Abstract
Introduction
Comparisons of healthcare utilization and expenditure among multiple sclerosis (MS) disease-modifying therapies (DMTs) are limited.
Methods
In this retrospective cohort study using commercial insurance claims data of a US population (2010–2019), we compared healthcare utilization and costs in MS across different DMTs. We assigned patients to different treatment arms: no DMT (ND), high-efficacy (HE) DMT (alemtuzumab, B cell depletion, cladribine, and natalizumab), and standard-efficacy (SE) DMT (dimethyl fumarate, glatiramer acetate, interferon beta, sphingosine-1-phosphate receptor modulator, and teriflunomide). We obtained healthcare costs and occurrences of healthcare services: outpatient visits, emergency room visits, hospitalizations, MS-related magnetic resonance imaging (MRI). We quantified relapses (based on MS-related hospitalizations, as well as outpatient visits with prescription of high-dose steroids) and medical complexity (based on unique drug classes of prescriptions). We calculated covariate-adjusted incidence rate ratio of healthcare services using negative binomial regression with ND as reference and covariate-adjusted mean cumulative healthcare costs using a generalized linear model with log-link function and gamma distribution.
Results
Among the 25,932 patients with MS (mean age 52.8 years, 75.2% women), both HE (mean age 54.0 years, 76.2% women) and SE (mean age 43.9 years, 75.6% women) groups had more non-pharmacy healthcare utilization than ND (mean age 57.6 years, 75.4% women), including overall outpatient doctor visits, neurology visits, and MS-related MRIs as well as relapses and medical complexities. Relative to ND, both HE and SE groups had higher pharmacy costs and overall healthcare costs 12 months after treatment initiation, despite having lower or equivalent non-pharmacy medical costs. In patients on DMT, pharmacy costs accounted for up to 65% of overall healthcare costs with over 85% of pharmacy costs attributable to DMT costs.
Conclusion
DMT cost is a key driver of the overall healthcare expenditure in MS. Future comparative and cost-effectiveness studies integrating claims and electronic health records data with better balancing of patient characteristics are warranted.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40120-022-00358-4.
Keywords: Multiple sclerosis, Disease-modifying therapy, DMT, Healthcare cost, Healthcare expenditure, Healthcare utilization, Health service, Administrative claims data, Pharmacoepidemiology
Key Summary Points
| Comparative studies of healthcare utilization and costs among multiple sclerosis (MS) disease-modifying therapies (DMTs) are limited. |
| In this study, we compared healthcare expenditure and utilization across DMTs in a commercially insured MS population in the USA during 2010–2019. |
| People with MS treated with both high- and standard-efficacy DMTs had significantly more overall outpatient doctor visits, neurology visits, MS-related brain and spine MRIs as well as occurrences of relapses and medical complexities than the untreated. |
| Both the high- and standard-efficacy DMT treatment groups had higher pharmacy costs and overall healthcare costs than the untreated group, despite having lower or equivalent non-pharmacy medical costs. |
| DMT cost is an important driver of healthcare expenditure in MS. |
Introduction
Multiple sclerosis (MS) is a chronic neurological disease with high socioeconomic burden [1–3]. Since the first approval of a disease-modifying therapy (DMT) in 1993, MS treatment has shifted from managing acute relapses to preventing disease activity and delaying disability as DMTs become integral to MS care [4]. Currently, there are more than 20 Food and Drug Administration (FDA)-approved DMTs.
People with MS (pwMS) access healthcare as they receive routine care and medications and when they experience relapses, disability, and comorbidities [3, 5, 6]. MS-related healthcare costs vary widely [7, 8]. One study estimated the annual DMT cost reaching (US) $70,000 per patient [9]. The growing DMT cost accounts for a major proportion of the MS healthcare expenditure [6, 10, 11]. Prior studies showed that DMTs reduce healthcare utilization, likely by reducing relapse and delaying disability [12, 13]. However, comparative examination of healthcare utilization and pharmacy costs across multiple DMTs or between DMT efficacy classes is limited [2, 14–17]. According to an economic analysis of 14 MS DMTs in 2017 by the non-profit Institute for Clinical and Economic Review [18], alemtuzumab was the only DMT to yield an economically attractive incremental cost-effectiveness ratio, at $38,277 per quality-adjusted life year ($48,787 per relapse avoided), though this DMT was rarely used because of serious side effects. Therefore, with more DMTs emerging in the past 5 years, there is a need for periodic reassessments of their real-world cost-effectiveness.
Claims data are ideal for examining healthcare utilization and expenditure. Here, we report the healthcare utilization and expenditure patterns in a large insured MS population in the USA from 2010 to 2019, across FDA-approved DMTs.
Methods
Study Population
We used anonymous patient-level commercial and Medicare Advantage claims data from Optum Clinformatics from January 1, 2010 to December 31, 2019 through a license to the University of Pittsburgh. The Institutional Review Board of the University of Pittsburgh determined the study as Non-Human Research and waived the need for ethical approval of study protocol and informed consent. The data include insurance claims for both medical (inpatient and outpatient) services and prescriptions [19].
We applied a published claims-based MS classification algorithm [20] to identify pwMS if the person had at least three separate MS-related claims (International Classification of Diagnosis 9 and 10 codes: ICD-9 = 340; ICD-10 = G35) on three separate days within 1 year, in any combination of inpatient, outpatient, or DMT-specific pharmacy claims. This practical method of identifying pwMS from claims has a positive predictive value of 95.4–97.8% for MS diagnosis in the prior study [20]. The study index date was the date of the first MS-related claim. A patient’s follow-up lasted until the end of either insurance coverage or study period, whichever occurred first.
Additional Inclusion Criteria
For the healthcare utilization and expenditure analysis, we included pwMS who met the additional criteria of having continuous enrollment for both medical and pharmacy benefits at least 3 months preceding and at least 12 months after the index date. We assigned patients to treatment groups based on DMT as identified from pharmacy dispensing claims.
We examined all FDA-approved DMTs during the study period: injectable (glatiramer acetate, interferon beta [all brands, including peginterferon beta-1a]), oral (cladribine, dimethyl fumarate, sphingosine-1-phosphate receptor [S1P-R modulators: fingolimod, siponimod], teriflunomide), and infusion (alemtuzumab, mitoxantrone, natalizumab, B cell depletion therapy [ocrelizumab, rituximab]). Although rituximab does not have approval for MS, it has been used off-label for MS in the USA. No patients with mitoxantrone prescriptions met the criteria. We excluded daclizumab given its voluntary withdrawal in 2018. We classified DMTs as high efficacy (HE) or standard efficacy (SE) [21]. HE DMTs included alemtuzumab, B cell depletion therapy, cladribine, and natalizumab. SE DMTs included dimethyl fumarate, glatiramer acetate, interferon beta, S1P-R modulator, and teriflunomide.
