoperative plasmapheresis | |||
| Yes | 1.00 | ||
| No | 0.88 | 0.48–1.64 | 0.699 |
| Surgical approach | |||
| TrUL | 1.00 | ||
| TS | 2.36 | 1.32–4.22 | 0.011 |
| TSX | 2.07 | 1.12–3.82 | 0.033 |
| Blood loss (ml) | 1.00 | 0.99–1.00 | 0.959 |
| Operation duration (min) | 1.00 | 0.99–1.00 | 0.402 |
| Ratio of thymus parenchyma | |||
| < 40% | 1.00 | ||
| > = 40% and < 70% | 0.83 | 0.59–1.22 | 0.347 |
| > = 70% | 0.73 | 0.31–1.68 | 0.457 |
ETT Ectopic thymic tissue, MG Myasthenia Gravis, MGFA Myasthenia Gravis Foundation of America, TS Transsternal, TrUL Thoracoscopic Right Unilateral, TSX Thoracoscopic Subxiphoid
Table 4.
Multivariate Logistic Regression Analysis (binomial) of Risk Factors associated with ETT Removal in Non-thymomatous MG Patients
| Variables | OR | 95% confidence interval | P-value |
|---|---|---|---|
| Sex | |||
| Male | 1.00 | ||
| Female | 1.27 | 0.81–1.89 | 0.327 |
| Age at surgery (years) | 0.99 | 0.96–1.02 | 0.622 |
| Juvenile or adult | |||
| Juvenile | 1.00 | ||
| Adult | 1.16 | 0.54–2.53 | 0.700 |
| MG onset | |||
| Early | 1.00 | ||
| Late | 0.99 | 0.40–2.46 | 0.979 |
| MGFA stage | |||
| Stage IA + IIA | 1.00 | ||
| Stage IIB | 0.86 | 0.49–1.50 | 0.588 |
| Stage IIIA | 1.04 | 0.66–1.64 | 0.867 |
| Stage IIIB + IVA + IVB | 0.88 | 0.51–1.51 | 0.643 |
| Preoperative Myasthenic Crisis | |||
| Yes | 1.00 | ||
| No | 1.2 | 0.40–3.42 | 0.731 |
| Time between disease onset and surgery (months) | 1.01 | 0.98–1.04 | 0.390 |
| Perioperative glucocorticoid usage | |||
| No | 1.00 | ||
| Yes | 0.96 | 0.63–1.48 | 0.861 |
| Preoperative immunoglobin usage | |||
| Yes | 1.00 | ||
| No | 1.11 | 0.69–1.80 | 0.660 |
| Preoperative plasmapheresis | |||
| Yes | 1.00 | ||
| No | 0.79 | 0.43–1.59 | 0.574 |
| Surgical approach | |||
| TrUL | 1.00 | ||
| TS | 2.02 | 1.10–3.71 | 0.024 |
| TSX | 1.67 | 0.95–2.93 | 0.076 |
| Blood loss (ml) | 1.00 | 0.99–1.00 | 0.405 |
| Operation duration (min) | 1.00 | 0.99–1.00 | 0.629 |
| Ratio of thymus parenchyma | |||
| < 40% | 1.00 | ||
| > = 40% and < 70% | 0.90 | 0.59–1.38 | 0.634 |
| > = 70% | 0.90 | 0.40–2.03 | 0.808 |
ETT Ectopic thymic tissue, MG Myasthenia Gravis, MGFA Myasthenia Gravis Foundation of America, TS Transsternal, TrUL Thoracoscopic Right Unilateral, TSX Thoracoscopic Subxiphoid
Discussion
Extended thymectomy is an effective treatment for MG [7]. Complete removal of thymus and adipose tissue in both anterior mediastinum and root of neck is critical of surgery [8]. Ectopic thymectomy is the purpose of adipose tissue clearance, as residual of ET is associated with poor response to extended thymectomy.
Due to excellent exposure in anterior mediastinum, TS has been widely accepted by thoracic surgeons and become the preferred surgical technique for MG treatment. However, patients undergoing thymectomy by TS have to suffer severe surgical wounds and pain. Therefore, it is of great value to find a suitable surgical technique that is as effective as TS but less invasive.
Since the introduction of thoracoscopic mini-invasive surgical technique, many studies have focused on thoracoscopic approaches applied in extended thymectomy [9–11]. Calvin S H Ng considered RUT as the better option because on one hand it had good vision on the junction of superior vena cava and the brachiocephalic vein, on the other hand, it was easier for right-handed surgeons performing thoracoscopic surgery to start at the inferior poles and work cephalad from the right side [12]. But Mineo advocated a left-sided approach and believed from the left the dissection maneuvers are safer, because the superior vena cava lies out of the surgical field, thus reducing risk of accidental injury [13]. In our opinion, Calvin S H Ng’s views is more acceptable, because on one hand, the good exposure conduces to avoid injury, on the other hand ET adjacent to the superior vena cava should not be ignored. However, when we applied RUT, for the better view of contralateral site, we usually needed to compress the heart which might cause arrhythmia or hypotension, even so the views of Q2 and Q4 quadrants were still poor. Chang Young Lee reported that bilateral thoracoscopic thymectomy has a surgical extent similar to that of transsternal approach with more favorable early surgical outcomes [14].
