Abstract
Introduction
Multiple sclerosis (MS) is a highly heterogeneous inflammatory disease of the central nervous system. Patient-reported outcomes (PROs) in a real-world clinical setting can provide detailed information about MS from the patient's perspective. PROs were used here to assess quality of life (QoL), treatment satisfaction, clinical efficacy, and safety outcomes in a Greek cohort of relapsing remitting MS (RRMS) patients treated with oral teriflunomide (14 mg/day).
Methods
AURELIO was a 2-year, prospective, observational study whose QoL primary endpoint was assessed with the Multiple Sclerosis Impact Scale (MSIS-29). Secondary endpoints included analyses of Patient Determined Disease Steps (PDDS), Treatment Satisfaction Questionnaire for Medication (TSQM), Expanded Disability Status Scale (EDSS), annualized relapse rate (ARR), adherence, and safety outcomes.
Results
AURELIO enrolled 282 patients (62.8% female; mean age 44.8 [SD ± 11] years; EDSS 2.0 [SD ± 1.6]; 44.6% treatment-naïve), with 212 patients (75%) remaining on treatment at study end. MSIS-29 total scores remained stable, while the MSIS-29 psychological scale showed significant improvement (p = 0.0015) at 2 years vs. baseline. TSQM scores at 2 years showed significant improvements in effectiveness (+ 6.6, p = 0.0001), convenience (+ 1.9, p = 0.0256), and global satisfaction (+ 8.1, p = 0.0001) vs. baseline. Disease progression was stable as indicated by non-significant changes in PDDS and EDSS vs. baseline. The ARR was low at 0.065, with a slightly higher ARR in previously treated (0.070) vs. naïve patients (0.058). Adherence was high at > 90%. Overall, 91 patients (32.3%) in the study reported a total of 215 safety events (32 serious, of which 21 were classified as mild–moderate). No new safety signals were observed.
Conclusions
These data highlight the importance of PROs to facilitate personalized treatment strategies in MS. In line with other teriflunomide studies, AURELIO showed stable QoL, efficacy and safety outcomes, and good treatment satisfaction both in treatment-naïve and previously treated patients in this Greek cohort of patients with RRMS.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40120-022-00384-2.
Keywords: Convenience, Efficacy, Multiple sclerosis, Quality of life, Teriflunomide, Treatment satisfaction
Key Summary Points
| Patient-reported outcomes (PROs) in a real-world setting provide a means for patients with relapsing–remitting multiple sclerosis (RRMS) to share important information about their disease status, quality of life (QoL), drug efficacy, and treatment satisfaction. |
| AURELIO was a 2-year study that assessed PROs in a Greek cohort of more than 280 patients with RRMS treated with once-daily oral teriflunomide. |
| Overall, QoL, and disability scores remained stable over the course of the study, while improved treatment satisfaction and efficacy outcomes versus baseline were observed. |
| Positive benefits of teriflunomide have now been reported in numerous real-world studies, with the AURELIO data highlighting the importance of PROs to facilitate personalized treatment strategies in MS. |
Introduction
Multiple sclerosis (MS) is an autoimmune-mediated chronic, demyelinating disease of the central nervous system (CNS) that affects approximately 2.8 million people worldwide [1]. The severity of disease activity, the location of affected regions in the CNS, and the extent of neurological reserve mean that MS affects people differently, with symptoms ranging from minor to severe and from physical to cognitive in nature [2]. The clinical course of relapsing–remitting MS (RRMS) is typically characterized by initial episodes of transient neurological compromise with full recovery, followed by a phase of cumulative physical disability that may increase with each new relapse [2, 3]. Most relapsing patients eventually develop secondary progressive MS (SPMS), which is characterized by chronic sequelae including profound muscle weakness, impaired gait and mobility, bladder and bowel dysfunction, and cognitive and visual impairments [2, 4, 5]. MS also encompasses neuropsychiatric symptoms that can manifest in the form of heightened anxiety, depression, cognitive impairment, irritability, and anger [6]. Taken together, it is evident that as the disease progresses, the potential exists for personal autonomy, independence, dignity, social interaction, and planning for the future to be severely compromised [4], thus impacting overall quality of life (QoL) [7–9].
Many of the current first-line disease-modifying therapies (DMTs) for RRMS consist of injectables, in which DMTs are administered subcutaneously or intramuscularly with pre-filled syringes or pens. Teriflunomide (AUBAGIO®; A771726, ATC-Code: L04AA31) is a once-daily oral treatment indicated for patients with RRMS, offering a more convenient mode of administration and avoiding adverse injection site reactions. It selectively and reversibly inhibits dihydro-orotate dehydrogenase (DHODH), a key mitochondrial enzyme for the de novo pyrimidine synthesis, which is required by rapidly dividing lymphocytes [10]. Teriflunomide has shown significant efficacy across key measures of MS disease, including reducing the number of relapses, slowing the progression of physical disability and brain volume loss, and reducing the number of brain lesions detected by magnetic resonance imaging (MRI) [11–15].
QoL measures based on patient-reported outcomes (PROs) are commonly assessed as secondary/tertiary endpoints in randomized control trials (RCTs). PROs can also provide highly relevant information about MS from the patient’s perspective in a real-life clinical setting where the broader MS patient population no longer conforms to strict age, baseline disease activity, and comorbidity restrictions seen in the realm of RCTs [16]. To this end, both the European Medicines Agency (EMA) and the US Food & Drug Administration (FDA) promote the collection of real-world data to answer questions that cannot be addressed in RCTs, and/or to provide ongoing benefit–risk analyses of approved drugs throughout the product lifecycle [17, 18]. PRO-based QoL measurements in the real world provide the means for patients to share important information about their disease status, medication use, adherence and/or persistence, and are considered increasingly important for healthcare professionals with regard to making treatment decisions, evaluating outcomes, anticipating disease progression, and providing comprehensive care to patients with MS. These measures also provide important socioeconomic data beyond the benefit–risk analysis and price of the drug, and are being requested more frequently by healthcare authorities, clinicians, and payers.
In addition to the ongoing clinical development program and long-term extension studies, real-world QoL outcomes with teriflunomide are currently being assessed in many countries around the world [19]. Indeed, positive findings showing stable or improved QoL, cognition, fatigue and treatment satisfaction outcomes have been reported from North America and rest-of-world (Teri-PRO) [20], France (Teri-FAST) [21], the Nordic region (Teri-LIFE) [22], Hungary (Teri-REAL) [23], and Germany (TAURUS) [24]. We report here results of the AURELIO study in Greece, situated on the Eastern Mediterranean Sea and with a population of about 10.5 million people. Greece has an MS prevalence of 197.8 persons per 100,000 population, with just over 73% of patients having received at least one DMT [25]. According to the guidelines of Hellenic Academy of Neuroimmunology (HELANI), patients without risk factors for poor prognosis and/or without high disease activity (in terms of numbers and severity of relapses and MRI active lesions or increased load) should be treated with first-line DMTs such as interferons, glatiramer acetate, teriflunomide, or dimethyl fumarate. Switching between first-line treatments can be done for reasons such as poor tolerance, adverse events, poor adherence to treatment, etc., while escalation to more potent drugs is recommended in the event of a lack of efficacy. To this end, AURELIO was undertaken to assess QoL, treatment satisfaction, efficacy, adherence, and safety outcomes in a cohort of more than 280 patients with RRMS recruited from Greek MS centers and treated with oral teriflunomide (14 mg/day) in a real-world setting.