Healthcare Utilization
For each DMT, we calculated the median treatment duration (treatment end date – treatment start date + days of supplies of the last prescription) and the associated healthcare utilization. Using the study index date (for the untreated pwMS) or the DMT initiation date (for the DMT-treated pwMS) as the reference date, we obtained the events of general (outpatient doctor visit of any specialty, emergency room [ER] visit, hospital admission) and MS-related healthcare activities (outpatient neurology visit, MS-related hospital admission with MS as the primary diagnosis, magnetic resonance imaging [MRI] of brain or [cervical or thoracic] spine) as well as acute MS relapses and medical complexity during the study period for untreated pwMS and during the DMT treatment period for pwMS on DMTs, respectively. We defined an occurrence of MS relapse as any inpatient hospital stay with MS as the primary diagnosis or any outpatient visit (either ER or office visit) with prescriptions of high-dose corticosteroid (daily dose of at least 500 mg of prednisone or methylprednisolone), adrenocorticotropic hormone, or plasma exchange within 30 days after the visit [22–24]. We computed medical complexity as the number of unique classes of medication according to the American Hospital Formulary System (AHFS) Pharmacologic Therapeutic Classification System [14, 25].
Healthcare Expenditure
We calculated three categories of healthcare expenditure using the claims data (in US dollars): medical costs, pharmacy costs (including DMT costs), and overall healthcare costs (sum of medical and pharmacy costs). For each category, we calculated two types of charges: total charges and out-of-pocket charges, the latter by summing copayments, coinsurance, and deductible payments. For untreated pwMS, we obtained the cumulative healthcare expenditure at 6 months and 12 months after cohort entry. For DMT-treated pwMS, we obtained the cumulative healthcare expenditure at 6 months and 12 months after DMT initiation. All healthcare expenditure was inflation-adjusted to 2019 dollars using the medical care component of the Consumer Price Index [26].
Covariates
To account for imbalance in patient characteristics in the healthcare utilization and expenditure analysis and potential confounders, we adjusted for key covariates readily available in the claims data: age, sex (male, female, or unknown), race (Asian, Black, Hispanic, White, or unknown), region in the USA (Northeast, South, Midwest, West, or unknown), time since MS cohort entry, and Elixhauser Comorbidity Index (ECI). ECI was computed using the R package “Comorbidity” with all patient-level ICD codes during follow-up. We used age, time since MS cohort entry, and ECI as continuous variables, and other covariates as categorical variables.
Statistical Analysis
We compared the mean treatment duration among DMT groups using Kruskal–Wallis test with post hoc Tukey test. For each treatment group, we calculated the incidence rates per 100 person-years of healthcare utilization activities (outpatient visits, ER visit, hospital admissions, MS-related MRI) as well as relapses and medical complexity. We then calculated the incidence rate ratio (IRR) of a given DMT-treated group during DMT treatment duration, when compared to the reference group of untreated pwMS receiving no DMT (ND) during cohort duration, using negative binomial regression (after confirming data overdispersion) and adjusting for age, sex, race, region, time since MS cohort entry, and ECI. We then estimated the mean cumulative healthcare expenditure at 6 and 12 months using a generalized linear model with the log-link function and gamma distribution, adjusting for age, sex, race, region of residence, time since MS cohort entry, and ECI. We used 0.05 significance level (two-sided) and presented Bonferroni-adjusted p values for multiple comparisons. We performed data analysis using R (version 4.0.3).
Results
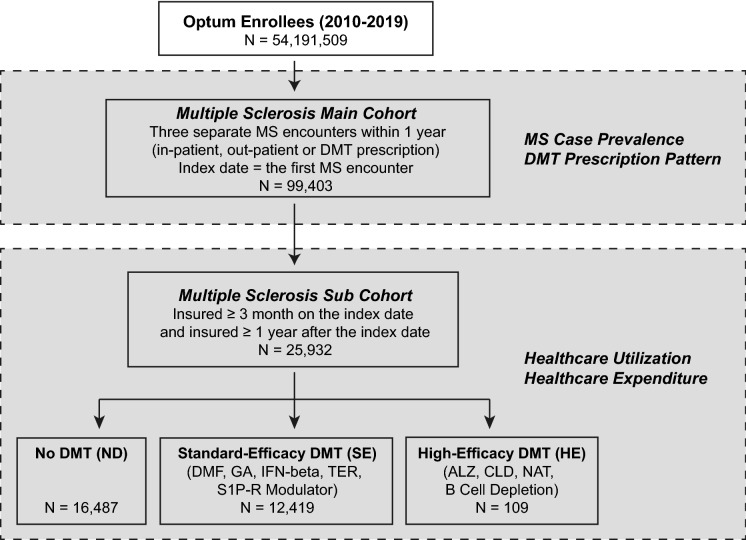
From 2010 to 2019, 99,403 people met the MS case definition from a commercially insured population of more than 54 million people in the USA (Fig. 1). To analyze healthcare utilization and expenditure, we identified a subset of 25,932 pwMS who met additional insurance enrollment criteria, forming the MS subcohort (see Table 1 for demographics). We described the MS case prevalence and DMT prescription pattern in the supplementary results (Fig. S1 and Table S1). Among patients in the subcohort during the follow-up period, 16,487 patients (63.6%) were not treated with any, 6897 patients (26.6%) were treated with only one DMT, 2548 patients (9.8%) were on more than one DMT. Patients who received more than one DMT were counted more than once in the following analysis of healthcare utilization and costs, though the claims attributed to a given DMT treatment were counted only after the treatment was started.
Fig. 1.
Patient selection criteria. DMT disease-modifying therapy, ICD International Classification of Diseases, ALZ alemtuzumab, CLD cladribine, DMF dimethyl fumarate, FGL fingolimod, GA glatiramer acetate, IFN-beta interferon beta (all brands), NAT natalizumab, S1P-R modulator sphingosine-1-phosphate receptor modulator (fingolimod, siponimod), TER teriflunomide. The “No DMT” group includes patients who were not treated with any DMT during the study period. Patients who had been on more than one DMT during the follow-up period belonged to multiple DMT groups, though the claims attributed to a given DMT treatment were counted only after the treatment was started
Table 1.