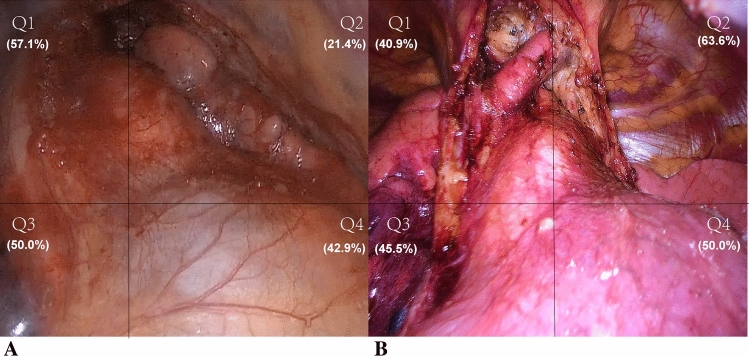
Extended thymectomy by TSX was first reported by C-P Hsu in 2002 [15]. He later reported a comparative study showed both RUT and TSX had satisfactory results for MG patients. Though C-P Hsu suggested TSX had better view of the bilateral pleural cavities, he did not mentioned the difference in ectopic thymectomy between RUT and TSX [16]. In this study, techniques of TSX (Three ports) was similar as the one described by Qiang Lu and colleagues in 2018 [17]. Though TSX spent longer operation time comparing with RUT, it had not only both the minimal volume of blood loss and thoracic drainage, but also the shortest time of thoracic drainage and hospitalization among three approaches. More importantly, surgical exposure of TSX was close to TS, especially the excellent view in both Q2 and Q4 quadrants, which was conducive to ectopic thymectomy comparing with RUT (Fig. 2).
Fig. 2.
A Incisions and view of surgery in right unilateral thoracoscopic approach. View of Q2 and Q4 quadrants was poor and the efficiency of ectopic thymectomy was 25.5%; B Incisions and view of surgery in thoracoscopic subxiphoid approach: View of Q2 and Q4 quadrants was better and the efficiency of ectopic thymectomy was 46.8%
ET in MG patients was first reported by Akira Masaoka [6]. Jaretzki III, a further described distribution of ET in 12 regions of anterior mediastinum [18]. Adverse impact of ET on effect of surgery for MG patients have been reported by many studies. Two phenomena support involvement of ET in the pathogenesis of MG. First, germinal centers which were considered being source of MG autoantibodies were also found in ET [19]. Second, several studies found that the presence of ET was significantly associated with poor response of thymectomy for MG [20–22]. Although some other studies have shown that there is no relationship between the discovery of ET and the effect of thymectomy in MG patients [13, 23], Zielinski and colleagues attributed this phenomenon to the residual of ET rather than the presence of it affecting the effect of thymectomy [24].
In this study, ET was found in 64 patients (41.3%), and the odds of finding ET were different in the 3 surgical methods. TS and TSX had close efficiency of ectopic thymectomy (49.1% and 46.8%, p = 0.397, not shown in the tables) which was superior to that of RUT (25.5%, p = 0.005).
Interestingly, the efficiency of ectopic thymectomy among the three surgical approaches were significantly different only in Q2 quadrant which located in upper left division of anterior mediastinum. In this area, ET were commonly distributed in the space above and below the left innominate vein where was difficult to be revealed by RUT (Fig. 3). As we mentioned above, major defect of RUT was the poor exposure of both Q2 and Q4 quadrants. When thoracic surgeons performed extended thymectomy by RUT, poor vision on the contralateral site might hesitate them from adipose tissue clearance and cause lower efficiency of ectopic thymectomy.
Fig. 3.
