Abstract
Introduction
Transcranial pulse stimulation (TPS) has been recently introduced as a novel clinical brain stimulation technique based on highly focused ultrasound pressure pulses. In a first pilot study on clinical effects of navigated and focused ultrasound neuromodulation, a dichotomy of functional effects was found: patients with Alzheimer’s disease improved cognition and language but deteriorated with visuo-constructive functions.
Methods
We analyzed changes in functional connectivity measured with functional magnetic resonance imaging (fMRI) using graph analysis of a visuo-constructive network in 18 patients with Alzheimer’s disease. We calculated the network’s global efficiency and tested for correlation with visuo-constructive test scores to explain this dichotomy.
Results
Important visuo-constructive network nodes were not stimulated in the pilot setting and correspondingly global efficiency of a visuo-constructive network was decreased after TPS therapy, compatible with a natural progress of the disease. A correlation between visuo-constructive scores and changes in global efficiency was found.
Conclusion
Results argue for a high functional specificity of ultrasound-based neuromodulation with TPS.
Keywords: Alzheimer’s disease, Functional magnetic resonance imaging, Non-invasive brain stimulation, Transcranial pulse stimulation, Ultrasound, Visuo-construction
Plain Language Summary
Over the last decade, there has been growing interest in ultrasound-based non-invasive brain stimulation techniques in neuroscience and as a potential therapy for disorders of the brain. Transcranial pulse stimulation (TPS) has been introduced as an innovative neuromodulation technique, applying ultrashort pressure pulses through the skull into neural tissue with 3D navigation in real time. In the first clinical pilot study, patients suffering from Alzheimer’s disease showed an increase in memory and language functions for up to 3 months after TPS therapy. However, visuo-constructive capacities (e.g., copying a geometrical figure) worsened. Notably, brain areas relevant for such processes had been left out during stimulation. This begged the question whether the brain areas that were targeted for brain stimulation as well as functional changes could explain this diverse response pattern. We therefore analyzed functional magnetic resonance data from patients. Specifically, we compared graph theoretical functional connectivity measures in a visuo-constructive network before and after TPS therapy. We found a decrease in connectivity in a central network node, which also correlated with visuo-constructive test scores. This deterioration is likely associated with normal disease progression. Together with the already reported improvement in global cognitive functions, these results argue for a functional specific effect of TPS.
Key Summary Points
| Why carry out this study? | |
| A first pilot study investigating clinical effects of transcranial pulse stimulation (TPS) in patients with Alzheimer’s disease (AD) showed a split functional effect pattern, namely improved cognition and language but worsened visuo-constructive capacities. | |
| This study investigated whether the targeted areas during brain stimulation and functional brain changes could illuminate these contrasting effects. | |
| What was learned from this study? | |
| Brain functions related to non-stimulated brain areas declined despite general cognitive improvement after AD stimulation therapy. | |
| The non-stimulated brain areas concerned visuo-constructive processing, which declined according to neuropsychological scores. This decline corresponded to reduced connectivity of the visuo-constructive network. | |
| These results underline the functional specificity of TPS with respect to the chosen stimulation targets. |
Introduction
Recent developments in novel transcranial ultrasound techniques have enabled new possibilities for precise neuromodulation as well as clinical add-on treatment [1–3]. Advantages over electromagnetic techniques concern precise targeting independent from pathological brain conductivities and non-invasive stimulation deep in the brain [4–6]. Transcranial pulse stimulation (TPS) has been recently introduced as a novel clinical brain stimulation technique applying navigated ultrashort pressure pulses [7, 8]. Stimulation of a broad memory network in a group of patients with Alzheimer’s disease (AD) (including language areas and extended dorsolateral prefrontal cortex) exhibited functional as well as behavioral improvements in memory, language, and mood [9]. Furthermore, a reduction in cortical atrophy measures (increase of cortical thickness) in central AD network areas after TPS intervention has been shown [10]. Notably, while these improvements were observable for up to 3 months after stimulation, there was a deterioration of visuo-constructive capacities after TPS treatment. Visuo-constructive deficits are among the most common behavioral symptoms in different dementias [11]. While there is limited information from neuroimaging studies investigating neuronal correlates of constructional deficits, structural as well as metabolic reductions in the posterior and temporoparietal brain related to visuo-constructive processing have been shown in patients with dementia [12]. Serra et al. [13] described a visuo-constructive network (VisNW) in a comparison between patients exhibiting AD with and without constructional deficits. Importantly, these network areas have been largely left out during the multicentric clinical pilot TPS study, which could explain the visuo-constructive deteriorations. The goal of this follow-up analysis was to investigate whether the visuo-constructive decline could be supported by an objective neurophysiological measure—a network specific connectivity decrease measured by functional magnetic resonance imaging (fMRI).