Methods
Study Design
AURELIO was a 2-year, multi-center, observational, prospective study, in which patients with RRMS were recruited from 26 hospitals in Greece. Patients who had been prescribed oral teriflunomide (14 mg/day) by treating physicians in accordance with local prescribing regulations were eligible for enrolment in this study, which took place from July 14, 2016 (first patient in) until March 9, 2020 (last patient out). Enrolled patients were required to make seven visits (V) over the course of the study: baseline (BL; V1), on day 1 of treatment (V2), and then after 1 (V3), 6 (V4), 12 (V5), 18 (V6), and 24 months (V7) of teriflunomide treatment.
As a real-world study, no medication was forbidden in AURELIO except for leflunomide (the parent drug/precursor of teriflunomide). In addition, teriflunomide had to be the primary therapy for RRMS, and not used an add-on therapy.
The study was conducted in accordance with the guidelines for Good Epidemiology Practice (2007) [26], and complied with local regulations, including local data protection regulations, individual hospital scientific committee approval, and the ethical principles of the Declaration of Helsinki (1964 and subsequent amendments). With respect to local regulations, no national ethics committee approval was required. However, in compliance with Greece’s National Medicines Organization (EOF) circular (EOF 82798/22-11-2012), scientific board approval from each study site was obtained and filed with the sponsor. AURELIO was registered as a non-interventional study in a local clinical trial registry, DHLON, which is supported by The Hellenic Association of Pharmaceutical Companies (SFEE), a local branch of the European Federation of Pharmaceutical Industries and Associations (EFPIA).
Patients
Patients were eligible for enrolment in AURELIO if they were ≥ 18 years and had been diagnosed with RRMS by a treating neurologist. As the study was real life and non-interventional, both 2010 and 2017 McDonald criteria [27, 28] could be used by physicians to establish a diagnosis at the time of recruitment. The decision to prescribe teriflunomide was in accordance with the recommendations outlined in the drug’s summary of product characteristics (AUBAGIO®, Summary of Product Characteristics) [10] and was made independently of and before entry of the patient into the study. All patients provided written informed consent and were willing and able to participate in the study and complete the study’s questionnaires.
Endpoints
The primary efficacy analysis for this study was the difference in QoL between BL and 24 months for the full patient cohort as measured by the Multiple Sclerosis Impact Scale (MSIS-29 v2.1) according to Hobart et al. [29]. A validated Greek translation of the test was used [30]. Data were analyzed descriptively using mean, median, standard deviation, quartiles, and extreme values.
Secondary efficacy analyses for the full patient cohort were the individual physical and psychological parts of the MSIS-29 test, and differences between BL and 24 months for the four components of the TSQM scale (efficacy, side effects, convenience, and global satisfaction), which were assessed according to Atkinson et al. [31]. Differences between BL and 12- and 24-month values for the Expanded Disability Status Scale (EDSS) score [32], the Patient Determined Disease Steps (PDDS), and Performance Scales (PS) [33–35] tests were also assessed. Greek translations of tests were used.
The annualized relapse rate (ARR) was calculated for the 24-month period. MRI scans were not performed routinely as part of the study, but if data were made available by participating investigators, then these were summarized for each visit. MRI results were characterized as “stable” at the discretion of the patient’s neurologist when no new lesions (T2, T1 or gadolinium-enhancing), no enlargement of pre-existing lesions (no change in size) and no increase in the total number of lesions were observed compared to baseline measures.
Adherence to teriflunomide was calculated at each visit according to Osterberg and Blaschke [36], where a patient was considered ‘adherent’ to teriflunomide if the number of tablets taken over a period of time was > 80% of the number of tablets prescribed by the physician to the patient in that time.
Safety and Tolerability Analysis
Adverse events (AEs) were analyzed in a descriptive manner, with the frequency and percentage of patients experiencing AEs, serious AEs (SAEs) and AEs of special interest (AESIs) during the 24 months of the study summarized by grade/intensity, relationship to teriflunomide, outcome, and action taken.
Data Collection
Data were collected via electronic clinical record files and by hard copies of patient questionnaires completed at each study visit. BL screening included MS disease history, and prior and concomitant medications (including those for MS).
Data collected at each visit (V1–V7) are summarized in Supplementary Table 1 (Supplemental Material). In addition to BL, the EDSS was repeated at 6, 12, 18, and 24 months, while MSIS-29 and PDDS measurements repeated at the 12- and 24-month study visits. TSQM data were obtained at BL for those patients switching from another DMT, and then at months 1, 12, and 24 for patients who remained in the study. BL values of the TSQM and its subscales were taken from the visit at month 1 for treatment-naïve patients and then months 12 and 24. MRI data, if available and performed as part of local standard of care, were collected at BL, and at any visit where available from 6 months onwards to assess disease progression. In addition, at each study visit, adherence to teriflunomide was assessed, as was clinical relapse, any adverse event, and any change in concomitant medications.
Subgroup Analysis of Patients Switching from Injectable DMTs
An additional aim of the AURELIO study was to perform a sub-group analysis to check for differences in QoL, treatment satisfaction and efficacy outcomes in patients switching from other injectable DMTs, which were defined as glatiramer acetate and all forms of interferon beta. Results will be shown for evaluations made at BL and 24 months after commencing teriflunomide treatment.
Additional Analyses
Further analyses were performed to assess the effect of several BL characteristics on MSIS-29 score across the entire cohort (years since MS diagnosis, presence of co-morbidities, EDSS score at BL, age < 55 years or > 55 years, co-medications). The effect of these characteristics was also evaluated relative to MRI results, TSQM subgroups, and EDSS score.
Statistical Methods
Sample Size and Power
There was no formal sample size determination for this study, but calculations based on Hobart et al. [29], assuming a correlation of 0.5 between the BL values of the physical/psychological component of the MSIS-29 and the post-BL values, suggested that 350 subjects would provide a 95% confidence interval for the mean change from BL in MSIS-29, which has a range of not more than 7 points. Nevertheless, given that this was a real-life observational study without comparator, a sample size determination of this nature is only informative. In light of the study by Bakirtzis et al. [25], as the prevalence of MS in Greece was found to be similar to those of other European countries, the sample population of 282 patients with RRMS in AURELIO should be considered representative of the Greek MS population.
Populations
The safety analysis set population (SASP) for this study comprised all subjects from the All Subjects Consented Population (ASCP) who consented to participate in the study, fulfilled the inclusion criteria and who received at least one dose of teriflunomide, The Full Analysis Set Population (FASP) included all patents in the SASP population who had a BL efficacy evaluation and at least one post-BL assessment of any efficacy measurement during follow-up.
Methodology
The Kolmogorov–Smirnov test was used to assess the MSIS-29 data to find any deviation from normality. The non-parametric Wilcoxon signed-rank test was then used to compare BL and 12- and 24-month visit values. The same test was applied to data from the separate parts of the MSIS-29 test (physical and psychological), the TSQM scales, EDSS, PDDS, and PS scores.