Study cohort characteristics
| Feature | Comparison between main and subcohort | |
|---|---|---|
| Main cohorta (N = 99,403) | Subcohortb (N = 25,932) | |
| Age, year (SD) | 52.1 (13.8) | 52.8 (12.7)) |
| Gender, n (%) | ||
| Female | 75,002 (75.3) | 19,499 (75.2) |
| Male | 24,541 (24.7) | 6429 (24.8) |
| Unknown | 0 (0.0) | 4 (0.0) |
| Race, n (%) | ||
| Asian | 1302 (1.3) | 380 (1.5) |
| Black | 10,064 (10.1) | 2578 (9.9) |
| Hispanic | 6554 (6.6) | 1887 (7.3) |
| White | 67,237 (67.5) | 18,303 (70.6) |
| Unknown | 14,396 (14.5) | 2784 (10.7) |
| Feature | Comparison among different DMT group | ||
|---|---|---|---|
| No DMT (N = 16,487) | Standard efficacy (N = 12,419) | High efficacy (N = 109) | |
| Age, year (SD) | 57.6 (14.5) | 43.9 (12.0) | 54.0 (11.9) |
| Gender, n (%) | |||
| Female | 12,427 (75.4) | 9380 (75.6) | 83 (76.2) |
| Male | 4058 (24.6) | 3036 (24.4) | 26 (23.8) |
| Unknown | 2 (0.0) | 3 (0.0) | 0 (0.0) |
| Race, n (%) | |||
| Asian | 228 (1.4) | 194 (1.6) | 0 (0.0) |
| Black | 1512 (9.2) | 1400 (11.2) | 14 (12.8) |
| Hispanic | 1116 (6.8) | 1009 (8.1) | 14 (12.8) |
| White | 11,642 (70.6) | 8831 (71.1) | 59 (54.2) |
| Unknown | 1989 (12.0) | 985 (8.0) | 22 (20.2) |
| Cohort duration, days (SD)c | 1342.1 (847.2) | 1490.7 (929.4) | 1828.5 (1040.9) |
| Monotherapy DMT, n (%) | 0 (0.0) | 6856 (55.1) | 51 (48.1) |
| Elixhauser Comorbidity Index, median (IQR)d | 11 (15) | 6 (9) | 10 (12) |
aWe classified an individual as having MS if the person had at least three separate MS-related claims on three separate days within a 1-year period, in any combination of inpatient, outpatient, or DMT-specific pharmacy claims (MS main cohort)
bTo analyze healthcare utilization and expenditure, we then identified from the MS main cohort a subset of 25,932 patients who additionally had continuous enrollment for both medical and pharmacy benefits for at least 3 months preceding and at least 12 months after the index date (MS subcohort). For patients on more than one DMT, the claims attributed to a given DMT treatment were counted only after the treatment was started in analysis of healthcare utilization and costs
cCohort duration was defined as time from the index date until either the termination of insurance coverage at United Healthcare or the end of the study period, whichever occurred first
dElixhauser Comorbidity Index was computed using the R package “comorbidity” and all ICD-9 and ICD-10 codes collected during the follow-up period
Healthcare Utilization According to DMT Efficacy Category
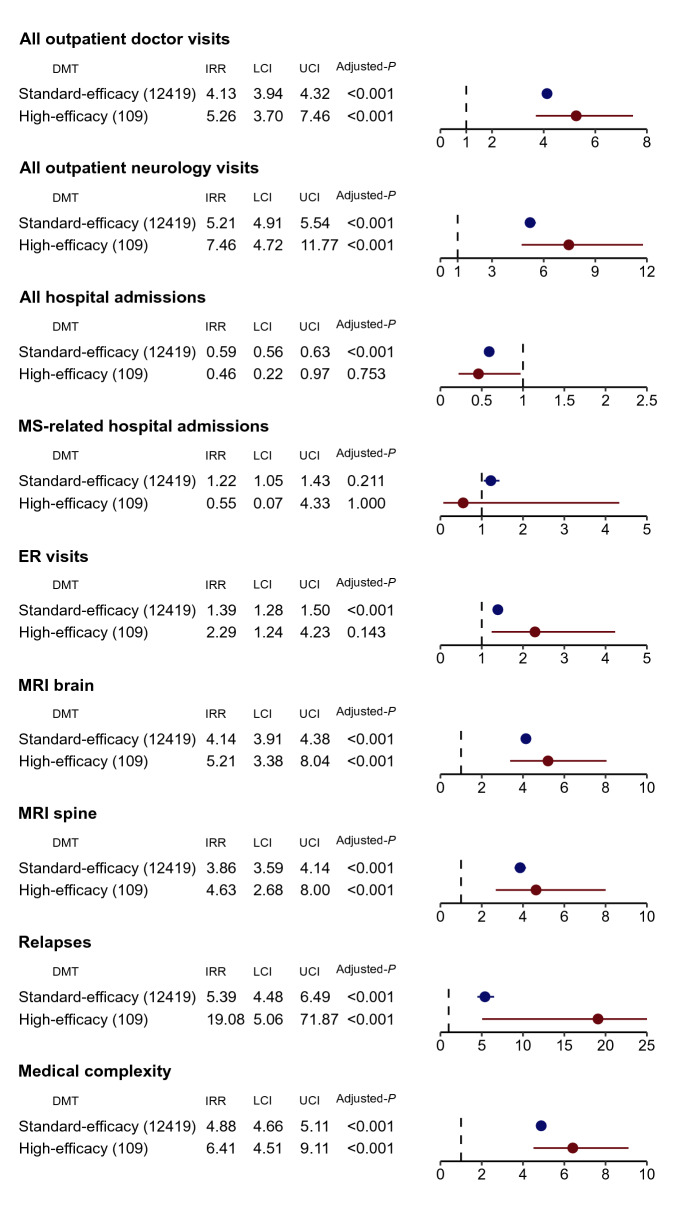
We assessed healthcare utilization of general (outpatient doctor visit of any specialty, ER visit, hospital admission) and MS-related healthcare activities (outpatient neurology visit, MS-related hospital admission with MS as the primary diagnosis, magnetic resonance imaging [MRI] of brain or [cervical or thoracic] spine) as well as acute MS relapses and medical complexity during the study period. To better capture comorbidities, which are known to affect MS outcomes, we included non-MS specific claims for all healthcare utilizations except for MS-related hospital admission, MS-related MRI studies, and acute MS relapses. Figure 2 summarizes the covariate-adjusted IRR of the healthcare utilization according to DMT efficacy categories. Relative to the ND group, pwMS receiving treatment with both HE DMTs and SE DMTs had more overall outpatient doctor visits (IRRHE = 5.26, 95% CI 3.70–7.46; IRRSE = 4.13, 95% CI 3.94–4.32) and more outpatient neurology visits (IRRHE = 7.46, 95% CI 4.72–11.77; IRRSE = 5.21, 95% CI 4.91–5.54) than the untreated. Further, pwMS on HE DMTs had similar ER visits (IRRHE = 2.29, 95% CI 1.24–4.23, padjusted = 0.143) and overall hospital admissions (IRRHE = 0.46, 95% CI 0.22–0.97, padjusted = 0.753) as the untreated, while pwMS on SE DMTs had more ER visits (IRRSE = 1.39, 95% CI 1.28–1.50) but fewer overall hospital admissions (IRRSE = 0.59, 95% CI 0.56–0.63) than the untreated. Notably, neither HE nor SE DMT group differed in MS-related hospitalizations from the ND group (IRRHE = 0.55, 95% CI 0.07–4.33, padjusted = 1.000; IRRSE = 1.22, 95% CI 1.05–1.43, padjusted = 0.211). PwMS on both HE and SE DMTs completed more brain (IRRHE = 5.21, 95% CI 3.38–8.04; IRRSE = 4.14, 95% CI 3.91–4.38) and (cervical and thoracic) spine (IRRHE = 4.63, 95% CI 2.68–8.00; IRRSE = 3.86, 95% CI 3.59–4.14) MRIs than the untreated.
Fig. 2.
Healthcare utilization and outcome across disease-modifying therapy (DMT) efficacy categories in the MS subcohort. Incidence rate ratio (IRR) of healthcare utilization among people with MS (pwMS) prescribed with the high-efficacy (HE) or standard-efficacy (SE) category of DMTs when compared to pwMS not on any DMT (ND) during the study period. We calculated the IRRs of overall outpatient doctor visits, neurology visit, emergency room (ER) visits, overall hospital admissions, hospital admissions for MS, MRI scan of the brain, MRI scan of the (cervical and thoracic) spine, occurrences of relapses, and medical complexity, using negative binomial regression and adjusting to age, sex, race, region of residence, time from MS cohort entry, and the Elixhauser Comorbidity Index. LCI lower boundary of 95% confidence interval, UCI upper boundary of 95% confidence interval, Adjusted P Bonferroni-adjusted p value for nine independent outcomes with two comparisons per outcome
Next, we examined an MS-related and a general health outcome during the follow-up period: (1) occurrence of acute MS relapse using a claims-based algorithm; (2) medical complexity based on the number of prescription classes (Fig. 2). PwMS on HE DMTs had more acute MS relapses (IRRHE = 19.08, 95% CI 5.06–71.87), followed by pwMS on SE DMTs (IRRSE = 5.39, 95% CI 4.48–6.49), relative to the untreated. Notably, pwMS on both HE and SE DMTs had higher medical complexity than untreated pwMS (IRRHE = 6.41, 95% CI 4.51–9.11; IRRSE = 4.88, 95% CI 4.66–5.11). This might explain the higher overall outpatient visits among pwMS on both HE and SE DMTs.