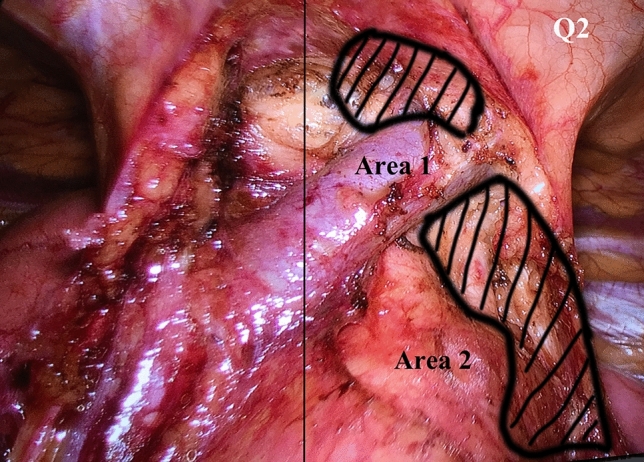
Areas of Q2 quadrant where ectopic thymus commonly distributed in the view of thoracoscopic subxiphoid approach: area 1 was the site above left innominate vein and area 2 was the site between aortic arch and left phrenic nerve
In this study, both TSX and RUT showed advantages of less blood loss and shorter operation time comparing with TS, however, TS had the best efficiency of ectopic thymectomy among the three approaches. Surgical injury caused by TS increased volume and time of thoracic drainage and hospitalization, therefore, it affected short-term recovery of the patients. However, TS also had the best efficiency of ectopic thymectomy which might be in favor of long-term outcome. In our opinion, at present TS is still an indispensable method of extended thymectomy in MG patients and cannot be replaced by thoracoscopic methods, especially unilateral thoracoscopic approach. However, it is noteworthy that TSX and bilateral thoracoscopic approaches have the similar surgical extent as TS. Both of them are potential to be as efficiency as TS in ectopic thymectomy. In this study, the different of ectopic thymectomy between TS and TSX was minor also supported this potentiality. Meanwhile, relative to the bilateral thoracoscopic approach, TSX showed advantage in more simplified procedure and easier conversion to TS while unpredicted injury happened. But nowadays, we still need more evidences to support that TSX could be an alternative way of surgical treatment in MG patients.
Prognosis data showed that overall response (CR + PR) to surgery in all patients was 70.3%. The differences of response rate among three groups were not statistically significant. However, TS and TSX approaches still showed the trend of better outcome comparing to RUT. As the median follow-up time was only less than 3 years, whether surgical approaches and their efficiency of ectopic thymectomy has affection on long-term outcome of MG is still unsettled.
Limitations of this study were shown below. First, it was a retrospective study, suggesting that the reliability of the results may be impaired by clinical characteristics bias. Second, Surgical process and outcome were strongly associated with surgeons’ experience and preference. The bias caused by that should not be ignored. Third, the size of cases was small and the time of follow-up was short, therefore, further study with larger sample with longer follow-up time may help us better understand the influence of surgical approaches on ectopic thymectomy.
Conclusively, Both RUT and TSX showed advantages in short-term recovery over TS in extended thymectomy. For the efficiency of ectopic thymectomy, TS was still the best option while TSX showed potential to be an alternative way and less invasive.
Funding
This study was supported by National Natural Science Foundation of China. No. 81701233.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shuishen Zhang, Zhenguang Chen and Bin Li equally contributed to this work and share first authorship.
References
- 1.Evoli A. Myasthenia gravis: new developments in research and treatment. Curr Opin Neurol. 2017;30(5):464–470. doi: 10.1097/WCO.0000000000000473. [DOI] [PubMed] [Google Scholar]
- 2.Harvey A, Lilienthal J, Talbot S. Observations on the nature of myasthenia gravis. The effect of thymectomy on neuro-muscular transmission. J Clin Investig. 1942;21(5):579–588. doi: 10.1172/JCI101336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klimek-Piotrowska W, et al. Ectopic thymic tissue in the mediastinum: limitations for the operative treatment of myasthenia gravis. Eur J Cardiothorac Surg. 2012;42(1):61–65. doi: 10.1093/ejcts/ezr268. [DOI] [PubMed] [Google Scholar]
- 4.Maurizi G, et al. Transsternal thymectomy. Thorac Cardiovasc Surg. 2015;63(03):178–186. doi: 10.1055/s-0034-1396083. [DOI] [PubMed] [Google Scholar]
- 5.Zou J, et al. Preoperative anxiety in patients with myasthenia gravis and risk for myasthenic crisis after extended transsternal thymectomy: a CONSORT study. Medicine (Baltimore) 2016;95(10):e2828. doi: 10.1097/MD.0000000000002828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masaoka A, Nagaoka Y, Kotake Y. Distribution of thymic tissue at the anterior mediastinum: current procedures in thymectomy. J Thorac Cardiovasc Surg. 1975;70(4):747–754. doi: 10.1016/S0022-5223(19)40309-7. [DOI] [PubMed] [Google Scholar]