The number and frequency of adherent patients were calculated by visit, including the 95% confidence intervals (CI) of frequency. ARR was calculated with its 95% Wald confidence interval [37] for all patients. MRI data were summarized at each visit by giving the number and frequency of cases with their 95% confidence intervals.
The effect of some BL characteristics (years from diagnosis [0–3, 3–6, > 6], presence of co-morbidities, EDSS score at BL [< 3, 3–4, 5 or > 4, 5], age [< 55 versus > 55 years], co-medications versus no co-medications, type of prior MS treatment [oral or injectable]) on the change in MSIS total score was also investigated. The Mann–Whitney–Wilcoxon test was used to compare two subgroups, and the Kruskal–Wallis ANOVA was used to compare three different subgroups.
Results
Patient Demographics
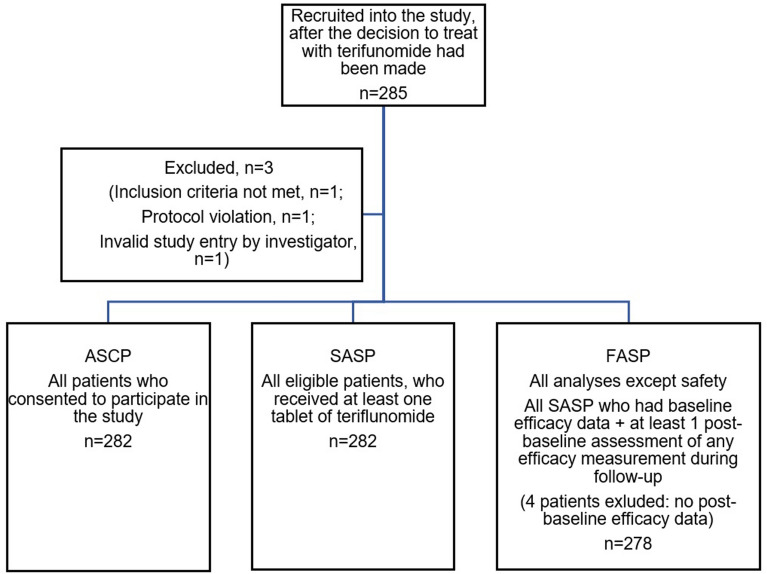
AURELIO enrolled 282 patients with RRMS (SASP) as per the flow diagram of study populations presented in Fig. 1. Baseline patient characteristics summarized in Table 1 show that 62.8% of patients were female and 44.6% of patients were treatment-naïve. The mean age of study participants was 44.8 (SD ± 11) years, and the mean BL EDSS score was 2.0 (SD ± 1.6). The most frequent concomitant medications prescribed for the study population were cholecalciferol (29 patients, 10.3%), methylprednisolone (31, 11%), levothyroxine sodium (24, 8.5%) and famipiridine (14, 5%), while the most common disorders (apart from RRMS) reported in patients’ medical histories at study entry were depression (14.2%) and dyslipidemia (5.3%).
Fig. 1.
Flow diagram of patients enrolled in the AURELIO study. ASCP all subjects consented population, FASP full analysis set population, SASP safety FASP analysis set population
Table 1.
Patient demographics and baseline PRO scores
| Parametera | Baseline score | Number |
|---|---|---|
| Age, mean (SD), range, years | 44.8 (11), 18–72 | 282b |
| Females, n (%) | 177 (62.8) | 282b |
|
Time since MS diagnosis Mean years (SD) |
6.87 (7.13) | 278c |
| Treatment naïve (n, %) | 126 (44.6) | 282 |
| Number of relapses in past 2 years | 0.74 (0.82) | 278c |
| EDSS score, mean (SD) | 2 (1.6) | 278c |
| PDDS score, mean (SD) | 1.2 (1.6) | 271c |
| Baseline QoL score (MSIS-29) | 48.3 (16.7) | 275c |
| Baseline QoL score (MSIS-29), psychological score | 25 (22.4) | 275c |
| Baseline QoL score (MSIS-29), physical score | 20.9 (20.5) | 275c |
| TSQM effectiveness | 66.1 (15.9) | 273c |
| TSQM side effects | 94.7 (14.8) | 272c |
| TSQM convenience | 85.2 (14.3) | 272c |
| TSQM global satisfaction | 63.6 (16.8) | 270c |
EDSS Expanded Disability Status Scale, MSIS Multiple Sclerosis Impact Scale, PDDS Patient Determined Disease Steps, QoL quality of life, SD standard deviation, TSQM Treatment Satisfaction Questionnaire for Medication
aMean (SD), unless otherwise stated
bSafety analysis set population (SASP)
cFull analysis set population (FASP)
Patient Disposition
Two hundred and twelve of the 282 enrolled patients (75.2%) completed the study, indicating good persistence over 2 years. Eleven patients (3.9%) discontinued due to efficacy (investigator’s decision) and 18 (6.4%) did not complete the study because of an AE (see below). Forty-one patients (14.5%) withdrew from the study for ‘other’ reasons or were lost to follow-up. In the case of 15 of these patients (5.3%), the investigator moved to another site and therefore the study data could not be completed; for a further five patients (1.8%) there was no response from the investigator. No pregnancies were reported during the study. One patient (0.4%) died during the study, but this event was deemed to be unrelated to teriflunomide treatment.
Primary Outcome: Quality of Life
The mean MSIS-29 total score improved slightly both at 12 and 24 months from the BL value of 48.3 ± 16.7 (mean ± SD), though the changes did not reach statistical significance (Fig. 2). In this way, the MSIS-29 total score improved by − 0.8 ± 11.3 after 12 months (p = 0.0836) and by − 0.8 ± 12.5 (p = 0.0614) after 24 months of teriflunomide treatment, thus showing that QoL remained stable during this period.
Fig. 2.
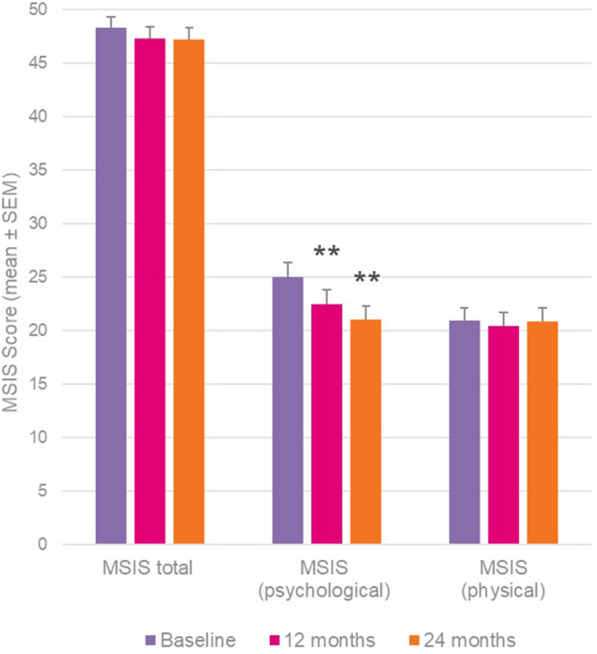
MSIS-29 outcomes at 12 and 24 months versus baseline. Wilcoxon signed-rank test; **p < 0.01 versus baseline
When the two parts of the MSIS-29 were examined separately, the psychological scale showed significant improvements over 2 years versus BL (change at 12 months: − 2.5 ± 18.9, p < 0.01; at 24 months: − 3.4 ± 19.7, p < 0.01), while the physical scale remained stable (Fig. 2).