Healthcare Utilization Across Individual DMTs
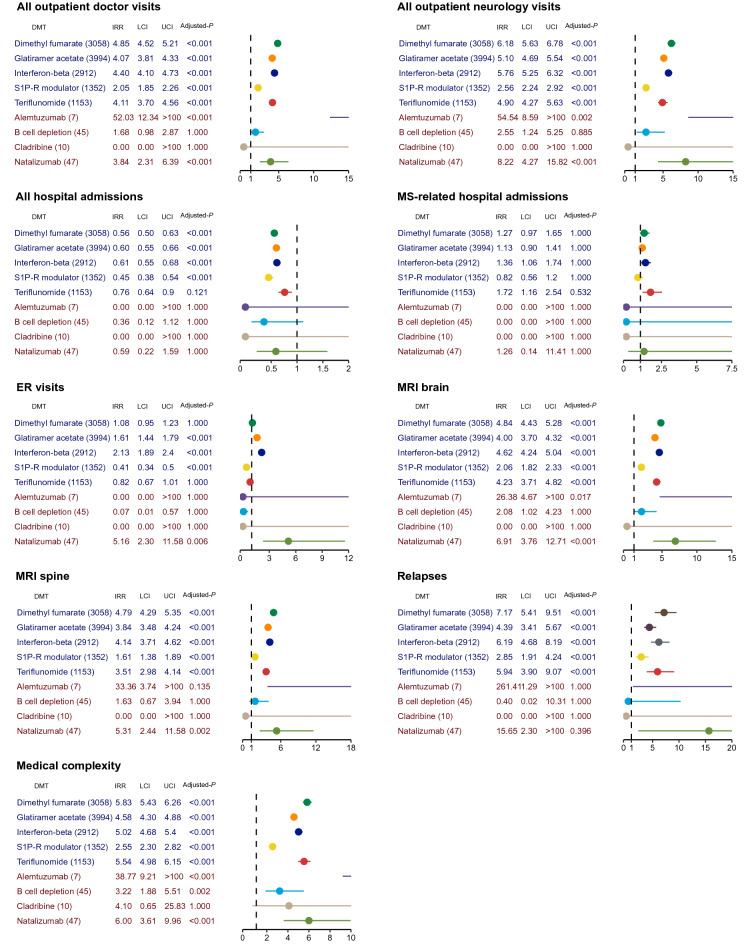
Finally, we assessed the healthcare utilizations across individual DMTs, again using the untreated group as reference (Fig. 3). The findings from pwMS on individual SE DMTs largely mirrored the analysis when dichotomizing DMTs as high or standard efficacy. However, some of the HE DMTs, particularly alemtuzumab (n = 7) and cladribine (n = 10), failed to produce meaningful results because of their modest sample size. Among the SE DMTs, pwMS treated with S1P-R modulator appeared to have the fewest healthcare utilizations in all categories. Moreover, this group was the only group having fewer ER visits (IRR = 0.41, 95% CI 0.34–0.50) than the untreated, in contrast to other SE standard-efficacy DMT groups. Among the HE DMTs, natalizumab seemed to drive the finding of greater overall outpatient doctor visits (IRR = 3.84, 95% CI 2.31–6.39), neurology visits (IRR = 8.22, 95% CI 4.27–15.82), ER visits (IRR = 5.16 95% CI 2.30–11.58), brain (IRR = 6.91, 95% CI 3.76–12.71) and spine (IRR = 5.31, 95% CI 2.44–11.58) MRIs as well as medical complexity (IRR = 6.00, 95% CI 3.61–9.96) of the HE DMT category than the untreated. However, the difference in acute relapses between natalizumab-treated pwMS and the untreated pwMS was not statistically significant (padjusted = 0.396).
Fig. 3.
Healthcare utilization and outcome across individual disease-modifying therapies (DMTs) in the MS subcohort. Incidence rate ratio (IRR) of healthcare utilization among people with MS (pwMS) prescribed with the individual disease-modifying therapy (DMT) when compared to pwMS not on any DMT (ND) during the study period. We calculated the IRRs of overall outpatient doctor visits, neurology visit, emergency room (ER) visits, overall hospital admissions, hospital admissions for MS, MRI scan of the brain, MRI scan of the (cervical and thoracic) spine, acute relapses, and medical complexity, using negative binomial regression and adjusting to age, sex, race, region of residence, time from MS cohort entry, and the Elixhauser Comorbidity Index. LCI lower boundary of 95% confidence interval, UCI upper boundary of 95% confidence interval, Adjusted P Bonferroni-adjusted p value for nine independent outcomes with nine comparisons per outcome
Healthcare Expenditure According to DMT Efficacy Category
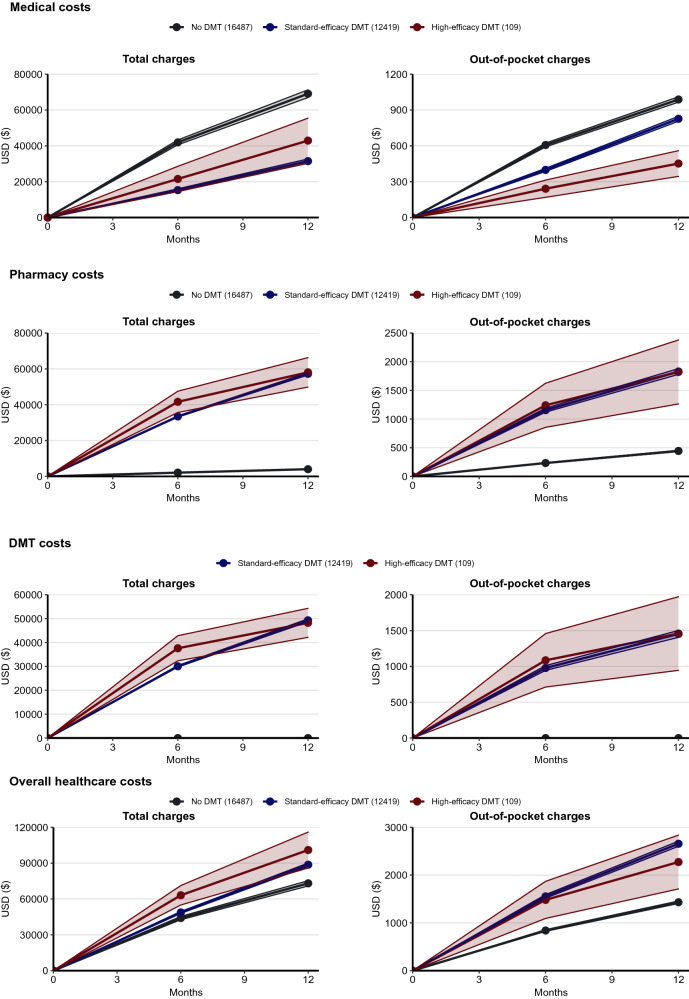
To ascertain the cumulative healthcare expenditure (in US dollars) in the MS subcohort at 6 and 12 months after the study index date for untreated pwMS or after DMT initiation for DMT-treated pwMS, we first estimated the total charges for the healthcare expenditure categories (medical, pharmacy, and overall costs) (Fig. 4).