Methods
Patients
All patients with AD and available functional MRI data from our previous study [7] were reanalyzed (n = 18, 11 female, mean age 69.9 years, mean Mini-Mental State Examination (MMSE) score 20.94, standard deviation 5.8, range 6–30). Recruitment was performed by independent neurologists, following the diagnosis criteria given in the ICD-10 (F00) and the NIA-AA criteria by an expert in cognitive neurology. Only patients receiving already optimized standard treatments were accepted. Inclusion criteria were clinically stable patients with probable AD, at least 3 months of stable antidementia therapy, age at least 18, signed informed consent. Exclusion criteria were noncompliance with the protocol, relevant intracerebral pathology unrelated to AD, hemophilia or blood clotting disorders or thrombosis, and corticosteroid treatment within the last 6 weeks before treatment onset.
Ethics Statement
All procedures in this study were approved and performed in accordance with the ethical code of the Ethical Committee of the Medical University of Vienna (EK1227/2015) and the Declaration of Helsinki. Written, informed consent was obtained from all patients.
Study Design
Magnetic resonance imaging (MRI) data acquisition and neuropsychological tests were performed the week before and after patients received TPS treatment for 4 weeks, with three TPS sessions per week (three patients for 2 weeks, one patient for 3 weeks).
TPS Treatment and Regions of Interest
Single ultrasound pressure pulses were applied using a NEUROLITH TPS generator (Storz Medical AG, Tägerwilen, Switzerland): duration about 3 µs, 0.2 mJ mm−2 energy flux density, pulse repetition frequency 5 Hz, pulse number per therapeutic session 6000. Individual regions of interest (ROIs) were defined by a neurologist (R.B.) to target brain areas relevant to AD. These include the classical AD and depression stimulation target dorsolateral prefrontal cortex, areas of the memory (including default mode network), and language networks. Specifically, ROIs comprised bilateral frontal cortex (dorsolateral prefrontal cortex and inferior frontal cortex extending to Broca’s area, ROI volume 136/164 cm3, 2 × 800 pulses per hemisphere), bilateral lateral parietal cortex (extending to Wernicke’s area, ROI volume 122/147 cm3, 2 × 400 pulses per hemisphere), and extended precuneus cortex (one bilateral volume with 66/92 cm3, 2 × 600 pulses). The goal was to distribute all pulses within the respective ROIs with a focus on the cortical tissue. Every ROI was stimulated twice per session. Two sets of standardized ROI sizes (ellipsoidal shape) were applied for either small or large sized patient brains (chosen according to pre-evaluation of brain size variability with in-house software for gross estimation of cerebrum size, based on their structural MRI). As previously described [7], individual real-time tracking allowed standardized focal brain stimulation across subjects.
MRI Parameters
T1-weighted structural images were recorded using a MPRAGE sequence (3 T Siemens PRISMA, TE/TR = 2.7/1800 ms, IT = 900 ms, FA = 9°, resolution 1 mm isotropic). Resting state fMRI covered the whole brain including cerebellum (GE-EPI sequence, 38 slices, TE/TR = 30/2500 ms, flip angle = 90°, in-plane acceleration = GRAPPA 2, field of view = 230 × 230 mm, voxel size = 1.8 × 1.8 × 3 mm, 25% gap). A total of 250 volumes (10 min 25 s) were recorded.