TSQM
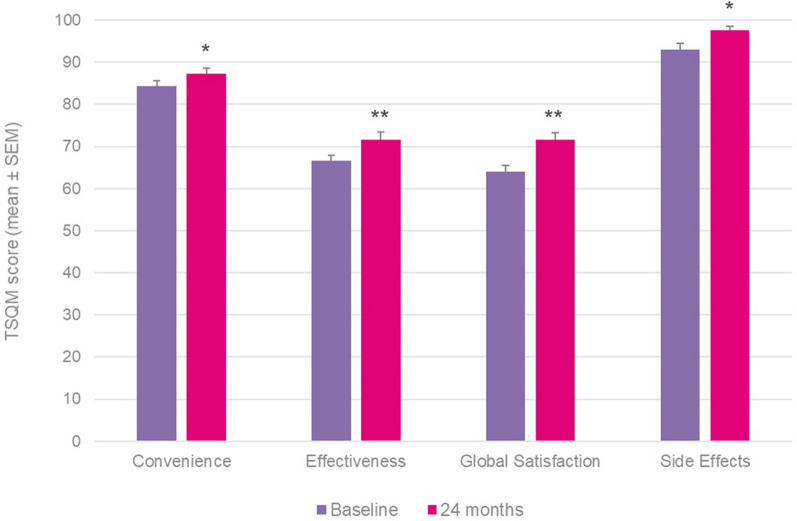
Results for the TSQM at 24 months showed significant improvements in convenience (+ 1.9, p = 0.0256), effectiveness (+ 6.6, p = 0.0001) and global satisfaction (+ 8.1, p = 0.0001), and a non-significant improvement in side effects (+ 1.1), with similar improvements and statistically significant outcomes also identified at the interim 12-month time-point (Fig. 3).
Fig. 3.
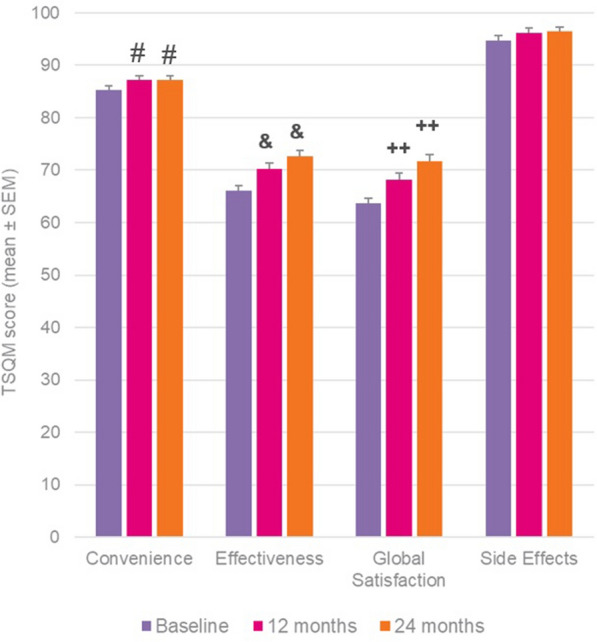
TSQM outcomes in whole patient cohort at 12 and 24 months versus baseline. Wilcoxon signed-rank test; #p = 0.02, ++p < 0.001 and &p < 0.0001 versus baseline
Efficacy Outcomes
Stability in disease progression was indicated by non-significant changes in EDSS, PDDS, and PS assessments versus BL readings at 12 and 24 months in all cases. Mean (± SD) EDSS scores were 2.0 (± 1.6) at BL compared to 2.0 (± 1.7) at 12 months (p = 0.577) and 2.0 (± 1.8) at 24 months (p = 0.7382). Likewise, the mean PDDS score at BL was 1.2 ± 1.6 compared to 1.1 ± 1.5 at the 12-month visit (p = 0.1937), and 1.2 ± 1.5 at the 24-month visit (p = 0.7316). In a similar manner, the mean PS sum of scores at 12 months (9.7 ± 8.7) and 24 months (9.9 ± 8.3) were not statistically significantly different from BL (10.2 ± 9.0; p = 0.4295 and 0.6643, respectively).
The ARR following treatment with teriflunomide was very low at 0.065 [95% CI 0.035–0.095]. Overall, 32 relapses were reported in 26 patients, while 256 patients (> 90%) did not suffer any relapses at all over the 24 months of the study period. Further evidence of disease stability over the 24 months of the study was provided by MRI data, where stable scans were available from 77 of 84 patients with RRMS (91.7%) with MRI scan data after 2 years of once daily oral teriflunomide treatment. Overall, 155 patients (55.8%) achieved NEDA-3 (no evidence of disease activity), defined as no relapse during the study, stable MRI and stable EDSS.
Adherence to teriflunomide was excellent and sustained throughout the 2-year study, with mean adherence above 97.6% for each study visit. That is to say, of all the study visits, no fewer than 97.6% of patients took at least 80% of the teriflunomide tablets prescribed to them by their neurologist.
Safety
The mean number of days of exposure to teriflunomide was 647 days (SD 203 days). In the 282 patients comprising the SASP who received at least one dose of teriflunomide, 91 (32.3%) reported 215 adverse events. The most frequently reported were (n, %) alopecia (24, 8.5%), diarrhea (16, 5.7%), and alanine aminotransferase (14, 5%). Adverse events occurring in more than 1% of patients and deemed by investigators as related to teriflunomide are listed in Table 2.
Table 2.
Adverse events occurring in more than 1% of patients and classified by investigators as related to teriflunomide
| Adverse event | Frequency, n (%) |
|---|---|
| Alopecia | 24 (8.5) |
| Diarrhea | 16 (5.7) |
| Elevated alanine aminotransferase | 14 (5) |
| Nausea | 4 (1.4) |
| Dizziness | 4 (1.4) |
| Elevated gamma-glutamyltransferase | 3 (1.1) |
| MS relapse (reported as an adverse event) | 3 (1.1) |
There were 32 SAEs reported in 27 patients (9.6%), the most common being MS relapse (17 events) and diarrhea (two events). Of the serious adverse events, six (2.1%) were considered drug-related and therapy was interrupted. Although not judged related to the study drug by the investigator, there was one fatal outcome—a 35-year-old man with acute lower respiratory tract infection and septic shock.
Twelve AESIs were reported during the study (autoimmune hepatitis [one event], infectious mononucleosis [one event], increase in alanine aminotransferase level [eight events], increase in transaminase level [one event], and hypertension [one event]).
Concomitant Medications
The most frequent concomitant mediations taken by participants in AURELIO are listed in Supplementary Table 2 (Supplementary Material).
Subgroup Analysis of Patients Switching from Injectable DMTs
We were interested to describe QoL and treatment satisfaction outcomes in patients who had switched from first-line injectable DMTs (glatiramer acetate and all forms of interferon beta) prior to enrolling in AURELIO given that lack of efficacy, AEs, or convenience are common reasons triggering a treatment change.