Fig. 4.
Healthcare expenditure in the MS subcohort. We assessed the mean and 95% confidence interval of healthcare costs among people with MS on high-efficacy DMTs (HE), standard-efficacy DMTs (SE), or no DMT (ND) at 6 months and 12 months after study index date (ND) or DMT initiation date (SE and HE). We obtained four categories of healthcare expenditure (in US dollars per person): medical costs, pharmacy costs, DMT costs (a subset of pharmacy costs), and overall healthcare costs (sum of medical costs and pharmacy costs). For each cost type, we calculated total charges and out-of-pocket charges, the latter by summing copayments, coinsurance, and deductible payments. All healthcare expenditure was inflation-adjusted to 2019 dollars using the medical care component of the Consumer Price Index
For non-pharmacy medical costs, pwMS on SE DMTs consistently had lower mean total charges than the untreated at 6 months (SE $14,418 [95% CI $13,593–15,293]; ND $35,585 [95% CI $28,038–45,158], padjusted < 0.001) and 12 months (SE $34,575 [95% CI $32,815–36,429]; ND $68,204 [95% CI $55,216–84,248], padjusted < 0.001) (Fig. 4, Table S2). While the mean total charges of medical costs of the HE DMT group appeared lower than the ND group at both 6 months (HE $21,089 [95%CI $13,454–33,056], punadjusted = 0.031, padjusted = 0.541) and 12 months (HE $51,115 [95% CI $34,318–76,134], punadjusted = 0.156, padjusted = 1.000), the differences were not statistically significant after correction for multiple testing. Given that hospital care costs accounted for more than 30% of the overall healthcare costs as of 2019 [27], the lower medical costs in the SE DMT group might be due to the fewer overall hospital admissions when compared to the untreated group, whereas the HE DMT and the untreated group did not differ in this measure (IRRSE = 0.59, padjusted < 0.001 and IRRHE = 0.46, padjusted = 0.753).
For pharmacy costs, the mean total charges were substantially higher for pwMS on DMTs than for the untreated pwMS at 6 months (HE $36,604 [95% CI $31,810–42,119], padjusted < 0.001; SE $31,167 [95% CI $27,760–34,992], padjusted < 0.001; versus ND $1977 [95% CI $1725–2266]) and 12 months (HE $48,962 [95% CI $42,535–56,360], padjusted < 0.001; SE $49,232 [95% CI $44,336–54,668], padjusted < 0.001; versus ND $3475 [95% CI $3057–3950]) (Fig. 4, Table S2). To better determine the contribution of DMT to pharmacy costs and overall healthcare costs, we further estimated the mean annual costs for DMTs. The mean total charges for the HE DMTs were $46,523 (95% CI $40,108–53,964) in the first year after DMT initiation, while those for the SE DMTs were $42,022 (95% CI $37,633–46,923). Notably, DMT costs accounted for over 85% of the annual pharmacy costs in the first year after DMT initiation.
For the overall healthcare costs after summing the medical costs and pharmacy costs, the SE DMT group consistently had higher mean total charges at both 6 months ($54,606, 95% CI $52,429–56,872, padjusted < 0.001) and 12 months ($100,910, 95% CI $97,144–104,821, padjusted < 0.001) (Fig. 4, Table S2). While there was no difference between the HE DMT group ($66,878, 95% CI $49,041–91,202, punadjusted = 0.0023, padjusted = 0.074) and the ND group ($41,870, 95% CI $35,520–49,356) at 6 months after correction for multiple testing, the HE DMT group ($115,470, 95% CI $86,397–154,327, padjusted = 0.020) had higher mean total charges than the ND group ($70,399, 95% CI $60,363–82,104) at 12 months. Importantly, pharmacy costs accounted for 71.5% of the overall healthcare costs in the SE DMT group and 65.4% in the HE DMT group 12 months after DMT initiation, in contrast to 10.5% in the ND group during the comparable period.
The patterns of the out-of-pocket charges of the healthcare expenditure categories among the DMT efficacy groups (Fig. 4, Table S2) largely mirrored the total charges.
Healthcare Expenditure Across Individual DMTs
We then assessed the healthcare expenditure across individual DMTs at 12 months after DMT initiation (Table 2). The findings largely mirrored the analysis when dichotomizing DMTs as high or standard efficacy. Given their modest sample size, alemtuzumab (n = 7) and cladribine (n = 10) failed to produce meaningful results as an individual DMT and their results were not reported in Table 2.
Table 2.
Analysis of healthcare expenditure across individual disease-modifying therapies (DMTs) in the MS subcohort
| Cost categorya | Treatment Groupb | 12 months | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total charges (USD)c | Out-of-pocket charges (USD)c | ||||||||
| Mean | 95% CI | Adjusted Pd | Mean | 95% CI | Adjusted Pd | ||||
| Medical costs | No DMT | $68,204 | $55,216 | $84,248 | NA | $988 | $968 | $1009 | NA |
| Dimethyl fumarate | $34,865 | $32,160 | $37,798 | < 0.001 | $803 | $754 | $855 | < 0.001 | |
| Glatiramer acetate | $37,077 | $34,478 | $39,871 | < 0.001 | $887 | $842 | $934 | 0.002 | |
| S1P-R modulator | $33,581 | $30,969 | $37,040 | < 0.001 | $789 | $739 | $842 | < 0.001 | |
| Teriflunomide | $27,873 | $24,895 | $31,207 | < 0.001 | $807 | $737 | $885 | 0.001 | |
| Interferon beta | $42,802 | $37,984 | $48,231 | < 0.001 | $804 | $728 | $887 | 0.002 | |
| B cell depletion | $48,504 | $26,386 | $89,162 | 1.000 | $450 | $187 | $1079 | 1.000 | |
| Natalizumab | $56,946 | $31,926 | $101,575 | 1.000 | $510 | $240 | $1086 | 1.000 | |
| Pharmacy costs | No DMT | $3475 | $3057 | $3950 | NA | $444 | $415 | $475 | NA |
| Dimethyl fumarate | $48,099 | $43,303 | $53,426 | < 0.001 | $1555 | $1434 | $1685 | < 0.001 | |
| Glatiramer acetate | $47,084 | $42,403 | $52,282 | < 0.001 | $1812 | $1681 | $1953 | < 0.001 | |
| S1P-R modulator | $45,677 | $41,118 | $50,742 | < 0.001 | $2078 | $1927 | $2240 | < 0.001 | |
| Teriflunomide | $59,263 | $53,307 | $65,885 | < 0.001 | $1701 | $1554 | $1863 | < 0.001 | |
| Interferon beta | $51,972 | $46,724 | $57,809 | < 0.001 | $2142 | $1,967 | $2332 | < 0.001 | |
| B cell depletion | $43,665 | $36,098 | $52,817 | < 0.001 | $1629 | $1141 | $2327 | < 0.001 | |
| Natalizumab | $45,524 | $38,119 | $54,368 | < 0.001 | $2086 | $1583 | $2748 | < 0.001 | |
| DMT costs | Dimethyl fumarate | $41,119 | $39,632 | $42,661 | NA | $1216 | $1102 | $1342 | NA |
| Glatiramer acetate | $38,666 | $37,355 | $40,024 | NA | $1400 | $1287 | $1523 | NA | |
| S1P-R modulator | $37,985 | $33,968 | $42,477 | NA | $1678 | $1582 | $1779 | NA | |
| Teriflunomide | $52,255 | $49,876 | $54,747 | NA | $1388 | $1232 | $1564 | NA | |
| Interferon beta | $45,710 | $43,497 | $48,036 | NA | $1774 | $1596 | $1972 | NA | |
| B cell depletion | $45,023 | $35,961 | $105,419 | NA | $1277 | $686 | $2377 | NA | |
| Natalizumab | $37,822 | $30,553 | $46,819 | NA | $1656 | $1034 | $2651 | NA | |