Behavioral Assessment
Principle component analysis (PCA) from CERAD Plus (German version) scores [14] were generated using the z-transformed values (corrected for age, gender, and formal education). The PCA on the CERAD subtests produced statistically independent factors that were able to explain individual test performance with an eigenvalue greater than 1, and therefore allowed grouping subtests according to cognitive components for memory, verbal processing, and visuospatial or constructional processing (FIGURAL) scores [7]. Specifically, the FIGURAL measure represented performance on a geometrical figure copy as well as a delayed figure recall task. Complete baseline and post-stimulation test data was available for 16 patients. FIGURAL test scores were normally distributed and further analyzed with paired t tests.
Preprocessing and Graph Analysis of a Visuo-constructive Network
Analysis was done with the CONN toolbox v19 [15]. Preprocessing comprised realignment, unwarping, slice-time correction, structural segmentation, normalization, and outlier detection (ART-based scrubbing). Following recommendation for fMRI group comparisons [16], images were smoothed with a kernel of 8 mm at full-width at half maximum, as the voxel size with gap was 3.75 mm. Subsequently, data was denoised using a band pass filter (0.008–0.09 Hz), removing motion confounds (six motion parameters and their first derivatives), applying aCompCor [17], and scrubbing. Bivariate correlations of the corrected time series of all voxels were calculated for first-level analysis. Graph theoretical measures were calculated within the a priori defined VisNW from Serra et al. [13]. The reported VisNW was largely in posterior and parietal brain areas and comprised the bilateral angular gyrus (AG), right intracalcarine cortex, right posterior medial temporal gyrus, posterior cingulate cortex, and right temporo-occipital fusiform cortex. These ROIs were defined in a nondirectional graph as nodes, with supra-threshold connections as edges. ROIs were taken from the Harvard–Oxford atlas. For each subject and condition, a graph adjacency matrix was then computed to calculate global efficiency (GE; a global connectedness measure of each ROI within a network [18]) and compare between baseline and post-stimulation sessions (correlation coefficient = 0.35, p-FDR = 0.05, paired t test, two-sided). We chose GE because it is an established metric indicating the capacity to pass information in a network and therefore is of particular interest in our investigation. Individual GE values showing a significant difference between sessions were extracted and used to correlate (Spearman rank) with FIGURAL test scores.
Results
Neuropsychological Assessment
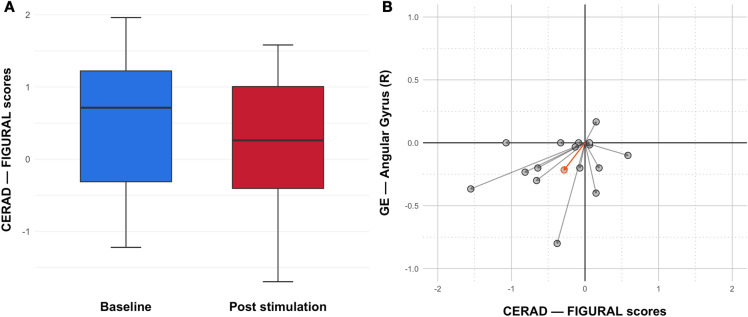
Comparing CERAD FIGURAL test scores between pre and post stimulation using a paired t test showed a trend in declining visuo-constructive capabilities after TPS treatment (Fig. 1a; p = 0.0558). Notably, this decline was significant 3 months after stimulation (p = 0.007) [7].
Fig. 1.