Of the 282 eligible patients enrolled in AURELIO, 123 had been previously treated with at least one injectable DMT. Baseline characteristics of this cohort are presented in Table 3. Compared to the full AURELIO cohort (Table 1), this subgroup on average was older, had higher disability, longer time since diagnosis and poorer QoL.
Table 3.
Baseline demographics and PRO scores for the subgroup of patients previously treated with first-line injectable therapies (glatiramer acetate, all forms of interferon beta)
| Parametera | Baseline score | Number |
|---|---|---|
| Age, years | 46.8 (10.4) | 123 |
| Females, n (%) | 77 (62.6) | 123 |
| EDSS score | 2.4 (1.7) | 116 |
| Time since diagnosis, years | 9.77 (6.08) | 123 |
| MSIS total score | 49.3 (17.8) | 122 |
| Number of relapses in 2 years prior to Visit 1 | 0.65 (0.77) | 123 |
| TSQM convenience score | 84.3 (14.1) | 120 |
| TSQM effectiveness score | 66.6 (15.1) | 120 |
| TSQM global satisfaction score | 64.1 (15.4) | 119 |
| TSQM side effects score | 92.9 (16.6) | 120 |
EDSS Expanded Disability Status Scale, MSIS Multiple Sclerosis Impact Scale, SD standard deviation, TSQM Treatment Satisfaction Questionnaire for Medication
aMean (SD) unless otherwise noted
Ninety-four of the 123 patients (76.4%) completed the study, indicating good persistence. Reasons for discontinuation were lack of efficacy (four patients), AEs (nine patients), and other reasons/lost to follow-up (16 patients). The mean MSIS-29 total score improved from 49.3 ± 17.8 (mean ± SD) at BL to 47.2 ± 16.2 at 24 months, although this trend did not reach statistical significance (p = 0.08).
Significant improvements in TSQM scores after 24 months versus BL were seen for all four domains of the measure (Fig. 4): global satisfaction and effectiveness (p < 0.0001), and for convenience and side effects (p < 0.05).
Fig. 4.
TSQM outcomes at 24 months versus BL in subgroup analysis of patients previously treated with first-line injectable DMTs. Wilcoxon signed-rank test; *p < 0.05; **p < 0.0001 versus baseline
The ARR for this subgroup was very low at 0.0644 (95% CI 0.0196–0.1093) and similar to that seen for the full cohort (0.0649; 95% CI 0.0349–0.0948). Disability remained stable for patients switching from injectables to teriflunomide, with no statistically significant change between BL and month 24 seen with respect to EDSS (p = 0.6066). Adherence at the 24-month visit for this subgroup was very high at 100%.
Additional Analyses
Further analyses were performed to assess the effect of several BL characteristics (years since MS diagnosis, presence of co-morbidities, EDSS score at BL, age < 55 years or > 55 years, co-medications) on MSIS-29 total score, TSQM subsections, EDSS, and MRI outcomes.
Significant differences between BL and month 24 were seen in MSIS-29 total score values with respect to age (≤ 55 versus > 55 years; p = 0.0015), while for TSQM subsections, significant differences between BL and month 24 were seen for ‘Effectiveness’ with respect to years since diagnosis (p = 0.0455).
The ratio of stable MRI results (descriptive data only) increased from BL to 24 months in all age groups (≤ 55 years; > 55 years), as well as in patients with concomitant medications, in patients with one or more than one co-morbidity, in all EDSS categories (3 ≤ EDSS ≤ 4.5; EDSS < 3; EDSS > 4.5); in patients diagnosed ≤ 3 years and > 6 years, independently from the administration route of previous MS treatments.
When we assessed all variables for potential differences with respect to previously treated versus treatment-naïve patients, the only differences observed were for the TSQM ‘Convenience’ score [where the mean score at 24 months was higher in the pre-treated subgroup (p = 0.0449)], and ARR, (where previously treated patients had a higher mean ARR (0.0701 [95% confidence interval (CI) 0.0283–0.1119]) compared with treatment-naïve patients (0.0584 [95% CI 0.0159–0.101]).
Discussion
AURELIO is the first real-world observational study to have analyzed treatment outcomes in a cohort of patients with RRMS prescribed teriflunomide in standard clinical practice in Greece. The study adds to an expanding body of PRO-related knowledge generated from real-world observational and registry studies showing evidence of solid efficacy and safety outcomes with this once-daily oral medication [20–24, 38–40].
Consistent with other real-world studies [20–24], the patient cohort here was older, had fewer relapses in the previous 2 years, and had less disability at BL than patients enrolled in the phase 3 TEMSO and TOWER clinical trials (age: 44.8 years in AURELIO versus around 38 years in TEMSO and TOWER; Mean relapses (previous 2 years): 0.7 in AURELIO; > 2 in TEMSO and TOWER; EDSS: 2.0 ± 1.6 (mean ± SD) in AURELIO versus 2.7 ± 1.2 (TEMSO) and 2.7 ± 1.4 (TOWER) in the 14 mg teriflunomide treatment arms) [12, 14]. This observation suggests that in this Greek cohort teriflunomide was being prescribed as a first-line treatment for patients with low disease activity and low disability levels.
The discontinuation rate recorded in AURELIO (24.8%) over two years was in line with that of other 2-year observational studies with teriflunomide such as Teri-PRO (21.4%) [20], Teri-LIFE (41%) [22], TAURUS (21.5%) [24], Teri-REAL (31%) [23], and Teri-FAST (34.6%) [21]. Although not taking into account that some patients may have left the study but remained on treatment, these values might partially reflect the plethora of alternative treatment options available to patients with RRMS should expectations concerning efficacy, safety, adherence, tolerance, or other factors not be fulfilled.
The primary efficacy outcome measured here was that of QoL as determined by MSIS-29 test scores. The mean MSIS-29 total score declined slightly at 12 and 24 months compared to BL, indicating a non-significant trend towards QoL improvement with teriflunomide treatment. On the other hand, the psychological subscale component of the MSIS-19 did show small though significant mean improvements (p < 0.01) at 12 and 24 months versus BL, while the physical subscale component remained stable. To the best of our knowledge, an analysis of what constitutes a clinically meaningful change in the psychological subscale has not been performed; most studies using the MSIS-29 subscales concentrate on the physical component where a change of at least 7.5 points is considered to be clinically meaningful [41–44]. Nevertheless, the results obtained here indicate stable QoL outcomes over 2 years in teriflunomide-treated patients, which is consistent with QoL results from other real-world studies in which PRO-based tests for QoL were reported [20–23].
The TSQM results at 12 and 24 months showed statistically significant improvements in convenience, effectiveness and global satisfaction, and non-significant improvements in side effects. Similarly positive outcomes in the TSQM for teriflunomide-treated patients were reported for the real-world Teri-PRO [20, 45] and TAURUS [24] studies. As pointed out by Haase and colleagues [46], improved treatment satisfaction in the PRO-based TSQM test is associated with improved drug adherence, and indeed in the AURELIO study we measured exceptional treatment adherence at levels close to 100%.