| Overall healthcare costs | No DMT | $70,399 | $60,363 | $82,104 | NA | $1432 | $1406 | $1458 | NA |
| Dimethyl fumarate | $101,195 | $95,409 | $107,331 | < 0.001 | $2357 | $2253 | $2467 | < 0.001 | |
| Glatiramer acetate | $100,891 | $95,686 | $106,379 | < 0.001 | $2699 | $2591 | $2811 | < 0.001 | |
| S1P-R modulator | $107,688 | $99,174 | $116,933 | < 0.001 | $2866 | $2737 | $3002 | < 0.001 | |
| Teriflunomide | $114,687 | $105,127 | $125,116 | < 0.001 | $2509 | $2351 | $2678 | < 0.001 | |
| Interferon beta | $95,907 | $90,410 | $101,739 | < 0.001 | $2946 | $2746 | $3160 | < 0.001 | |
| B cell depletion | $103,839 | $66,626 | $161,837 | 1.000 | $2079 | $1474 | $2931 | 0.545 | |
| Natalizumab | $117,491 | $77,058 | $179,139 | 1.000 | $2596 | $1854 | $3634 | 0.030 | |
aUsing study index date (for the untreated patients) or DMT initiation date (for the DMT-treated patients) as the reference date, we obtained four categories of healthcare expenditure (in US dollars per person): medical costs, pharmacy costs, DMT costs (a subset of pharmacy costs), and overall healthcare costs (sum of medical costs and pharmacy costs) at 12 months
bWe compared the differences in medical, pharmacy, DMT costs, and overall healthcare costs among patients on individual DMTs during the study period, using the untreated group as reference
cFor each cost category, we calculated two types of charges: total charges and out-of-pocket charges, the latter by summing copayments, coinsurance, and deductible payments. All healthcare expenditure was inflation-adjusted to 2019 dollars using the medical care component of the Consumer Price Index
dAdjusted P = Bonferroni-adjusted p value for eight independent outcomes with seven comparisons per outcome
First, we evaluated the non-pharmacy medical costs at 12 months after DMT initiation (Table 2). When compared to the untreated pwMS ($68,204, 95% CI $55,216–84,248), pwMS on all SE DMTs had significantly lower mean total charges, with interferon beta having the lowest mean total charges. For the two HE DMTs, the mean total charges in pwMS treated with B cell depletion agent ($48,504, 95% CI $26,386–89,162, padjusted = 1.000) and natalizumab ($56,946, 95% CI $31,926–101,575, padjusted = 1.000) appeared lower than untreated pwMS, but the differences were not statistically significant after correction for multiple testing.
Next, we evaluated pharmacy costs at 12 months after DMT initiation (Table 2). PwMS on all individual DMTs had substantially higher mean total charges than untreated pwMS ($3475, 95% CI $3057–3950). Among the DMTs, the B cell depletion group appeared to have the lowest mean total charges ($43,665, 95% CI $36,098–52,817, padjusted < 0.001 relative to ND), while the group on teriflunomide had the highest mean total charges $59,263, 95% CI $53,307–65,885, padjusted < 0.001 relative to ND).
We further examined the DMT costs at 12 months after DMT initiation (Table 2). DMT costs represent a subset of pharmacy costs. PwMS on natalizumab had the lowest mean total charges ($37,882, 95% CI $30,553–46,819), while pwMS on teriflunomide had the highest mean total charges ($52,255, 95% CI $49,876–54,747).
Finally, we analyzed the overall healthcare costs at 12 months after DMT initiation by summing the medical costs and pharmacy costs (Table 2). Similar to the findings when dichotomizing DMTs as high or standard efficacy, pwMS treated with SE DMTs had higher mean cumulative total than the untreated ($70,399, 95% CI $60,363–82,104). Among the SE DMTs, interferon beta had the lowest mean total charges ($95,907, 95% CI $90,410–101,739, padjusted < 0.001 relative to ND), while teriflunomide had the highest mean total charges ($114,687, 95% CI $105,127–125,116, padjusted < 0.001 relative to ND). The modest sample sizes likely explained the finding that the mean total charges for pwMS on the two HE DMTs (B cell depletion agent and natalizumab) did not significantly differ from the untreated patients.
For the out-of-pocket charges for medical costs, pharmacy costs, and overall healthcare costs among the individual DMT groups (Table 2), the patterns largely mirrored the total charges, while the mean out-of-pocket charges of DMT costs ranged from the lowest (dimethyl fumarate: $1216, 95% CI $1102–1342) to the highest (S1P-R modulator: $1678, 95% CI $1582–1779).
Discussion
In this examination of the healthcare utilization and expenditure patterns from 2010 to 2019 in a large commercially insured MS population in the USA, pwMS on both HE and SE DMTs unsurprisingly had significantly higher healthcare utilizations in overall outpatient doctor visits, neurology visits, MS-related brain and spine MRIs as well as occurrences of acute relapses and medical complexities than the untreated pwMS, after accounting for confounders. Relative to the untreated group, the SE DMT group had significantly fewer overall hospital admissions but more ER visits, while the HE DMT group had similar overall hospital admissions and ER visits. Neither group differed in MS-related hospitalizations from the untreated. Further, pwMS treated with SE and HE DMTs incurred higher pharmacy costs and overall healthcare costs, but a lower (non-pharmacy) or equivalent medical cost compared to the untreated pwMS 12 months after treatment initiation. Our study supports that DMT cost is an important component of the high healthcare cost facing people with MS in the USA.
The study has several strengths. First, we included a large MS population identified using a validated claims-based MS-classification algorithm [20]. The claims data objectively and comprehensively captured DMT prescriptions and healthcare utilizations (during the insured period), avoiding recall bias and missing data. Second, to compare healthcare utilization across DMTs, we used both general and MS-specific healthcare activities. Because these healthcare utilization measures are not equivalent to the standard measures in MS clinical trials, we examined two additional validated claims-based outcomes to better explain the findings: acute relapse occurrence [23, 24] and medical complexity [14, 25]. Third, our study population likely represented a predominantly community-based population in contrast to most observational studies primarily based on data from academic MS centers. The overall low DMT prescription rates support the possibility that most patients in this data set received care at community practices where particularly high-efficacy DMT prescription rates are expected to be lower than academic practices. Findings based on this complementary patient population contribute to the real-world evidence of healthcare utilization and expenditure in pwMS.