Visuo-constructive performance decreases together with deterioration of functional connectivity. a CERAD FIGURAL test scores exhibit a trend to decline 1 week after the completed 4-week-long TPS treatment (p = 0.0558; paired t test, two-sided). This effect reached significance 3 months post treatment. b Individual differences of FIGURAL test scores and global efficiency values of the right angular gyrus between baseline and post stimulation decreased together in the majority of patients. Arrows indicate changes for individual patients, with group mean difference in orange
Graph Analysis of Visuo-constructive Network and Correlation Analysis
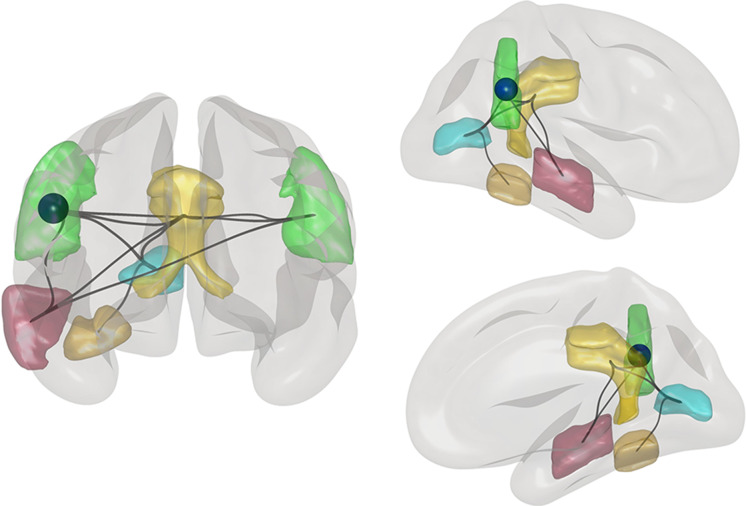
Analyzing the visuo-constructive network revealed a significant decrease in GE for the VisNW (p = 0.016) with the right AG also showing an individual decline in GE (Fig. 2; p-FDR = 0.0153; corrected for multiple comparisons for each network node). CERAD FIGURAL test scores positively correlate with right AG GE values (p = 0.046; rho = 0.356) and plotting the differences in individual GE values and corresponding FIGURAL scores together showed an overall non-significant trend of a joint decrease (Fig. 1b). This tendency is also reflected in the group mean. The VisNW as described by Serra et al. [13] also included parts of the precuneus, which, in fact, was among the targeted areas during TPS treatment. In the current analysis, this area was excluded to specifically test a network of nodes that have been left out during stimulation. However, we also tested the network including the precuneus and still found a significant GE decrease in the right AG (p-FDR = 0.0262), indicating a robust decline in network connectivity despite one node receiving stimulation.
Fig. 2.
Functional decline in a node of a visuo-constructive network. The global efficiency significantly decreased in the right angular gyrus (AG; indicated by the blue sphere) after TPS stimulation compared to the baseline. The visuo-constructive network consists of bilateral AG (green), right intracalcarine gyrus (blue), posterior cingulate cortex (yellow), right posterior medio-temporal gyrus (red), and right temporo-occipital fusiform cortex (gold). Lines indicate all suprathreshold connections of the network
Discussion
The novel brain stimulation technique TPS can generate improvements for memory, language, and mood when targeting functionally relevant brain regions [7, 9]. In contrast, there was a specific decrease in visuo-constructive test scores while network areas for a VisNW had been omitted during stimulation. The present results support the decline in visuo-constructive abilities by the documented functional decrease in a VisNW, with a specific decrease in GE in the right AG. Owing to the high sensitivity of fMRI for detection of small functional brain changes, network changes were already significant immediately after the stimulation phase. Although a general correlation between visuo-constructive scores and network connectivity was found, analysis of individual pre-/post-treatment differences did not reach significance, most likely because of our limited number of subjects. However, the group mean clearly supported this trend (Fig. 1b). Overall, the results indicate an associated functional and cognitive deterioration associated with brain areas that were not treated with TPS. Most likely this represents the natural progress of the disease [19].
The AG has been implicated in constructional processing [20–22], so a possible hypothesis is that because it is functionally more relevant in the VisNW than, e.g., the precuneus, network function decreased together with corresponding visuo-constructive capacities despite stimulation of the precuneus. Recently, TPS has been shown to induce long-term effects on functional and structural connectivity in connected network areas after stimulation of a somatosensory cortex area [23]. In a similar vein, Verhagen et al. [24] reported that in macaques not only stimulated brain regions but also their closest connected neighboring areas show modulated activity patterns. In contrast, networks functionally unrelated to the stimulation target did not exhibit significant changes, arguing for a functionally specific network effect. Together with previous results [7], the current study suggests, likewise, a confined, functionally specific response to TPS treatment with matching behavioral effects.