Overall, more than 90% of patients were relapse-free over the 24 months of the study period, meaning that teriflunomide treatment was also associated with extremely low rates of relapse in the AURELIO study. An ARR of 0.065 [95% CI 0.0349–0.0948] is much lower than that reported in phase 2 and 3 clinical trials and other real-world studies in the teriflunomide clinical development program. However, the result is not unprecedented given that an ARR of 0.08 ± 0.31 (mean ± SD) was seen in the 2-year Teri-REAL study in Hungary [23]. This may reflect the profile of patients and the clinical reality of teriflunomide prescribing practices in Greece and Hungary, with both AURELIO and Teri-REAL enrolling patients with low relapse activity, low disability, and 45–50% being treatment naïve (this study [23]. Stability in disease progression was also indicated by non-significant changes in EDSS, PDDS, and PS assessments versus BL readings at 12 and 24 months in all cases. This fits with teriflunomide’s favorable impact on disease progression data (disability (EDSS), brain atrophy) seen during the clinical trial program [12, 15, 47, 48].
Thirty-two percent of patients reported adverse events during the treatment period, with just under 10% of patients reporting serious AEs. The most frequent AEs were alopecia, diarrhea, and elevated liver enzyme levels, which is consistent with AE profiles reported in clinical trial and RWE study program [11, 12, 14, 15, 20–24]. No new safety signals were seen.
In the AURELIO study we were also interested in examining treatment satisfaction treatment satisfaction and QoL outcomes in patients who were previously treated with injectable DMTs and who then switched to teriflunomide. These patients had switched to teriflunomide for reasons based on tolerance, safety, convenience, and patient’s preference. Results showed significant improvements in TSQM scores after 24 months versus BL in all four domains of the measure. This was accompanied by an improved mean MSIS-29 total score versus BL, although this did not reach statistical significance. Similar TSQM results in teriflunomide-treated patients who switched from first-line injectables (glatiramer acetate or interferon-beta) were reported by Kallmann et al. [24] for the TAURUS study in Germany.
AURELIO, like all RWE studies, has inherent limitations which should be considered when interpreting the study’s outcomes. Being a single-arm, open label study, there is no comparator group for reference. Moreover, although patients who were lost to follow-up may have continued teriflunomide treatment, it is reasonable to assume that some discontinuations were for efficacy or safety reasons. Discontinuations for such motives would have the effect of enriching the cohort for patients who were doing well on the drug.
Conclusions
Overall, the AURELIO study showed good QoL, treatment satisfaction, efficacy, safety, and adherence outcomes in this cohort of Greek patients with RRMS. Data from patients switching to teriflunomide from first-line injectable drugs also suggest that teriflunomide is associated with a more convenient route of administration as well as greater perceived efficacy and tolerability. These results should be of particular interest to MS neurologists in Greece, a country with one of the highest physician-to-patient ratios in the world [25].
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the participants of the study and their families/carers for their efforts. The AURELIO study investigators (listed below) are also thanked for enrolling patients in the study.
Funding
This research and the journal’s Rapid Service Fee were funded by Sanofi.
Editorial Assistance
Editorial assistance for the preparation of this article was provided by Dr. Michael Patterson of Sciencedit S.L. and funded by Sanofi.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conceptualization: Efthymios Dardiotis, Georgia Perpati, Nikolaos Grigoriadis; methodology: Efthymios Dardiotis, Georgia Perpati, Nikolaos Grigoriadis; formal analysis: Mariann Borsos, Georgia Perpati; writing—original draft preparation: Georgia Perpati. All authors read and approved the final manuscript.
List of Investigators
In addition to the authors of this article, the following AURELIO investigators enrolled at least one patient in the study: Sotirios Papagiannopoulos (General Hospital of Thessaloniki G. Papanikolaou); Anastasios Orologas (University Hospital of Thessaloniki AHEPA); Panagiotis Aggelidakis (General Hospital of Athens EVAGELISMOS); Antonios Kodounis (Hellenic Air force General Hospital); Nicolaos Fakas (General Military Hospital of Athens 401); Alexandros Papadimitriou (Henry Dunant General Hospital); Ioannis Iliopoulos (University General Hospital of Alexandroupolis); Vasiliki Kostadima (University General Hospital of Ioannina); Klimentini Karageorgiou (Athens Medical Center); Sotirios Lamprakopoulos (University General Hospital of Alexandroupolis); Dimitrios Nikiforidis (General Hospital of Xanthi); Konstantinos Voumvourakis (General University Hospital Attikon); Afroditi Kaponi (ATHENS EUROCLINIC); Maria Maltezou (Oncology Hospital of Kifissia ''AG. ANARGYROI''); Tereza Pasqua Acquaviva ("THRIASIO" General Hospital of Elefsina); Elli Kerezoudi (PAMMAKARISTOS Hospital); Georgios Balamoutsos (EUROMEDICA Kyanous Stavros General Hospital); Antonios Kerasnoudis (St. Luke's Hospital); Panayiotis Mitsias (University General Hospital of Herakleion «PAGNI»); Triantafyllos Doskas (Naval Hospital of Athens); Thomas Maris (General Hospital of Herakleion "VENIZELEIO PANANEIO"); Christos Baltogiannis (T.Y.P.E.T. Clinic "Hygeias Melathron"); Elisavet Chroni (University General Hospital of Patra)
Prior Presentation
Some data from this article were previously presented in poster communication form at the 37th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), October 13–15, 2021, Vienna, Austria (P854 and P876).
Disclosures
Efthymios Dardiotis: Consulting, speaking fees, principal investigator, grants/research support (Allergan, Novartis, Genesis, ELPEN, Bayer, Teva, Merck-Serono, Sanofi Genzyme, Roche, UCB, IPSEN); Georgia Perpati: Employed as Medical Lead MS by Sanofi Greece & Cyprus; Mariann Borsos: Nothing to declare; Ioannis Nikolaidis: Consulting, speaking fees, grants/research support (Sanofi Genzyme, Specifar-Teva, Genesis Pharma, Novartis; Hellenic Foundation for Research and Innovation (H.F.R.I.) and General Secretariat for Research and Innovation (GSRI)); Dimitrios Tzanetakos: Consulting, speaking fees, travel grants (Roche, Teva, Sanofi Genzyme, Novartis, and Genesis Pharm); Georgia Derentzi: Consulting, speaking fees, principal investigator (Merck, Genesis Pharma, Mylan, Teva, Sanofi Genzyme); Evangelos Koutlas: Consulting, speaking fees, principal investigator (Genesis, Merck, Schering, Sanofi, Teva, Novartis); Constantinos Kilidireas: Consulting fees (Roche, Merck, Genesis, Teva, Sanofi); Dimos Dimitrios Mitsikostas: Consulting, speaking fees, principal investigator, and grants/research support (Allergan, Amgen, Bayer, Biogen, Cefaly, Genesis Pharma, GlaxoSmithKline, ElectroCore, Eli Lilly, Merck-Serono, Merz, Mylan, Lundbeck, Novartis, Roche, Sanofi Genzyme, Specifar, Teva); Georgios Hadjigeorgiou: Nothing to declare; Nikolaos Grigoriadis: Consulting, speaking fees, principal investigator, grants/research support (Biogen Idec, Biologix, Novartis, TEVA, Bayer, Merck Serono, Genesis Pharma, Sanofi Genzyme, Roche, Cellgene, ELPEN).