We used untreated pwMS during the study period as reference to enable critical comparisons across DMTs. The untreated pwMS likely have different MS disease status and course from those on DMTs. One clue was that the untreated pwMS were older and had fewer acute MS relapses. The significantly older age in the untreated group than the SE DMT group might explain more overall hospitalizations, though not more MS-specific hospitalizations. Alternatively, untreated pwMS might also have inadequate neurological access (independent of health insurance) as the untreated pwMS had significantly fewer outpatient neurology visits and MS-relevant MRIs than DMT-treated pwMS. Findings that incorporated the untreated pwMS (despite the differences when compared to the treated pwMS) yielded a more comprehensive assessment of the healthcare utilization and cost in pwMS.
Although DMT-treated pwMS did not have more MS-related hospital admissions, they consistently used more healthcare resources for MS management, e.g., more outpatient neurology visits and MS-related MRIs, than the untreated. There are two potential explanations. First, DMT-treated pwMS required more frequent monitoring, which was particularly pronounced for those on HE DMTs (e.g., natalizumab, and to a lesser extent B cell depleting agents). Second, pwMS with high disease activity were more likely to receive DMTs, while those with benign disease [28] likely remained untreated. The higher IRR of acute relapses in DMT-treated pwMS, especially in the HE DMT group, again supports this explanation. Our conclusions are not causal given the difficulty to temporally dissociate DMTs and relapse in a relatively short follow-up period.
The mean annual healthcare cost exceeded $70,000 in the first year after MS classification, consistent with prior reports [1]. Importantly, we further reported that the mean annual healthcare cost exceeded $100,000 in the first year after DMT initiation. Previous studies reported that prescription drugs account for 10–15% of overall healthcare expenditure in the general population [29], while pharmacy spending accounts for more than two-thirds of overall healthcare expenditures in pwMS [2, 30, 31]. Comparably, pharmacy costs in this study accounted for 10% of the overall healthcare costs in pwMS not receiving DMT, but more than 65% of the overall healthcare costs in DMT-treated pwMS, with DMT costs accounting for more than 85% of the pharmacy costs. This data set was queried before biosimilars of rituximab (Ruxience, Truxima), fumarates (Vumerity, Bafierta), and S1P-R modulator (Ponvory, Zeposia) became widely prescribed. Future analysis incorporating these additional DMTs will be informative.
In this study, B cell depletion therapy seemed to be associated with lower rates of relapse when compared to SE DMTs, while the healthcare expenditure (medical costs, pharmacy costs, and overall healthcare costs) for this group was comparable to other SE DMTs. Given the increasing interest in early initiation of the HE DMTs in pwMS at risk for worse prognosis, future studies examining the cost-effectiveness of HE DMTs versus SE DMTs as first-line treatment are warranted.
Overall, when examining healthcare utilization in parallel to expenditure, pwMS on DMTs used more MS-related healthcare resources, while DMT prescriptions were associated with lower or equivalent non-pharmacy medical costs (excluding pharmacy costs) but higher overall healthcare costs. DMT costs contributed to the higher pharmacy costs and the higher overall healthcare costs among both HE and SE DMT groups relative to the untreated. Thus, our findings support that DMT cost is a key driver of high healthcare expenditure in MS.
Limitations
This study has limitations. First, while the study population was large, it comprised employed people or dependents and Medicare Advantage recipients with commercial insurance. (Medicare is available to individuals 65 years or older in the USA, while Medicare Advantage offers additional coverage through private insurance.) The findings might not be generalizable to other populations. Second, health insurance continuation (~36% with at least 3 years coverage) and the cohort follow-up duration were relatively short, but comparable to other claims-based studies. To reduce potential bias, we examined the subgroup with a reasonable minimum follow-up duration in the healthcare utilization and cost analysis. Third, while claims data have the unique advantages of comprehensively capturing DMT prescriptions, healthcare utilizations, and expenditures over other data sources, claims data have limited features to allow for full accounting of imbalance in patient characteristics among treatment groups or further exploration of the reasons leading to differential healthcare utilizations across different treatment groups. However, nearly all claims-based MS studies face this limitation. In our analysis, we adjusted for key covariates (age, sex, race, region, time since MS cohort entry, and ECI) readily available from the claims data. Our recent study highlighted the value of incorporating electronic health record (EHR) data as high-dimensional covariates in real-world comparative analysis of MS [32]. In future studies, we will perform integrated analysis of claims and EHR data to compare long-term healthcare utilization, expenditure, and disability (based on computed phenotype from claims and/or EHR data) across the treatment groups after better accounting for confounders. Finally, the costs in this study reflected the covered benefit amount provided by the insurance and owed by the patients but did not include discount mechanisms such as rebates or other cost offsets. Our healthcare cost estimates may be higher than the actual payments.
Conclusion
This large-scale study of commercially insured pwMS highlighted the healthcare utilization patterns that contributed to the high healthcare expenditure. Specifically, DMT cost is an important driver of healthcare expenditure in MS. As more DMTs emerge, there is a need for periodic reassessments of their real-world cost-effectiveness.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
The study has no specific funding. Zongqi Xia is supported by NINDS R01NS098023 and NIH R01NS124882. The corresponding author Zongqi Xia paid for the journal’s Rapid Service Fee.
Author Contributions
Wen Zhu, Kangho Suh, John D. Seeger, and Zongqi Xia contributed to the concept and study design. Wen Zhu and Xiaoyu Tang, contributed to the data acquisition and analysis. Wen Zhu and Zongqi Xia contributed to the drafting of the manuscript and figures. Wen Zhu, Xiaoyu Tang, Rock A. Heyman, Tianxi Cai, Kangho Suh, John D. Seeger, and Zongqi Xia contributed to the editing and revision of the manuscript.
Prior Presentation
Poster presentation at the 2022 Annual Meeting of American Academy of Neurology (Seattle) on April 7, 2022.
Disclosures
Zongqi Xia serves on the scientific advisory board of Roche/Genentech, and has a research agreement with Octave Biosciences, none of which is related to the current study. Wen Zhu serves on the scientific advisory board of Roche/Genentech. John D. Seeger is an employee of Optum. Xiaoyu Tang, Rock A. Heyman, Tianxi Cai, and Kangho Suh have nothing to disclose.
Compliance with Ethics Guidelines
We used anonymous patient-level commercial and Medicare Advantage claims data from Optum Clinformatics from January 1, 2010 to December 31, 2019 through a license to the University of Pittsburgh. The Institutional Review Board of the University of Pittsburgh determined the study as Non-Human Research and waived the need for ethical approval of study protocol and informed consent.
Data Availability
Wen Zhu and Zongqi Xia had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Supplementary material is available on the journal website. Code for analysis and figures is available at https://github.com/wzhupitt/MS-Healthcare-Utilization-and-Expenditure.git. The Optum license does not permit data distribution.