A number of limitations need to be addressed. There was no sham control included, and thus results need to be interpreted with care. However, as already discussed, the complex functional and behavioral responses shown here and elsewhere [7] argue against a global placebo response [25]. The small sample size limits any premature conclusions and larger studies are needed to elucidate individual treatment responses in more detail. Further, fMRI measurements were taken only at one time point about 5–6 weeks after the baseline fMRI, so longer-term fMRI behavior (more than 6 weeks) could not be investigated.
Conclusions
This study presents evidence for the spatial and functional specificity of ultrasound-based neuromodulation in a therapeutic context. Modern fMRI techniques allow one to elucidate the complex response patterns after TPS interventions, which will help to design stimulation protocols tailored to a specific clinical or neuroscientific interest. Future investigations may build on this functional specificity of TPS brain stimulation to develop optimized stimulation protocols also for visuo-constructional deficits in patients.
Acknowledgements
We thank the participants of the study.
Funding
This work was supported by a research-cluster grant from the Medical University of Vienna and University of Vienna (SO10300020) and by research grants from STORZ Medical and by the Herzfelder Stiftung (to R.B.). MRI methodology was partially developed via support of the Austrian Science Fund (FWF KLIF455, to R.B.). The authors funded the journal’s Rapid Service Fee.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the study conception and methodology. Formal analysis was performed by Gregor Dörl and Eva Matt. Project administration, funding acquisition and supervision were performed by Roland Beisteiner. The original draft of the manuscript was written by Gregor Dörl and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
This work was supported by research grants from STORZ Medical (including equipment, to Roland Beisteiner). Roland Beisteiner is President of the Organization for Human Brain Mapping Alpine Chapter and the Austrian Society for fMRI (unpaid). Eva Matt received travel grants from the Austrian Research Association (ÖFG). Gregor Dörl has nothing to declare.
Compliance with Ethic Guidelines
All procedures in this study were approved and performed in accordance with the ethical code of the Ethical Committee of the Medical University of Vienna (EK1227/2015) and the Declaration of Helsinki. Written, informed consent was obtained from all patients.
Data Availability
The datasets analyzed for the current study are not publicly available due to patient confidentiality and participant privacy restrictions.
References
- 1.Beisteiner R, Lozano AM. Transcranial ultrasound innovations ready for broad clinical application. Adv Sci. 2020;7(23):2002026. doi: 10.1002/advs.202002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meng Y, Hynynen K, Lipsman N. Applications of focused ultrasound in the brain: from thermoablation to drug delivery. Nat Rev Neurol. 2021;17(1):7–22. doi: 10.1038/s41582-020-00418-z. [DOI] [PubMed] [Google Scholar]
- 3.Beisteiner R. Human neuromodulation: state of the art. Brain Sci. 2022;12:208. doi: 10.3390/brainsci12020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabut C, Yoo S, Hurt RC, et al. Ultrasound technologies for imaging and modulating neural activity. Neuron. 2020;108(1):93–110. doi: 10.1016/j.neuron.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minjoli S, Saturnino GB, Blicher JU, et al. The impact of large structural brain changes in chronic stroke patients on the electric field caused by transcranial brain stimulation. NeuroImage Clin. 2017;15:106–117. doi: 10.1016/j.nicl.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li LM, Violante IR, Zimmerman K, et al. Traumatic axonal injury influences the cognitive effect of non-invasive brain stimulation. Brain. 2019;142(10):3280–3293. doi: 10.1093/brain/awz252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beisteiner R, Matt E, Fan C, et al. Transcranial pulse stimulation with ultrasound in Alzheimer’s disease—a new navigated focal brain therapy. Adv Sci. 2019;7:1902583. doi: 10.1002/advs.201902583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beisteiner R, Hallett M. Transcranial pulse stimulation (TPS)—a highly focused brain stimulation therapy with 3D navigation. Clin Neurophysiol. 2022;1388–2457(22):00145–146. doi: 10.1016/j.clinph.2022.01.127. [DOI] [PubMed] [Google Scholar]