- 7.Sanders DB, Wolfe GI, Narayanaswami P. Developing treatment guidelines for myasthenia gravis. Ann N Y Acad Sci. 2018;1412(1):95–101. doi: 10.1111/nyas.13537. [DOI] [PubMed] [Google Scholar]
- 8.Masaoka A, et al. Extended thymectomy for myasthenia gravis patients: a 20-year review. Ann Thorac Surg. 1996;62(3):853–859. doi: 10.1016/S0003-4975(96)00376-1. [DOI] [PubMed] [Google Scholar]
- 9.Granone P, et al. Thymectomy in myasthenia gravis via video-assisted infra-mammary cosmetic incision. Eur J Cardiothorac Surg. 1999;15(6):861–863. doi: 10.1016/S1010-7940(99)00120-7. [DOI] [PubMed] [Google Scholar]
- 10.Pompeo E, et al. Thoracoscopic completion thymectomy in refractory nonthymomatous myasthenia. Ann Thorac Surg. 2000;70(3):918–923. doi: 10.1016/S0003-4975(00)01566-6. [DOI] [PubMed] [Google Scholar]
- 11.Savcenko M, et al. Video-assisted thymectomy for myasthenia gravis: an update of a single institution experience. Eur J Cardiothorac Surg. 2002;22(6):978–983. doi: 10.1016/S1010-7940(02)00593-6. [DOI] [PubMed] [Google Scholar]
- 12.Ng CS, Wan IY, Yim AP. Video-assisted thoracic surgery thymectomy: the better approach. Ann Thorac Surg. 2010;89(6):S2135–S2141. doi: 10.1016/j.athoracsur.2010.02.112. [DOI] [PubMed] [Google Scholar]
- 13.Mineo TC, et al. Thoracoscopic thymectomy in autoimmune myasthenia: results of left-sided approach. Ann Thorac Surg. 2000;69(5):1537–1541. doi: 10.1016/S0003-4975(00)01237-6. [DOI] [PubMed] [Google Scholar]
- 14.Lee CY, et al. Bilateral video-assisted thoracoscopic thymectomy has a surgical extent similar to that of transsternal extended thymectomy with more favorable early surgical outcomes for myasthenia gravis patients. Surg Endosc. 2011;25(3):849–854. doi: 10.1007/s00464-010-1280-y. [DOI] [PubMed] [Google Scholar]
- 15.Hsu C-P. Subxiphoid approach for thoracoscopic thymectomy. Surg Endosc. 2002;16(7):1105. doi: 10.1007/s00464-001-4255-1. [DOI] [PubMed] [Google Scholar]
- 16.Hsu C-P, et al. Comparison between the right side and subxiphoid bilateral approaches in performing video-assisted thoracoscopic extended thymectomy for myasthenia gravis. Surg Endosc Other Interv Tech. 2004;18(5):821–824. doi: 10.1007/s00464-003-9146-1. [DOI] [PubMed] [Google Scholar]
- 17.Lu Q, et al. Subxiphoid and subcostal arch “Three ports” thoracoscopic extended thymectomy for myasthenia gravis. J Thorac Dis. 2018;10(3):1711. doi: 10.21037/jtd.2018.02.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaretzki A, III, Wolff M. “Maximal” thymectomy for myasthenia gravis: surgical anatomy and operative technique. J Thorac Cardiovasc Surg. 1988;96(5):711–716. doi: 10.1016/S0022-5223(19)35177-3. [DOI] [PubMed] [Google Scholar]
- 19.Ambrogi V, Mineo TC. Active ectopic thymus predicts poor outcome after thymectomy in class III myasthenia gravis. J Thorac Cardiovasc Surg. 2012;143(3):601–606. doi: 10.1016/j.jtcvs.2011.04.050. [DOI] [PubMed] [Google Scholar]
- 20.Li F, et al. Unraveling the role of ectopic thymic tissue in patients undergoing thymectomy for myasthenia gravis. J Thorac Dis. 2019;11(9):4039. doi: 10.21037/jtd.2019.08.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ponseti JM, et al. Influence of ectopic thymic tissue on clinical outcome following extended thymectomy in generalized seropositive nonthymomatous myasthenia gravis. Eur J Cardiothorac Surg. 2008;34(5):1062–1067. doi: 10.1016/j.ejcts.2008.07.049. [DOI] [PubMed] [Google Scholar]
- 22.Ashour M. Prevalence of ectopic thymic tissue in myasthenia gravis and its clinical significance. J Thorac Cardiovasc Surg. 1995;109(4):632–635. doi: 10.1016/S0022-5223(95)70343-8. [DOI] [PubMed] [Google Scholar]
- 23.Marulli G, et al. Surgical and neurologic outcomes after robotic thymectomy in 100 consecutive patients with myasthenia gravis. J Thorac Cardiovasc Surg. 2013;145(3):730–736. doi: 10.1016/j.jtcvs.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 24.Zieliński M, et al. Comparison of late results of basic transsternal and extended transsternal thymectomies in the treatment of myasthenia gravis. Ann Thorac Surg. 2004;78(1):253–258. doi: 10.1016/j.athoracsur.2003.11.040. [DOI] [PubMed] [Google Scholar]