References
- 1.Asche CV, Singer ME, Jhaveri M, Chung H, Miller A. All-cause health care utilization and costs associated with newly diagnosed multiple sclerosis in the United States. J Manag Care Pharm. 2010;16:703–712. doi: 10.18553/jmcp.2010.16.9.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartung DM, Bourdette DN, Ahmed SM, Whitham RH. The cost of multiple sclerosis drugs in the US and the pharmaceutical industry: too big to fail? Neurology. 2015;84:2185–2192. doi: 10.1212/WNL.0000000000001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378:169–180. doi: 10.1056/NEJMra1401483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology. 2018;90:777–788. doi: 10.1212/WNL.0000000000005347. [DOI] [PubMed] [Google Scholar]
- 5.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 6.Kobelt G, Berg J, Atherly D, Hadjimichael O. Costs and quality of life in multiple sclerosis: a cross-sectional study in the United States. Neurology. 2006;66:1696–1702. doi: 10.1212/01.wnl.0000218309.01322.5c. [DOI] [PubMed] [Google Scholar]
- 7.Ernstsson O, Gyllensten H, Alexanderson K, et al. Cost of illness of multiple sclerosis—a systematic review. PLoS One. 2016;11:e0159129. doi: 10.1371/journal.pone.0159129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saguil A, Kane S, Farnell E. Multiple sclerosis: a primary care perspective. Am Fam Physician. 2014;90:644–652. [PubMed] [Google Scholar]
- 9.Hartung DM. Economics and cost-effectiveness of multiple sclerosis therapies in the USA. Neurotherapeutics. 2017;14:1018–1026. doi: 10.1007/s13311-017-0566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carroll CA, Fairman KA, Lage MJ. Updated cost-of-care estimates for commercially insured patients with multiple sclerosis: retrospective observational analysis of medical and pharmacy claims data. BMC Health Serv Res. 2014;14:286. doi: 10.1186/1472-6963-14-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Owens GM. Managed care aspects of managing multiple sclerosis. Am J Manag Care. 2013;19:s307–312. [PubMed] [Google Scholar]
- 12.Bonafede MM, Johnson BH, Watson C. Health care-resource utilization before and after natalizumab initiation in multiple sclerosis patients in the US. Clinicoecon Outcomes Res. 2013;6:11–20. doi: 10.2147/CEOR.S55779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noyes K, Bajorska A, Chappel A, et al. Cost-effectiveness of disease-modifying therapy for multiple sclerosis: a population-based study. Neurology. 2011;77:355–363. doi: 10.1212/WNL.0b013e3182270402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wijnands JMA, Kingwell E, Zhu F, et al. Health-care use before a first demyelinating event suggestive of a multiple sclerosis prodrome: a matched cohort study. Lancet Neurol. 2017;16:445–451. doi: 10.1016/S1474-4422(17)30076-5. [DOI] [PubMed] [Google Scholar]
- 15.Sharac J, McCrone P, Sabes-Figuera R. Pharmacoeconomic considerations in the treatment of multiple sclerosis. Drugs. 2010;70:1677–1691. doi: 10.2165/11538000-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 16.Ng HS, Zhu F, Kingwell E, et al. Disease-modifying drugs for multiple sclerosis and subsequent health service use. Mult Scler. 2021 doi: 10.1177/13524585211063403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobelt G, Thompson A, Berg J, et al. New insights into the burden and costs of multiple sclerosis in Europe. Mult Scler. 2017;23:1123–1136. doi: 10.1177/1352458517694432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Institute for Clinical and Economic Review. Disease-modifying therapies for relapsing remitting and primary-progressive multiple sclerosis: effectiveness and value. 2017. https://icer.org/assessment/multiple-sclerosis-2017. Accessed 14 Mar 2022.
- 19.Seeger J, Daniels G. Commercial databases. In: Strom B, Hennessey S, Kimmel S, editors. Pharmacoepidemiology. 5. Chichester: Wiley; 2012. pp. 189–208. [Google Scholar]
- 20.Culpepper WJ, Marrie RA, Langer-Gould A, et al. Validation of an algorithm for identifying MS cases in administrative health claims datasets. Neurology. 2019;92:e1016–e1028. doi: 10.1212/WNL.0000000000007043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harding K, Williams O, Willis M, et al. Clinical outcomes of escalation vs early intensive disease-modifying therapy in patients with multiple sclerosis. JAMA Neurol. 2019;76:536–541. doi: 10.1001/jamaneurol.2018.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chastek BJ, Oleen-Burkey M, Lopez-Bresnahan MV. Medical chart validation of an algorithm for identifying multiple sclerosis relapse in healthcare claims. J Med Econ. 2010;13:618–625. doi: 10.3111/13696998.2010.523670. [DOI] [PubMed] [Google Scholar]
- 23.Chen C, Wu N, Watson C. Multiple sclerosis patients who are stable on interferon therapy show better outcomes when staying on same therapy than patients who switch to another interferon. Clinicoecon Outcomes Res. 2018;10:723–730. doi: 10.2147/CEOR.S163907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freeman L, Kee A, Tian M, Mehta R. Retrospective claims analysis of treatment patterns, relapse, utilization, and cost among patients with multiple sclerosis initiating second-line disease-modifying therapy. Drugs Real World Outcomes. 2021 doi: 10.1007/s40801-021-00251-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higdon R, Stewart E, Roach J, et al. Predictive analytics in healthcare: medications as a predictor of medical complexity. Big Data. 2013;1:237–244. doi: 10.1089/big.2013.0024. [DOI] [PubMed] [Google Scholar]
- 26.Dunn A, Grosse SD, Zuvekas SH. Adjusting health expenditures for inflation: a review of measures for health services research in the United States. Health Serv Res. 2018;53:175–196. doi: 10.1111/1475-6773.12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sisko AM, Keehan SP, Poisal JA, et al. National health expenditure projections, 2018–27: economic and demographic trends drive spending and enrollment growth. Health Aff (Millwood) 2019;38:491–501. doi: 10.1377/hlthaff.2018.05499. [DOI] [PubMed] [Google Scholar]
- 28.Correale J, Peirano I, Romano L. Benign multiple sclerosis: a new definition of this entity is needed. Mult Scler. 2012;18:210–218. doi: 10.1177/1352458511419702. [DOI] [PubMed] [Google Scholar]
- 29.Hartung DM. Health economics of disease-modifying therapy for multiple sclerosis in the United States. Ther Adv Neurol Disord. 2021;14:1756286420987031. doi: 10.1177/1756286420987031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim Y, Krause TM, Blum P, Freeman L. Disease modifying therapies continue to drive up health care cost among individuals with multiple sclerosis. Mult Scler Relat Disord. 2019;30:69–75. doi: 10.1016/j.msard.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 31.San-Juan-Rodriguez A, Good CB, Heyman RA, et al. Trends in prices, market share, and spending on self-administered disease-modifying therapies for multiple sclerosis in medicare part D. JAMA Neurol. 2019 doi: 10.1001/jamaneurol.2019.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hou J, Kim N, Cai T, et al. Comparison of dimethyl fumarate vs fingolimod and rituximab vs natalizumab for treatment of multiple sclerosis. JAMA Netw Open. 2021;4:e2134627. doi: 10.1001/jamanetworkopen.2021.34627. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Wen Zhu and Zongqi Xia had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Supplementary material is available on the journal website. Code for analysis and figures is available at https://github.com/wzhupitt/MS-Healthcare-Utilization-and-Expenditure.git. The Optum license does not permit data distribution.