Compliance with Ethics Guidelines
The study was conducted in accordance with the guidelines for Good Epidemiology Practice (2007), and complied with local regulations, including local data protection regulations, individual hospital scientific committee approval, and the ethical principles of the Declaration of Helsinki (1964 and subsequent amendments). All patients provided written informed consent and were willing and able to participate in the study and complete the study’s questionnaires. With respect to local regulations, no national ethics committee approval was required. However, in compliance with Greece’s National Medicines Organization (EOF) circular (EOF 82798/22-11-2012), scientific board approval from each study site was obtained and filed with the sponsor.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. Some data are not publicly available due to the potential for patient privacy/consent to be compromised.
Footnotes
The members of the AURELIO investigators are listed in the Acknowledgements section.
Contributor Information
Nikolaos Grigoriadis, Email: ngrigoriadis@auth.gr.
The AURELIO investigators:
Sotirios Papagiannopoulos, Anastasios Orologas, Panagiotis Aggelidakis, Antonios Antonios, Nicolaos Fakas, Alexandros Papadimitriou, Ioannis Iliopoulos, Vasiliki Kostadima, Klimentini Karageorgiou, Sotirios Lamprakopoulos, Dimitrios Nikiforidis, Konstantinos Voumvourakis, Afroditi Kaponi, Maria Maltezou, Tereza Pasqua Acquaviva, Elli Kerezoudi, Georgios Balamoutsos, Antonios Kerasnoudis, Georgia Deretzi, Panayiotis Mitsias, Triantafyllos Doskas, Thomas Maris, Christos Baltogiannis, and Elisavet Chroni
References
- 1.Multiple Sclerosis International Federation. Atlas of MS 3rd edition. Mult Scler Int Fed. 2020;1–37.
- 2.Giovannoni G, Butzkueven H, Dhib-Jalbut S, Hobart J, Kobelt G, Pepper G, et al. Brain health: time matters in multiple sclerosis. Mult Scler Relat Disord. 2016;9:S5–48. doi: 10.1016/j.msard.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Ziemssen T, Derfuss T, De SN, Giovannoni G. Optimizing treatment success in multiple sclerosis. J Neurol. 2016;263(6):1053–1065. doi: 10.1007/s00415-015-7986-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boeije HR, Duijnstee MS, Grypdonck MHPA. Encountering the downward phase: biographical work in people with multiple sclerosis living at home. Soc Sci Med. 2002;55(6):881–893. doi: 10.1016/S0277-9536(01)00238-6. [DOI] [PubMed] [Google Scholar]
- 5.Cree BAC, Arnold DL, Chataway J, Chitnis T, Fox RJ, Pozo Ramajo A, et al. Secondary progressive multiple sclerosis: new insights. Neurology. 2021;97(8):378–388. doi: 10.1212/WNL.0000000000012323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feinstein A. The neuropsychiatry of multiple sclerosis. Can J Psychiatry. 2004;49(3):157–163. doi: 10.1177/070674370404900302. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell AJ, Benito-León J, González JMM, Rivera-Navarro J. Quality of life and its assessment in multiple sclerosis: Integrating physical and psychological components of wellbeing. Lancet Neurol. 2005;4(9):556–566. doi: 10.1016/S1474-4422(05)70166-6. [DOI] [PubMed] [Google Scholar]
- 8.Noble JG, Osborne LA, Jones KH, Middleton RMFD. Commentary on “disability outcome measures in multiple sclerosis clinical trials”. Mult Scler. 2012;18(12):1718–1720. doi: 10.1177/1352458512457847. [DOI] [PubMed] [Google Scholar]
- 9.Baumstarck K, Boyer L, Boucekine M, Michel P, Pelletier J, Auquier P. Measuring the quality of life in patients with multiple sclerosis in clinical practice: a necessary challenge. Mult Scler Int. 2013;1–8. [DOI] [PMC free article] [PubMed]
- 10.Sanofi-Aventis. AUBAGIO (teriflunomide) Summary of Product Characteristics. 2013. https://www.ema.europa.eu/en/documents/product-information/aubagio-epar-product-information_en.pdf. Accessed May 2022.
- 11.O’Connor PW, Li D, Freedman MS, Bar-Or A, Rice GPA, Confavreux C, et al. A phase II study of the safety and efficacy of teriflunomide in multiple sclerosis with relapses. Neurology. 2006;66(6):894–900. doi: 10.1212/01.wnl.0000203121.04509.31. [DOI] [PubMed] [Google Scholar]
- 12.O’Connor P, Wolinsky JS, Confavreux C, Comi G, Kappos L, Olsson TP, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med. 2011;365(14):1293–1303. doi: 10.1056/NEJMoa1014656. [DOI] [PubMed] [Google Scholar]
- 13.Wolinsky JS, Narayana PA, Nelson F, Datta S, O’Connor P, Confavreux C, et al. Magnetic resonance imaging outcomes from a phase III trial of teriflunomide. Mult Scler J. 2013;19(10):1310–1319. doi: 10.1177/1352458513475723. [DOI] [PubMed] [Google Scholar]
- 14.Confavreux C, O’Connor P, Comi G, Freedman MS, Miller AE, Olsson TP, et al. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13(3):247–256. doi: 10.1016/S1474-4422(13)70308-9. [DOI] [PubMed] [Google Scholar]
- 15.Miller AE. An updated review of teriflunomide’s use in multiple sclerosis. Neurodegener Dis Manag. 2021;11(5):387–409. doi: 10.2217/nmt-2021-0014. [DOI] [PubMed] [Google Scholar]
- 16.Trojano M, Tintore M, Montalban X, Hillert J, Kalincik T, Iaffaldano P, et al. Treatment decisions in multiple sclerosis-insights from real-world observational studies. Nat Rev Neurol. 2017;13(2):105–118. doi: 10.1038/nrneurol.2016.188. [DOI] [PubMed] [Google Scholar]
- 17.Moseley J. Regulatory Perspective on Real World Evidence (RWE) in scientific advice. https://www.ema.europa.eu/en/documents/presentation/presentation-regulatory-perspective-real-world-evidence-rwe-scientific-advice-emas-pcwp-hcpwp-joint_en.pdf. Accessed May 2022.
- 18.U.S. Food & Drug Administration. Framework for FDA’s Real-World Evidence Program. https://www.fda.gov/media/120060/download. Accessed May 2022.
- 19.Chan A, Gobbi C, Maurer M, Rufi R, Poole E M-LJ. Teriflunomide Use in European Clinical Practice in Patients with Relapsing Forms of Multiple Sclerosis: An Overview of Regional Real-World Studies. In: European academy of neurology annual congress. 2018. p. Poster EPR3094.
- 20.Coyle PK, Khatri B, Edwards KR, Meca-Lallana JE, Cavalier S, Rufi P, et al. Patient-reported outcomes in relapsing forms of MS: real-world, global treatment experience with teriflunomide from the Teri-PRO study. Mult Scler Relat Disord. 2017;17(July):107–115. doi: 10.1016/j.msard.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 21.de Sèze J, Devy R, Planque E, Delabrousse-Mayoux JP, Vandhuick O, Kabir M, et al. Fatigue in teriflunomide-treated patients with relapsing remitting multiple sclerosis in the real-world Teri-FAST study. Mult Scler Relat Disord. 2020;2021(47):102659. doi: 10.1016/j.msard.2020.102659. [DOI] [PubMed] [Google Scholar]
- 22.Hestvik ALK, Frederiksen J, Nielsen HH, Eek C, Huang-link Y, Haghighi S, et al. Teri-LIFE: an observational study of quality of life in patients with relapsing remitting multiple sclerosis treated with teriflunomide in the Nordic region. ECTRIMS P452. 2019.