- 9.Matt E, Dörl G, Beisteiner R. Transcranial pulse stimulation (TPS) improves depression in AD patients on state-of-the-art treatment. Alzheimers Dement. 2022;8(1):e12245. doi: 10.1002/trc2.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Popescu T, Pernet C, Beisteiner R. Transcranial ultrasound pulse stimulation reduces cortical atrophy in Alzheimer's patients: a follow-up study. Alzheimers Dement. 2021;7(1):e12121. doi: 10.1002/trc2.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trojano L, Gainotti G. Drawing disorders in Alzheimer’s disease and other forms of dementia. J Alzheimers Dis. 2016;53(1):31–52. doi: 10.3233/JAD-160009. [DOI] [PubMed] [Google Scholar]
- 12.Gainotti G, Trojano L. Constructional apraxia. Handb Clinic Neurol. 2018;151:331–348. doi: 10.1016/B978-0-444-63622-5.00016-4. [DOI] [PubMed] [Google Scholar]
- 13.Serra L, Fadda L, Perri R, et al. Constructional apraxia as a distinctive cognitive and structural brain feature of pre-senile Alzheimer's disease. J Alzheimers Dis. 2014;38(2):391–402. doi: 10.3233/JAD-130656. [DOI] [PubMed] [Google Scholar]
- 14.Ehrensperger MM, Berres M, Taylor KI, Monsch AU. Early detection of Alzheimer’s disease with a total score of the German CERAD. J Int Neuropsychol. 2010;16(5):910–920. doi: 10.1017/S1355617710000822. [DOI] [PubMed] [Google Scholar]
- 15.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- 16.Mikl M, Mareček R, Hluštík P, et al. Effects of spatial smoothing on fMRI group inferences. Magn Reson Imaging. 2008;26(4):490–503. doi: 10.1016/j.mri.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37(1):90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Achard S, Bullmore E. Efficiency and cost of economical brain functional networks. PLoS Comput Biol. 2007;3:0174–183. doi: 10.1371/journal.pcbi.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sendi MS, Zendehrouh E, Miller RL, et al. Alzheimer’s disease projection from normal to mild dementia reflected in functional network connectivity: a longitudinal study. Front Neural Circuits. 2021;14:87. doi: 10.3389/fncir.2020.593263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramanan S, Piguet O, Irish M. Rethinking the role of the angular gyrus in remembering the past and imagining the future: the contextual integration model. Neuroscientist. 2018;24(4):342–352. doi: 10.1177/1073858417735514. [DOI] [PubMed] [Google Scholar]
- 21.Seghier ML. The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist. 2013;19(1):43–61. doi: 10.1177/1073858412440596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biesbroek JM, van Zandvoort MJ, Kuijf HJ, et al. The anatomy of visuospatial construction revealed by lesion-symptom mapping. Neuropsychologia. 2014;62:68–76. doi: 10.1016/j.neuropsychologia.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Matt E, Kaindl L, Tenk S, et al. First evidence of long-term effects of transcranial pulse stimulation (TPS) on the human brain. J Transl Med. 2022;20(1):1–13. doi: 10.1186/s12967-021-03222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verhagen L, Gallea C, Folloni D, et al. Offline impact of transcranial focused ultrasound on cortical activation in primates. eLife. 2019;8:1–28. doi: 10.7554/eLife.40541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito K, Corrigan B, Romero K, et al. Understanding placebo responses in Alzheimer's disease clinical trials from the literature meta-data and CAMD database. J Alzheimers Dis. 2013;37(1):173–183. doi: 10.3233/JAD-130575. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed for the current study are not publicly available due to patient confidentiality and participant privacy restrictions.