- 23.Bencsik K, Dobos E, Jobbágy Z, Birkás AJ, Kovács K, Sátori M, et al. Patient-reported outcomes to assess quality of life in teriflunomide-treated patients with relapsing–remitting multiple sclerosis: results of teri-real—a real-world study from Hungary. In: ECTRIMS. 2021. p. P863. [DOI] [PMC free article] [PubMed]
- 24.Kallmann BA, Tiel-Wilck K, Kullmann JS, Engelmann U, Chan A. Real-life outcomes of teriflunomide treatment in patients with relapsing multiple sclerosis: TAURUS-MS observational study. Ther Adv Neurol Disord. 2019;12(January):1–14. doi: 10.1177/1756286419835077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bakirtzis C, Grigoriadou E, Boziki MK, Kesidou E, Siafis S, Moysiadis T, et al. The administrative prevalence of multiple sclerosis in Greece on the basis of a nationwide prescription database. Front Neurol. 2020;11(September):1–9. doi: 10.3389/fneur.2020.01012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Good Epidemiological Practice—IEA guidelines for proper conduct of epidemiological research.
- 27.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162–173. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- 29.Hobart J, Lamping D, Fitzpatrick R, Riazi A, Thompson A. The multiple sclerosis impact scale (MSIS-29) a new patient-based outcome measure. Brain. 2001;124(5):962–973. doi: 10.1093/brain/124.5.962. [DOI] [PubMed] [Google Scholar]
- 30.Osmani D, Rajagopalan K, Hobart J. Pnd33 linguistic validation of the multiple sclerosis impact scale (Msis-29) for use in 29 languages. Value Health. 2009;12(7):A371. doi: 10.1016/S1098-3015(10)74827-0. [DOI] [Google Scholar]
- 31.Atkinson MJ, Sinha A, Hass SL, Colman SS, Kumar RN, Brod M, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004;2:1–13. doi: 10.1186/1477-7525-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurtzke J. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33(11):1444–1452. doi: 10.1212/WNL.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 33.Hohol MJ, Orav EJWH. Disease steps in multiple sclerosis: a simple approach to evaluate disease progression. Neurology. 1995;45(2):251–255. doi: 10.1212/WNL.45.2.251. [DOI] [PubMed] [Google Scholar]
- 34.Hohol MJ, Orav EJWH. Disease steps in multiple sclerosis: a longitudinal study comparing disease steps and EDSS to evaluate disease progression. Mult Scler. 1999;5(5):349–354. doi: 10.1177/135245859900500508. [DOI] [PubMed] [Google Scholar]
- 35.Marrie RA, Goldman M. Validity of performance scales for disability assessment in multiple sclerosis. Mult Scler. 2007;13(9):1176–1182. doi: 10.1177/1352458507078388. [DOI] [PubMed] [Google Scholar]
- 36.Osterberg L, Blaschke T. Drug therapy—adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 37.Tanusit M. Two-side confidence intervals for the Poisson means. Int J Model Optim. 2012;2(5):589–591. doi: 10.7763/IJMO.2012.V2.189. [DOI] [Google Scholar]
- 38.Alnajashi HA, Alshamrani FJ, Freedman MS. Tolerability and discontinuation rates in teriflunomide-treated patients: a real-world clinical experience. Neurosciences. 2018;23(3):204–207. doi: 10.17712/nsj.2018.3.20180003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bucello S, Annovazzi P, Ragonese P, Altieri M, Barcella V, Bergamaschi R, et al. Real world experience with teriflunomide in multiple sclerosis: the TER-Italy study. J Neurol. 2021;268(8):2922–2932. doi: 10.1007/s00415-021-10455-3. [DOI] [PubMed] [Google Scholar]
- 40.Papp V, Buron MD, Siersma V, Rasmussen PV, Illes Z, Kant M, et al. Real-world outcomes for a complete nationwide cohort of more than 3200 teriflunomide-treated multiple sclerosis patients in the Danish Multiple Sclerosis Registry. PLoS ONE. 2021;16:1–16. doi: 10.1371/journal.pone.0250820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Costelloe L, Rourke KO, Kearney H, Mcguigan C, Gribbin L, Daly L, et al. The patient knows best: significant change in the physical component of the Multiple Sclerosis Impact Scale (MSIS-29 physical) J Neurol Neurosurg Psychiatry. 2007;78:841–844. doi: 10.1136/jnnp.2006.105759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phillips GA, Wyrwich KW, Guo S, Medori R, Altincatal A, Wagner L, et al. Responder definition of the Multiple Sclerosis Impact Scale physical impact subscale for patients with physical worsening. Mult Scler. 2014;20(13):1753–1760. doi: 10.1177/1352458514530489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gasperini C, Hupperts R, Lycke J, Short C, McNeill M, Zhong J, et al. Prolonged-release fampridine treatment improved subject-reported impact of multiple sclerosis: item-level analysis of the MSIS-29. J Neurol Sci. 2016;370:123–131. doi: 10.1016/j.jns.2016.08.052. [DOI] [PubMed] [Google Scholar]
- 44.Hobart J, Ziemssen T, Feys P, Linnebank M, Goodman AD, Farrell R, et al. Assessment of clinically meaningful improvements in self-reported walking ability in participants with multiple sclerosis: results from the randomized, double-blind, phase III ENHANCE trial of prolonged-release fampridine. CNS Drugs. 2019;33(1):61–79. doi: 10.1007/s40263-018-0586-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coyle PK, Khatri B, Edwards KR, Meca-Lallana JE, Cavalier S, Rufi P, et al. Patient-reported outcomes in patients with relapsing forms of MS switching to teriflunomide from other disease-modifying therapies: results from the global Phase 4 Teri-PRO st. Mult Scler Relat Disord. 2018;26:211–218. doi: 10.1016/j.msard.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 46.Haase R, Kullmann JS, Ziemssen T. Therapy satisfaction and adherence in patients with relapsing–remitting multiple sclerosis: the THEPA-MS survey. Ther Adv Neurol Disord. 2016;9(4):250–263. doi: 10.1177/1756285616634247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Radue EW, Sprenger T, Gaetano L, Mueller-Lenke N, Cavalier S, Thangavelu K, et al. Teriflunomide slows BVL in relapsing MS. Neurol Neuroimmunol NeuroInflamm. 2017;4(5):1–7. doi: 10.1212/NXI.0000000000000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zivadinov R, Dwyer MG, Carl E, Poole EM, Cavalier S, Briassouli P, et al. Slowing of brain atrophy with teriflunomide and delayed conversion to clinically definite MS. Ther Adv Neurol Disord. 2020;13:1–13. doi: 10.1177/1756286420970754. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. Some data are not publicly available due to the potential for patient privacy/consent to be compromised.